Abstract
Mitochondria play important roles in generation of free radicals, ATP formation, and in apoptosis. We studied the levels of mitochondrial electron transport chain (ETC) complexes, that is, complexes I, II, III, IV, and V, in brain tissue samples from the cerebellum and the frontal, parietal, occipital, and temporal cortices of subjects with autism and age-matched control subjects. The subjects were divided into two groups according to their ages: Group A (children, ages 4–10 years) and Group B (adults, ages 14–39 years). In Group A, we observed significantly lower levels of complexes III and V in the cerebellum (p < 0.05), of complex I in the frontal cortex (p < 0.05), and of complexes II (p < 0.01), III (p<0.01), and V (p < 0.05) in the temporal cortex of children with autism as compared to age-matched control subjects, while none of the five ETC complexes was affected in the parietal and occipital cortices in subjects with autism. In the cerebellum and temporal cortex, no overlap was observed in the levels of these ETC complexes between subjects with autism and control subjects. In the frontal cortex of Group A, a lower level of ETC complexes was observed in a subset of autism cases, that is, 60% (3/5) for complexes I, II, and V, and 40% (2/5) for complexes III and IV. A striking observation was that the levels of ETC complexes were similar in adult subjects with autism and control subjects (Group B). A significant increase in the levels of lipid hydroperoxides, an oxidative stress marker, was also observed in the cerebellum and temporal cortex in the children with autism. These results suggest that the expression of ETC complexes is decreased in the cerebellum and the frontal and temporal regions of the brain in children with autism, which may lead to abnormal energy metabolism and oxidative stress. The deficits observed in the levels of ETC complexes in children with autism may readjust to normal levels by adulthood.
Keywords: autism, electron transport chain complexes, energy, mitochondria, oxidative stress
Autism is a complex pervasive developmental disorder that is characterized by impaired language, communication, and social skills, as well as by repetitive and stereotypic patterns of behavior, all occurring by the age of 3 years (Lord et al. 2000). It is a heterogeneous disorder, belonging to a group of neurodevelopmental disorders, known as the autism spectrum disorders (ASDs) that include Asperger syndrome and pervasive developmental disorder-not otherwise specified. According to a recent report from the Centers for Disease Control and Prevention, the prevalence of autism by the age of 8 years is 1 in 110 children (Rice 2009). The onset of autism is gradual in many children. However, functional regression has been reported in early childhood in some autism cases (Goldberg et al. 2003; Lord et al. 2004; Ozonoff et al. 2005; Hansen et al. 2008). Accumulating evidence supports a prenatal onset for developmental abnormalities leading to autism (Kolevzon et al. 2007; Kinney et al. 2008). Postmortem assessments of the brains of individuals with autism have unveiled early neurodevelopmental alterations, including reduced programed cell death and/or increased cell proliferation, altered cell migration, abnormal cell differentiation with reduced neuronal size, and altered synaptogenesis (Bauman and Kemper 2005; Wegiel et al. 2009, 2010).
Mitochondria are central to many cellular functions, including the generation of energy in the form of ATP and the maintenance of intracellular calcium homeostasis. They are the primary source of free radicals, that is, reactive oxygen species (ROS) and trigger apoptosis (Cadenas and Davies 2000; Lenaz 2001; Szewczyk and Wojtczak 2002; Polster and Fiskum 2004). Neurons in particular rely on the mitochondria because of neurons’ high levels of metabolism and subsequent need for energy. Mitochondria are localized in synapses, and alterations of the number, morphology, or function of synaptic mitochondria can be detrimental to synaptic transmission (Polster and Fiskum 2004). Extensive evidence suggests that mitochondrial dysfunction, oxidative stress, and reduced neurotransmission occur in the early stages of several major neurodegenerative diseases, such as Alzheimer’s disease (Reddy 2008; Reddy and Beal 2008; Aliev et al. 2009; Wang et al. 2009), Parkinson’s disease (Schapira et al. 1990; Navarro et al. 2009), Huntington disease (Gu et al. 1996), and amyotrophic lateral sclerosis (Wiedemann et al. 2002). Mitochondrial decay has also been suggested to be major contributor to aging (Ames 2004; Reddy 2008). In addition, mitochondrial dysfunction in the brain of some individuals with schizophrenia has been reported (Bubber et al. 2004). However, brain mitochondria have not yet been studied in autism, although altered energy metabolism as evidenced by alterations in peripheral markers, such as increased plasma lactate levels has been suggested in autism (Filipek et al. 2004; Correia et al. 2006).
Mitochondria are responsible for most of the energy production through oxidative phosphorylation, a process requiring the action of various respiratory enzyme complexes, the mitochondrial electron transport chain (ETC) located in the inner mitochondrial membrane (Szewczyk and Wojtczak 2002; Boekema and Braun 2007). Mitochondria produce ATP by generating a protons gradient (membrane potential) with the help of five ETC complexes, that is, complex I (NADH dehydrogenase), complex II (succinate dehydrogenase), complex III (cytochrome bc1 complex), complex IV (cytochrome c oxidase), and ATP synthase, also known as complex V, where the electron transport couples with translocation of protons from the mitochondrial matrix to the intermembrane space. The generated proton gradient is used by ATP synthase to catalyze the formation of ATP by the phosphorylation of ADP (Scholes and Hinkle 1984; Bertram et al. 2006). The number of mitochondria per cell is roughly related to the energy demands of the cell. The brain has a high demand for energy, and neurons contain a large number of mitochondria. The ETC in mitochondria is also a prime mechanism for free radicals generation (Cadenas and Davies 2000; Lenaz 2001). The changes in the mitochondrial ETC have been suggested to be an important factor in the pathogenesis of several diseases, including neuropsychiatric (Rezin et al. 2009) and neurodegenerative disorders (Burchell et al. 2010; Moreira et al. 2010).
In this study, we compared the protein levels of various mitochondrial respiratory ETC complexes in different regions of the brain from subjects with autism and age-matched control subjects. Although children with autism showed a decrease in protein levels of ETC complexes in the cerebellum and the frontal and temporal cortices, no change was observed in the occipital and parietal cortices. Interestingly, when we analyzed the data as a function of age, children with autism (4–10 years of age) but not adults with autism (14–39 years of age) showed lower protein levels of brain ETC complexes, suggesting that developmental mitochondrial abnormalities resulting in mitochondrial dysfunction, oxidative stress, and abnormal energy metabolism may contribute to autistic phenotype.
Materials and methods
Materials
Samples of postmortem frozen brain regions, that is, the cerebellum, and cortices from the frontal, temporal, parietal, and occipital lobes (N = 7–8 for different brain regions) from subjects with autism and age-matched control subjects were obtained from the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders at the University of Maryland Donors with autism fit the diagnostic criteria of the Diagnostic and Statistical Manual-IV, as confirmed by the Autism Diagnostic Interview-Revised. All brain samples were stored at −70°C. This study was approved by the Institutional Review Board of the New York State Institute for Basic Research in Developmental Disabilities. The case history (diagnosis, age, postmortem interval, and cause of death) for the subjects with autism and control subjects is summarized in Table S1.
Preparation of brain homogenates
The tissue samples were homogenized (10% w/v) in cold buffer containing 50 mm Tris-HCl (pH 7.4), 8.5% sucrose, 2 mm EDTA, 10 mm β-mercaptoethanol, and protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) in a Downs homogenizer with five strokes at 4°C. The protein concentration was assayed by bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL, USA).
