Abstract
Beige adipocytes emerge postnatally within the white adipose tissue in response to certain environmental cues, such as chronic cold exposure. Because of its highly recruitable nature and relevance to adult humans, beige adipocytes have gained much attention as an attractive cellular target for antiobesity therapy. However, molecular circuits that preferentially promote beige adipocyte biogenesis remain poorly understood. We report that a combination of mild cold exposure at 17°C and capsinoids, a nonpungent analog of capsaicin, synergistically and preferentially promotes beige adipocyte biogenesis and ameliorates diet-induced obesity. Gain- and loss-of-function studies show that the combination of capsinoids and cold exposure synergistically promotes beige adipocyte development through the β2-adrenoceptor signaling pathway. This synergistic effect on beige adipocyte biogenesis occurs through an increased half-life of PRDM16, a dominant transcriptional regulator of brown/beige adipocyte development. We document a previously unappreciated molecular circuit that controls beige adipocyte biogenesis and suggest a plausible approach to increase whole-body energy expenditure by combining dietary components and environmental cues.
Introduction
Obesity develops from a chronic imbalance in energy homeostasis between energy intake and energy expenditure. Currently, all the available antiobesity medications act by limiting energy intake through suppression of appetite or inhibition of intestinal lipid absorption; however, long-term use of such medications often is associated with adverse effects, such as depression and steatorrhoea (1). Over the past few years, a growing body of evidence from studies on rodent models and adult humans has indicated that activating thermogenesis in brown adipose tissue (BAT) is a plausible alternative approach to modulate whole-body energy balance (2).
Brown adipocytes dissipate chemical energy and produce heat through the BAT-specific mitochondrial protein uncoupling protein 1 (UCP1). The thermogenic capacity of UCP1 to uncouple cellular respiration from ATP synthesis is highly regulated by the activation of β-adrenoreceptors (β-ARs) through the sympathetic nervous system. At a molecular level, catecholamines released from sympathetic nerve terminals bind to β-ARs in response to cold exposure, leading to the production of free fatty acids by lipolysis. The produced free fatty acids are a critical switch for the proton uncoupling activity of UCP1 (3). Extensive efforts have been made to pharmacologically activate BAT thermogenesis by using synthetic β-AR agonists. Recently, a selective β3-AR agonist, mirabegron, has been demonstrated to powerfully activate BAT metabolic activity, as assessed by 18F-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography scans, which leads to an increased resting metabolic rate in healthy adult humans who possess detectable BAT depots (4). On the other hand, the efficacy of the β3-AR agonists was marginal or absent in the obese population (5,6). Furthermore, impaired expression and functional activity of β3-AR are reported in obese animals and humans. For example, a variant allele of the human β3-AR gene (64 Trp/Arg) is associated with reduced β3-AR signaling (7,8) and increased BMI and adiposity (9,10). Hence, understanding the regulatory circuits that enhance the β-AR signaling pathway within adipose tissues is important such that clinically significant efficacy can be achieved even in obese subjects.
Rodents and humans possess two distinct forms of UCP1-positive thermogenic adipocytes: classical brown adipocytes and beige adipocytes (also referred to as brite adipocytes). Whereas classical brown adipocytes and beige adipocytes share many functional characteristics (i.e., thermogenesis), they are distinct cell types at developmental, anatomical, and molecular levels. Classical brown adipocytes are prenatally derived from a subset of dermomyotome, whereas beige/brite adipocytes postnatally emerge within white adipose tissue (WAT) in response to certain environmental cues, such as chronic cold exposure, exercise, and long-term treatment with peroxisome proliferator–activated receptor-γ (PPARγ) agonists (11–13). The environmental cue–induced beige adipocyte biogenesis in WAT often is referred to as WAT browning. Of note, molecular signatures of adult human BAT have been shown to resemble mouse beige adipocytes (12,14–17). We recently found that clonally derived adult human brown adipocytes possess beige-like characteristics based on unbiased RNA sequencing analyses (17). Furthermore, chronic cold exposure for up to 6 weeks recruits new active BAT depots in adult humans who did not possess appreciable levels of BAT depots before cold exposure (18–20). An emergence of the newly recruited BAT has been associated with an increase in cold-stimulated energy expenditure or with improved postprandial insulin sensitivity. The findings from these studies suggest that adult human BAT largely comprises the recruitable form of thermogenic adipocytes, that is, beige adipocytes. Hence, understanding molecular circuits that preferentially promote beige adipocyte biogenesis may provide a new opportunity for antiobesity therapies for obese or older individuals who do not possess active BAT depots.
Capsinoids are capsaicin analogs found in a nonpungent type of chili pepper, CH-19 Sweet (21,22). Although capsinoids differ from capsaicin only in their chemical structure at an ester bond in the center linkage, they possess substantially less pungency than capsaicin by at least 1,000-fold. Dietary supplementation with capsinoids leads to an increase in energy expenditure and reduced body weight gain in animal models as well as in adult humans (23,24). For example, Yoneshiro et al. (20) showed that prolonged dietary supplementation with capsinoids for 6 weeks increased cold-induced thermogenesis in adult humans. Given the relevance of beige adipocytes in adult humans, it is hypothesized that long-term capsinoids treatment promotes beige adipocyte biogenesis. However, the underlying mechanisms remain poorly understood.
We report that dietary supplementation of capsinoids in a mild cold environment (17°C) powerfully and preferentially promotes beige adipocyte biogenesis in rodent models. This WAT browning effect by capsinoids was well associated with an increase in whole-body energy expenditure. Gain- and loss-of-function studies showed that beige adipocyte biogenesis by capsinoids is largely mediated through an activation of the β2-AR signaling pathway. Of note, we found that an activation of the β2-AR signaling pathway enhanced the protein half-life of PRDM16, a dominant transcriptional regulator of brown and beige adipocyte development. These results illuminate a molecular circuit that preferentially promotes beige adipocyte development in vivo. This study also suggests a plausible approach to increasing whole-body energy expenditure through dietary components.
Research Design and Methods
Animals
Male C57BL/6J mice were purchased from Charles River at 7 weeks of age. Triple knockout mice of the β1-, β2-, and β3-ARs (β-less mice) were a gift from B.B. Lowell of Harvard Medical School. Mice were kept at 17 ± 1°C (17°C) or 25 ± 1°C (25°C) in a controlled light environment (12-h cycle). The temperature was precisely controlled by an animal environmental control system (LP-30CCFL-8CTAR; NKsystem).
After a 1-week acclimation at 17°C or 25°C on a regular diet, the mice were randomly assigned to two groups. One group (n = 6) was allowed ad libitum access to a high-fat diet (HFD), and the other group (n = 6) received an HFD supplemented with 0.3% (weight for weight) capsinoids, which contained capsiate (62.7%), dihydrocapsiate (32.2%), and nordihydrocapsiate (5.5%) (Yoyu-Lab) for 8 weeks. Nutritional content of the HFD containing capsinoids is provided in Supplementary Table 1. The experiments in β-less mice were performed for 4 weeks.
