Abstract
Multiple differentially methylated sites and regions associated with adiposity have now been identified in large-scale cross-sectional studies. We tested for replication of associations between previously identified CpG sites at HIF3A and adiposity in ∼1,000 mother-offspring pairs from the Avon Longitudinal Study of Parents and Children (ALSPAC). Availability of methylation and adiposity measures at multiple time points, as well as genetic data, allowed us to assess the temporal associations between adiposity and methylation and to make inferences regarding causality and directionality. Overall, our results were discordant with those expected if HIF3A methylation has a causal effect on BMI and provided more evidence for causality in the reverse direction (i.e., an effect of BMI on HIF3A methylation). These results are based on robust evidence from longitudinal analyses and were also partially supported by Mendelian randomization analysis, although this latter analysis was underpowered to detect a causal effect of BMI on HIF3A methylation. Our results also highlight an apparent long-lasting intergenerational influence of maternal BMI on offspring methylation at this locus, which may confound associations between own adiposity and HIF3A methylation. Further work is required to replicate and uncover the mechanisms underlying the direct and intergenerational effect of adiposity on DNA methylation.
Introduction
The notion that epigenetic processes are linked to variation in adiposity is well established (1). Genome-wide quantification of site-specific DNA methylation has led to the identification and validation of multiple adiposity-associated differentially methylated sites and regions (2–8).
A large-scale epigenome-wide association study (EWAS) of BMI, undertaken using the Infinium HumanMethylation450 BeadChip array (Illumina Inc., San Diego, CA), found robust associations between BMI and DNA methylation at three neighboring probes in intron 1 of HIF3A, which were confirmed in two additional independent cohorts (6). The site locus has also been associated with adiposity since then in four further studies (7–10). Furthermore, HIF3A methylation has been found to be associated with weight but not height, and methylation at this locus in adipose tissue has been found to be strongly associated with BMI (6,7), indicating that methylation at this locus might be related to some component of adiposity.
HIF3A and other hypoxia-inducible transcription factors regulate cellular and vascular responses to decreased levels of oxygen, and studies in mice suggest they may play key roles in metabolism, energy expenditure, and obesity (11–14). This lends support for a role of this gene in the development of obesity and its consequent comorbidities. However, it is also possible that greater BMI induces changes in HIF3A methylation because the direction of the effect is difficult to discern in these cross-sectional studies (6).
Further research is required to determine the directionality of the association and strengthen the inference regarding causality. A large-scale longitudinal design is warranted to investigate the temporal relationship between baseline adiposity and follow-up methylation, and vice versa (15–17).
Mendelian randomization uses genetic variants as instrumental variables (IVs) to investigate the causal relationship between an exposure and outcome of interest (18–21). The assumptions of this approach are that the IV is robustly related to the exposure, is related to the outcome only through its robust association with the exposure of interest, and is not related to confounding factors for the exposure-outcome association and not influenced by the development of the outcome. If these assumptions are true, then any association observed between the IV and outcome is best explained by a true causal effect of the exposure on the outcome (22). It has been shown that genetic variants are not likely to be related to confounding factors that explain nongenetic associations and are unaffected by disease (23) and, therefore, may be used to strengthen causal inference.
In the context of methylation, Mendelian randomization may be facilitated by the strong cis-effects that allow the isolation of specific loci influencing methylation (24) and has been applied elsewhere to assess causal effects (25,26). In the study that identified differential methylation at HIF3A (6), cis-genetic variants robustly associated with DNA methylation at this locus were used as causal anchors in a pseudo-Mendelian randomization approach to assert no causal effect of methylation at HIF3A on adiposity. However, no attempt was made to investigate causality in the reverse direction (i.e., the causal effect of adiposity on HIF3A methylation). Bidirectional Mendelian randomization may be used to elucidate the causal direction between HIF3A and adiposity by using valid IVs for each trait (21,27,28).
Investigating a possible intergenerational intrauterine effect of maternal BMI on offspring methylation could further strengthen causal inference because it is plausible that maternal BMI could influence offspring methylation through intrauterine effects independent of the offspring’s own BMI (29). Indeed, a recent study postulated and found some evidence for a confounding effect of the prenatal environment on the association between adiposity and HIF3A methylation through an assessment of birth weight (9). Alternatively, confounding by familial socioeconomic and lifestyle characteristics may explain the observed associations between adiposity on HIF3A methylation, and this was not fully assessed in the previous study (6).
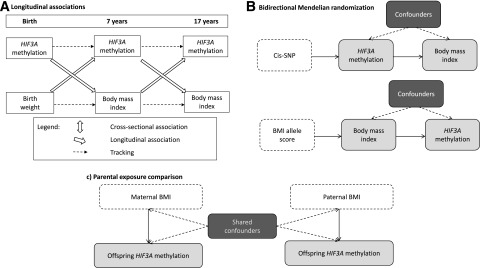
We aimed to investigate associations between methylation at HIF3A and BMI at different ages using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) as part of the Accessible Resource for Integrated Epigenomics Studies (ARIES) project. We first estimated effect sizes for the three previously identified probes in HIF3A, with and without adjustment for a number of potential confounding factors. Given evidence of an association between HIF3A methylation and components of adiposity specifically, we also investigated associations between methylation at HIF3A and fat mass index (FMI) (6,7). To further investigate the dominant direction of causality in any observed associations, we undertook the following additional analyses: 1) investigating longitudinal associations between BMI and methylation, 2) performing bidirectional Mendelian randomization analysis, and 3) determining whether there is an intergenerational effect of parental BMI on offspring methylation through an intrauterine effect of maternal BMI or a postnatal effect of paternal/maternal BMI through shared familial lifestyle or genetic factors (Fig. 1). The results of the various analyses that would be expected under the different hypotheses being tested are outlined in Supplementary Table 1.
Figure 1.
Schematic diagrams of the causal inference methods being implemented in this study. A: Investigating longitudinal associations between BMI and HIF3A methylation. B: Investigating the dominant direction of causality in the association between BMI and HIF3A methylation with the use of bidirectional Mendelian randomization analysis. C: Investigating the intrauterine effect of maternal smoking on offspring DNA methylation with the use of a parental comparison design.
Research Design and Methods
Participants
ALSPAC is a large, prospective birth cohort study based in the South West of England. The study recruited 14,541 pregnant women residents in Avon, U.K., with expected dates of delivery from 1 April 1991 to 31 December 1992, and detailed information has been collected on these women and their offspring at regular intervals (30,31). The study website contains details of all the data that are available through a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/).
As part of the ARIES project (32), the Illumina Infinium HumanMethylation450K (HM450) BeadChip (33) has been used to generate epigenetic data on 1,018 mother-offspring pairs in the ALSPAC cohort (v1; data release 2014). A web portal has been constructed to allow openly accessible browsing of aggregate ARIES DNA methylation data (ARIES-Explorer, http://www.ariesepigenomics.org.uk/).
The ARIES participants were selected based on the availability of DNA samples at two time points for the mother (antenatal and at follow-up when the offspring were adolescents) and three time points for the offspring (neonatal, childhood [mean age 7.5 years], and adolescence [mean age 17.1 years]). We focused our analyses on offspring in the ARIES study who have more detailed longitudinal and parental exposure data available. Therefore, this project uses methylation data from the three time points in the offspring. A detailed description of the data available in ARIES is available in a data resource profile for the study (32).
Written informed consent was obtained from all ALSPAC participants. Ethical approval for the study was obtained from the ALSPAC Ethics and Law Committee and the local research ethics committees.
