Abstract
Site-specific conjugation technologies enable the production of homogeneous antibody-drug conjugates (ADCs) with improved therapeutic indices compared to conventional ADCs. However, current site-specific conjugation methods can only attach one type of drug to a single antibody. Given the emergence of acquired resistance to current ADCs, arming single antibodies with different drugs may provide an attractive option in the development of next-generation ADCs. Here, we describe a site-specific dual conjugation strategy as a platform for dual warhead ADCs.
Keywords: Antibody-drug conjugates, selenocysteine, THIOMAB, dual antibody conjugation, site-specific
Graphical abstract
INTRODUCTION
Antibody-drug conjugates (ADCs) are powerful “smart bombs” which deliver highly potent cytotoxic drugs directly to targeted tumor tissues while avoiding other healthy tissues 1–3. An ADC is comprised of three components: a monoclonal antibody, a cytotoxic drug, and a linker that connects antibody and drug. All three components play critical roles in the generation of clinically successful ADCs 3, 4. Additionally, conjugation strategies which determine the stoichiometry, drug loading, and placement of conjugation sites can greatly influence therapeutic indices and outcomes 5, 6.
Conventional lysine or inter-chain cysteine (Cys) conjugation methods generate heterogeneous products, which have variable pharmacokinetics, pharmacodynamics, affinity, and toxicity profiles 6–8. In addition, maintaining batch-to-batch consistency is a challenge to regulation and manufacturing of ADCs. To overcome these limitations, a number of site-specific conjugation methods have been developed to achieve homogeneous ADCs 9, 10. Among these methods, the THIOMAB technology introduces Cys residues at certain positions in the heavy or light chains of antibodies. Drugs were specifically conjugated to the engineered Cys without disruption of the structural disulfide bonds 11. THIOMAB-drug conjugates displayed equivalent efficacy and greater safety than conventional ADCs and therefore have an improved therapeutic index 11–13. We previously reported site-specific antibody conjugation through an engineered selenocysteine (Sec) residue 14, 15. Sec, the 21st natural amino acid 16, is structurally identical to Cys except for containing a selenium atom in place of the sulfur atom. The selenolate group has a pKa of 5.2 and is a more reactive nucleophile than its thiolate counterpart (pKa 8.3). The different chemical reactivities of Sec and Cys prompted us to investigate a combination of THIOMAB and SELENOMAB technology for site-specific dual conjugation of antibodies (THIO-SELENOMABs).
Most treated patients develop acquired drug resistance to trastuzumab emtansine 17, an FDA-approved ADC for the treatment of patients with HER2-positive metastatic breast cancer. While the exact mechanisms of resistance are still under investigation, recent studies reveal that ADC-resistant tumor cells retain sensitivity to other ADCs and standard-of-care chemotherapy 18, 19. Therefore, arming a monoclonal antibody with multiple drugs that have different mechanisms of action is a promising approach for improving the potency of ADCs and preventing drug resistance. The THIO-SELENOMAB dual labeling technology we present here provides a method for site-specific conjugation of two different drugs to the same antibody, affording an antibody engineering platform for next-generation ADCs.
