Abstract
The progression to advanced stage cancer requires changes in many characteristics of a cell. These changes are usually initiated through spontaneous mutation. As a result of these mutations, gene expression is almost invariably altered allowing the cell to acquire tumor-promoting characteristics. These abnormal gene expression patterns are in part enabled by the posttranslational modification and remodeling of nucleosomes in chromatin. These chromatin modifications are established by a functionally diverse family of enzymes including histone and DNA-modifying complexes, histone deposition pathways, and chromatin remodeling complexes. Because the modifications these enzymes deposit are essential for maintaining tumor-promoting gene expression, they have recently attracted much interest as novel therapeutic targets. One class of enzyme that has not generated much interest is the chromatin remodeling complexes. In this review, we will present evidence from the literature that these enzymes have both causal and enabling roles in the transition to advanced stage cancers; as such, they should be seriously considered as high-value therapeutic targets. Previously published strategies for discovering small molecule regulators to these complexes are described. We close with thoughts on future research, the field should perform to further develop this potentially novel class of therapeutic target.
1. INTRODUCTION—THE IMPORTANCE OF GENE EXPRESSION TO CANCER BIOLOGY
Advanced stage cancer occurs when normal cells acquire several tumor-promoting characteristics. These characteristics are broadly organized into eight categories that have been widely accepted as hallmarks of cancer (Hanahan & Weinberg, 2011). At a fundamental level, acquiring the hallmarks of cancer is a consequence of deregulating any one of a number of basic cellular properties including motility, differentiation, proliferation, and viability. In one theory, the acquisition of hallmarks occurs spontaneously as a result of somatic mutation. At a molecular level, these mutations deregulate cellular properties to promote tumor growth (Nowell, 1976). In some cases, these somatic mutations directly cause abnormal gene expression patterns favoring tumor cell growth. A classic example is the t(8;14)(q24; q32) translocation that juxtaposes the immunoglobulin heavy chain locus with the MYC protooncogene resulting in elevated MYC expression (Erikson, ar-Rushdi, Drwinga, Nowell, & Croce, 1983; Taub et al., 1982). In other cases, mutations deregulate circuits resulting in the abnormal regulation of a large number of genes to favor tumor cell growth. Mutations in Ras (RasG12V), which result in tumor-promoting gene regulation, are a classic example (Ayllon & Rebollo, 2000; Wong-Staal, Dalla-Favera, Franchini, Gelmann, & Gallo, 1981). Once established, abnormal gene expression profiles maintain the tumor cell phenotype through a process of oncogene addiction (Weinstein, 2002). Because abnormal gene expression first promotes, and can later become essential for the tumor cell phenotype, it can be assumed that correcting abnormal gene expression would be disadvantageous to the cancer cell, and therefore, a viable therapeutic strategy (Yeh, Toniolo, & Frank, 2013).
Several approaches have been proposed to correct abnormal gene expression in tumors. By changing the DNA sequence, one can attempt to correct abnormal gene expression (Perez-Pinera, Ousterout, & Gersbach, 2012). This can occur by correcting the mutations that promote abnormal gene expression or by changing essential regulatory sequences necessary for tumor-promoting gene expression. Correcting DNA sequences in a patient’s tumor cells is certainly plausible but is not yet a practical approach to correcting abnormal gene expression. Alternatively, abnormal gene expression can be corrected by altering any one of several posttranslational modifications found on the chromatin of tumor cells that are important for gene expression (Plass et al., 2013). These diverse post-translational modifications are commonly, but not accurately, referred to as epigenetic regulatory mechanisms (Ptashne, 2013). In contrast to genetic regulatory mechanisms that are stable, posttranslational modifications on chromatin are dynamic, and in theory, reversible. Posttranslational modifications to chromatin that accompany regulated transcription do not change the DNA sequence but rather are deposited onto chromatin by enzyme catalyzed reactions (Allis, Jenuwein, & Reinberg, 2007). Because posttranslational modifications on chromatin are required for regulating procancer gene expression in tumor cells, do not change the DNA sequence, are in theory reversible, and are established by the actions of enzymes, makes them attractive targets to correct abnormal gene expression as a means of therapy. In this review, we will focus on the therapeutic potential of one class of epigenetic regulator—chromatin remodeling. We will present a growing body of evidence that the enzymes that catalyze ATP-dependent chromatin remodeling not only play an important role as drivers of cancer biology but also operate in concert with oncogenes and tumor suppressors to promote the abnormal gene expression necessary to maintain cancer biology. Current strategies used to develop small molecule regulators to chromatin remodeling complexes are discussed, and we close with a discussion on how the field can further develop this class of enzymes as viable therapeutic targets for cancer treatment.
2. AN OVERVIEW OF EPIGENETIC REGULATORY MECHANISMS
In higher eukaryotes, posttranslational modification regulates the function of chromatin. Chromatin is composed of nucleosomes, which in turn are composed of two copies of each of the conical histones H2A, H2B, H3, and H4 wrapped by ~150 bp of DNA (Luger, Mader, Richmond, Sargent, & Richmond, 1997). Nucleosomes are positioned along DNA in a “beads on a string” configuration to create a 10-nm fiber that through internucleosome contacts creates a variety of higher ordered chromatin structures including a molten globule with short stretches of a 30-nm fiber (Kornberg, 1974; Woodcock & Ghosh, 2010). In most cases, these chromatin structures repress DNA accessibility and therefore must be modified to promote essential nuclear processes like transcription, DNA replication, and DNA repair (Laybourn & Kadonaga, 1991). These modifications include histone posttranslational modifications, DNA methylation, incorporation of histone variants, and chromatin remodeling (Allis et al., 2007). Small RNA regulation is another major category of epigenetic regulation that does not operate on chromatin (at least in mammals) and is not discussed in this review. The interplay between chromatin modifications participate as part of a larger “epigenetic code” which is essential for the function of DNA regulatory elements in chromatin. These modifications arise and are subsequently maintained on chromatin through interactions between the enzymes which deposit them and sequence-specific DNA-binding factors (Kouzarides, 2007).
The posttranslational modification of histones occurs by histone modifying enzymes. And just as histones can be modified, the modifications can be removed by de-modifying enzymes. Histones are primarily modified on the N-terminal and C-terminal ends but can be modified throughout their sequence (Kouzarides, 2007). The effect of histone modifications is to alter chromatin structure or recruit additional chromatin-modifying complexes, which in turn regulate nuclear processes like transcription. Common modifications include acetylation, methylation, and phosphorylation, and many of the enzymes which add and remove these modifications have been identified and characterized. Histone modifications are usually found in combinations in chromatin (e.g., H3K9me1 with H3K4me3) so as to provide redundancy in their ability to direct nuclear processes (Wang et al., 2008).
Just like histones, DNA can be modified to provide a stable method of regulating gene expression. In mammals, modifications to DNA usually occur on CpG dinucleotides, but they have also been observed outside of this context (Lister et al., 2009; Ziller et al., 2011). The most common modification to CpG dinucleotides is the methylation of the fifth carbon of cytosine (5mC), catalyzed by the DNMT family of enzymes. 5mC is removed by both passive and active means, the latter including the TET family of enzymes and to a lesser extent APOBEC (Shen & Zhang, 2013). DNA methylation largely represses gene expression by altering nucleosome positioning and stability and by recruiting chromatin-modifying complexes containing subunits that bind methylated DNA (Bartke et al., 2010; Lee & Lee, 2012).
To similar effect, the incorporation of histone variants serves to alter chromatin structure and stability, and it provides unique contact surfaces for the recruitment of chromatin-modifying complexes (Skene & Henikoff, 2013). The incorporation of histone variants can occur through both ATP-dependent and ATP-independent mechanisms. ATP-independent mechanisms include histone chaperones dedicated to the deposition of specific histone variants. Histone chaperones support replication dependent (coupled with DNA replication during S-phase) and independent (outside S-phase) histone deposition pathways. Examples include the ATRX/DAXX pathway for the deposition of histone H3.3 and the HJURP pathway for the deposition of CENP-A (Skene & Henikoff, 2013). The incorporation of histone variants by ATP-dependent mechanisms usually occurs through chromatin remodeling complexes and is the focus of this review.
3. ATP-DEPENDENT CHROMATIN REMODELING
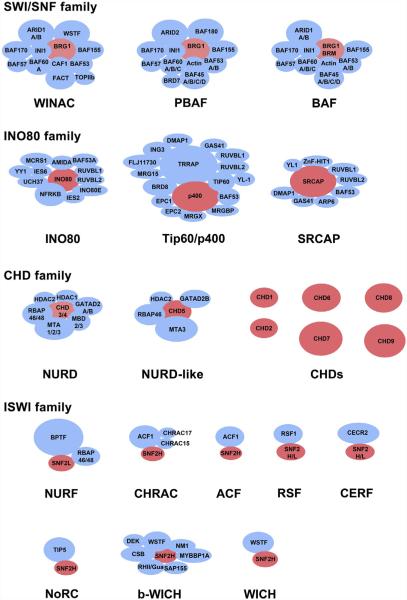
Chromatin remodeling is the process wherein the position, occupancy, or the histone composition of a nucleosome is altered in the chromatin. These activities occur by both ATP-dependent and ATP-independent mechanisms (Aalfs & Kingston, 2000). ATP-independent mechanisms can occur from transcription factors shifting the position of nucleosomes as a consequence of DNA binding, or by the action of histone chaperones, depositing or removing histones from chromatin (De Koning, Corpet, Haber, & Almouzni, 2007; Workman & Kingston, 1992). In contrast to ATP-independent mechanisms, ATP-dependent mechanisms are enzymatic and constitute the majority of the remodeling activity in the cell (Zhang et al., 2011). These activities are usually catalyzed by multisubunit ATP-dependent chromatin remodeling complexes which are organized into SWI/SNF, ISWI, CHD, and INO80 families (Fig. 5.1). ATPases are organized into these families based on the sequence homology of the incorporated ATPase (Becker & Workman, 2013). Each family of ATPase catalyzes distinct remodeling activities: incremental nucleosome sliding on DNA in cis; the creation of DNA loops on the surface of the nucleosome; eviction of histone H2A/H2B dimers; eviction of the histone octamer; or the exchange of histone octamer subunits within the nucleosome to change its composition. Each of these activities alters the accessibility of DNA in the chromatin to DNA-binding factors, which in turn regulates essential nuclear processes like transcription, DNA replication, and DNA repair.
Figure 5.1. Chromatin remodeling complexes are segregated into distinct families.
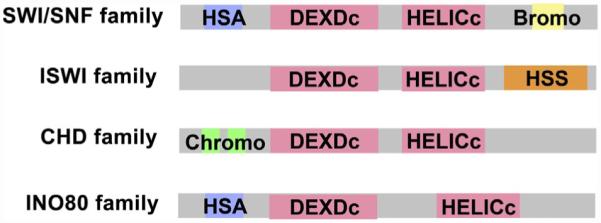
The ATPase containing subunits found in chromatin remodeling complexes have common functional domains allowing their segregation into distinct families. All ATPase subunits have distinctive DEXDc ATP-binding domains and a HELICc helicase domain. The combination of these domains convert the chemical energy stored in ATP into mechanical energy used to remodel nucleosomes. The space between these two domains varies among family members and is distinctively long for the INO80 family of ATPases. Other domains which are diagnostic to specific families include the HSA domain (HSA) found in SWI/SNF and INO80 families, bromodomains (bromo) found in SWI/SNF family members, chromodomains (chromo) found in the CHD family, and the HAND–SANT–SLIDE (HSS) cluster found in ISWI family members (Becker & Workman, 2013).
The chromatin remodeling activity of an ATPase usually occurs in the context of a large multisubunit complex, but on rare occasions, they are known to function as single ATPase subunits (Fig. 5.2; Becker & Workman, 2013). Complexes in the SWI/SNF family include the large multisubunit BAF, PBAF, and WINAC complexes which function as coregulators of transcription, and they also aid in the repair of DNA damage (Oya et al., 2009; Wang et al., 1996). Remodeling reactions catalyzed by SWI/SNF family members include simple nucleosome sliding reactions, but they can include more dramatic reactions like creating DNA loops on the surface of nucleosomes or the eviction of H2A/H2B dimers from the nucleosome structure (Bowman, 2010). Like SWI/SNF, the members of the INO80 family of complexes are also large multisubunit complexes which regulate transcription, but they have more prominent functions in DNA damage repair (Morrison & Shen, 2009). These complexes are unique among ATP-dependent remodeling complexes because they can catalyze the exchange of histones from the nucleosome structure. Complexes with the best characterized functions include SRCAP and Tip60/p400, which exchange the H2A/H2B histone dimer found in a canonical nucleosome for a variant H2A.Z/H2B dimer (Gevry, Chan, Laflamme, Livingston, & Gaudreau, 2007; Ruhl et al., 2006; Wong, Cox, & Chrivia, 2007). In contrast to most chromatin remodeling enzymes, these complexes are dedicated histone exchange enzymes, with little nucleosome sliding activities. Like Tip60/p400 and SRCAP, the INO80 complex has been reported to exchange a H2A.Z/H2B histone dimer found in a variant nucleosome for a canonical H2A/H2B dimer but also has significant nucleosome sliding activities (Papamichos-Chronakis, Watanabe, Rando, & Peterson, 2011; Udugama, Sabri, & Bartholomew, 2011). The CHD family contains nine different ATPases, the most numerous of the remodeling families (Hall & Georgel, 2007). The best characterized complex in this family is NURD. NURD contains both ATP-dependent chromatin remodeling and histone deacetylase activities (Tong, Hassig, Schnitzler, Kingston, & Schreiber, 1998). A subset of the NURD complexes is unique in its ability to bind 5mC DNA through the MBD2 subunit (Hendrich & Bird, 1998). Once recruited by 5mC enriched DNA, the MBD2–NURD complex promotes the repression of genes through its remodeling and histone deacetylase activities. However, alternative NURD complexes exist that replace the MBD2 subunit for MBD3, decoupling NURD from 5mC recruitment, and promote its functions as an activator of transcription (Gunther et al., 2013). In addition to NURD, a NURD-like complex containing CHD5 has been identified in the brain and acts as a coregulator of transcription (Potts et al., 2011). Most of the complexes containing other CHD ATPases await characterization. As opposed to the large SWI/SNF, INO80, and CHD complexes, most ISWI complexes are comparatively small, consisting of only two or three subunits (Erdel & Rippe, 2011). At a minimum, each of these complexes contains a single large subunit with several histone-binding domains (including PHD and bromodomains) and an ISWI family ATPase. ISWI family chromatin remodeling complexes catalyze the sliding of nucleosomes in short increments without DNA looping, histone exchange, or nucleosome displacement. These complexes have diverse functions including the spacing of nucleosomes after DNA replication (CHRAC, ACF), RNA polymerase elongation (RSF), serving as coregulators of transcription (CERF, NURF, NoRC, b-WICH), and regulating DNA damage repair (WICH) (Banting et al., 2005; Barak et al., 2003; Cavellan, Asp, Percipalle, & Farrants, 2006; LeRoy, Loyola, Lane, & Reinberg, 2000; LeRoy, Orphanides, Lane, & Reinberg, 1998; Poot et al., 2000, 2004; Strohner et al., 2001).
Figure 5.2. Chromatin remodeling complexes vary subunit composition to increase diversity.
The subunit composition of major characterized chromatin remodeling complexes from the SWI/SNF, INO80, CHD, and ISWI families are shown in cartoon. Complexes are organized into rows based on family. ATPase subunits are shown in red; all other subunits are shown in blue. Subunits which can be differentially incorporated into complexes are separated by a backslash symbol (/).
Understanding how these complexes function in vivo continues to be an active area of basic research. Key to this endeavor includes understanding how they are recruited to discrete regions of the genome at distal regulatory elements, promoters, and sites of DNA damage. Recruitment commonly occurs through direct interactions with a combination of transcription factors, histone modifications, and DNA sequences. These interactions occur through domains on the surfaces of subunits in the complex, and they likely operate in a combinatorial fashion to confer the necessary affinity and specificity for meaningful recruitment (Lange, Demajo, Jain, & Di Croce, 2011). Domains found in subunits that recognize specific histone modifications include a variety of well-characterized PHD, chromo, and bromodomains, among others. How these domains bind modified histones has been reviewed extensively (Yun, Wu, Workman, & Li, 2011). The incorporation of individual subunits into chromatin remodeling complexes varies based on availability, thus providing a means to generate distinct complexes with unique interaction surfaces. The cell-type-restricted expression of BAF45 and BAF60 subunits from the SWI/SNF family of complexes in embryonic stem cells, neural progenitor cells, and more differentiated neurons presents the best example of this mechanism (Ho & Crabtree, 2010). Like that for histone modifications, the interactions between transcription factors and chromatin remodeling complexes are specific, and in many cases they have been shown to depend on the activation state of the transcription factor (ligand binding as an example) (e.g., see Badenhorst et al., 2005; Fujiki et al., 2005). While quite specific in nature, the interaction surfaces on the chromatin remodeling complexes that participate in these interactions are not as well defined as those that interact with modified histones.
