Abstract
Objectives
The authors investigated aging effects on the envelope of the frequency following response to dynamic and static components of speech. Older adults frequently experience problems understanding speech, despite having clinically normal hearing. Improving audibility with hearing aids provides variable benefit, as amplification cannot restore the temporal precision degraded by aging. Previous studies have demonstrated age-related delays in subcortical timing specific to the dynamic, transition region of the stimulus. However, it is unknown whether this delay is mainly due to a failure to encode rapid changes in the formant transition because of central temporal processing deficits or as a result of cochlear damage that reduces audibility for the high-frequency components of the speech syllable. To investigate the nature of this delay, the authors compared subcortical responses in younger and older adults with normal hearing to the speech syllables /da/ and /a/, hypothesizing that the delays in peak timing observed in older adults are mainly caused by temporal processing deficits in the central auditory system.
Design
The frequency following response was recorded to the speech syllables /da/ and /a/ from 15 younger and 15 older adults with normal hearing, normal IQ, and no history of neurological disorders. Both speech syllables were presented binaurally with alternating polarities at 80 dB SPL at a rate of 4.3 Hz through electromagnetically shielded insert earphones. A vertical montage of four Ag–AgCl electrodes (Cz, active, forehead ground, and earlobe references) was used.
Results
The responses of older adults were significantly delayed with respect to younger adults for the transition and onset regions of the /da/ syllable and for the onset of the /a/ syllable. However, in contrast with the younger adults who had earlier latencies for /da/ than for /a/ (as was expected given the high-frequency energy in the /da/ stop consonant burst), latencies in older adults were not significantly different between the responses to /da/ and /a/. An unexpected finding was noted in the amplitude and phase dissimilarities between the two groups in the later part of the steady-state region, rather than in the transition region. This amplitude reduction may indicate prolonged neural recovery or response decay associated with a loss of auditory nerve fibers.
Conclusions
These results suggest that older adults’ peak timing delays may arise from decreased synchronization to the onset of the stimulus due to reduced audibility, though the possible role of impaired central auditory processing cannot be ruled out. Conversely, a deterioration in temporal processing mechanisms in the auditory nerve, brainstem, or midbrain may be a factor in the sudden loss of synchronization in the later part of the steady-state response in older adults.
Keywords: Aging, Frequency following response, Temporal processing
INTRODUCTION
The number of adults 60 years and older will dramatically increase in the next 10 years, bringing about a greater prevalence of communication problems (Lin et al. 2011). Older adults frequently report they can hear what is said but cannot understand the meaning, especially in noise. These communication difficulties have a significant social impact on older adults, as several studies have shown strong correlations among hearing loss and depression (Kay et al. 1964; Herbst & Humphrey 1980; Laforge et al. 1992; Carabellese et al. 1993) and cognitive impairment (Uhlmann et al. 1989; Gates et al. 1996; Lin et al. 2013). Although amplification benefits hearing aid users (Humes et al. 2001), it may not improve speech understanding in noise for older adults, as increased audibility does not restore temporal precision degraded by aging (Tremblay et al. 2003).
Evidence of age-related deficits in auditory temporal processing has been found in psychoacoustics studies (Pichora-Fuller & Schneider 1991; Fitzgibbons & Gordon-Salant 1996; Frisina & Frisina 1997; Schneider & Hamstra 1999; Gordon-Salant et al. 2006; He et al. 2008), at the single neuron level from various nuclei of the auditory pathway in animal models (Walton et al. 1998; Schatteman et al. 2008; Recanzone et al. 2011) and in electrophysiological studies in humans and animals (Lister et al. 2011; Parthasarathy & Bartlett 2011; Anderson et al. 2012; Clinard & Tremblay 2013; Clinard & Cotter 2015). A number of rodent studies support the theory that this degradation of temporal precision may be attributed to a significant decrease of inhibitory functions and a consequent loss of balance between excitatory and inhibitory processes in the dorsal cochlear nuclei (Caspary et al. 2005; Schatteman et al. 2008; Wang et al. 2009), inferior colliculi (IC) (Caspary et al. 1995), spiral ganglion neurons (Tang et al. 2014), and auditory cortices (de Villers-Sidani et al. 2010; Hughes et al. 2010; Juarez-Salinas et al. 2010).
Anderson et al. (2012) found peak latency delays in older adults’ responses evoked by a consonant–vowel (CV) syllable, but only in responses to the formant transition region of the syllable and not in responses to the steady state. What remains to be clarified is if this delay arises from central temporal processing deficits that reduce the precision of encoding the rapidly changing formants in the CV transition or from an onset delay originating in the cochlea. The stop consonant burst in the /da/ syllable used in previous studies contains high-frequency energy (Vander Werff & Burns 2011; Anderson et al. 2012; Clinard & Tremblay 2013). Because older adults typically have higher audiometric thresholds than younger adults in the high frequencies, even when their hearing would be classified as clinically normal, the delay in latency may be due to reduced audibility of the /da/ consonant.
We hypothesized that impaired auditory temporal processing is the main cause of the delayed neural timing for the rapidly changing formants in the dynamic regions of speech in older adults. To test our hypothesis, we recorded frequency following responses (FFRs) in younger and older adults to the speech syllables /da/ and /a/. We expected to find latency delays for the CV transition of the /da/, but not for the same time region of the /a/, in older adults compared with younger adults. If decreased audibility is a factor in the delayed latencies for the /da/, however, we would expect that latencies for the /da/ would be earlier than those for the /a/ in the younger group given cochlear tonotopicity, but in the older group, damage in the basal end of the cochlea would lead to similar latencies for the /da/ and /a/.
MATERIALS AND METHODS
Participants
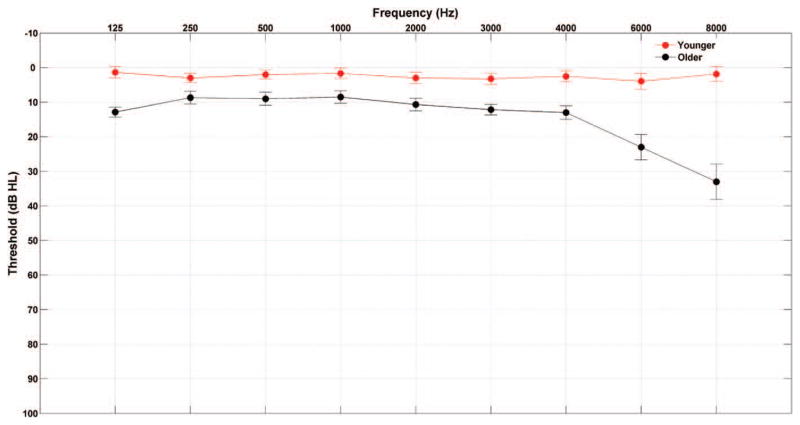
Participants comprised 15 younger adults (21 to 30 years old; mean ± standard error [SE], 24.4 ± 0.65 years; two males) and 15 older adults (60 to 68 years old; mean ± SE, 63.73 ± 0.66 years; four males) recruited from the Maryland, Washington, DC, and Virginia areas. All participants had clinically normal hearing defined as follows: (1) air conduction thresholds ≤ 25 dB HL from 125 to 4000 Hz bilaterally and (2) no interaural asymmetry > 15 dB HL at two or more adjacent frequencies. In addition, all subjects had normal click-evoked brainstem response latencies (wave V < 6.8 msec; Otto & McCandless 1982) measured using a 100-μs click stimulus presented at 80 dB peSPL at a rate of 31.3 Hz. No participants reported a history of neurological disorders, and all were native speakers of English. Because of the established effects of musicianship on subcortical auditory processing (Bidelman & Krishnan 2010; Parbery-Clark et al. 2012), professional musicians were excluded and the groups were matched on self-rated instrument proficiency [t(28) = 0.874, p = 0.389]. The two groups had normal IQs (≥ 85 on the Wechsler Abbreviated Scale of Intelligence; Zhu & Garcia 1999) and were matched on IQ [t(28) = 1.691, p = 0.102] and sex (Fisher exact, p = 0.651). In addition, the older adults were screened for dementia using the Montreal Cognitive Assessment (Nasreddine et al. 2005). See Figure 1 for average audiometric thresholds and Table 1 for mean and SEs of the test scores, audiometric thresholds, and click-evoked response latencies. All procedures were reviewed and approved by the Institutional Review Board of the University of Maryland. Participants gave informed consent and were paid for their time.