RESULTS AND DISCUSSION
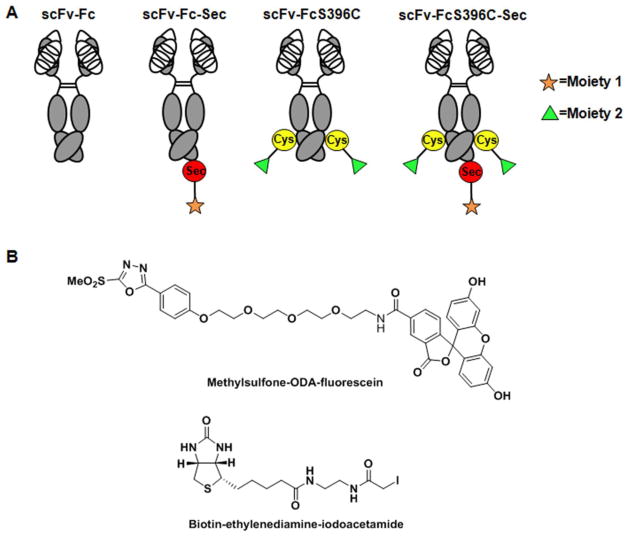
To make a dual labeled antibody, we introduced Sec at the C-terminus of trastuzumab scFv-FcS396C THIOMAB. The resulting antibody is scFv-FcS396C-Sec (THIO-SELENOMAB). To evaluate the specificity of dual conjugation, trastuzumab scFv-FcS396C THIOMAB, scFv-Fc-Sec SELENOMAB, and scFv-Fc were included as control antibodies (Figure 1A). THIO-SELENOMAB was expected to site-specifically conjugate to compounds containing two different moieties under optimized conjugation conditions, while control antibodies THIOMAB and SELENOMAB should conjugate to only one of two compounds, and trastuzumab scFv-Fc should conjugate to neither one (Figure 1A). To analyze the effectiveness of dual conjugation, compounds containing two different detectable reporter groups, biotin and fluorescein, were used for labeling. We previously reported that a methylsulfone phenyloxadiazole (ODA) linker site-specifically labeled engineered Cys and Sec residues in THIOMAB and SELENOMAB 20. Sulfone conjugates showed improved human plasma stability relative to maleimide conjugates, especially at sites with high predicted fractional solvent accessibility such as FcS396C 20. Therefore, methylsulfone-ODA-fluorescein was used as one of the labeling compounds in the current study (Figure 1B). Based on our previous studies that revealed that iodoacetamide derivatives can be specifically and efficiently conjugated to Sec residues in SELENOMAB 14, 21, biotin-ethylenediamine-iodoacetamide was used as the other labeling compound (Figure 1B).
Figure 1.
(A) HER2-targeting monoclonal antibody trastuzumab in scFv-Fc format was engineered with or without selenocysteine (Sec) or cysteine (Cys) resulting in four different constructs, scFv-Fc, scFv-Fc-Sec, scFv-FcS396C, and scFv-FcS396C-Sec. Engineered Sec and Cys enabled site-specific modification of the antibody with dual modalities. (B) Structures of methylsulfone-ODA-fluorescein and biotin-ethylenediamine-iodoacetamide.
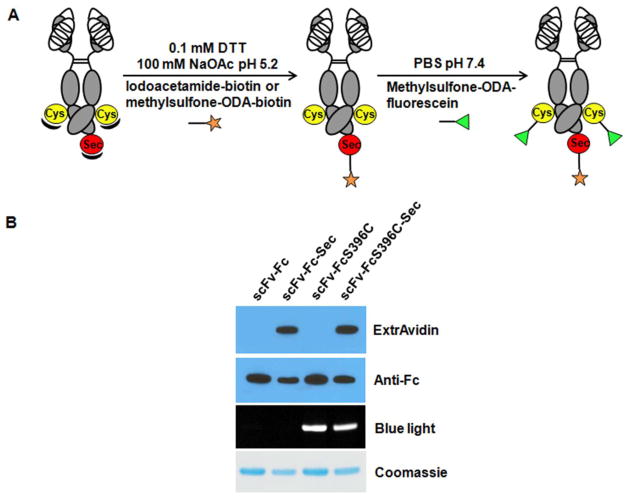
We previously demonstrated that engineered Fc-Sec, IgG-Sec, Fab-Sec, and scFv-Fc-Sec display unique chemical reactivity, allowing selective conjugation at the Sec interface under mildly acidic and reducing conditions 14, 15, 21. Here, using these conjugation conditions, we first labeled the antibodies at the Sec residue with biotin-ethylenediamine-iodoacetamide at pH 5.2 in the presence of 0.1 mM dithiothreitol (DTT). Next, we simultaneously removed excess DTT and unreacted biotin-ethylenediamine-iodoacetamide while changing the conjugation conditions to make them suitable for THIOMAB labeling with methylsulfone-ODA-fluorescein at the engineered Cys residues (FcS396C) (Figure 2A).
