Abstract
Background
Recently, several combined therapeutic strategies and targeted agents have been under investigation for their potential role in lung cancer. The combined administration of dendritic cells (DCs) and immune-adjuvant cytidyl guanosyl oligodeoxynucleotide (CpG-ODN) after cryosurgery has proven to be an effective strategy for treating lung cancer. However, whether the application of CpG-ODN could affect the therapeutic results remained to be further explored.
Material/Methods
The Lewis lung cancer (LLC)−bearing mice received cryoablation and injection of ex vivo-cultured DCs into the peritumoral zone. Subsequently, CpG-ODN was administered to experimental animals 6 hours, 12 hours, and 24 hours after DC injection. The mice in the control group received coadministration of DCs and CpG-ODN simultaneously. Therapeutic effects were evaluated by survival rates. The resistance to rechallenge of LLC cell was assessed by lung metastasis and in vitro cytotoxicity of splenocytes. Furthermore, T-cell subsets and multiple cytokines (interleukin [IL]-4, -10, and-12; interferon [IFN]-γ; tumor necrosis factor [TNF]-α) in the blood were assessed to elucidate the underlying mechanisms.
Results
Higher ratios of CD4+ and CD8+ T cells and higher levels of IL-12, IFN-γ, and TNF-α were found in the blood of the mice that received CpG-ODN therapy 12 h after DC injection. The cytotoxicity potency of the splenocytes of these mice was significantly higher compared with the mice in other groups. Moreover, the mice receiving CpG-ODN therapy 12 h after DC injection showed significantly better resistance to rechallenge. Compared with the mice in other groups, the mice receiving CpG-ODN therapy 12 h after DC injection were superior in survival rates and antimetastatic effects.
Conclusions
Our study suggested that the therapeutic efficacy was closely associated with CpG-ODN administration in the combined therapeutic protocol of cryoablation, DCs, and immune adjuvant. In situ administration of CpG-ODN 12 h after DC injection might be considered the optimum application.
MeSH Keywords: Cryosurgery, Dendritic Cells, Immunotherapy
Background
Lung cancer is an aggressive tumor of the lung with a tendency to metastasize widely during the course of the disease. Currently, the standard therapeutic strategies for lung cancer include surgery, chemoradiation, and chemotherapy [1,2]. However, only a quarter of patients will be cured with current standard treatments and the majority of patients ultimately succumb to their disease. A very complex genetic landscape of lung cancer accounts for its resistance to conventional therapy and a high recurrence rate. In recent years, several combined therapeutic strategies and targeted agents have been under investigation for their potential role in lung cancer. Several of them, including DNA repair inhibitors, cellular developmental pathway inhibitors, antibody drug conjugates, immune therapy with vaccines, immune modulators, and immune checkpoint inhibitors are being tested [2,3]. Although the systemic antitumor immune effect of cryosurgery has been proven experimentally, the clinical results are not absolutely satisfactory [4]. The key factor contributing to the generation of cryoimmune response is the uptake and presentation of tumor antigens, which are released by the cryoablated tumor cells [5]. To enhance the cryoimmune effect, some researchers combined cryoablation with in situ administration of dendritic cells (DCs), which is proven to work synergistically [6]. DCs are considered the most potent antigen-presenting cells (APCs) with the capacity to initiate a primary immune response and the only APC type that can cross-present the captured antigens on major histocompatibility complex (MHC) molecules to CD8+ T cells. These unique properties are crucial for the induction of antitumor immune response. Immature DCs reside in the periphery tissues until antigen uptake and subsequently change into the mature state in a stimulatory environment. Maturation is accompanied by the down-regulation of antigen-uptake ability and the up-regulation of antigen-presenting ability [7,8]. An important procedure in the DC-based immunotherapy (imDCs) is to promote the maturation of DCs because only mature DCs can initiate the naive immune response (immature DCs would lead to immune tolerance) [9,10].