Fig. 1.
Audiogram (mean ± 1 SE) of the grand averages of hearing thresholds in younger and older adults.
TABLE 1.
Group means (with standard errors) for the younger and older adults for pure tone averages (0.125–4 kHz), age, click latency wave I and wave V, Wechsler Abbreviated Scale of Intelligence IQ, and Montreal Cognitive Assessment scores
| Pure Tone Average for 0.125–8 kHz (dB HL) | Age (yr) | Click Latency Wave I (msec) | Click Latency Wave V (msec) | Wechsler Abbreviated Scale of Intelligence IQ (Standard Score) | Montreal Cognitive Assessment (Standard Score) | |
|---|---|---|---|---|---|---|
| Younger (n = 15) | 2.519 (0.390) | 24.40 (0.65) | 1.69 (0.03) | 5.65 (0.05) | 110.80 (3.14) | Not applicable |
| Older (n = 15) | 14.552 (0.774) | 63.73 (0.66) | 1.73 (0.06) | 6.04 (0.07) | 119.13 (3.79) | 25.86 (0.44) |
| Older (n = 6) | 10.093 (0.702) | 63.33 (1.22) | 1.67 (0.09) | 5.95 (0.10) | 114.33 (7.07) | 25.83 (0.40) |
Electrophysiology
Stimuli
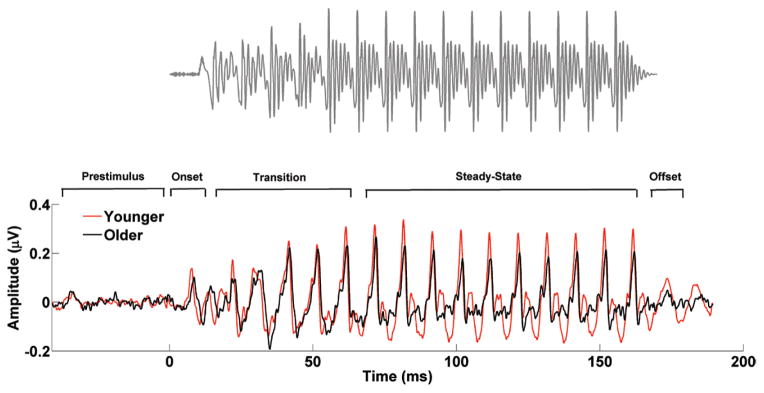
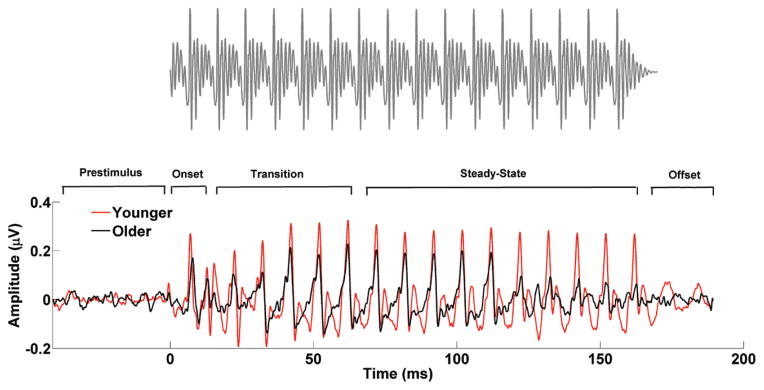
Two speech syllables were used. (1) A 170-msec / da/ was synthesized at a 20-kHz sampling rate with a Klatt-based synthesizer (Klatt 1980). After an initial 10-msec stop burst in the syllable, voicing remained constant with a fundamental frequency (F0) of 100 Hz. During the 50-msec transition from the /d/ to the /a/, the lower three formants shifted (F1, 400 → 720 Hz; F2, 1700 → 1240 Hz; F3, 2580 → 2500 Hz) but stabilized for the 120-msec steady-state vowel portion. The fourth to sixth formants (F4 to F6) remained constant over 170 msec at 3300, 3750, and 4900 Hz, respectively. A waveform of the /da/ is presented in Figure 2 along with average responses in younger and older adults. (2) A 170-msec /a/ was synthesized at a 20-kHz sampling rate with a Klatt-based synthesizer (Klatt 1980). This syllable had a fundamental frequency (F0) of 100 Hz, and in contrast with the /da/, all the first six formants stayed constant throughout the whole presentation of the stimulus (F1 = 720 Hz; F2 = 1240 Hz; F3 = 2500 Hz; F4 = 3300 Hz; F5 = 3750 Hz; F6 = 4900 Hz). A waveform of the /a/ is presented in Figure 3 along with average responses in younger and older adults. Despite the absence of a transition region in the /a/, the peaks occurring between 22 and 62 msec were marked as transition to compare this time window with the equivalent transition segment observed in the /da/. The /da/ was chosen because it includes a formant-changing transition and a steady-state region, and the /a/ was chosen because the formants are constant throughout the waveform. Both speech syllables were presented binaurally using Intelligent Hearing Systems SmartEP system (Miami, FL) with alternating polarities at 80 dB SPL at a rate of 4.3 Hz through electromagnetically shielded insert earphones (ER-3; Etymotic Research) at a sampling rate of 13.3 kHz.
Fig. 2.
Stimulus waveform (top) and average brainstem responses to /da/ in the younger (red) and older (black) adults (bottom). The prestimulus and response regions are labeled with respect to the onset, formant transition, and steady-state vowel of the stimulus.
Fig. 3.
Stimulus waveform (top) and average brainstem responses to /a/ in the younger (red) and older (black) adults (bottom). The steady-state region in the /a/ response starts at ~20 msec, but for comparison purposes with /da/, the transition region, in addition to the prestimulus, onset, steady state, and offset regions, is also marked.
Recording
Subcortical responses were recorded in an electrically shielded double-walled sound-attenuating chamber. The online filter was set at 50 to 3000 Hz. A vertical montage of four Ag–AgCl electrodes (Cz active, forehead ground, average earlobes as reference) was used. During the recording session (~1 hr), participants sat in a reclining chair and watched a silent, captioned movie of their choice to facilitate a relaxed yet wakeful state. The order of syllable presentation was randomized. Six thousand artifact-free sweeps were recorded for each speech syllable from each participant.