Methylation Assay: Laboratory Methods, Quality Control, and Preprocessing
We examined DNA methylation in relation to BMI using methylation data from the Infinium HM450 BeadChip (33). The Infinium HM450 BeadChip assay detects the proportion of molecules methylated at each CpG site on the array. For the samples, the methylation level at each CpG site was calculated as a β-value, which is the ratio of the methylated probe intensity and the overall intensity and ranges from 0 (no cytosine methylation) to 1 (complete cytosine methylation) (34,35). All analyses of DNA methylation used these β-values.
Cord blood and peripheral blood samples (whole blood, buffy coats, or blood spots) were collected according to standard procedures, and the DNA methylation wet-laboratory and preprocessing analyses were performed as part of the ARIES project, as previously described (32). In brief, samples from all time points in ARIES were distributed across slides using a semirandom approach to minimize the possibility of confounding by batch effects. The main batch variable was the bisulfite conversion plate number. Samples failing quality control (average probe P value ≥0.01, those with sex or genotype mismatches) were excluded from further analysis and scheduled for repeat assay, and probes that contained <95% of signals detectable above background signal (detection P value <0.01) were excluded from analysis. Methylation data were preprocessed using R 3.0.1 software, with background correction and subset quantile normalization performed using the pipeline described by Touleimat and Tost (36). In the offspring, 914 samples at birth, 973 samples at follow-up in childhood, and 974 samples at follow-up in adolescence passed the quality control.
Anthropometry
In childhood (mean age 7.5 years) and adolescence (mean age 17.1 years), offspring attended follow-up clinics where weight and height were measured with the participant in light clothing and without shoes. Weight was measured to the nearest 0.1 kg with Tanita scales and height to the nearest 0.1 cm using a Harpenden stadiometer. BMI (kg/m2) was then calculated. At the adolescent clinic, fat mass (kg) and lean mass (kg) were also assessed by a Lunar Prodigy dual-energy X-ray absorptiometry (DXA) scanner (GE Medical Systems Lunar, Madison, WI). The scans were visually inspected and realigned where necessary. Once complete, the tester examined the scan to ensure its quality and, if necessary, repeated the scan. The FMI (kg/m2) was calculated.
After recruitment, mothers were asked to report their height and prepregnancy weight in a questionnaire administered at 12 weeks’ gestation, which were then used to calculate prepregnancy maternal BMI. Reported weight was highly correlated with the first antenatal clinic measure of weight (Pearson correlation coefficient 0.95). Partners reported their own heights and weights in questionnaires at 12 weeks’ gestation, which were used to determine paternal BMI. For this study, data for partners who were not confirmed as being the biological father of the child by the mothers’ report were excluded.
Other Variables
Age, sex, birth weight, gestational age, maternal education, household social class, maternal smoking and alcohol consumption in pregnancy, and own smoking and alcohol consumption were also considered potential confounders. Sex, gestational age, and infant birth weight were recorded in the delivery room and abstracted from obstetric records and/or birth notifications. Gestational age was based on the date of the mother’s last menstrual period, clinical records, or ultrasound examinations. The highest occupation of the mother or her partner on the questionnaire responses in pregnancy was used to define family social class as manual or nonmanual (using the 1991 British Office of Population and Census Statistics classification). The highest educational qualification for the mothers was collapsed into whether they had achieved a university degree. Mothers were asked about their smoking during pregnancy, and these data were used to generate a binary variable of any smoking during pregnancy. In addition, mothers were asked whether they had drunk any alcohol during the first trimester, and these data were used to generate a binary variable: never or ever drank alcohol during the first trimester. Offspring smoking was obtained from a questionnaire administered at the clinic when DNA was extracted for methylation at age 15–17 years, and this was categorized into never/less than weekly, weekly and daily. Adolescent alcohol intake was obtained from the same questionnaires and categorized into whether or not they consumed alcohol at least weekly.
Genotypes
ALSPAC offspring were genotyped using the Illumina HumanHap550-Quad genome-wide single nucleotide polymorphism (SNP) genotyping platform by the Wellcome Trust Sanger Institute (Cambridge, U.K.) and the Laboratory Corporation of America (Burlington, NC), with support from 23andMe. ALSPAC mothers were genotyped on the Illumina 660K-Quad chip at the Centre National de Génotypage (Paris, France). DNA extraction, quality control, SNP genotyping, and imputation were done separately in the ALSPAC mothers and offspring and have been described in detail elsewhere (37,38).
Statistical Analysis
Cross-sectional Analysis
We performed multivariable regression analysis of log-transformed BMI with the concurrently measured methylation level (β-values) at each of the three CpG sites in HIF3A identified (6) in both mothers and offspring in ARIES. Main models were adjusted for age, sex, and bisulfite conversion batch in the analyses of offspring childhood BMI and for age, sex, smoking status, and bisulfite conversion batch in the analyses of offspring adolescent BMI and maternal BMI. All covariates, including bisulfite conversion batch, were included as fixed effects. BMI was treated as the outcome variable by Dick et al. (6); thus, we present coefficients as the percentage change per 0.1-increase in methylation so that the magnitude of the observational estimates can be compared directly with those reported. DXA-measured FMI was also investigated as the outcome variable in a secondary analysis of the individuals at adolescence, which was similarly log-transformed.
Secondary models were adjusted for age, sex, bisulfite conversion batch, birth weight, gestational age, maternal education, household social class, maternal smoking and alcohol consumption in pregnancy, and own smoking and alcohol consumption. In addition, it has been demonstrated that differences in methylation can arise as a result of variability of cell composition in whole blood (39). To ensure that the results are not influenced by variation in cell type fraction between samples, we estimated the fraction of CD8+T, CD4+T, natural killer, and B cells and monocytes and granulocytes in the samples using the estimateCellCounts function in the minfi Bioconductor package implemented in R software (40,41). This approach uses as a reference a data set presented by Reinius et al. (39) that identified differentially methylated regions that could discriminate cell types in flow-sorted leukocytes from six adult samples. Analyses were repeated adjusting for cell composition by including each blood cell fraction as a covariate in the multivariable linear regression.
Additional Analyses
To further investigate the dominant direction of causality in any observed associations, we undertook the following additional analyses (Fig. 1).
Longitudinal Analysis
Multiple linear regression models were next used to establish the association of methylation with future adiposity and of adiposity with future methylation in the offspring, with adjustments made for sex, age, and batch and for baseline adiposity or methylation, respectively. Specifically, BMI in adolescence was regressed on childhood methylation, and methylation in adolescence on childhood BMI. Childhood methylation was also regressed on birth weight, and childhood BMI on cord blood methylation at birth. Secondary models were adjusted for age, sex, batch, baseline adiposity or methylation, birth weight (where birth weight or methylation at birth was not the outcome or main exposure), gestational age, maternal education, household social class, maternal smoking and alcohol consumption in pregnancy, and own smoking and alcohol consumption.
Mendelian Randomization Analysis
That genetic factors regulate variation in methylation is now well established (42), and two SNPs, rs8102595 and rs3826795, were found to have strong cis-effects on methylation at HIF3A (6). These same SNPs were not associated with BMI in the previous study cohorts or in a large-scale meta-analysis of genome-wide association studies (GWAS) for BMI (43), implying that increasing methylation at the HIF3A CpG sites does not have a causal effect on BMI. We aimed to perform formal Mendelian randomization analysis to establish a causal effect of methylation at HIF3A on BMI using these previously identified cis-SNPs combined in a weighted allele score by using the weights from a meta-analysis of the discovery and replication cohorts in Dick et al. (6) as a proxy for methylation levels.
We also performed reciprocal Mendelian randomization analysis to investigate whether there was evidence of a causal effect of BMI on HIF3A methylation using genetic variants found to be robustly associated with BMI in large-scale GWAS (43,44). For this, a weighted allele score was created from 97 SNPs that are reliably associated with BMI (44) and was used as a genetic instrument for adiposity. The dose of the effect allele at each locus was weighted by the effect size of the variant in this independent meta-analysis, and these doses were summed to reflect the average number of BMI-increasing alleles carried by an individual. Analyses were performed using a standardized allele score.