Toll-like receptor 9 (TLR9), one of the pattern recognition receptors, is extensively expressed in both murine and human plasmacytoid DCs. Once stimulated, the TLR9 can initiate the signaling cascades, resulting in the maturation of DCs, which eventually activates the Th1-biased immune response [11]. As a TLR9 agonist, cytidyl guanosyl oligodeoxynucleotide (CpG-ODN) has been used in the treatment of tumors and is proven to be an efficient immune adjuvant [12–14]. Zoya Alteber et al. found that the combination of cryosurgery with coadministration of DCs and CpG-ODN was effective in eliciting antitumor immune response [15]. In viewing the functional changes of DCs during the maturing process, we postulated that the consecutive administration of DCs and CpG-ODN after cryotherapy might induce a more potent immune response, by which the immature DCs would have adequate time to uptake antigens before they are promoted to the mature state by CpG-ODN.
The Lewis lung cancer (LLC)-bearing mouse is typically used as an animal model to explore the pathology and therapeutics of lung cancer [16,17]. In the present study, we adopted this mouse model to verify whether the application of CpG-ODN could affect the therapeutic results of the combined administration of cryoablation and DCs. Our study suggested that therapeutic efficacy was closely associated with the time of CpG-ODN administration. Furthermore, T-cell subsets and multiple cytokines (interleukin [IL]-4, -10, and -12; interferon [IFN]-γ; tumor necrosis factor [TNF]-α) in the blood of the experimental animals were assessed to elucidate the underlying mechanisms. To our knowledge, this is the first study to explore the therapeutic effects of the application of CpG-ODN in a combined protocol with DCs.
Material and Methods
Animals and cell lines
Animals
The LLC-bearing C57BL/6 mice (female, 6–8 weeks old) and LLC cell line were provided by the Cancer Institute & Hospital, Chinese Academy of Medical Sciences (Animal Certificate of Conformity: SCXK, Beijing, 2010–2015). The subcutaneous tumor with the volume of 200–220 mm3 was on the femur. Normal C57BL/6 mice (6–8 weeks old) were provided by Experimental Animal Center of the PLA General Hospital. All animals were maintained under specified pathogen-free (SPF1) conditions (temperature, 19–23°C [66–73°F]; humidity, 40–65%; lighting, 350 lux, 12-h dark/light cycle [lights on at 7:00 AM and off at 7:00 PM]). The animals were housed in 590×400×200-mm cages (Zhongke Animal Tec, ZX210001, Beijing, China) with food and water available ad lib. The experimental animals were housed in groups of 4 and given 6 days to acclimate to the housing facility.
All sections of this report adhere to the ARRIVE Guidelines for reporting animal research. A completed ARRIVE guidelines checklist is included in Checklist S1. All procedures regarding the use and the handling of the animals were conducted as approved by the Institutional Animal Care and Use Committee of the Military Medical Sciences Academy (No. 15007719XL) in accordance with the Animals (Scientific Procedures) Act, 1986 (UK, amended 2013). The overall physiology and food intake of the experimental animals were monitored every 24 hours. All efforts were made to minimize the number of animals used and their suffering. The experimenters were blinded to the pharmacologic or surgical treatment while processing data and making exclusion decisions.
DC culture
Bone marrow-derived dendritic cell culture was performed following the previously described method with minor modifications [18]. Briefly, the bone marrow cells were obtained from the femurs and tibias of C57BL/6 mice and then cultured in the 1640 medium supplemented with 8% heat-inactivated FBS (fetal bovine serum) in the 6-well plates at the density of 1×106 cells/5 mL per well. Recombinant mouse granulocyte macrophage colony-stimulating factor (rmGM-CSF; R&D, Minneapolis, MN, USA) 20 ng/mL and recombinant mouse IL-4 (rmIL-4; R&D, Minneapolis, MN, USA) 20 ng/mL were added to the culture medium every day. To maintain the immature state, DCs were collected on the sixth day and used in subsequent experiments. CD11c, CD80, CD86, and CD83 surface molecules were analyzed by flow cytometry.
Therapeutic administration and animal grouping
Grouping
Totally 120 LLC-bearing C57BL/6 mice were randomly assigned to 4 groups (30 mice per group) according to the applied timing of CpG-ODN administration. All the mice were treated by cryoablation and followed by the peritumoral injections of immature DCs. Then, CpG-ODN was injected in the same peritumoral zone 6 h (6-h group), 12 h (12-h group) and 24 h (24-h group) after cryoablation, respectively. Coadministration of DCs and CpG-ODN (0-h group) was performed on the mice of control group.