Figure 2.
(A) Reaction scheme for sequential modification of scFv-FcS396C-Sec with dual modalities. Either iodoacetamide or methylsulfone ODA derivatives of biotin can be used in the first reaction. Mass spectrometry analysis revealed that the engineered free Cys and Sec residues in scFv-FcS396C-Sec are shielded prior to addition of DTT. Note that excess DTT and unreacted biotin derivatives are simultaneously removed in an intermediate purification step prior to the second reaction. (B) Western blot and SDS-PAGE analysis of biotin and fluorescein labeled antibody conjugates. All four antibodies were subjected to the conjugation conditions shown in (A). Biotin-ethylenediamine-iodoacetamide was used in the first step and During cell culture, the engineered Cys residues in THIOMAB are usually capped with a methylsulfone-ODA-fluorescein in the second step. HRP-conjugated ExtrAvidin and donkey anti-human IgG Fcγ pAbs were employed to detect biotin labeling (top two lanes). Fluorescent and Coomassie-stained gels were used to detect fluorescein labeling (bottom two lanes).
During expression, the engineered Cys residues in THIOMAB are usually capped with a cysteine, a glutathione, or a homocysteine through a disulfide bond 11, 22. This may also apply to engineered Sec residues in SELENOMAB. Therefore, THIOMAB labeling requires reducing the antibody first to remove the attached cap. However, unlike SELENOMAB conjugation, the reducing reagent must be removed before adding electrophilic compounds to label the THIOMAB 11. Our dual labeling strategy removes reducing agent and the first unconjugated compound simultaneously. In contrast to the reported THIOMAB labeling procedure 11, no additional step is required. Moreover, in the first step of our conjugation sequence, other electrophilic groups including those with poor reactivity for Cys residues can also be used due to the fact that selenolates are 100–1000 fold more nucleophilic than thiolates 20, 23, 24.