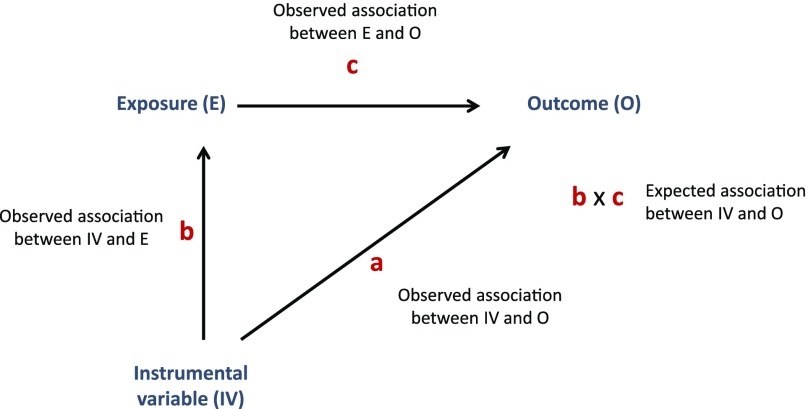
We used the approach of “triangulation” for the Mendelian randomization analyses (45–47). This approach involves a comparison of the observed association between the instrument and the outcome with the association that would be expected if the observed exposure-outcome association were causal (Fig. 2). The expected association is calculated by multiplying the observed instrument-exposure association with the observed exposure-outcome association, whereas the SE for the expected effect size is calculated using a second-order Taylor series expansion of the product of two means, where the covariance of the estimated parameters was estimated using a bootstrapping procedure with 200 replications (48).
Figure 2.
Triangulation approach for IV analyses used in this study. The observed association between the IV and the outcome (a) is compared with that expected given the association between the IV and the exposure (b) and the association between the exposure and the outcome (c).
Here we estimated the expected effect of the instrument-outcome association based on the effect estimates for the instrument-exposure and exposure-outcome associations and compared this with the observed association of instrument with outcome (DNA methylation), performing a z-test for the difference between the observed and expected estimates, where again, the covariance of the estimated parameters was estimated using a bootstrapping procedure. Where the observed and expected estimates are consistent, this suggests that there is unlikely to be marked residual confounding in the association between exposure and outcome (i.e., it supports a causal effect), assuming there is adequate statistical power for this comparison. The only covariate included in the main model was bisulfite conversion batch.
Intergenerational Analysis
We next performed multivariable linear regression analysis to investigate associations between log-transformed maternal prepregnancy BMI and offspring HIF3A methylation at birth, childhood, and adolescence. These models were adjusted for maternal age at delivery, maternal smoking status in pregnancy, offspring sex, and bisulfite conversion batch. Analyses assessing the association of maternal BMI with childhood and adolescent methylation at HIF3A were also adjusted for offspring’s age at methylation measurement.
Our primary interest was in the direction of any causal effect, and this intergenerational design effectively rules out an effect of offspring methylation on maternal BMI. Had any robust associations of maternal BMI with offspring DNA methylation at HIF3A been identified, we planned to use causal inference strategies to investigate whether these associations were likely to be caused by an intrauterine effect of maternal BMI or rather by confounding due to shared familial lifestyles and/or genetic factors.
Specifically, these strategies were a negative control design and Mendelian randomization. In the negative control design, associations of maternal exposure and paternal exposure (the negative control) with the offspring outcome are compared. If these are similar, it suggests that confounding by shared familiar factors, shared epigenetic inheritance, or parental genotypes is likely, whereas a stronger maternal-offspring association (even after adjustment for paternal exposure) would provide support for a causal intrauterine effect (49,50). Associations of maternal prepregnancy BMI and offspring methylation at HIF3A were therefore compared, visually and formally, using incremental F tests, to associations of paternal BMI and offspring methylation, with and without mutual adjustment.
For the Mendelian randomization analysis, genetic variants in the mothers were used to create a weighted allele score for maternal BMI and the IV approach of triangulation was applied to infer a causal effect on offspring DNA methylation at HIF3A. In this case, however, an obvious violation of the IV assumption is the relationship of maternal genotype to offspring (fetal) genotype, which could provide a pathway from the instrument (maternal genotype) to the outcome (offspring DNA methylation at HIF3A) that is not via the exposure of interest (maternal BMI) and hence would bias our findings (51). Therefore, the analysis was adjusted for the offspring’s BMI allele score. All analyses were also adjusted for bisulfite conversion batch.
All statistical analyses were performed in R 3.0.1 software.
Results
Basic Characteristics
Methylation data were available for 973 children at the mean age of age 7.5 (SD 0.1) years and 974 adolescents at the mean age of 17.1 (1.0) years, with data available at both time points for 940 individuals. For the three HIF3A probes identified previously, mean methylation levels were lower in adolescence than in childhood (Table 1). Methylation in childhood was positively associated with methylation in the same individuals assessed in adolescence (Pearson correlation coefficients: 0.72 at cg22891070, 0.57 at cg27146050, and 0.68 at cg16672562). The R2 values for regressions of methylation in adolescence on methylation in childhood showed that childhood methylation explained 52.3%, 32.4%, and 46.8% of variation in methylation in adolescence at cg22891070, cg27146050, and cg16672562, respectively.
Table 1.
Characteristics of ARIES participants included in analyses
| ARIES participants | ||
|---|---|---|
| Childhood (n = 970) | Adolescence (n = 845) | |
| Age (years) | 7.5 (0.1) | 17.1 (1.0) |
| Males, n (%) | 485 (49.8) | 474 (48.7) |
| Height (m) | 1.26 (0.05) | 1.72 (0.09) |
| Weight (kg) | 25.9 (4.6) | 66.2 (9.1) |
| BMI (kg/m2) | 16.2 (2.1) | 22.3 (3.9) |
| FMI (kg/m2) | — | 5.9 (3.5) |
| Fat mass (%) | — | 25.1 (11.0) |
| Smoke at least weekly, n (%) | — | 130 (15.2) |
| Methylation of cg22891070 (β-value) | 0.664 (0.102, 0.281–0.918) | 0.578 (0.120, 0.200–0.884) |
| Methylation of cg27146050 (β-value) | 0.182 (0.035, 0.080–0.538) | 0.167 (0.033, 0.083–0.399) |
| Methylation of cg16672562 (β-value) | 0.660 (0.131, 0.200–0.930) | 0.536 (0.147, 0.122–0.925) |
Continuous data are shown as mean (SD) or mean (SD, range) and categoric data as indicated.
Cross-sectional Analysis
No cross-sectional associations were found between methylation at cg22891070 and cg16672562 and BMI in childhood or adolescence (Table 2). There was also no robust association between methylation at cg27146050 and childhood BMI (Table 2), although there was some suggestive evidence of an association between and methylation across the HIF3A region and childhood BMI (Supplementary Fig. 1). An association between methylation at cg27146050 and BMI in adolescence withstood Bonferroni correction; a 0.1 increase in the methylation β-value at cg27146050 was associated with a 4.7% (95% CI 1.0, 8.3; P = 0.012) increase in BMI, which is in line with previously reported adult BMI effect estimates (6).
Table 2.