For the pharmacological experiments using β-AR antagonists, 9-week-old male C57BL/6J mice were kept at 17°C and fed an HFD or HFD supplemented with 0.3% capsinoids. Mice were injected subcutaneously with a β2-AR antagonist (ICI 118,551; Tocris) at a dose of 2 mg/kg/day or a β3-AR antagonist (SR59230A; Sigma-Aldrich) at a dose of 1 mg/kg/day for 4 weeks. For the experiments using β-AR agonists, 8-week-old male C57BL/6J mice were injected intraperitoneally with a β2-AR agonist (formoterol; Santa Cruz Biotechnology) at a dose of 1 mg/kg or a β3-AR agonist (CL316243; Sigma-Aldrich) at a dose of 1 mg/kg for 1 week. The dose of each compound was based on the previous studies that used the β3-AR agonist for the induction of beige adipocyte development in mice (15). All animal protocols were approved by the Animal Committee of Ajinomoto Co., Inc., or by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Metabolic Parameters
Whole-body energy expenditure of mice was assessed with a metabolic chamber (ARCO2000-RAT/ANI System; Arco) as described previously (23). Mice were injected intraperitoneally with vehicle (saline) or CL316243 at a dose of 0.01 mg/kg during the measurements. The respiratory quotient (VCO2/VO2) was calculated based on Weir (25). Spontaneous activity of the mice was simultaneously measured with an activity sensor (NS-AS01; Neuroscience). All measurements were performed at 4.5-min intervals.
For the glucose tolerance test, mice were injected with glucose 1 g/kg i.p. after a 6-h fast. An insulin tolerance test was performed by injecting insulin 0.75 units/kg after an overnight fast. Blood glucose levels were measured with blood glucose test strips (Arkary) postinjection at 15, 30, 60, and 120 min. Fasting plasma glucose levels were determined with FUJI DRI-CHEM (Fujifilm). Plasma insulin levels were measured with a commercially available ELISA (Morinaga). Plasma triglyceride (TG) levels were measured with a Wako TG test kit. Total liver lipids were extracted with a chloroform:methanol mixture (2:1 volume for volume) as described by Folch et al. (26). The liver TG concentration in the lipid extracts was also measured with the Wako TG test.
Cell Culture
Stromal vascular (SV) fraction was isolated as described by Ohno et al. (27). Adipocyte differentiation was induced in advanced DMEM/F12 medium (d-glucose 25 mmol/L) containing 10% FBS, 0.5 mmol/L isobutylmethylxanthine, 125 nmol/L indomethacin, 5 μmol/L dexamethasone, 850 nmol/L insulin, and 1 nmol/L triiodothyronine. Two days later, the cells were placed in maintenance medium containing 10% FBS, 850 nmol/L insulin, and 1 nmol/L triiodothyronine.
For PRDM16 knockdown experiments in cultured adipocytes, we infected inguinal WAT–derived SV cells with adenoviral vectors expressing short hairpin RNA targeting PRDM16 (sh-PRDM16) or a scramble control (sh-scr) (27). The cells were allowed to differentiate for 7 days into mature adipocytes in the absence or presence of 1 μmol/L of the β2-AR agonist formoterol.
Gene Expression Analysis
Total RNA was isolated using RiboZol (AMRESCO) according to the manufacturer’s protocol. Reverse transcription reactions were performed with an iScript cDNA Synthesis Kit (Bio-Rad). The primer sequences used in the amplification are shown in Supplementary Table 2. Quantitative RT-PCR (qRT-PCR) was performed with iTaq Fast SYBR Green Supermix (Bio-Rad) using an ABI ViiA 7 PCR machine (Life Technologies). Relative mRNA expression was determined by the ΔΔ-Ct method, using TATA-binding protein (TBP) as an internal control.
Western Blotting
Total tissue lysates from BAT and WAT were prepared in radioimmunoprecipitation assay buffer containing 25 mmol/L Tris-HCl (pH 7.6), 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, and 150 mmol/L NaCl supplemented with 1% protease inhibitor (Nacalai Tesque). The protein content was determined by using a Bradford protein assay (Bio-Rad). Proteins were separated by using 7.5% or 10% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad). The membranes were subsequently probed with antibodies for UCP1 (ab10983 1:2,000 dilution; Abcam) or Prdm16 (ab106410 1:2,000 dilution; Abcam). A β-actin antibody (ab8228 1:5,000 dilution; Abcam) was used as a loading control. Proteins were detected using horseradish peroxidase (HRP)–conjugated IgG secondary antibody (goat anti-rabbit IgG-HRP, 4010-05 1:10,000 dilution; Southern Biotech) and Chemi-Lumi One Super (Nacalai Tesque).
Immunohistochemistry
Tissues were fixed overnight at 4°C in 4% paraformaldehyde, washed three times in PBS, infiltrated with 12.5–30% sucrose followed by optimal cutting temperature compound, and frozen in liquid nitrogen. Using a Leica CM3050 S cryostat (Leica Microsystems), 8–12-μm-thick sections were cut, fixed, and permeabilized in 10 mmol/L citric acid (pH 6.0) for 20 min at 121°C and then incubated for 20 min with 2% BSA in PBS. They were incubated for 1 h with an antibody for UCP1 (ab10983 1:2,000 dilution) followed by incubation with secondary HRP-conjugated antibodies (goat anti-rabbit IgG-HRP, 4010-05 1:200 dilution) and detected with the AEC Chromogen Kit (Sigma).
Protein Stability Assay
Inguinal WAT–derived SV cells were differentiated into mature adipocytes in the presence or absence of 1 μmol/L formoterol. Subsequently, the differentiated adipocytes were incubated in medium containing cycloheximide (20 μg/mL) for 1, 8, 16, and 24 h. Cell lysates were subjected to Western blotting to quantify the endogenous PRDM16 protein levels. β-Actin was used as a loading control. ImageJ software was used to quantify the signal intensity.
Oxygen Consumption Assays
Mice were anesthetized and transcardially perfused with Krebs-Ringer bicarbonate HEPES buffer (KRBH) containing 120 mmol/L NaCl, 4 mmol/L KH2PO4, 1 mmol/L MgSO4, 0.75 mmol/L CaCl2, 10 mmol/L NaHCO3, and 30 mmol/L HEPES (pH 7.4). Adipose depots were carefully dissected and minced in KRBH supplemented with 0.45 mg/mL d-glucose, 10 mg/mL fatty acid–free BSA, and 1 mg/mL collagenase II (Sigma). After 1 h of incubation at 37°C, adipose tissues were filtered through a 200-μm nylon mesh and centrifuged at 200g for 1 min at ambient temperature. The oxygen consumption rate (OCR) of the isolated adipocytes (4–6 × 105 cells from inguinal WAT) was measured with a Clark-type oxygen electrode at 37°C in buffer containing KRBH supplemented with 0.486 mg/mL d-glucose and 40 mg/mL fatty acid–free BSA in a total volume of 2 mL. Saline or norepinephrine (100 nmol/L) was added to assess thermogenic response to cAMP stimuli. OCR was measured every 0.5 s up to 10 min.
Statistical Analysis
Statistical analysis was performed with JMP version 10.0 software (SAS Institute, Inc.). Repeated-measures ANOVA was applied to compare the time courses. Statistical significance was determined with the unpaired two-tailed Student t test for single- and two-way ANOVAs followed by Bonferroni posttests for multiple variables. Values are shown as the mean ± SEM unless otherwise stated. P < 0.05 was considered significant.
Results
A Synergistic Antiobesity Effect by Capsinoids and Mild Cold Exposure
We first examined whether the effect of capsinoids on whole-body energy metabolism is influenced by temperature. To this end, C57BL/6J male mice were fed an HFD containing capsinoids at a concentration of 0.3% or vehicle under ambient temperature (25°C) and mild cold conditions (17°C) for 8 weeks. Consistent with previous studies (23), mice gained modest, but significantly less body weight on a capsinoids-supplemented diet compared with mice on a control diet when kept at ambient temperature. The antiobesity effect by capsinoids was significantly enhanced under mild cold at 17°C (Fig. 1A). This antiobesity effect by the combination of capsinoids and mild cold exposure was synergistic because capsinoids supplementation under 17°C led to a 31% suppression of diet-induced body weight gain compared with control mice, whereas capsinoids or mild cold exposure alone reduced body weight gain by 14% and 12%, respectively. The synergy is statistically significant according to the analysis by interaction plot (P = 0.03). This synergistic antiobesity effect was independent of changes in energy intake and behavior because no major difference was observed in food intake and locomotor activity between the vehicle- and capsinoids-treated groups both at ambient and cold temperature (Supplementary Fig. 1A and B). We also found that the antiobesity effect by capsinoids was completely blunted under thermoneutral conditions at 30°C (Fig. 1B). These data indicate that dietary supplementation of capsinoids promotes cold-induced thermogenesis in vivo.