The biological functions of chromatin remodeling complexes during normal development have been extensively studied using mouse models (Ho & Crabtree, 2010). As expected, the subunits of many chromatin remodeling complexes have essential functions during mammalian development. Translating these phenotypes into an understanding of how specific chromatin remodeling complexes function during development has remained a challenge. This challenge occurs largely because subunits of chromatin remodeling complexes are often found in several chromatin-associated complexes. As such, the deletion of a single subunit likely compromises several complexes resulting in pleotropic effects. As an example, RUVBL1 and RUVBL2 are utilized by three chromatin remodeling complexes and at least three other chromatin-associated complexes (Huen et al., 2010). However, studies have been done on several subunits for which all the evidence suggests that they are unique to a single chromatin remodeling complex. Examples include BPTF (ISWI family NURF complex) and INO80 (INO80 family INO80 complex) (Barak et al., 2003; Chen et al., 2011; Jin et al., 2005; Landry et al., 2008; Min et al., 2013). In somewhat of a surprise, several ATPases are not essential for the cell: SWI/SNF family members BRG1 and BRM, CHD family member CHD4, and ISWI family member SNF2L (Bultman et al., 2000; Reyes et al., 1998; Yip et al., 2012; Yoshida et al., 2008). In other cases including the INO80 and p400 ATPases from the INO80 family and SNF2H ATPase from the ISWI family, the ATPase is important for cell viability (Fujii, Ueda, Nagata, & Fukunaga, 2010; Min et al., 2013; Stopka & Skoultchi, 2003). The importance of chromatin remodeling complexes for development, but not necessarily for cell viability, suggests that their mutation can result in a viable cell with abnormal developmental pathways. Because deregulated developmental pathways are known to contribute to the transition to cancer, it is reasonable to suggest that the mutation of subunits in chromatin remodeling complexes could contribute to cancer-related phenotypes (Hanahan & Weinberg, 2011; Plass et al., 2013).
4. EVIDENCE OF WIDESPREAD ROLES FOR CHROMATIN REMODELING IN HUMAN CANCER
Until recently, chromatin remodeling complexes were not thought to have widespread roles in the establishment and progression of human cancers. The recent availability of comprehensive genome-wide datasets, in the form of exon sequencing and genome-wide expression arrays for many human cancers, has provided an opportunity to discover novel connections between chromatin remodeling complexes and human cancer (Chang et al., 2013).
Mutation is thought to be the driving force behind the progression from normal tissue to advanced stage human cancer. Tumor-promoting mutations can be inherited, but they usually occur spontaneously in the form of somatic mutations that activate oncogenes and repress tumor suppressors in pathways important for tumor development. Frequently mutated genes are identified as driver mutations because of their inferred tumor-promoting potential (Kandoth et al., 2013; Tamborero et al., 2013). Driver mutations frequently occur in upstream components of signaling pathways (receptors), likely because of their potential for widespread effects on cell physiology. With less frequency, mutations in downstream components of signaling pathways (transducers) are identified as driving mutations. Included in this category are mutations in the subunits of chromatin remodeling complexes. As an exercise to quantify the mutation frequency for each chromatin remodeling complex subunit from a wide array of tumors, we mined the COSMIC database of somatic mutations in human cancers (Forbes et al., 2011). Consistent with what was published by others, we observed that subunits of the SWI/SNF family of remodeling complexes are the most prominently mutated of the chromatin remodeling complex families (Fig. 5.3; Table 5.1; Kadoch et al., 2013; Shain & Pollack, 2013). Mutations in the INI1 (also known as SNF5) and ARID1A (also known as BAF250A) components are frequently observed in tumors from diverse tissues including central nervous system, stomach, large intestine, bone, endometrium, liver, soft tissue, ovary, and urinary tract. Additional SWI/SNF subunits include the BRG1 ATPase, which is frequently mutated in tumors from ganglia and to a lesser extent the esophagus, large intestine, lung, and urinary tract. The PBAF-specific BAF180 (also known as PBRM1) and ARID2 subunits are frequently mutated in kidney cancer and to a lesser extent in a variety of tumors from the skin, esophagus, large intestine, cervix, liver, and lung. Interestingly, several of the relatively uncharacterized CHD ATPases were found modestly mutated in a variety of cancers including the stomach, large intestine, cervix, endometrium, lung, and urinary tract.
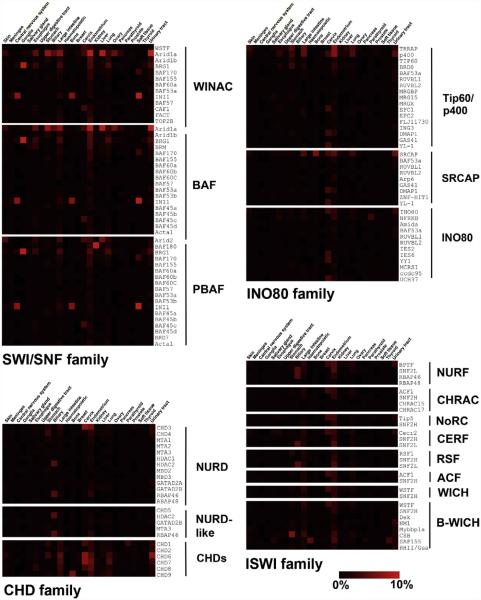
Figure 5.3. Heat map representation of somatic mutation rate for subunits of chromatin remodeling complexes in human cancers.
The COSMIC database was queried using each of the subunits found in the chromatin remodeling complexes depicted in Fig. 5.2 (Forbes et al., 2011). The frequency of somatic mutation is expressed as a percentage of sequenced tumors from each tissue type. Percentages are not corrected for background mutation rates which vary with individual tumors and tumors from tissue types. The heat map range is shown on the lower right and varies from 0% to 10% of sequenced tumors in the COSMIC database.
Table 5.1.
Summary of chromatin remodeling subunits identified with probable or potential driver somatic mutations in human tumors
| Remodeling family |
Subunit | Mutated cancer tissue types | Complexes |
|---|---|---|---|
| SWI/SNF family |
WSTF | Large intestine, cervix, endometrium | WINAC, b-WICH, WICH |
|
| |||
| ARID1A | Skin, esophagus, stomach, biliary tract, large intestine, cervix, endometrium, liver, ovary, urinary tract, lung |
WINAC, BAF | |
|
| |||
| BRG1 | Autonomic ganglia, esophagus, lung, urinary tract, large intestine, endometrium |
WINAC, PBAF, BAF |
|
|
| |||
| ARID2 | Skin, esophagus, large intestine, cervix, endometrium, liver, lung |
PBAF | |
|
| |||
| BAF180 | Endometrium, kidney | PBAF | |
|
| |||
| INO80 family |
SRCAP | Large intestine, cervix, bone, endometrium, lung, urinary tract |
SRCAP |
|
| |||
| CHD family | CHD3 | Cervix, endometrium | NURD |
|
| |||
| CHD4 | Stomach, endometrium | NURD | |
|
| |||
| CHD7 | Large intestine, cervix, endometrium, lung, urinary tract |
CHD7 | |
|
| |||
| CHD8 | Large intestine, cervix, endometrium | CHD8 | |
|
| |||
| ISWI family | Tip5 | Cervix, endometrium | NoRC |
|
| |||
| SAP55 | Hematopoietic and lymphoid tissue, endometrium, thyroid |
b-WICH | |
Subunits of chromatin remodeling complexes whose somatic mutation was identified as a probable or potential driver of human cancer are shown (Kandoth et al., 2013; Tamborero et al., 2013). The tissues in which the gene encoding the subunit was mutated in greater than 5% of the sequenced tumors from the COSMIC database are shown. Known chromatin remodeling complexes that each subunit can assemble into are also listed.
In addition to somatic mutation rates, the abnormal expression of genes in cancers compared to their normal tissues of origin can indicate roles in tumor development. While changes in gene expression are not considered driving events, they can provide necessary changes to cellular physiology that support cancer phenotypes. Toward this end, we surveyed genomewide expression datasets from primary tumors using ONCOMINE to determine the frequency of deregulation for each subunit for chromatin remodeling complexes (Fig. 5.4; Table 5.2) (Rhodes et al., 2004). Consistent with their frequent somatic mutation in tumor samples, SWI/SNF family members are frequently deregulated in expression in tumors, thus underscoring their likely importance to cancer biology. The BRG1 ATPase, BAF155, BAF53A, and INI1 are overexpressed in a variety of tumor types including bladder, liver, ovarian, melanoma, leukemia, and myeloma cancers. In addition to the prominent representation of SWI/SNF subunits, subunits from the ISWI, CHD, and INO80 complexes are also deregulated. Subunits of the INO80 family of chromatin remodeling complexes including BAF53A, RUVBL1, and RUVBL2 subunits are overexpressed in bladder, cervix, myeloma, colon, and ovarian cancers. The NURD subunits MTA1 (but not MTA2 or 3), HDAC1 and 2, and pRBAP48 are modestly deregulated in cervix, pancreatic, leukemia, colon, and esophagus cancers. Compared to the other families of remodeling complexes, the ISWI families are only modestly deregulated in cancers including overexpression in head, cervix, and kidney cancers.
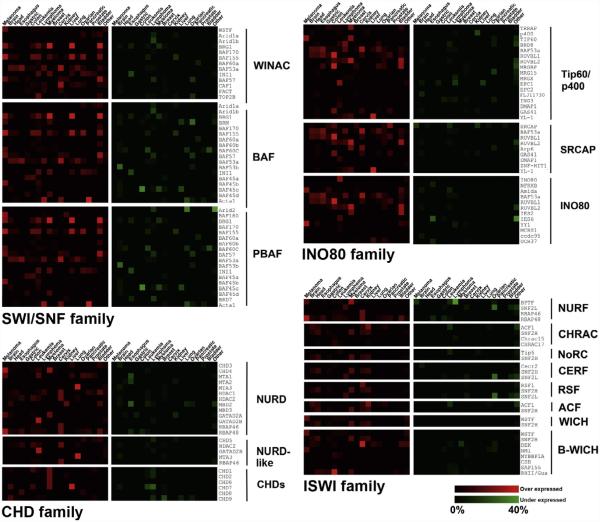
Figure 5.4. Heat map representation of gene expression changes for subunits of chromatin remodeling complexes in human cancers.
The ONCOMINE database was queried using each of the subunits found in the chromatin remodeling complexes depicted in Fig. 5.2 (Rhodes et al., 2004). Data is expressed as a percentage of unique analyses for which transcript levels of the subunit differ by greater than twofold between tumor and control normal tissue (P value≥1.0×10−4). Transcripts for which overexpression was observed in tumor tissue are colored in red, and those where an underexpression is observed are shown in green. The heat map range is shown on the lower right and varies from 0% to 40% of unique analyses found in the ONCOMINE database.
Table 5.2.
Summary of chromatin remodeling subunits identified with frequent deregulated expression in human tumors
| Remodeling family |
Subunit | Overexpressed | Underexpressed | Complexes |
|---|---|---|---|---|
| SWI/SNF family |
CAF1 | Cervix | WINAC | |
|
| ||||
| BAF180 | Melanoma | PBAF | ||
|
| ||||
| Arid2 | Lung | PBAF | ||
|
| ||||
| Acta1 | Lymphoma, pancreatic |
PBAF, BAF | ||
|
| ||||
| BAF45b | Melanoma | PBAF, BAF | ||
|
| ||||
| BAF53b | Brain | PBAF, BAF | ||
|
| ||||
| BAF45c | Colon | PBAF, BAF | ||
|
| ||||
| BAF155 | Melanoma, liver |
WINAC, PBAF, BAF |
||
|
| ||||
| BAF57 | Bladder | WINAC, PBAF, BAF |
||
|
| ||||
| BRG1 | Melanoma, myeloma, liver, bladder |
WINAC, PBAF, BAF |
||
|
| ||||
| BRM | Ovarian | WINAC, PBAF, BAF |
||
|
| ||||
| BAF53a | Cervix | WINAC, PBAF, BAF, Tip60/p400, SRCAP INO80 |
||
|
| ||||
| INO80 family |
SRCAP | Pancreatic | SRCAP | |
|
| ||||
| Amida | Melanoma | INO80 | ||
|
| ||||
| IES2 | Bladder | INO80 | ||
|
| ||||
| IES6 | Esophagus | INO80 | ||
|
| ||||
| INO80 | Leukemia | INO80 | ||
|
| ||||
| YY1 | Melanoma | INO80 | ||
|
| ||||
| EPC1 | Leukemia | Tip60/p400 | ||
|
| ||||
| MRGBP | Colon, bladder |
Tip60/p400 | ||
|
| ||||
| TIP60 | Melanoma, myeloma |
Tip60/p400 | ||
|
| ||||
| TRRAP | Myeloma | Tip60/p400 | ||
|
| ||||
| YL-1 | Liver | Tip60/p400, SRCAP |
||
|
| ||||
| RUVBL2 | Myeloma, bladder |
INO80, Tip60/ p400, SRCAP |
||
|
| ||||
| RUVBL1 | Colon | INO80, Tip60/ p400, SRCAP |
||
|
| ||||
| CHD family | CHD4 | Melanoma, myeloma |
NURD | |
|
| ||||
| MTA3 | Lung | NURD | ||
|
| ||||
| MBD2 | Esophagus | NURD | ||
|
| ||||
| GATAD2B | Leukemia | NURD-Like | ||
|
| ||||
| CHD7 | Liver | Lymphoma | CHD7 | |
|
| ||||
| ISWI family | BPTF | Myeloma | Lymphoma | NURF |
|
| ||||
| DEK | Cervix | b-WICH | ||
|
| ||||
| DDX21 | Colon | b-WICH | ||
|
| ||||
| ACF1 | Kidney | CHRAC, ACF | ||
|
| ||||
| RBAP48 | Melanoma | NURF, NURD | ||
|
| ||||
| SNF2L | Ovarian | NURF, RSF, CERF |
||
Subunits of chromatin remodeling complexes whose expression is deregulated in at least 20% of the unique analyses from each tissue type from Fig. 5.4 are listed. Known chromatin remodeling complexes that each subunit can assemble into are also listed.
The combination of somatic mutation frequency and deregulated expression of the subunits of chromatin remodeling complexes highlights their widespread potential for functional roles in human cancer. Just like characterizing their knockout phenotypes during mouse development, the molecular consequences of mutation or deregulated expression for some of these subunits are complicated by the fact that many are found in multiple protein complexes. In fact, many subunits from the SWI/SNF family, which are frequently mutated or deregulated in expression, are incorporated into several complexes (Tables 5.1 and 5.2). This is less of a problem for the CHD and the INO80 family because many mutated and deregulated subunits are only known to assemble into a single chromatin remodeling complex. However, it must be emphasized that many of these subunits are found in other nuclear complexes. For example, the Tip60 and TRRAP subunits are found in several histone acetyltransferase complexes (Murr, Vaissiere, Sawan, Shukla, & Herceg, 2007).
In support of these genome-wide analyses, independent reports in the literature have accumulated for several decades and have been used to establish correlative (germ line and somatic mutation, deregulated expression, copy number changes), causative (mouse models), and mechanistic (physical and functional connections to cancer relevant pathways) connections between chromatin remodeling complexes and cancers. Using the significance of genome-wide datasets as a backdrop, we summarize this body of literature, highlighting studies contributing mechanistic insight to the roles for chromatin remodeling complexes in pathways of cancer.
5. A REVIEW OF THE LITERATURE ON CHROMATIN REMODELING AND CANCER
5.1. SWI/SNF family of chromatin remodeling complexes
5.1.1 BRG1/BRM
The catalytic subunits of the SWI/SNF family of chromatin remodeling complexes are the BRG1 and BRM ATPases. Targeted studies reported in the literature from a variety of tumor types observed sporadic mutation of either BRG1 or BRM in human primary tumors (Endo et al., 2013; Gunduz et al., 2005; Medina et al., 2004; Rodriguez-Nieto et al., 2011; Valdman et al., 2003). Because many of the mutations found in primary tumors are missense and heterozygous, their effect on the protein function is unknown. A recent report showed that two BRG1 missense mutations found in human cancers resulted in reduced ATPase activity and increased genome instability, suggesting that at least some of these missense mutations could be detrimental to BRG1 function (Dykhuizen et al., 2013). In contrast to tumors, BRG1 and BRM are frequently mutated in cell lines from several tumor sources including lung, prostate, breast, pancreas, and colon (Decristofaro et al., 2001; Medina et al., 2008; Reisman, Sciarrotta, Wang, Funkhouser, & Weissman, 2003; Wong et al., 2000). Many of these mutations are homozygous truncating or nonsense mutations resulting in an inactive protein. The combination of these results suggest that growth of cancer cells in tissue culture, as opposed to the primary tumor, more frequently selects for the loss of BRG1 and BRM activity.