Western blotting
The brain homogenates of subjects with autism and control subjects were mixed with loading buffer and boiled in a water bath for 5 min. Fifty micrograms of total protein of each sample was separated using a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (0.45 µm; Bio-Rad Laboratories, Hercules, CA, USA) using 100 V for 40 min. The membrane was blocked with Tris-buffered saline containing 5% fat-free dried milk for 1 h at 22°C, and further incubated overnight at 4°C with mouse monoclonal OXPHOS antibody (dilution 1 : 1500; MitoSciences, Eugene, OR, USA) against mitochondrial ETC complexes I–V. The membrane was then washed with Tris-buffered saline-0.05% Tween 20 three times and incubated with horseradish peroxidase-conjugated secondary antibody (dilution 1 : 5000; Thermo Scientific) for 45 min at 22°C. The membrane was washed again, and the immunoreactive proteins were visualized using the ECL substrate (Thermo Scientific). The levels of β-actin (the loading control) were determined by stripping and reprobing the membrane with anti-β-actin antibody (dilution 1 : 20 000; Abcam, Cambridge, MA, USA). While the two top bands for ETC complexes II and V were clearly visible, other complexes were relatively faint on the same blot. Therefore, to enhance visualization of the remaining ETC complexes, the membrane was re-exposed for a longer period of time.
The film was scanned and the bands were analyzed by using Image J software (NIH, Bethesda, MD, USA). The densities of different mitochondrial ETC complexes and β-actin were estimated. The relative densities of the mitochondrial complexes versus β-actin in autism and control groups were compared by unpaired Student’s t-test
Measurement of lipid hydroperoxide (LOOH)
The levels of LOOH were measured in the brain homogenates, as described by Patsoukis and Georgiou 2007. The photometric assay of LOOH measurement is based on the reaction of Fe3+ with LOOH, which converts Fe3+ to Fe2+. The reaction of Fe2+ with the reagent dye xylenol orange results in the formation of chromogenic product, which is measured at 560 nm.
Results
The levels of different ETC complexes were measured in the cerebellum and the frontal, parietal, occipital, and temporal cortices of autism and age-matched controls by western blotting. The relative density of the bands of different mitochondrial complexes versus β-actin (loading control) is plotted as a histogram, and scattered plots show overall distribution of the data. Analysis of the data revealed lower levels of the ETC complexes in the cerebellum and the frontal and temporal cortices in the children with autism of ages 4–10 years than in age-matched controls, but not in autistic group of 14–39 years of age. None of the ETC complexes showed any difference in parietal and occipital cortices between subjects with autism and control subjects in any age group, suggesting that there are brain region-specific changes in mitochondrial ETC complexes in children with autism. Therefore, we divided the samples into two groups: Group A (children, 4–10 years) and Group B (adults, 14–39 years). The densitometric data of all ETC complexes normalized to β-actin are shown for all brain regions in Group A, Group B, and the entire group, that is, Group A + Group B. The scattered plot of samples is only shown when statistically significant changes in ETC complexes between autism and control groups were observed.
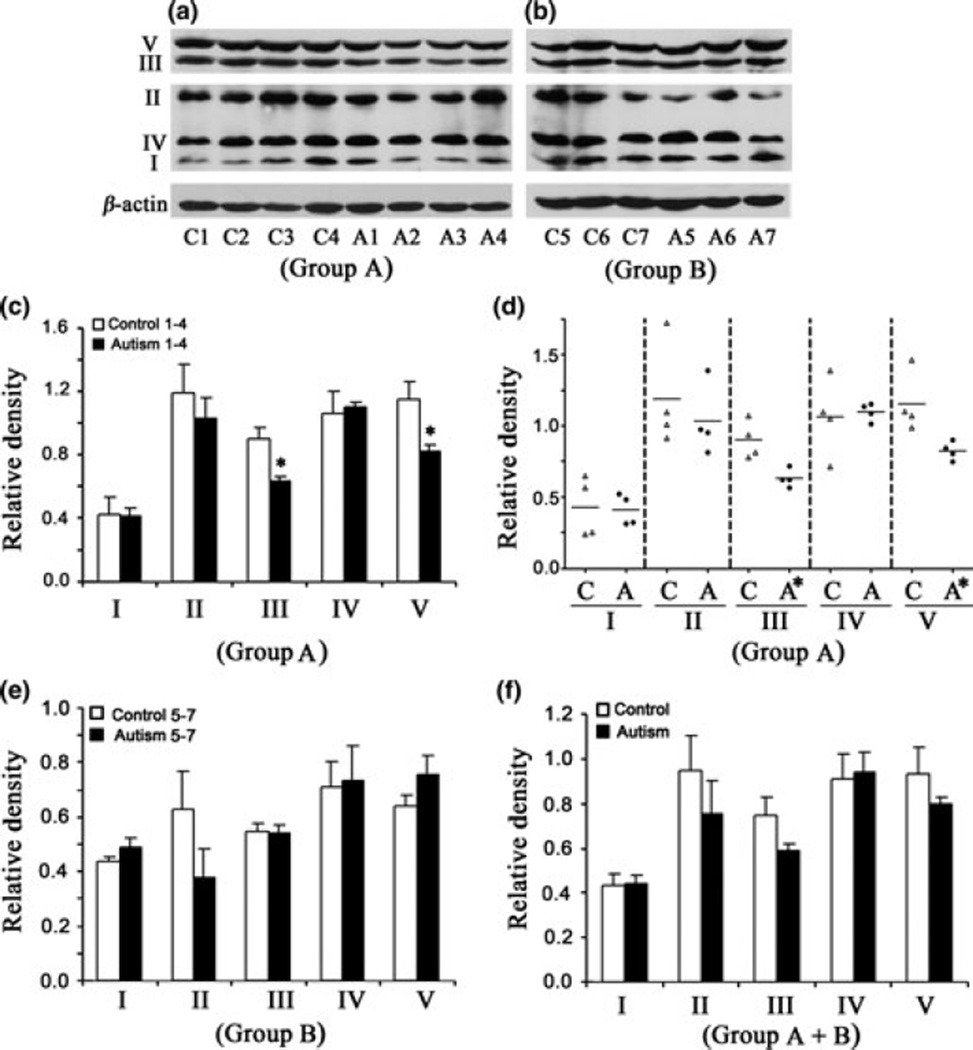
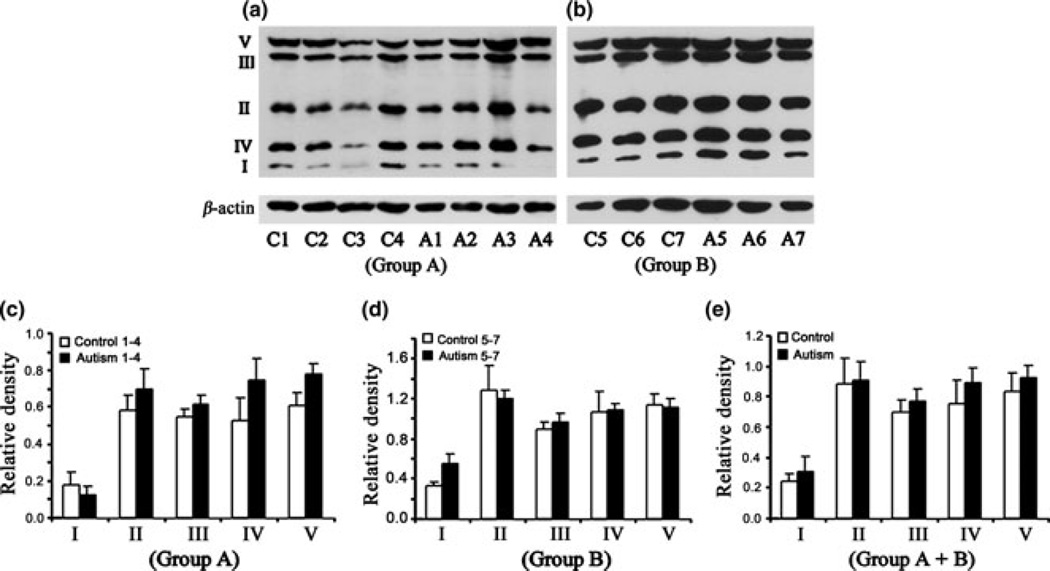
Lower levels of ETC complexes III and V in the cerebellum of children with autism
