Abstract
Adipose tissue (AT) inflammation contributes to impaired insulin action, which is a major cause of type 2 diabetes. RBP4 is an adipocyte- and liver-derived protein with an important role in insulin resistance, metabolic syndrome, and AT inflammation. RBP4 elevation causes AT inflammation by activating innate immunity, which elicits an adaptive immune response. RBP4-overexpressing mice (RBP4-Ox) are insulin resistant and glucose intolerant and have increased AT macrophages and T-helper 1 cells. We show that high-fat diet–fed RBP4−/− mice have reduced AT inflammation and improved insulin sensitivity versus wild type. We also elucidate the mechanism for RBP4-induced macrophage antigen presentation and subsequent T-cell activation. In RBP4-Ox, AT macrophages display enhanced c-Jun N-terminal kinase, extracellular signal–related kinase, and p38 phosphorylation. Inhibition of these pathways and of NF-κB reduces activation of macrophages and CD4 T cells. MyD88 is an adaptor protein involved in proinflammatory signaling. In macrophages from MyD88−/− mice, RBP4 fails to stimulate secretion of tumor necrosis factor, IL-12, and IL-6 and CD4 T-cell activation. In vivo blockade of antigen presentation by treating RBP4-Ox mice with CTLA4-Ig, which blocks costimulation of T cells, is sufficient to reduce AT inflammation and improve insulin resistance. Thus, MyD88 and downstream mitogen-activated protein kinase and NF-κB pathways are necessary for RBP4-induced macrophage antigen presentation and subsequent T-cell activation. Also, blocking antigen presentation with CTLA4-Ig improves RBP4-induced insulin resistance and macrophage-induced T-cell activation.
Introduction
Serum retinol-binding protein (RBP)4 is the main vitamin A (retinol)–transport protein in the blood (1). Many clinical studies link increased serum RBP4 levels to metabolic disease, including obesity (2,3), insulin resistance (3–5), type 2 diabetes (T2D) (3), and cardiovascular disease (6). Moreover, large epidemiologic studies demonstrate that elevated circulating RBP4 levels are a biomarker for these diseases (6–8). Mice with genetic or pharmacologic elevation of RBP4 levels develop insulin resistance (4,9), whereas lowering RBP4 levels improves insulin sensitivity (10). Serum RBP4 levels are elevated in people with prediabetes even before overt hyperglycemia occurs (3,7). The possibility that RBP4 causes insulin resistance in humans is supported by data showing that a single human nucleotide polymorphism that increases RBP4 promoter activity confers a twofold increased risk of T2D (11,12).
The immune system plays an important role in obesity-related insulin resistance (13,14). The mechanism for RBP4-induced insulin resistance involves inflammation. In humans, RBP4 levels in serum and adipose tissue (AT) strongly correlate with subclinical inflammation, including proinflammatory cytokine levels (15,16). RBP4 impairs insulin signaling in adipocytes indirectly by inducing proinflammatory cytokine production from macrophages through the c-Jun N-terminal kinase (JNK)-dependent pathway (17). Transgenic mice overexpressing RBP4 (RBP4-Ox) have increased numbers of AT macrophages and CD4 T cells. Transfer of antigen-presenting cells (APCs) treated with RBP4 ex vivo is sufficient to cause insulin resistance in normal mice (9). These effects are not mediated through a known RBP4 receptor, stimulated by retinoic acid 6 (STRA6), but, rather, involve Toll-like receptor 4 (17).
Several immune cell types regulate AT immune responses and insulin sensitivity (18). In AT from lean mice and humans, alternatively activated macrophages (M2), which primarily promote AT remodeling, are the major population of macrophages (18–20). Obesity triggers the accumulation of classically activated (M1) macrophages (18–21) and increases antigen presentation in AT, which stimulates proinflammatory T cells (T-helper 1 [Th]1 cells), thereby contributing to insulin resistance (22). AT inflammation and insulin resistance are improved in Th1 lineage–defining transcription factor–deficient mice on a high-fat diet (HFD), which demonstrates an important role for Th1 cells in AT inflammation–induced insulin resistance (23).
Here, we show that genetic deletion of RBP4 in obese mice reduces the number of AT proinflammatory macrophages and Th1 cells, thereby improving insulin resistance. This is consistent with our observation that RBP4 induces antigen presentation by macrophages, which activates T cells toward a Th1 profile (9). It is not known whether blocking antigen presentation improves insulin resistance. In addition, the signaling pathways that mediate RBP4 effects on antigen presentation and the importance of MyD88, the canonical adaptor for inflammatory signaling pathways downstream of Toll-like and interleukin (IL)-1 receptor families, are unknown. We now show that MyD88 and its downstream mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) pathways are necessary for macrophage activation by RBP4 and the resulting T-cell activation. This suggests that blockade of these signaling pathways or of antigen presentation itself could have beneficial effects. In fact, we show that blockade of antigen presentation with cytotoxic T-cell–associated antigen 4–Ig (CTLA4-Ig) is sufficient to improve systemic insulin resistance and AT inflammation in RBP4-Ox mice. This indicates that the RBP4-induced insulin resistance associated with obesity could be improved by blocking antigen presentation and subsequent T-cell activation.