BRG1 has well-characterized roles in regulating several tumor suppressor and oncogene pathways. BRG1 tumor suppressing roles include directly interacting with and stabilizing p53. These interactions likely recruit BRG1 to the p21 promoter to regulate its expression during DNA damage (Naidu, Love, Imbalzano, Grossman, & Androphy, 2009). In addition to p21, BRG1 is essential for the expression of BRCA1, another known p53 regulated gene (Bochar et al., 2000). BRG1 also directly interacts with BRCA1 but is not necessary for its functions during homologous recombination (Hill, de la Serna, Veal, & Imbalzano, 2004). In addition to its role as a coregulator of p53, BRG1 is known to directly interact with RB to regulate cell cycle arrest (Bartlett, Orvis, Rosson, & Weissman, 2011; Dunaief et al., 1994). Subsequent studies showed that BRG1 regulation of E2F2 requires RB and that the recruitment of BRG1 to the E2F2 promoter requires the RB interacting protein, prohibitin (Wang, Zhang, & Faller, 2002). BRG1 also interacts with other cell cycle regulators including LKB1 (where LKB1 is important for BRG1-dependent growth arrest) and MYC (where it is important for its transactivation activity) (Marignani, Kanai, & Carpenter, 2001; Romero et al., 2012). BRG1 can also regulate Cyclin D1 expression through less well-characterized interactions (Rao et al., 2008). In addition to regulating the cell cycle, BRG1 regulates genes important for metastasis. In these functions, BRG1 interacts with the ZEB1 transcription factor to regulate E-cadherin expression and regulates CD44, a metastasis-associated gene, by an unknown mechanism (Sanchez-Tillo et al., 2010; Strobeck et al., 2001).
Mouse models have demonstrated that BRG1, but not BRM, has tumor suppressing activity. The increased tumor incidences observed in heterozygous BRG1 knockout mice demonstrates that BRG1 haploinsufficiency can promote tumor development (Bultman et al., 2000; Bultman et al., 2008). Because the rate of tumor incidence was not increased by a BRM homozygous knockout background demonstrates that BRG1 is more important of the two SWI/SNF ATPases for tumor development. It is important to note that a functional BRG1 allele is retained in BRG1 heterozygous tumors suggesting that while haploinsufficiency of BRG1 can promote tumor development, there is a selection against loss of heterozygosity (LOH) at BRG1 (Bultman et al., 2008).
5.1.2 INI1
The first clear connection between chromatin remodeling complexes and human cancer was observed in tumors from patients with childhood rhabdoid tumors. These tumors frequently arise in soft tissues of children (brain, kidney, and other soft tissues of the body), are aggressive and poorly responsive to therapies, and as a result are frequently lethal (Haas, Palmer, Weinberg, & Beckwith, 1981). In almost all cases, rhabdoid tumors acquire a somatic mutation in one INI1 allele, followed by a LOH event that inactivates a second functional INI1 allele. There is little other evidence of genome instability or mutation in these tumors (Lee et al., 2012). In more rare circumstances, familial cases can occur as the result of inheriting a defective INI1 allele and acquiring a LOH event at the functional INI1 allele (Biegel et al., 1999, 2000; Sevenet et al., 1999).
The use of mouse models has presented strong evidence that INI1 is a tumor suppressor. As in humans, mice heterozygous for INI1 develop sarcomas closely resembling the human malignant rhabdoid tumors (Klochendler-Yeivin et al., 2000; Roberts, Galusha, McMenamin, Fletcher, & Orkin, 2000). In mice, as in humans, tumors arise by inactivating the second functional INI1 allele through an LOH event (Klochendler-Yeivin et al., 2000). When allele inversion is used to create biallelic inactivation of INI1 in a mosaic of cells, the result is a fully penetrant cancer phenotype inducing rapid onset lymphomas and rhabdoid tumors (Roberts, Leroux, Fleming, & Orkin, 2002). Studies using these mouse models have identified several genes that influence INI1 tumor incidence. Because tumor onset depends on BRG1, aberrant remodeling activity from one or more SWI/SNF family complexes appears to be necessary to promote tumor development. Tumor onset also requires the Cyclin D1 oncogene, a positive regulator of the G1/S-phase transition, suggesting that tumor development promotes a defective G1/S-phase check point (Tsikitis, Zhang, Edelman, Zagzag, & Kalpana, 2005). For less clear reasons, tumor onset requires the polycomb subunit EZH2 (Wilson et al., 2010). The requirement of EZH2 for tumor onset is likely because polycomb promotes protumor gene expression in the absence of INI1. Polycomb and SWI/SNF complexes are known to oppose each other during gene expression in a number of model systems (Francis, Saurin, Shao, & Kingston, 2001; Kennison & Tamkun, 1988; Kia, Gorski, Giannakopoulos, & Verrijzer, 2008). In addition, the cell cycle regulator p53, but not p16Ink4a or RB, inhibits tumor onset suggesting that INI1 loss of function (LOF) triggers p53-dependent cell cycle checkpoints (Isakoff et al., 2005).
In addition to in vivo experiments with the mouse, further mechanistic insight into INI1 function has been obtained using tissue culture. The knockout of INI1 in wild-type MEFs results in G2 cell cycle arrest and apoptosis. Correlated with these findings, INI1 knockout regulates the E2F target gene p16Ink4a and upregulates the expression of Cyclin D1, p53, and p21 (Isakoff et al., 2005). Initial work reintroducing INI1 in knockout tumor cells showed that it regulates mitotic checkpoints and chromosome stability via the p16-Cyclin D1/CDK4-pRB-E2F pathway (Vries et al., 2005). Subsequent work reintroducing INI1 into knockout MEFs resulted in increased p21 and p16 expression and G1 arrest. The study showed that INI1 directly interacts with the p21 and p16INK4a promoters, and the study showed that the upregulation of these genes is essential for G1 arrest (Kuwahara, Charboneau, Knudsen, & Weissman, 2010). However, some of these cell cycle defects could be a consequence of INI1 functioning as an essential coregulator of MYC (Cheng et al., 1999). In addition to the cell cycle regulators, INI1 directly regulates the expression of the proapoptotic factor NOXA, possibly contributing to the apoptosis observed with INI1 knockout (Kuwahara, Wei, Durand, & Weissman, 2013).
5.1.3 ARID1A
The SWI/SNF subunit ARID1A (also known as BAF250A) is frequently mutated in several types of human cancer. In some tumor types, AIRID1A is the most frequently mutated gene. For example, AIRID1A mutations approach 50% of the sequenced ovarian clear cell tumors and 30% of sequenced ovarian endometrioid cancers (Jones et al., 2010; Wiegand et al., 2010). In contrast to BRG1 mutations that are frequently missense mutations, many ARID1A mutations may result in LOF gene products as they are nonsense or truncating mutations (Jones et al., 2012). When an ARID1A transgene is used to restore function in ovarian cancer cell lines with ARID1A LOF, the transgene expression suppresses cell line growth both in culture and in mice. In follow-up experiments, this same group showed that ARID1A regulates the expression of the p53 target genes CDKN1A (p21) and SMAD3, and at least part of the suppression of tumor growth by ARID1A involves p21 upregulation. The function of ARID1A in these pathways could be through a direct interaction with p53, an interaction that recruits a SWI/SNF complex to p53 target gene promoters (Guan, Wang, & Shih Ie, 2011). Similar effects were observed in gastric cancer cell lines where expression of ARID1A in cell lines with ARID1A LOF resulted in reduced growth (Zang et al., 2012). In an earlier study, knockdown of ARID1A, but not ARID1B, in immortalized preosteoblast cell line resulted in reduced differentiation and cell cycle arrest. The cell cycle arrest with ARID1A knockdown occurred in the absence of increased p21 expression and downregulation of cyclins (Nagl et al., 2005). These results demonstrate that ARID1A containing complexes are functionally distinct from ARID1B containing complexes with regard to regulation of the cell cycle. This difference could be through differential interactions between ARID1A and ARID1B containing SWI/SNF complexes for E2F transcription factors which are key regulators of the cell cycle (Nagl, Wang, Patsialou, Van Scoy, & Moran, 2007). How mutations in ARID1A can promote the development of tumors de novo is largely unknown.
Work using mouse models has not yet shown ARID1A to be involved in cancer. It is known that ARID1A is important for differentiation of ESC (Gao et al., 2008). An ENU mutagenesis screen has identified a point mutant in ARID1A which prevents the SWI/SNF complex from binding to DNA; this mutation is associated with neural tube closure, heart defects, and extra embryonic vasculature that likely occur through an inability to regulate gene expression (Chandler et al., 2013).
5.1.4 BAF180
At a reduced frequency compared to ARID1A, truncating mutations in BAF180 have been identified in a high percentage of renal cell carcinomas suggesting that it functions as a tumor suppressor in this cancer type (Cancer Genome Atlas Research Network, 2013). Supporting its role as a tumor suppressor, the loss of expression of the BAF180 protein has been confirmed to be a poor prognosis event in renal cell cancer (Pawlowski et al., 2013). LOH events appear to be specific to mutations as promoter hypermethylation of BAF180 does not occur (Ibragimova, Maradeo, Dulaimi, & Cairns, 2013). Like ARID1A, BAF180 is an essential regulator of the p53 target gene p21, likely through direct interactions with p53 and functioning to inhibit the cell cycle and promote senescence (Burrows, Smogorzewska, & Elledge, 2010; Xia et al., 2008). Possibly as a result of its interactions with p53, BAF180 has also been shown to regulate genome stability through functions in regulating DNA damage repair (Niimi, Chambers, Downs, & Lehmann, 2012).
5.1.5 BAF53/BAF57
Both BAF53 and BAF57 are frequently deregulated in several cancer cell types with possible significance to cancer biology. BAF53 is a common subunit to several chromatin remodeling complexes from both the SWI/SNF and INO80 families. In addition to its functions in these complexes, it copurifies with MYC in a complex containing RUVBL1, RUVBL2, and actin. In this same study, the overexpression of BAF53 with deletions in several functional domains has a dominant negative effect on MYC transformation suggesting that these domains are important for this function (Park, Wood, & Cole, 2002). BAF53 is also necessary for the expression and transformation capability of the human papilloma virus E6 and E7 oncogenes. In this function, BAF53 is thought to regulate a unique chromatin structure established at the E6/E7 oncogene integration site and to consequently regulate their expression (Lee, Lee, Kwon, & Kwon, 2011).
Like BAF53, BAF57 is found in each of the SWI/SNF chromatin remodeling complexes. Knockdown of BAF57 in Hela cells results in G2/M arrest, reduced colony formation, and impaired growth in soft agar. Genome-wide microarray experiments in these cells showed that BAF57 knockdown results in the deregulation of genes (e.g., CCNB1, CENPE, CENPF, and MYC) involved in regulating the G2/M transition (Hah et al., 2010). In prostate cancer cell lines, BAF57 is recruited to androgen receptor (AR)-binding sites in a ligand-dependent manner and regulates many AR gene targets (Link et al., 2005). Through its interactions with AR, BAF57 is thought to regulate key aspects of prostate cancer cell biology. In prostate cancer cell lines, BAF57 overexpression increases cell motility in an alpha 2 integrin-dependent manner and is required for AR-dependent proliferation (Balasubramaniam et al., 2013). The interaction surface on the AR that is important for BAF57 binding was mapped to the DNA-binding domain-hinge region (Link et al., 2008). The function of BAF57 as a coregulator of estrogen receptor alpha (ERα) in breast cancer parallels closely those of the AR in prostate cancer. Like AR, BAF57 directly interacts with the ERα receptor hinge region, and this interaction is stimulated by ligand (Garcia-Pedrero, Kiskinis, Parker, & Belandia, 2006). Also as in AR, BAF57 is required for ERα regulated transcription. Interestingly, the ectopic expression of BAF57 truncation mutants cloned from human breast cancer cells abnormally activates both AR and ERα reporter constructs (Villaronga et al., 2011). These results suggest that BAF57 truncation mutations found in human cancers lead to artificially elevated ER- and AR-regulated gene expression and provide a plausible molecular mechanism for their functions in regulating prostate and breast cancer cell biology.
5.2. INO80 family of chromatin remodeling complexes
5.2.1 TRAAP/p400
TRRAP and p400 are essential subunits of the Tip60/p400 chromatin remodeling complex (Cai et al., 2003). Recently, TRRAP was discovered in an RNAi screen as an essential factor for brain tumor-initiating cellular differentiation (Wurdak et al., 2010). TRRAP knockdown in both tissue culture and mouse tumor models resulted in increased tumor cell differentiation, reduced cell proliferation, and increased sensitivity to apoptotic stimuli. TRRAP has well-documented functions in several pathways relevant to human cancer. TRRAP was originally shown to interact with the MYC and E2F oncogenes, and likely through these direct interactions TRRAP is essential for MYC and E1A transformation (Deleu, Shellard, Alevizopoulos, Amati, & Land, 2001; McMahon, Van Buskirk, Dugan, Copeland, & Cole, 1998). Later, it was discovered that the TRRAP-containing complex essential for E1A transformation contains p400, and that the p400 subunit is also necessary for the E1A transformation (Fuchs et al., 2001). Functional connections between TRRAP and MYC were later reinforced using shRNA screens which again identified TRRAP as an essential factor for MYC transformation activity (Toyoshima et al., 2012). In addition to its functions as a coregulator of MYC and E1A, TRRAP also interacts with acetylated p53 and is essential for p53 to regulate gene expression (Ard et al., 2002; Barlev et al., 2001). In a somewhat surprising role, TRRAP influences β-catenin stability by regulating β-catenin interactions with SKP1/SCF, an E2/E3 ubiquitin ligase complex important for the degradation of β-catenin (Finkbeiner, Sawan, Ouzounova, Murr, & Herceg, 2008). TRRAP regulated stability of β-catenin in turn influences canonical Wnt signaling, a pathway frequently deregulated in human cancers (Clevers, 2006; Polakis, 2000).
In addition to its role as a regulator of transcription, the Tip60/p400 complex has important functions in repairing DNA damage, and through these functions the Tip60/p400 complex could contribute to cancer (Xu & Price, 2011). Defects in DNA damage repair pathways are known to contribute to the progression of cancer by promoting somatic mutations and genome instability (Luijsterburg & van Attikum, 2011). The TRRAP-containing Tip60/p400 complex is recruited to DNA breaks in mammalian cells; once recruited, the Tip60/p400 complex acetylates histones at sites of DNA damage (Ikura et al., 2000). p400 then remodels chromatin by loosening the chromatin structure and promoting access for repair factors like 53BP1, RAD51, and BRCA1 (Murr et al., 2006; Xu et al., 2010).
5.2.2 INO80
While none of the INO80 remodeling complex subunits are significantly mutated in human cancers, they do have functions in telomere stability and DNA damage repair pathways. Through its interactions with the YY1 transcription factor, INO80 has essential functions in homologous recombination repair pathways (Wu et al., 2007). At sites of DNA breaks, INO80 is important for the recruitment of 53BP1 and the 5′-3′ resection of the DNA double-strand break ends (Gospodinov et al., 2011). At least some of these functions are believed to be through the ability of the INO80 complex to regulate gene expression, because the expression of DNA damage repair genes, including RAD54B and XRCC3, are INO80 dependent (Park, Hur, & Kwon, 2010).
Conditional knockout of INO80 in mouse embryonic fibroblasts inhibits cellular proliferation and results in p21-dependent cellular senescence. The decrease in proliferation was not rescued by expressing large T antigen suggesting that its regulation of growth is not p53 or RB dependent. Knockout cells had increased single-stranded DNA, phosphorylation of CHK1, and defects in telomere structure. INO80 is important for the generation of single-stranded DNA at the telomeres resulting in defects in homology driven DNA repair. INO80 heterozygous knockout mice are not tumor prone, but they do have an increased incidence of soft tissue sarcomas compared to INO80 wild-type mice on a p53 knockout background (Min et al., 2013).
5.2.3 RUVBL1/RUVBL2
The INO80 family members RUVBL1 and RUVBL2 are overexpressed in cancers from many different tissue types (see references in Grigoletto, Lestienne, & Rosenbaum, 2011). Each of the RUVBL proteins directly interacts with the MYC, β-catenin, and E2F1 oncogenes (Bauer et al., 2000; Dugan, Wood, & Cole, 2002; Wood, McMahon, & Cole, 2000). These physical interactions could be significant to the observation that RUVBL1 is necessary for transformation by E1A, β-catenin, and MYC (Dugan et al., 2002; Feng, Lee, & Fearon, 2003; Wood et al., 2000). As a consequence of these interactions, RUVBL proteins regulate the expression of TERT, p21, and KAI-1, which could play a significant role in cancer cell growth. Inducible knockdown of RUVBL2 in tissue culture and xenograft animal tumor models shows that RUVBL2 is essential for growth of several cancer cell types (Menard et al., 2010; Schlabach et al., 2008). The mechanism of these effects could be in part through functions in INO80 family chromatin remodeling complexes; however, the cellular consequences of a RUVBL2 knockdown are likely to be pleotropic due to its widespread use in many signal transduction pathways including mTOR, SMG-1, ATM, ATR, DNA-PKcs to regulate translation, energy metabolism, mRNA decay, and the DNA damage response (Rosenbaum et al., 2013).
5.3. CHD family of chromatin remodeling complexes
5.3.1 CHD3/CHD4
CHD3 and CHD4 are the essential ATPase subunits of the NURD complex. The CHD3 and CHD4 genes themselves are not frequently mutated in human cancers, with the exception that some increased incidence was observed in cancers of the cervix, stomach, and endometrium (Fig. 5.3; Table 5.1). The CHD3/4 remodeling subunits directly interact with NAB2, a corepressor of the EGR family of transcription factors (Srinivasan, Mager, Ward, Mayer, & Svaren, 2006). In prostate cancer, in part by regulating the expression of IGF2, TGFB1, and PDGFA (each of which have roles in tumor progression), EGR1 regulates cell growth, differentiation, and apoptosis (Gitenay & Baron, 2009). In addition to its interactions with NAB2, CHD4 has been shown to interact with ZIP, a transcriptional repressor of genes involved in cell proliferation, survival, and migration (Li, Zhang, et al., 2009).