Data Reduction
Sweeps with amplitudes in the ±31 μV range were retained and averaged in real time and then processed off-line using MATLAB (Mathworks, version R2011b). Responses were digitally band-pass-filtered off-line from 70 to 2000 Hz using a Butterworth filter to minimize the effects of low-frequency oscillations that originate from the cortex, given the subcortical origin of the FFR (Smith et al. 1975; Galbraith et al. 2000). Although some neurons in the auditory cortex are capable of phase locking up to 100 Hz (Wallace et al. 2000), the number of these neurons is much smaller than can be found in subcortical structures (Bartlett et al. 2007; Wang et al. 2008), and therefore, the cortical contribution to the response is likely to be minimal. The time window for each sweep was −41 to 189 msec referenced to the stimulus onset. The final average was computed by averaging the 6000 sweeps (3000 of each polarity). One final average response was created for subsequent analysis, for which the two polarities were averaged to minimize the influence of cochlear microphonic and stimulus artifact on the response. Averaging alternating polarities also minimizes the temporal fine structure while maximizing the envelope response (Chimento & Schreiner 1990; Aiken & Picton 2008; Campbell et al. 2012). Adding alternating polarities to extract the envelope introduces rectification-related distortion at the level of the cochlea into the response, which may produce energy at integer multiples of the F0. Previous studies have shown that energy at the F0 makes the strongest contribution to the envelope of the FFR (Greenberg et al. 1987; Aiken & Picton 2006). Added responses were used to analyze latency, amplitude, frequency and time–frequency representation of the F0 and lower harmonics, and phase-locking value (PLV).
Analyses
Timing
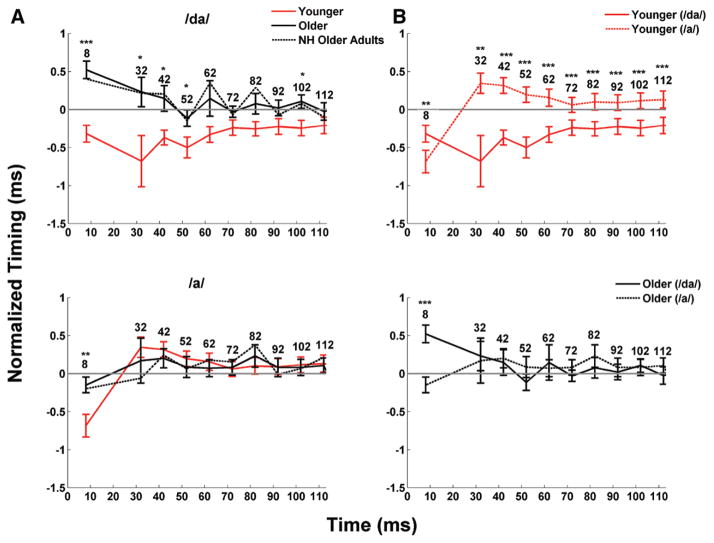
To analyze aging effects on neural timing, we extracted peaks in the subcortical responses in two steps. First, a function written in MATLAB identified the peaks that were closest to the expected latency. These latencies were chosen based on the peaks extracted from the group average and from latencies obtained in previous studies (Anderson et al. 2012; Parbery-Clark et al. 2012). Then, a trained peak picker, blind to participant group, confirmed each peak identification and made changes where appropriate. This identification provided the latency and amplitude of each peak. Peaks were labeled according to a reference latency of the speech syllable /a/ (i.e., a peak occurring 32 to 33 msec after onset would be called “peak 32”; Fig. 4). The onset peak was identified as peak 8, transition peaks were 32, 42, and 52, and steady-state peaks were 62, 72, 82, 92, 102, and 112.
Fig. 4.
Neural delays (mean ± 1 SE) in the aging population for the /da/ and /a/. The x axis represents the peak analyzed for each subject, while the y axis represents the normalized peak latency for each subject. To facilitate visualization of the data, peak latencies on the y axis were normalized. Normalization was obtained by subtracting the expected latency from the /da/ (8, 32, 42, 52, 62, etc.) from the actual response latency until 112 msec for the transition and the steady state. For instance, if the latencies detected at the onset for two different subjects were 7 and 9 msec, the corresponding normalized values would be −1 and 1 msec, respectively. Negative values indicate that the peaks were early with respect to the expected latency, while positive values indicate that the peaks were late with respect to the expected latency. A, In response to the /da/, older adults show a shift in neural response timing with respect to the younger adults for both the onset and transition (peaks 32–52) but not for the steady state, with the exception of peak 102. In response to the /a/, the only shift in neural response timing with respect to younger adults was observed at the onset. Dashed lines represent the latencies for the six older adults that had hearing thresholds < 30 dB HL (average between right and left ears) at 6 and 8 kHz. The exclusion of these subjects with greater hearing loss did not result in latency changes. B, Latency differences within age groups between the /da/ and /a/ syllables. The younger adults’ latencies were earlier for the /da/ than for the /a/ throughout the whole response (all p < 0.01), but there were no latency differences between syllables in the older adults’ responses (all p > 0.05), except at the onset. *p < 0.05, **p < 0.01, ***p < 0.001, NS = not significant. NH indicates normal hearing.
Response and Spectral Magnitudes
Root mean square (RMS) amplitude was used to objectively quantify the overall magnitude of response and prestimulus (i.e., nonresponse) activity. RMS amplitudes were computed for the prestimulus period (−41 to 0 msec), transition (18 to 68 msec), steady-state I (SS-I = 68 to 117 msec), steady-state II (SS-II = 117 to 169), and offset response (169 to 188 msec). The steady-state region was divided into two sections because of an observed drop in amplitude in the older adults’ responses after ~115 msec. Average spectral amplitudes over 40 Hz bins were calculated from each response using a fast Fourier transform with zero padding and 1-Hz frequency resolution over the two steady-state time regions (68 to 117 msec and 117 to 169 msec) for the F0 and second and third harmonics.
Time–Frequency Analysis
Wavelets were used to analyze the grand average of the responses of the envelope in the time–frequency domain. The complex Morlet wavelets, w(t, f0), were used to decompose the signal between 80 and 1000 Hz (Tallon-Baudry et al. 1997). Morlet wavelets expressed as have Gaussian shapes both in time and frequency domains with f0 denoting central frequency. Time and frequency standard deviations σt and σf are related to each other as σf =1/ (2 πσt), and the factor is used to normalize the total energy of the wavelets to 1. This wavelet family is characterized by f0/σf = 7. The time-varying amplitude was calculated by taking the absolute values of the convolution of the complex wavelet with the signal: Ampl(t, f0) = |w(t, f0) × s(t)|.
PLV Analysis
The PLV in the 80 to 1000 Hz range of the group averages of younger versus older adults for the /a/ and /da/ was calculated using the same procedure and equations as reported by Lachaux et al. (2002). In contrast with the phase-locking analysis performed by Anderson et al. (2012), this PLV was not applied to single trials but rather to the group average waveforms of younger and older participants. This analysis was used to evaluate the level of phase synchronization between responses of younger and older adults to /da/ and /a/. The range of values of the PLV goes from 0 (absence of phase coherence between the two signals analyzed) to 1 (perfect phase coherence between the two signals analyzed). The advantage of using this mathematical model is that only the phase of each continuous wavelet transform contributes to the final value of the phase coherence, as the amplitude has been removed. Moreover, wavelets ensure an optimal compromise time–frequency resolution for our frequency range.
