Abstract
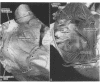
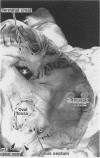
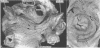
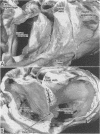
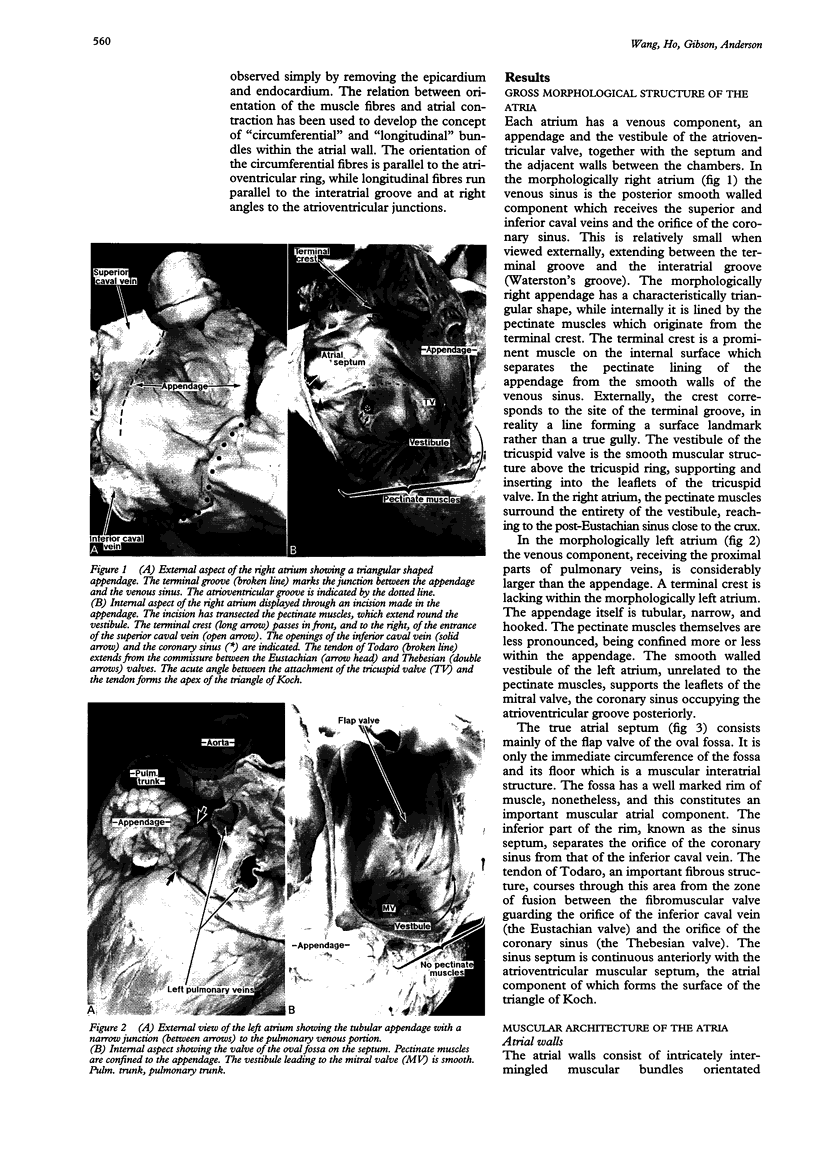
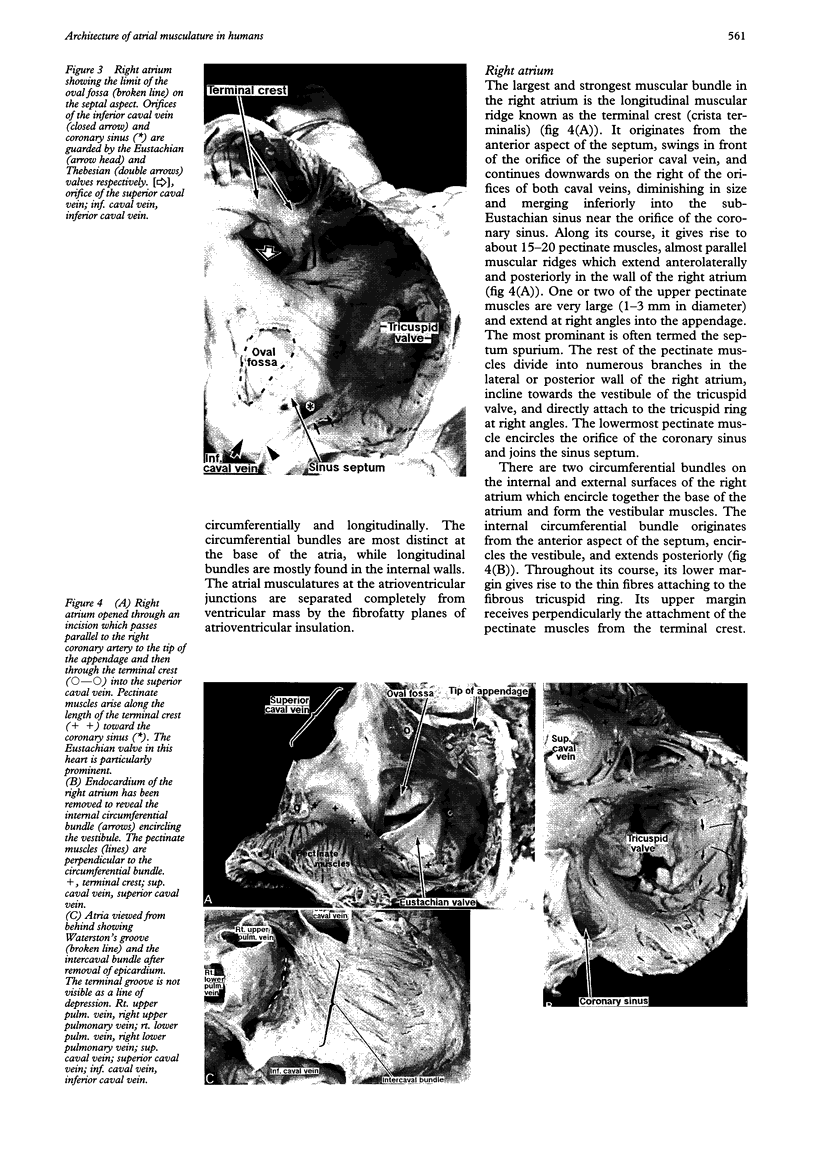
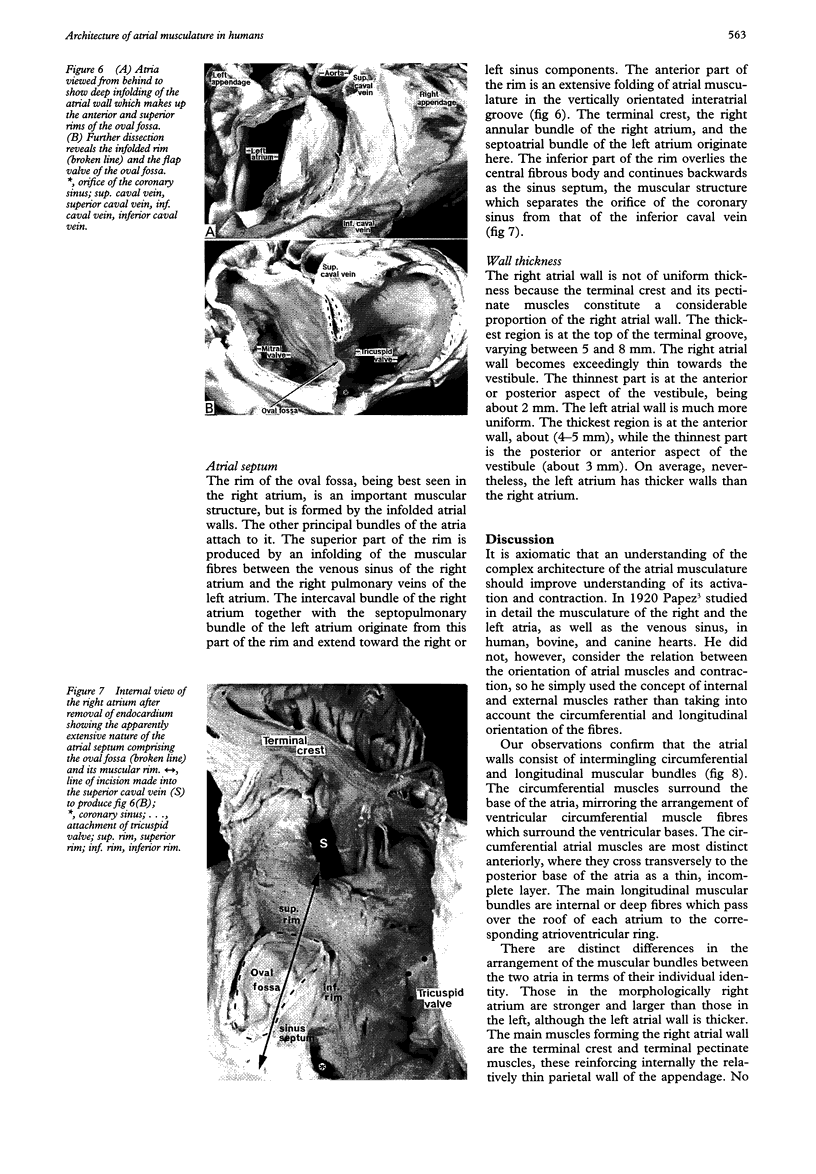
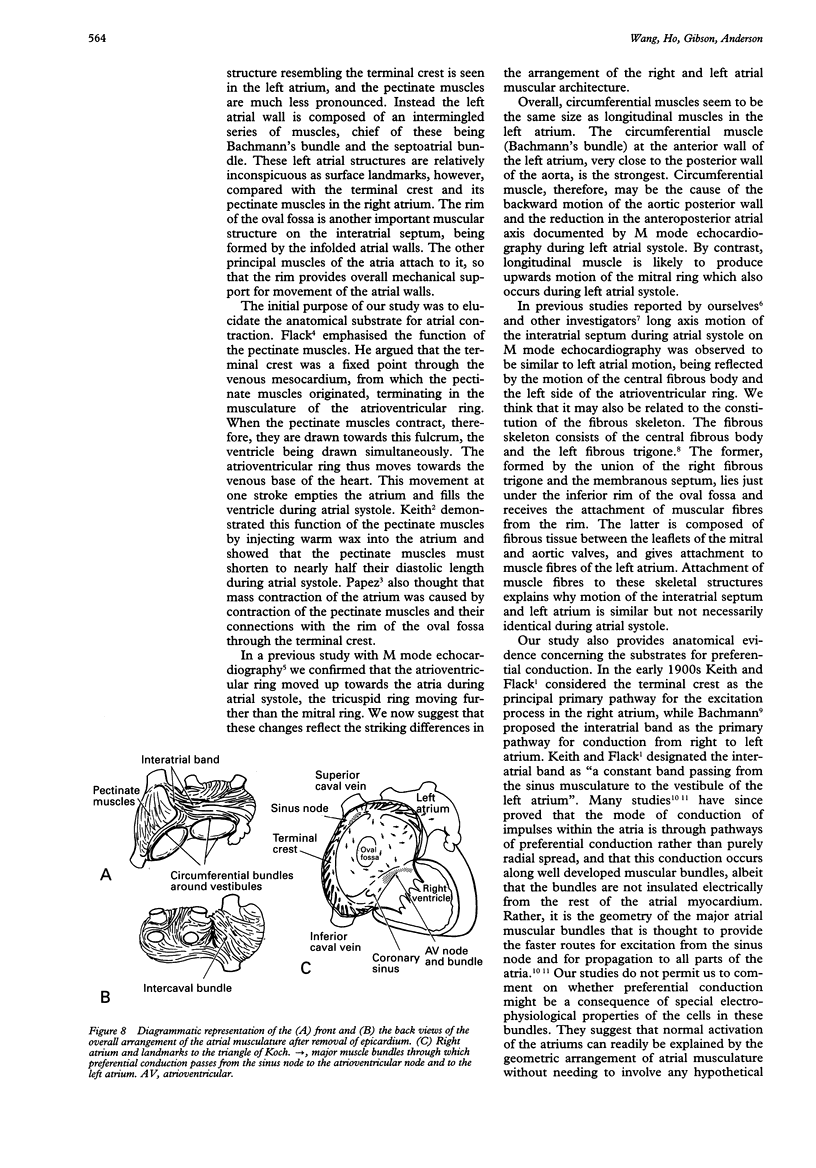
OBJECTIVE--To investigate the gross arrangement of the principal muscular bundles of the two atria, and to suggest how it may contribute to contraction and spread of atrial excitation. DESIGN--A prospective analysis based on anatomical examination of adult human hearts. SETTING--A national heart and lung institute and a tertiary referral centre for cardiac disease. MATERIAL--9 normal postmortem human hearts. METHODS--Dissection of atrial muscles with macrophotography. RESUltS--The atrial walls consist of circumferential and longitudinal muscular bundles, the former being arranged at the base of the atria with the latter predominating in the parietal walls. The muscular bundles in the right atrium are larger than those in the left. The main muscles forming the right atrial wall are the terminal crest and terminal pectinate muscles. The terminal crest, the most obvious muscle, is arranged longitudinally with its pectinate muscles connecting to the musculature of the atrioventricular vestibule. No structure