The CHD4 ATPase, as a component of the NURD remodeling complex, has been shown by several groups to be important for DNA damage repair. Knockdown experiments have shown that CHD4 is essential for the repair of breaks and the survival of the cell after DNA damage (Smeenk et al., 2010). The CHD4 containing NURD complex is recruited to sites of DNA damage by poly ADP-ribose, and once recruited CHD4 is phosphorylated by the ATM kinase. As part of this process, the authors have shown that NURD is important for the deacetylation of p53 to control the G1/S checkpoint through regulated expression of p21, likely through its HDAC1 and HDAC2 subunits (Polo, Kaidi, Baskcomb, Galanty, & Jackson, 2010). At the sites of DNA damage, CHD4 promotes ubiquitination of the BRCA1 complex, possibly through direct interactions with BRCA1, to promote the assembly of the BRCA complex (Larsen et al., 2010; Pan et al., 2012). The inability to assemble functional repair complexes results in elevated ionizing radiation-induced DNA breakage, reduced efficiency of DNA repair, G2/M arrest, and decreased survival.
5.3.2 MTA1
MTA1 is widely reported to be overexpressed in a variety of human cancers and in metastatic tumor cells (especially breast cancer) (see references in Li, Pakala, Nair, Eswaran, & Kumar, 2012). In contrast to its frequent over-expression, there is little evidence that it is significantly mutated in human cancers. Transformation assays have shown that MTA1 is required for MYC-induced transformation (Zhang et al., 2005). Using similar assays, MTA1 overexpression promotes transformation by downregulating Gi12 alpha, a negative regulator of the G protein activation cycle (Ohshiro et al., 2010). The repression of Gi12 alpha results in constitutively active G proteins and as a result, a hyper-stimulation of the mitogenic and procell invasion Ras–Raf pathway. In addition to its roles in promoting MAPK signaling, MTA1 is also a positive regulator of the canonical Wnt signaling pathway (Kumar, Balasenthil, Manavathi, Rayala & Pakala, 2010; Kumar, Balasenthil, Pakala, et al., 2010). MTA1 stimulates the expression of the Wnt1 gene, a ligand of the canonical Wnt signaling pathway, through the repression of the Six3 repressor. The importance of MTA1 for MYC and MAPK-dependent transformation and for Wnt signaling supports its proposed function as a tumor promoter.
Beyond its functions in promoting protumor pathways, MTA1 over-expression also represses tumor suppressor pathways. MTA1 is recruited to the ERα, BRAC1, p21, HIC1, and p19ARF promoters to repress gene expression as a subunit of the NURD complex (Fu et al., 2012; Li et al., 2010, 2011; Marzook et al., 2012; Molli, Singh, Lee, & Kumar, 2008; Van Rechem et al., 2010). Significant mechanistic insight into how MTA1 regulates these promoters was reported recently. MTA1 is methylated by the G9a methyltransferase and demethylated by the LSD1 demethylase (Nair, Li, & Kumar, 2013). The methylation state of MTA1 determines whether it will recruit repressive NURD (methylated MTA1) or activating NURF chromatin remodeling activities (demethylated MTA1). In most cases, MTA1 is recruited to promoters through interactions with transcription factors. For example, MTA1 interacts with the BCL11B repressor in T cell leukemia cells and ERα in breast cancer cells to regulate gene expression (Cismasiu et al., 2005; Mazumdar et al., 2001). Genes important to metastasis are also known targets of MTA1 regulatory activity. MTA1, recruited by c/EBPa to the RNF144A promoter and by TWIST to the E-cadherin promoter, silences the genes and in doing so promotes metastasis (Fu et al., 2011; Marzook et al., 2012). Repression of BRCA1 by MTA1 promotes anchorage-independent invasive growth and metastasis (Molli et al., 2008). The ability of MTA1 to directly interact with p53 provides a mechanism for regulating the p53 target genes p21 and HIC1 (Luo, Su, Chen, Shiloh, & Gu, 2000; Moon, Cheon, & Lee, 2007).
Beyond acting as a coregulator of transcription, there is evidence for MTA1 using posttranscription mechanisms to promote tumor progression. MTA1 overexpression stabilizes p53 independently of the NURD complex by acting as a competing substrate for the COP1 E3 ligase. The stabilization of p53 by MTA1 overexpression promotes the repair of DNA damage in cancer cells (Li, Divijendra Natha Reddy, et al., 2009; Li, Ohshiro, et al., 2009). By recruiting HDAC1, MTA1 stabilizes HIF1a. HIF1a deacetylation by HDAC1 stabilizes the transcription factor and promotes hypoxia-stimulated gene expression, which includes proangiogenesis genes (Moon et al., 2006; Yoo, Kong, & Lee, 2006).
The mouse models used to investigate the functions of MTA1 in cancer have supported much of the work performed in tissue culture. Overexpression of MTA1 in mouse breast epithelial cells promotes ductal hyperbranching, hyperplasic nodules, and eventually adenocarcinoma development suggesting that it has a causal role in the development of breast cancer (Bagheri-Yarmand, Talukder, Wang, Vadlamudi, & Kumar, 2004). The increase in cell proliferation with MTA1 overexpression in breast epithelial cells results in stimulation of the Wnt pathway, a known positive regulator of the cell cycle (Kumar, Balasenthil, Pakala, et al., 2010). Overexpression of MTA1 using a transgene results in an increase in B-cell lymphomas through the downregulation of p27 and the upregulation of Bcl2 and Cyclin D1 (Bagheri-Yarmand et al., 2007). These results have relevance to humans as MTA1 is frequently overexpressed in human B-cell lymphomas.
5.3.3 HDAC1/HDAC2
Like the MTAs, HDAC1 and HDAC2 are frequently overexpressed but rarely mutated in human cancers. HDAC1 and HDAC2 are known to function as regulators of the cell cycle, angiogenesis, apoptosis, differentiation, and metastasis (Hagelkruys, Sawicka, Rennmayr, & Seiser, 2011). Understanding the context through which HDAC1 and HDAC2 regulate these pathways is complicated by the fact that they are found in many complexes, only a few of which have chromatin remodeling activities (NURD, NURD-like, NoRC). Relevant to its roles in chromatin remodeling complexes, HDAC1 and HDAC2 repress the p21 promoter, likely in context with the NURD complex to regulate the cell cycle (Zupkovitz et al., 2010). Consistent with these observations, knockdown of HDAC1 and HDAC2 in several human cancer cell lines results in upregulation of p21 followed by cell cycle arrest both in tissue culture and in xenograft tumor models (see references in Hagelkruys et al., 2011). In addition to its roles in regulating p21 expression, HDAC1 and HDAC2 play prominent roles in promoting the activity of oncogenic-DNA-binding fusion proteins in hematologic malignancies. In many cases, these fusion proteins bind to promoters of genes to recruit HDAC1 and HDAC2 and repress genes with tumor suppressor activity. NURD directly interacts with the PML–RARα fusion product in human acute promyelocytic leukemias (Morey et al., 2008). PML–RARα recruits NURD with its HDAC activities to the tumor suppressor gene RARB2, promoting its repression.
5.3.4 MBD2
MBD2 is infrequently mutated in human cancers and is not significantly deregulated in expression. It does have important functions in the development of cancers through its ability to recruit the coregulatory activities of the NURD complex to the hypermethylated promoters of tumor suppressor genes. This mechanism is well characterized in several cancer types including colon, lung, prostate, and renal cancers and with a variety of genes including CDKN2A (p21), BubR1, beta-defensin-2, 14-3-3 sigma, and BTG3 tumor suppressors (Magdinier & Wolffe, 2001; Majid et al., 2009; Park et al., 2007; Pulukuri & Rao, 2006; Shukeir, Pakneshan, Chen, Szyf, & Rabbani, 2006; Zhuravel, Shestakova, Glushko, Soldatkina, & Pogrebnoy, 2008). These data suggest the cooperation between DNMTs, MBD2, and HDACs represses tumor suppressor genes.
5.3.5 pRBAP48
pRBAP46/48 proteins are members of a family of WD repeat proteins, proteins which have a highly conserved propeller-like structure. The NURD subunit pRBAP48 is frequently overexpressed in a variety of human cancers (Fig. 5.4). As with several of the other subunits discussed in this review, it is difficult to understand the consequences of its overexpression because pRBAP48 is a subunit of several complexes including histone chaperones, polycomb complexes, the RB family of complexes, and chromatin remodeling complexes like NURD and NURF (Migliori, Mapelli, & Guccione, 2012). When RBAP46 is overexpressed in breast cancer cell lines both colony formation in vitro and tumor growth in mice is suppressed (Li, Guan, & Wang, 2003). In addition to these studies, pRBAP48 is a key regulator of the transformation ability of the HPV16 transcription factor in cervical cancer (Kong et al., 2007). The nature of the increased transformation ability is likely through deregulation of the RB and p53 complexes and not through chromatin remodeling.
5.3.6 CHD5
The CHD5 gene is found on chromosome 1q36. This chromosome interval is the most common deletion found in a variety of human cancers, most prominently cancers of the CNS, hematopoietic system, and epithelium (thyroid, colon, cervix, and breast). In an elegant series of chromosome engineering experiments, the CHD5 gene located in this interval was identified as a tumor suppressor (Bagchi et al., 2007). However, in addition to CHD5, several other laboratories have identified additional tumor suppressors in the 1q36 chromosome interval (Henrich, Schwab, & Westermann, 2012). The presence of several tumor suppressors at 1q36 means that the deletion of this interval can result in the loss of several divergent tumor suppressor pathways. Mechanistically, CHD5 tumor suppressor activity was found to be a positive regulator of the p19ARF/p53 pathway (Bagchi et al., 2007). Point mutations in the PHD finger show that the ability to bind unmodified histone H3 is essential for tumor suppressing functions of CHD5 (Paul et al., 2013).
Reduced CHD5 tumor suppressor activities usually occurs by heterozygous mutation of CHD5 or through the repression of its transcription. Rarely is a homozygous deletion of CHD5 found in human cancers. A common method of suppressing CHD5 expression is through promoter CpG hypermethylation (Qu, Dang, & Hou, 2013; Wang et al., 2011; Zhao et al., 2012). Less common methods include the overexpression of microRNA-211 that targets the CHD5 transcript (Cai et al., 2012). CHD5 is predominantly expressed in the brain where it assembles into a NURD-like complex with HDAC2, MTA3, GATAD2B, and RBAP46 and has important functions as a positive and negative regulator of gene expression in neurons (Potts et al., 2011).
5.3.7 CHD1/CHD2/CHD7
Compared with CHD3, CHD4, and CHD5, little is known about the functions of the other CHD ATPases in cancer. CHD1 is frequently mutated in a rare subset of EGR fusion negative prostate cancers (Liu et al., 2012). The selectivity for CHD1 mutation in this subset of prostate cancers could be due to its role as an essential cofactor for AR receptor transcription activity (Burkhardt et al., 2013). AR-stimulated transcription is essential for the expression of EGR, that in turn promotes genome instability and gene fusions to the region. Consequences of CHD1 deletion in prostate cancer cell lines have been investigated and it was shown to promote cell invasiveness (Huang et al., 2012). Mice heterozygous for the hypermorphic allele of CHD2 have defects in hematopoietic stem cell differentiation, accumulation of DNA damage, and increased incidences of lymphomas (Nagarajan et al., 2009). Beyond that, little is known of their molecular mechanisms in cancers. CHD7 was discovered to be significantly mutated in lung tumors from heavy smokers (Pleasance et al., 2010). In these tumors, CHD7 is mutated by either fusions or partial duplications. The significance of these mutations to lung cancers is unknown.
Mouse mutants of CHD7 are embryonic lethal at E10.5 dpc and heterozygous mice have phenotypes similar to humans with CHARGE syndrome (Adams et al., 2007). No cancer phenotypes have been described for these mice.
5.4. ISWI family of chromatin remodeling complexes
5.4.1 SNF2H/SNF2L
The catalytic subunits of the ISWI family of chromatin remodeling complexes are the SNF2H and SNF2L ATPases. Two reports document that SNF2L expression, in contrast to SNF2H, is important for the growth of human cancer cell lines. In these studies, SNF2L was found to be expressed in a wide variety of normal tissues, tumors, and cancer cell lines. Knockdown of SNF2L, but not SNF2H, in several cancer cell lines resulted in reduced cell proliferation, arrest in G2/M, increased DNA damage and activation of DNA damage repair pathways (phosphorylation of CHK1/2, ATM, and p53), and apoptosis (Ye et al., 2009). Follow-up studies identified SNF2LT as a splice variant of SNF2L that lacks its HAND–SANT–SLIDE domains. In these follow-up studies, they observed that knockdown of SNF2LT or the full-length SNF2L gene resulted in similar cancer cell-specific phenotypes (Ye et al., 2012). Similar knockdown studies have shown that SNF2L is an essential regulator of the Wnt signaling pathway (Eckey et al., 2012). In these studies, in contradiction to previous SNF2L studies, SNF2L depletion results in increased proliferation and chemotaxis. This study used genome-wide gene expression profiling to discover that many targets of the Wnt signaling pathway are upregulated with SNF2L knockdown.
Few studies have specifically investigated the role of SNF2H in cancer cell biology. Knockdown studies in several cancer cell lines show that it is not essential for cell proliferation (Ye et al., 2009). SNF2H is a component of several chromatin remodeling complexes with important functions in DNA damage repair, and as such it has been reported to be present at sites of DNA damage (Poot, Bozhenok, van den Berg, Hawkes, & Varga-Weisz, 2005). One study found that the NAD-dependent deacetylase SIRT6 is required for the recruitment of SNF2H to sites of DNA damage (Toiber et al., 2013). Through the use of an SIRT6 knockdown, inefficient DNA damage repair was shown to occur when these factors are not recruited to sites of DNA damage. At sites of DNA damage, SIRT6 deacetylates histone H3K56ac and recruits SNF2H, and the combination of these two events remodels nucleosomes to loosen chromatin structure. The remodeling of chromatin promotes the binding of 53BP1, BRCA1, and RPA which are necessary for DNA repair.
5.4.2 BPTF
BPTF is the largest subunit of the NURF complex. BPTF resides on 17q and is frequently duplicated in several cancer types, with the greatest frequency in neuroblastoma (Bown et al., 1999). A nonreciprocal translocation of BPTF on 17q was characterized in a human lung cell line following continuous culturing (Buganim et al., 2010). This translocation resulted in increased BPTF expression and correlated with increased cell proliferation. Knockdown of BPTF in these cells reduced proliferation. Consistent with the frequent duplication of 17q in human tumors, the BPTF gene was found to be amplified in various human tumors, especially neuroblastomas and lung cancers and many human cancer cell lines. Whether BPTF duplication provides an advantage to cancer cells, or is just a benign consequence of the 17q duplication, needs to be specifically addressed.
5.4.3 RSF1
RSF1, located on chromosome 11q13.5, is also frequently duplicated in human tumors. Several studies have documented that RSF1 is overexpressed in human cancers. In oral squamous cell cancers, RSF1 was found to be overexpressed relative to normal tissue, which correlates with decreased survival (Fang et al., 2011). Knockdown of RSF1 in these cancer cells results in decreased proliferation and increased cell death. In addition to oral cancer, the duplication of this region is also associated with paclitaxel resistance in ovarian cancers (Choi et al., 2009). A shRNA knockdown screen showed that the RSF1 gene located in the duplicated region is essential for paclitaxel resistance. The knockdown of either subunit of the RSF complex, RSF1 or the ATPase SNF2H, results in increased sensitivity to paclitaxel. Interestingly, the expression of a RSF1 mutant with SNF2H-binding capacity, but not remodeling activity, resulted in the same sensitivity to paclitaxel. These results suggest that the loss of RSF remodeling activity, rather than the redistribution of SNF2H to other remodeling complexes, is what causes sensitivity to paclitaxel. Candidate pathways involved in RSF mediated paclitaxel resistance include NF-kB, ERK, Akt, and EGR1. How RSF1 regulates these pathways is unknown. Pull down and mass spectrometry experiments from an ovarian carcinoma cell line showed that RSF1 physically interacts with Cyclin E1, an important G1/S-phase regulator (Sheu et al., 2013). Overexpression of both RSF1 and Cyclin E1 promotes cellular proliferation but only in a p53 null background. Ectopic expression of a dominant negative truncation of RSF1 in mouse xenograft tumor models results in reduced cancer cell proliferation and tumor size. In combination, these results suggest that a RSF1–Cyclin E1 interaction promotes p53 null ovarian tumors. Similar RSF1 overexpression studies in nontransformed ovarian cells results in increased DNA damage, activation of DNA damage repair pathways, and growth arrest (Sheu et al., 2010). The growth arrest accompanies upregulation of p53 and p21 and depends on an active p53 allele. shRNA knockdown experiments showed that the increase in DNA damage observed with RSF1 overexpression requires SNF2H suggesting that it is dependent on the formation of the RSF complex. In lung cancers, RSF1 is also frequently overexpressed, and knockdown of RSF1 in lung cancer cell lines resulted in reduced proliferation and increased apoptosis [222]. In addition to defects in proliferation and apoptosis, there was an accompanying decrease in Cyclin D1 and phospho-ERK levels in these cells suggesting that RSF1 overexpression promotes G1/S-phase transition and mitogenic ERK signaling.