Research Design and Methods
Animal Studies and Measurement of Metabolic Parameters
The RBP4-Ox mice were generated as previously described (46). Male RBP4-Ox mice on a C57BL6 background were bred with female C57BL6/J mice (The Jackson Laboratory) to generate RBP4-Ox mice and control littermates as previously described (4). Male and female Rbp4+/− mice were bred to obtain Rbp4−/− mice as previously described (4). MyD88−/− and WT C57BL6 mice were purchased from The Jackson Laboratory. RBP4-Ox mice and their WT littermates were fed a standard chow diet (Formulab 5008, 15 IU vitamin A/g). RBP4-knockout (KO) mice and their WT littermates were fed a chow diet (Harlan-Teklad 1810541, 4 IU vitamin A/g) or HFD (55% fat calories) (Harlan-Teklad 130873, 4 IU vitamin A/g) for 18 weeks. The RBP4 KO mice line was on a low (4 IU) vitamin A diet for four to five generations before they were studied. Metabolic studies were performed as previously described (9). Mice were treated with CTLA4-Ig (20 mg/kg i.p.) 3 days a week for 6 weeks, and metabolic and immunology studies were performed. Mouse studies were conducted in accordance with federal guidelines. The Institutional Animal Care and Use Committee (Beth Israel Deaconess Medical Center) approved all studies. All studies were performed on age- and sex-matched littermates.
Recombinant RBP4 Preparation
Human RBP4 was expressed in Escherichia coli and purified as described previously (4). Endotoxin was removed by sequential affinity adsorption to Endotrap matrix (Hyglos GmbH) and Detoxigel (Pierce). Recombinant RBP4 protein purity was determined by Coomassie staining of SDS-PAGE gels and mass spectrometry with and without a strong cation exchange cleanup. Proteomic and lipidomic mass spectrometry analysis of our RBP4 preparation confirmed its purity and showed no contaminating endotoxin (lipopolysaccharide [LPS]), other lipopolysaccharides, lipoproteins, lipids, or additional proteins (9). Absence of potential lipopolysaccharide contamination was further demonstrated by the fact that boiling, which would denature the RBP4 protein, removed the effect of RBP4 to stimulate tumor necrosis factor (TNF) secretion from bone marrow–derived dendritic cells but not the effect of LPS (9). If LPS was present, the proinflammatory effect of RBP4 would persist after boiling. In addition, recombinant RBP4 generated from mammalian cells and human RBP4 purified from human blood, where the potential exposure to LPS is minimal, had the same inflammatory effect as bacterially derived RBP4 (9).
Preparation of Stromal Vascular Fraction, Adipocyte Isolation, and Determination of Adipocyte Number
Stromal vascular fraction (SVF) was prepared as previously described (47). Briefly, mice were perfused with PBS and perigonadal fat pads were obtained, minced, and collagenase treated (Sigma-Aldrich) for 30 min at 37°C with shaking. The cell suspensions were filtered through a 40-μm filter and centrifuged at 1,800 rpm for 5 min. After that, the SVF cells were incubated with red blood cell lysis buffer (Biolegend) for 3 min. Media was added prior to centrifugation at 1,800 rpm for 5 min and resuspended in PBS buffer. The adipocytes were isolated from AT as previously described (48,49). Briefly, perigonadal AT was digested with collagenase (1 mg/mL) at 30°C in Krebs-Ringer–HEPES buffer (pH 7.4) with 3% BSA and 200 nmol/L adenosine. A longitudinal piece of AT was fixed in osmium, and cell size and number were determined with a Coulter counter as previously described.
Generation and RBP4 Treatment of Bone Marrow–Derived Macrophages
Mouse bone marrow cells were obtained as previously described (50). Macrophage colony-stimulating factor at a concentration of 50 ng/mL was used for bone marrow–derived macrophage (BMDM) differentiation. RBP4 was used at a final concentration of 50 μg/mL. The dialysate buffer was used as a vehicle control in experiments where indicated. For signaling pathway inhibition, BMDMs were pretreated for 30 min with inhibitors of JNK (5 μmol/L), Erk (10 μmol/L), p38 (10 μmol/L), and NF-κB (10 μmol/L) for 30 min and then incubated with RBP4 for 18 h.
Bead Purification, Coculture Assay, and Flow Cytometry
CD4+ T cells from the spleen of donor male age-matched mice of WT mice were purified as previously described (51). BMDMs were cocultured with cell-trace violet-labeled bead-purified splenic syngeneic CD4+ T cells from WT mice as previously described (9). For flow cytometry analysis, AT SVF cells or BMDMs were resuspended in PBS supplemented with 2% FCS, and surface markers (CD45, CD11b, F4/80, CD206, CD11c, CD4, and CD69) were stained with monoclonal antibody for multicolor flow cytometry. For intracellular cytokine staining (IL-1β, TNF, and interferon-γ [IFN-γ]), cells were stimulated for 4 h with leukocyte activation cocktail (BD Bioscience) and stained as previously described (51). The phosphorylation of JNK, p38, and Erk was performed in AT CD45+CD11b+ and CD45+CD11c+ cells. All antibodies (p-JNK, p-p38, p-Erk, CD45, CD11c, and CD11b) were obtained from BD Biosciences, and PhosFlow staining was performed as recommended by the manufacturer. The cells were acquired on an LSR II flow cytometer (BD Biosciences) at the Beth Israel Deaconess Medical Center flow cytometry core and analyzed with FlowJo 9.5.3 software (Treestar).
Western Blotting
Cells were lysed with 20 mmol/L Tris-HCl (pH 7.5), 137 mmol/L NaCl, 1 mmol/L MgCl2, 1 mmol/L CaCl2, 1% NP-40, 10% glycerol, 1 mmol/L sodium orthovanadate, 5 mmol/L NaF, 5 mmol/L β-glycerophosphate, and protease inhibitor, including 1 mmol/L phenylmethylsulfonyl fluoride and 10 μg/mL aprotinin, and Western blotting was performed as previously described (17). The following antibodies from Cell Signaling Technology were used: JNK (catalog no. 9252), p-JNK (catalog no. 9251), p-p38 (catalog no. 9211), p38 (catalog no. 9212), p-Erk (catalog no. 9101), Erk (catalog no. 9102), and IκBα (catalog no. 9242).
Analytical Procedures
Triglyceride levels were measured by colorimetric enzyme assays, and free fatty acid (FFA) levels were measured in serum using the NEFA-C kit (Wako, Richmond, VA). Cytokines (Biolegend) and insulin (Crystal Chem, Inc.) were measured by ELISA kits.