Cryoablation
The mice were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.006 mL/kg). Then, hair on the operative zone was shaved and the skin was sterilized with iodophor. Argon-helium cryoablation was performed using Cryocare Surgical System (Cryo-Hit, Israel). A 1.47-mm-diameter cryoprobe was inserted into the center of the tumor, with the inserting depth of 3±0.5 mm, freezing for 20 s at −140°C and then rewarming to 10°C for 10 s. Subsequently, another cycle was performed to simulate the double cycled clinical protocols [19]. A 1.5±3-mm-diameter iceball was formed and the whole tumor was absolutely covered in the iceball.
Administration of DCs and CpG-ODN
Immature DCs (4×106/mouse, in 100 μL phosphate-buffered saline solution [PBS]) were injected in the peritumoral zone immediately after cryosurgery. CpG-ODN (50 μg/mouse; Sangon Biological Engineering Technology Company, Shanghai, China) was dissolved in 100 μL PBS and injected into peritumoral zone 6, 12 h and 24 h after DC administration. Coadministration of immature DCs (4×106/mouse) and CpG-ODN (50 μg/mouse) was performed on the mice in the control group.
Survival rate analysis
Ten mice from each group were randomly selected for the survival rate analysis. The day that mice received cryoablation was considered as day 0. Ten untreated LLC-bearing mice were used as control subjects.
Cytokines and T lymphocyte subsets assessment
Ten mice in each group were randomly sacrificed on the seventh day after cryoablation and their eyeballs were picked for blood sampling. Cytokines, including the IFN-γ, IL-12/IL-23p40, IL-4, IL-10, and TNF-α, in the serum were measured by the BD™ Cytometric Bead Array (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) according to the manufacturer’s directions. Briefly, cytokine standard solutions were serially diluted and different concentrations were measured by BD LSRII (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) flow cytometry. Standard curves were generated by the BD FCAP software. Serum (50 μL) was incubated with capture beads (50 μL) for 1 h in the dark at room temperature. Then the phycoerythrin (PE)-labeled detection antibodies (50 μL) were added to the sample and incubated for 1 h. The sample was washed twice with buffer lotion by centrifugation before assay. Results were obtained using BD LSRII flow cytometry and the BD FCAP Array™ software.
In order to analyze the T cell subsets in the blood serum, the PerCP anti-mouse CD3e, FITC, anti-CD4, and PE anti-CD8a (BioLegend, USA) were added and incubated for 20 minutes in the darkness according to the manufacturer’s directions. Red blood cells (RBCs) were lysed by RBC Lysis Buffer (BioLegend, USA) before the samples were analyzed by the BD LSRII flow cytometry.
Rechallenge analysis
Ten mice in each group were re-challenged by the subcutaneous injections of LLC cells (5×105 cells/mice, in 0.2 mL PBS) to the contralateral femur 4 weeks after cryosurgery. The tumor size was assessed with a vernier caliper twice a week. Four weeks after rechallenge, the mice were sacrificed. The tumor volume was calculated by the following formula: V=4/3πr3 [5].
The lung was weighed after extirpation to evaluate the metastasis. In vitro cytotoxicities of the splenic lymphocytes were estimated with the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA). Splenic lymphocytes were isolated by the mouse lymphocytic separation medium (EZ-Sep™, Dakewe Biotech, Shenzhen, China) and incubated with LLC cells (2×105/well) at different effector/target ratios (25: 1, 50: 1, 100: 1) in the 96-well culture plates for 4 hours, according to the manufacturer’s directions. The supernatants were collected and the absorbance at 490nm was tested to evaluate the lactate dehydrogenase (LDH). The percentage of LDH release was calculated by the following formula:
Statistic analysis
The statistics of survival rate was analyzed by Kaplan-Meier curves and compared with the log-rank test. The statistics of the levels of serum cytokines, T-cell subsets, and lung weight were analyzed with 1-way analysis of variance (ANOVA) followed by post hoc test. Tumor growth kinetics and in vitro cytotoxicity assay were analyzed by 2-way ANOVA. Bonferroni multiple comparison posttest was used to determine the significance of difference. Graph Pad Prism version 5.0 was used for the statistical analyses. P<0.05 was considered to be significant.
