Abstract
Fibroblast growth factor 23 (FGF23) circulates as active protein and inactive fragments. Low iron status increases FGF23 gene expression, and iron deficiency is common. We hypothesized that in healthy premenopausal women, serum iron influences C-terminal and intact FGF23 concentrations, and that iron and FGF23 associate with bone mineral density (BMD).
Serum iron, iron binding capacity, percent iron saturation, phosphorus, and other biochemistries were measured in stored fasting samples from healthy premenopausal white (n=1898) and black women (n= 994), age 20–55 years. Serum C-terminal and intact FGF23 were measured in a subset (1631 white and 296 black women). BMD was measured at the lumbar spine and femur neck.
Serum phosphorus, calcium, alkaline phosphatase and creatinine were lower in white women than black women (p<0.001). Serum iron (p<0.0001) and intact FGF23 (p< 0.01) were higher in white women. C-terminal FGF23 did not differ between races. Phosphorus correlated with intact FGF23 (white women, r=0.120, p<0.0001; black women r=0.163, p<0.01). However, phosphorus correlated with C-terminal FGF23 only in black women (r=0.157, p<0.01). Intact FGF23 did not correlate with iron. C-terminal FGF23 correlated inversely with iron (white women r=−0.134, p<0.0001; black women r=−0.188, p<0.01), having a steeper slope at iron <50 mcg/dl than >50 mcg/dl. Longitudinal changes in iron predicted changes in C-terminal FGF23.
Spine BMD correlated with iron negatively (r=−0.076, p<0.01) in white women; femur neck BMD correlated with iron negatively (r=−0.119, p<0.0001) in black women. Both relationships were eliminated in weight-adjusted models. BMD did not correlate with FGF23.
Serum iron did not relate to intact FGF23, but was inversely related to C-terminal FGF23. Intact FGF23 correlated with serum phosphorus. In weight-adjusted models, BMD was not related to intact FGF23, C-terminal FGF23 or iron. The influence of iron on FGF23 gene expression is not important in determining bone density in healthy premenopausal women.
Keywords: iron, fibroblast growth factor 23, FGF23, bone mineral density, DXA, premenopausal women
1. Introduction
Fibroblast growth factor 23 (FGF23) is a hormone produced in osteocytes, which in the renal tubule decreases expression of sodium phosphate co-transporters and of vitamin D 1α-hydroxylase, thus decreasing phosphorus reabsorption and serum phosphorus and 1,25-dihydroxyvitamin D [1,25(OH)2D] levels(1). High serum phosphorus and 1,25(OH)2D increase production of FGF23, providing regulation via a feedback loop. FGF23 is secreted both as inactive fragments and as active intact protein (2). Serum FGF23 is currently measured using two different assays: an assay detecting only the biologically active intact FGF23 (intact assay) and an assay that detects the combination of intact hormone plus biologically inactive C-terminal fragments (C-terminal assay)(3, 4). Disorders of increased intact FGF23 cause hypophosphatemic rickets/osteomalacia (5–7), while disorders of decreased intact FGF23 cause hyperphosphatemia with ectopic calcifications(2, 8). FGF23 has been proposed also to directly impair skeletal mineralization, independent of hypophosphatemia(9). Studies on the influence of FGF23 on bone mineral density (BMD), independent of its effect on phosphorus and bone mineralization, have produced conflicting results. Studies investigating the relationships of FGF23 on BMD and/or fracture risk in post-menopausal women or older men have reported either a positive or no relationship between C-terminal or intact FGF23 and lumbar spine or proximal femur BMD (10–14).
In autosomal dominant hypophosphatemic rickets (ADHR), a genetic mutation causes FGF23 to resist proteolytic cleavage(15). Disease activity waxes and wanes, with high intact FGF23 and hypophosphatemia occurring during times of low serum iron concentrations (3, 7). Low iron status increases FGF23 gene expression (3, 4), and in the absence of a mutation, intact FGF23 concentrations remain normal as measured by the intact assay, but C-terminal fragments increase as detected by the C-terminal assay (3, 4). Low iron status is associated with high incidence of stress fractures in females (16). On the other hand, iron overload, such as occurs in hemochromatosis, is associated with osteoporosis, even in the absence of hypogonadism which commonly occurs in hemochromatosis(17). Furthermore, in post-menopausal women, higher quartiles of ferritin, a marker of higher iron stores, are associated with lower bone mineral density (BMD) and greater risk of vertebral fracture(18, 19). The relationships among iron status, serum FGF23 levels and BMD in premenopausal women remain to be established.
If iron influences BMD, one possible mechanism could be through changes in serum intact FGF23 or its C-terminal fragments. We sought to test the following hypotheses in healthy premenopausal white and black women: 1) Serum iron concentration is negatively associated with both C-terminal and intact FGF23 concentrations; and 2) Serum iron and FGF23 concentrations are associated with BMD at the proximal femur and lumbar spine (L2–4) in both white and black women.
2. Materials and Methods
2.1 Subjects
Healthy premenopausal adult white and black women in Indiana were recruited into a study to discover genes and other predictors underlying peak bone mass(20). Serum iron and FGF23 concentrations and additional mineral metabolism biochemistries related to FGF23 activity were measured in samples from 2,892 subjects, age 20–55 years. Both white women and black women were assessed due to racial differences in BMD. Due to cost, FGF23 was measured in a subset of white women who had been part of a previous GWAS(20). FGF23 was measured in a subset of the black women to identify racial differences and validate the FGF23 iron relationships in black women. Serum samples from return visits (median 5.7 years) (21) were available in 356 white women for measures of changes in iron and FGF23. This research was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Indiana University Institutional Review Board, and all subjects signed informed consent prior to participating.
2.2 Measurements
Fasting blood samples were collected in all subjects after an overnight fast and stored at −80° C until analysis. Serum biochemistries were measured with the Randox Daytona clinical chemistry analyzer (Randox Laboratories; Antrim, Northern Ireland, United Kingdom) for calcium, phosphorus, creatinine, total alkaline phosphatase, iron, and unbound iron binding capacity (UIBC). Iron and UIBC were measured using a spectrophotometric method. Total iron binding capacity (TIBC) was calculated as the sum of serum iron and UIBC. Iron saturation was calculated as serum iron ÷ TIBC and expressed as a percentage. Iron deficiency was defined as serum iron concentration < 50 mcg/dl (22). Parathyroid hormone (PTH), 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D [1,25(OH)2D] were measured using radioimmunoassays from DiaSorin (Stillwater, MN) in a subset of white women.