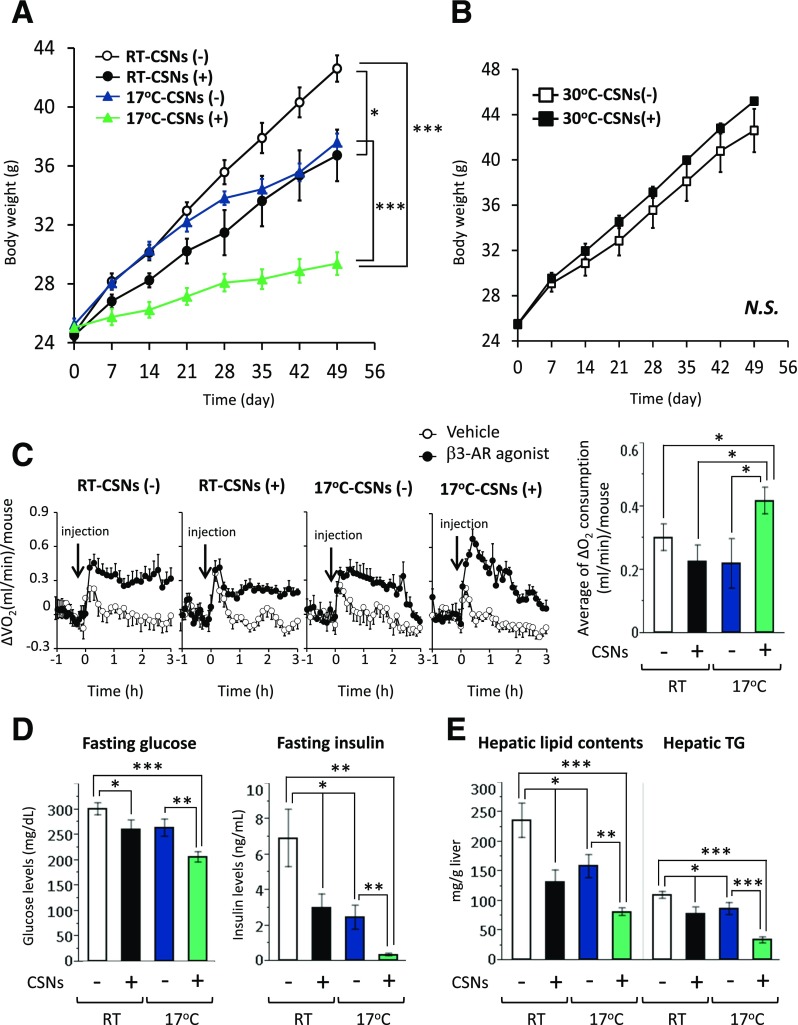
Figure 1.
Capsinoids (CSNs) and mild cold exposure synergistically suppressed body weight gain and increased whole-body energy expenditure on an HFD. A: Changes in body weight of C57BL/6J male mice (n = 6). Mice were fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 8 weeks under ambient temperature (RT) or 17°C. Data are mean ± SEM. *P < 0.05, ***P < 0.001. B: Changes in body weight of C57BL/6J mice kept under thermoneutral conditions (30°C) (n = 6). C57BL/6J male mice were fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 8 weeks. C: Whole-body OCR (mL/min) per mouse was measured in C57BL/6J male mice fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) at RT or at 17°C (n = 6). To stimulate BAT thermogenesis, mice were injected with vehicle (saline) or the β3-AR agonist (CL316243) at a dose of 0.01 mg/kg body weight. OCR was monitored for 3 h after the injection. Quantification of changes in VO2 in response to CL316243 is also shown (right). These changes were calculated by subtracting the VO2 of vehicle-treated mice from the VO2 of β3-AR agonist–treated mice. *P < 0.05. D: Fasting blood glucose levels and fasting insulin levels in mice in panel A. Mice were fasted for 3 h before blood collection. *P < 0.05, **P < 0.01, ***P < 0.001. E: Total lipid content and TG content were measured in the liver of mice in panel A. *P < 0.05, **P < 0.01, ***P < 0.001. N.S., not significant.
We next measured changes in whole-body OCR of mice treated with vehicle and capsinoids in response to the β3-AR agonist CL316243 to mimic cold-induced activation of β3-AR signaling. As shown in Fig. 1C, CL316243 significantly increased the whole-body OCR, representing BAT-mediated thermogenesis (28). The β3-AR agonist–induced whole-body OCR was increased in mice treated with capsinoids compared with vehicle-treated mice under 17°C (Fig. 1C and Supplementary Fig. 2).
To further examine metabolic consequences of capsinoids supplementation and mild cold exposure, we assessed systemic glucose homeostasis and lipid metabolism by examining fasting glucose levels, insulin levels, and hepatic lipid contents. Consistent with the changes in body weight, we found that fasting plasma concentrations of glucose and insulin were significantly lower in mice treated with capsinoids under 17°C than in vehicle-treated mice and in mice kept under ambient temperature (Fig. 1D). In addition, glucose tolerance and insulin sensitivity, as assessed by glucose and insulin tolerance tests, respectively, were significantly improved in mice treated with capsinoids under 17°C (Supplementary Fig. 3A and B). We also found that hepatic steatosis was improved by the combination of capsinoids and mild cold because total lipid and TG content in the liver was significantly reduced in mice treated with capsinoids under 17°C (Fig. 1E).
Capsinoids and Mild Cold Exposure Synergistically Promote Beige Adipocyte Biogenesis in Inguinal WAT
On the basis of the aforementioned observation that capsinoids supplementation under mild cold temperature at 17°C synergistically increases energy expenditure, we hypothesized that this increase was through an activation of the thermogenic program in the interscapular BAT depots and/or through recruitment of new beige adipocytes in the subcutaneous WAT depots. To this end, we first measured mRNA expression of the BAT-selective genes and beige adipocyte–selective genes in the inguinal WAT and in the interscapular BAT depots by qRT-PCR. Expression of the BAT-selective genes, such as Ucp1, Pgc1α, and Cidea, and beige adipocyte–selective genes, such as Cd137 and Tmem26, was significantly higher in the inguinal WAT of mice treated with capsinoids under 17°C than in vehicle-treated mice and mice kept under ambient temperature (Fig. 2A and Supplementary Fig. 4A, left panel). No significant change was observed in the expression of a general adipogenic marker, adiponectin (Adipoq) (Supplementary Fig. 5). We did not observe statistically significant changes in mRNA expression of the thermogenic genes and beige adipocyte–selective markers in the interscapular BAT depots by capsinoids treatment, although the UCP1 mRNA levels were ∼100-fold higher in the BAT than in the inguinal WAT (Fig. 2A and Supplementary Fig. 4A, right panel, and B).