Statistical Analyses
All statistical analyses were conducted in IBM SPSS statistics software, version 21.0. A doubly multivariate repeated-measures analysis of variance (ANOVA) was used to test group differences between transition and steady-state regions and between /a/ and /da/ in the time domain. This design was adopted because it permits analyses in which the dependent variables represent measurements of several variables for the different levels of the within-subjects factors. One-way ANOVAs were used for peak latencies (younger versus older) and for RMS amplitudes, the F0, and its harmonics. Levene test was used to ensure homogeneity of variance for all measures. Paired t tests were used to test RMS amplitude and latency differences within age groups in the time domain. Because the requirements for the Levene test were not met for all our analyses, the nonparametric Mann–Whitney U and Friedman tests were applied to replace one-way ANOVA and one-way ANOVA with repeated measures, respectively.
RESULTS
Timing
The timing analysis partially refuted our hypothesis that older adults have impaired auditory processing for dynamic but not static regions of the speech stimuli used in this experiment. Overall, we found evidence suggesting that the age-related differences in the transition region of the /da/ were primarily due to reduced encoding of the high-frequency energy of the /d/ consonant rather than to impaired temporal processing. This conclusion is based on the following analyses: we first tested differences between transition and steady-state regions in the two syllables between younger and older adults by using a doubly multivariate repeated-measures model with 4 levels (/da/ versus /a/, transition versus steady-state regions) and 3 peaks (32-, 42-, and 52-msec peaks in the transition or 72, 82, and 92 msec in the steady-state regions). Results indicated a significant Group × Region × Syllable interaction [F(1,26)= 3.171, p = 0.041]. This interaction was driven by both Group × Region [F(1,26) = 3.869, p = 0.021] and Region × Syllable interactions [F(1,26) = 4.38, p = 0.011].
To determine the factors that led to these interactions, we performed follow-up between-group and within-group testing. Follow-up between-group analyses for peak latencies were performed by using one-way ANOVAs (Fig. 4A). Younger adults had earlier latencies than older adults for the onset of both the /a/ and /da/ stimuli [F(1,28) = 8.737, p = 0.006 and F(1,28) = 27.636, p < 0.001, respectively]. Results showed a group latency difference that is trending toward significance in the transition region in the /da/ [F(1,28) = 8.014, p = 0.064], but not in /a/ [F(1,28) = 0.162, p = 0.921], and no significant group latency differences in the steady-state region for either the /da/ [F(1,23) = 2.150, p = 0.0860] or the /a/ [F(1,28) = 0.381, p = 0.629].
Although these results might support the hypothesis that impaired temporal processing resulted in delayed latencies for the dynamic region of the syllable, the follow-up within-group testing suggested a primarily peripheral cause. Within-group testing showed earlier latencies for the /da/ than for the /a/ in the younger group for both the transition [F(1,12) = 22.678, p < 0.001] and steady-state regions [F(1,12) = 18.761, p < 0.001], as expected given cochlear tonotopicity. However, the /da/ latencies were not earlier than the /a/ latencies in the older group for either the transition [F(1,12) = 0.970, p = 0.439] or the steady-state regions [F(1,12) = 0.618, p = 0.616], suggesting that reduced audibility from damage to the basal end of the cochlea may have resulted in latency delays for the /da/ (Fig. 4B). The expected time delay between syllables was only observed in older adults at the onset of the stimulus [t(14) = 6.686, p < 0.001, paired t test].
The click analysis suggested that temporal processing deficits might also be a contributing factor to delayed latencies. The click-evoked auditory brainstem response is dominated by contributions from the basal end of the cochlea (Don & Eggermont 1978). The latencies for wave I should be affected by the audibility of the click, yet no significant differences were found in wave I [F(1,28) = 1.036, p = 0.317]. On the contrary, click latencies for wave V were significantly different between groups [F(1,28) = 11.48, p = 0.002]. Therefore, although audibility appears to be a primary factor for the age-related latency delays for the /da/, we cannot rule out the possibility of central contributions to these delays.
Effects of High-Frequency Hearing Loss on Latency
Given the presence of high-frequency hearing loss in many of our older adults, we repeated the analysis after removing the subjects whose averaged right and left hearing threshold at both 6000 and 8000 Hz was greater than 25 dB HL. One-way ANOVAs between younger adults (n = 15) and the subset of older adults (n = 6) also showed significant differences at the onset and at peaks 42, 62, and 82 msec in response to /da/ (all p values < 0.05), while no significant differences were found in response to the /a/ (all p values > 0.05) as seen in Figure 4A (black dashed lines). However, there was also a similar pattern for the between-syllable comparison within older adults, in whom significant differences between the two speech syllables were observed only at the onset [t(14) = 8, p < 0.001] and at peak 112 msec [t(14) = −3.882, p = 0.012]. Therefore, age-related cochlear changes were a factor in the delayed /da/ latencies even in this subset of older adults with relatively better hearing.
Response Magnitude: Time and Frequency Domains Time Domain
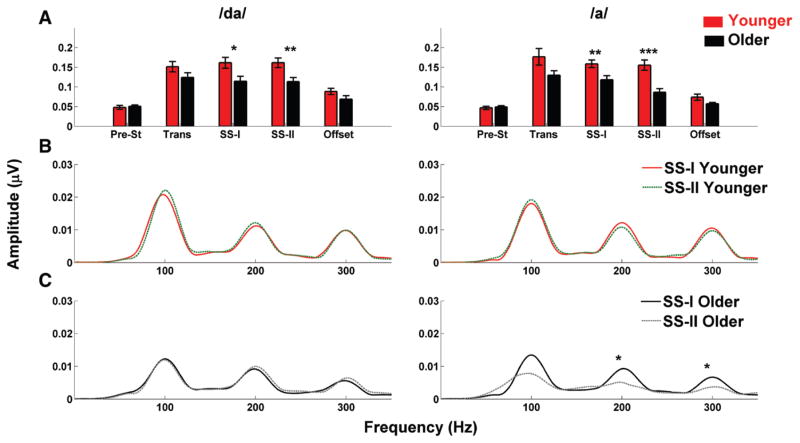
One-way ANOVAs were applied to the RMS values in the prestimulus, transition, SS-I, SS-II (only in response to the /da/), and offset (only in response to the /da/) regions between age groups. Because Levene test of equality of error of variance failed for SS-II and offset in response to /a/, the nonparametric Mann–Whitney U test was used for these two cases. Results represented in Figure 5A showed that the response amplitudes of the younger adults were significantly higher than the older adults in the SS-I and SS-II regions for both the /a/ [F(1,28) = 7.656, p = 0.009, U(28) = 22, Z = −3.754, p < 0.001, respectively] and the /da/ [F(1,28) = 6.641, p = 0.015, F(1,28) = 8.979, p = 0.005, respectively], while no significance differences were found in the transition region [F(1,28) = 3.855, p = 0.059 for the /a/ and F(1,28) = 2.629, p = 0.116 for the /da/] and in the offset [U(28) = 82, Z = −1.265, p = 0.216 for the /a/ and F(1,28) = 2.696, p = 0.111 for the /da/].
Fig. 5.
A, Bar graphs showing the root mean square (RMS) values (mean ± 1 SE) for younger (red) and older (black) adults in response to /da/ and /a/. Older adults have lower RMS values in response to the steady-state regions in both the /da/ and /a/. B and C, Fast Fourier transforms calculated for the steady state (SS)-I and SS-II regions for the younger (B) and older (C) participants. Solid lines (red for younger and black for older adults) represent the spectral amplitudes for the SS-I, while dashed lines (green for younger and gray for older adults) represent spectral amplitudes for the SS-II. Note the difference between the SS-I and SS-II regions in older adults in response to the /a/ stimulus, only. *p < 0.05, **p < 0.01, ***p < 0.01.