The reporter groups we employed facilitated an analysis of the specificity for dual conjugation. Only antibody conjugates containing Sec were detectable by Western blotting using horse radish peroxidase (HRP)-conjugated ExtrAvidin, indicating that biotin was site-specifically conjugated at the Sec residues (Figure 2B upper panel). Furthermore, analyzing the fluorescence of antibody conjugates following SDS-PAGE, we confirmed that only THIOMAB and THIO-SELENOMAB were labeled in the second step (Figure 2B lower panel).
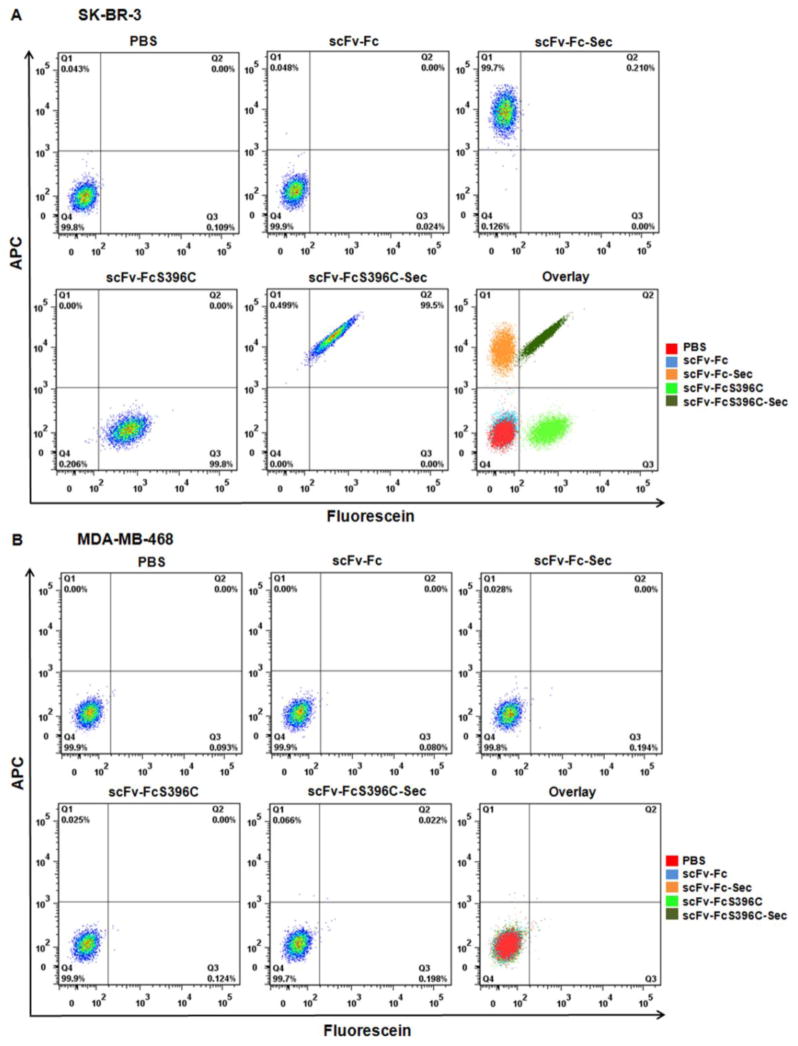
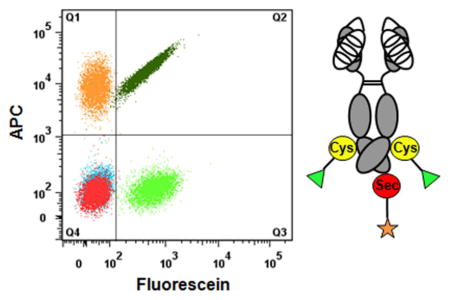
Both THIOMAB and SELENOMAB maintained antigen recognition activity and high affinity after conjugation in our previous studies 20, 21. We next evaluated the antigen binding activity of dual labeled THIO-SELENOMAB on HER2-positive SK-BR-3 and HER2-negative MDA-MB-468 cells by flow cytometry using APC-conjugated streptavidin. SK-BR-3 cells stained with dual labeled THIO-SELENOMAB were detected in both APC and fluorescein channels, confirming the presence of both biotin and fluorescein. Dual labeled THIO-SELENOMAB bound to SK-BR-3 cells with an avidity similar to single labeled THIOMAB and SELENOMAB (Figure 3A). As expected, MDA-MB-468 cells stained with each antibody or antibody conjugate were not detected by either color (Figure 3B). We used surface plasmon resonance to confirm that dual labeled THIO-SELENOMAB and unmodified parental antibody bind HER2 with comparable avidities (Figure S1 and Table S1).
Figure 3.
Flow cytometry analyses of the staining for HER2-positive SK-BR-3 cells (A) and HER2-negative MDA-MB-468 cells (B) with antibody conjugates and controls.