FGF23 was measured using two different ELISAs: the intact FGF23 ELISA from Kainos Laboratories (Tokyo, Japan), measured only intact (biologically active) FGF23; and the C-terminal FGF23 ELISA (Immutopics, San Clemente, CA) measures a combination of both C-terminal fragments and intact FGF23. The intra-assay and inter-assay coefficients of variation (CV) were 7.6% and 13.5% for C-terminal FGF23. The intra-assay and inter-assay CV were 6.7% and 4.4% for intact FGF23.
Areal BMD (g/cm2) at the lumbar spine (L2–L4) and femoral neck were measured by DXA, using two DPX-L and one Prodigy machines (GE Lunar Corp., Madison, WI, USA), which were cross-calibrated weekly with a step-wedge phantom. There were no systematic differences among the three devices. The CV of duplicate measurements after subject repositioning was 1.0% for femoral neck and 0.52% for lumbar spine. Height was measured using a Harpenden stadiometer and weight with a Scale-Tronix scale.
2.3 Statistical Analysis
All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Categorical variables were summarized by counts and percentages and were compared using Chi-square tests. Continuous variables were summarized by medians and 1st and 3rd quartiles. The ratio of intact FGF23 to C-terminal FGF23 was calculated to identify whether similar proportions of intact FGF23 and fragments occurred between black women and white women. Wilcoxon rank sum tests were used to compare variables between the two race groups, since normality assumptions were violated for most variables. Logarithmic transformations of base 10 were applied to both C-terminal and intact FGF23 before all analyses. Pearson’s correlation, Spearman’s correlation and scatter plots with linear fits were used to explore pair-wise relationships. Since Pearson’s and Spearman’s correlation gave similar results for this data set, Pearson’s correlations were presented to be consistent with the linear fits. Given that there were two major hypotheses with sub-hypotheses under each and relatively large sample sizes, as a conservative threshold, we adopted a Type I error level of 0.01 instead of the conventional 0.05 level.
The relationships between FGF23 and iron were evaluated using change-point models with the change-point at an iron concentration of 50 mcg/dl. These models allow the relationships between FGF23 and iron, i.e., the slopes of the linear regressions, to be different for iron <50 mcg/dl and for iron ≥50 mcg/dl as suggested by the exploratory plots. The models were adjusted for factors involved in iron metabolism, or bone and mineral metabolism, as well as race, age, height, weight. Since UIBC increases in the setting of iron deficiency (23, 24), UIBC was included in these models as a measure of iron binding capacity. UIBC was chosen because UIBC was directly measured, as opposed to TIBC or iron saturation, which were calculated based on serum iron and the UIBC. Two main adjusted regression models for the relationship between iron and FGF23 were used. Model 1 adjusted for race, age, height, weight, serum UIBC, phosphorus, calcium, creatinine, and alkaline phosphatase. Model 2 was applied to only white women and adjusted for variables in Model 1, plus PTH, 25OHD and 1,25(OH)2D. The relationships between FGF23 and vitamin D metabolites (25OHD and 1,25(OH)2D) were evaluated using partial correlations, adjusting for the other variables in Model 2 including iron. The relationships between longitudinal changes in iron and FGF23 were evaluated by linear regression.
The BMD relationships with iron were first evaluated using bivariate linear regressions; while adjusting for age, race, height, weight, serum UIBC (Model A) and the addition of intact and C-terminal FGF23 (Model B). Since weight interacted with serum iron concentration in the models for BMD, in Model C an interaction term between iron and obesity was added (BMI<30 vs ≥30 kg/m2) to the variables in Model B. Models D and E, applied to white women only, added PTH, 25OHD and 1,25(OH)2D to Models B and C, respectively. All models were examined separately for black and white women.
3. Results
3.1 Cohort description and biochemistries
Cohort characteristics and differences between the premenopausal white and black women are shown in Table 1. White women had lower weight and were taller. White women had lower median serum phosphorus, calcium, alkaline phosphatase and creatinine levels than black women, and higher iron, TIBC and iron saturation. More black women than white women had serum iron concentration <50 mcg/dl (26.5% versus 10.0%, respectively, p<0.0001). Serum PTH, 25OHD and 1,25(OH)2D3, measured in only white women, were normal. BMD at the femur neck and spine was lower in white women than in black women (p<0.0001).
Table 1.
Descriptive statistics.
| White Women n= 1898 (65.63%) |
Black Women n= 994 (34.37%) |
P value | |||
|---|---|---|---|---|---|
| n | Descriptive | n | Descriptive | ||
| Age, years | 1898 | 32.8 (25.1, 41.0) | 994 | 32.9 (26.8, 39.8) | 0.84 |
| Weight, kg | 1890 | 66.3 (59.0, 78.3) | 984 | 80.2 (67.6, 96.9) | <0.0001 |
| Height, cm | 1890 | 165.0 (161.0, 169.2) | 984 | 164.1 (160.0, 168.1) | <0.0001 |
| Calcium mg/dl | 1898 | 9.71 (9.42, 10.00) | 994 | 9.76 (9.54, 9.98) | <0.001 |
| Phosphorus mg/dl | 1898 | 3.66 (3.37, 3.97) | 994 | 3.73 (3.42, 4.06) | <0.001 |
| Creatinine mg/dl | 1897 | 0.90 (0.83, 0.97) | 994 | 0.92 (0.86, 1.01) | <0.0001 |
| Alkaline phosphatase U/L | 1898 | 56 (46, 69) | 994 | 64 (51, 79) | <0.0001 |
| Iron mcg/dl | 1898 | 94.5 (68.5, 124.5) | 994 | 68.0 (49.0, 92.2) | <0.0001 |
| Iron <50 mcg/dl (n,%) | 1898 | 189 (10.0%) | 994 | 263 (26.5%) | <0.0001 |
| UIBC mcg/dl | 1893 | 271.1 (228.0, 317.8) | 990 | 282.8 (244.0, 322.2) | <0.0001 |
| TIBC mcg/dl | 1893 | 371.7 (332.0, 413.9) | 990 | 355.1 (324.0, 391.9) | <0.0001 |
| Iron saturation % | 1893 | 26 (19, 34) | 990 | 19 (14, 26) | <0.0001 |
| PTH pg/ml a | 1055 | 27.1 (20.2, 35.0) | - | - | |
| 25OHD ng/ml a | 1067 | 29.7 (21.8, 38.4) | - | - | |
| 1,25(OH)2D pg/ml a | 858 | 34.7 (25.2, 47.4) | - | - | |
| C-terminal FGF23 RU/mlb | 1631 | 13.0 (8.5, 20.0) | 296 | 12.9 (9.2, 18.6) | 0.82 |
| Intact FGF23 pg/ml b | 1631 | 38.0 (29.3, 48.2) | 296 | 35.7 (27.5, 44.4) | <0.01 |
| Ratio of Intact/C-terminal FGF23 b |
1631 | 2.83 (1.78,4.57) | 296 | 2.64 (1.75, 4.01) | 0.12 |
| BMD Femur neck, g/cm2 | 1890 | 1.03(0.94,1.12) | 983 | 1.11 (1.01, 1.20) | <0.0001 |
| BMD Spine (L2–4), g/cm2 | 1665 | 1.25(1.17,1.36) | 898 | 1.31 (1.21, 1.43) | <0.0001 |
Values listed are median (first quartile, third quartile) unless otherwise indicated.