Statistics
All values are given as means ± SEM. Differences among groups were compared using ANOVA with Tukey posttest for multiple comparisons and Student t test when there were only two groups. All statistical analyses were performed using GraphPad PRISM 5 software, and the differences were considered significant when P < 0.05.
Results
RBP4 Is Necessary for AT Inflammation and Insulin Resistance With HFD-Induced Obesity
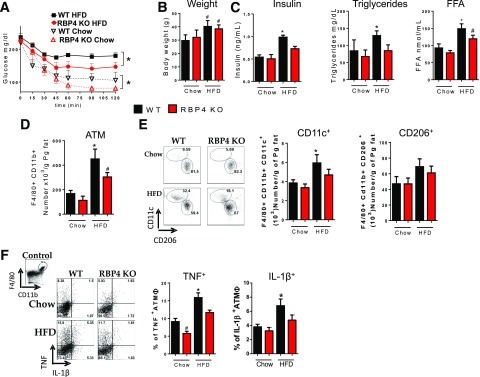
RBP4 KO mice fed chow or HFD are more insulin sensitive than wild-type (WT) mice on the same diet, with no difference in body weight between genotypes on the same diet (Fig. 1A and B). As expected, WT mice on HFD have higher serum insulin, triglyceride, and FFA levels than chow-fed mice (Fig. 1C). In RBP4 KO mice on HFD, insulin and triglyceride levels are not elevated (Fig. 1C). To investigate the effects of RBP4 KO on HFD-induced AT inflammation, we selected CD45+ cells from perigonadal AT SVF and analyzed the expression of surface markers for specific subtypes of immune cells. HFD increases the total number of macrophages (Supplementary Fig. 1), CD11c+ macrophages, and macrophages producing proinflammatory cytokines (IL-1β and TNF) (Fig. 1D–F). In HFD-fed RBP4 KO mice, the total number of AT macrophages is lower than in WT HFD mice (Fig. 1D). We evaluated AT macrophage polarization by selecting F4/80+CD11b+ cells. HFD-fed RBP4 KO mice display a lower number of CD11c+ proinflammatory (9,19,24–26) macrophages and no change in CD206+ anti-inflammatory macrophages compared with HFD-fed WT mice (Fig. 1E). However, CD206+ macrophages were still more abundant in AT of both genotypes. The intracellular content of IL-1β and TNF in AT macrophages from HFD-fed WT mice is increased compared with that in lean mice and HFD-fed RBP4 KO mice (Fig. 1F). To confirm the pro- and anti-inflammatory phenotype, we analyzed the expression of Mgl1/2 and arginase-1. These markers are upregulated in anti-inflammatory CD206+ AT macrophages (9,19,24–26). In both WT and RBP4 KO mice, CD206+ AT macrophages have increased expression of both arginase-1 and Mgl1/2 compared with CD11c+ macrophages, confirming the alternative activated macrophage phenotype (Supplementary Fig. 1). Together, our data indicate that deletion of RBP4 reduces insulin resistance and AT inflammation in HFD-fed mice.
Figure 1.
Deletion of RBP4 improves HFD-induced AT inflammation and insulin resistance. A: Insulin tolerance test. B: Body weight. C: Serum insulin, triglycerides, and FFA levels. D: AT macrophage numbers in perigonadal fat. E: Flow cytometry representation (left panel) and number (right panel) of CD11c+ and CD206+ AT macrophages. F: Flow cytometry representation (left panel) and percentage (right panel) of intracellularly stained TNF+ and IL-1β+ AT macrophages. (n = 8 mice/group.) Male mice, 18 weeks on HFD. Values are means ± SEM. *P < 0.05 vs. all other groups or as indicated. #P < 0.05 vs. chow-fed mice, same genotype. ATM, adipose tissue macrophage; Pg, perigonadal.
RBP4 Is Necessary for Macrophage-Induced CD4 T-Cell Activation and Th1 Polarization
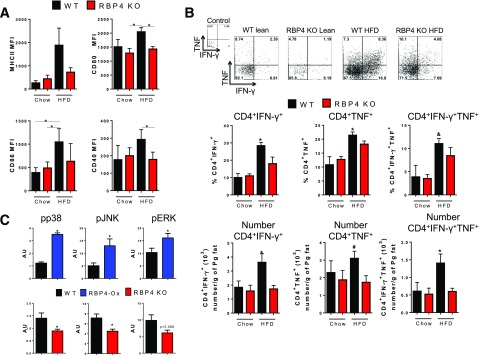
HFD-fed RBP4 KO mice display decreased expression of the costimulatory molecules CD80, CD86, and CD40 in AT macrophages compared with HFD-fed WT mice (Fig. 2A). Moreover, CD80, CD86, and CD40 expression is decreased in CD11c+ AT macrophages from HFD-fed RBP4 KO mice compared with HFD-fed WT mice and is similar to levels in chow-fed RBP4 KO and WT mice (Supplementary Fig. 2A). The expression of these costimulatory molecules in CD206+ AT macrophages is similar between RBP4 KO and WT mice fed an HFD, although it is increased compared with chow-fed mice. In contrast, MHCII expression is reduced in CD206+ AT macrophages from RBP4 KO compared with HFD-fed WT mice (Supplementary Fig. 2A). No difference in the percent or number of AT CD4 T cells intracellularly stained for IFN-γ and TNF was observed between chow-fed RBP4 KO and WT mice (Fig. 2B). HFD-fed WT mice have increased percentage and number of AT CD4 T cells expressing IFN-γ and TNF, and this increase is reduced in RBP4 KO on HFD (Fig. 2B). Thus, RBP4 is an important factor regulating the effect of AT macrophages on proinflammatory CD4 T cells.
Figure 2.