Western blot analysis of the levels of different ETC complexes in the cerebellum of subjects with autism and age-matched control subjects is shown in Fig. 1(a) (Group A, age: 4–10 years) and Fig. 1(b) (Group B, age: 14–39 years). The relative densities of different ETC complexes normalized to that of β-actin (loading control) are presented in Fig. 1(c) (Group A), Fig. 1(e) (Group B), and Fig. 1(f) (Groups A + B). In Group A, significantly lower levels were observed for complex III [mean ± SE = 0.629 ± 0.032 (autism), 0.899 ± 0.067 (control), p < 0.05)] and complex V [mean ± SE = 0.823 ± 0.032 (autism), 1.154 ±0.105 (control), p < 0.05)] in subjects with autism as compared with age-matched controls (Fig. 1c). Scattered plot of the data in Group A showed that there was no overlap for complexes III and V between subjects with autism and control subjects (Fig. 1d). A trend toward lower levels of complex II was also observed in subjects with autism compared to control subjects, but it was not significant (Fig. 1c), while the levels of complexes I and IV were similar between subjects with autism and control subjects. In adults, that is, Group B, there was no change in the levels of ETC complexes in subjects with autism compared with those in age-matched controls. However, a decrease in complex II was observed in 66% of subjects with autism (mean ± SE = 0.38 ± 0.102) compared with control subjects (mean ± SE = 0.626 ± 0.139), but it was not significant. When the data were analyzed for Group A + Group B, lower levels of complexes II, III, and V were observed in subjects with autism, but it was not significant (Fig. 1f).
Fig. 1.
Electron transport chain (ETC) complexes in the cerebellum from subjects with autism and age-matched control subjects in Group A (age: 4–10 years) and Group B (age: 14–39 years). The Group A samples were A1-A4 for subjects with autism, and C1-C4 for control subjects (a), whereas Group B samples were A5-A7 for subjects with autism, and C5-C7 for control subjects (b). Western blots are represented in (a) (Group A) and (b) (Group B). The relative densities of different ETC complexes normalized to β-actin are shown in (c) (Group A), (e)(Group B), and (f) (combined Groups A + B). Scattered plot of the data for Group A is shown in (d). *p < 0.05, unpaired t-test.
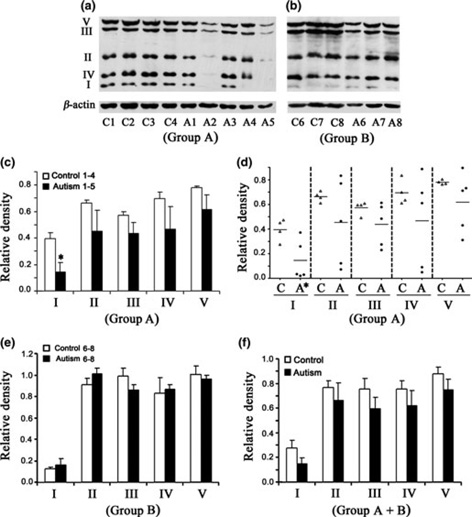
Lower levels of ETC complexes in the frontal cortex of children with autism
Western blot analysis of the levels of ETC complexes in the frontal cortex of subjects with autism and age-matched control subjects is shown in Fig. 2(a) for Group A, and Fig. 2(b) for Group B. Histogram analysis of the relative density of the data of ETC complexes is shown in Fig. 2(c) for Group A, Fig. 2(e) for Group B, and Fig. 2(f) for the entire Group A + B. When data in Group A were analyzed, a significant decrease in levels was observed for only complex I [(mean ± SE = 0.143 ±0.073 (autism), 0.395 ± 0.044 (control), p < 0.05)]; however, a general trend toward decreases in levels of the other complexes, that is, II–V was also observed (Fig. 2c). It was interesting to observe from the scattered plot that 60% (3/5) of complexes I, II, and V, and 40% (2/5) of complexes III and IV in the autism group had levels below the cutoff lower range for the control group, suggesting that a subset of autism cases has decreased levels of all ETC complexes in the frontal cortex.
Fig. 2.
Electron transport chain (ETC) complexes in the frontal cortex from subjects with autism and age-matched control subjects in Group A (age: 4–10 years) and Group B (age: 14–39 years). The Group A samples were A1-A5 for subjects with autism, and C1-C4 for control subjects (a), whereas Group B samples were A6-A8 for subjects with autism, and C5-C7 for control subjects (b). Western blots are represented in (a) (Group A) and (b) (Group B). The relative densities of different ETC complexes normalized to β-actin are shown in (c) (Group A), (e) (Group B), and (f) (combined Groups A + B). Scattered plot of the data for Group A is shown in (d). *p < 0.05, unpaired t-test.
In Group B, no change was observed in the levels of ETC complexes (Fig. 1e), except that a non-significant decrease was observed for complex III, where 66% of subjects with autism had decreased levels. When both Groups A and B were analyzed together (Fig. 2f), a general trend toward decreases in the levels of all ETC complexes was observed in subjects with autism, but it was not significant.