PTH, 25-hydroxyvitamin D (25OHD) and 1,25-dihydroxyvitamin D [1,25(OH)2D] were measured in white women only.
C-terminal FGF23 and intact FGF23 were measured in only a subset.
Median serum intact FGF23 concentration was higher in white women than black women (p<0.01, Table 1), but median serum C-terminal FGF23 did not differ between groups. The median ratios of intact to C-terminal FGF23 did not differ between white women and black women. FGF23 values were log transformed for analyses. C-terminal FGF23 correlated positively with intact FGF23 in white women (r=0.122, p<0.0001), but not in black women (r=0.041, p=0.48). The slope for the line (y=log10[C-terminal FGF23], x=log10[intact FGF23]) was also numerically steeper (0.22) for white women than for black women (0.08), but the slopes were not significantly different (p=0.3).
In white women, phosphorus was positively related to intact FGF23 (r=0.120, p<0.0001) but not to C-terminal FGF23 (r=0.047, p=0.06). However, phosphorus was positively correlated with both intact FGF23 (r=0.163, p<0.01) and C-terminal FGF23 (r=0.157 p<0.01) in black women. Serum phosphorus was inversely related to serum iron concentration in both white women (r=−0.061, p<0.01) and black women (r=−0.113, p<0.001).
In white women, phosphorus correlated negatively with PTH (r=−0.099, p<0.01), but not with 1,25(OH)2D or 25OHD. PTH correlated negatively with 25OHD (r= −0.148, p<0.0001) but not with 1,25(OH)2D. PTH was not related to C-terminal FGF23 or intact FGF23. C-terminal FGF23 correlated negatively with 25OHD (r=−0.105, p<0.001), but not with 1,25(OH)2D (r= −0.044, p=0.2). However, intact FGF23 correlated negatively with 1,25(OH)2D (r=−0.113, p<0.01), but not with 25OHD (r=0.043, p=0.17). Of note, iron weakly correlated with 25OHD (r=0.083, p<0.01), but not with 1,25(OH)2D (r=0.064, p=0.06).
Intact FGF23 correlated positively with weight to a similar degree in both groups (white women r= 0.109, p <0.001; black women r= 0.156, p <0.01). However, C-terminal FGF23 inversely correlated with weight in black women (r= −0.151, p < 0.01) but not in white women (p=0.9). Serum iron also inversely correlated with weight, similarly in white women (r=−0.197, p<0.0001) and black women (r=−0.210, p<0.0001).
3.2 FGF23 and Iron
Serum intact FGF23 concentration did not relate to iron in either white or black women (Figure 1). However, in both groups, serum C-terminal FGF23 concentration was related inversely to serum iron concentration (white women r= −0.134, p<0.0001; black women r= −0.188, p<0.01). UIBC correlated inversely with serum iron concentration similarly in white and black women (white women r=−0.483, p<0.0001; black women r=−0.557, p<0.0001). Consistent with this relationship, UIBC was positively correlated with C-terminal FGF23 (white women r=0.105, p<0.0001; black women r=0.205, p<0.001), but not with intact FGF23. Whereas TIBC was not related to C-terminal or intact FGF23 in either group.
Figure 1.
The relationships of serum intact FGF23 (panel A and B) and C-terminal FGF23 (panel C and D) concentrations to serum iron concentration are shown for black women (panels A and C) and white women (panel B and D). The solid line indicates the linear relationship. The dashed line is a curve fitted to the datapoints, which in panel C and D indicates an apparent change-point at an iron level of approximately 50 mcg/dl, with the slope of a fitted line for C- terminal FGF23 becoming steeper at iron concentrations below 50 mcg/dl. Significance is set at p<0.01.
The relationship between C-terminal FGF23 and serum iron had a steeper slope at iron <50 mcg/dl, the value taken as indicating iron deficiency, than at iron ≥50 mcg/dl (Figure 1 and Table 2). Incorporating a slope change-point for iron at 50 mcg/dl, the relationship of C-terminal FGF23 to iron was not present when iron values were ≥50 mcg/dl, but was highly significantly negative when serum iron was <50 mcg/dl, in both white women (r=−0.284, p<0.0001) and black women (r=−0.299, p<0.001) (Table 2, Base Model). The slope was somewhat steeper when iron was <50 mcg/dl in white women than in black women (p=0.01 for differences between race groups). When iron is <50 mcg/dl, a 10mcg/dl decrease in iron corresponded to approximately a 20% increase in C-terminal FGF23 in black women and a 42% increase in C-terminal FGF23 in white women. These slopes remained similar in adjusted models, but the difference between groups lost significance (below). The relationship of iron to C-terminal FGF23 also varied according to serum phosphorus concentration (Supplemental Figure 1), such that high serum phosphorus decreased the relationship of iron with C-terminal FGF23.
Table 2.