6. THERAPEUTIC POTENTIAL OF CHROMATIN REMODELING COMPLEXES IN HUMAN CANCER
Targeting epigenetic regulators for the treatment of human cancers has proved successful for histone deacetylases and DNA methyltransferases. In each case, the therapeutics developed are approved for difficult to treat cancers providing valuable assets to oncologists (Arrowsmith, Bountra, Fish, Lee, & Schapira, 2012; Dawson & Kouzarides, 2012). Examples include: vorinostat and romidepsin, FDA-approved HDAC inhibitors used to treat cutaneous T cell lymphoma; and azacitidine and decitabine, FDA-approved DNMT inhibitors for the treatment of myelodysplastic syndromes (Dawson, Kouzarides, & Huntly, 2012; Nebbioso, Carafa, Benedetti, & Altucci, 2012). Next generation, more specific HDAC and DNA methyltransferase inhibitors are currently being developed to improve on these successes. Because of these successes, many other small molecules are actively being pursued which target posttranslational modifications on chromatin for therapeutic benefit. These therapies primarily target a large number of histone modifying enzymes and small RNA regulatory pathways. Histone modifying enzymes currently being targeted include deacetylases, acetyltransferases, and demethylases. In some cases, small molecules targeting these regulators have made it into Phase II clinical trials (Arrowsmith et al., 2012; Dawson et al., 2012; Nebbioso et al., 2012; Plass et al., 2013). These molecules are tested alone and in combination chemotherapy and include HDAC inhibitors (entinostat, mocetinostat, panobinostat, bellinostat, valproic acid, givinostat, CHR-3996, CHR-2845, and SB939), the KDM1A demethylase inhibitor tranylcypromine, and the HAT inhbitior curcumin (Arrowsmith et al., 2012; Dawson et al., 2012; Nebbioso et al., 2012; Plass et al., 2013). Phase II trials revealed promising results for mocetinostat and bellinostat. The considerable efforts invested into developing small molecules targeting these mechanisms highlights their widely perceived therapeutic potential. This potential can also be realized by developing small regulators to chromatin remodeling complexes, another major class of chromatin regulator.
The discovery that chromatin remodeling complexes regulate gene expression important for cancer progression has stimulated interest in screening them for small molecule regulators. Using completely different approaches, two successful screens have been performed for the BRG1 and RUVBL1 ATPases (Dykhuizen, Carmody, Tolliday, Crabtree, & Palmer, 2012; Elkaim et al., 2012). To discover small molecule regulators of BRG1, a live cell screen was performed by measuring the expression of Bmi, a known target of BRG1 in embryonic stem cells, by high-throughput qRT-PCR. Follow-up assays were performed using embryonic stem cell lines with a knock-in Bmi-luciferase reporter allele. Several compounds (which were not described) were discovered with this approach and are being investigated further. In contrast to the live cell approach used for BRG1, a structure-based biochemical screen was performed to discover small molecule regulators for the RUVBL1 ATPase. To identify inhibitors of the RUVBL1 ATPase activity, the authors used its known structure to model molecules from several libraries to the ATP-binding site. Promising compounds that could bind the ATP-binding site were assayed for the ability to inhibit a DNA-dependent ATPase activity for recombinant RUVBL1. This approach yielded several compounds, some of which inhibited the proliferation of cancer cells.
In addition to discovering small molecules targeting the ATPase activity as was performed for RUVBL1, resources can be invested to discover molecules to inhibit key protein–protein interactions essential for the function of chromatin remodeling complexes. The inhibition of the BAF57–AR interaction is a successful example of this strategy. BAF57 is known to physically interact with AR, and is required for AR binding to chromatin and AR-regulated gene expression in prostate cancer cells. The ectopic expression of a peptide mimic to the sequence on BAF57 that interacts with AR resulted in inhibited AR binding to chromatin, reduced AR-stimulated transcription, and inhibited AR-dependent prostate cancer cell proliferation (Link et al., 2008). These results are consistent with the possibility that the peptide is inhibiting the BAF57–AR interaction surface and preventing BAF57-dependent AR binding and transactivation. Additional means to inhibit chromatin remodeling complexes with this approach can also include targeting essential subunit interactions within a remodeling complex. While this approach benefits from the focus of a structure-based design, it will suffer from the difficulty of delivering peptides to intracellular targets in a patient’s tumor.
Another strategy to therapeutically regulate chromatin remodeling is to identify small molecules that can regulate the expression of genes encoding essential subunits of chromatin remodeling complexes. This has successfully been done to reexpress the BRM ATPase in cancer cell lines that have silenced its expression without a deletion (Gramling & Reisman, 2011; Gramling, Rogers, Liu, & Reisman, 2011). This screen used a glucocorticoid receptor-regulated luciferase reporter that is induced in the presence of BRM. As a result of this screen, two compounds were identified which increased the expression of BRM, inducing the expression of BRM target genes, and decreased the growth of cancer cell lines. Each of these compounds regulates BRM expression through an unknown mechanism.
These different strategies provide excellent models on how to approach small molecule discovery screens for chromatin remodeling complexes. Biochemical screens can be performed for individual ATPase subunits or chromatin remodeling complexes which can be produced recombinantly. Conversely, live cell assays can be used for chromatin remodeling complexes with many subunits, which are not feasible to produce recombinantly. Once candidate compounds are identified, they can be then characterized using biochemical assays requiring small amounts of purified complex.
7. CONCLUDING REMARKS
Many changes occur to the cell during the transition from normal tissue to advanced stage cancer. In many cases, the changes result in abnormal gene expression patterns, which in turn establish and maintain key aspects of cancer cell biology. Over the past decade, chromatin remodeling complexes have been shown to establish and maintain abnormal gene expression in cancer cells. In addition, the recent explosion in sequencing cancer cell genomes has documented that subunits of chromatin remodeling complexes are frequently mutated in several cancer cell types suggesting that some of these mutations can drive the cancer cell phenotype. The sum of these results demonstrates that chromatin remodeling complexes have the potential to be a novel class of targets for developing cancer therapeutics.
To rigorously explore if chromatin remodeling complexes are viable therapeutic targets, several avenues of research should be pursued. The sequence data from cancer cell genomes suggests that subunits of chromatin remodeling complexes might be drivers of cancer development. To investigate the potential driver status of these mutations, conditional mouse alleles must be developed and used in longevity studies. These studies should be done in combination with common oncogene transgenes (MYC and RasG12V) or the knockout of other tumor suppressor pathways (p53 or pRB) to study their tumor-promoting potential in context with other common tumor-promoting mutations. Proof of principle studies have been performed with the INI1 mutation showing that its tumor-promoting activities require a functional BRG1, polycomb, and Cyclin D1. A BRG1 requirement for tumor development with INI mutations suggests that they result from deregulated SWI/SNF complexes lacking the INI1 subunit. Similar studies should be performed for mutations in other SWI/SNF accessory subunits including ARID1A and BAF180. If tumor-promoting activities for these mutations require an active ATPase, then small molecules could be designed to BRG1 for therapeutic advantage for these types of tumors.
Chromatin remodeling activities are perceived to be essential for cell viability. While it is almost universal that they are required for embryonic development, many reports document that at least a handful of remodeling complexes are not cell essential, and therefore may not be required for adult organisms. In order to justify the design of small molecule regulators for these complexes, it will be important to know the function of these complexes in adult cells. These studies are relevant from a therapeutic standpoint because in a majority of cases the targeting of chromatin remodeling complexes with therapies will be performed in an adult human. The use of tissue-specific knockout alleles to deplete subunits in specific tissues should promote these studies. In combination, conditional organism-wide knockouts should be pursued using ligand-inducible knockout alleles in adults. These experiments are critical for determining the possible consequences to the adult animal from targeting these complexes with small molecule regulators.
In addition to oncogenes and tumor suppressors, which have important functions for establishing cancer, many genes are necessary to maintain progrowth signals in cancer cells. These genes are not essential for normal cells but are essential for the cancer cell to maintain its phenotype. This class of nononcogenes has been actively investigated by several groups using shRNA knockdown screening strategies (Luo, Solimini, & Elledge, 2009). Relevant to chromatin remodeling, preliminary evidence shows that SNF2L containing chromatin remodeling complexes like NURF, RSF, and CERF could participate in these nononcogene pathways. It would be useful to the field to conduct shRNA knockdown screens specifically looking for nononcogene function for chromatin remodeling complexes in multiple cancer types. From a practical sense, these studies are only useful in combination with studies showing that the remodeling complexes are not essential for normal cell survival in adult organisms (see above).
In order to rationally approach the design of small molecule regulators for these complexes, their atomic structures must be solved. Much success has been realized over several years solving the low-resolution structure of chromatin remodeling complexes by electron microscopy (Leschziner, 2011). While these structures are a major step forward, their resolution must be improved. The size and heterogeneity of chromatin remodeling complexes, particularly in complex with a nucleosome, will likely prevent their crystallization. However, atomic resolution structures can be assembled using integrated structural techniques (Ward, Sali, & Wilson, 2013). In this approach, a low-resolution EM structure serves as a scaffold with which to model atomic resolution structures of individual subunits or domains. Recent success using this approach has been realized for the INO80 and SWR1 complexes (Nguyen et al., 2013; Tosi et al., 2013). With these structures, we can begin to study the molecular contacts these complexes make between subunits and the nucleosome, with the hope of rationally designing small molecules to disrupt these interactions. Significant progress into rationally designing small molecules to the ATPase-binding sites can also be realized by solving the crystal structures of the ATPase containing subunit.
Chromatin remodeling complexes should be feasible drug targets because, like histone modifying enzymes and DNA methyltransferases, they are enzymes. Druggable active sites which would have the most profound effect on the activity of the complexes include the ATP and nucleosome-binding sites. To discover small molecules for these sites, rational approaches would be preferred which will require the atomic resolution structure (see above) but can also be approached with live cell or biochemical screens for small molecule inhibitors. These approaches have been used successfully in the past (see Section 6). Off target effects for small molecules targeting the nucleosome-binding site are less of a concern than the ATP-binding site because significant nucleosome contacts are established by subunits unique to each complex (Leschziner, 2011; Nguyen et al., 2013; Tosi et al., 2013). Beyond targeting the ATPase and nucleosome-binding sites, these complexes have many binding sites for histone modifications including chromo, PHD, and bromodomains. Targeting histone-binding domains with small molecules might provide a means to inhibit their ability to bind chromatin (Dawson et al., 2012).
The explosion of research into the field of epigenetics has yielded much understanding on how these mechanisms operate under normal circumstances to regulate gene expression. While much more work needs to be done to further our understanding of these mechanisms, we are in a position to begin to seriously explore whether epigenetic mechanisms can be viable drug targets for the treatment of human disease. As outlined in this review, there is potential for these complexes to participate in cancer as driving mutations, regulating oncogenes and tumor suppressor pathways, and participating as nononcogenes. As such, chromatin remodeling complexes have a great potential to be successfully targeted for therapeutic benefit. The next decade of research on chromatin remodeling complexes should yield many discoveries, some of which may have value in the clinic.
ACKNOWLEDGMENTS
The authors would like to thank the V Foundation for Cancer Research, the Jeffress Memorial Fund, Virginia Commonwealth University School of Medicine, and the Massey Cancer Center for financial support. The authors would also like to thank Kevin Hogan for editorial review of the manuscript.
Footnotes
Conflict of Interest: The authors do not declare any conflicts of interest.
REFERENCES
- Aalfs JD, Kingston RE. What does ‘chromatin remodeling’ mean? Trends in Biochemical Sciences. 2000;25(11):548–555. doi: 10.1016/s0968-0004(00)01689-3. [DOI] [PubMed] [Google Scholar]
- Adams ME, Hurd EA, Beyer LA, Swiderski DL, Raphael Y, Martin DM. Defects in vestibular sensory epithelia and innervation in mice with loss of Chd7 function: Implications for human CHARGE syndrome. The Journal of Comparative Neurology. 2007;504(5):519–532. doi: 10.1002/cne.21460. [DOI] [PubMed] [Google Scholar]
- Allis CD, Jenuwein T, Reinberg D. Epigenetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2007. [Google Scholar]
- Ard PG, Chatterjee C, Kunjibettu S, Adside LR, Gralinski LE, McMahon SB. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Molecular and Cellular Biology. 2002;22(16):5650–5661. doi: 10.1128/MCB.22.16.5650-5661.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: A new frontier for drug discovery. Nature Reviews Drug Discovery. 2012;11(5):384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- Ayllon V, Rebollo A. Ras-induced cellular events (review) Molecular Membrane Biology. 2000;17(2):65–73. doi: 10.1080/09687680050117093. [DOI] [PubMed] [Google Scholar]
- Badenhorst P, Xiao H, Cherbas L, Kwon SY, Voas M, Rebay I, et al. The Drosophila nucleosome remodeling factor NURF is required for Ecdysteroid signaling and metamorphosis. Genes & Development. 2005;19(21):2540–2545. doi: 10.1101/gad.1342605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi A, Papazoglu C, Wu Y, Capurso D, Brodt M, Francis D, et al. CHD5 is a tumor suppressor at human 1p36. Cell. 2007;128(3):459–475. doi: 10.1016/j.cell.2006.11.052. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Balasenthil S, Gururaj AE, Talukder AH, Wang YH, Lee JH, et al. Metastasis-associated protein 1 transgenic mice: A new model of spontaneous B-cell lymphomas. Cancer Research. 2007;67(15):7062–7067. doi: 10.1158/0008-5472.CAN-07-0748. [DOI] [PubMed] [Google Scholar]
- Bagheri-Yarmand R, Talukder AH, Wang RA, Vadlamudi RK, Kumar R. Metastasis-associated protein 1 deregulation causes inappropriate mammary gland development and tumorigenesis. Development. 2004;131(14):3469–3479. doi: 10.1242/dev.01213. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S, Comstock CE, Ertel A, Jeong KW, Stallcup MR, Addya S, et al. Aberrant BAF57 signaling facilitates prometastatic phenotypes. Clinical Cancer Research. 2013;19(10):2657–2667. doi: 10.1158/1078-0432.CCR-12-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banting GS, Barak O, Ames TM, Burnham AC, Kardel MD, Cooch NS, et al. CECR2, a protein involved in neurulation, forms a novel chromatin remodeling complex with SNF2L. Human Molecular Genetics. 2005;14(4):513–524. doi: 10.1093/hmg/ddi048. [DOI] [PubMed] [Google Scholar]
- Barak O, Lazzaro MA, Lane WS, Speicher DW, Picketts DJ, Shiekhattar R. Isolation of human NURF: A regulator of Engrailed gene expression. The EMBO Journal. 2003;22(22):6089–6100. doi: 10.1093/emboj/cdg582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Molecular Cell. 2001;8(6):1243–1254. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell. 2010;143(3):470–484. doi: 10.1016/j.cell.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett C, Orvis TJ, Rosson GS, Weissman BE. BRG1 mutations found in human cancer cell lines inactivate Rb-mediated cell-cycle arrest. Journal of Cellular Physiology. 2011;226(8):1989–1997. doi: 10.1002/jcp.22533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A, Chauvet S, Huber O, Usseglio F, Rothbacher U, Aragnol D, et al. Pontin52 and reptin52 function as antagonistic regulators of beta-catenin signalling activity. The EMBO Journal. 2000;19(22):6121–6130. doi: 10.1093/emboj/19.22.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker PB, Workman JL. Nucleosome remodeling and epigenetics. Cold Spring Harbor Perspectives in Biology. 2013;5(9) doi: 10.1101/cshperspect.a017905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegel JA, Fogelgren B, Wainwright LM, Zhou JY, Bevan H, Rorke LB. Germline INI1 mutation in a patient with a central nervous system atypical teratoid tumor and renal rhabdoid tumor. Genes, Chromosomes & Cancer. 2000;28(1):31–37. doi: 10.1002/(sici)1098-2264(200005)28:1<31::aid-gcc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Research. 1999;59(1):74–79. [PubMed] [Google Scholar]