Associations between methylation at three CpG sites at HIF3A and BMI
| Childhood | Adolescence | |||||||
|---|---|---|---|---|---|---|---|---|
| Basic model (n = 970)* | Adjusted model (n = 918)† | Basic model (n = 845)‡ | Adjusted model (n = 804)† | |||||
| Percentage change in BMI§ | P value | Percentage change in BMI§ | P value | Percentage change in BMI§ | P value | Percentage change in BMI§ | P value | |
| cg22891070 | 0.44 (−0.35, 1.23) | 0.27 | 0.45 (−0.32, 0.12) | 0.25 | 0.66 (−0.31, 1.63) | 0.19 | 0.30 (−0.67, 1.28) | 0.54 |
| cg27146050 | 0.62 (−1.69, 2.93) | 0.60 | 0.34 (−1.89, 2.56) | 0.77 | 4.66 (1.04, 8.29) | 0.01 | 3.49 (−0.12, 7.10) | 0.06 |
| cg16672562 | 0.31 (−0.32, 0.93) | 0.34 | 0.32 (−0.29, 0.93) | 0.30 | 0.40 (−0.41, 1.20) | 0.34 | 0.24 (−0.56, 1.05) | 0.55 |
Data are % (95% CI) unless stated otherwise.
*Childhood analyses are adjusted for age, sex, and batch.
†Adolescent analyses are adjusted for age, sex, smoking, and batch.
‡Basic model additionally adjusted for smoking, alcohol, maternal education, social class, maternal smoking, maternal alcohol, birth weight, and gestational age.
§Coefficients have been converted into percentage change in BMI for every 0.1 unit increase in methylation β-value.
We investigated whether the observed association between adolescent BMI and cg27146050 methylation could be explained by additional confounding factors (Supplementary Table 2). The association between methylation at cg27146050 and BMI in adolescence was attenuated by 25% upon adjustment for these, indicating some potential confounding in the observational association (Table 2). DNA was extracted from buffy coats in adolescence. To establish the effect of correcting for buffy coat cell type, predicted cell type components were added as covariates to the main and secondary models. Evidence for association strengthened after this adjustment (Supplementary Table 3).
Effect estimates for associations between adolescent methylation and FMI were consistently larger for all three of the CpG sites compared with those for BMI, particularly at cg27146050, where an increase in the methylation β-value of 0.1 was associated with an 11.8% (95% CI −0.1, 23.7) increase in FMI (P = 0.053); however, the CIs were wider, and the P values for the associations did not withstand Bonferroni correction. We also investigated whether the observed associations could be explained by additional confounding factors that may exist in the context of adiposity and methylation by assessing the effect of adjusting for potential confounders on the observational effect estimates. The association between methylation at cg27146050 and FMI in adolescence was similarly attenuated by 25%, indicating some potential confounding in the observational association (Supplementary Table 4).
Longitudinal Associations
We next investigated the prospective associations between HIF3A methylation at birth and childhood BMI, between birth weight and childhood HIF3A methylation, between childhood HIF3A methylation and adolescent BMI, and between childhood BMI and HIF3A methylation in adolescence, with and without adjustment for adiposity or methylation at the earlier time point (Table 3). We observed positive associations between birth weight and childhood methylation at all three sites, which was not attenuated with adjustment for cord blood methylation at birth (P = 0.0019–0.019). Although there was weak evidence of inverse associations between HIF3A methylation at birth and childhood BMI, these associations were attenuated after adjusting for birth weight (Table 3).
Table 3.
Prospective associations between cord blood methylation at birth and childhood BMI, between birth weight and childhood methylation, between childhood methylation and adolescent BMI, and between childhood BMI and adolescent methylation
| Exposure | Outcome | CpG site | N | Association without adjustment for the outcome at baseline* |
N | Association with adjustment for the outcome at baseline* |
||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | P value | β (95% CI) | P value | |||||
| Birth methylation | Childhood BMI | cg22891070 | 890 | −1.70 (−3.66, 0.30)a | 0.10 | 874 | −1.65 (−3.52, 0.26)a | 0.09 |
| cg27146050 | 890 | −0.03 (−1.04, 1.00)a | 0.96 | 874 | 0.21 (−0.77, 1.21)a | 0.67 | ||
| cg16672562 | 890 | −2.66 (−5.10, −0.16)a | 0.04 | 874 | −2.23 (−4.58, 0.17)a | 0.07 | ||
| Birth weight | Childhood methylation | cg22891070 | 957 | 0.02 (0.01, 0.04)b | 0.01 | 871 | 0.02 (0.003, 0.035)b | 0.02 |
| cg27146050 | 957 | 0.01 (0.001, 0.012)b | 0.02 | 871 | 0.01 (0.003, 0.014)b | 0.04 | ||
| cg16672562 | 957 | 0.03 (0.01, 0.05)b | 0.01 | 871 | 0.03 (0.006, 0.046)b | 0.01 | ||
| Childhood methylation | Adolescent BMI | cg22891070 | 922 | 0.68 (−0.40, 1.76)a | 0.22 | 919 | 0.14 (−0.64, 0.91)a | 0.73 |
| cg27146050 | 922 | 2.30 (−0.83, 5.43)a | 0.15 | 919 | 1.33 (−0.91, 3.57)a | 0.24 | ||
| cg16672562 | 922 | 0.31 (−0.54, 1.15)a | 0.48 | 919 | −0.04 (−0.64, 0.57)a | 0.90 | ||
| Childhood BMI | Adolescent methylation | cg22891070 | 971 | 0.005(−0.002, 0.011)c | 0.17 | 937 | 0.001 (−0.004, 0.005)c | 0.78 |
| cg27146050 | 971 | 0.003 (0.001, 0.005)c | 0.001 | 937 | 0.003 (0.001, 0.004)c | 0.001 | ||
| cg16672562 | 971 | 0.005 (−0.003, 0.013)c | 0.21 | 937 | 0.002 (−0.004, 0.008)c | 0.60 | ||
*Also adjusted for age at childhood/adolescence, sex, and batch.
aCoefficients have been converted into percentage change in BMI for every 0.1-unit increase in methylation β-value.
bCoefficients are change in methylation per 1-kg increase in birth weight.
cCoefficients are change in methylation per 10% increase in BMI.
We also observed a positive association between childhood BMI and cg27146050 methylation in adolescence (0.003 [95% CI 0.001, 0.005]) increase in methylation β-value per 10% increase in BMI (P = 0.001), which was not attenuated with adjustment for childhood methylation at this site. The effect remained unchanged with adjustment for a number of potential confounders (Supplementary Table 5). However, no prospective associations were found between childhood BMI and adolescent methylation at cg22891070 or cg16672562.
Mendelian Randomization Analysis
To investigate the potential effect of methylation at cg27146050 on BMI, we first assessed genetic associations with methylation using a score composed of two SNPs, rs8102595 and rs3826795, found to have strong cis-effects on methylation at HIF3A in an independent study (6). There was a 0.2 (95% CI 0.16, 0.25; R2 = 7.4%, P < 10−10) increase in the methylation β-value at cg27146050 per unit increase in the cis-SNP score (Supplementary Table 6). Unlike for the adiposity and methylation measures, there was no strong evidence of association between the cis-SNP score and a number of potential confounding factors (Supplementary Table 8).
Given the strength of the association with methylation at cg27146050 and the lack of association with confounding factors, we used the cis-SNP score as an instrument for methylation in a Mendelian randomization analysis. There was little association between the cis-SNPs and BMI compared with the expected association if methylation on BMI was causal (Table 4). However, wide 95% CIs for the observed estimates meant that there was no strong evidence of a difference between the observed and expected effect estimates (observed effect = −0.04 [−0.29, 0.22]; expected effect = 0.10 [0.03, 0.17]; P = 0.30 for difference).
Table 4.