Multivariable model for FGF23 concentration
| C-terminal FGF23 (Log10) | |||||
|---|---|---|---|---|---|
| Effect | Race | Slope Estimate | SE | P value for slope | |
| Base Model | Iron <50 mcg/dl | Black | −8.08×10−3 | 2.19×10−3 | <0.001 a |
| White | −15.10×10−3 | 1.90×10−3 | <0.0001 | ||
| Iron ≥50 mcg/dl | Black | −0.70×10−3 | 0.48× 10−3 | 0.14 | |
| White | −0.43×10−3 | 0.201×10−3 | 0.04 | ||
| Model 1 | Iron <50 mcg/dl | Black | −7.91×10−3 | 2.23×10−3 | <0.001 |
| White | −13.92×10−3 | 1.92×10−3 | <0.0001 | ||
| Iron ≥50 mcg/dl | Black | −0.24×10−3 | 0.50×10−3 | 0.62 | |
| White | −0.28×10−3 | 0.24×10−3 | 0.24 | ||
| Model 2 | Iron <50 mcg/dl | White | −14.26×10−3 | 2.76×10−3 | <0.0001 |
| Iron ≥50 mcg/dl | White | −0.81×10−3 | 0.30×10−3 | <0.01 | |
| Intact FGF23 (Log10) | |||||
| Effect | Race | Slope Estimate | SE | P value for slope | |
| Base Model | Iron <50 mcg/dl | Black | 1.04×10−3 | 7.38×10−3 | 0.40 |
| White | 1.94×10−3 | 1.06×10−3 | 0.07 | ||
| Iron ≥50 mcg/dl | Black | −0.67×10−3 | 0.27×10−3 | 0.014 | |
| White | −0.14×10−3 | 0.11×10−3 | 0.23 | ||
| Model 1 | Iron <50 mcg/dl | Black | 1.71×10−3 | 1.23×10−3 | 0.16 |
| White | 1.57×10−3 | 1.06×10−3 | 0.14 | ||
| Iron ≥50 mcg/dl | Black | −0.69×10−3 | 0.27×10−3 | 0.012b | |
| White | 0.04×10−3 | 0.13×10−3 | 0.74 | ||
| Model 2 | Iron <50 mcg/dl | White | 1.53×10−3 | 1.69×10−3 | 0.37 |
| Iron ≥50 mcg/dl | White | −0.17×10−3 | 0.19×10−3 | 0.36 | |
The slope estimate and standard error are shown for iron concentrations in similarly structured multivariable regression models for C-terminal and intact FGF23. P-values <0.01 were considered statistically significant. The listed p values indicate significance of the slope as listed.
Indicates p=0.01 for differences in the slope between white women and black women.
Indicates p<0.01 for differences in the slope between white women and black women.
Base Model: A change-point model allowing different slopes when iron is <50 and ≥50 mcg/dl. Adjusted Model 1: In white and black women, adjusted for race, age, height, weight, serum UIBC, phosphorus, calcium, creatinine and alkaline phosphatase.
Adjusted Model 2: In white women only, adjusted for variables in Model 1 plus PTH, 25OHD and 1,25(OH)2D.
3.3 Multivariable models for FGF23 and iron
In a multivariable regression model (Table 2, adjusted Model 1), the negative relationship of C-terminal FGF23 to iron when serum iron is <50 mcg/dl was significant (p<0.001) in both white women and black women after adjusting for race, age, height, weight, serum UIBC, phosphorus, calcium, creatinine and alkaline phosphatase. In this model age and serum phosphorus remained independently associated with C-terminal FGF23 (each p<0.01). Adjusted Model 2 was fitted in white women only, adding PTH, 25OHD, and 1,25(OH)2D to the variables in Model 1 (Table 2), as these variables were not measured in black women. In Model 2, C-terminal FGF23 remained significantly inversely related to iron <50 mcg/dl, without decrease in effect size (compared to the Base Model or to adjusted Model 1). In addition C-terminal FGF23 was now also inversely related to serum iron at iron concentrations ≥50 mcg/dl (p<0.01). In contrast, intact FGF23 was not significantly related to iron in similarly adjusted models.
3.4 Multivariable modeling for FGF23 and vitamin D
Using Pearson correlations, C-terminal FGF23 was negatively correlated to 25OHD, while intact FGF23 was negatively correlated to 1,25(OH)2D (see above). However, after adjusting for iron and Model 2 variables, C-terminal FGF23 was no longer significantly related to either 25OHD or 1,25(OH)2D (p>0.4). In contrast, intact FGF23 remained significantly negatively related to 1,25(OH)2D (r=−0.092, p<0.01), and was now positively related to 25OHD (r=0.124, p<0.01).
3.5 BMD relationship to iron and FGF23
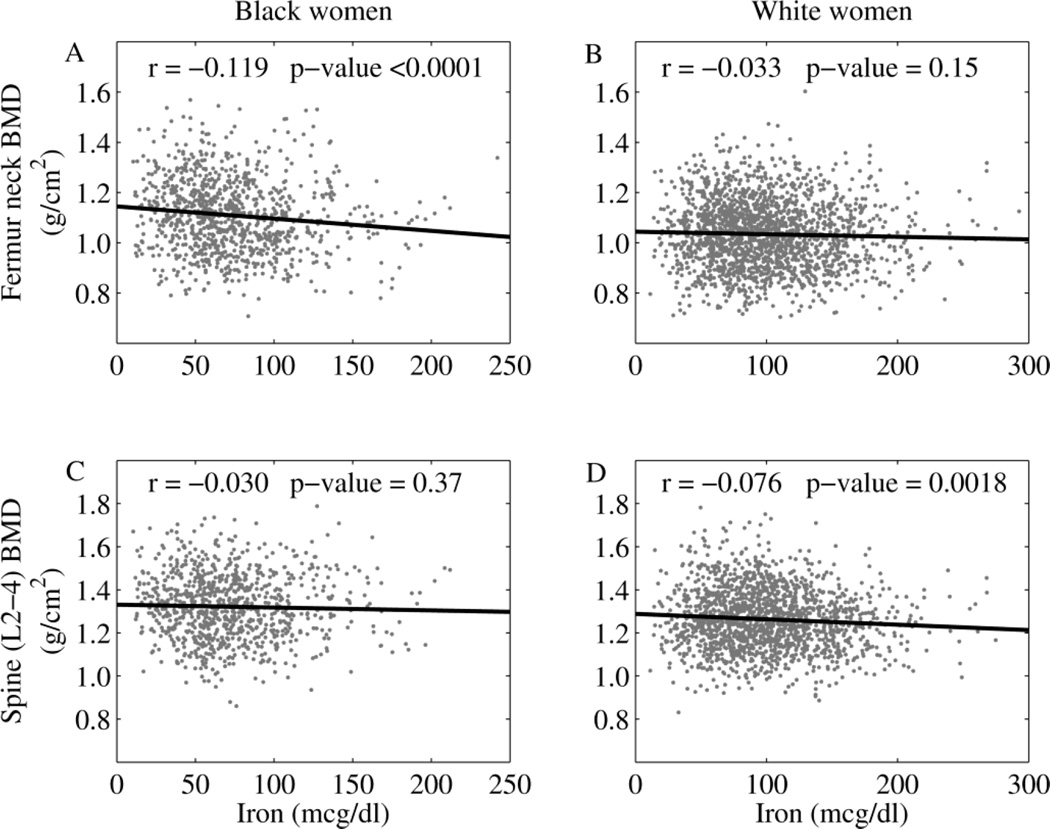
Spine BMD was related inversely to serum iron (r= −0.076, p<0.01) only in white women; whereas femur neck BMD was related inversely to serum iron (r= −0.119, p<0.0001) only in black women (Figure 2). Similarly, spine BMD correlated inversely to percent iron saturation only in white women (p<0.01), while femur neck BMD correlated inversely to percent iron saturation only in black women (p<0.001) (Supplemental Table 1).