Deletion of RBP4 reduces CD4 T-cell activation and AT macrophage JNK, Erk, and p38 phosphorylation. A: Expression of MHCII and costimulatory molecules (CD80, CD86, and CD40) in AT macrophages (CD11b+F4/80+). B: Flow cytometry representation (upper panel) and percentage (bottom panel) of intracellularly stained IFN-γ+ and TNF+ AT CD4 T cells. (n = 8 mice/group.) C: Phosphorylated (p)-p38, p-JNK, and p-Erk levels normalized to isotype control antibody in AT C45+CD11b+ cells from RBP4-Ox, RBP4 KO, and WT mice. (n = 4 mice/group.) Male mice, 23 weeks of age on HFD for 18 weeks for RBP4 KO and 18 weeks of age on chow diet for RBP4-Ox. Values are means ± SEM. *P < 0.05 vs. all other groups or as indicated. #P < 0.05 vs. chow-fed mice, same genotype. AU, arbitrary units; MFI, median fluorescence intensity; Pg, perigonadal.
Antigen Presentation by Macrophages Activated by RBP4 Is Dependent on the MAPK and NF-κB Pathways
To determine the signaling pathways involved in RBP4-induced macrophage activation in vivo, we investigated the phosphorylation of p38, JNK, and extracellular signal–related kinase (Erk) in AT CD11b+ and CD11c+ cells from both RBP4-Ox and RBP4 KO mice compared with WT mice. Gating strategy for the analysis of the phosphorylation of p38, JNK, and Erk in RBP4-Ox AT CD11b+ and CD11c+ cells is shown in Supplementary Fig. 3A. We used the same gating strategy shown for RBP4-Ox (Supplementary Fig. 3A) also for RBP4 KO and WT mice. CD11b+ (Fig. 2C) and CD11c+ (Supplementary Fig. 3B) cells from AT of RBP4-Ox have increased phosphorylation of these molecules compared with cells from AT of WT mice. This phosphorylation was reduced in CD11b+ (Fig. 2C, bottom panel), and the phosphorylation of p38 and JNK tended to be lower in CD11c+ cells (Supplementary Fig. 3B) from AT of RBP4 KO mice. Thus, RBP4 effects in macrophages and induction of CD4 T-cell activation may be dependent on the activation of p38, JNK, and Erk signaling pathways.
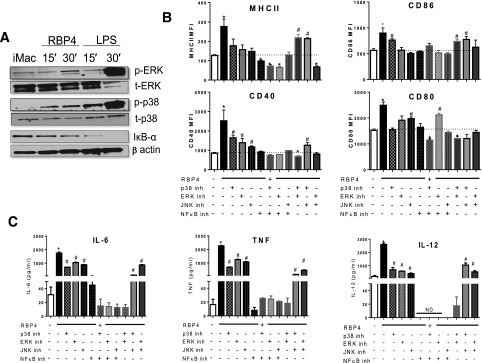
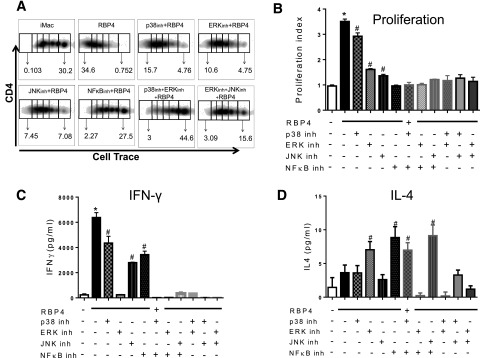
Next, we determined whether these pathways are required for RBP4-induced macrophage activation. RBP4 induces the phosphorylation of Erk and p38 and decreases inhibitor of κB (IκB)-α disappearance, indicating increased NF-κB activation (Fig. 3A). Inhibition of JNK, p38, Erk, or NF-κB reduced RBP4-induced expression of MHCII and costimulatory molecules (Fig. 3B) and of TNF, IL-12, and IL-6 (Fig. 3C). NF-κB inhibition is more effective in reducing MHCII, costimulatory molecules, and cytokine expression compared with the other inhibitors alone or in combination (Fig. 3B and C). Together, these data indicate that the NF-κB pathway is more effective than the MAPK pathway for RBP4-induced macrophage activation. Then, we investigated the importance of these RBP4-activated pathways in macrophages for macrophage-induced T-cell activation. RBP4-activated macrophages induce CD4 T-cell proliferation, which is reduced by inhibiting p38, Erk, or JNK (Fig. 4A–B). The inhibition of NF-κB or of Erk concurrent with p38 or Erk and JNK resulted in lower CD4 T-cell proliferation. Inhibitors for any of these pathways reduce IFN-γ levels (Fig. 4C), but the inhibition of Erk, NF-κB, or a combination of two MAPK inhibitors (p38, Erk, or JNK) completely abrogated the RBP4 effect on macrophage-induced IFN-γ secretion by CD4 T cells. Furthermore, treatment with MAPK or NF-κB inhibitors increased the secretion of an AT anti-inflammatory cytokine, IL-4 (Fig. 4D). These data indicate that full activation of macrophages by RBP4 and the subsequent induction of CD4 T-cell proliferation and IFN-γ secretion are dependent on the p38, JNK, Erk, and NF-κB pathways. Since MyD88 is upstream of all these pathways, we investigated whether MyD88 is necessary for RBP4 effects on macrophage activation and the subsequent CD4 T-cell activation.
Figure 3.