A within-group comparison of RMS amplitude differences between the /da/ and /a/ syllables was consistent with our latency analysis. The older adults showed no significant amplitude differences between responses to /da/ and /a/ (p > 0.05, paired t test) in any of the time regions except for SS-II [t(14) = −3.557, p = 0.003, paired t test]. Conversely, in younger adults, significant differences were found in the transition and offset regions [t(14) = 2.353, p = 0.034 and t(14) = −3.848, p = 0.002, respectively; paired t test].
Frequency Domain
One-way ANOVAs were used to investigate differences in spectral amplitudes between younger and older adults in response to /da/ in the SS-I and SS-II regions and in response to /a/ in the SS-I region. The Mann–Whitney U test was used for the response to /a/ in the SS-II region. Results indicated that younger adults had significantly higher amplitudes than older adults in the F0 and in the third harmonic (H3), but not in the second harmonic (H2) in response to /da/ in the SS-I [F0: F(1,28) = 4.523, p = 0.042; H2: F(1,28) = 1.641, p = 0.211; H3: F(1,28) = 8.302, p = 0.007] and SS-II [F0: p = 0.007; H2: p = 0.412; H3: F(1,28) = 7.609, p = 0.010] regions. Significantly higher amplitudes in response to /a/ were found in younger adults only in the H3 in the SS-I region [F0: F(1,28) = 2.248, p = 0.145; H2: F(1,28) = 3.817, p = 0.061; H3: F(1,28) = 8.281, p = 0.007] and in the F0 and the second and third harmonics in SS-II region [F0: U(28) = 44, Z = −2.841, p = 0.003; H2: U(28) = 39, Z = −3.049, p = 0.001; H3: U(28) = 38, Z = −3.09, p = 0.001].
Within-group comparisons between the SS-I and SS-II regions showed that older adults had higher amplitudes in the SS-I compared with the SS-II regions for both the F0 [t(14) = 3.031, p = 0.009] and for the first two harmonics [t(14) = 3.231, p = 0.006 and t(14) = 2.877, p = 0.012, for H2 and H3, respectively] in response to the /a/, while no significant differences were found in response to the /da/ (all p values > 0.05). No significant within-group differences between the SS-I and SS-II regions were found in the F0 and in the harmonics in response to /da/ and /a/ in younger adults (all p values > 0.05) as seen in F igure 5B, C.
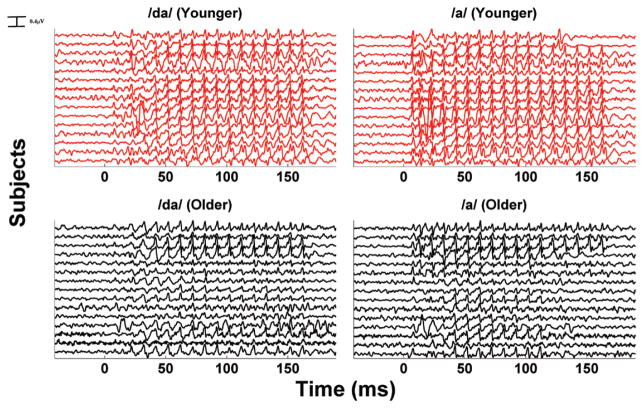
The percentage differences between the SS-I and SS-II regions for the F0, H2, and H3 in both /da/ and /a/ were also calculated in younger and older adults. Given the fact that the requirements for the Levene test were not met, the nonparametric Friedman test was used to estimate the following: (1) if the percentage differences in /da/ and /a/ were significantly different within age groups and (2) if the percentage differences in /da/ and /a/ were significantly different between age groups. Results reported in Table 2 showed that significant differences within groups (/da/ versus /a/) were found in older adults ( , p = 0.007) but not in younger adults ( , p = 0.664), while significant differences between groups were found in response to /a/ ( , p < 0.001) but not to /da/ ( , p = 0.940). Figure 6 shows the waveforms for each subject in response to /da/ and /a/.
TABLE 2.
Group means (with standard errors) for the younger and older adults for the percentage difference of the fundamental and of the first two harmonics and for the percentage difference of the root mean square values
| F0 (/da/) | F1 (/da/) | F2 (/da/) | F0 (/a/) | F1 (/a/) | F2 (/a/) | RMS (/da/) | RMS (/a/) | |
|---|---|---|---|---|---|---|---|---|
| Younger | −6.89 ± 7.14 | −6.43 ± 6.68 | −0.53 ± 7.53 | 6.47 ± 13.58 | 34.27 ± 18.75 | 38.66 ± 29.78 | 0.33 ± 2.57 | 5.95 ± 4.52 |
| Older | 9.16 ± 13.72 | 0.15 ± 9.28 | −6.42 ± 7.68 | 74.24 ± 18.46 | 164.12 ± 61.18 | 122.01 ± 41.36 | 1.24 ± 3.14 | 40.18 ± 9.88 |
RMS, root mean square.
Fig. 6.
Time series of for each subject in response to /da/ and /a/. Note how the steady state II tends to desynchronize in the majority of older adults. This phenomenon is observed only in response to /a/.
The percentage differences between the RMS values of the SS-I and SS-II regions were also calculated to estimate the effect of loss of synchronization for each subject. As in the case of the RMS values, because Levene test of equality of error of variance failed for percentage difference for the SS-II region in response to /a/, the nonparametric Mann–Whitney U test was used. Results reported in Table 2 show that there was no significant difference between younger and older adults in response to /da/ [F(1,28) = 0.051, p = 0.823]. However, the loss of synchronization in SS-II in the majority of older adults in response to /a/ resulted in a dramatic decrease in amplitude, which was not observed in younger adults. This is reflected by significant differences between the two age groups [U(28) = 45, Z = −2.8, p = 0.004].
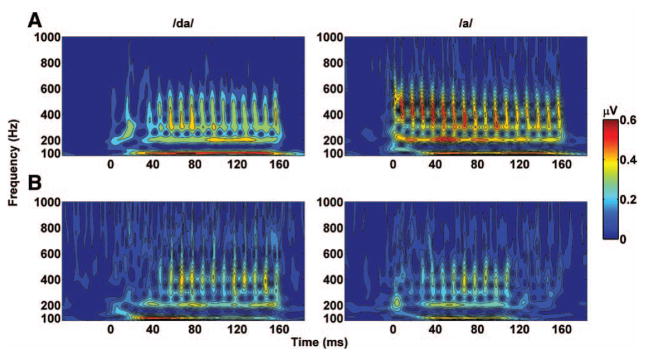
Time–Frequency Analysis
The time–frequency analysis for the grand average of younger and older adults in response to /da/ and /a/ was carried out using Morlet wavelets. Results displayed in Figure 7 suggest that higher harmonics (H2 to H4) in younger and older adults synchronized later to the /da/ than to the /a/. In response to the /da/, the younger and older adults synchronized at approximately 45 and 55 msec, respectively, and in response to the /a/, they synchronized at 10 and 25 msec, respectively. Synchronization to the steady-state region was preserved throughout the whole stimulation time in both age groups in response to the /da/ but faded around ~115 ms in the older adults only in response to the /a/. The spectrogram also showed higher amplitude in younger adults at all frequencies for both /da/ and /a/.
Fig. 7.
Amplitude of the time–frequency analysis of the grand averages of the younger (A) and older populations (B). “0” indicates the stimulus onset. Note how the amplitude of the older population in response to /a/ drastically decreases at ~120 msec.