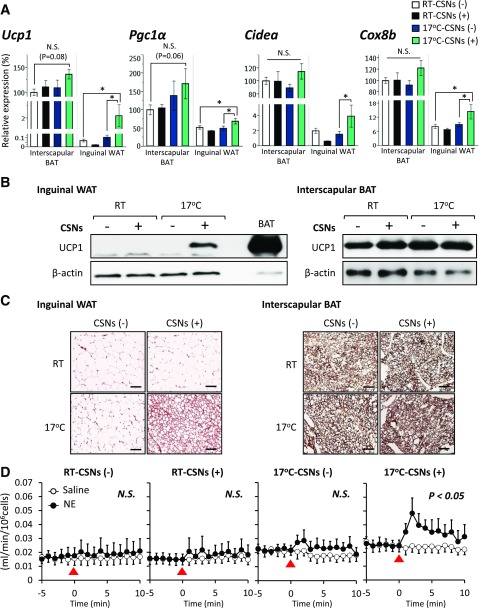
Figure 2.
Capsinoids (CSNs) and mild cold exposure synergistically promote beige adipocyte biogenesis in inguinal WAT. A: Relative mRNA expression levels of Ucp1, Pgc1α, Cidea, and Cox8b were measured by qRT-PCR in the inguinal WAT and the interscapular BAT of mice kept under ambient temperature (RT) or 17°C (n = 6). Mice were fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 8 weeks. Data are mean ± SEM. *P < 0.05. B: UCP1 protein levels were analyzed by Western blotting in the inguinal WAT and the interscapular BAT from mice in panel A. β-Actin was used as a loading control. C: Immunohistochemistry of UCP1 in the inguinal WAT and the interscapular BAT from mice in panel A. Scale bar = 100 μm. D: Cellular respiration was measured in isolated adipocytes from the inguinal WAT of mice in panel A. To stimulate thermogenesis, the cells were treated with saline or norepinephrine (NE) at a dose of 1 μmol/L at the 0 time point (indicated by arrowheads). N.S., not significant.
Consistent with the increase in Ucp1 mRNA expression, we also observed a striking increase in UCP1 protein expression in the inguinal WAT of mice treated with capsinoids under 17°C (Fig. 2B, left panel). In contrast, we did not observe major changes in UCP1 protein expression in the interscapular BAT by capsinoids and mild cold exposure (Fig. 2B, right panel), although UCP1 expression in BAT was 50-fold higher than in inguinal WAT when normalized to β-actin (Fig. 2B and Supplementary Fig. 6). This significant increase in UCP1 expression in the inguinal WAT was tightly associated with an increase in the number of UCP1-positive beige adipocytes containing multilocular lipid droplets (Fig. 2C, left panels). We did not observe major changes in UCP1 density in the interscapular BAT depots, although adipocyte size appeared smaller in the BAT from mice treated with capsinoids (Fig. 2C, right panels).
To examine thermogenic function of the newly recruited beige adipocytes, we measured cellular respiration of adipocytes isolated from the inguinal WAT of mice treated with capsinoids under 17°C or ambient temperature. As shown in Fig. 2D, adipocytes from mice treated with capsinoids under 17°C exhibited significantly higher cellular respiration in response to norepinephrine stimulation, whereas no major change was seen in adipocytes from vehicle-treated mice and from mice under ambient temperature. Together, these results suggest that a combination of capsinoids supplementation and mild cold exposure synergistically and preferentially promotes beige adipocyte biogenesis in vivo.
Although capsinoids are hydrolytically unstable under aqueous conditions and quickly degraded in the gastrointestinal tract (29), we tested the possibility that capsinoids in the circulation may directly promote beige adipocyte biogenesis by using the plasma from mice fed capsinoids. The plasma samples were isolated from the mice that exhibited higher levels of UCP1 and other beige adipocyte–selective gene expression by capsinoids treatment. As shown in Supplementary Fig. 7, the plasma from mice fed capsinoids did not affect the expression of thermogenic genes in inguinal WAT–derived primary adipocytes.
The β-AR Pathway Is Required for the Capsinoids-Induced Antiobesity Effect and Beige Adipocyte Biogenesis
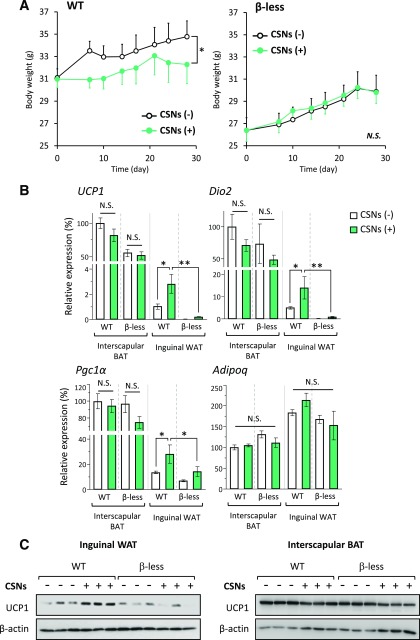
We have previously shown that capsinoids activate the sympathetic nervous system through the gastrointestinal transient receptor potential vanilloid type 1 (TRPV1) channel (30). Because β-ARs play a dominant role in the control of brown and beige adipocyte biogenesis, we hypothesized that capsinoids act through the β-adrenergic signaling pathway to promote beige adipocyte development. To test this hypothesis, we treated β-less mice (31) that lack all three forms of β-ARs (i.e., β1, β2, and β3) with capsinoids under 17°C. Because β-less mice were bred in a mixed genetic background (FVB/C57BL/6J/DBA/2/129SvJ) (31), we used the corresponding age-matched wild-type (WT) mice (6 weeks old) in a mixed genetic background as a control group. Hereafter, we performed all metabolic studies under 17°C. Consistent with the results in the C57BL/6J strain, capsinoids treatment for 4 weeks significantly (although modestly) suppressed HFD-induced body weight gain in WT mice (Fig. 3A, left panel). In contrast, the suppression in body weight gain by capsinoids treatment was completely abolished in β-less mice (Fig. 3A, right panel). Expression of BAT-selective genes, such as Ucp1, Pgc1α, and Dio2, was significantly higher in the inguinal WAT of WT mice treated with capsinoids under 17°C, whereas this induction was largely impaired in β-less mice (Fig. 3B, top panel). Consistent with the previous study (31), the basal level of Ucp1 expression was significantly lower in the interscapular BAT of β-less mice than in WT mice. However, capsinoids treatment did not alter the BAT-selective gene expression in the interscapular BAT of WT and β-less mice (Fig. 3B, bottom panel). Similarly, capsinoids treatment increased UCP1 protein expression in the inguinal WAT of WT mice but not in that of β-less mice (Fig. 3C). These results suggest that the β-adrenergic pathway is required for capsinoids-induced beige adipocyte biogenesis.
Figure 3.
The β-AR pathway is required for the capsinoids (CSNs)-induced antiobesity effect and beige adipocyte biogenesis. A: Changes in body weight of WT and β-less mice fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 4 weeks (n = 6–8). Mice were kept under 17°C throughout the experiments. Data are mean ± SEM. *P < 0.05. B: Relative mRNA expression levels of Ucp1, Pgc1α, Dio2, and Adipoq were measured by qRT-PCR in the inguinal WAT and the interscapular BAT from mice shown in panel A. *P < 0.05, **P < 0.01. C: UCP1 protein expression was analyzed by Western blotting in the inguinal WAT and interscapular BAT from mice shown in panel A. β-Actin was used as a loading control. N.S., not significant.