Results
Phenotype of immature DCs
DCs were successfully cultured and harvested in the in vitro environment. The morphology and phenotype of these in vitro-generated DCs were absolutely typical (Figure 1A). The expression levels of the CD80, CD86, and CD83 molecules were remarkably low in these cultured DCs, indicating that they were in the immature state (Figure 1C).
Figure 1.
(A) The morphology and phenotype of the in vitro generated DCs. (B) The expression levels of the CD80, CD86 and CD83 molecules are remarkably low in these cultured DCs. (C) Survival rates of the mice treated by different protocols. The survival rate in the 12-h group was significantly higher than in the other 3 groups. (Analysis of variance followed by post hoc test, n=10; ** P<0.001 for differences between groups).
Survival analysis
Ten mice from each group were randomly selected for the survival rate analysis. It was found that the survival rate of all 4 treated groups (0-h group, 6-h group, 12-h group, 24-h group) were substantially higher than the untreated group (P<0.01, ANOVA followed by post hoc test, n=10). Moreover, the survival rate of the 12-h group was higher than other 3 groups and the differences were statistically significant (P<0.01, ANOVA followed by post hoc test, n=10; Figure 1B).
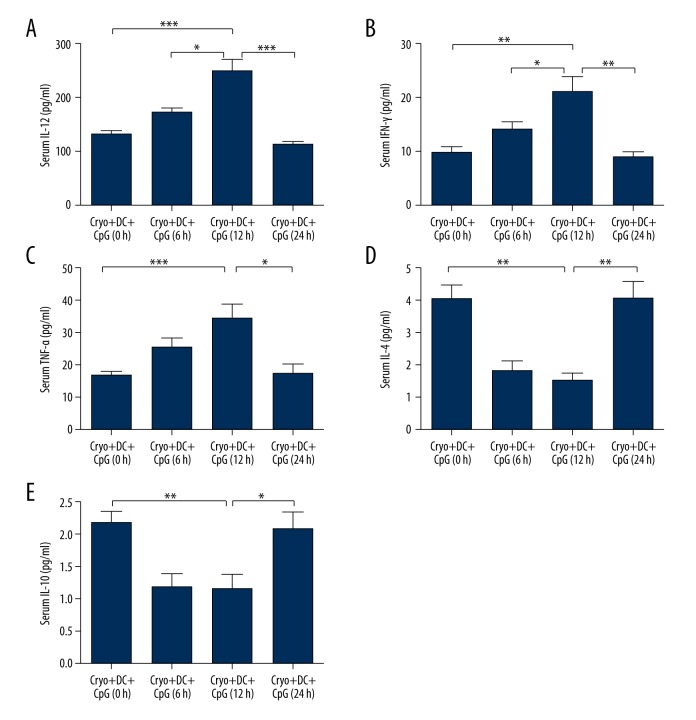
Levels of cytokines in blood serum
Multiple cytokines in the serum of the experimental animal in different groups were tested. Our results suggested that the expression levels of serum IL-12 and IFN-γ of the 12-h group were significantly higher than the other 3 groups (IL-12: P<0.001, 12-h group vs. 24-h group, 12-h group vs. 0-h group; P<0.05, 12-h group vs. 6-h group; IFN-γ: P<0.01, 12-h group vs. 24-h group, 12-h group vs. 0-h group; P<0.05, 12-h group vs. 6-h group, ANOVA followed by post hoc test, n=10; Figure 2A, 2B). The TNF-α level of the 12-h group was also significantly higher than that of the 0-h group and 24-h group (P<0.05, 12-h group vs. 24-h group; P<0.05, 12-h group vs. 0-h group; ANOVA followed by post hoc test, n=10; Figure 2C). However, no significant difference was found between the 12-h group and the 6-h group (P>0.05, 12-h group vs. 6-h group; ANOVA followed by post hoc test, n=10). On the other hand, the IL-4 and IL-10 levels of the 12-h group were significantly lower than those of the 0-h and 24-h groups (IL-4: P<0.01, 12-h group vs. 24-h group, 12-h group vs. 0-h group; IL-10: P<0.05, 12-h group vs. 24-h group; P<0.01, 12-h group vs. 0-h group, ANOVA followed by post hoc test, n=10; Figure 2D, 2E). Meanwhile, no significant difference was found between the 12-h group and the 6-h group in terms of the IL-4 or IL-10 levels (P>0.05, 12-h group vs. 6-h group; ANOVA followed by post hoc test, n=10).