- Bochar DA, Wang L, Beniya H, Kinev A, Xue Y, Lane WS, et al. BRCA1 is associated with a human SWI/SNF-related complex: Linking chromatin remodeling to breast cancer. Cell. 2000;102(2):257–265. doi: 10.1016/s0092-8674(00)00030-1. [DOI] [PubMed] [Google Scholar]
- Bowman GD. Mechanisms of ATP-dependent nucleosome sliding. Current Opinion in Structural Biology. 2010;20(1):73–81. doi: 10.1016/j.sbi.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown N, Cotterill S, Lastowska M, O’Neill S, Pearson AD, Plantaz D, et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. The New England Journal of Medicine. 1999;340(25):1954–1961. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- Buganim Y, Goldstein I, Lipson D, Milyavsky M, Polak-Charcon S, Mardoukh C, et al. A novel translocation breakpoint within the BPTF gene is associated with a pre-malignant phenotype. PLoS One. 2010;5(3):e9657. doi: 10.1371/journal.pone.0009657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, et al. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Molecular Cell. 2000;6(6):1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Bultman SJ, Herschkowitz JI, Godfrey V, Gebuhr TC, Yaniv M, Perou CM, et al. Characterization of mammary tumors from Brg1 heterozygous mice. Oncogene. 2008;27(4):460–468. doi: 10.1038/sj.onc.1210664. [DOI] [PubMed] [Google Scholar]
- Burkhardt L, Fuchs S, Krohn A, Masser S, Mader M, Kluth M, et al. CHD1 is a 5q21 tumor suppressor required for ERG rearrangement in prostate cancer. Cancer Research. 2013;73(9):2795–2805. doi: 10.1158/0008-5472.CAN-12-1342. [DOI] [PubMed] [Google Scholar]
- Burrows AE, Smogorzewska A, Elledge SJ. Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14280–14285. doi: 10.1073/pnas.1009559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Ashktorab H, Pang X, Zhao Y, Sha W, Liu Y, et al. MicroRNA-211 expression promotes colorectal cancer cell growth in vitro and in vivo by targeting tumor suppressor CHD5. PLoS One. 2012;7(1):e29750. doi: 10.1371/journal.pone.0029750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Jin J, Tomomori-Sato C, Sato S, Sorokina I, Parmely TJ, et al. Identification of new subunits of the multiprotein mammalian TRRAP/TIP60-containing histone acetyltransferase complex. The Journal of Biological Chemistry. 2003;278(44):42733–42736. doi: 10.1074/jbc.C300389200. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499(7456):43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavellan E, Asp P, Percipalle P, Farrants AK. The WSTF-SNF2h chromatin remodeling complex interacts with several nuclear proteins in transcription. The Journal of Biological Chemistry. 2006;281(24):16264–16271. doi: 10.1074/jbc.M600233200. [DOI] [PubMed] [Google Scholar]
- Chandler RL, Brennan J, Schisler JC, Serber D, Patterson C, Magnuson T. ARID1a-DNA interactions are required for promoter occupancy by SWI/SNF. Molecular and Cellular Biology. 2013;33(2):265–280. doi: 10.1128/MCB.01008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Creighton CJ, Davis C, Donehower L, Drummond J, Wheeler D, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nature Genetics. 2013;45(10):1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Cai Y, Jin J, Florens L, Swanson SK, Washburn MP, et al. Subunit organization of the human INO80 chromatin remodeling complex: An evolutionarily conserved core complex catalyzes ATP-dependent nucleosome remodeling. The Journal of Biological Chemistry. 2011;286(13):11283–11289. doi: 10.1074/jbc.M111.222505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SW, Davies KP, Yung E, Beltran RJ, Yu J, Kalpana GV. c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function. Nature Genetics. 1999;22(1):102–105. doi: 10.1038/8811. [DOI] [PubMed] [Google Scholar]
- Choi JH, Sheu JJ, Guan B, Jinawath N, Markowski P, Wang TL, et al. Functional analysis of 11q13.5 amplicon identifies Rsf-1 (HBXAP) as a gene involved in paclitaxel resistance in ovarian cancer. Cancer Research. 2009;69(4):1407–1415. doi: 10.1158/0008-5472.CAN-08-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismasiu VB, Adamo K, Gecewicz J, Duque J, Lin Q, Avram D. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene. 2005;24(45):6753–6764. doi: 10.1038/sj.onc.1208904. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Kouzarides T. Cancer epigenetics: From mechanism to therapy. Cell. 2012;150(1):12–27. doi: 10.1016/j.cell.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. The New England Journal of Medicine. 2012;367(7):647–657. doi: 10.1056/NEJMra1112635. [DOI] [PubMed] [Google Scholar]
- Decristofaro MF, Betz BL, Rorie CJ, Reisman DN, Wang W, Weissman BE. Characterization of SWI/SNF protein expression in human breast cancer cell lines and other malignancies. Journal of Cellular Physiology. 2001;186(1):136–145. doi: 10.1002/1097-4652(200101)186:1<136::AID-JCP1010>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- De Koning L, Corpet A, Haber JE, Almouzni G. Histone chaperones: An escort network regulating histone traffic. Nature Structural & Molecular Biology. 2007;14(11):997–1007. doi: 10.1038/nsmb1318. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Deleu L, Shellard S, Alevizopoulos K, Amati B, Land H. Recruitment of TRRAP required for oncogenic transformation by E1A. Oncogene. 2001;20(57):8270–8275. doi: 10.1038/sj.onc.1205159. [DOI] [PubMed] [Google Scholar]
- Dugan KA, Wood MA, Cole MD. TIP49, but not TRRAP, modulates c-Myc and E2F1 dependent apoptosis. Oncogene. 2002;21(38):5835–5843. doi: 10.1038/sj.onc.1205763. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, Strober BE, Guha S, Khavari PA, Alin K, Luban J, et al. The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest. Cell. 1994;79(1):119–130. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- Dykhuizen EC, Carmody LC, Tolliday N, Crabtree GR, Palmer MA. Screening for inhibitors of an essential chromatin remodeler in mouse embryonic stem cells by monitoring transcriptional regulation. Journal of Biomolecular Screening. 2012;17(9):1221–1230. doi: 10.1177/1087057112455060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen EC, Hargreaves DC, Miller EL, Cui K, Korshunov A, Kool M, et al. BAF complexes facilitate decatenation of DNA by topoisomerase IIalpha. Nature. 2013;497(7451):624–627. doi: 10.1038/nature12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckey M, Kuphal S, Straub T, Rummele P, Kremmer E, Bosserhoff AK, et al. Nucleosome remodeler SNF2L suppresses cell proliferation and migration and attenuates Wnt signaling. Molecular and Cellular Biology. 2012;32(13):2359–2371. doi: 10.1128/MCB.06619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkaim J, Castroviejo M, Bennani D, Taouji S, Allain N, Laguerre M, et al. First identification of small-molecule inhibitors of Pontin by combining virtual screening and enzymatic assay. The Biochemical Journal. 2012;443(2):549–559. doi: 10.1042/BJ20111779. [DOI] [PubMed] [Google Scholar]
- Endo M, Yasui K, Zen Y, Gen Y, Zen K, Tsuji K, et al. Alterations of the SWI/SNF chromatin remodelling subunit-BRG1 and BRM in hepatocellular carcinoma. Liver International. 2013;33(1):105–117. doi: 10.1111/liv.12005. [DOI] [PubMed] [Google Scholar]
- Erdel F, Rippe K. Chromatin remodelling in mammalian cells by ISWI-type complexes—Where, when and why? The FEBS Journal. 2011;278(19):3608–3618. doi: 10.1111/j.1742-4658.2011.08282.x. [DOI] [PubMed] [Google Scholar]
- Erikson J, ar-Rushdi A, Drwinga HL, Nowell PC, Croce CM. Transcriptional activation of the translocated c-myc oncogene in burkitt lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(3):820–824. doi: 10.1073/pnas.80.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FM, Li CF, Huang HY, Lai MT, Chen CM, Chiu IW, et al. Overexpression of a chromatin remodeling factor, RSF-1/HBXAP, correlates with aggressive oral squamous cell carcinoma. The American Journal of Pathology. 2011;178(5):2407–2415. doi: 10.1016/j.ajpath.2011.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Lee N, Fearon ER. TIP49 regulates beta-catenin-mediated neoplastic transformation and T-cell factor target gene induction via effects on chromatin remodeling. Cancer Research. 2003;63(24):8726–8734. [PubMed] [Google Scholar]
- Finkbeiner MG, Sawan C, Ouzounova M, Murr R, Herceg Z. HAT cofactor TRRAP mediates beta-catenin ubiquitination on the chromatin and the regulation of the canonical Wnt pathway. Cell Cycle. 2008;7(24):3908–3914. doi: 10.4161/cc.7.24.7354. [DOI] [PubMed] [Google Scholar]
- Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, et al. COSMIC: Mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Research. 2011;39(Database issue):D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NJ, Saurin AJ, Shao Z, Kingston RE. Reconstitution of a functional core polycomb repressive complex. Molecular Cell. 2001;8(3):545–556. doi: 10.1016/s1097-2765(01)00316-1. [DOI] [PubMed] [Google Scholar]
- Fu J, Qin L, He T, Qin J, Hong J, Wong J, et al. The TWIST/Mi2/NuRD protein complex and its essential role in cancer metastasis. Cell Research. 2011;21(2):275–289. doi: 10.1038/cr.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Zhang L, He T, Xiao X, Liu X, Wang L, et al. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. International Journal of Biological Sciences. 2012;8(4):522–532. doi: 10.7150/ijbs.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M, Gerber J, Drapkin R, Sif S, Ikura T, Ogryzko V, et al. The p400 complex is an essential E1A transformation target. Cell. 2001;106(3):297–307. doi: 10.1016/s0092-8674(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Fujii T, Ueda T, Nagata S, Fukunaga R. Essential role of p400/mDomino chromatin-remodeling ATPase in bone marrow hematopoiesis and cell-cycle progression. The Journal of Biological Chemistry. 2010;285(39):30214–30223. doi: 10.1074/jbc.M110.104513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki R, Kim MS, Sasaki Y, Yoshimura K, Kitagawa H, Kato S. Ligand-induced transrepression by VDR through association of WSTF with acetylated histones. The EMBO Journal. 2005;24(22):3881–3894. doi: 10.1038/sj.emboj.7600853. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z. ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(18):6656–6661. doi: 10.1073/pnas.0801802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pedrero JM, Kiskinis E, Parker MG, Belandia B. The SWI/SNF chromatin remodeling subunit BAF57 is a critical regulator of estrogen receptor function in breast cancer cells. The Journal of Biological Chemistry. 2006;281(32):22656–22664. doi: 10.1074/jbc.M602561200. [DOI] [PubMed] [Google Scholar]
- Gevry N, Chan HM, Laflamme L, Livingston DM, Gaudreau L. p21 transcription is regulated by differential localization of histone H2A.Z. Genes & Development. 2007;21(15):1869–1881. doi: 10.1101/gad.1545707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitenay D, Baron VT. Is EGR1 a potential target for prostate cancer therapy? Future Oncology. 2009;5(7):993–1003. doi: 10.2217/fon.09.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodinov A, Vaissiere T, Krastev DB, Legube G, Anachkova B, Herceg Z. Mammalian Ino80 mediates double-strand break repair through its role in DNA end strand resection. Molecular and Cellular Biology. 2011;31(23):4735–4745. doi: 10.1128/MCB.06182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramling S, Reisman D. Discovery of BRM targeted therapies: Novel reactivation of an anti-cancer gene. Letters in Drug Design & Discovery. 2011;8(1):93–99. doi: 10.2174/157018011793663840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramling S, Rogers C, Liu G, Reisman D. Pharmacologic reversal of epigenetic silencing of the anticancer protein BRM: A novel targeted treatment strategy. Oncogene. 2011;30(29):3289–3294. doi: 10.1038/onc.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoletto A, Lestienne P, Rosenbaum J. The multifaceted proteins Reptin and Pontin as major players in cancer. Biochimica et Biophysica Acta. 2011;1815(2):147–157. doi: 10.1016/j.bbcan.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Guan B, Wang TL, Shih Ie M. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Research. 2011;71(21):6718–6727. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunduz E, Gunduz M, Ouchida M, Nagatsuka H, Beder L, Tsujigiwa H, et al. Genetic and epigenetic alterations of BRG1 promote oral cancer development. International Journal of Oncology. 2005;26(1):201–210. [PubMed] [Google Scholar]
- Gunther K, Rust M, Leers J, Boettger T, Scharfe M, Jarek M, et al. Differential roles for MBD2 and MBD3 at methylated CpG islands, active promoters and binding to exon sequences. Nucleic Acids Research. 2013;41(5):3010–3021. doi: 10.1093/nar/gkt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Human Pathology. 1981;12(7):646–657. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- Hagelkruys A, Sawicka A, Rennmayr M, Seiser C. The biology of HDAC in cancer: The nuclear and epigenetic components. Handbook of Experimental Pharmacology. 2011;206:13–37. doi: 10.1007/978-3-642-21631-2_2. [DOI] [PubMed] [Google Scholar]
- Hah N, Kolkman A, Ruhl DD, Pijnappel WW, Heck AJ, Timmers HT, et al. A role for BAF57 in cell cycle-dependent transcriptional regulation by the SWI/SNF chromatin remodeling complex. Cancer Research. 2010;70(11):4402–4411. doi: 10.1158/0008-5472.CAN-09-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Georgel PT. CHD proteins: A diverse family with strong ties. Biochemistry and Cell Biology. 2007;85(4):463–476. doi: 10.1139/O07-063. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hendrich B, Bird A. Identification and characterization of a family of mammalian methyl-CpG binding proteins. Molecular and Cellular Biology. 1998;18(11):6538–6547. doi: 10.1128/mcb.18.11.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich KO, Schwab M, Westermann F. 1p36 tumor suppression—A matter of dosage? Cancer Research. 2012;72(23):6079–6088. doi: 10.1158/0008-5472.CAN-12-2230. [DOI] [PubMed] [Google Scholar]
- Hill DA, de la Serna IL, Veal TM, Imbalzano AN. BRCA1 interacts with dominant negative SWI/SNF enzymes without affecting homologous recombination or radiation-induced gene activation of p21 or Mdm2. Journal of Cellular Biochemistry. 2004;91(5):987–998. doi: 10.1002/jcb.20003. [DOI] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463(7280):474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Gulzar ZG, Salari K, Lapointe J, Brooks JD, Pollack JR. Recurrent deletion of CHD1 in prostate cancer with relevance to cell invasiveness. Oncogene. 2012;31(37):4164–4170. doi: 10.1038/onc.2011.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huen J, Kakihara Y, Ugwu F, Cheung KL, Ortega J, Houry WA. Rvb1-Rvb2: Essential ATP-dependent helicases for critical complexes. Biochemistry and Cell Biology. 2010;88(1):29–40. doi: 10.1139/o09-122. [DOI] [PubMed] [Google Scholar]
- Ibragimova I, Maradeo ME, Dulaimi E, Cairns P. Aberrant promoter hypermethylation of PBRM1, BAP1, SETD2, KDM6A and other chromatinmodifying genes is absent or rare in clear cell RCC. Epigenetics. 2013;8(5):486–493. doi: 10.4161/epi.24552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, et al. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102(4):463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- Isakoff MS, Sansam CG, Tamayo P, Subramanian A, Evans JA, Fillmore CM, et al. Inactivation of the Snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17745–17750. doi: 10.1073/pnas.0509014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Cai Y, Yao T, Gottschalk AJ, Florens L, Swanson SK, et al. A mammalian chromatin remodeling complex with similarities to the yeast INO80 complex. The Journal of Biological Chemistry. 2005;280(50):41207–41212. doi: 10.1074/jbc.M509128200. [DOI] [PubMed] [Google Scholar]
- Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Human Mutation. 2012;33(1):100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, Roden R, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330(6001):228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nature Genetics. 2013;45(6):592–601. doi: 10.1038/ng.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennison JA, Tamkun JW. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(21):8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia SK, Gorski MM, Giannakopoulos S, Verrijzer CP. SWI/SNF mediates polycomb eviction and epigenetic reprogramming of the INK4b-ARF-INK4a locus. Molecular and Cellular Biology. 2008;28(10):3457–3464. doi: 10.1128/MCB.02019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klochendler-Yeivin A, Fiette L, Barra J, Muchardt C, Babinet C, Yaniv M. The murine SNF5/INI1 chromatin remodeling factor is essential for embryonic development and tumor suppression. EMBO Reports. 2000;1(6):500–506. doi: 10.1093/embo-reports/kvd129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L, Yu XP, Bai XH, Zhang WF, Zhang Y, Zhao WM, et al. RbAp48 is a critical mediator controlling the transforming activity of human papillomavirus type 16 in cervical cancer. The Journal of Biological Chemistry. 2007;282(36):26381–26391. doi: 10.1074/jbc.M702195200. [DOI] [PubMed] [Google Scholar]