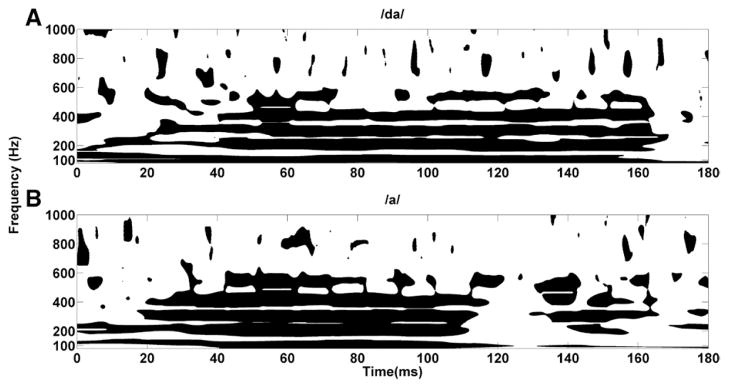
Phase-Locking Value
Results from the PLV analysis of the grand average are displayed in Figure 8, where differences in phase locking between the two age groups are shown. A significance threshold of ≥ 0.95 was chosen according to the values calculated by Lachaux et al. (2002). PLV values confirmed that the harmonics (H2 to H4) of older and younger adults synchronized earlier in response to the /a/ stimulus than in response to the /da/ stimulus. This is evident when looking at the first 40 msec, where robust synchronization between younger and older adults’ responses in the first three harmonics (up to 400 Hz) was observed in response to /a/, while a significant level of synchronization (black color) between the two age groups was not achieved until after 40 msec in response to / da/. This delay in synchronization to the /da/ is consistent with the peak latency delays in responses to /da/ resulting from reduced audibility. The synchronization to the /a/ was consistent up to ~115 msec, beyond which point the harmonics and the F0 tended to desynchronize. Conversely, the synchronization in response to the /da/ was stable through 160 msec. These observations were consistent with both the frequency and the time–frequency analysis, where a clear reduction of amplitude was observed at around 115 msec in older adults in response to the /a/ and at around 160 msec in both younger and older adults in response to the /da/.
Fig. 8.
Phase-locking value (PLV) for /da/ (younger vs. older) (A) and PLV for /a/ (younger vs. older) (B). The black color signifies that the responses of the younger and older adults are significant in phase (values ≥ 0.95), while the white color signifies that the responses of the two groups are out of phase (values < 0.95). The threshold value of 0.95 has been chosen based on the parameters used to calculate the PLV.
DISCUSSION
The results did not entirely support the hypothesis that aging effects on subcortical responses are due to central rather than peripheral causes; rather, both peripheral and central factors appeared to contribute to the findings. The older adults’ latency delays in the CV transition were likely driven by reduced audibility (i.e., peripheral), while the loss of synchronization that was present in the late stage of the steady-state region in response to the speech-syllable /a/ in older adults may arise from impaired central processing.
Timing
Although we found the same latency delays for the transition region of the /da/ that were found in Anderson et al. (2012), our results suggest that these delays are at least partly due to a reduction of audibility for the high-frequency components of the /d/ consonant in older adults resulting from slight high-frequency hearing loss. There were significant group differences in hearing thresholds across the frequency range, with the greatest differences occurring at frequencies above 4 kHz. These hearing threshold differences likely account for the finding that responses of younger adults showed earlier peak latencies for the syllable /da/ than /a/ (as expected giving cochlear tonotopicity), while the same latency differences were not seen in the responses of older adults. Although the participants had clinically normal hearing, subclinical loss of outer hair cells may have contributed to the 1-msec difference in response latencies to the /da/ observed between younger and older adults.
However, this mild high-frequency hearing loss did not result in delayed wave I click latencies in older adults compared with younger adults, while we did find age-related delays for wave V. This finding of delays for responses arising from the brainstem (wave V) but not from responses from the auditory nerve (wave I) provides evidence for a temporal processing deficit in addition to the delay caused by cochlear damage.
Response Magnitude: Time and Frequency Domain
Static Versus Dynamic Regions
Aging affects response amplitudes both in the time and frequency domains, reflecting decreased encoding of the periodicity envelope derived from speech fine structure, consistent with what was previously reported using a similar 170-msec speech syllable /da/ (Anderson et al. 2012). Similar age effects, even though limited to the transition region, were found by Vander Werff and Burns (2011) and Clinard and Tremblay (2013), who used a 40-msec /da/. In contrast with the studies using a 40-msec /da/, the amplitude differences between younger and older adults reached significant values in the steady-state region, but not in the transition and offset regions, for both speech syllables. Because amplitude and latency should both be affected by temporal precision, these results appear to suggest that different neural mechanisms may underlie these age-related changes. It is possible that peripheral changes, such as damage to the cochlea, may be primarily responsible for the time delay observed in the responses of older adults. However, a loss of temporal precision at higher levels in the auditory system, such as the midbrain, may be the most plausible explanation for a loss of synchronization in the late stage of the steady-state response. It is most likely that both peripheral and central factors contributed to our findings.
A reduction in auditory nerve fibers and loss of neural synchrony would presumably affect both latency and amplitude of the entire response. Furthermore, decreased inhibitory neurotransmission and other age-related changes may also have an impact on subcortical auditory processing in the midbrain (Walton et al. 1997; Caspary et al. 2005; Caspary et al. 2006). One of the functions of inhibitory neurotransmitters is to sharpen neural responses to rapidly varying acoustic stimuli (reviewed in Caspary et al. 2008); therefore, a reduction in inhibitory neurotransmission may lead to a timing deficit specific to the changing formant transition region of the speech syllable. Because of the frequency differences in our stimuli, however, we are not able to make this determination based on our results.
Loss of Synchronization in SS-II
A different mechanism may be responsible for the loss of amplitude noted in the SS-II region of the response of older adults to /a/. Synchronization appears to significantly decrease after ~95 msec (corresponding to ~115 msec from stimulus onset) of sustained phase locking, and this decrease is evident both in the time and frequency domains. Although surprising, this finding may arise from a loss of auditory nerve fibers that leads to an inability to sustain neural firing, such as may be found in abnormal acoustic reflex decay or tone decay test findings associated with VIIIth nerve lesions (Lidén & Korsan-Bengtsen 1973; Olsen et al. 1975). Another possible explanation for the older adults’ inability to sustain encoding a stimulus as efficiently as younger adults is prolonged neural refraction and loss of temporal synchronization among the neurons devoted to encoding that particular acoustic stimulus, as suggested by Walton et al. (1998). In that study, the integrity of temporal processing in the auditory midbrain was investigated by comparing detection of brief silent intervals in younger versus older CBA mice. The number of IC neurons that encoded short gap durations was reduced by approximately 50% in older mice. Furthermore, older mice had slower neural recovery times after previous stimulation with respect to younger mice, leading to poorer performance in detecting silent gaps. This ~50% reduction of IC neurons devoted to the encoding of short gap durations may be related in part to the loss of fibers with low spontaneous discharge rates from noise-induced cochlear neuropathy reported by Furman et al. (2013). Furman et al. speculated that this significant reduction in nerve fibers may cause hyperactivity that contributes to difficulty in processing auditory information in noisy environments by decreasing the signal-to-noise ratio. This effect was not found in response to /da/, possibly because its steady-state region was shorter in duration. Our results seem to contrast with those of Bidelman et al. (2014), who used a speech vowel continuum that did not include a transition region (similar to the /a/ used in our experiment) and did not report loss of synchronization. However, the length of the stimulus in that experiment was only 100 msec, and the effects observed in our study occurred after approximately 112 msec. Further studies that extend the steady-state region of the /da/ need to be conducted to explore the underlying mechanisms of this phenomenon.