RBP4-induced expression of MHCII and costimulatory molecules and secretion of proinflammatory cytokines are dependent on Erk, JNK, p38, and NF-κB signaling pathways. A: BMDMs from WT mice were stimulated with RBP4 for 15 or 30 min, and phosphorylation of JNK, p38, and Erk and IκBα disappearance were evaluated by Western blot. Data are representative of 3 independent experiments. B: BMDMs were stimulated with RBP4 (50 μg/mL for 24 h) in the presence or absence of p38, Erk, JNK, and NF-κB inhibitors (inh). MHCII and costimulatory molecule (CD80, CD86, and CD40) levels. C: TNF, IL-6, and IL-12 levels. n = 4 mice/group. Values are means ± SEM. *P < 0.05 vs. all other groups. #P < 0.05 vs. nonstimulated control group. MFI, median fluorescence intensity; t-ERK, total ERK.
Figure 4.
RBP4-induced activation of macrophages and the resulting activation of CD4 T cells are dependent on Erk, JNK, p38, and NF-κB signaling pathways. A: BMDMs stimulated with RBP4 and treated with inhibitors (inh) for p38, Erk, JNK, and NF-κB were cocultured with cell trace–labeled WT CD4 T cells, and proliferation was evaluated by flow cytometry. B: Representative flow cytometry plots. Proliferation index was calculated with FlowJo software. C: IFN-γ secretion in the coculture assay. D: IL-4 secretion in the coculture assay. n = 4 mice/group. Values are means ± SEM. *P < 0.05 vs. all other groups. #P < 0.05 vs. nonstimulated control group. iMAC, not activated, immature macrophage.
MyD88 Is Required for RBP4-Induced Macrophage Activation and Antigen Presentation
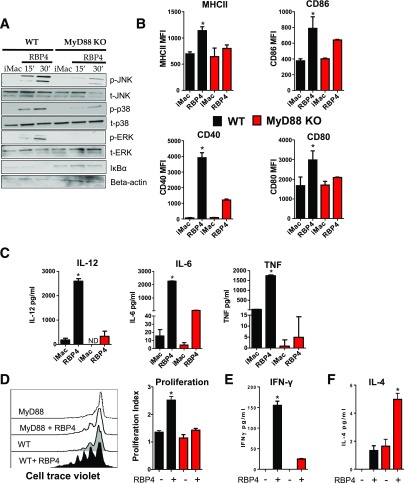
MyD88 is an upstream adaptor that is required for the effects of inflammatory receptors in macrophages on the NF-κB and MAPK pathways. We generated macrophages from MyD88−/− mice to determine whether MyD88 is necessary for RBP4-induced activation of these signaling pathways. MyD88−/− macrophages treated with RBP4 displayed reduced JNK, p38, and Erk phosphorylation and increased IκBα levels compared with macrophages from WT mice (Fig. 5A). RBP4-induced expression of MHCII, CD40, CD80, and CD86 in macrophages was also reduced (Fig. 5B). In macrophages from MyD88−/− mice, the RBP4-induced secretion of IL-6, TNF, and IL-12 was also reduced (Fig. 5C). RBP4 primes macrophages to promote CD4 T-cell proliferation and IFN-γ secretion. These effects were also reduced in MyD88−/− macrophages (Fig. 5D and E). Moreover, the coculture of CD4 T cells with macrophages from MyD88−/− treated with RBP4 showed increased secretion of IL-4 (Fig. 5F) as observed with inhibition of p38, Erk, JNK, and NF-κB (Fig. 4D). Thus, RBP4-induced activation of macrophages and subsequent CD4 T-cell activation are dependent on the MyD88 pathway.
Figure 5.
RBP4-induced activation of macrophages and the resulting activation of CD4 T cells are dependent on the MyD88 pathway. A: BMDMs from WT and MyD88−/− mice were stimulated with RBP4 for 15 or 30 min, and phosphorylation of JNK, p38, and Erk and IκBα disappearance were evaluated by Western blot. Data are representative of 3 independent experiments. B: MHCII and costimulatory molecule (CD80, CD86, and CD40) levels. C: TNF, IL-6, and IL-12 levels. D: BMDMs from WT and MyD88−/− were stimulated with RBP4 and cocultured with cell trace–labeled WT CD4 T cells, and proliferation of CD4 T cells was evaluated by cell trace dilution. Representative histograms indicating CD4 T-cell proliferation (left panel) and proliferation index (right panel). E: IFN-γ secretion in the coculture assay. F: IL-4 secretion in the coculture assay. n = 4 mice/group. Values are means ± SEM. *P < 0.05 vs. all other groups. #P < 0.05 vs. nonstimulated control group. iMAC, not activated, immature macrophage; MFI, median fluorescence intensity.
Blockade of Antigen Presentation Reduces RBP4-Induced AT Inflammation and Insulin Resistance In Vivo
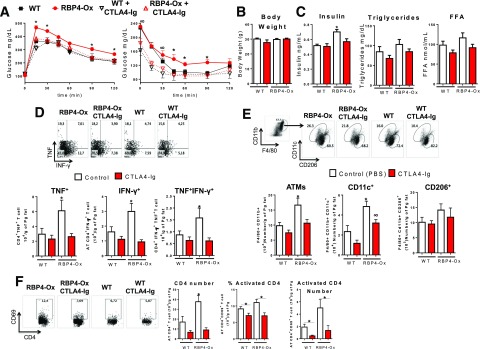
Blocking antigen presentation in AT may result in reduced AT inflammation and increased insulin sensitivity. CTLA-4 is a receptor that acts as an “off” switch when bound to CD80 or CD86 on the surface of APCs, preventing APC-induced T-cell activation. Fusion proteins of CTLA4 and antibodies (CTLA4-Ig) showed success in clinical trials for autoimmune diseases (27). We hypothesized that CTLA4-Ig treatment would reduce insulin resistance in RBP4-Ox mice. After 6 weeks of CTLA4-Ig treatment, RBP4-Ox mice displayed increased glucose tolerance (Fig. 6A, left panel) and improved insulin sensitivity (Fig. 6A, right panel). The body weights of CTLA4-Ig–treated RBP4-Ox and WT mice were comparable to those treated with PBS (Fig. 6B). CTLA4-Ig treatment reduced insulin levels with no effect on serum triglyceride and FFA concentrations (Fig. 6C). Since CTLA-4 reduces inflammation by blocking T-cell activation, we investigated AT CD4 T-cell activation. Treatment with CTLA4-Ig reduced the RBP4-induced increase in the number of AT CD4 T cells intracellularly stained for TNF and IFN-γ to levels observed in PBS-treated WT mice (Fig. 6D). In RBP4-Ox mice, the number of total and CD11c+ AT macrophages is increased compared with WT, and CTLA4-Ig treatment lowered the numbers to PBS-treated WT levels (Fig. 6E). CTLA4-Ig treatment also tended to decrease the number of CD11c+ AT macrophages in WT mice (Fig. 6E). Moreover, CTLA4-Ig–treated RBP4-Ox and WT mice have a reduced number of CD4 T cells and reduced percentage and number of activated (CD4+CD69+) CD4 T cells compared with PBS-treated controls (Fig. 6F). Thus, disruption of antigen presentation with CTLA4-Ig treatment improves insulin resistance and AT inflammation induced by increased RBP4 levels.