- Kornberg RD. Chromatin structure: A repeating unit of histones and DNA. Science. 1974;184(4139):868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kumar R, Balasenthil S, Manavathi B, Rayala SK, Pakala SB. Metastasis-associated protein 1 and its short form variant stimulates Wnt1 transcription through promoting its derepression from Six3 corepressor. Cancer Research. 2010;70(16):6649–6658. doi: 10.1158/0008-5472.CAN-10-0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Balasenthil S, Pakala SB, Rayala SK, Sahin AA, Ohshiro K. Metastasis-associated protein 1 short form stimulates Wnt1 pathway in mammary epithelial and cancer cells. Cancer Research. 2010;70(16):6598–6608. doi: 10.1158/0008-5472.CAN-10-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara Y, Charboneau A, Knudsen ES, Weissman BE. Reexpression of hSNF5 in malignant rhabdoid tumor cell lines causes cell cycle arrest through a p21 (CIP1/WAF1)-dependent mechanism. Cancer Research. 2010;70(5):1854–1865. doi: 10.1158/0008-5472.CAN-09-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara Y, Wei D, Durand J, Weissman BE. SNF5 reexpression in malignant rhabdoid tumors regulates transcription of target genes by recruitment of SWI/SNF complexes and RNAPII to the transcription start site of their promoters. Molecular Cancer Research. 2013;11(3):251–260. doi: 10.1158/1541-7786.MCR-12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sharov AA, Piao Y, Sharova LV, Xiao H, Southon E, et al. Essential role of chromatin remodeling protein Bptf in early mouse embryos and embryonic stem cells. PLoS Genetics. 2008;4(10):e1000241. doi: 10.1371/journal.pgen.1000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Demajo S, Jain P, Di Croce L. Combinatorial assembly and function of chromatin regulatory complexes. Epigenomics. 2011;3(5):567–580. doi: 10.2217/epi.11.83. [DOI] [PubMed] [Google Scholar]
- Larsen DH, Poinsignon C, Gudjonsson T, Dinant C, Payne MR, Hari FJ, et al. The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. The Journal of Cell Biology. 2010;190(5):731–740. doi: 10.1083/jcb.200912135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybourn PJ, Kadonaga JT. Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase II. Science. 1991;254(5029):238–245. doi: 10.1126/science.254.5029.238. [DOI] [PubMed] [Google Scholar]
- Lee JY, Lee TH. Effects of histone acetylation and CpG methylation on the structure of nucleosomes. Biochimica et Biophysica Acta. 2012;1824(8):974–982. doi: 10.1016/j.bbapap.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Lee AY, Kwon YK, Kwon H. Suppression of HPV E6 and E7 expression by BAF53 depletion in cervical cancer cells. Biochemical and Biophysical Research Communications. 2011;412(2):328–333. doi: 10.1016/j.bbrc.2011.07.098. [DOI] [PubMed] [Google Scholar]
- Lee RS, Stewart C, Carter SL, Ambrogio L, Cibulskis K, Sougnez C, et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. The Journal of Clinical Investigation. 2012;122(8):2983–2988. doi: 10.1172/JCI64400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRoy G, Loyola A, Lane WS, Reinberg D. Purification and characterization of a human factor that assembles and remodels chromatin. The Journal of Biological Chemistry. 2000;275(20):14787–14790. doi: 10.1074/jbc.C000093200. [DOI] [PubMed] [Google Scholar]
- LeRoy G, Orphanides G, Lane WS, Reinberg D. Requirement of RSF and FACT for transcription of chromatin templates in vitro. Science. 1998;282(5395):1900–1904. doi: 10.1126/science.282.5395.1900. [DOI] [PubMed] [Google Scholar]
- Leschziner AE. Electron microscopy studies of nucleosome remodelers. Current Opinion in Structural Biology. 2011;21(6):709–718. doi: 10.1016/j.sbi.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Divijendra Natha Reddy S, Pakala SB, Wu X, Zhang Y, Rayala SK, et al. MTA1 coregulator regulates p53 stability and function. The Journal of Biological Chemistry. 2009;284(50):34545–34552. doi: 10.1074/jbc.M109.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Guan LS, Wang ZY. Overexpression of RbAp46 facilitates stress-induced apoptosis and suppresses tumorigenicity of neoplastigenic breast epithelial cells. International Journal of Cancer. 2003;105(6):762–768. doi: 10.1002/ijc.11148. [DOI] [PubMed] [Google Scholar]
- Li DQ, Ohshiro K, Reddy SD, Pakala SB, Lee MH, Zhang Y, et al. E3 ubiquitin ligase COP1 regulates the stability and functions of MTA1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(41):17493–17498. doi: 10.1073/pnas.0908027106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Pakala SB, Nair SS, Eswaran J, Kumar R. Metastasis-associated protein 1/nucleosome remodeling and histone deacetylase complex in cancer. Cancer Research. 2012;72(2):387–394. doi: 10.1158/0008-5472.CAN-11-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Pakala SB, Reddy SD, Ohshiro K, Peng SH, Lian Y, et al. Revelation of p53-independent function of MTA1 in DNA damage response via modulation of the p21 WAF1-proliferating cell nuclear antigen pathway. The Journal of Biological Chemistry. 2010;285(13):10044–10052. doi: 10.1074/jbc.M109.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DQ, Pakala SB, Reddy SD, Ohshiro K, Zhang JX, Wang L, et al. Bidirectional autoregulatory mechanism of metastasis-associated protein 1-alternative reading frame pathway in oncogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8791–8796. doi: 10.1073/pnas.1018389108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhang H, Yu W, Chen Y, Gui B, Liang J, et al. ZIP: A novel transcription repressor, represses EGFR oncogene and suppresses breast carcinogenesis. The EMBO Journal. 2009;28(18):2763–2776. doi: 10.1038/emboj.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link KA, Balasubramaniam S, Sharma A, Comstock CE, Godoy-Tundidor S, Powers N, et al. Targeting the BAF57 SWI/SNF subunit in prostate cancer: A novel platform to control androgen receptor activity. Cancer Research. 2008;68(12):4551–4558. doi: 10.1158/0008-5472.CAN-07-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link KA, Burd CJ, Williams E, Marshall T, Rosson G, Henry E, et al. BAF57 governs androgen receptor action and androgen-dependent proliferation through SWI/SNF. Molecular and Cellular Biology. 2005;25(6):2200–2215. doi: 10.1128/MCB.25.6.2200-2215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Lindberg J, Sui G, Luo J, Egevad L, Li T, et al. Identification of novel CHD1-associated collaborative alterations of genomic structure and functional assessment of CHD1 in prostate cancer. Oncogene. 2012;31(35):3939–3948. doi: 10.1038/onc.2011.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Luijsterburg MS, van Attikum H. Chromatin and the DNA damage response: The cancer connection. Molecular Oncology. 2011;5(4):349–367. doi: 10.1016/j.molonc.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: Oncogene and non-oncogene addiction. Cell. 2009;136(5):823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Su F, Chen D, Shiloh A, Gu W. Deacetylation of p53 modulates its effect on cell growth and apoptosis. Nature. 2000;408(6810):377–381. doi: 10.1038/35042612. [DOI] [PubMed] [Google Scholar]
- Magdinier F, Wolffe AP. Selective association of the methyl-CpG binding protein MBD2 with the silent p14/p16 locus in human neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):4990–4995. doi: 10.1073/pnas.101617298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid S, Dar AA, Ahmad AE, Hirata H, Kawakami K, Shahryari V, et al. BTG3 tumor suppressor gene promoter demethylation, histone modification and cell cycle arrest by genistein in renal cancer. Carcinogenesis. 2009;30(4):662–670. doi: 10.1093/carcin/bgp042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marignani PA, Kanai F, Carpenter CL. LKB1 associates with Brg1 and is necessary for Brg1-induced growth arrest. The Journal of Biological Chemistry. 2001;276(35):32415–32418. doi: 10.1074/jbc.C100207200. [DOI] [PubMed] [Google Scholar]
- Marzook H, Li DQ, Nair VS, Mudvari P, Reddy SD, Pakala SB, et al. Metastasis-associated protein 1 drives tumor cell migration and invasion through transcriptional repression of RING finger protein 144A. The Journal of Biological Chemistry. 2012;287(8):5615–5626. doi: 10.1074/jbc.M111.314088. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, et al. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nature Cell Biology. 2001;3(1):30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Van Buskirk HA, Dugan KA, Copeland TD, Cole MD. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94(3):363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- Medina PP, Carretero J, Fraga MF, Esteller M, Sidransky D, Sanchez-Cespedes M. Genetic and epigenetic screening for gene alterations of the chromatin-remodeling factor, SMARCA4/BRG1, in lung tumors. Genes, Chromosomes & Cancer. 2004;41(2):170–177. doi: 10.1002/gcc.20068. [DOI] [PubMed] [Google Scholar]
- Medina PP, Romero OA, Kohno T, Montuenga LM, Pio R, Yokota J, et al. Frequent BRG1/SMARCA4-inactivating mutations in human lung cancer cell lines. Human Mutation. 2008;29(5):617–622. doi: 10.1002/humu.20730. [DOI] [PubMed] [Google Scholar]
- Menard L, Taras D, Grigoletto A, Haurie V, Nicou A, Dugot-Senant N, et al. In vivo silencing of Reptin blocks the progression of human hepatocellular carcinoma in xenografts and is associated with replicative senescence. Journal of Hepatology. 2010;52(5):681–689. doi: 10.1016/j.jhep.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Migliori V, Mapelli M, Guccione E. On WD40 proteins: Propelling our knowledge of transcriptional control? Epigenetics. 2012;7(8):815–822. doi: 10.4161/epi.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JN, Tian Y, Xiao Y, Wu L, Li L, Chang S. The mINO80 chromatin remodeling complex is required for efficient telomere replication and maintenance of genome stability. Cell Research. 2013;23:1396–1413. doi: 10.1038/cr.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molli PR, Singh RR, Lee SW, Kumar R. MTA1-mediated transcriptional repression of BRCA1 tumor suppressor gene. Oncogene. 2008;27(14):1971–1980. doi: 10.1038/sj.onc.1210839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HE, Cheon H, Chun KH, Lee SK, Kim YS, Jung BK, et al. Metastasis-associated protein 1 enhances angiogenesis by stabilization of HIF-1alpha. Oncology Reports. 2006;16(4):929–935. [PubMed] [Google Scholar]
- Moon HE, Cheon H, Lee MS. Metastasis-associated protein 1 inhibits p53-induced apoptosis. Oncology Reports. 2007;18(5):1311–1314. [PubMed] [Google Scholar]
- Morey L, Brenner C, Fazi F, Villa R, Gutierrez A, Buschbeck M, et al. MBD3, a component of the NuRD complex, facilitates chromatin alteration and deposition of epigenetic marks. Molecular and Cellular Biology. 2008;28(19):5912–5923. doi: 10.1128/MCB.00467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AJ, Shen X. Chromatin remodelling beyond transcription: The INO80 and SWR1 complexes. Nature Reviews Molecular Cell Biology. 2009;10(6):373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr R, Loizou JI, Yang YG, Cuenin C, Li H, Wang ZQ, et al. Histone acetylation by Trrap-Tip60 modulates loading of repair proteins and repair of DNA double-strand breaks. Nature Cell Biology. 2006;8(1):91–99. doi: 10.1038/ncb1343. [DOI] [PubMed] [Google Scholar]
- Murr R, Vaissiere T, Sawan C, Shukla V, Herceg Z. Orchestration of chromatin-based processes: Mind the TRRAP. Oncogene. 2007;26(37):5358–5372. doi: 10.1038/sj.onc.1210605. [DOI] [PubMed] [Google Scholar]
- Nagarajan P, Onami TM, Rajagopalan S, Kania S, Donnell R, Venkatachalam S. Role of chromodomain helicase DNA-binding protein 2 in DNA damage response signaling and tumorigenesis. Oncogene. 2009;28(8):1053–1062. doi: 10.1038/onc.2008.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl NG, Jr., Patsialou A, Haines DS, Dallas PB, Beck GR, Jr., Moran E. The p270 (ARID1A/SMARCF1) subunit of mammalian SWI/SNF-related complexes is essential for normal cell cycle arrest. Cancer Research. 2005;65(20):9236–9244. doi: 10.1158/0008-5472.CAN-05-1225. [DOI] [PubMed] [Google Scholar]
- Nagl NG, Jr., Wang X, Patsialou A, Van Scoy M, Moran E. Distinct mammalian SWI/SNF chromatin remodeling complexes with opposing roles in cell-cycle control. The EMBO Journal. 2007;26(3):752–763. doi: 10.1038/sj.emboj.7601541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu SR, Love IM, Imbalzano AN, Grossman SR, Androphy EJ. The SWI/SNF chromatin remodeling subunit BRG1 is a critical regulator of p53 necessary for proliferation of malignant cells. Oncogene. 2009;28(27):2492–2501. doi: 10.1038/onc.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SS, Li DQ, Kumar R. A core chromatin remodeling factor instructs global chromatin signaling through multivalent reading of nucleosome codes. Molecular Cell. 2013;49(4):704–718. doi: 10.1016/j.molcel.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbioso A, Carafa V, Benedetti R, Altucci L. Trials with ‘epigenetic’ drugs: An update. Molecular Oncology. 2012;6(6):657–682. doi: 10.1016/j.molonc.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VQ, Ranjan A, Stengel F, Wei D, Aebersold R, Wu C, et al. Molecular architecture of the ATP-dependent chromatin-remodeling complex SWR1. Cell. 2013;154(6):1220–1231. doi: 10.1016/j.cell.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi A, Chambers AL, Downs JA, Lehmann AR. A role for chromatin remodellers in replication of damaged DNA. Nucleic Acids Research. 2012;40(15):7393–7403. doi: 10.1093/nar/gks453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ohshiro K, Rayala SK, Wigerup C, Pakala SB, Natha RS, Gururaj AE, et al. Acetylation-dependent oncogenic activity of metastasis-associated protein 1 co-regulator. EMBO Reports. 2010;11(9):691–697. doi: 10.1038/embor.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya H, Yokoyama A, Yamaoka I, Fujiki R, Yonezawa M, Youn MY, et al. Phosphorylation of Williams syndrome transcription factor by MAPK induces a switching between two distinct chromatin remodeling complexes. The Journal of Biological Chemistry. 2009;284(47):32472–32482. doi: 10.1074/jbc.M109.009738. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pan MR, Hsieh HJ, Dai H, Hung WC, Li K, Peng G, et al. Chromodomain helicase DNA-binding protein 4 (CHD4) regulates homologous recombination DNA repair, and its deficiency sensitizes cells to poly(ADP-ribose) polymerase (PARP) inhibitor treatment. The Journal of Biological Chemistry. 2012;287(9):6764–6772. doi: 10.1074/jbc.M111.287037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichos-Chronakis M, Watanabe S, Rando OJ, Peterson CL. Global regulation of H2A.Z localization by the INO80 chromatin-remodeling enzyme is essential for genome integrity. Cell. 2011;144(2):200–213. doi: 10.1016/j.cell.2010.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Hur SK, Kwon J. Human INO80 chromatin-remodelling complex contributes to DNA double-strand break repair via the expression of Rad54B and XRCC3 genes. The Biochemical Journal. 2010;431(2):179–187. doi: 10.1042/BJ20100988. [DOI] [PubMed] [Google Scholar]
- Park HY, Jeon YK, Shin HJ, Kim IJ, Kang HC, Jeong SJ, et al. Differential promoter methylation may be a key molecular mechanism in regulating BubR1 expression in cancer cells. Experimental & Molecular Medicine. 2007;39(2):195–204. doi: 10.1038/emm.2007.22. [DOI] [PubMed] [Google Scholar]
- Park J, Wood MA, Cole MD. BAF53 forms distinct nuclear complexes and functions as a critical c-Myc-interacting nuclear cofactor for oncogenic transformation. Molecular and Cellular Biology. 2002;22(5):1307–1316. doi: 10.1128/mcb.22.5.1307-1316.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Kuo A, Schalch T, Vogel H, Joshua-Tor L, McCombie WR, et al. Chd5 requires PHD-mediated histone 3 binding for tumor suppression. Cell Reports. 2013;3(1):92–102. doi: 10.1016/j.celrep.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski R, Muhl SM, Sulser T, Krek W, Moch H, Schraml P. Loss of PBRM1 expression is associated with renal cell carcinoma progression. International Journal of Cancer. 2013;132(2):E11–E17. doi: 10.1002/ijc.27822. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Ousterout DG, Gersbach CA. Advances in targeted genome editing. Current Opinion in Chemical Biology. 2012;16(3–4):268–277. doi: 10.1016/j.cbpa.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plass C, Pfister SM, Lindroth AM, Bogatyrova O, Claus R, Lichter P. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nature Reviews Genetics. 2013;14(11):765–780. doi: 10.1038/nrg3554. [DOI] [PubMed] [Google Scholar]
- Pleasance ED, Stephens PJ, O’Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463(7278):184–190. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes & Development. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- Polo SE, Kaidi A, Baskcomb L, Galanty Y, Jackson SP. Regulation of DNA-damage responses and cell-cycle progression by the chromatin remodelling factor CHD4. The EMBO Journal. 2010;29(18):3130–3139. doi: 10.1038/emboj.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poot RA, Bozhenok L, van den Berg DL, Hawkes N, Varga-Weisz PD. Chromatin remodeling by WSTF-ISWI at the replication site: Opening a window of opportunity for epigenetic inheritance? Cell Cycle. 2005;4(4):543–546. doi: 10.4161/cc.4.4.1624. [Research Support, Non-U.S. Gov’t Review] [DOI] [PubMed] [Google Scholar]