Time–Frequency Analysis and PLV
Time–frequency analysis was applied to the grand averages of the younger and older adults to investigate representation of the fundamental frequency (100 Hz) and its harmonics. The results differed for the two stimuli. As expected, both younger and older adults showed a delay in synchronization in response to /da/ because of the presence of the stop consonant /d/. This delay was represented by smaller amplitudes at all frequencies in the onset region. However, in younger adults, the response became robust at all frequencies (higher amplitude) earlier (~30 msec) than in older adults (~46 msec). Consistent with what was observed in the frequency and time-domain analyses and with previous studies (Clinard et al. 2010; Anderson et al. 2012), differences in amplitude between the two age groups (Fig. 7) were present throughout the entire time region of stimulation and in all harmonics, while phase differences (Fig. 8A) were present in the transition region only. These phase differences observed in the transition region could be explained by the high-frequency components present in the consonant /d/ that older adults might have failed to encode due to cochlear damage. Failure to encode the high-frequency components of the speech syllable might have caused a delay in the response to the transition rather than poorer phase locking to the stimulus. Conversely, in response to the /a/ stimulus, both younger and older adults show rapid synchronization, possibly because of the absence of high-frequency components present in the dynamic region of the /da/ (~20 and ~30 msec, respectively). Differences in amplitude were still evident throughout the entire stimulation time, suggesting that aging effects on amplitudes in midbrain were not limited to the dynamic encoding of speech. However, as observed in the time and frequency analysis, there was a drastic and significant loss of activity in the later region of the steady state of older adults, which was denoted by loss of amplitude (Fig. 7) and phase synchronization (Fig. 8B) in the last ~50 msec.
Limitations
We did not design this study to evaluate aging effects on sustained phase locking to a steady-state auditory signal. Therefore, a limitation of this study is our inability to elucidate the mechanism that underlies the sudden loss of synchronization in the SS-II region in response to the /a/. Furthermore, because of the higher frequency energy in the /da/ that is not present in the /a/, we were unable to rule out a peripheral contribution to the latency delays. To further investigate these phenomena, a follow-up experiment should compare responses to /a/ with responses to a CV speech syllable that has identical starting formants and steady-state regions of equivalent durations. In addition, it would be useful to include a group of older adults with significant hearing loss to further determine the extent to which loss of audibility contributes to latency delays.
CONCLUSIONS
Altogether, our findings of delayed latencies and reduced ability to sustain steady-state activity in older adults may be in line with results obtained with histological studies and single-neuron and single-fiber recordings. Animal models have shown that cochlear hair cell loss, decrease in synchronization, and prolonged neural refraction result in poor performance in tasks that involve rapid changes of the stimulus, such as in short gap detection tasks (Walton et al. 1998; Altschuler et al. 2015). These age-related changes may contribute to the older adult’s difficulty with understanding someone who is talking rapidly or following a conversation in which there are multiple speakers. The results are in agreement with several studies that reported age-related changes in the peripheral and central auditory systems that affect the performance of older human and animals in tasks in which temporal processing plays a critical role, such as when measuring auditory perception with distorted speech (Goŕdon-Salant & Fitzgibbons 1993) or variation of inter-onset intervals (Fitzgibbons & Gordon-Salant 2001) or when collecting physiological gap detection in a mouse model (Walton et al. 1997).
We have replicated the set of findings reported by Anderson et al. (2012) of diminished temporal precision in the FFRs in older adults who had normal hearing across the audiometric frequency range. Our study extends these findings and suggests that two different mechanisms could be responsible for the latency delay and loss of synchronization: cochlear damage and a loss of temporal precision in the midbrain. The decreased amplitude in the steady-state regions for both syllables and the sudden and unexpected loss of activity observed in the last 50 msec of the steady-state region in the older adults in response to the /a/ suggest that multiple mechanisms contribute to reduced temporal precision and consequent impairments in speech perception. Aging affects auditory neural mechanisms in the form of loss of neurons and loss of synaptic connections (Willott 1996) and a decrease in inhibitory neurotransmission (Caspary et al. 2008). This impoverishment of neural networks may make the processing of some characteristics of sound harder to elaborate, such as tracking the CV transition or the sustained steady-state activity in a vowel. Imprecise representation of an auditory signal reduces the ability to selectively attend to that signal and extract meaning from it (Ding & Simon 2012). These findings advance understanding of the difficulties experienced by older adults when trying to understand speech in noisy conditions.
Acknowledgments
The authors thank the participants who participated in this study and Travis White-Schwoch and Trent Nicol for their useful comments on the manuscript.
This study was supported by the Department of Hearing and Speech Sciences at the University of Maryland.
Footnotes
S.A. designed the experiments; A.P., K.J., and R.L. collected the data; A.P. analyzed the data; A.P. and S.A. wrote the article.
The authors declare no other conflict of interest.
References
- Aiken SJ, Picton TW. Envelope following responses to natural vowels. Audiol Neurootol. 2006;11:213–232. doi: 10.1159/000092589. [DOI] [PubMed] [Google Scholar]
- Aiken SJ, Picton TW. Envelope and spectral frequency-following responses to vowel sounds. Hear Res. 2008;245:35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Altschuler RA, Dolan DF, Halsey K, et al. Age-related changes in auditory nerve-inner hair cell connections, hair cell numbers, auditory brain stem response and gap detection in UM-HET4 mice. Neuroscience. 2015;292:22–33. doi: 10.1016/j.neuroscience.2015.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, et al. Aging affects neural precision of speech encoding. J Neurosci. 2012;32:14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol. 2007;97:1005–1017. doi: 10.1152/jn.00593.2006. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Krishnan A. Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Res. 2010;1355:112–125. doi: 10.1016/j.brainres.2010.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM, Weiss MW, Moreno S, et al. Coordinated plasticity in brainstem and auditory cortex contributes to enhanced categorical speech perception in musicians. Eur J Neurosci. 2014;40:2662–2673. doi: 10.1111/ejn.12627. [DOI] [PubMed] [Google Scholar]