Mendelian randomization analysis for associations between adolescent BMI and methylation at cg27146050
| IV | Exposure | Outcome (O) | Observed association between IV and O (c)* | Expected association between IV and O (a × b) |
Difference between observed (c) and expected (a × b) estimates |
|
|---|---|---|---|---|---|---|
| N | β (95% CI) | β (95% CI) | P value | |||
| Adolescent cis-SNP score | Adolescent methylation at cg27146050 | Adolescent log BMI | 831 | −0.0381 (−0.2937, 0.2176) | 0.1027 (0.0315, 0.1739) | 0.30 |
| Adolescent standardized 97 SNP allele score | Adolescent log BMI | Adolescent methylation at cg27146050 | 849 | 0.0014 (−0.0009, 0.0037) | 0.0008 (0.0002, 0.0013) | 0.55 |
*Analyses are adjusted for bisulfite conversion batch only.
We calculated that we would need a sample of 25,369 to confidently detect an association (at P < 0.001) between the cis-SNP allele score that explained 0.1% of the variance in log-BMI with 95% power. Therefore, we also tested for associations between the cis-SNPs and BMI by performing a look-up of the SNPs in the publically available results of the most recent Genetic Investigation of Anthropometric Traits (GIANT) consortium meta-analysis (44). In this sample, there was no strong evidence of association between either of the SNPs and BMI (rs3826795: n = 224,403, β = 0.002 [SE 0.005], P = 0.63; rs8102595: n = 223,534, β = −0.002 [0.007], P = 0.78), in accordance with previous findings using data from a smaller meta-analysis in GIANT (6). In addition, we performed two-sample Mendelian randomization (52), using SNP-methylation association estimates obtained from the ARIES data set and SNP-BMI association estimates obtained from the GIANT results, to derive a Wald ratio estimate for the causal effect of methylation on BMI. An inverse-weighted variance meta-analysis of the estimates derived using the two SNPs showed a 1-unit increase in methylation was associated with a −0.021 (95% CI −0.55, 0.51; P = 0.94) decrease in inverse-normally transformed BMI residuals, thus providing further evidence against a causal effect of methylation at HIF3A on BMI (Supplementary Table 10).
To investigate the potential effect of BMI on methylation at cg27146050, we confirmed the expected association between a weighted allele score composed of 97 BMI variants identified in an independent study (44) and log-transformed BMI in our sample (β = 0.036 [95% CI 0.025, 0.046]; R2 = 5.2%; P < 10−10) (Supplementary Table 7). Unlike for the adiposity and methylation measures, there was no evidence of association between the BMI allele score and a number of potential confounding factors (Supplementary Table 8). Although there was some evidence for a difference in the mean allele score between groups based on adolescent own smoking, this was driven by a small number of individuals in the group who smoked weekly (n = 29), and no linear trend was observed.
We applied this instrument to investigate the potential causal effect of BMI on HIF3A methylation (Table 4). The direction of effect observed was consistent with that expected if the effect were causal. In addition, there was little evidence of a difference between the observed and expected effect estimates (observed effect = 0.0014 [95% CI −0.0009, 0.0037]; expected effect = 0.0008 [0.0002, 0.0013]; P = 0.55 for difference). However, no robust evidence of an association between the allele score and methylation was observed because of the wide CIs. To confidently detect an association between the BMI allele score and HIF3A methylation (at P < 0.001) that explained 0.1% of the variance in log BMI with 95% power, we calculated that we would need a sample of 30,523. Unfortunately, no publically available methylation quantitative trait locus data of this sample size are currently available to investigate this.
Intergenerational Analysis
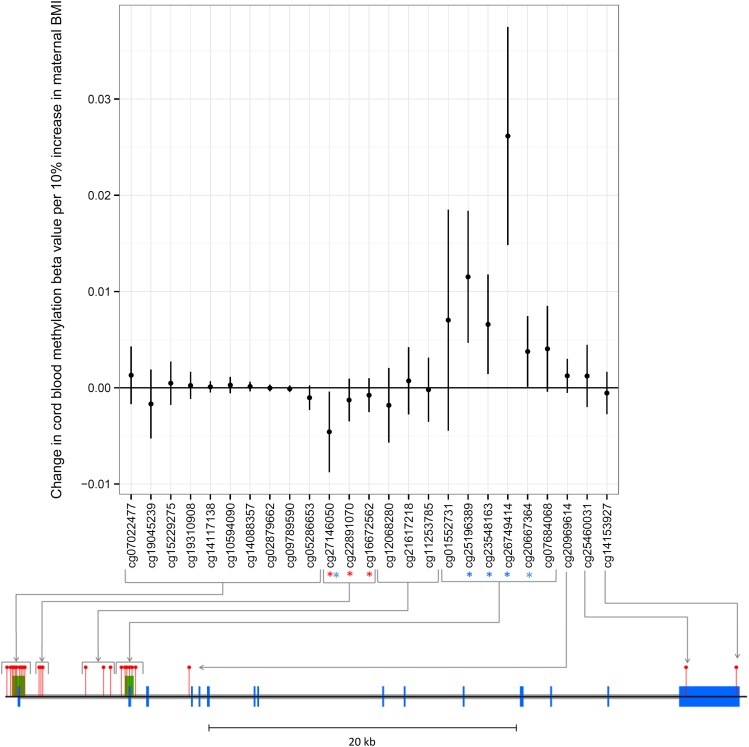
We next performed an intergenerational analysis to investigate a potential intrauterine effect of maternal BMI on offspring methylation at cg27146050 from birth to adolescence. Maternal prepregnancy BMI was associated with offspring cord blood methylation at cg27146050 (P = 0.027). However, whereas own BMI was positively associated with methylation at this site, maternal BMI was inversely associated with offspring DNA methylation at cg27146050 in cord blood (−0.0048 [95% CI −0.0092, 0.0004]) change in methylation per 10% increase in maternal BMI) (Fig. 3).
Figure 3.
Associations between maternal BMI and offspring methylation at birth at HIF3A CpG sites. Associations of maternal BMI and offspring cord blood methylation at birth at all 25 CpG sites at the HIF3A locus (mean change in methylation per unit increase in log-maternal prepregnancy BMI; error bars indicate 95% CIs). The locations of CpG sites on the HIF3A gene are mapped on the diagram below the graph. Blue blocks are exons, gray blocks are introns, green blocks are CpG islands, and red pins are CpG sites. The three sites previously identified in adult peripheral blood as associated with own BMI are highlighted with a red *. All sites associated with maternal BMI with a P value <0.05 in our analyses are highlighted with a blue *.
Maternal BMI was also associated with cord blood methylation at four other CpG sites at HIF3A (cg20667364, cg26749414, cg25196389, and cg23548163; P values ranging from 7.5 × 10−6 to 4.6 × 10−2) (Fig. 3). These sites in the second CpG island were positively associated with maternal BMI in contrast to cg27146050, which was negatively associated. A heat map of the correlation between methylation β-values at HIF3A (Supplementary Fig. 2) shows that the sites in the second CpG island are inversely correlated with cg27146050.
Associations between maternal BMI and offspring methylation at birth at the additional sites in the second CpG island did not persist at later ages (birth, n = 795; childhood, n = 845; adolescence, n = 851) (Supplementary Fig 3). The inverse association of maternal prepregnancy BMI with methylation at cg27146050 in cord blood reversed to a positive one in adolescence, in line with the association of own BMI with methylation at this site.