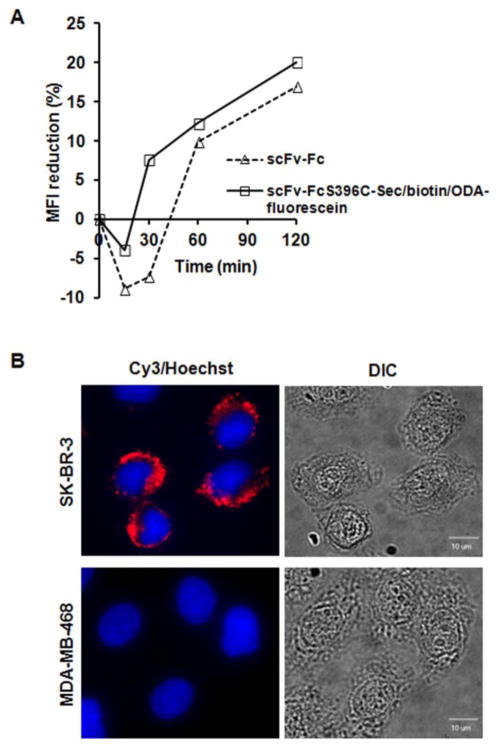
To investigate whether dual conjugation influences internalization, which is a critical parameter of payload delivery, we used flow cytometry and confocal immunofluorescence microscopy. Compared to unmodified parental antibody, the internalization rate of dual labeled THIO-SELENOMAB was slightly increased (Figure 4A). Internalized dual labeled THIO-SELENOMAB was detectable in HER2-positive SK-BR-3 cells but not in HER2-negative MDA-MB-468 cells (Figure 4B).
Figure 4.
Internalization of dual labeled THIO-SELENOMAB. (A) HER2-positive SK-BR-3 cells were incubated with 20 ng/ml of unlabeled scFv-Fc or dual labeled scFv-FcS396C-Sec/biotin/ODA-fluorescein at 37°C at the indicated time points. The mean fluorescence intensity (MFI) of the cells was measured by flow cytometry using an APC-conjugated goat anti-human Fcγ antibody. (B) SK-BR-3 and MDA-MB-468 cells were incubated with 2 μg/ml dual labeled scFv-FcS396C-Sec/biotin/ODA-fluorescein at 37 °C for 4 h. Internalized antibody was detected by Cy3-conjugated goat anti-human Fcγ antibody. Cell nuclei were stained with Hoechst 33342. DIC represents the image from differential interference contrast.
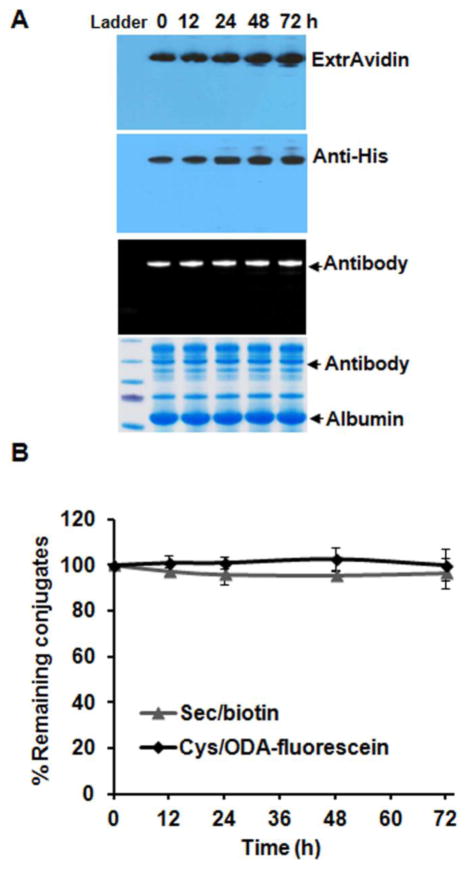
The stability of an ADC in circulation greatly influences its safety and efficacy 25. We previously reported a significant improvement of the plasma stability of THIOMAB sulfone conjugates relative to maleimide conjugates 20. Demonstrating the robustness of both methylsulfone ODA conjugation to Cys and iodoacetamide conjugation to Sec, the dual labeled THIO-SELENOMAB revealed excellent stability in human plasma without significant decay after three days (Figures 5A and 5B).
Figure 5.