Figure 2.
The serum cytokine levels of the experimental animal in different groups. The expression levels of serum IL-12 (A) and IFN-γ (B) of the 12-h group were significantly higher than those of the other 3 groups. The TNF-α (C) level of the 12-h group was also significantly higher than those of the 0-h and 24-h groups. The IL-4 (D) and IL-10 (E) levels of the 12-h group were significantly lower than those of the 0-h and 24-h groups. (ANOVA followed by post hoc test, n=10; * P<0.05; ** P<0.01; *** P<0.001for differences between groups).
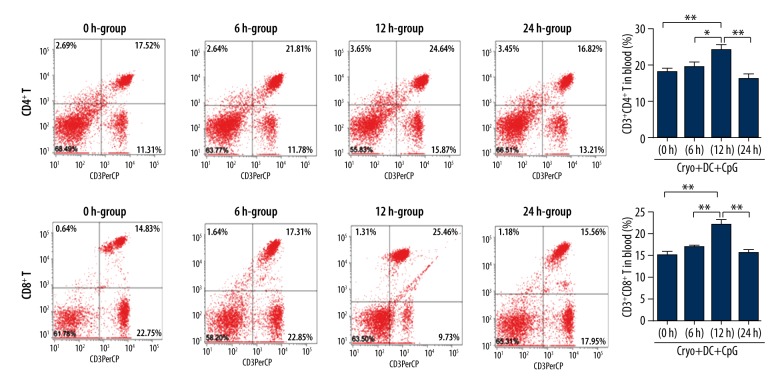
CD4+ and CD8+ T lymphocytes in the blood
The CD4+ and CD8+ T lymphocytes in the blood of the experimental animal were analyzed by flow cytometry. It was found that the CD4+ and CD8+ T lymphocytes in blood of the 12-h group were both significantly higher than the other 3 groups (ANOVA followed by post hoc test, n=10; Figure 3).
Figure 3.
Levels of CD4+ and CD8+ T lymphocytes in blood of the 12-h group were both significantly higher than in the other 3 groups. (ANOVA followed by post hoc test, n=10, * P<0.05; ** P<0.01 for differences between groups).
Tumor volume and lung weight of the rechallenged mice
Four weeks after rechallenge, the mice were sacrificed and the tumor volume was calculated. It was found that the tumor volume of the 12-h group was significantly smaller than that of the 24-h group and 0-h group (P<0.001, 12-h group vs. 24-h group; P<0.001, 12-h group vs. 0-h group; ANOVA followed by post hoc test, n=10; Figure 4A). Although the tumor volume of the of the 12-h group is also smaller than that of the 6-h group, it was not statistically significant (P>0.05, 12-h group vs. 6-h group; ANOVA followed by post hoc test, n=10).
Figure 4.
(A) The tumor volume of the 12-h group was significantly smaller than that of the 0- and 24-hour groups. (B) The lung weight of the 12-h group was significantly less than that of the 0- and 24-h groups. The lung weight of the 12-h group was also less than that of the 6-h group, though this was not statistically significant. (C) The 12-h group showed more potent in vitro cytotoxicity of splenic lymphocytes than the 0- and 24-h groups. (ANOVA followed by post hoc test, n=10; * P<0.05; ** P<0.01; *** P<0.001 for differences between groups).
Moreover, the lung of the experimental animal was weighed after extirpation to evaluate the metastasis. It was found that the lung weight of the 12-h group was the lowest among the 4 groups, indicating the best resistance to tumor metastasis. The lung weight of the 12-h group was significantly smaller than that of the 24-h group and 0-h group (P<0.001, 12-h group vs. 24-h group; P<0.001, 12-h group vs. 0-h group; ANOVA followed by post hoc test, n=10; Figure 4B). The lung weight of the 12-h group was also smaller than that of the 6-h group, while it was not statistically significant (P>0.05, 12-h group vs. 6-h group; ANOVA followed by post hoc test, n=10).