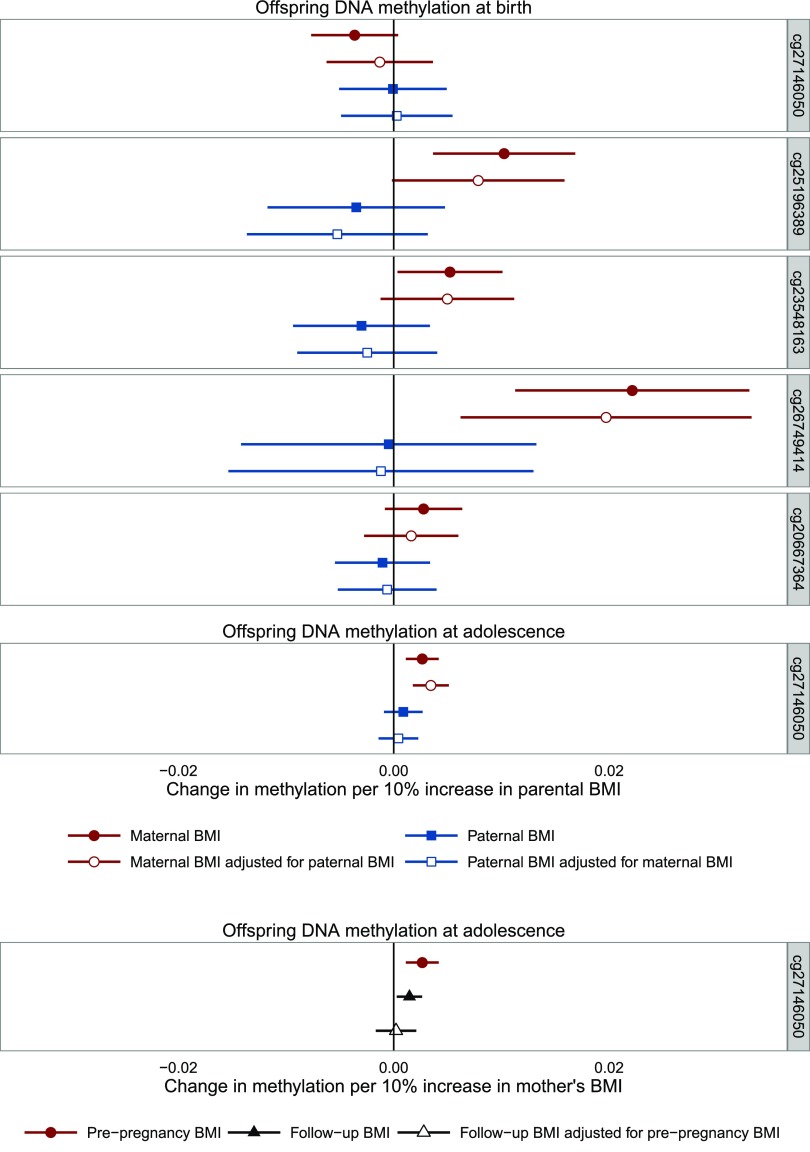
Using a negative control design, we found that the association between maternal BMI and offspring methylation at the sites identified in cord blood tended to be stronger than the association with paternal BMI (maternal, n = 797; paternal, n = 655; mutually adjusted, n = 625) (Fig. 4), but after mutual adjustment of maternal and paternal BMI, there was only robust evidence that they differed at cg25196389 (the difference between maternal and paternal associations with mutual adjustment by Wald test P value was 0.031 for cg25196389; all other probes, P > 0.05). We also found that, for cg27146050 in adolescence, the association with prepregnancy maternal BMI was stronger than the association with paternal BMI, with and without mutual adjustment (P= 0.009 by Wald test) (Fig. 4), and was also stronger than the association with maternal BMI measured postnatally when their offspring were approximately age 15 (P = 0.050 by Wald test in adjusted model) (Fig. 4).
Figure 4.
Associations between parental BMI and offspring DNA methylation at HIF3A. The error bars indicate the 95% CI. Maternal antenatal: n = 849 (birth) 904 (adolescence); paternal: n = 694 (birth) 742 (adolescence); mutually adjusted: n = 662 (birth) 708 (adolescence); maternal at follow-up: n = 819 (adolescence); maternal antenatal adjusted for maternal at follow-up: n = 763 (adolescence).
In the Mendelian randomization analyses of maternal BMI on cord blood methylation (Supplementary Table 9), the observed associations between the IV and offspring methylation were stronger than the expected estimates, although the 95% CIs were wide and included the null value at most sites. There was little evidence that the expected and observed associations of the maternal BMI allele score with offspring methylation differed. Adjusting for offspring allelic score slightly strengthened the observed maternal allelic score–methylation relationship, but conclusions were generally the same. However, in the Mendelian randomization analysis of maternal BMI on cg27146050 methylation in adolescence, no association was observed between maternal genotype and offspring methylation, which we would expect to find if the effect of maternal BMI on offspring methylation in adolescence were causal. However, again, effect estimates were imprecise (Supplementary Table 9).
Discussion
In this study, we tested for replication of a previous investigation of the association between BMI and DNA methylation at HIF3A in childhood and adolescence in a subset of individuals from the ALSPAC (6). Although no clear cross-sectional associations were observed between childhood BMI and methylation, we found evidence of a positive association between adolescent BMI and methylation at cg27146050 in HIF3A, with a magnitude of effect similar to that seen previously (6).
We also examined the association between HIF3A methylation and DXA-derived FMI in adolescence and found positive associations at all three CpG sites. Effect estimates were larger than those observed in the associations with BMI, although the associations were imprecisely estimated with wide CIs that included the null value.
We performed several additional analyses to investigate the dominant direction of causality in any observed associations (Fig. 1). In longitudinal analysis, we found an association between childhood BMI and methylation in adolescence, but childhood methylation was not robustly associated with BMI in adolescence, implying that the direction of any possible effect is from adiposity to methylation at this locus, rather than the other way round.
For the Mendelian randomization analysis, we confirmed associations between two cis-SNPs and methylation at HIF3A and, in line with the aforementioned study (6), did not find associations between these SNPs and BMI, suggesting that variation in methylation at HIF3A does not causally affect BMI. This was supported by our finding that the observed effect estimate of the SNPs on BMI was different from that expected if methylation at HIF3A had a causal effect on BMI in the ARIES sample as well as a null effect estimate for the causal effect of HIF3A methylation on BMI in the GIANT data set (44) established using a two-sample Mendelian randomization approach.
We were able to extend the analysis by using instruments for BMI to investigate causality of the reciprocal effect. We used an allele score composed of variants robustly associated with BMI in an independent GWAS (44) and assessed the magnitude of association between this score and methylation at HIF3A in adolescence. Although this analysis showed no robust evidence of an association between the allele score and methylation, the CIs were wide, and here the observed effect estimate was in the same direction and exceeded the expected magnitude of a causal effect.
Several studies have shown that maternal adiposity during pregnancy is associated with offspring DNA methylation (53–56). We performed intergenerational analysis and identified associations between maternal prepregnancy BMI and offspring cord blood methylation at cg27146050 as well as four novel CpG sites at HIF3A. Because the association of maternal BMI with offspring DNA methylation could not be explained by reverse causality, this lends further plausibility to an effect of adiposity on DNA methylation at HIF3A.
Associations of maternal BMI and offspring methylation at the novel sites at HIF3A were stronger at birth than in childhood and adolescence, suggesting that any effect of maternal BMI on neonatal DNA methylation at these sites does not persist into later life. This seemingly transient effect of maternal BMI on offspring cord blood methylation at HIF3A may be indicative of changes in the regulation of hypoxia-inducible transcription factors specific to pregnancy (57). Meanwhile, an association between maternal BMI and offspring methylation was evident for cg27146050 at all three time points, although the direction of the association changed over time.