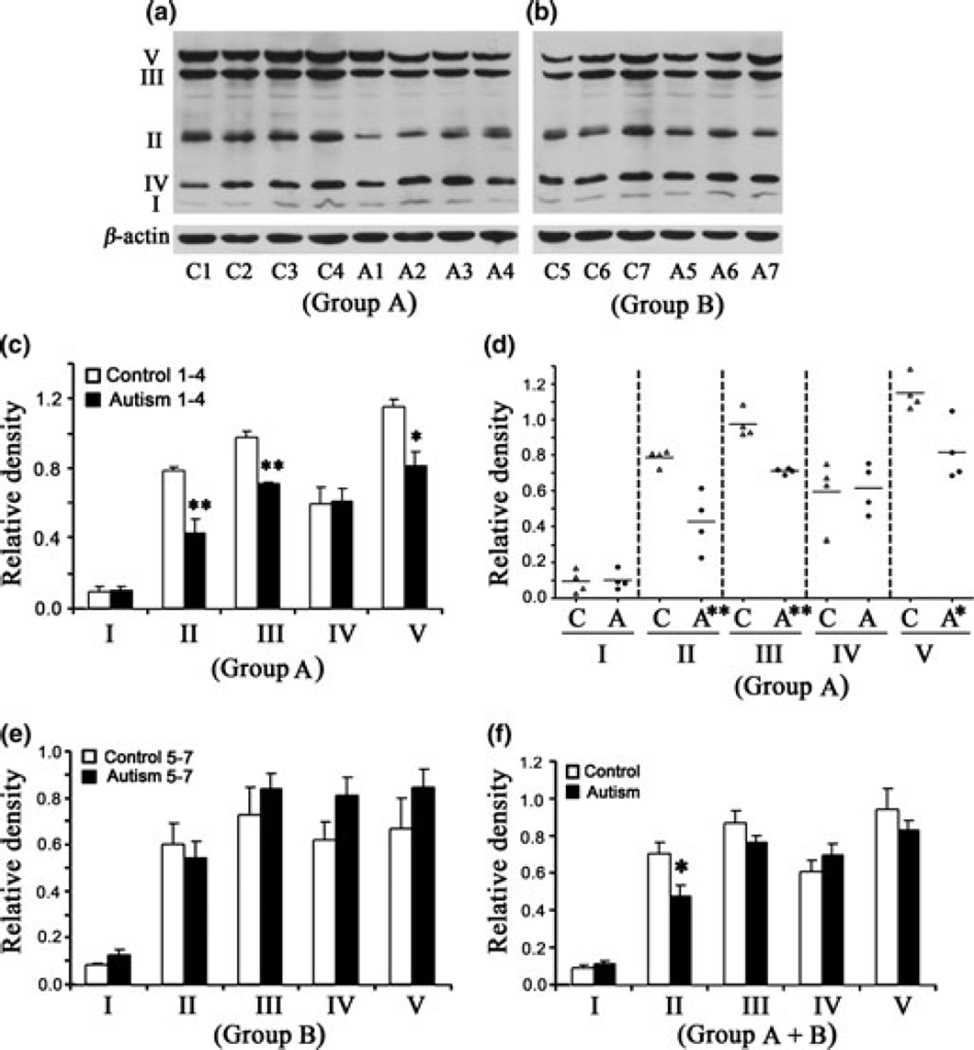
Lower levels of ETC complexes II, III and V in the temporal cortex of children with autism
Western blot analysis of the levels of ETC complexes in the temporal cortex of subjects with autism and age-matched control subjects is shown in Fig. 3(a) (Group A) and Fig. 3(b) (Group B). Data analysis in Group A showed that the levels of complexes II, III, and V were significantly lower in subjects with autism as compared with age-matched control subjects (Fig. 3c). The mean values ± SE were as follows: for complex II in autism, 0.425 ± 0.082, in control, 0.787 ± 0.022 (p < 0.01); complex III in autism, 0.710 ± 0.008, and in control, 0.972 ± 0.038 (p < 0.01); and complex V in autism, 0.813 ± 0.083, and in control, 1.147 ± 0.048 (p < 0.05). Scattered plot analysis showed that there was no overlap in the levels of these ETC complexes between subjects with autism and control subjects. In contrast, no significant change in any of the ETC complexes was observed in Group B (Fig. 5f). When both Groups A and B were combined, only complex II was significantly decreased (p < 0.05) in subjects with autism (mean ± SE = 0.474 ± 0.057) as compared to control subjects (mean ± SE = 0.706 ± 0.053) (Fig. 3f).
Fig. 3.
Electron transport chain (ETC) complexes in the temporal cortex from subjects with autism and age-matched control subjects in Group A (age: 4–10 years) and Group B (age: 14–39 years). The Group A samples were A1-A4 for subjects with autism, and C1-C4 for control subjects (a), whereas Group B samples were A5-A7 for subjects with autism, and C5-C7 for control subjects (b). Western blots are represented in (a) (Group A) and (b) (Group B). The relative densities of different ETC complexes normalized to β-actin are shown in (c) (Group A), (e) (Group B), and (f) (combined Groups A + B). Scattered plot of the data for Group A is shown in (d).*p < 0.05, **p < 0.01, unpaired t-test.
Fig. 5.
Electron transport chain (ETC) complexes in the occipital cortex from subjects with autism and age-matched control subjects in Group A (age: 4–10 years) and Group B (age: 14–39 years). The Group A samples were A1-A4 for subjects with autism, and C1-C4 for control subjects (a), whereas group B samples were A5-A7 for subjects with autism and C5-C7 for control subjects (b). Western blots are represented in (a) (Group A) and (b) (Group B). The relative densities of different ETC complexes normalized to β-actin are shown in (c) (Group A), (d) (Group B), and (e) (combined Groups A + B).
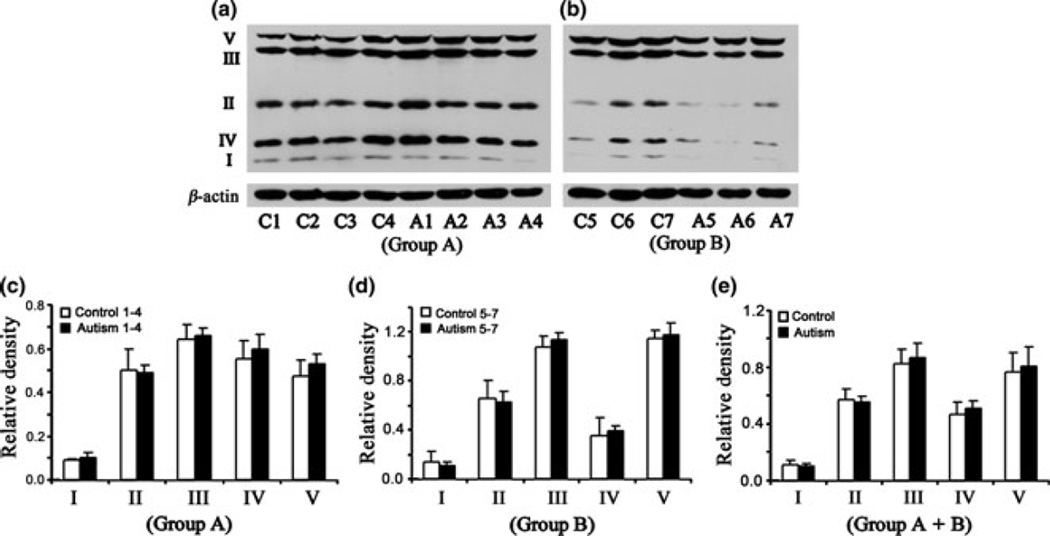
The levels of ETC complexes are not affected in parietal and occipital cortices of subjects with autism
Western blots of ETC complexes in the parietal cortex (Fig. 4a and b) and occipital cortex (Fig. 5a and b) and histograms of relative densities (parietal cortex, Fig. 4c–e; occipital cortex, Fig. 5c–e) showed that the levels of ETC complexes are not affected in Group A as well as in Group B of subjects with autism as compared to age-matched control subjects. These results suggest that there is a brain region-specific decrease in the levels of ETC complexes in the cerebellum and the frontal and temporal cortices but not in the parietal and occipital cortices of subjects with autism. Because the parietal and occipital cortices were not affected in subjects with autism in comparison with control subjects, while the frontal and temporal cortices and the cerebellum from subjects with autism were affected, our results indirectly suggest that postmortem interval is not a contributing factor toward the observed brain mitochondrial abnormalities in autism.
Fig. 4.
Electron transport chain (ETC) complexes in the parietal cortex from subjects with autism and age-matched control subjects in Group A (age: 4–10 years) and Group B (age: 14–39 years). The Group A samples were A1-A4 for subjects with autism, and C1-C4 for control subjects (a), whereas Group B samples were A5-A7 for subjects with autism and C5-C7 for control subjects (b). Western blots are represented in (a) (Group A) and (b) (Group B). The relative densities of different ETC complexes normalized to β-actin are shown in (c) (Group A), (d) (Group B), and (e) (combined Groups A + B).