resembling the terminal crest is seen in the left atrium. Instead the left atrial wall is composed of intermingled series of muscles, chief of these being the interatrial band and the septoatrial bundle. The former is arranged circumferentially at the atrial base, while the latter is mainly longitudinal. The wall of the right atrium is not of uniform thickness because of the presence of the terminal crest and its pectinate muscles on its internal surface. By contrast, the left atrial wall is much more uniform and its average thickness is greater than that of the right atrium. The rim of the oval fossa is the most important muscular structure on the septal surface and is formed by the infolded atrial walls. The other principal muscles of the atria attach to it, so that the rim provides mechanical support for overall movement of the atrial walls. Comparison of the gross arrangement of the atrial musculature with earlier echocardiographic measurements showed that this arrangement of the muscle explains movement of the atrioventricular ring and overall atrial contraction, and provides a suitable substrate for preferential conduction. CONCLUSION--The anatomical features of the atrial musculature explain the known facts concerning atrial contraction and preferential conduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol. 1976 Feb;255(2):335–346. doi: 10.1113/jphysiol.1976.sp011283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henein M. Y., Priestley K., Davarashvili T., Buller N., Gibson D. G. Early changes in left ventricular subendocardial function after successful coronary angioplasty. Br Heart J. 1993 Jun;69(6):501–506. doi: 10.1136/hrt.69.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse M. K., Anderson R. H., van Capelle F. J., Durrer D. A combined electrophysiological and anatomical study of the human fetal heart. Am Heart J. 1976 May;91(5):556–562. doi: 10.1016/s0002-8703(76)80139-1. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Song G. J., Gibson D. G. An echocardiographic assessment of atrial mechanical behaviour. Br Heart J. 1991 Jan;65(1):31–36. doi: 10.1136/hrt.65.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith A. An Account of the Structures concerned in the Production of the Jugular Pulse. J Anat Physiol. 1907 Oct;42(Pt 1):1–25. [PMC free article] [PubMed] [Google Scholar]
- Keith A, Flack M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J Anat Physiol. 1907 Apr;41(Pt 3):172–189. [PMC free article] [PubMed] [Google Scholar]
- Keren G., Sonnenblick E. H., LeJemtel T. H. Mitral anulus motion. Relation to pulmonary venous and transmitral flows in normal subjects and in patients with dilated cardiomyopathy. Circulation. 1988 Sep;78(3):621–629. doi: 10.1161/01.cir.78.3.621. [DOI] [PubMed] [Google Scholar]
- Roberts D. E., Hersh L. T., Scher A. M. Influence of cardiac fiber orientation on wavefront voltage, conduction velocity, and tissue resistivity in the dog. Circ Res. 1979 May;44(5):701–712. doi: 10.1161/01.res.44.5.701. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C., Sommer J. R. Discontinuous propagation: an hypothesis based on known cardiac structural complexities. Int J Cardiol. 1985 Feb;7(2):167–174. doi: 10.1016/0167-5273(85)90361-4. [DOI] [PubMed] [Google Scholar]
- Spach M. S., King T. D., Barr R. C., Boaz D. E., Morrow M. N., Herman-Giddens S. Electrical potential distribution surrounding the atria during depolarization and repolarization in the dog. Circ Res. 1969 Jun;24(6):857–873. doi: 10.1161/01.res.24.6.857. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Lieberman M., Scott J. G., Barr R. C., Johnson E. A., Kootsey J. M. Excitation sequences of the atrial septum and the AV node in isolated hearts of the dog and rabbit. Circ Res. 1971 Aug;29(2):156–172. doi: 10.1161/01.res.29.2.156. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Dolber P. C., Kootsey J. M., Sommer J. R., Mosher C. E., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res. 1982 Feb;50(2):175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]