- Poot RA, Bozhenok L, van den Berg DL, Steffensen S, Ferreira F, Grimaldi M, et al. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nature Cell Biology. 2004;6(12):1236–1244. doi: 10.1038/ncb1196. [DOI] [PubMed] [Google Scholar]
- Poot RA, Dellaire G, Hulsmann BB, Grimaldi MA, Corona DF, Becker PB, et al. HuCHRAC, a human ISWI chromatin remodelling complex contains hACF1 and two novel histone-fold proteins. The EMBO Journal. 2000;19(13):3377–3387. doi: 10.1093/emboj/19.13.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts RC, Zhang P, Wurster AL, Precht P, Mughal MR, Wood WH, 3rd., et al. CHD5, a brain-specific paralog of Mi2 chromatin remodeling enzymes, regulates expression of neuronal genes. PLoS One. 2011;6(9):e24515. doi: 10.1371/journal.pone.0024515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Epigenetics: Core misconcept. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(18):7101–7103. doi: 10.1073/pnas.1305399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulukuri SM, Rao JS. CpG island promoter methylation and silencing of 14-3-3sigma gene expression in LNCaP and Tramp-C1 prostate cancer cell lines is associated with methyl-CpG-binding protein MBD2. Oncogene. 2006;25(33):4559–4572. doi: 10.1038/sj.onc.1209462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Dang S, Hou P. Gene methylation in gastric cancer. Clinica Chimica Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Rao M, Casimiro MC, Lisanti MP, D’Amico M, Wang C, Shirley LA, et al. Inhibition of cyclin D1 gene transcription by Brg-1. Cell Cycle. 2008;7(5):647–655. doi: 10.4161/cc.7.5.5446. [DOI] [PubMed] [Google Scholar]
- Reisman DN, Sciarrotta J, Wang W, Funkhouser WK, Weissman BE. Loss of BRG1/BRM in human lung cancer cell lines and primary lung cancers: Correlation with poor prognosis. Cancer Research. 2003;63(3):560–566. [PubMed] [Google Scholar]
- Reyes JC, Barra J, Muchardt C, Camus A, Babinet C, Yaniv M. Altered control of cellular proliferation in the absence of mammalian brahma (SNF2alpha) The EMBO Journal. 1998;17(23):6979–6991. doi: 10.1093/emboj/17.23.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Galusha SA, McMenamin ME, Fletcher CD, Orkin SH. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13796–13800. doi: 10.1073/pnas.250492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CW, Leroux MM, Fleming MD, Orkin SH. Highly penetrant, rapid tumorigenesis through conditional inversion of the tumor suppressor gene Snf5. Cancer Cell. 2002;2(5):415–425. doi: 10.1016/s1535-6108(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Nieto S, Canada A, Pros E, Pinto AI, Torres-Lanzas J, Lopez-Rios F, et al. Massive parallel DNA pyrosequencing analysis of the tumor suppressor BRG1/SMARCA4 in lung primary tumors. Human Mutation. 2011;32(2):E1999–E2017. doi: 10.1002/humu.21415. [DOI] [PubMed] [Google Scholar]
- Romero OA, Setien F, John S, Gimenez-Xavier P, Gomez-Lopez G, Pisano D, et al. The tumour suppressor and chromatin-remodelling factor BRG1 antagonizes Myc activity and promotes cell differentiation in human cancer. EMBO Molecular Medicine. 2012;4(7):603–616. doi: 10.1002/emmm.201200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J, Baek SH, Dutta A, Houry WA, Huber O, Hupp TR, et al. The emergence of the conserved AAA + ATPases Pontin and Reptin on the signaling landscape. Science Signaling. 2013;6(266):mr1. doi: 10.1126/scisignal.2003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl DD, Jin J, Cai Y, Swanson S, Florens L, Washburn MP, et al. Purification of a human SRCAP complex that remodels chromatin by incorporating the histone variant H2A.Z into nucleosomes. Biochemistry. 2006;45(17):5671–5677. doi: 10.1021/bi060043d. [DOI] [PubMed] [Google Scholar]
- Sanchez-Tillo E, Lazaro A, Torrent R, Cuatrecasas M, Vaquero EC, Castells A, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene. 2010;29(24):3490–3500. doi: 10.1038/onc.2010.102. [DOI] [PubMed] [Google Scholar]
- Schlabach MR, Luo J, Solimini NL, Hu G, Xu Q, Li MZ, et al. Cancer proliferation gene discovery through functional genomics. Science. 2008;319(5863):620–624. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenet N, Sheridan E, Amram D, Schneider P, Handgretinger R, Delattre O. Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. American Journal of Human Genetics. 1999;65(5):1342–1348. doi: 10.1086/302639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain AH, Pollack JR. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One. 2013;8(1):e55119. doi: 10.1371/journal.pone.0055119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Zhang Y. 5-Hydroxymethylcytosine: Generation, fate, and genomic distribution. Current Opinion in Cell Biology. 2013;25(3):289–296. doi: 10.1016/j.ceb.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JJ, Choi JH, Guan B, Tsai FJ, Hua CH, Lai MT, et al. Rsf-1, a chromatin remodelling protein, interacts with cyclin E1 and promotes tumour development. The Journal of Pathology. 2013;229(4):559–568. doi: 10.1002/path.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu JJ, Guan B, Choi JH, Lin A, Lee CH, Hsiao YT, et al. Rsf-1, a chromatin remodeling protein, induces DNA damage and promotes genomic instability. The Journal of Biological Chemistry. 2010;285(49):38260–38269. doi: 10.1074/jbc.M110.138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukeir N, Pakneshan P, Chen G, Szyf M, Rabbani SA. Alteration of the methylation status of tumor-promoting genes decreases prostate cancer cell invasiveness and tumorigenesis in vitro and in vivo. Cancer Research. 2006;66(18):9202–9210. doi: 10.1158/0008-5472.CAN-06-1954. [DOI] [PubMed] [Google Scholar]
- Skene PJ, Henikoff S. Histone variants in pluripotency and disease. Development. 2013;140(12):2513–2524. doi: 10.1242/dev.091439. [DOI] [PubMed] [Google Scholar]
- Smeenk G, Wiegant WW, Vrolijk H, Solari AP, Pastink A, van Attikum H. The NuRD chromatin-remodeling complex regulates signaling and repair of DNA damage. The Journal of Cell Biology. 2010;190(5):741–749. doi: 10.1083/jcb.201001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Mager GM, Ward RM, Mayer J, Svaren J. NAB2 represses transcription by interacting with the CHD4 subunit of the nucleosome remodeling and deacetylase (NuRD) complex. The Journal of Biological Chemistry. 2006;281(22):15129–15137. doi: 10.1074/jbc.M600775200. [DOI] [PubMed] [Google Scholar]
- Stopka T, Skoultchi AI. The ISWI ATPase Snf2h is required for early mouse development. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14097–14102. doi: 10.1073/pnas.2336105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobeck MW, DeCristofaro MF, Banine F, Weissman BE, Sherman LS, Knudsen ES. The BRG-1 subunit of the SWI/SNF complex regulates CD44 expression. The Journal of Biological Chemistry. 2001;276(12):9273–9278. doi: 10.1074/jbc.M009747200. [DOI] [PubMed] [Google Scholar]
- Strohner R, Nemeth A, Jansa P, Hofmann-Rohrer U, Santoro R, Langst G, et al. NoRC—A novel member of mammalian ISWI-containing chromatin remodeling machines. The EMBO Journal. 2001;20(17):4892–4900. doi: 10.1093/emboj/20.17.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamborero D, Gonzalez-Perez A, Perez-Llamas C, Deu-Pons J, Kandoth C, Reimand J, et al. Comprehensive identification of mutational cancer driver genes across 12 tumor types. Scientific Reports. 2013;3:2650. doi: 10.1038/srep02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1982;79(24):7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toiber D, Erdel F, Bouazoune K, Silberman DM, Zhong L, Mulligan P, et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Molecular Cell. 2013;51(4):454–468. doi: 10.1016/j.molcel.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong JK, Hassig CA, Schnitzler GR, Kingston RE, Schreiber SL. Chromatin deacetylation by an ATP-dependent nucleosome remodelling complex. Nature. 1998;395(6705):917–921. doi: 10.1038/27699. [DOI] [PubMed] [Google Scholar]
- Tosi A, Haas C, Herzog F, Gilmozzi A, Berninghausen O, Ungewickell C, et al. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell. 2013;154(6):1207–1219. doi: 10.1016/j.cell.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Toyoshima M, Howie HL, Imakura M, Walsh RM, Annis JE, Chang AN, et al. Functional genomics identifies therapeutic targets for MYC-driven cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(24):9545–9550. doi: 10.1073/pnas.1121119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsikitis M, Zhang Z, Edelman W, Zagzag D, Kalpana GV. Genetic ablation of Cyclin D1 abrogates genesis of rhabdoid tumors resulting from Ini1 loss. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12129–12134. doi: 10.1073/pnas.0505300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama M, Sabri A, Bartholomew B. The INO80 ATP-dependent chromatin remodeling complex is a nucleosome spacing factor. Molecular and Cellular Biology. 2011;31(4):662–673. doi: 10.1128/MCB.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdman A, Nordenskjold A, Fang X, Naito A, Al-Shukri S, Larsson C, et al. Mutation analysis of the BRG1 gene in prostate cancer clinical samples. International Journal of Oncology. 2003;22(5):1003–1007. [PubMed] [Google Scholar]
- Van Rechem C, Boulay G, Pinte S, Stankovic-Valentin N, Guerardel C, Leprince D. Differential regulation of HIC1 target genes by CtBP and NuRD, via an acetylation/SUMOylation switch, in quiescent versus proliferating cells. Molecular and Cellular Biology. 2010;30(16):4045–4059. doi: 10.1128/MCB.00582-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaronga MA, Lopez-Mateo I, Markert L, Espinosa E, Fresno Vara JA, Belandia B. Identification and characterization of novel potentially oncogenic mutations in the human BAF57 gene in a breast cancer patient. Breast Cancer Research and Treatment. 2011;128(3):891–898. doi: 10.1007/s10549-011-1492-4. [DOI] [PubMed] [Google Scholar]
- Vries RG, Bezrookove V, Zuijderduijn LM, Kia SK, Houweling A, Oruetxebarria I, et al. Cancer-associated mutations in chromatin remodeler hSNF5 promote chromosomal instability by compromising the mitotic checkpoint. Genes & Development. 2005;19(6):665–670. doi: 10.1101/gad.335805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen H, Fu S, Xu ZM, Sun KL, Fu WN. The involvement of CHD5 hypermethylation in laryngeal squamous cell carcinoma. Oral Oncology. 2011;47(7):601–608. doi: 10.1016/j.oraloncology.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Wang W, Cote J, Xue Y, Zhou S, Khavari PA, Biggar SR, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. The EMBO Journal. 1996;15(19):5370–5382. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nature Genetics. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang B, Faller DV. Prohibitin requires Brg-1 and Brm for the repression of E2F and cell growth. The EMBO Journal. 2002;21(12):3019–3028. doi: 10.1093/emboj/cdf302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AB, Sali A, Wilson IA. Biochemistry. Integrative structural biology. Science. 2013;339(6122):913–915. doi: 10.1126/science.1228565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein IB. Cancer. Addiction to oncogenes—The Achilles heal of cancer. Science. 2002;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. The New England Journal of Medicine. 2010;363(16):1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BG, Wang X, Shen X, McKenna ES, Lemieux ME, Cho YJ, et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell. 2010;18(4):316–328. doi: 10.1016/j.ccr.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Cox LK, Chrivia JC. The chromatin remodeling protein, SRCAP, is critical for deposition of the histone variant H2A.Z at promoters. The Journal of Biological Chemistry. 2007;282(36):26132–26139. doi: 10.1074/jbc.M703418200. [DOI] [PubMed] [Google Scholar]
- Wong AK, Shanahan F, Chen Y, Lian L, Ha P, Hendricks K, et al. BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Research. 2000;60(21):6171–6177. [PubMed] [Google Scholar]
- Wong-Staal F, Dalla-Favera R, Franchini G, Gelmann EP, Gallo RC. Three distinct genes in human DNA related to the transforming genes of mammalian sarcoma retroviruses. Science. 1981;213(4504):226–228. doi: 10.1126/science.6264598. [DOI] [PubMed] [Google Scholar]
- Wood MA, McMahon SB, Cole MD. An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-Myc. Molecular Cell. 2000;5(2):321–330. doi: 10.1016/s1097-2765(00)80427-x. [DOI] [PubMed] [Google Scholar]
- Woodcock CL, Ghosh RP. Chromatin higher-order structure and dynamics. Cold Spring Harbor Perspectives in Biology. 2010;2(5):a000596. doi: 10.1101/cshperspect.a000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Kingston RE. Nucleosome core displacement in vitro via a metastable transcription factor-nucleosome complex. Science. 1992;258(5089):1780–1784. doi: 10.1126/science.1465613. [DOI] [PubMed] [Google Scholar]
- Wu S, Shi Y, Mulligan P, Gay F, Landry J, Liu H, et al. A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair. Nature Structural & Molecular Biology. 2007;14(12):1165–1172. doi: 10.1038/nsmb1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Zhu S, Romero A, Lorger M, Watson J, Chiang CY, et al. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 2010;6(1):37–47. doi: 10.1016/j.stem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Xia W, Nagase S, Montia AG, Kalachikov SM, Keniry M, Su T, et al. BAF180 is a critical regulator of p21 induction and a tumor suppressor mutated in breast cancer. Cancer Research. 2008;68(6):1667–1674. doi: 10.1158/0008-5472.CAN-07-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Price BD. Chromatin dynamics and the repair of DNA double strand breaks. Cell Cycle. 2011;10(2):261–267. doi: 10.4161/cc.10.2.14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Sun Y, Jiang X, Ayrapetov MK, Moskwa P, Yang S, et al. The p400 ATPase regulates nucleosome stability and chromatin ubiquitination during DNA repair. The Journal of Cell Biology. 2010;191(1):31–43. doi: 10.1083/jcb.201001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Xiao Y, Wang W, Gao JX, Yearsley K, Yan Q, et al. Singular v dual inhibition of SNF2L and its isoform, SNF2LT, have similar effects on DNA damage but opposite effects on the DNA damage response, cancer cell growth arrest and apoptosis. Oncotarget. 2012;3(4):475–489. doi: 10.18632/oncotarget.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Xiao Y, Wang W, Wang Q, Yearsley K, Wani AA, et al. Inhibition of expression of the chromatin remodeling gene, SNF2L, selectively leads to DNA damage, growth inhibition, and cancer cell death. Molecular Cancer Research. 2009;7(12):1984–1999. doi: 10.1158/1541-7786.MCR-09-0119. [DOI] [PubMed] [Google Scholar]
- Yeh JE, Toniolo PA, Frank DA. Targeting transcription factors: Promising new strategies for cancer therapy. Current Opinion in Oncology. 2013;25(6):652–658. doi: 10.1097/01.cco.0000432528.88101.1a. [DOI] [PubMed] [Google Scholar]
- Yip DJ, Corcoran CP, Alvarez-Saavedra M, DeMaria A, Rennick S, Mears AJ, et al. Snf2l regulates Foxg1-dependent progenitor cell expansion in the developing brain. Developmental Cell. 2012;22(4):871–878. doi: 10.1016/j.devcel.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo YG, Kong G, Lee MO. Metastasis-associated protein 1 enhances stability of hypoxia-inducible factor-1alpha protein by recruiting histone deacetylase 1. The EMBO Journal. 2006;25(6):1231–1241. doi: 10.1038/sj.emboj.7601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Hazan I, Zhang J, Ng SY, Naito T, Snippert HJ, et al. The role of the chromatin remodeler Mi-2beta in hematopoietic stem cell self-renewal and multilineage differentiation. Genes & Development. 2008;22(9):1174–1189. doi: 10.1101/gad.1642808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M, Wu J, Workman JL, Li B. Readers of histone modifications. Cell Research. 2011;21(4):564–578. doi: 10.1038/cr.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nature Genetics. 2012;44(5):570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- Zhang XY, DeSalle LM, Patel JH, Capobianco AJ, Yu D, Thomas-Tikhonenko A, et al. Metastasis-associated protein 1 (MTA1) is an essential downstream effector of the c-MYC oncoprotein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):13968–13973. doi: 10.1073/pnas.0502330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wippo CJ, Wal M, Ward E, Korber P, Pugh BF. A packing mechanism for nucleosome organization reconstituted across a eukaryotic genome. Science. 2011;332(6032):977–980. doi: 10.1126/science.1200508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Yan Q, Lv J, Huang H, Zheng W, Zhang B, et al. CHD5, a tumor suppressor that is epigenetically silenced in lung cancer. Lung Cancer. 2012;76(3):324–331. doi: 10.1016/j.lungcan.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Zhuravel E, Shestakova T, Glushko N, Soldatkina M, Pogrebnoy P. Expression patterns of murine beta-defensin-2 mRNA in Lewis lung carcinoma cells in vitro and in vivo. Experimental Oncology. 2008;30(3):206–211. [PubMed] [Google Scholar]
- Ziller MJ, Muller F, Liao J, Zhang Y, Gu H, Bock C, et al. Genomic distribution and inter-sample variation of non-CpG methylation across human cell types. PLoS Genetics. 2011;7(12):e1002389. doi: 10.1371/journal.pgen.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupkovitz G, Grausenburger R, Brunmeir R, Senese S, Tischler J, Jurkin J, et al. The cyclin-dependent kinase inhibitor p21 is a crucial target for histone deacetylase 1 as a regulator of cellular proliferation. Molecular and Cellular Biology. 2010;30(5):1171–1181. doi: 10.1128/MCB.01500-09. [DOI] [PMC free article] [PubMed] [Google Scholar]