Figure 2.
The relationships of BMD at the femur neck (A and B) and the lumbar spine (C and D) to serum iron concentration are shown for black women (A and C) and white women (B and D). Significance is set at p<0.01.
The relationships between FGF23 and BMD are shown in Figure 3. At the femur neck, BMD did not correlate significantly with intact FGF23 or C-terminal FGF23 in either white women or black women. Spine BMD did not correlate significantly with intact FGF23 in white women (r=0.037, p=0.16) or in black women (r= 0.149, p=0.011). There were no significant correlations between spine BMD and C-terminal FGF23.
Figure 3.
FGF23 and BMD. Intact FGF23 concentrations (A, B, E, F) and C-terminal FGF23 concentrations (C, D, G, H) are plotted on the x-axis with femur neck BMD (A–D) and spine BMD (E–H) on the y-axis. Significance is set at p<0.01. There were no significant relationships.
Correlations between BMD and additional variables in white women and black women are shown in Supplemental Table 1. BMD at the femur neck correlated negatively with age (p<0.01) in white and black women, while spine BMD correlated positively with age in white women (p<0.0001). BMD at both the femur neck and spine correlated most strongly (and positively) with weight and height in white women and black women (p<0.0001). Femur neck BMD was related positively to serum phosphorus, creatinine, UIBC and TIBC (p<0.01) only in white women. Femur neck BMD correlated negatively to serum calcium in black women (p<0.01). Spine BMD did not correlate with phosphorus, UIBC, TIBC, creatinine or calcium in white women or black women. BMD did not correlate with alkaline phosphatase levels.
3.6 Multivariable models for BMD
Although there were negative correlations between BMD and serum iron on univariate analysis (at the femur neck for black women, and at the spine for white women; Figure 2), adjusting for race, age, height, weight and UIBC, eliminated these inverse relationships (Model A, Supplemental Table 2). After adding C-terminal and intact FGF23 to the model (Model B), femur neck BMD was now positively associated with serum iron (p<0.01) in white women, contrasting with the negative correlation in univariate analysis of black women. Spine BMD was not significantly related to serum iron in any of the adjusted models.
The loss of the negative relationship of BMD with iron in multivariate models was primarily due to weight. The associations of BMD with iron for obese (BMI≥30 kg/m2) and non-obese ((BMI<30) subjects were assessed after adjusting for race, age, height, weight, UIBC, C-terminal FGF23 and intact FGF23 (Model C, Supplemental Table 2). Model C indicated no relationships between BMD and iron, except a small positive association remaining between femur neck BMD and iron, only in non-obese white women (p<0.01) with small effect size (slope 0.0022 g/cm2 per 10 mcg/dl change in serum iron). This relationship was not present in obese subjects or in black women, though the slopes were not significantly different between white and black women. Thus in adjusted models, the negative univariate relationship of BMD to serum iron did not persist.
Neither C-terminal nor intact FGF23 were related significantly to BMD in any adjusted models (Supplemental Table 2), including models adjusting for iron and obesity in white and black women; and in models in white women adjusting for PTH, 25OHD and 1,25(OH)2D.
3.7 Longitudinal changes in iron and FGF23 in white women
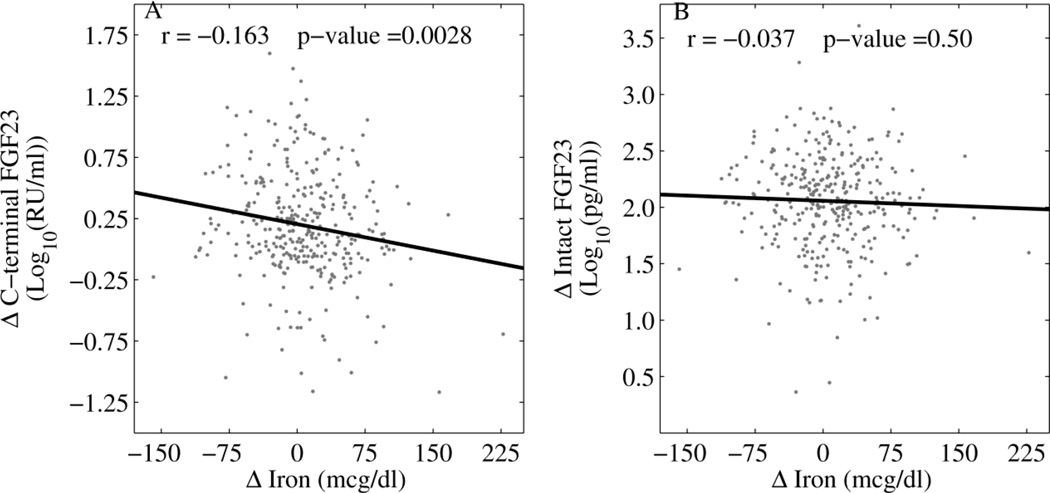
Samples from two visits were analyzed from 338 white women, with a median interval of 5.7 years between visits, to assess changes in biochemistries (Supplemental Table 3). The baseline age at the initial visit in this subset was 35.7 years (first quartile 30.8 years; third quartile 40.3 years). Changes in C-terminal FGF23 correlated inversely to changes in serum iron (r=−0.163, p <0.01) (Figure 4), but intact FGF23 changes did not relate to iron changes (Supplemental Figure 2).
Figure 4.
The relationship of changes in serum iron concentration over a median interval of 5.7 years to changes in serum C-terminal FGF23 (panel A) and intact FGF23 (panel B) concentrations are plotted in white women. Δ indicates the change in the value of the variable. Significance is set at p<0.01.
4. Discussion
This study evaluated the relationship of iron to intact and C-terminal FGF23 in a large cohort of healthy premenopausal white and black women. Serum iron concentration was independently related to C-terminal FGF23 but was not related to intact FGF23, even after adjustment for several biochemical variables. Likewise changes in serum iron predicted changes in C-terminal FGF23 but not intact FGF23 over a median of 5.7 years. The relationship of C-terminal FGF23 to iron varied with serum phosphorus (Supplemental Figure 1). Thus the highest C-terminal FGF23 concentrations occurred in subjects with the lowest serum iron regardless of phosphorus; while the lowest C-terminal FGF23 concentrations occurred in those subjects with the highest serum iron and lowest serum phosphorus. Importantly, the increase in C-terminal FGF23 with lower iron concentrations was most pronounced in the iron deficient range, as evidenced by the steeper slope in this range.