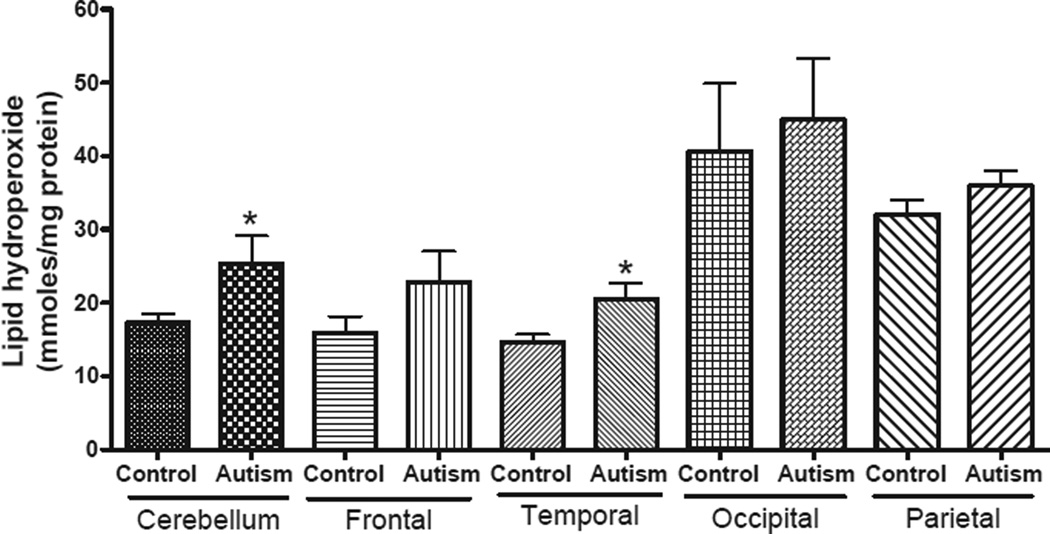
Increased levels of LOOHs in specific brain regions in children with autism
To assess whether changes in mitochondrial ETC in the children with autism also results in increased free radical generation and oxidative stress, we measured the levels of LOOH, a product of fatty acid oxidation, in the frontal, temporal, occipital and parietal cortices, and cerebellum from children with autism and age-matched controls (Fig. 6). The levels of LOOH were significantly increased in the cerebellum and temporal cortex of subjects with autism as compared with age-matched control subjects in Group A. An increase in the levels of LOOH was also observed in the frontal cortex in autism group, but it was not statistically significant. No change in the levels of LOOH was observed in the parietal and occipital cortices between autism and control groups.
Fig. 6.
Levels of lipid hydroperoxides in different regions of brain from subjects with autism and age-matched controls in Group A (age: 4–10 years). Lipid hydroperoxides were measured in the brain homogenates from frontal, temporal, occipital and parietal cortices, and cerebellum of subjects with autism and age-matched controls in Group A. The data represent mean ± SE. *p < 0.05, unpaired t-test.
Discussion
Although the cause of autism remains elusive, it is considered a multifactorial disorder that is influenced by genetic, environmental, and immunological factors as well as increased vulnerability to oxidative stress (Chauhan and Chauhan 2006). In this study, we report two interesting observations: (i) brain region-specific changes occur in the levels of ETC complexes in the cerebellum and the frontal and temporal cortices but not in the parietal and occipital cortices in subjects with autism, and (ii) the changes above are observed only in young children with autism but not in adults with autism. We recently reported that the activities of Ca2+-Mg2+-ATPase and Na+-K+-ATPase were also affected in the cerebellum and frontal cortex, suggesting that the cerebellum and frontal cortex may have biochemical changes in autism (Ji et al. 2009). Mitochondria are vulnerable to a wide array of endogenous and exogenous factors, which appear to be linked by excessive production of free radicals. The free radicals are generated endogenously during oxidative metabolism and energy production by mitochondria (Cadenas and Davies 2000; Lenaz 2001). Mitochondria are not only the source of free radicals, but they are also the target of oxidative damage. In addition to producing more oxidants, damaged mitochondria are also vulnerable to oxidative stress. Increasing evidence from our and other groups suggests a role of oxidative stress in the development and clinical manifestation of autism (McGinnis 2004; Chauhan and Chauhan 2006). Levels of oxidative stress markers are increased in the blood (Chauhan et al. 2004; James et al. 2004; Zoroglu et al. 2004; Chauhan and Chauhan 2006), urine (Ming et al. 2005), and brains (Lopez-Hurtado and Prieto 2008; Evans et al. 2009; Muthaiyah et al. 2009; Sajdel-Sulkowska et al. 2009) of individuals with autism as compared with controls. In this study, increased levels of LOOH were also observed in the children with autism in same brain regions where mitochondrial ETC abnormalities were observed. As ETC in mitochondria is a prime source for ROS generation, these results also support the findings on mitochondrial dysfunction in children with autism.
Mitochondria play a central role in the energy-generating process through the transfer of electrons with the help of five ETC complexes and generation of a proton gradient in the inner membrane of the cell. Although the end product of the respiratory chain is water that is generated in a four-electron reduction of molecular oxygen by complex IV, a minor proportion of O2 can be involved in the one-electron reduction processes generating ROS, in particular, superoxide anion radical (·O−2), hydrogen peroxide (H2O2), and the extremely reactive hydroxyl radical (OH·). Generation of ROS occurs mainly at complex III as a result of proton cycling between ubiquinone, cytochromes b and c1, and iron-sulfur protein (Sugioka et al. 1988). Some contribution of complex I to this process has also been found. Consequently, abnormalities in the levels of ETC complexes may be responsible for the observed oxidative stress in autism. The brain is highly vulnerable to oxidative stress, as it represents only 2% of the total body weight, but it accounts for 20% of all oxygen consumption, reflecting its high rate of metabolic activity (Juurlink and Paterson 1998; Shulman et al. 2004). Mitochondria have a crucial role in the supply of energy to the brain. Damaged ETC complexes compromise ATP synthesis and accelerate the generation of free radicals. Therefore, the mitochondrial ETC defects observed in the brains of young individuals with autism may have important, detrimental consequences on the function and plasticity of neurons in autism.
Mitochondrial diseases have been linked to poor growth, loss of muscle coordination, muscle weakness, developmental delays, learning disabilities, mental retardation, gastrointestinal disorders, neurological problems, seizures, and dementia (Read and Calnan 2000; Xu et al. 2005; Aliev et al. 2009). Depending on how severe the mitochondrial disorder is, the illness can range in severity from mild to fatal. It should be noted that some of the symptoms of mitochondrial diseases, such as learning disabilities, mental retardation, seizures, neurological problems, and gastrointestinal disturbances, are also present in a subset of individuals with autism.
Although this study is the first to report on brain mitochondrial abnormalities in autism, a few case reports of mitochondrial disorder have been reported in individuals with autism on the basis of blood analysis and/or muscle biopsy. These case reports include a child with autism with documented complex IV deficiency (Laszlo et al. 1994); a boy with autism with complex IV defect and a mtDNA G8363A mutation (Graf et al. 2000); two children with autism with deficiencies in several respiratory chain enzymes, including complexes I–III and coenzyme Q (Tsao and Mendell 2007); and five individuals with autism and mtDNA mutations or a mtDNA deletion (Pons et al. 2004). Anatomical and neuroradiographical studies of the brains of individuals with autism have also suggested that a disturbance of energy metabolism may be present (Lombard 1998; Chugani et al. 1999). 31P-Magnetic resonance spectroscopy showed increased membrane degradation and decreased synthesis of ATP in autism (Minshew et al. 1993). In addition, carnitine deficiency in plasma, accompanied by elevations in lactate, alanine, and ammonia levels in autism, findings suggestive of mild mitochondrial dysfunction was reported in autism (Filipek et al. 2004). Another study also showed a high frequency of increased plasma lactate levels and increased lactate/pyruvate ratio in individuals with autism (Correia et al. 2006). Although the mechanism of hyperlactacidemia remains unknown, these case reports support dysfunction of mitochondrial oxidative phosphorylation in autism.