In vitro cytotoxicity assessment
Four weeks after rechallenge, in vitro cytotoxicity was assessed at different effector/target ratios. It was found that the 12-h group showed more potent in vitro cytotoxicity of splenic lymphocytes in comparison with the 0-h and 24-h groups (1: 100: P<0.001, 12-h group vs. 24-h group; P<0.001, 12-h group vs. 0-h group; 1: 25: P<0.05, 12-h group vs. 24-h group; 1: 50: P<0.01, 12-h group vs. 24-h group; ANOVA followed by post hoc test, n=10; Figure 4C). Meanwhile, no statistical significance was found between the 6-h group and the 12-h group (P>0.05, 12-h group vs. 6-h group; ANOVA followed by post hoc test, n=10).
Discussion
In the present study, we systematically explored the therapeutic efficacy of the combined administration DCs and immune adjuvant CpG-ODN after cryosurgery in the LLC mode. Initially, the double-cycled cryoablation was performed to the experimental animals to mimic the clinical procedures [19]. Subsequently, in vitro cultured imDCs with minimum expression levels of the CD80, CD86 and CD83 molecules, were injected in the peritumoral area (but not the cryoablated area) to maintain their vitality, because the permeation of tumor antigens from the necrotic cells to the injection site is time consuming. Because the coadministration of DCs and CpG-ODN might result in the maturation of DCs before antigen uptake, the CpG-ODN was administered at different time points after DC injection. Our results suggested that the application of CpG-ODN can significantly influence the therapeutic effect of the combined protocol. Administration of CpG-ODN 12 h after DC injection could efficiently prolong the survival of mice and enhance their antirechallenge ability. The expression levels of CD4+ and CD8+ T cells, IL-12, IFN-γ, and TNF-α in the 12-h group were substantially higher than the other groups, indicating a more potent Th1 type immune response. Antitumor immune response was gradually enhanced with the prolonged time intervals between DC and CpG-ODN administration and peaked at the 12-h time point. Therefore, our study suggested that therapeutic efficacy is closely associated with CpG-ODN administration in the combined therapeutic protocol of cryoablation, DCs, and immune adjuvant. In situ administration of CpG-ODN 12 hours after DC injection might be considered the optimum application.
Lung cancer is the most common cause of cancer death worldwide. Most cases have progressed to advanced-stage disease by the time of diagnosis and thus are no longer eligible for surgical therapy. Cryosurgery is an alternative therapeutic strategy that combines the procedures of freezing and thawing, resulting in tissue destruction and in situ absorption [21]. It has been extensively used in ablating malignancies in the breast, prostate, esophagus, bone, kidney, skin, lung, and liver [22]. Hitherto, cryosurgery was suited for patients with (a) advanced-stage cancer, (b) poor general health or poor respiratory function, (c) tumor recurrence, or (d) localized lung cancer who refused surgery [20]. The related merits of cryosurgery have been discovered by abundant research. Besides the local destruction of tumors, another potential advantage is the secondary antitumor immune response generated by the tumor antigens that get released from the necrotic tumor cells. Several experiments have shown that the cryoimmune response is stimulatory [4]. Successful induction of cryoimmune response depends on (a) the release of tumor antigens from the necrotic tumor cells; (b) the smooth uptake of antigens by APCs; (c) the maturation of APCs in a stimulatory environment; (d) the migration of APCs to draining lymph nodes and activation of naive T cells. Furthermore, the functional impairment of DCs in the tumor microenvironment should be partially responsible for the failure of cryosurgery-induced antitumor immune response [23,24].