Figure 6.
CTLA4-Ig treatment of RBP4-Ox mice reduces RBP4-induced AT inflammation and insulin resistance. RBP4-Ox mice were treated with CTLA4-Ig (intraperitoneally) 3 times a week for 6 weeks. A: Glucose tolerance test (left panel) and insulin tolerance test (right panel). Body weight (B) and serum insulin, triglycerides, and FFA (C). D: Flow cytometry representation of gated AT CD4 T cells intracellularly stained with IFN-γ and TNF (top panel). Number of CD4 T cells intracellularly stained for IFN-γ and TNF (bottom panel). E: Flow cytometry representation (top panel) and number (bottom panel) of total, CD11c+, and CD206+ AT macrophages. F: Flow cytometry representation (left panel) and number (right panel) of total CD4+ and CD4+CD69+ (activated) T cells. n = 10/group. Values are means ± SEM. *P < 0.05 vs. all other groups or as indicated. ∞P < 0.05 vs. chow-fed, same genotype.
Because inflammation can impair adipogenesis (28–31), we investigated whether there were alterations in adipocyte number and adipocyte cell size. Perigonadal fat pad weight, adipocyte number, and adipocyte size were comparable between WT and RBP4-Ox (Supplementary Fig. 4), suggesting that RBP4 overexpression does not influence adipogenesis in vivo.
Discussion
Here, we show that knockout of RBP4 results in lower HFD-induced inflammation in AT and improves insulin resistance. This may have implications for AT inflammation and insulin resistance in people, since RBP4 is elevated in insulin resistant states and a single human nucleotide polymorphism in the RBP4 gene, which confers increased RBP4 expression in AT in humans, is associated with a twofold increased risk of T2D (11). We also show that MyD88 is necessary for RBP4-induced activation of JNK, Erk, p38, and NF-κB pathways in macrophages. These pathways are required for RBP4-induced macrophage proinflammatory cytokine secretion, MHCII, and costimulatory molecule expression and the subsequent CD4 T-cell activation. Blocking the interaction of APCs, such as macrophages, and T cells with CTLA4-Ig treatment is sufficient to improve insulin resistance, glucose homeostasis, and AT inflammation caused by elevated RBP4 levels. This indicates that approaches modulating the cross talk between innate and adaptive immune cells could lead to new therapies for insulin resistance in T2D.
Antigen presentation to T cells involves costimulation of CD28 by CD80/CD86, which provides a built-in fail-safe that prevents T cells from being autoreactive (32). T cells that recognize a peptide MHC complex in the absence of costimulation become anergic (33). Thus, the recognition of MHCII in the absence of the costimulation could have beneficial effects by reducing proinflammatory T cell activation. We show that mice deficient in RBP4 fed an HFD have reduced levels of MHCII and costimulatory molecules and lower number of CD4 T cells producing IFN-γ compared with WT HFD-fed mice. Furthermore, the decreased expression of molecules required for T-cell activation is associated with improved insulin sensitivity in RBP4 KO mice fed an HFD. This could have clinical relevance because MHCII, CD80, and CD86 expression in AT is increased in obese humans (34,35). Furthermore, RBP4 is elevated in insulin resistant people (3), and overexpression of RBP4 in mice causes a similar increase in MHCII, CD80, and CD86 in AT macrophages (9). This resulted in increased antigen presentation and induction of CD4 T-cell proliferation and Th1 polarization (9).
RBP4-Ox mice fed a chow diet had increased numbers of CD206+ AT macrophages, which express higher levels of costimulatory molecules, MHCII, and proinflammatory cytokines and, thus, contribute to AT inflammation (9). They also had increased numbers of CD11c+ (proinflammatory) AT macrophages, but these macrophages do not have an increase in MHCII or costimulatory molecule expression compared with WT (9). On the other hand, the RBP4 KO mice were fed an HFD, which may explain why most of the changes were observed in CD11c+ macrophages. WT HFD-fed mice have a switch from an M2 (CD206+) to M1 (CD11c+) macrophage phenotype, resulting in more proinflammatory AT macrophages (19,26,36). RBP4 KO fed an HFD showed decreased CD11c+ AT macrophage polarization and activation (proinflammatory cytokines) compared with WT on HFD. Thus, activation of macrophages drives AT inflammation with HFD in WT mice or with RBP4 overexpression in mice on a chow diet, but the population of macrophages contributing to AT inflammation is different. Importantly, knockout of RBP4 in HFD-fed mice reduces AT inflammation.