This is consistent with previous reports that while low iron status increases gene expression of FGF23, FGF23 protein levels are further regulated by cleavage into inactive N- and C-terminal fragments to maintain normal phosphate homeostasis (3, 4, 25, 26). Other authors have noted an inverse association of iron stores assessed by ferritin and hemoglobin to C-terminal FGF23 in iron deficient Gambian children, with responsiveness of C-terminal FGF23 to treatment of iron deficiency(26, 27). In a small number of women, iron status was correlated inversely to C-terminal FGF23 (14). Recently iron levels were also noted to be inversely related to C-terminal FGF23 in elderly patients (28). However these authors also found an inverse relationship between serum iron and intact FGF23 concentrations, using the Immutopics intact FGF23 ELISA. Differences from the present study may be due to assay differences or to population differences. Although males were not included in the current study, intact FGF23 using the Kainos assay did not correlate to serum iron in a small number of healthy non-elderly males (6).
Serum iron was lower in black than white women, and more black women were iron deficient. The median C-terminal FGF23 did not differ between white and black women, but the distribution was positively skewed in both groups, and the inverse relationship between C-terminal FGF23 and serum iron was similar. C-terminal FGF23 correlated with intact FGF23 in white women but not black women. The differences between black and white women for relationships of intact FGF23 with C-terminal FGF23 (or for relationships between FGF23 and other variables) are unexplained. In part these findings may be due to the smaller sample size and an increased incidence of iron deficiency in black women, since iron deficiency influences the amount of C-terminal FGF23.
Intact FGF23 was positively and similarly associated with phosphorus in both groups (black women r=0.163; white women r=0.120, each p<0.01). Although the ratio of intact to C-terminal FGF23 was similar between groups, the C-terminal FGF23 was related to serum phosphorus in black women but not in white women. However, black women had slightly, but significantly, lower intact FGF23 with slightly higher serum phosphorus than white women (each p<0.01), suggesting a possible racial difference in the regulation of serum phosphorus concentration. In addition, only intact FGF23 was related to 1,25(OH)2D (measured in white women). However, C-terminal FGF23 was influenced by serum iron in both white and black women. Overall, these data are biologically consistent with the intact FGF23 being the functional protein in regards to regulating phosphorus metabolism. Thus measurements of intact FGF23 are more likely to provide biologically important information regarding phosphorus metabolism.
Phosphorus was inversely related to iron concentrations, but intact FGF23 is not affected by serum iron concentrations in the absence of mutations of FGF23 impairing cleavage(3, 4). One possible explanation for this finding is that high dietary iron intake could serve as a phosphate binder, inhibiting intestinal phosphate absorption. Indeed oral iron has been administered as a phosphate binder in chronic kidney disease patients(29).
Although BMD was inversely associated with serum iron at the spine in white women and femur neck in black women on univariate analysis, a biologically important systemic factor such as iron is not likely to have important effects only at different BMD sites in different race groups. Furthermore these small inverse correlations were eliminated (or reversed on one subgroup) when adjusting for other variables, especially weight. In adjusted analyses, BMD also did not correlate to intact or C-terminal FGF23 in white or black premenopausal women. Consequently there does not appear to be a direct impact of FGF23 concentrations on BMD within the ranges experienced in normal healthy premenopausal women.
Strengths of this study include the large sample size and the inclusion of both white and black premenopausal women, along with the assessment of both intact and C-terminal FGF23 and BMD. In addition this was also the first study to demonstrate longitudinally that changes in serum iron correlate to changes in C-terminal FGF23 in a large number of healthy premenopausal women, confirming the nature of relationship of C-terminal FGF23 and iron.
Limitations included that PTH, 25OHD and 1,25(OH)2D were only measured in white women and that serum iron and percent iron saturation were the only measures of iron status. Thus the models including PTH, 25OHD and 1,25(OH)2D are only generalizable to white women. The analysis also did not account for other measures of iron status such as ferritin or hemoglobin. However the inclusion of UIBC in models, which increases with iron deficiency and has been used as an indicator of iron status (23, 24), enables the models to account for iron binding capacity in assessing degree of iron deficiency. FGF23 was measured in serum rather than in plasma, and the C-terminal FGF23 concentrations were lower than previous reported ranges measured in plasma(3). Thus C-terminal FGF23 concentrations cannot be directly compared to studies using plasma C-terminal FGF23. However, despite this difference in serum versus plasma C-terminal FGF23 concentrations, the present study demonstrated a similar though smaller relationship between serum iron and serum C-terminal FGF23 (r=−0.134 in white women and r=−0.188 in black women), to that previously reported between serum iron and plasma C-terminal FGF23 in white women (r=−0.278) (3). In the previous study using plasma FGF23, we also could not identify a relationship between C-terminal FGF23 and serum phosphorus. These indicate some consistency of these relationships when using either plasma or serum C-terminal FGF23, even though the absolute values were smaller when measured in serum. On the other hand intact FGF23 concentrations in serum were similar to that previously reported in plasma from healthy subjects(3).
Studies investigating the influence of iron on BMD have predominantly focused on dietary iron intake (30–32), or on iron overload states (17, 33). Notably, iron restriction impairs calcium phosphate mineral accrual in female rats, which is exacerbated by concomitant calcium deficiency(34). Dietary iron intake may have a protective effect on bone loss at the spine(30), and is positively associated with BMD in adult women, especially among those receiving estrogen replacement therapy (31, 32).
Among Korean women under age 45 and among men, iron stores were not related to proximal femur BMD (18). These results are similar to our femur neck BMD findings in premenopausal white women, though an inverse relationship with serum iron and femur neck BMD was present in black women. Our study indicated a small inverse relationship of iron to spine BMD in white women, but in adjusted models BMD was no longer inversely related to iron and instead had a small positive relationship at the femur neck (only in non-obese white women). Conversely, prevalent osteoporosis and fractures were 1.55-fold higher in postmenopausal women with high versus low iron stores (18), and high iron stores associated with declines in proximal femur BMD in Korean postmenopausal women and middle aged men(19).
Low BMD occurs with elevated risk of vertebral fractures in iron excess states due to genetic hemochromatosis (17, 33) or treatment of beta-thalassemia (35). Low BMD occurs in these conditions even in the absence of complications such as hypogonadism or cirrhosis, which may independently contribute to risk. BMD in beta-thalassemia improves after iron chelation therapy (35). However, in our study few women had high serum iron concentration, limiting the ability to assess iron overload.