Stability of dual labeled THIO-SELENOMAB in human plasma. (A) Western blot and SDS-PAGE analysis of scFv-FcS396C-Sec/biotin/ODA-fluorescein in human plasma at 37°C at the indicated time points. HRP-conjugated ExtrAvidin and a mouse anti-His mAb were employed to detect the stability of biotin labeling (top two lanes). Fluorescent and Coomassie-stained gels were used to detect the stability of fluorescein labeling (bottom two lanes). (B) Quantitative analysis of the band intensity in (A) using Image J software.
To further characterize the conjugated antibody by MALDI-TOF mass spectrometry, we synthesized a biotin iodoacetamide derivative with a long PEG linker (Figure S2). This compound has a molecular weight of 1,645 Da and was used to increase the accuracy of mass analysis compared to biotin-ethylenediamine-iodoacetamide (454 Da). Because of the cellular production and secretion process, as discussed above, the unpaired Cys and Sec residues in our engineered antibodies may be capped by cysteine, glutathione, or homocysteine 11, 22. The mass spectrometry analysis of unconjugated THIO-SELENOMAB indicated that this antibody is capped with one cysteine at each engineered Cys and Sec residue, given that the observed mass is 372 Da larger than its calculated value, which is close to the mass of the antibody attached to three cysteines (Figure S3A). After conjugation to biotin-PEG-iodoacetamide, the antibody gained the molecular weight of one biotin-PEG (Figure S3B). As expected, one molecule of biotin-PEG conjugated antibody added two molecules of ODA-fluorescein in the second step of our conjugation sequence (Figure S3C). Notably, both fluorescence analysis after SDS-PAGE and MALDI-TOF suggested that the elongated biotin-PEG-iodoacetamide compound (unlike biotin-ethylenediamine-iodoacetamide) conjugated in the first step sterically blocked the conjugation of methylsulfone-ODA-fluorescein in the second step (data not shown). Accordingly, when attaching cytotoxic drugs to THIO-SELENOMABs, careful consideration should be given to the linker and to the spacing of Cys and Sec residues.
CONCLUSIONS
Utilizing the unique chemical reactivity of selenocysteine relative to cysteine, we developed a site-specific THIO-SELENOMAB dual labeling technology. Proof-of-concept experiments demonstrated that scFv-FcS396C-Sec THIO-SELENOMAB was site-specifically labeled in two steps without compromising avidity and internalization. Furthermore, the dual labeled antibody revealed excellent stability in human plasma. Collectively, our technology provides a robust strategy for the attachment of two different drugs to the same antibody, which broadens the scope of ADC technologies.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (U01 CA174844 and R01 CA181258), the Lymphoma Research Foundation, and the Klorfine Foundation. We thank Alex R. Nanna and Henry D. Wilson for comments on the manuscript and Dr. Haiyong Peng for assistance with the Biacore X100 instrument. This is manuscript 29100 from The Scripps Research Institute.
Footnotes
Dedicated to the memory of Carlos F. Barbas III
NOTES
The authors declare no competing financial interests.
ASSOCIATED CONTENT
Experimental procedures are available free of charge via the internet at http://pubs.acs.org.