β2-AR Mediates Capsinoids-Induced Beige Adipocyte Biogenesis
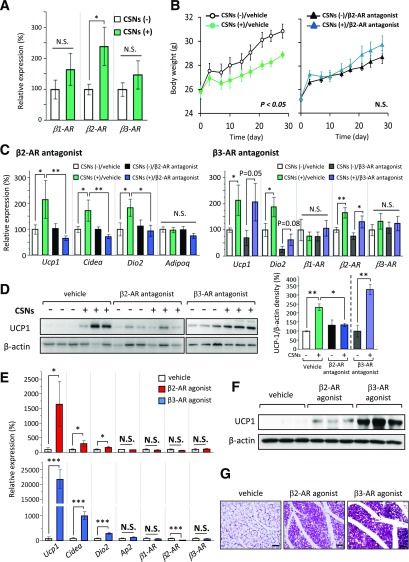
Because three types of β-ARs (β1, β2, and β3) are expressed in WAT, we next asked which form of the β-ARs mediates the biological effect of capsinoids on the browning of WAT in vivo. We found that expression of β2-AR was significantly higher in the inguinal WAT of mice treated with capsinoids, whereas no significant difference was observed in the expression of β1-AR and β3-AR (Fig. 4A). Hence, we hypothesized that capsinoids promote beige adipocyte biogenesis through the β2-AR pathway. To this end, we examined the requirement of β2-AR signaling on the WAT browning by using a specific β2-AR antagonist (ICI 118,551). As shown in Fig. 4B (left panel), mice cotreated with capsinoids and the β2-AR antagonist ICI 118,551 at a dose of 2 mg/kg/day nearly completely abolished the antiobesity effect of capsinoids in mice, even under 17°C. Expression of the BAT-selective genes, such as Ucp1, Cidea, and Dio2, in the inguinal WAT was significantly increased by the capsinoids treatment under 17°C; however, such induction was completely blunted when the β2-AR antagonist was coadministered (Fig. 4C). Consistent with the changes in gene expression levels, cotreatment with the β2-AR antagonist blocked the increase in UCP1 protein expression by capsinoids (Fig. 4D). Of note, we found that the β3-AR antagonist (SR59230A) did not block the increase in UCP1 protein and mRNA expression by capsinoids, indicating that a selective requirement of β2-AR for the capsinoids-induced beige adipocyte biogenesis (Fig. 4B, right panel, and D).
Figure 4.
β2-AR mediates the capsinoids (CSNs)-induced beige adipocyte biogenesis. A: Relative mRNA expression levels of β1-AR, β2-AR, and β3-AR were measured by qRT-PCR in the inguinal WAT of mice fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 8 weeks (n = 6). Mice were kept under 17°C throughout the experiments. Data are mean ± SEM. *P < 0.05. B: Changes in body weight of WT mice fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 4 weeks (n = 6). The mice under 17°C were injected daily with vehicle (saline) or the β2-AR antagonist (ICI 118,551) at a dose of 2 mg/kg/day. C: Relative mRNA expression levels of Ucp1, Cidea, Dio2, Adipoq, β1-AR, β2-AR, and β3-AR were measured by qRT-PCR in the inguinal WAT of mice in panel B and mice injected with vehicle (saline) or a β3-AR antagonist (SR59230A) at a dose of 1 mg/kg/day. *P < 0.05, **P < 0.01. D: UCP1 protein expression was analyzed by Western blotting in the inguinal WAT of mice shown in panel B and mice injected with the β3-AR antagonist (SR59230A) at a dose of 1 mg/kg/day. β-Actin was used as a loading control. Quantification of the UCP1/β-actin is also shown (right). Density of UCP1/β-actin was calculated by ImageJ software. Data are mean ± SEM. *P < 0.05, **P < 0.01. E: Relative mRNA expression levels of Ucp1, Cidea, Dio2, aP2, β1-AR, β2-AR, and β3-AR were measured by qRT-PCR in the inguinal WAT of mice injected with vehicle (saline) or the β2-AR agonist (formoterol) at a dose of 1 mg/kg/day or β3-AR agonist (CL316234) at a dose of 1 mg/kg/day under ambient temperature for 1 week (n = 6). *P < 0.05, ***P < 0.001. F: UCP1 protein expression was analyzed by Western blotting in the inguinal WAT of mice shown in panel E. β-Actin was used as a loading control. G: Hematoxylin and eosin staining of the inguinal WAT of mice shown in panel E. Scale bar = 100 μm. Note that β2-AR agonist (formoterol) treatment led to a striking increase in the number of multilocular adipocytes in the inguinal WAT. N.S., not significant.
We next tested whether activation of the β2-AR pathway is sufficient to promote beige adipocyte biogenesis in vivo. Mice were treated with the β2-AR agonist formoterol or the β3-AR agonist CL316243 at a dose of 1 mg/kg for 1 week. Formoterol is known to selectively bind and activate β2-AR compared with β3-AR by 645.7-fold (32). As shown in Fig. 4E, the β2-AR agonist significantly increased mRNA expression of the BAT-selective genes, such as Ucp1, Cidea, and Dio2, in the inguinal WAT without affecting mRNA expression of β-ARs. Additionally, other β2-AR agonists (selectivity to β2-AR compared with β3-AR: procaterol 1,318.36-fold, salmeterol 851.1-fold) also recruited browning (Supplementary Fig. 8). Formoterol powerfully increased UCP1 protein expression (Fig. 4F) as well as the number of multilocular beige adipocytes in inguinal WAT (Fig. 4G). These data support our hypothesis that the β2-AR pathway largely mediates the effects of capsinoids in promoting beige adipocyte biogenesis in vivo.
Capsinoids Stimulate a Stabilization of the PRDM16 Protein Through the β2-AR Pathway
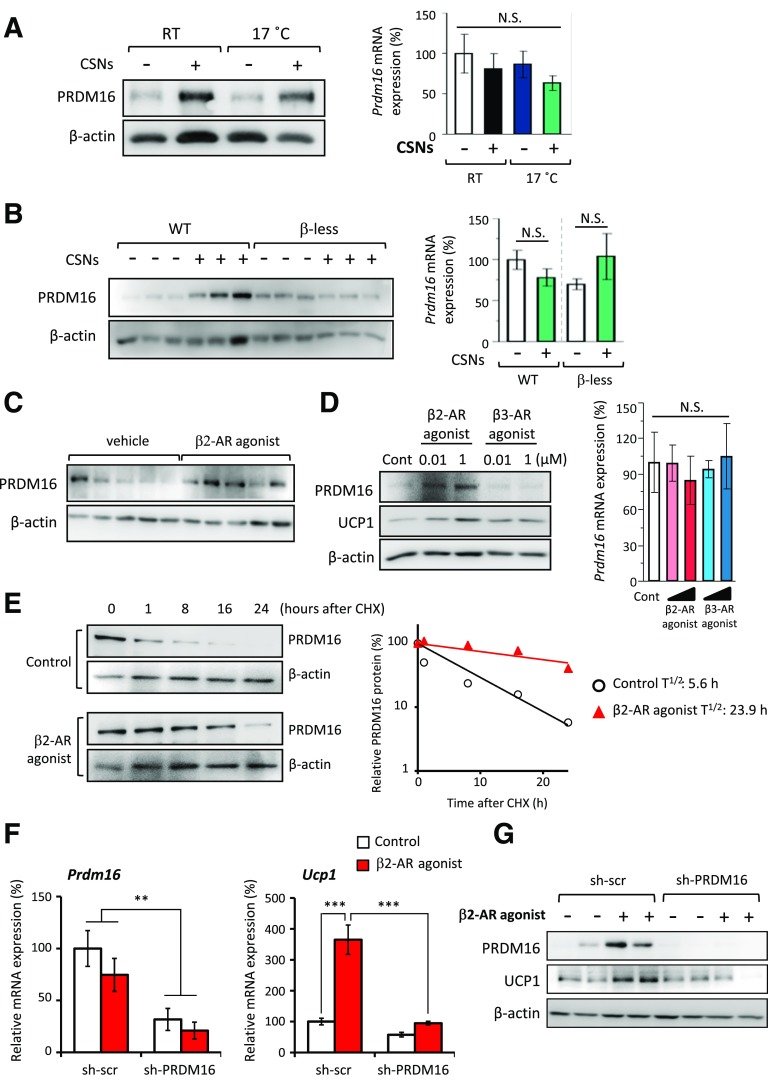
PRDM16 functions as a dominant transcriptional regulator of brown and beige adipocyte development (33–37). We have previously shown that protein stabilization of PRDM16 is a crucial event for PPARγ agonist–induced beige adipocyte development (27). We found that capsinoids robustly increased PRDM16 protein expression in the inguinal WAT of mice under ambient temperature and 17°C without affecting its mRNA levels (Fig. 5A). Furthermore, the increase in PRDM16 protein expression by capsinoids was completely abolished in β-less mice (Fig. 5B) as well as in mice injected with the β2-AR antagonist ICI 118,551 (Supplementary Fig. 9). These results indicate that capsinoids stimulate PRDM16 protein accumulation through the β2-AR pathway.