Previous studies report conflicting data regarding the relationship of BMD to FGF23. Using various unadjusted or adjusted models, a positive association has been reported between spine BMD or hip BMD and either C-terminal FGF23 or intact FGF23 in older men(11, 12) and postmenopausal women(10, 14). However some studies indicate no relationship between BMD and C-terminal FGF23 in women(11), or between BMD and intact FGF23 in men(13). We found no significant relationship between BMD and intact (or C-terminal) FGF23 in either white women or black women, using unadjusted and adjusted models.
In one study, older men with the highest intact FGF23 concentrations also had increased fracture risk independent of BMD (36), but other authors found that intact FGF23 increased fracture risk only in men with chronic kidney disease(13). Our study did not assess fracture risk, but the absence of effect of FGF23 on BMD would decrease the likelihood of effects on fracture risk in premenopausal women.
Of the measured variables, BMD was most strongly correlated to weight (positively) in both groups. In contrast, iron was inversely associated with weight. Both C-terminal FGF23 and intact FGF23 positively associated with obesity in other studies (14, 37, 38), and the inclusion of body mass index in an adjusted model abolished a positive univariate relationship of BMD with C-terminal FGF23 in women (14). We also demonstrated a positive association between body weight and intact FGF23 in premenopausal black and white women. However, C-terminal FGF23 was negatively associated with weight in black women, with no association in white women. After inclusion of weight in adjusted models, BMD was not related to iron, intact FGF23 or C-terminal FGF23, except for a slight positive femur neck BMD relationship with iron in the subgroup of non-obese white women. However, the relationship of iron to C-terminal FGF23 was robust and did persist after weight adjustment.
In conclusion, in a large sample of healthy premenopausal white and black women, serum iron concentrations were inversely related to C-terminal FGF23, especially at low iron concentrations, without relationship to intact FGF23. Changes of serum iron concentration over time were also inversely related to changes in serum C-terminal FGF23, but not intact FGF23. However, BMD was not significantly associated with serum iron, C-terminal FGF23 or intact FGF23 concentrations in adjusted models. Thus it appears that neither iron, C-terminal FGF23 nor intact FGF23 have significant effects on BMD at the levels generally experienced in healthy premenopausal women.
Supplementary Material
Highlights.
Serum iron correlated negatively with C-terminal FGF23.
Serum iron did not correlate with intact FGF23.
In adjusted models BMD was not consistently related to intact FGF23, C-terminal FGF23 or iron.
Acknowledgments
This work was supported by National Institutes of Health grants R21 AR061078 (MJE), K23AR057096 (EAI), R01 AG041517 (MJE), and M01 RR-00750 (MP), and by a donation from the Scottish Rite of Indianapolis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
EA Imel (Kyowa Hakko Kirin Pharma, Inc.: Research Grant for clinical trials, Consulting; Ultragenyx: Research Grant for clinical trials, Consulting);
M Peacock (Kyowa Hakko Kirin Pharma, Inc.: Research Grant for clinical trials, Consulting; Ultragenyx: Research Grant for clinical trials, Consulting);
MJ Econs (FGF23 patent).
All other authors (ZL, AKM, DA, AA, and LRP) report no disclosures.
Authors’ roles: Study design: EAI, MP, MJE; Study conduct: EAI, AKM, DA, AA, LRP, MP, MJE; Data collection: EAI, AKM, DA, AA, LRP; Data analysis: ZL; Data interpretation: EAI, ZL, MP, MJE; Drafting manuscript: EAI and ZL; Revising manuscript content: EAI, ZL, AKM, DA, AA, LRP, MP, MJE; Approving final version of manuscript: EAI, ZL, AKM, DA, AA, LRP, MP, MJE; All authors take responsibility for the integrity of the data and ZL takes responsibility for integrity of the data analysis.
References
- 1.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 2.Larsson T, Davis SI, Garringer HJ, Mooney SD, Draman MS, Cullen MJ, et al. Fibroblast growth factor-23 mutants causing familial tumoral calcinosis are differentially processed. Endocrinology. 2005;146(9):3883–3891. doi: 10.1210/en.2005-0431. [DOI] [PubMed] [Google Scholar]
- 3.Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96(11):3541–3549. doi: 10.1210/jc.2011-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):1146–1155. doi: 10.1073/pnas.1110905108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87(11):4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 6.Imel EA, Gray A, Padgett L, Econs MJ. Iron and fibroblast growth factor 23 in X-linked hypophosphatemia. Bone. 2014;60:87–92. doi: 10.1016/j.bone.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imel EA, Hui SL, Econs MJ. FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res. 2007;22(4):520–526. doi: 10.1359/jbmr.070107. [DOI] [PubMed] [Google Scholar]
- 8.Ichikawa S, Baujat G, Seyahi A, Garoufali AG, Imel EA, Padgett LR, et al. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am J Med Genet A. 2010;152A(4):896–903. doi: 10.1002/ajmg.a.33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalhoub V, Ward SC, Sun B, Stevens J, Renshaw L, Hawkins N, et al. Fibroblast growth factor 23 (FGF23) and alpha-klotho stimulate osteoblastic MC3T3.E1 cell proliferation and inhibit mineralization. Calcif Tissue Int. 2011;89(2):140–150. doi: 10.1007/s00223-011-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bansal N, Katz R, de Boer IH, Kestenbaum B, Siscovick DS, Hoofnagle AN, et al. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: The MESA study. J Clin Endocrinol Metab. 2013;98(12):4890–4898. doi: 10.1210/jc.2013-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jovanovich A, Bùžková P, Chonchol M, Robbins J, Fink HA, de Boer IH, et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: The Cardiovascular Health Study. J Clin Endocrinol Metab. 2013;98(8):3323–3331. doi: 10.1210/jc.2013-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsell R, Mirza MA, Mallmin H, Karlsson M, Mellstrom D, Orwoll E, et al. Relation between fibroblast growth factor-23, body weight and bone mineral density in elderly men. Osteoporos Int. 2009;20(7):1167–1173. doi: 10.1007/s00198-008-0780-2. [DOI] [PubMed] [Google Scholar]