AT antigen presentation integrates the AT inflammatory responses (9,22,24,34,35) and regulates AT T-cell activation and polarization (24). The improvement in insulin resistance that results from blocking costimulatory signals with CTLA4-Ig indicates that the mechanism for RBP4-induced AT inflammation and insulin resistance involves antigen presentation and the resulting T-cell activation. We demonstrate the importance of antigen presentation to T cells for RBP4-induced insulin resistance by showing that treatment of RBP4-Ox mice with CTLA4-Ig improves AT inflammation and insulin resistance. In WT mice fed a chow diet, the level of AT inflammation is low. This explains why the effect of CTLA4-Ig treatment is small in WT compared with RBP4-Ox mice fed a chow diet. A certain degree of inflammation is needed so that there is increased antigen presentation that CTLA4-Ig can inhibit. CTLA4-Ig fusion protein binds to CD80/CD86, thereby blocking the engagement of CD28 on T cells and preventing T-cell activation. The effectiveness of this fusion protein is evident from its success in treating rheumatoid arthritis and graft rejection in humans (37,38). The possibility that this approach could be beneficial for insulin resistant people is supported by observational studies showing that patients with rheumatoid arthritis who are treated with CTLA4-Ig show improved whole-body insulin sensitivity and glucose tolerance (39,40). Our data indicate that CTLA4-Ig treatment reduces CD4 T-cell activation in AT and proinflammatory AT macrophage numbers, resulting in improved overall glucose homeostasis, which could be the mechanism for improved insulin sensitivity in rheumatoid arthritis patients treated with this compound.
Here, we elucidated the pathways triggered by RBP4 that are required for macrophage-induced CD4 T-cell activation. JNK expression in macrophages is necessary for both AT inflammation and obesity-induced insulin resistance (25). We find that in addition to the JNK pathway, Erk, p38, and NF-κB pathways are required for macrophage activation by RBP4 and subsequent induction of CD4 T-cell proliferation and IFN-γ secretion. Moreover, the activation of these pathways by RBP4 is MyD88 dependent. The importance of MyD88 in obesity is demonstrated by the finding that endogenous molecules (ceramides, high-mobility group box 1, fetuin-A, and modified LDLs) that activate Toll-like receptor 4 signaling require MyD88 to activate the downstream NF-κB and MAPK pathways (41). These pathways inhibit insulin action by impairing insulin receptor signaling in adipocytes and induce the transcription of proinflammatory cytokines (TNF, IL-6, and IL-1β) in immune cells (42). We observed that MyD88 deletion in macrophages impairs RBP4-induced macrophage activation and subsequent CD4 T-cell Th1 polarization. This is important, since Th1 cells play a critical role in AT inflammation. Deletion of Th1 cells or their product (IFN-γ) reduces AT inflammation and improves insulin resistance during obesity (23,43–45). Thus, the proinflammatory effects of RBP4 in macrophages involve MyD88 and its downstream pathways, which could be therapeutic targets in insulin resistant states in which RBP4 levels are elevated. In addition, RBP4 increases the capacity of AT macrophages to induce a proinflammatory CD4 T-cell response, and when this is blocked in vivo, AT inflammation and insulin resistance are improved.
Supplementary Material
Article Information
Acknowledgments. The authors thank Juanjuan Zhao for technical support.
Funding. This study was supported by National Institutes of Health grants R37-DK-43051 and P30-DK-57521 (to B.B.K.), a fellowship from FAPESP 2011/12682-4 and 2014/02218-6 (to A.C.), and a grant from the JPB Foundation (to B.B.K.).
Duality of Interest. O.D.P. and B.B.K. are inventors on a patent on RBP4. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. P.M.M.-V. and A.C. conducted experiments, acquired data, and analyzed data. P.A. and K.W. conducted experiments and acquired data. P.M.M.-V., A.C., O.D.P., and B.B.K. designed research studies. P.M.M.-V. and B.B.K. wrote the manuscript. B.B.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1696/-/DC1.
A.C. is currently affiliated with the Department of Immunology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil.
References
- 1.Andrade-Oliveira V, Camara NO, Moraes-Vieira PM: Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res 2015;2015:681612 [DOI] [PMC free article] [PubMed]
- 2.Aeberli I, Biebinger R, Lehmann R, L’allemand D, Spinas GA, Zimmermann MB. Serum retinol-binding protein 4 concentration and its ratio to serum retinol are associated with obesity and metabolic syndrome components in children. J Clin Endocrinol Metab 2007;92:4359–4365 [DOI] [PubMed] [Google Scholar]
- 3.Graham TE, Yang Q, Blüher M, et al. . Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med 2006;354:2552–2563 [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Graham TE, Mody N, et al. . Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–362 [DOI] [PubMed] [Google Scholar]
- 5.Faghihi T, Radfar M, Abdoli E, Amini H, Hemami MR, Larijani B. Association of serum retinol-binding protein 4 with insulin resistance and metabolic parameters during olanzapine therapy. Clin Endocrinol (Oxf) 2012;76:207–211 [DOI] [PubMed] [Google Scholar]
- 6.Sun Q, Kiernan UA, Shi L, et al. . Plasma retinol-binding protein 4 (RBP4) levels and risk of coronary heart disease: a prospective analysis among women in the nurses’ health study. Circulation 2013;127:1938–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meisinger C, Rückert IM, Rathmann W, et al. . Retinol-binding protein 4 is associated with prediabetes in adults from the general population: the Cooperative Health Research in the Region of Augsburg (KORA) F4 Study. Diabetes Care 2011;34:1648–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi Q, Yu Z, Ye X, et al. . Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in Chinese people. J Clin Endocrinol Metab 2007;92:4827–4834 [DOI] [PubMed] [Google Scholar]
- 9.Moraes-Vieira PM, Yore MM, Dwyer PM, Syed I, Aryal P, Kahn BB. RBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistance. Cell Metab 2014;19:512–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am J Physiol Endocrinol Metab 2009;297:E1420–E1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munkhtulga L, Nagashima S, Nakayama K, et al. . Regulatory SNP in the RBP4 gene modified the expression in adipocytes and associated with BMI. Obesity (Silver Spring) 2010;18:1006–1014 [DOI] [PubMed] [Google Scholar]