Figure 5.
Capsinoids (CSNs) stimulate a stabilization of the PRDM16 protein through the β2-AR pathway. A: Endogenous PRDM16 protein and relative mRNA expression of Prdm16 in the inguinal WAT of mice kept under ambient temperature (RT) or 17°C (n = 6). Mice were fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 8 weeks. β-Actin was used as a loading control for Western blotting. The Prdm16 mRNA expression data are mean ± SEM. B: Endogenous PRDM16 protein and relative mRNA expression of Prdm16 in the inguinal WAT of WT mice and β-less mice under 17°C (n = 6). Mice were fed an HFD supplemented with 0.3% CSNs (+) or vehicle (CSNs [−]) for 4 weeks. β-Actin was used as a loading control for Western blotting. C: PRDM16 protein expression was analyzed by Western blotting in the inguinal WAT of mice injected with vehicle (saline) or β2-AR agonist (formoterol) at a dose of 1 mg/kg/day for 1 week (n = 5). β-Actin was used as a loading control. D: Protein expression of PRDM16 and UCP1 was analyzed by Western blotting in primary inguinal WAT–derived adipocytes. The cells were cultured in the absence or presence of β2-AR agonist (formoterol) or β3-AR agonist (CL316243) at doses of 0.01 and 1 μmol/L throughout adipocyte differentiation (n = 3). β-Actin was used as a loading control. Relative Prdm16 mRNA expression in these cells is also shown. E: Cycloheximide (CHX) chase experiment in primary inguinal WAT–derived adipocytes. Endogenous PRDM16 protein expression was analyzed by Western blotting. Differentiated adipocytes were treated with vehicle or β2-AR agonist (formoterol) at a dose of 1 μmol/L in the presence of CHX for 1, 8, 16, and 24 h. β-Actin was used as a loading control. Regression analysis of PRDM16 protein stability is also shown. F: Relative mRNA expression of Prdm16 and Ucp1 was measured by qRT-PCR in primary inguinal WAT–derived adipocytes infected with sh-scr or sh-PRDM16 (n = 3). The adipocytes were treated with vehicle (control) and β2-AR agonist (formoterol) at a dose of 1 μmol/L throughout adipocyte differentiation. **P < 0.01, ***P < 0.001. G: PRDM16 and UCP1 protein expression was analyzed by Western blotting in the primary inguinal WAT–derived adipocytes shown in panel F. β-Actin was used as a loading control. Cont, control; N.S., not significant; T1/2, half-life.
To test whether an activation of the β2-AR pathway is sufficient to increase PRDM16 protein expression, we injected WT mice with the specific β2-AR agonist formoterol at 1 mg/kg for 1 week. As shown in Fig. 5C, treatment with the β2-AR agonist led to a robust increase in PRDM16 protein expression in the inguinal WAT. This increase in PRDM16 protein expression was tightly correlated with increases in BAT-selective gene expression and the number of UCP1-positive beige adipocytes (Fig. 4E–G). Furthermore, we found that the β2-AR agonist increased PRDM16 protein expression in a cell-autonomous manner because formoterol treatment in cultured inguinal WAT–derived primary adipocytes increased PRDM16 protein expression without affecting its mRNA expression (Fig. 5D). Consistent with the animal experiments, the β2-AR agonist–induced PRDM16 protein expression was highly correlated with an increase in BAT-selective gene expression in vitro (Supplementary Fig. 10). The β3-AR agonist did not increase PRDM16 protein expression (Fig. 5D), indicating that β3-AR activation may induce browning of WAT through a distinct mechanism from the β2-AR agonist.
We next tested whether the accumulation of PRDM16 protein by β2-AR stimulation was due to changes in the rate of protein degradation. To this end, we measured the protein half-life of endogenous PRDM16 by cycloheximide chase experiments in inguinal WAT–derived primary adipocytes. As shown in Fig. 5E, the β2-AR agonist formoterol powerfully extended the protein half-life of PRDM16 from 5.6 to 23.9 h. Finally, we tested the requirement of PRDM16 for the β2-AR agonist–induced browning effect. To this end, inguinal WAT–derived primary preadipocytes were infected with adenoviral vectors expressing sh-PRDM16 or sh-scr in the presence or absence of formoterol at a dose of 1 μmol/L throughout adipocyte differentiation. As shown in Fig. 5F and G, the β2-AR agonist formoterol did not increase UCP1 expression when adipocytes were depleted with PRDM16. Altogether, these data indicate that capsinoids act through the β2-AR pathway to stimulate PRDM16 protein stabilization, leading to a powerful activation of the beige adipocyte gene program in inguinal WAT.
Discussion
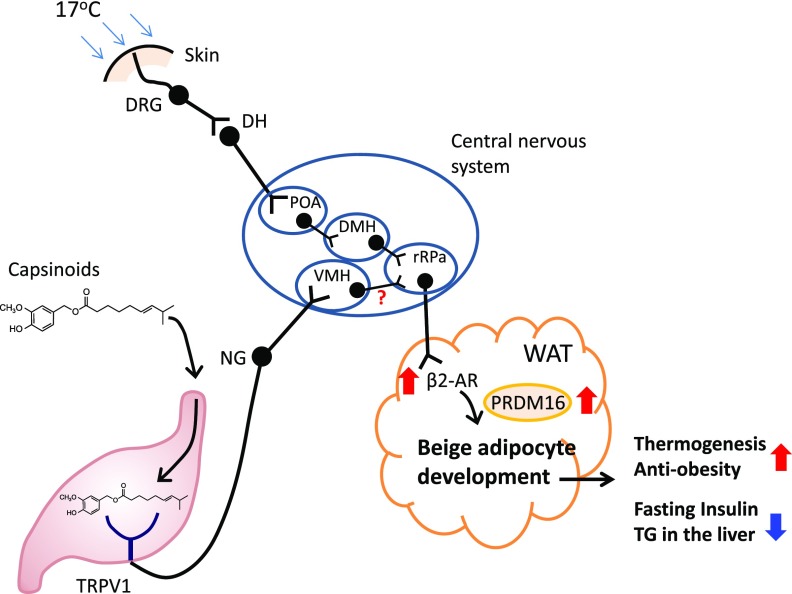
Capsinoids, including capsiate, dihydrocapsiate, and nordihydrocapsiate, are unique capsaicin analogs found abundantly in a nonpungent type of chili pepper, CH-19 Sweet (21,22). The three forms of capsinoids can directly bind to TRPV1 at a similar binding affinity to capsaicin; however, the potency of the capsinoids to increase intercellular Ca2+ levels was ∼10 times lower than capsaicin (38). In addition, the chemical structure of capsinoids is distinct from capsaicin at an ester bond, making capsinoids hydrolytically unstable in an aqueous environment such that they are quickly degraded in the gastrointestinal tract and not detectable in circulation (29). Hence, capsinoids promote the browning of WAT largely through CNS-mediated signaling rather than direct action through the circulation (Supplementary Fig. 5). How do capsinoids synergistically promote BAT-mediated thermogenesis together with mild cold exposure in vivo? As illustrated in Fig. 6, previous studies have shown that capsinoids act on TRPV1 in the gut, leading to an activation of vagal afferent nerves that project into the ventromedial hypothalamus (VMH) (30). Capsinoids also activate several brain regions, including the VMH, in a TRPV1-dependent fashion (39). Of note, the VMH is known to control BAT-mediated thermogenesis by stimulating sympathetic efferent (40,41) and promoting beige adipocyte biogenesis in WAT (42), likely through rostral raphe pallidus (rRPa) neurons (43). In addition, we found that capsinoids increased β2-AR expression in the inguinal WAT. On the other hand, cold exposure acts on distinct neuronal circuits from capsinoids to activate thermogenesis. In response to cold exposure, thermal sensory receptors transmit signals to second-order thermal sensory neurons in the dorsal horn. These neurons activate the signal to the preoptic area where gabanergic neurons control the outputs to the neurons at the dorsomedial nucleus of the hypothalamus and subsequently in the rRPa nucleus (44). Hence, it is conceivable that capsinoids and mild cold exposure stimulate the respective neuronal circuits independently such that a combination of the two independent stimuli promotes an additive thermogenic response more potently than single stimulation alone.