Some evidence for a causal intrauterine effect of maternal BMI on offspring cord blood was supported with the use of both a parental negative control comparison analysis, where no association was seen between paternal BMI (the negative control) and offspring cord blood methylation, and Mendelian randomization using a BMI allele score in the mothers. For the latter, conclusions were similar even after adjustment for offspring genotype. A parental comparison analysis also provided support for a possible legacy from the intrauterine effect of maternal BMI on offspring DNA methylation into adolescence, as has been previously identified in the case of maternal smoking in pregnancy (58,59). However, this could be influenced by parental differences in the proportion of environmental factors shared with offspring postnatally, and although maternal BMI in pregnancy was more strongly associated with offspring methylation than maternal BMI postnatally, Mendelian randomization did not provide strong support for a causal intrauterine effect at this later time point.
Strengths of this analysis include the extension of a previous study, with the aim of replicating identified associations between BMI and methylation at the HIF3A locus in a younger cohort. We obtained similar findings in the direction of effect between BMI and methylation at the identified CpG sites in HIF3A, although associations were weaker, as has been found previously (9). In addition, more thorough consideration has been given to a number of potential confounding factors, and longitudinal and Mendelian randomization analysis have both been used to assess causality in the observed association.
The main limitation of this analysis was the limited power to detect a difference between the observed and expected triangulation estimates between the BMI allele score and DNA methylation, and further exploration in additional large studies is warranted. Other possible limitations of Mendelian randomization include population stratification, canalization, pleiotropy, and linkage disequilibrium (18,21,60). Major population stratification is unlikely because this analysis was completed in unrelated individuals of European ancestry. However, a pleiotropic association of a cis-SNP with BMI or the BMI allele score with HIF3A methylation, or linkage disequilibrium between these genotypes and a functional variant independently associated with the outcome, would violate the assumptions of the Mendelian randomization analysis.
Although the genetic variants included in the cis-SNP score were robustly associated with cg27146050 methylation levels, in a previous study, they were associated with methylation at the neighboring CpG, cg22891070, implying nonspecificity of these genetic instruments, which instead proxy for regional HIF3A methylation levels rather than methylation at individual CpG sites. To investigate specificity of the BMI SNPs, we performed a look-up of the 97 SNPs in a large-scale methylation quantitative trait locus analysis within the ARIES data set and did not find any SNP-CpG associations that surpassed genome-wide significance, indicating that the BMI SNPs are unlikely to have a pleiotropic influence on methylation independent of BMI.
Canalization (or developmental compensation) could potentially bias the Mendelian randomization analysis assessing causality in the adolescent BMI–methylation association but is not an issue in the intergenerational analysis because the mother’s genetic instrument will only influence the developmental environment of the offspring through the exposure of interest (61). Nonetheless, the intergenerational Mendelian randomization estimates are potentially biased with adjustment for offspring BMI genotype, which might introduce a different pathway between the maternal BMI genotype and the paternal BMI genotype (a form of collider bias). However, as we have already stated, paternal BMI is unlikely to have a direct effect on offspring methylation, and adjusting for offspring BMI genotype did not substantially alter effect estimates for this Mendelian randomization analysis.
Further limitations of the study include missing data for BMI, FMI, and some of the potential confounders that reduced the complete case sample size. It should be noted that we found no CpG sites in HIF3A that were associated with offspring or maternal BMI with a P value <1 × 10−7 (the widely used Bonferroni cutoff for genome-wide significance on the HM450 array); therefore, an EWAS of own or maternal BMI in ARIES would not have identified any sites in HIF3A. However, given the existence of correlation structure and comethylation in this region, correction for multiple testing based on independent tests in an EWAS would likely be too stringent. In addition, 8 of the 25 Illumina 450K probes at HIF3A appear on a comprehensive list of probes that provide noisy or inaccurate signals (62). This list includes two (cg22891070 and cg16672562) of the sites previously identified as being associated with own BMI, so these findings are at a high risk of being false discoveries. In addition, although not the primary focus of our analyses, we did not find strong associations between HIF3A methylation at any of the three sites and BMI in the ARIES mothers at the time of pregnancy or ∼17 years later at follow-up, although the direction of effect was consistent with that found previously at these sites (6) (Supplementary Table 11).
An additional limitation is that cord blood or peripheral blood may not be the most appropriate tissues in which to study associations with BMI, and a more pronounced association of BMI with HIF3A methylation has been observed in adipose tissue (6,7). Furthermore, this analysis was limited to blood samples with mixed cell composition. Although no differences were found in the analysis with estimated cell-type correction, how effective the method used to correct for cell-type proportions is in these samples is unclear because the reference data sets are available only for adult peripheral blood (39).
Overall, our results do not support a causal effect of HIF3A methylation on BMI and are more suggestive of a causal effect in the reverse direction (i.e., an effect of higher BMI on higher HIF3A methylation). Use of a range of causal inference techniques, including longitudinal analysis, Mendelian randomization, and a parental comparison design, provided findings largely consistent with a causal effect of own BMI on methylation at HIF3A as well as an independent intrauterine effect of maternal BMI on offspring cord blood methylation at HIF3A (Supplementary Fig. 1). Further work is required to uncover the mechanisms underlying both a direct and intrauterine effect of adiposity on DNA methylation in this gene and to investigate its role in the downstream effects of adiposity, given that methylation changes have been shown to influence gene expression at this locus (6).
Supplementary Material
Article Information
Acknowledgments. The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Funding. This work was supported by the U.K. Medical Research Council Integrative Epidemiology Unit and the University of Bristol (MC_UU_12013_1, MC_UU_12013_2, MC_UU_12013_5, and MC_UU_12013_8), the Wellcome Trust (WT088806), Cancer Research UK (C18281/A19169), and the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-10324). A.F. is funded by a U.K. Medical Research Council research fellowship (MR/M009351/1). R.C.R. and M.E.W. are funded by a Wellcome Trust 4-year PhD studentship (WT097097MF and 099873/Z/12/Z). G.D.S. and C.L.R. are partially supported by the Economic and Social Research Council (RES-060-23-0011, “The biosocial archive: transforming lifecourse social research through the incorporation of epigenetic measures”). The U.K. Medical Research Council and the Wellcome Trust (102215/2/13/2) and the University of Bristol provide core support for ALSPAC. ARIES was funded by the U.K. Biotechnology and Biological Sciences Research Council (BB/I025751/1 and BB/I025263/1).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.C.R., G.C.S., M.E.W., A.F., D.A.L., G.D.S., and C.L.R. conceived and designed the experiments and wrote the manuscript. R.C.R., G.C.S., and M.E.W. analyzed the data. O.L., W.L.M., S.M.R., and T.R.G. contributed to data production and management. R.C.R., G.C.S., and M.E.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-0996/-/DC1.