- 12.van Hoek M, Dehghan A, Zillikens MC, Hofman A, Witteman JC, Sijbrands EJ. An RBP4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia 2008;51:1423–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moraes-Vieira PM, Bassi EJ, Araujo RC, Câmara NO. Leptin as a link between the immune system and kidney-related diseases: leading actor or just a coadjuvant? Obes Rev 2012;13:733–743 [DOI] [PubMed] [Google Scholar]
- 14.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–246 [DOI] [PubMed] [Google Scholar]
- 15.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J Clin Endocrinol Metab 2007;92:1971–1974 [DOI] [PubMed] [Google Scholar]
- 16.Yao-Borengasser A, Varma V, Bodles AM, et al. . Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab 2007;92:2590–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norseen J, Hosooka T, Hammarstedt A, et al. . Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:2010–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell 2015;161:146–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008;57:3239–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013;339:172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris DL, Cho KW, Delproposto JL, et al. . Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 2013;62:2762–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stolarczyk E, Lord GM, Howard JK. The immune cell transcription factor T-bet: A novel metabolic regulator. Adipocyte 2014;3:58–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho KW, Morris DL, DelProposto JL, et al. . An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Reports 2014;9:605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han MS, Jung DY, Morel C, et al. . JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013;339:218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes 2007;56:16–23 [DOI] [PubMed] [Google Scholar]
- 27.Cutolo M, Nadler SG. Advances in CTLA-4-Ig-mediated modulation of inflammatory cell and immune response activation in rheumatoid arthritis. Autoimmun Rev 2013;12:758–767 [DOI] [PubMed] [Google Scholar]
- 28.Xing H, Northrop JP, Grove JR, Kilpatrick KE, Su JL, Ringold GM. TNF alpha-mediated inhibition and reversal of adipocyte differentiation is accompanied by suppressed expression of PPARgamma without effects on Pref-1 expression. Endocrinology 1997;138:2776–2783 [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Sethi JK, Hotamisligil GS. Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J Biol Chem 1999;274:26287–26295 [DOI] [PubMed] [Google Scholar]
- 30.Nov O, Shapiro H, Ovadia H, et al. . Interleukin-1β regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS One 2013;8:e53626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todoric J, Strobl B, Jais A, et al. . Cross-talk between interferon-γ and hedgehog signaling regulates adipogenesis. Diabetes 2011;60:1668–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roche PA, Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat Rev Immunol 2015;15:203–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macián F, Im SH, García-Cózar FJ, Rao A. T-cell anergy. Curr Opin Immunol 2004;16:209–216 [DOI] [PubMed] [Google Scholar]
- 34.Deng T, Lyon CJ, Minze LJ, et al. . Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab 2013;17:411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatzigeorgiou A, Chung KJ, Garcia-Martin R, et al. . Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology 2014;60:1196–1210 [DOI] [PubMed] [Google Scholar]
- 36.Castoldi A, Naffah de Souza C, Câmara NO, Moraes-Vieira PM. The Macrophage Switch in Obesity Development. Front Immunol 2015;6:637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisman MH, Durez P, Hallegua D, et al. . Reduction of inflammatory biomarker response by abatacept in treatment of rheumatoid arthritis. J Rheumatol 2006;33:2162–2166 [PubMed] [Google Scholar]
- 38.Satyananda V, Shapiro R. Belatacept in kidney transplantation. Curr Opin Organ Transplant 2014;19:573–577 [DOI] [PubMed] [Google Scholar]
- 39.Ursini F, Russo E, Letizia Hribal M, et al. . Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore) 2015;94:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ursini F, Mauro D, Naty S, Gagliardi D, Grembiale RD. Improvement in insulin resistance after short-term treatment with abatacept: case report and short review. Clin Rheumatol 2012;31:1401–1402 [DOI] [PubMed] [Google Scholar]
- 41.Jialal I, Kaur H, Devaraj S. Toll-like receptor status in obesity and metabolic syndrome: a translational perspective. J Clin Endocrinol Metab 2014;99:39–48 [DOI] [PubMed] [Google Scholar]
- 42.Prajapati B, Jena PK, Rajput P, Purandhar K, Seshadri S. Understanding and modulating the Toll like Receptors (TLRs) and NOD like Receptors (NLRs) cross talk in type 2 diabetes. Curr Diabetes Rev 2014;10:190–200 [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Youm YH, Vandanmagsar B, et al. . Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol 2010;185:1836–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18:1918–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winer S, Chan Y, Paltser G, et al. . Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quadro L, Blaner WS, Hamberger L, et al. . Muscle expression of human retinol-binding protein (RBP). Suppression of the visual defect of RBP knockout mice. J Biol Chem 2002;277:30191–30197 [DOI] [PubMed] [Google Scholar]
- 47.Nguyen MT, Favelyukis S, Nguyen AK, et al. . A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 2007;282:35279–35292 [DOI] [PubMed] [Google Scholar]
- 48.Zemany L, Bhanot S, Peroni OD, et al. . Transthyretin antisense oligonucleotides lower circulating RBP4 levels and improve insulin sensitivity in obese mice. Diabetes 2015;64:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zemany L, Kraus BJ, Norseen J, et al. . Downregulation of STRA6 in adipocytes and adipose stromovascular fraction in obesity and effects of adipocyte-specific STRA6 knockdown in vivo. Mol Cell Biol 2014;34:1170–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moraes-Vieira PM, Larocca RA, Bassi EJ, et al. . Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur J Immunol 2014;44:794–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moraes-Vieira PM, Bassi EJ, Larocca RA, et al. . Leptin deficiency modulates allograft survival by favoring a Th2 and a regulatory immune profile. [corrected]. Am J Transplant 2013;13:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.