Figure 6.
Proposed mechanisms by which capsinoids and mild cold temperature promote beige adipocyte biogenesis and energy expenditure. Capsinoids bind to TRPV1 in gut. The signal is transmitted to the CNS through vagal nerves that project into the VMH. The signal is subsequently transmitted to subcutaneous WAT depots through the β2-AR in inguinal WAT. Capsinoids increase the expression of β2-AR. However, cold is sensed by skin and transmitted to the somatosensory nerves through second-order thermal sensory neurons in the dorsal horn (DH). These neurons activate the signal to the preoptic area (POA) where gabanergic neurons control the outputs to the neurons at the dorsomedial nucleus of the hypothalamus (DMH) and subsequently in the rRPa nucleus. These stimuli synergistically promote beige adipocyte biogenesis through stabilizing PRDM16 protein in inguinal WAT, leading to an increase in whole-body energy expenditure and a decrease in fasting insulin levels and hepatic TG content. DRG, dorsal root ganglion; NG, nodose ganglion.
Within the inguinal WAT of mice treated with capsinoids, we found that the PRDM16 protein level was robustly increased without affecting its mRNA expression. PRDM16 functions as a dominant transcriptional regulator of brown and beige adipocyte development (33–37). A requirement of PRDM16 for beige adipocyte development has been demonstrated by studies in which adipose-specific depletion of PRDM16 significantly impaired beige adipocyte biogenesis in response to cold exposure or to long-term treatment with PPARγ agonists (27,33). We have shown that PPARγ agonists, such as rosiglitazone, promote beige adipocyte differentiation by powerfully extending the protein half-life of PRDM16 through inhibiting E3 ligase–mediated ubiquitination (27). In addition, rosiglitazone deacetylates PPARγ on Lys268 and Lys293 by Sirt1, leading to an enhanced complex formation between PRDM16 and PPARγ (45). In the current study, we found that capsinoids treatment induces the protein stabilization of PRDM16 through β2-AR signaling. It is known that an activation of β2-AR leads to phosphorylation of protein kinase A, p38 mitogen–activated protein kinase, extracellular signal–regulated kinase, and AMPK (46–48). It is possible that PRDM16 protein is phosphorylated in response to an activation of β2-AR signaling, leading to an increase in PRDM16 protein stability. Future studies are warranted to better understand the molecular mechanisms by which the β2-AR signal extends the half-life of PRDM16 protein.
Previous studies have shown that obese propensity in response to an HFD is highly strain dependent (49). In addition, browning capacity in the subcutaneous WAT depot is highly variable among mouse strains (50–52). Although we found that antiobesity and browning effects by mild cold exposure and capsinoids were more potent in the C57BL/6J background than in the mixed background, these effects were consistently observed in both strains. On the other hand, in previous studies, the effects of capsinoids on hepatic steatosis were observed even under ambient temperature. For instance, treatment with capsinoids alone was sufficient to reduce hepatic lipid and TG content by 44% and 29%, respectively. The current study also found that capsinoids treatment increased the expression of genes involved in hepatic fatty acid oxidation, such as Hsl, Atgl, and Aco, under ambient temperature (Supplementary Fig. 11). This increase in hepatic fatty acid oxidation may contribute to the improvement in lipid metabolism in the liver (e.g., reduced hepatic lipid and TG content) by capsinoids treatment. Relative contribution of liver versus adipose tissue to the improvement of systemic glucose homeostasis remains to be determined.
The biological significance of beige fat in whole-body metabolism has been a topic of discussion because total expression level of UCP1 protein in beige fat is substantially lower than that found in interscapular BAT depots, even though beige adipocytes are functionally thermogenic (Supplementary Fig. 6) (53). In the current study, we found that an increased beige fat mass by capsinoids and mild cold exposure was tightly associated with an increase in whole-body energy expenditure. Of note, the increase in energy expenditure leads to a robust reduction in body weight gain while on an HFD, indicating that beige fat significantly contributes to the regulation of whole-body energy expenditure. This is consistent with several genetic mouse models in which selective modulation of beige fat mass is sufficient to alter whole-body metabolism. For example, transgenic expression of PRDM16 driven by the Fabp4 gene promoter preferentially stimulates the formation of beige adipocytes in the subcutaneous WAT depots without major changes in the interscapular BAT depots. The Fabp4-PRDM16 transgenic mice exhibit increased whole-body energy expenditure and reduced body weight gain while on an HFD (54). Conversely, adipose-selective genetic deletion of Prdm16 or its cofactor EHMT1 by using Adiponectin-Cre mice selectively attenuates beige adipocyte development and reduces whole-body energy expenditure (33,55). Also of note, beige fat plays a major role in systemic glucose and lipid homeostasis. Because brown and beige adipocytes possess high oxidative phosphorylation capacity, they function as a metabolic sink for glucose and fatty acids. Indeed, we found that liver TG level was strikingly lower in mice treated with capsinoids under mild cold in parallel with an increased beige fat mass. Conversely, a developmental defect in brown/beige adipocytes is sufficient to cause hepatic steatosis and insulin resistance in mice (33,55). Hence, the therapeutic benefit of increased beige fat mass not only is limited to antiobesity effects but also can include improvements in hepatic steatosis and insulin resistance.
In summary, the current study found a previously unappreciated molecular circuit that preferentially promotes beige adipocyte biogenesis in vivo. Given the relevance of this cell type in adult humans, this study also illuminates a plausible approach to increase whole-body energy expenditure and to improve lipid and glucose homeostasis by combining dietary components and environmental cues. It has been reported that chronic supplementation of capsinoids at a dose of 9 mg/day for 6 weeks was able to increase cold-stimulated whole-body energy expenditure even in adult humans who lacked detectable active BAT depots before the treatment (20). Because 1 g dry weight of CH-19 Sweet contains ∼5 mg of capsinoids (56), we speculate that supplementary intake would be a realistic approach to achieve the levels of capsinoids that can activate BAT thermogenesis. Future studies aim to establish practical approaches to activate BAT thermogenesis by capsinoids supplementation and mild cold with no deleterious effects.