- 13.Lane NE, Parimi N, Corr M, Yao W, Cauley JA, Nielson CM, et al. Association of serum fibroblast growth factor 23 (FGF23) and incident fractures in older men: The Osteoporotic Fractures in Men (MrOS) study. J Bone Miner Res. 2013;28(11):2325–2332. doi: 10.1002/jbmr.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández-Real JM, Puig J, Serrano M, Sabater M, Rubió A, Moreno-Navarrete JM, et al. Iron and obesity status-associated insulin resistance influence circulating fibroblast-growth factor-23 concentrations. PloS one. 2013;8(3):e58961. doi: 10.1371/journal.pone.0058961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–2086. doi: 10.1046/j.1523-1755.2001.00064.x. [DOI] [PubMed] [Google Scholar]
- 16.Merkel D, Moran DS, Yanovich R, Evans RK, Finestone AS, Constantini N, et al. The association between hematological and inflammatory factors and stress fractures among female military recruits. Med Sci Sports Exerc. 2008;40(11 Suppl):S691–S697. doi: 10.1249/MSS.0b013e318189560c. [DOI] [PubMed] [Google Scholar]
- 17.Valenti L, Varenna M, Fracanzani AL, Rossi V, Fargion S, Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporosis Int. 2009;20(4):549–555. doi: 10.1007/s00198-008-0701-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim BJ, Lee SH, Koh JM, Kim GS. The association between higher serum ferritin level and lower bone mineral density is prominent in women ≥45 years of age (KNHANES 2008–2010) Osteoporosis Int. 2013;24(10):2627–2637. doi: 10.1007/s00198-013-2363-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim BJ, Ahn SH, Bae SJ, Kim EH, Lee SH, Kim HK, et al. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: A 3-year retrospective longitudinal study. J Bone Miner Res. 2012;27(11):2279–2290. doi: 10.1002/jbmr.1692. [DOI] [PubMed] [Google Scholar]
- 20.Koller DL, Ichikawa S, Lai D, Padgett LR, Doheny KF, Pugh E, et al. Genome-wide association study of bone mineral density in premenopausal European-American women and replication in African-American women. J Clin Endocrinol Metab. 2010;95(4):1802–1809. doi: 10.1210/jc.2009-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui SL, Koller DL, Foroud TM, Econs MJ, Johnston CC, Peacock M. Heritability of changes in bone size and bone mass with age in premenopausal white sisters. J Bone Miner Res. 2006;21(7):1121–1125. doi: 10.1359/jbmr.060412. [DOI] [PubMed] [Google Scholar]
- 22.Dale JC, Burritt MF, Zinsmeister AR. Diurnal variation of serum iron, iron-binding capacity, transferrin saturation, and ferritin levels. American journal of clinical pathology. 2002;117(5):802–808. doi: 10.1309/2YT4-CMP3-KYW7-9RK1. [DOI] [PubMed] [Google Scholar]
- 23.Boinska J, Zekanowska E, Kwapisz J. Pro-hepcidin and iron metabolism parameters in multi-time blood donors. International journal of laboratory hematology. 2010;32(5):483–490. doi: 10.1111/j.1751-553X.2009.01207.x. [DOI] [PubMed] [Google Scholar]
- 24.Asberg A, Thorstensen K, Mikkelsen G, Asberg AE. The diagnostic accuracy of unbound iron binding capacity (UIBC) as a test for empty iron stores. Scand J Clin Lab Invest. 2013;73(3):208–213. doi: 10.3109/00365513.2013.765029. [DOI] [PubMed] [Google Scholar]
- 25.Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;29(2):361–369. doi: 10.1002/jbmr.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braithwaite V, Prentice AM, Doherty C, Prentice A. FGF23 is correlated with iron status but not with inflammation and decreases after iron supplementation: a supplementation study. International journal of pediatric endocrinology. 2012;2012(1):27. doi: 10.1186/1687-9856-2012-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braithwaite V, Jarjou L, Goldberg G, Prentice A. Iron status and fibroblast growth factor-23 in Gambian children. Bone. 2012;50(6):1351–1356. doi: 10.1016/j.bone.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozentowicz-Wikarek M, Kocelak P, Owczarek A, Olszanecka-Glinianowicz M, Mossakowska M, Skalska A, et al. Plasma fibroblast growth factor 23 concentration and iron status. Does the relationship exist in the elderly population? Clin Biochem. 2015;48(6):431–436. doi: 10.1016/j.clinbiochem.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham R, Walton J, Russell L, Wolman R, Wardley-Smith B, Green JR, et al. Dietary determinants of post-menopausal bone loss at the lumbar spine: a possible beneficial effect of iron. Osteoporosis Int. 2006;17(8):1165–1173. doi: 10.1007/s00198-005-0033-6. [DOI] [PubMed] [Google Scholar]
- 31.Harris MM, Houtkooper LB, Stanford VA, Parkhill C, Weber JL, Flint-Wagner H, et al. Dietary iron is associated with bone mineral density in healthy postmenopausal women. The Journal of nutrition. 2003;133(11):3598–3602. doi: 10.1093/jn/133.11.3598. [DOI] [PubMed] [Google Scholar]
- 32.Maurer J, Harris MM, Stanford VA, Lohman TG, Cussler E, Going SB, et al. Dietary iron positively influences bone mineral density in postmenopausal women on hormone replacement therapy. The Journal of nutrition. 2005;135(4):863–869. doi: 10.1093/jn/135.4.863. [DOI] [PubMed] [Google Scholar]
- 33.Guggenbuhl P, Deugnier Y, Boisdet JF, Rolland Y, Perdriger A, Pawlotsky Y, et al. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int. 2005;16(12):1809–1814. doi: 10.1007/s00198-005-1934-0. [DOI] [PubMed] [Google Scholar]
- 34.Parelman M, Stoecker B, Baker A, Medeiros D. Iron restriction negatively affects bone in female rats and mineralization of hFOB osteoblast cells. Exp Biol Med. 2006;231(4):378–386. doi: 10.1177/153537020623100403. [DOI] [PubMed] [Google Scholar]
- 35.Di Stefano M, Chiabotto P, Roggia C, Garofalo F, Lala R, Piga A, et al. Bone mass and metabolism in thalassemic children and adolescents treated with different iron-chelating drugs. J Bone Miner Metab. 2004;22(1):53–57. doi: 10.1007/s00774-003-0449-z. [DOI] [PubMed] [Google Scholar]
- 36.Mirza MAI, Karlsson MK, Mellström D, Orwoll E, Ohlsson C, Ljunggren Ö, et al. Serum fibroblast growth factor-23 (FGF-23) and fracture risk in elderly men. J Bone Miner Res. 2011;26(4):857–864. doi: 10.1002/jbmr.263. [DOI] [PubMed] [Google Scholar]
- 37.Mirza MA, Alsio J, Hammarstedt A, Erben RG, Michaelsson K, Tivesten A, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arteriosclerosis, thrombosis, and vascular biology. 2011;31(1):219–227. doi: 10.1161/ATVBAHA.110.214619. [DOI] [PubMed] [Google Scholar]
- 38.Grethen E, Hill KM, Jones R, Cacucci BM, Gupta CE, Acton A, et al. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin d, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J Clin Endocrinol Metab. 2012;97(5):1655–1662. doi: 10.1210/jc.2011-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.