References
- 1.Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl. 2014;53:3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 2.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 3.Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. doi: 10.1016/j.cbpa.2010.06.170. [DOI] [PubMed] [Google Scholar]
- 4.Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjugate chem. 2010;21:5–13. doi: 10.1021/bc9002019. [DOI] [PubMed] [Google Scholar]
- 5.Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, Ho WH, Farias S, Casas MG, Abdiche Y, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20:161–167. doi: 10.1016/j.chembiol.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10:7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Amphlett G, Blattler WA, Lambert JM, Zhang W. Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci. 2005;14:2436–2446. doi: 10.1110/ps.051478705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boswell CA, Mundo EE, Zhang C, Bumbaca D, Valle NR, Kozak KR, Fourie A, Chuh J, Koppada N, Saad O, et al. Impact of drug conjugation on pharmacokinetics and tissue distribution of anti-STEAP1 antibody-drug conjugates in rats. Bioconjugate Chem. 2011;22:1994–2004. doi: 10.1021/bc200212a. [DOI] [PubMed] [Google Scholar]
- 9.Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal P, Bertozzi CR. Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjugate Chem. 2015;26:176–192. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 12.Junutula JR, Flagella KM, Graham RA, Parsons KL, Ha E, Raab H, Bhakta S, Nguyen T, Dugger DL, Li G, et al. Engineered thio-trastuzumab-DM1 conjugate with an improved therapeutic index to target human epidermal growth factor receptor 2-positive breast cancer. Clin Cancer Res. 2010;16:4769–4778. doi: 10.1158/1078-0432.CCR-10-0987. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey SC, Burke PJ, Lyon RP, Meyer DW, Sussman D, Anderson M, Hunter JH, Leiske CI, Miyamoto JB, Nicholas ND, et al. A potent anti-CD70 antibody- drug conjugate combining a dimeric pyrrolobenzodiazepine drug with site-specific conjugation technology. Bioconjugate Chem. 2013;24:1256–1263. doi: 10.1021/bc400217g. [DOI] [PubMed] [Google Scholar]
- 14.Hofer T, Thomas JD, Burke TR, Jr, Rader C. An engineered selenocysteine defines a unique class of antibody derivatives. Proc Natl Acad Sci USA. 2008;105:12451–12456. doi: 10.1073/pnas.0800800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer T, Skeffington LR, Chapman CM, Rader C. Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry. 2009;48:12047–12057. doi: 10.1021/bi901744t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 2014;39:112–120. doi: 10.1016/j.tibs.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barok M, Joensuu H, Isola J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 2014;16:209. doi: 10.1186/bcr3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loganzo F, Tan X, Sung M, Jin G, Myers JS, Melamud E, Wang F, Diesl V, Follettie MT, Musto S, et al. Tumor cells chronically treated with a trastuzumab- maytansinoid antibody-drug conjugate develop varied resistance mechanisms but respond to alternate treatments. Mol Cancer Thet. 2015;14:952–963. doi: 10.1158/1535-7163.MCT-14-0862. [DOI] [PubMed] [Google Scholar]
- 19.Yu SF, Zheng B, Go MA, Lau J, Spencer S, Raab H, Soriano R, Jhunjhunwala S, Cohen R, Caruso M, et al. A novel anti-CD22 anthracycline-based antibody-drug conjugate (ADC) that overcomes resistance to auristatin based ADCs. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-14-2035. [DOI] [PubMed] [Google Scholar]
- 20.Patterson JT, Asano S, Li X, Rader C, Barbas CF., 3rd Improving the serum stability of site-specific antibody conjugates with sulfone linkers. Bioconjugate Chem. 2014;25:1402–1407. doi: 10.1021/bc500276m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Yang J, Rader C. Antibody conjugation via one and two C-terminal selenocysteines. Methods. 2014;65:133–138. doi: 10.1016/j.ymeth.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dengl S, Hoffmann E, Grote M, Wagner C, Mundigl O, Georges G, Thorey I, Stubenrauch KG, Bujotzek A, Josel HP, et al. Hapten-directed spontaneous disulfide shuffling: a universal technology for site-directed covalent coupling of payloads to antibodies. FASEB J. 2015;29:1763–1779. doi: 10.1096/fj.14-263665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B, Ge C, Yao J, Liu Y, Xie H, Fang J. Selective selenol fluorescent probes: design, synthesis, structural determinants, and biological applications. J Am Chem Soc. 2015;137:757–769. doi: 10.1021/ja5099676. [DOI] [PubMed] [Google Scholar]
- 24.Steinmann D, Nauser T, Koppenol WH. Selenium and sulfur in exchange reactions: a comparative study. J Org Chem. 2010;75:6696–6699. doi: 10.1021/jo1011569. [DOI] [PubMed] [Google Scholar]
- 25.Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, Parsons-Reponte KL, Tien J, Yu SF, Mai E, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30:184–189. doi: 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.