Various immunotherapeutic methods, such as the in situ administration of DCs and CpG-ODN, are adjunctively combined with cryoablation to enhance the cryoimmune effect [25]. Den Brok et al. coadministered cryoablation with CpG-ODN to generate an in vivo DC vaccine, resulting in the eradication of primary and recurrent melanoma tumors [26]. Machlenkin et al. combined cryoablation with DCs and successfully induced the antitumor immune response [27]. Intriguingly, a novel study found that coadministration of DCs and CpG-ODN after cryosurgery was superior to the administration of DCs or CpG-ODN alone [15]. In imDCs, promoting the maturation of DCs is of great importance because imDCs exert immunosuppressive, rather than immunostimulatory, effects [28, 29]. However, whether the application of CpG-ODN affects the therapeutic results of the combined administration of cryoablation and DCs. Generally, 2 signals are necessary for the full activation of T cells: signal 1 is the recognition of the peptide/MHC by the T-cell receptor, and signal 2 is delivered by the costimulatory molecules [30]. Naive T cells receiving signal 1 without signal 2 will lead to the down-regulation or deletion of T cells [4]. With the stimulation of CpG-ODN, DCs secrete type 1 cytokine and increase the expressional levels of costimulatory molecules such as CD80 and CD86, which are crucial for the initiation of cell-mediated Th1 and T lymphocyte responses [27]. With all the effort made to promote the maturation of DCs, one fact should not be neglected: that the maturation of DCs is accompanied by the down-regulation of phagocytosis-associated receptors, which results in the weakened antigen-uptake capacity of DCs. Therefore, it is critical to maintain the immature state of DCs in the therapy before antigen uptake. On the contrary, when the time interval of administration was extended to 24 h, the therapeutic results and antitumor immune response became weakened in comparison with those of the 12-h group. Consequently, we hypothesize that the migration of DCs might have happened before the injection of CpG-ODN if the time window was as long as 24 h, subsequently resulting in abatement interactions.
Based on the aforementioned findings, we conclude that administration of CpG-ODN 12 h after DC injection after cryoablation should be considered the optimum application. The combination of cryosurgery and DCs have the potential to present the entire repertoire of antigens harbored by autologous tumor cells to the host immune system, although much detailed work is still to be done to optimize the combined protocols. Our novel findings could provide the basis for further formulation of a reasonable clinical strategy [31]. Another key factor for the successful induction of antitumor immune response is the maturation and homing of DCs to the draining lymph nodes, a topic that will be investigated in further studies [32,33].
Conclusions
The therapeutic efficacy is closely associated with CpG-ODN administration in the combined therapeutic protocol of cryoablation, DCs, and immune adjuvant. In situ administration of CpG-ODN 12 hours after DC injection might be considered the optimum application.
Footnotes
Conflicts of interest statement
The authors declare that there is no conflict of interests.
Source of support: This study is sponsored by grant Z13146806813023 from the capital civilian health program
References
- 1.Johnson BE, Grayson J, Makuch RW, et al. Ten-year survival of patients with small-cell lung cancer treated with combination chemotherapy with or without irradiation. J Clin Oncol. 1990;8:396–401. doi: 10.1200/JCO.1990.8.3.396. [DOI] [PubMed] [Google Scholar]
- 2.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28:3–13. [PubMed] [Google Scholar]
- 3.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83:355–67. doi: 10.4065/83.3.355. [DOI] [PubMed] [Google Scholar]
- 4.Sabel MS. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 5.Yin Z, Lu G, Xiao Z, et al. Antitumor efficacy of argon-helium cryoablation-generated dendritic cell vaccine in glioma. Neuro Report. 2014;125:199–204. doi: 10.1097/WNR.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 6.Machlenkin A, Goldberger O, Tirosh B, et al. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res. 2005;11:4955–61. doi: 10.1158/1078-0432.CCR-04-2422. [DOI] [PubMed] [Google Scholar]