A population-based study in Portugal examining medical conditions in 120 children with autism found a disproportionately high prevalence (7%) of mitochondrial diseases in individuals with autism (Oliveira et al. 2005). However, these children did not have any known mtDNA mutations and/or deletions associated with known mitochondrial disorders. This report suggests that a substantial percentage of subgroups of autism may have a mitochondrial disorder.
The risk of sudden death of individuals who have inverted duplication of chromosome 15q (idic 15) is approximately 1% per year (Cleary 2009). This abnormality occurs in 1–5% of individuals with autism (Gillberg 1998; Schroer et al. 1998). Children with autism with a chromosome 15q11-q13 inverted duplication have been found to have motor delay, lethargy, severe hypotonia, and modest lactic acidosis. It is of interest to note that two children with autism and idic 15 showed mitochondrial hyperproliferation and complex III defect (Filipek et al. 2003), and two autism cases associated with sudden infant death syndrome showed mild mitochondrial hyperproliferation and a possible complex II defect (Gargus and Imtiaz 2008). These studies suggest that candidate gene loci for autism within the critical region may affect pathways influencing mitochondrial function (Filipek et al. 2003).
In regressive autism, children first show signs of normal social and language development through the first year of life but lose these developmental skills at 15–24 months and develop autistic behavior (Ozonoff et al. 2005). The rate of regressive autism varies from 15% to 62% of cases (Goldberg et al. 2003; Lord et al. 2004; Hansen et al. 2008). A recent study examined a group of 25 individuals with autism who also had confirmed mitochondrial disorders (Weissman et al. 2008). They reported that 40% of this group demonstrated unusual pattern of regression (multiple episodes, loss of motor skills, and regression after the age of 3). In this cohort, the deficiency of ETC complexes I and III was observed in 64% and 20% of individuals with autism, respectively, and two had a rare mtDNA mutation. Another case report implicated mitochondrial dysfunction as a factor contributing to vaccine-related regression (Poling et al. 2006; Zecavati and Spence 2009). A recent report also suggests that fever in children with mitochondrial disease is a risk to autistic regression (Shoffner et al. 2010).
This study suggests that abnormalities in the mitochondrial ETC complex levels may be one of the factors in the etiology of autism. This will lead to oxidative stress and abnormal energy metabolism in autism. In our studies, deficiency of mitochondrial ETC complexes was observed in children with autism (ages 4–10 years) but not in adults with autism (14–39 years of age). Age also seems to play a critical role in determining brain growth in autism. Enlarged brain size (megaloencephaly), particularly in the temperoparietal region, is the most consistent observation in young children with autism (Goldberg et al. 1999). The initial accelerated brain growth in young children is followed by abnormal slowness and growth arrest that results in normalization of brain size in late childhood and in adults (Hardan et al. 2001; Aylward et al. 2002; Courchesne 2004; Herbert 2005). Head circumference measurements have also shown increased brain volume in young children, later returning to normal volume. Thus, very large differences between children with autism and normal children are evident at early ages, but differences are not seen in adult cases (Aylward et al. 2002; Courchesne 2004). In addition, age-related changes in cerebellar nuclei and inferior olives have also been reported in autism (Palmen et al. 2004). The pattern of age-related changes in the severity of autism symptoms also suggests that causative factors determine both developmental and age-associated modifications. While age-related increases in the severity of autism symptoms have been reported among individuals with idic 15 syndrome (Rineer et al. 1998), significant improvement of communication and social behaviors with increasing age has been reported in other autistic cohorts (Mesibov et al. 1989; Piven et al. 1996). Recent evidence suggests that 3–25% children with a previous diagnosis of ASD recover and show normal ranges of cognitive, adaptive, and social skills (Helt et al. 2008).
Neuropathological studies in autism suggest prenatal and postnatal developmental abnormalities in multiple regions of the brain, including the cerebellum, frontal and temporal cortices, cortical white matter, amygdala, and brainstem (particularly the olivary nuclei) (Palmen et al. 2004; Bauman and Kemper 2005; Pickett and London 2005; Schmitz and Rezaie 2008; Wegiel et al. 2009, 2010). There is substantial evidence from neuroimaging studies that dysfunctions in the cerebellum and possibly the temporal lobe and association cortex result in autism symptoms. Loss of Purkinje and granule cells has been reported throughout the cerebellar hemispheres in autism (Bauman and Kemper 1985, 2005; Kern 2003; Casanova 2007). Alterations in neuronal size, density, and dendritic branching in the cerebellum and limbic structures (hippocampus and amygdala) have also been reported in autism.
The prevalence rate of mitochondrial disease is about one in 5000–10 000 children (Skladal et al. 2003; Schaefer et al. 2004). In contrast, the prevalence rate for autism is 1 in 110 children (Rice 2009). As we observed a high percentage of changes in complexes I–III, and V in the cerebellum and frontal and temporal cortices of individuals with autism, it seems that autism is associated with mitochondrial dysfunction, although clinical symptoms of mitochondrial disease may be lacking. Therefore, mitochondrial dysfunctions rather than mitochondrial disorders may be more relevant in autism. The clinical diagnosis of mitochondrial disease is often made with biochemical analysis of lactate, pyruvate, and alanine in blood, urine, or cerebrospinal fluid. However, the analysis of biochemical metabolites to diagnose mitochondrial disease may not be sufficient, as these analyses seem to be frequently normal, even in some severe cases of the disease. The clinical symptoms of mitochondrial disease are increased when ASD has comorbidity, such as hypotonia and motor delay, fatigue, metabolic abnormalities, and epilepsy (Fillano et al. 2002). The genetics of autism is complex, with the involvement of multiple genes. However, no gene has been identified that follows the typical Mendelian laws of inheritance. ASDs may have mild changes in the levels of ETC complexes that may or may not be related to a gene mutation. The mild form of mitochondrial abnormalities observed in autism may also be linked to other abnormalities such as the excessive Ca2+ observed in the mitochondria in autism. Excessive levels of Ca2+ in the mitochondria can affect the mitochondrial metabolism and increase the oxidative stress in the brains of individuals with autism (Palmieri et al. 2010).
The mechanism by which mitochondrial dysfunction may occur and affect development of autism is not entirely clear. It is possible that in comparison with classical mitochondrial disease, mitochondrial dysfunction may show less severe symptoms and may not show the classical mitochondrial pathology on muscle biopsy (Lombard 1998). Further research with larger sample sizes is needed to determine the association between mitochondrial dysfunction and severity, clinical phenotypes, regression, and/or idic 15 in autism.
Supplementary Material
Acknowledgments
This work was supported in part by funds from the New York State Office of People with Developmental Disabilities, Department of Defense Autism Spectrum Disorders Research Program AS073224P2, Autism Research Institute, Autism Speaks, and Autism Collaboration.