Supplementary Material
Article Information
Acknowledgments. The authors thank the colleagues of Ajinomoto Co., Inc., S. Takahashi and N. Nishikawa, for technical assistance. They also thank the labmates of Kajimura Lab, H. Hong, L.Z. Sharp, and D. Low, for their assistance.
Funding. K.Sh. is supported by a fellowship from the Japan Society for the Promotion of Science. This work was supported by the National Institutes of Health (DK-087853 and DK-097441), a Diabetes Endocrinology Research Center grant (DK-63720), the Pew Charitable Trusts, and PRESTO from Japan Science and Technology Agency to S.K.
Duality of Interest. This work was supported by grants from Ajinomoto Co., Inc. K.O., Y.N., K.Su., and M.B. are employees of Ajinomoto Co., Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.O. contributed to the data research, discussion, and writing and review/editing of the article. Y.N. and K.Sh. contributed to the data research and review of the article. K.Su. contributed to the review of the article. M.B. and S.K. contributed to the discussion and writing and review/editing of the article. M.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0662/-/DC1.
References
- 1.Cheung BM, Cheung TT, Samaranayake NR. Safety of antiobesity drugs. Ther Adv Drug Saf 2013;4:171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 2014;76:225–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012;151:400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cypess AM, Weiner LS, Roberts-Toler C, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 2015;21:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carey AL, Formosa MF, Van Every B, et al. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 2013;56:147–155 [DOI] [PubMed] [Google Scholar]
- 6.Redman LM, de Jonge L, Fang X, et al. Lack of an effect of a novel beta3-adrenoceptor agonist, TAK-677, on energy metabolism in obese individuals: a double-blind, placebo-controlled randomized study. J Clin Endocrinol Metab 2007;92:527–531 [DOI] [PubMed] [Google Scholar]
- 7.Kimura K, Sasaki N, Asano A, et al. Mutated human beta3-adrenergic receptor (Trp64Arg) lowers the response to beta3-adrenergic agonists in transfected 3T3-L1 preadipocytes. Horm Metab Res 2000;32:91–96 [DOI] [PubMed]
- 8.Piétri-Rouxel F, St. John Manning B, Gros J, Strosberg AD. The biochemical effect of the naturally occurring Trp64→Arg mutation on human beta3-adrenoceptor activity. Eur J Biochem 1997;247:1174–1179 [DOI] [PubMed]
- 9.Mitchell BD, Blangero J, Comuzzie AG, et al. A paired sibling analysis of the beta-3 adrenergic receptor and obesity in Mexican Americans. J Clin Invest 1998;101:584–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sipiläinen R, Uusitupa M, Heikkinen S, Rissanen A, Laakso M. Polymorphism of the beta3-adrenergic receptor gene affects basal metabolic rate in obese Finns. Diabetes 1997;46:77–80 [DOI] [PubMed] [Google Scholar]
- 11.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013;19:1252–1263 [DOI] [PubMed] [Google Scholar]
- 12.Lidell ME, Betz MJ, Dahlqvist Leinhard O, et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013;19:631–634 [DOI] [PubMed] [Google Scholar]
- 13.McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR. Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell 2015;160:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes 2014;38:170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp LZ, Shinoda K, Ohno H, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 2012;7:e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu J, Boström P, Sparks LM, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012;150:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinoda K, Luijten IH, Hasegawa Y, et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med 2015;21:389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 2014;19:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Lans AA, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013;123:3395–3403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013;123:3404–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobata K, Sutoh K, Todo T, Yazawa S, Iwai K, Watanabe T. Nordihydrocapsiate, a new capsinoid from the fruits of a nonpungent pepper, Capsicum annuum. J Nat Prod 1999;62:335–336 [DOI] [PubMed] [Google Scholar]
- 22.Kobata KTT, Yazawa S, Iwai K, Watanabe T. Novel capsaicinoid-like substances, capsiate and dihydrocapsiate, from the fruits of a nonpungent cultivar, CH-19 Sweet, of pepper (Capsicum annuum L.). J Agric Food Chem 1998;46:1695–1697 [Google Scholar]
- 23.Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Biochem 2001;65:2735–2740 [DOI] [PubMed] [Google Scholar]
- 24.Snitker S, Fujishima Y, Shen H, et al. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr 2009;89:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 27.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012;15:395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359 [DOI] [PubMed] [Google Scholar]
- 29.Shirai Y, Ueno S, Nakayama A, et al. Studies of the toxicological potential of capsinoids, XII: pharmacokinetic study of capsinoid-containing CH-19 Sweet extract in rats. Int J Toxicol 2010;29(Suppl.):15S–21S [DOI] [PubMed] [Google Scholar]
- 30.Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, et al. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol (1985) 2011;110:789–798 [DOI] [PubMed] [Google Scholar]
- 31.Bachman ES, Dhillon H, Zhang CY, et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002;297:843–845 [DOI] [PubMed] [Google Scholar]
- 32.Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol 2010;160:1048–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014;156:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajimura S, Seale P, Kubota K, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009;460:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajimura S, Seale P, Tomaru T, et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 2008;22:1397–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008;454:961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 2007;6:38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasahara I, Furuhata Y, Iwasaki Y, et al. Assessment of the biological similarity of three capsaicin analogs (capsinoids) found in non-pungent chili pepper (CH-19 Sweet) fruits. Biosci Biotechnol Biochem 2010;74:274–278 [DOI] [PubMed] [Google Scholar]
- 39.Tsurugizawa T, Nogusa Y, Ando Y, Uneyama H. Different TRPV1-mediated brain responses to intragastric infusion of capsaicin and capsiate. Eur J Neurosci 2013;38:3628–3635 [DOI] [PubMed] [Google Scholar]
- 40.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature 1981;289:401–402 [DOI] [PubMed] [Google Scholar]
- 41.Saito M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation accelerates norepinephrine turnover in brown adipose tissue of rats. Life Sci 1987;41:193–197 [DOI] [PubMed] [Google Scholar]
- 42.Beiroa D, Imbernon M, Gallego R, et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014;63:3346–3358 [DOI] [PubMed] [Google Scholar]
- 43.Martínez de Morentin PB, González-García I, Martins L, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab 2014;20:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 2014;19:741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qiang L, Wang L, Kon N, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012;150:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collins S, Cao W, Robidoux J. Learning new tricks from old dogs: beta-adrenergic receptors teach new lessons on firing up adipose tissue metabolism. Mol Endocrinol 2004;18:2123–2131 [DOI] [PubMed] [Google Scholar]
- 47.Gauthier MS, Miyoshi H, Souza SC, et al. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: potential mechanism and physiological relevance. J Biol Chem 2008;283:16514–16524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omar B, Zmuda-Trzebiatowska E, Manganiello V, Göransson O, Degerman E. Regulation of AMP-activated protein kinase by cAMP in adipocytes: roles for phosphodiesterases, protein kinase B, protein kinase A, Epac and lipolysis. Cell Signal 2009;21:760–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montgomery MK, Hallahan NL, Brown SH, et al. Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 2013;56:1129–1139 [DOI] [PubMed] [Google Scholar]
- 50.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998;102:412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007;48:41–51 [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Bolze F, Fromme T, Klingenspor M. Intrinsic differences in BRITE adipogenesis of primary adipocytes from two different mouse strains. Biochim Biophys Acta 2014;1841:1345–1352 [DOI] [PubMed] [Google Scholar]
- 53.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Reports 2013;5:1196–1203 [DOI] [PubMed] [Google Scholar]
- 54.Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 2011;121:96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 2013;504:163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanaka Y. Research on capsiconinoid contents, nonpungent capsaicinoid analogues, in Capsicum cultivars. Sci Rep Faculty of Agriculture, Okayama University 2014;103:37–43
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.