- Campbell T, Kerlin JR, Bishop CW, et al. Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography. Ear Hear. 2012;33:144–150. doi: 10.1097/AUD.0b013e3182280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Am Geriatr Soc. 1993;41:401–407. doi: 10.1111/j.1532-5415.1993.tb06948.x. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Hughes LF, Schatteman TA, et al. Age-related changes in the response properties of cartwheel cells in rat dorsal cochlear nucleus. Hear Res. 2006;216–217:207–215. doi: 10.1016/j.heares.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, et al. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211(Pt 11):1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Schatteman TA, Hughes LF. Age-related changes in the inhibitory response properties of dorsal cochlear nucleus output neurons: Role of inhibitory inputs. J Neurosci. 2005;25:10952–10959. doi: 10.1523/JNEUROSCI.2451-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimento TC, Schreiner CE. Selectively eliminating cochlear microphonic contamination from the frequency-following response. Electroencephalogr Clin Neurophysiol. 1990;75:88–96. doi: 10.1016/0013-4694(90)90156-e. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Cotter CM. Neural representation of dynamic frequency is degraded in older adults. Hear Res. 2015;323:91–98. doi: 10.1016/j.heares.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL. Aging degrades the neural encoding of simple and complex sounds in the human brainstem. J Am Acad Audiol. 2013;24:590–599. doi: 10.3766/jaaa.24.7.7. quiz 643. [DOI] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, Krishnan AR. Aging alters the perception and physiological representation of frequency: Evidence from human frequency-following response recordings. Hear Res. 2010;264:48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, et al. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci U S A. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Emergence of neural encoding of auditory objects while listening to competing speakers. Proc Natl Acad Sci U S A. 2012;109:11854–11859. doi: 10.1073/pnas.1205381109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M, Eggermont JJ. Analysis of the click-evoked brainstem potentials in man unsing high-pass noise masking. J Acoust Soc Am. 1978;63:1084–1092. doi: 10.1121/1.381816. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Auditory temporal processing in elderly listeners. J Am Acad Audiol. 1996;7:183–189. [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S. Aging and temporal discrimination in auditory sequences. J Acoust Soc Am. 2001;109:2955–2963. doi: 10.1121/1.1371760. [DOI] [PubMed] [Google Scholar]
- Frisina DR, Frisina RD. Speech recognition in noise and presbycusis: relations to possible neural mechanisms. Hear Res. 1997;106:95–104. doi: 10.1016/s0378-5955(97)00006-3. [DOI] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith GC, Threadgill MR, Hemsley J, et al. Putative measure of peripheral and brainstem frequency-following in humans. Neurosci Lett. 2000;292:123–127. doi: 10.1016/s0304-3940(00)01436-1. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cobb JL, Linn RT, et al. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch Otolaryngol Head Neck Surg. 1996;122:161–167. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- Goŕdon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Hear Res. 1993;36:1276–1285. doi: 10.1044/jshr.3606.1276. [DOI] [PubMed] [Google Scholar]
- Goŕdon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, et al. Age-related differences in identification and discrimination of temporal cues in speech segments. J Acoust Soc Am. 2006;119:2455–2466. doi: 10.1121/1.2171527. [DOI] [PubMed] [Google Scholar]
- Greenberg S, Marsh JT, Brown WS, et al. Neural temporal coding of low pitch. I. Human frequency-following responses to complex tones. Hear Res. 1987;25:91–114. doi: 10.1016/0378-5955(87)90083-9. [DOI] [PubMed] [Google Scholar]
- He NJ, Mills JH, Ahlstrom JB, et al. Age-related differences in the temporal modulation transfer function with pure-tone carriers. J Acoust Soc Am. 2008;124:3841–3849. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst KG, Humphrey C. Hearing impairment and mental state in the elderly living at home. Br Med J. 1980;281:903–905. doi: 10.1136/bmj.281.6245.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, et al. Processing of broadband stimuli across A1 layers in young and aged rats. Hear Res. 2010;264:79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Garner CB, Wilson DL, et al. Hearing-aid outcome measured following one month of hearing aid use by the elderly. J Speech Lang Hear Res. 2001;44:469–486. doi: 10.1044/1092-4388(2001/037). [DOI] [PubMed] [Google Scholar]
- Juarez-Salinas DL, Engle JR, Navarro XO, et al. Hierarchical and serial processing in the spatial auditory cortical pathway is degraded by natural aging. J Neurosci. 2010;30:14795–14804. doi: 10.1523/JNEUROSCI.3393-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DW, Beamish P, Roth M. Old age mental disorders in Newcastle upon Tyne. I. A study of prevalence. Br J Psychiatry. 1964;110:146–158. doi: 10.1192/bjp.110.465.146. [DOI] [PubMed] [Google Scholar]
- Klatt DH. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–995. [Google Scholar]
- Lachaux JP, Lutz A, Rudrauf D, et al. Estimating the time-course of coherence between single-trial brain signals: An introduction to wavelet coherence. Neurophysiol Clin. 2002;32:157–174. doi: 10.1016/s0987-7053(02)00301-5. [DOI] [PubMed] [Google Scholar]
- Laforge RG, Spector WD, Sternberg J. The relationship of vision and hearing impairment to one-year mortality and functional decline. J Aging Health. 1992;4:126–148. [Google Scholar]
- Lidén G, Korsan-Bengtsen M. Audiometric manifestations of retrocochlear lesions. Adv Otorhinolaryngol. 1973;20:271–287. doi: 10.1159/000393104. [DOI] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, et al. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, et al. Health ABC Study Group. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ, et al. Auditory evoked response to gaps in noise: Older adults. Int J Audiol. 2011;50:211–225. doi: 10.3109/14992027.2010.526967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Olsen WO, Noffsinger D, Kurdziel S. Acoustic reflex and reflex decay. Occurrence in patients with cochlear and eighth nerve lesions. Arch Otolaryngol. 1975;101:622–625. doi: 10.1001/archotol.1975.00780390036009. [DOI] [PubMed] [Google Scholar]
- Otto WC, McCandless GA. Aging and the auditory brain stem response. Audiology. 1982;21:466–473. doi: 10.3109/00206098209072759. [DOI] [PubMed] [Google Scholar]
- Parbery-Clark A, Anderson S, Hittner E, et al. Musical experience offsets age-related delays in neural timing. Neurobiol Aging. 2012;33:1483.e1–1483.e4. doi: 10.1016/j.neurobiolaging.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA. Masking-level differences in the elderly: A comparison of antiphasic and time-delay dichotic conditions. J Speech Hear Res. 1991;34:1410–1422. doi: 10.1044/jshr.3406.1410. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Engle JR, Juarez-Salinas DL. Spatial and temporal processing of single auditory cortical neurons and populations of neurons in the macaque monkey. Hear Res. 2011;271:115–122. doi: 10.1016/j.heares.2010.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatteman TA, Hughes LF, Caspary DM. Aged-related loss of temporal processing: Altered responses to amplitude modulated tones in rat dorsal cochlear nucleus. Neuroscience. 2008;154:329–337. doi: 10.1016/j.neuroscience.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Hamstra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. J Acoust Soc Am. 1999;106:371–380. doi: 10.1121/1.427062. [DOI] [PubMed] [Google Scholar]
- Smith JC, Marsh JT, Brown WS. Far-field recorded frequency-following responses: Evidence for the locus of brainstem sources. Electroencephalogr Clin Neurophysiol. 1975;39:465–472. doi: 10.1016/0013-4694(75)90047-4. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, et al. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–734. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Zhu X, Ding B, et al. Age-related hearing loss: GABA, nicotinic acetylcholine and NMDA receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience. 2014;259:184–193. doi: 10.1016/j.neuroscience.2013.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Uhlmann RF, Larson EB, Rees TS, et al. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261:1916–1919. [PubMed] [Google Scholar]
- Vander Werff KR, Burns KS. Brain stem responses to speech in younger and older adults. Ear Hear. 2011;32:168–180. doi: 10.1097/AUD.0b013e3181f534b5. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Shackleton TM, et al. Phaselocked responses to pure tones in guinea pig auditory cortex. Neuroreport. 2000;11:3989–3993. doi: 10.1097/00001756-200012180-00017. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, et al. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol A. 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lu T, Bendor D, et al. Neural coding of temporal information in auditory thalamus and cortex. Neuroscience. 2008;154:294–303. doi: 10.1016/j.neuroscience.2008.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, et al. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience. 2009;160:227–239. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF. Anatomic and physiologic aging: A behavioral neuroscience perspective. J Am Acad Audiol. 1996;7:141–151. [PubMed] [Google Scholar]
- Zhu J, Garcia E. The Wechsler Abbreviated Scale of Intelligence (WASI) New York, NY: Psychological Corporation; 1999. [Google Scholar]