Abbreviations used
- ASDs
autism spectrum disorders
- ETC
electron transport chain
- LOOH
lipid hydroperoxide
- ROS
reactive oxygen species
Footnotes
Supporting information
Additional supporting information may be found in the online version of this article:
Table S1. Case history of autism and control brain samples.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Aliev G, Palacios HH, Walrafen B, Lipsitt AE, Obrenovich ME, Morales L. Brain mitochondria as a primary target in the development of treatment strategies for Alzheimer disease. Int. J. Biochem. Cell Biol. 2009;41:1989–2004. doi: 10.1016/j.biocel.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Ames BN. Mitochondrial decay, a major cause of aging, can be delayed. J. Alzheimers Dis. 2004;6:117–121. doi: 10.3233/jad-2004-6202. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–874. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int. J. Dev. Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bertram R, Gram PM, Luciani DS, Sherman A. A simplified model for mitochondrial ATP production. J. Theor. Biol. 2006;243:575–586. doi: 10.1016/j.jtbi.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Braun HP. Supramolecular structure of the mitochondrial oxidative phosphorylation system. J. Biol. Chem. 2007;282:1–4. doi: 10.1074/jbc.R600031200. [DOI] [PubMed] [Google Scholar]
- Bubber P, Tang J, Haroutunian V, Xu H, Davis KL, Blass JP, Gibson GE. Mitochondrial enzymes in schizophrenia. J. Mol. Neurosci. 2004;24:315–321. doi: 10.1385/JMN:24:2:315. [DOI] [PubMed] [Google Scholar]
- Burchell VS, Gandhi S, Deas E, Wood NW, Abramov AY, Plun-Favreau H. Targeting mitochondrial dysfunction in neurodegenerative disease: part II. Expert Opin. Ther Targets. 2010;14:497–511. doi: 10.1517/14728221003730434. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Casanova MF. The neuropathology of autism. Brain Pathol. 2007;17:422–433. doi: 10.1111/j.1750-3639.2007.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V. Oxidative stress in autism. Pathophysiology. 2006;13:171–181. doi: 10.1016/j.pathophys.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Chauhan A, Chauhan V, Brown WT, Cohen I. Oxidative stress in autism: increased lipid peroxidation and reduced serum levels of ceruloplasmin and transferrin-the antioxidant proteins. Life Sci. 2004;75:2539–2549. doi: 10.1016/j.lfs.2004.04.038. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Sundram BS, Behen M, Lee ML, Moore GJ. Evidence of altered energy metabolism in autistic children. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1999;23:635–641. doi: 10.1016/s0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- Cleary N. Sudden death in chromosome 15qll-ql3 duplication syndrome. 2010 http://www.idic15.org/PhysicianAdvisory_Feb2009.pdf.
- Correia C, Coutinho AM, Diogo L, et al. Brief report: high frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J. Autism Dev. Disord. 2006;36:1137–1140. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- Evans TA, Perry G, Smith MA, Salomon RG, McGinnis WR, Sajdel-Sulkowska EM, Zhu X. Evidence for oxidative damage in the autistic brain. In: Chauhan A, Chauhan V, Brown WT, editors. Autism: Oxidative Stress, Inflammation and Immune Abnormalities. Florida: CRC Press, Taylor and Francis groups; 2009. pp. 35–46. [Google Scholar]
- Filipek PA, Juranek J, Smith M, et al. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53:801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- Filipek PA, Juranek J, Nguyen MT, Cummings C, Gargus JJ. Relative carnitine deficiency in autism. J. Autism Dev. Disord. 2004;34:615–623. doi: 10.1007/s10803-004-5283-1. [DOI] [PubMed] [Google Scholar]
- Fillano JJ, Goldenthal MJ, Rhodes CH, Marin-Garcia J. Mitochondrial dysfunction in patients with hypotonia, epilepsy, autism, and developmental delay: HEADD syndrome. J. Child Neurol. 2002;17:435–439. doi: 10.1177/088307380201700607. [DOI] [PubMed] [Google Scholar]
- Gargus JJ, Imtiaz FI. Mitochondrial energy-deficient endophenotype in autism. Am. J. Biochem. Biotech. 2008;4:198–207. [Google Scholar]
- Gillberg C. Chromosomal disorders and autism. J. Autism Dev Disord. 1998;28:415–425. doi: 10.1023/a:1026004505764. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Szatmari P, Nahmias C. Imaging of autism: lessons from the past to guide studies in the future. Can. J. Psychiatry. 1999;44:793–801. doi: 10.1177/070674379904400806. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, Flodman P, Spence MA. Language and other regression: assessment and timing. J. Autism Dev. Disord. 2003;33:607–616. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- Graf WD, Marin-Garcia J, Gao HG, Pizzo S, Naviaux RK, Markusic D, Barshop BA, Courchesne E, Haas RH. Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J. Child Neurol. 2000;15:357–361. doi: 10.1177/088307380001500601. [DOI] [PubMed] [Google Scholar]
- Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- Hansen RL, Ozonoff S, Krakowiak P, Angkustsiri K, Jones C, Deprey LJ, Le DN, Croen LA, Hertz-Picciotto I. Regression in autism: prevalence and associated factors in the CHARGE Study. Ambul. Pediatr. 2008;8:25–31. doi: 10.1016/j.ambp.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J. Child Neurol. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Helt M, Kelley E, Kinsbourne M, Pandey J, Boorstein H, Herbert M, Fein D. Can children with autism recover? If so, how? Neuropsychol. Rev. 2008;18:339–366. doi: 10.1007/s11065-008-9075-9. [DOI] [PubMed] [Google Scholar]
- Herbert MR. Large brains in autism: the challenge of pervasive abnormality. The Neuroscientist. 2005;11:417–440. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- Ji L, Chauhan A, Brown WT, Chauhan V. Increased activities of Na+/K+-ATPase and Ca2 + /Mg2 + -ATPase in the frontal cortex and cerebellum of autistic individuals. Life Sci. 2009;85:788–793. doi: 10.1016/j.lfs.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juurlink BH, Paterson PG. Review of oxidative stress in brain and spinal cord injury: suggestions for pharmacological and nutritional management strategies. J. Spinal Cord Med. 1998;21:309–334. doi: 10.1080/10790268.1998.11719540. [DOI] [PubMed] [Google Scholar]
- Kern JK. Purkinje cell vulnerability and autism: a possible etiological connection. Brain Dev. 2003;25:377–382. doi: 10.1016/s0387-7604(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Munir KM, Crowley DJ, Miller AM. Prenatal stress and risk for autism. Neurosci. Biobehav Rev. 2008;32:1519–1532. doi: 10.1016/j.neubiorev.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch. Pediatr. Adolesc. Med. 2007;161:326–333. doi: 10.1001/archpedi.161.4.326. [DOI] [PubMed] [Google Scholar]
- Laszlo A, Horvath E, Eck E, Fekete M. Serum serotonin, lactate and pyruvate levels in infantile autistic children. Clin. Chim. Acta. 1994;229:205–207. doi: 10.1016/0009-8981(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–164. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- Lombard J. Autism: a mitochondrial disorder? Med. Hypotheses. 1998;50:497–500. doi: 10.1016/s0306-9877(98)90270-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Hurtado E, Prieto JJ. A microscopic study of language-related cortex in autism. Am. J. Biochem. Biotech. 2008;4:130–145. [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Shulman C, DiLavore P. Regression and word loss in autistic spectrum disorders. J. Child Psychol. Psychiatry. 2004;45:936–955. doi: 10.1111/j.1469-7610.2004.t01-1-00287.x. [DOI] [PubMed] [Google Scholar]
- McGinnis WR. Oxidative stress in autism. Altern. Ther. Health Med. 2004;10:22–36. [PubMed] [Google Scholar]