References
- 1.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS; Members of EpiSCOPE. Epigenics and human obesity. Int J Obes (London) 2015;39:85–97 [DOI] [PubMed] [Google Scholar]
- 2.Wang XL, Zhu HD, Snieder H, et al. Obesity related methylation changes in DNA of peripheral blood leukocytes. BMC Med 2010;8;87 [DOI] [PMC free article] [PubMed]
- 3.Feinberg AP, Irizarry RA, Fradin D, et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med 2010;2:49ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almén MS, Jacobsson JA, Moschonis G, et al. Genome wide analysis reveals association of a FTO gene variant with epigenetic changes. Genomics 2012;99:132–137 [DOI] [PubMed] [Google Scholar]
- 5.Xu XJ, Su SY, Barnes VA, et al. A genome-wide methylation study on obesity Differential variability and differential methylation. Epigenetics 2013;8:522–533 [DOI] [PMC free article] [PubMed]
- 6.Dick KJ, Nelson CP, Tsaprouni L, et al. DNA methylation and body-mass index: a genome-wide analysis. Lancet 2014;383:1990–1998 [DOI] [PubMed] [Google Scholar]
- 7.Agha G, Houseman EA, Kelsey KT, Eaton CB, Buka SL, Loucks EB. Adiposity is associated with DNA methylation profile in adipose tissue. Int J Epidemiol 2014;44:1277–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demerath EW, Guan W, Grove ML, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet 2015;24:4464–4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan H, Lin X, Wu Y, et al.; GUSTO Study Group . HIF3A association with adiposity: the story begins before birth. Epigenomics 2015;7:937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang T, Zheng Y, Qi Q, et al. DNA methylation variants at HIF3A locus, B vitamins intake, and long-term weight change: gene-diet interactions in two US cohorts. Diabetes 2015;464:3146–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YS, David AE, Huang Y, et al. In vivo delivery of cell-permeable antisense hypoxia-inducible factor 1α oligonucleotide to adipose tissue reduces adiposity in obese mice. J Control Release 2012;161:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Zhang G, Gonzalez FJ, Park SM, Cai D. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol 2011;9:e1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang C, Qu A, Matsubara T, et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 2011;60:2484–2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shin MK, Drager LF, Yao Q, et al. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One 2012;7:e46562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Relton CL, Davey Smith G. Epigenetic epidemiology of common complex disease: prospects for prediction, prevention, and treatment. PLoS Med 2010;7:e1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Relton CL, Groom A, St Pourcain B, et al. DNA methylation patterns in cord blood DNA and body size in childhood. PLoS One 2012;7:e31821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng JW, Barrett LM, Wong A, Kuh D, Davey Smith G, Relton CL. The role of longitudinal cohort studies in epigenetic epidemiology: challenges and opportunities. Genome Biol 2012;13:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22 [DOI] [PubMed] [Google Scholar]
- 19.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res 2007;16:309–330 [DOI] [PubMed] [Google Scholar]
- 20.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133–1163 [DOI] [PubMed] [Google Scholar]
- 21.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014;23(R1):R89–R98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Didelez V, Meng S, Sheehan NA. Assumptions of IV methods for observational epidemiology. Stat Sci 2010;25:22–40 [Google Scholar]
- 23.Davey Smith G, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. PLoS Med 2007;4:e352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Relton CL, Davey Smith G. Two-step epigenetic Mendelian randomization: a strategy for establishing the causal role of epigenetic processes in pathways to disease. Int J Epidemiol 2012;41:161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang L, Willis-Owen SA, Laprise C, et al. An epigenome-wide association study of total serum immunoglobulin E concentration. Nature 2015;520:670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allard C, Desgagne V, Patenaude J, et al. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics 2015;10:342–351 [DOI] [PMC free article] [PubMed]
- 27.Timpson NJ, Nordestgaard BG, Harbord RM, et al. C-reactive protein levels and body mass index: elucidating direction of causation through reciprocal Mendelian randomization. Int J Obes 2011;35:300–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh P, Polisecki E, Robertson M, et al. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab 2010;95:93–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol 2012;8:679–688 [DOI] [PubMed] [Google Scholar]
- 30.Boyd A, Golding J, Macleod J, et al. Cohort profile: the ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraser A, Macdonald-Wallis C, Tilling K, et al. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol 2013;42:97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relton CL, Gaunt T, McArdle W, et al. Data resource profile: Accessible Resource for Integrated Epigenomic Studies (ARIES). Int J Epidemiol 2015;44:1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dedeurwaerder S, Defrance M, Calonne E, Denis H, Sotiriou C, Fuks F. Evaluation of the Infinium Methylation 450K technology. Epigenomics 2011;3:771–784 [DOI] [PubMed] [Google Scholar]
- 34.Du P, Zhang X, Huang CC, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 2010;11:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bibikova M, Barnes B, Tsan C, et al. High density DNA methylation array with single CpG site resolution. Genomics 2011;98:288–295 [DOI] [PubMed] [Google Scholar]
- 36.Touleimat N, Tost J. Complete pipeline for Infinium Human Methylation 450K BeadChip data processing using subset quantile normalization for accurate DNA methylation estimation. Epigenomics 2012;4:325–341 [DOI] [PubMed] [Google Scholar]
- 37.Paternoster L, Zhurov AI, Toma AM, et al. Genome-wide association study of three-dimensional facial morphology identifies a variant in PAX3 associated with nasion position. Am J Hum Genet 2012;90:478–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans DM, Zhu G, Dy V, et al. Genome-wide association study identifies loci affecting blood copper, selenium and zinc. Hum Mol Genet 2013;22:3998–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinius LE, Acevedo N, Joerink M, et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 2012;7:e41361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaffe AE, Irizarry RA. Accounting for cellular heterogeneity is critical in epigenome-wide association studies. Genome Biol 2014;15:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houseman EA, Kelsey KT, Wiencke JK, Marsit CJ. Cell-composition effects in the analysis of DNA methylation array data: a mathematical perspective. BMC Bioinformatics 2015;16:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang D, Cheng L, Badner JA, et al. Genetic control of individual differences in gene-specific methylation in human brain. Am J Hum Genet 2010;86:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Locke AE, Kahali B, Berndt SI, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freathy RM, Timpson NJ, Lawlor DA, et al. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes 2008;57:1419–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Silva NM, Freathy RM, Palmer TM, et al. Mendelian randomization studies do not support a role for raised circulating triglyceride levels influencing type 2 diabetes, glucose levels, or insulin resistance. Diabetes 2011;60:1008–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fall T, Hägg S, Mägi R, et al.; European Network for Genetic and Genomic Epidemiology (ENGAGE) consortium . The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med 2013;10:e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas DC, Lawlor DA, Thompson JR. Re: Estimation of bias in nongenetic observational studies using “Mendelian triangulation” by Bautista et al. Ann Epidemiol 2007;17:511–513 [DOI] [PubMed] [Google Scholar]
- 49.Davey Smith G. Negative control exposures in epidemiologic studies. Epidemiology 2012;23:350–351; author reply 351–352 [DOI] [PubMed] [Google Scholar]
- 50.Richmond RC, Al-Amin A, Davey Smith G, Relton CL. Approaches for drawing causal inferences from epidemiological birth cohorts: a review. Early Hum Dev 2014;90:769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lawlor DA, Timpson NJ, Harbord RM, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med 2008;5:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 2013;178:1177–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guénard F, Tchernof A, Deshaies Y, et al. Methylation and expression of immune and inflammatory genes in the offspring of bariatric bypass surgery patients. J Obes 2013;2013:492170 [DOI] [PMC free article] [PubMed]
- 54.Liu X, Chen Q, Tsai HJ, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen 2014;55:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morales E, Groom A, Lawlor DA, Relton CL. DNA methylation signatures in cord blood associated with maternal gestational weight gain: results from the ALSPAC cohort. BMC Res Notes 2014;7:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharp G, Lawlor DA, Richmond RC, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2015;44:1288–1304 [DOI] [PMC free article] [PubMed]
- 57.Rajakumar A, Conrad KP. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol Reprod 2000;63:559–569 [DOI] [PubMed] [Google Scholar]
- 58.Lee KW, Richmond R, Hu P, et al. Prenatal exposure to maternal cigarette smoking and DNA methylation: epigenome-wide association in a discovery sample of adolescents and replication in an independent cohort at birth through 17 years of age. Environ Health Perspect 2015;123:193–199 [DOI] [PMC free article] [PubMed]
- 59.Richmond RC, Simpkin AJ, Woodward G, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: Findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet 2014;24:2201–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davey Smith G, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol 2004;33:30–42 [DOI] [PubMed] [Google Scholar]
- 61.Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr 2011;6:27–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naeem H, Wong NC, Chatterton Z, et al. Reducing the risk of false discovery enabling identification of biologically significant genome-wide methylation status using the HumanMethylation450 array. BMC Genomics 2014;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.