- 7.Hammer GE, Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013;31:743–79. doi: 10.1146/annurev-immunol-020711-074929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzurro GA, Barrio MM. Dendritic cell-based vaccine efficacy: Aiming for hot spots. Front Immunol. 2015;6:91. doi: 10.3389/fimmu.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cintolo JA, Datta J, Mathew SJ, et al. Dendritic cell-based vaccines: Barriers and opportunities. Future Oncol. 2012;8(10):1273–99. doi: 10.2217/fon.12.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandholm J, Selander KS. Toll-like receptor 9 in breast cancer. Front Immunol. 2014;5:330. doi: 10.3389/fimmu.2014.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerkovnik P, Novakovic BJ, Stegel V, Novakovic S. Tumor vaccine composed of C-class CpG oligodeoxynucleotides and irradiated tumor cells induces long-term antitumor immunity. BMC Immunol. 2010;11:45. doi: 10.1186/1471-2172-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahrsdörfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update Cancer Ther. 2008;3(1):27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nierkens S, den Brok MH, Garcia Z. Immune adjuvant efficacy of CpG oligonucleotide in cancer treatment is founded specifically upon TLR9 function in plasmacytoid dendritic cells. Cancer Res. 2011;71(20):6428–37. doi: 10.1158/0008-5472.CAN-11-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai MS, Chang CC, Kuo ML, Wu YT. Vascular endothelial growth factor-A and changes in a tumor-bearing mouse model with Lewis lung cancer. Oncol Lett. 2011;2(6):1143–47. doi: 10.3892/ol.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi G, Wang H, Zhuang X. Myeloid-derived suppressor cells enhance the expression of melanoma-associated antigen A4 in a Lewis lung cancer murine model. Oncol Lett. 2016;11(1):809–16. doi: 10.3892/ol.2015.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alteber Z, Azulay M, Cafri G, et al. Cryoimmunotherapy with local co-administration of ex vivo generated dendritic cells and CpG-ODN immune adjuvant, elicits a specifc antitumor immunity. Cancer Immunol Immunother. 2014;63:369–80. doi: 10.1007/s00262-014-1520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawano M, Nishida H, Nakamotoet Y, et al. Cryoimmunologic antitumor effects enhanced by dendritic cells in osteosarcoma. Clin Orthop Relat Res. 2010;468(5):1373–83. doi: 10.1007/s11999-010-1302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maccini M, Sehrt D, Pompeo A, et al. Biophysiologic considerations in cryoablation: a practical mechanistic molecular review. International Braz J Urol. 2011;37(6):693–96. doi: 10.1590/s1677-55382011000600002. [DOI] [PubMed] [Google Scholar]
- 20.Niu L, Xu K, Mu F. Cryosurgery for lung cancer. J Thorac Dis. 2012;4(4):408–19. doi: 10.3978/j.issn.2072-1439.2012.07.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baust JG, Gage AA, Bjerklund Johansen TE, Baust JM. Mechanisms of cryoablation: Clinical consequences on malignant tumors. Cryobiology. 2014;68(1):1–11. doi: 10.1016/j.cryobiol.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baust JG, Gage AA, Bjerklund Johansen TE, et al. Mechanisms of cryoablation: Clinical consequences on malignant tumors. Cryobiology. 2014;68(1):1–11. doi: 10.1016/j.cryobiol.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legitimo A, Consolini R, Failli A, et al. Dendritic cell defects in the colorectal cancer. Hum Vaccin Immunother. 2014;10(11):3224–35. doi: 10.4161/hv.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma Y, Shurin GV, Gutkin DW, et al. Tumor associated regulatory dendritic cells. Semin Cancer Biol. 2012;22(4):298–306. doi: 10.1016/j.semcancer.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidana A. Cancer immunotherapy using tumor cryoablation. Immunotherapy. 2014;6(1):85–93. doi: 10.2217/imt.13.151. [DOI] [PubMed] [Google Scholar]
- 26.den Brok MH, Sutmuller RP, Nierkens S, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–92. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 27.Machlenkin A, Goldberger O, Tirosh B, et al. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res. 2005;11:4955–61. doi: 10.1158/1078-0432.CCR-04-2422. [DOI] [PubMed] [Google Scholar]
- 28.Linette GP, Carreno BM. Dendritic cell-based vaccines: Shining the spotlight on signal 3. Oncoimmunology. 2013;2:e26512. doi: 10.4161/onci.26512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonifaz L, Bonnyay D, Mahnke K, et al. Effcient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capece D, Verzella D, Fischietti M, et al. Targeting costimulatory molecules to improve antitumor immunity. J Biomed Biotechnol. 2012;2012:926321. doi: 10.1155/2012/926321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janikashvili N, Larmonier N, Katsanis E. Personalized dendritic cell-based tumor immunotherapy. Immunotherapy. 2010;2(1):57–68. doi: 10.2217/imt.09.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Li W, Procissi D. Antigen-loaded dendritic cell migration: MR imaging in a pancreatic carcinoma model. Radiology. 2015;274(1):192–200. doi: 10.1148/radiol.14132172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turnis ME, Rooney CM. Enhancement of dendritic cells as vaccines for cancer. Immunotherapy. 2010;2(6):847–62. doi: 10.2217/imt.10.56. [DOI] [PMC free article] [PubMed] [Google Scholar]