- Mesibov GB, Schopler E, Schaffer B, Michal N. Use of the childhood autism rating scale with autistic adolescents and adults. J. Am. Acad. Child Adolesc. Psychiatry. 1989;28:538–541. doi: 10.1097/00004583-198907000-00012. [DOI] [PubMed] [Google Scholar]
- Ming X, Stein TR, Brimacombe M, Johnson WG, Lambert GH, Wagner GC. Increased excretion of a lipid peroxidation biomarker in autism. Prostaglandins Leukot. Essent. Fatty Acids. 2005;73:379–384. doi: 10.1016/j.plefa.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Goldstein G, Dombrowski SM, Panchalingam K, Pettegrew JW. A preliminary 31P MRS study of autism: evidence for undersynthesis and increased degradation of brain membranes. Biol. Psychiatry. 1993;33:762–773. doi: 10.1016/0006-3223(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Zhu X, Wang X, Lee HG, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: a therapeutic target in neurodegeneration. Biochim. Biophys. Acta. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthaiyah B, Essa MM, Chauhan V, Brown WT, Wegiel J, Chauhan A. Increased lipid peroxidation in cerebellum and temporal cortex of brain in autism. J. Neurochem. 2009;108(Suppl. 1):73. [Google Scholar]
- Navarro A, Boveris A, Bandez MJ, Sanchez-Pino MJ, Gomez C, Muntane G, Ferrer I. Human brain cortex: mitochondrial oxidative damage and adaptive response in Parkinson disease and in dementia with Lewy bodies. Free Radic. Biol. Med. 2009;4:1574–1580. doi: 10.1016/j.freeradbiomed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Oliveira G, Diogo L, Grazina M, Garcia P, Ataide A, Marques C, Miguel X, Borges L, Vicente AM, Oliveira CR. Mitochondrial dysfunction in autism spectrum disorders: a population-based study. Dev. Med. Child Neurol. 2005;47:185–189. doi: 10.1017/s0012162205000332. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Williams BJ, Landa R. Parental report of the early development of children with regressive autism: the delays-plus-regression phenotype. Autism. 2005;9:461–486. doi: 10.1177/1362361305057880. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Papaleo V, Porcelli V, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol. Psychiatry. 2010;15:38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- Patsoukis N, Georgiou D. Effect of sulfite-hydrosulfite and nitrite on thiol redox state, oxidative stress and sclerotial differentiation of filamentous phytopathogenic fungi. Pesticide Biochem. Physiol. 2007;88:226–235. [Google Scholar]
- Pickett J, London E. The neuropathology of autism: a review. J. Neuropathol. Exp. Neurol. 2005;64:925–935. doi: 10.1097/01.jnen.0000186921.42592.6c. [DOI] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. J. Am. Acad. Child Adolesc. Psychiatry. 1996;35:523–529. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Poling JS, Frye RE, Shoffner J, Zimmerman AW. Developmental regression and mitochondrial dysfunction in a child with autism. J. Child Neurol. 2006;21:170–172. doi: 10.2310/7010.2006.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J. Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- Pons R, Andreu AL, Checcarelli N, Vila MR, Engelstad K, Sue CM, Shungu D, Haggerty R, de Vivo DC, DiMauro S. Mitochondrial DNA abnormalities and autistic spectrum disorders. J. Pediatr. 2004;144:81–85. doi: 10.1016/j.jpeds.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Read CY, Calnan RJ. Mitochondrial disease: beyond etiology unknown. J. Pediatr. Nurs. 2000;15:232–241. doi: 10.1053/jpdn.2000.8042. [DOI] [PubMed] [Google Scholar]
- Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res. Brain Res. Rev. 2008;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem. Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- Rice C. Centers for Disease Control and Prevention. Prevalence of Autism Spectrum Disorders- Autism and Developmental Disabilities Monitoring Network, United States, 2006. Summaries: Morbidity and Mortality Weekly Report (December 18, 2009) 2009;58:1–20. [PubMed] [Google Scholar]
- Rineer S, Finucane B, Simon EW. Autistic symptoms among children and young adults with isodicentric chromosome 15. Am. J. Med. Genet. 1998;81:428–433. doi: 10.1002/(sici)1096-8628(19980907)81:5<428::aid-ajmg12>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in cerebellar neurotrophin-3 and oxidative stress markers in autism. Cerebellum. 2009;8:366–372. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders-past, present and future. Biochim. Biophys. Acta. 2004;1659:115–120. doi: 10.1016/j.bbabio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Rezaie P. The neuropathology of autism: where do we stand? Neuropathol. Appl. Neurobiol. 2008;3:4–11. doi: 10.1111/j.1365-2990.2007.00872.x. [DOI] [PubMed] [Google Scholar]
- Scholes TA, Hinkle PC. Energetics of ATP-driven reverse electron transfer from cytochrome c to fumarate and from succinate to NAD in submitochondrial particles. Biochemistry. 1984;23:3341–3345. doi: 10.1021/bi00309a035. [DOI] [PubMed] [Google Scholar]
- Schroer RJ, Phelan MC, Michaelis RC, et al. Autism and maternally derived aberrations of chromosome 15q. Am. J. Med. Genet. 1998;76:327–336. doi: 10.1002/(sici)1096-8628(19980401)76:4<327::aid-ajmg8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Shoffher J, Hyams L, Langley GN, et al. Fever plus mitochondrial disease could be risk factors for autistic regression. J. Child Neurol. 2010;25:429–434. doi: 10.1177/0883073809342128. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL, Hyder F. Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Skladal D, Halliday J, Thorburn DR. Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain. 2003;126:1905–1912. doi: 10.1093/brain/awg170. [DOI] [PubMed] [Google Scholar]
- Sugioka K, Nakano M, Totsune-Nakano H, Minakami H, Tero-Kubota S, Ikegami Y. Mechanism of O2- generation in reduction and oxidation cycle of ubiquinones in a model of mitochondrial electron transport systems. Biochim. Biophys. Ada. 1988;936:377–385. doi: 10.1016/0005-2728(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Wojtczak L. Mitochondria as a pharmacological target. Pharmacol. Rev. 2002;54:101–127. doi: 10.1124/pr.54.1.101. [DOI] [PubMed] [Google Scholar]
- Tsao CY, Mendell JR. Autistic disorder in 2 children with mitochondrial disorders. J. Child Neurol. 2007;22:1121–1123. doi: 10.1177/0883073807306266. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2009;109:153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Wisniewski T, Chauhan A, et al. Type, topology, and sequelae of neuropathological changes shaping clinical phenotype of autism. In: Chauhan A, Chauhan V, Brown WT, editors. Autism: Oxidative Stress, Inflammation and Immune Abnormalities. Florida: CRC Press, Taylor and Francis groups; 2009. pp. 1–34. [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, et al. The neuropathology of autism: defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman JR, Kelley RI, Bauman ML, Cohen B, Murray KF, Mitchell RL, Kern RL, Natowicz MR. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3:e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann FR, Manfredi G, Mawrin C, Beal MF, Schon EA. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J. Neurochem. 2002;80:616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu P, Li Y. Impaired development of mitochondria plays a role in the central nervous system defects of fetal alcohol syndrome. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:83–91. doi: 10.1002/bdra.20110. [DOI] [PubMed] [Google Scholar]
- Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Curr. Neurol. Neurosci Rep. 2009;9:129–136. doi: 10.1007/s11910-009-0021-x. [DOI] [PubMed] [Google Scholar]
- Zoroglu SS, Armutcu F, Ozen S, Gurel A, Sivasli E, Yetkin O, Meram I. Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254:143–147. doi: 10.1007/s00406-004-0456-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.