Abstract
Rapid evaluation of therapies designed to preserve β cells in persons with type 1 diabetes (T1D) is hampered by limited availability of sensitive β-cell health biomarkers. In particular, biomarkers elucidating the presence and degree of β-cell stress are needed. We characterized β-cell secretory activity and stress in 29 new-onset T1D subjects (10.6 ± 3.0 years, 55% male) at diagnosis and then 8.2 ± 1.2 weeks later at first clinic follow-up. We did comparisons with 16 matched healthy controls. We evaluated hemoglobin A1c (HbA1c), β-cell function (random C-peptide [C] and proinsulin [PI]), β-cell stress (PI:C ratio), and the β-cell stress marker heat shock protein (HSP)90 and examined these parameters’ relationships with clinical and laboratory characteristics at diagnosis. Mean diagnosis HbA1c was 11.3% (100 mmol/mol) and 7.6% (60 mmol/mol) at follow-up. C-peptide was low at diagnosis (P < 0.001 vs controls) and increased at follow-up (P < 0.001) to comparable with controls. PI did not differ from controls at diagnosis but increased at follow-up (P = 0.003) signifying increased release of PI alongside improved insulin secretion. PI:C ratios and HSP90 concentrations were elevated at both time points. Younger subjects had lower C-peptide and greater PI, PI:C, and HSP90. We also examined islets isolated from prediabetic nonobese diabetic mice and found that HSP90 levels were increased ~4-fold compared with those in islets isolated from matched CD1 controls, further substantiating HSP90 as a marker of β-cell stress in T1D. Our data indicate that β-cell stress can be assessed using PI:C and HSP90. This stress persists after T1D diagnosis. Therapeutic approaches to reduce β-cell stress in new-onset T1D should be considered.
INTRODUCTION
Type 1 diabetes (T1D) is characterized by autoimmune pancreatic β-cell destruction. After diagnosis and insulin therapy initiation, persons with new-onset T1D frequently enter a transient partial recovery of β-cell function known as the “honeymoon” period. The maximum recovery is typically reached 2–4 months after diagnosis and characterized by increased endogenous insulin secretion, decreased exogenous insulin demands, and improved glycemic control.1–3 The honeymoon period has been characterized in numerous ways, with all definitions including 1 or more of the following parameters: hemoglobin A1c (HbA1c) < 8.0% (64 mmol/mol), daily total insulin dose <0.5 units per Kg body weight per day (U/kg/d), or stimulated insulin connecting peptide (C-peptide) concentrations > 300 pM.1,3 However, this remission is nearly always relatively short lived.
Emerging data from rodent and human models suggest that activation of intrinsic β-cell stress pathways such as endoplasmic reticulum (ER) stress, oxidative stress, and mitochondrial dysfunction contribute to T1D pathogenesis.4–6 These pathways likely become activated early during the progression toward T1D and may either trigger autoimmunity through neoantigen formation or act independently to accelerate autoimmune-mediated β-cell death.7–11 Robustly and noninvasively identifying activation of these processes and monitoring their progression after T1D diagnosis are not currently feasible. Moreover, whether the honeymoon period is associated with modulation of β-cell stress remains undefined.
Insulin is synthesized in β cells as the precursor molecule preproinsulin, which consists of an N-terminal signal peptide, the insulin B chain, C-peptide, and the insulinA chain. As the newly synthesized insulin protein is translocated into the ER lumen to undergo folding and maturation, the signal peptide is removed generating a proinsulin (PI) molecule. PI cleavage (into insulin and C-peptide) occurs in secretory granules before β-cell exocytosis. Under normal conditions, little intact PI is secreted.12 An elevation in the proportional secretion of PI relative to fully processed, mature insulin (assessed using C-peptide) is indicative of β-cell dysfunction and is primarily thought to reflect alterations in insulin protein folding and processing that originate in the ER.12,13 Under inflammatory conditions, isolated islets release of PI increases.14 Because pre-PI is the most abundant protein produced by the β cell, alterations in this molecule’s processing not only provide insight into β-cell secretory capacity but may also provide an assessment of overall ER health.
When proteins fail to fold correctly within the ER lumen, an unfolded protein response (UPR) is activated. 7,8 The UPR decreases new protein delivery to the ER, restores cellular homeostasis, and ultimately increases ER protein-folding capacity through key chaperone protein synthesis such as protein disulfide isomerase and heat shock proteins (HSPs), for example, HSP90.7,15,16 If the inciting stress is unresolved, continual UPR stimulation can lead to activation of proapoptotic pathways and eventual β-cell death. This transition is referred to as ER stress.7,8 ER health alterations may arise from a variety of perturbations relevant to T1D pathophysiology including autoimmunity and inflammation, intracellular calcium homeostasis alterations, oxidative stress, and hyperglycemia. Islets from nonobese diabetic (NOD) mice demonstrate increased activation of these pathways before and at T1D onset.4 Moreover, pancreatic section analyses from humans with T1D, obtained through the Network of Pancreatic Organ Donors with Diabetes program, demonstrate increased expression of ER stress molecules such as CCAAT/enhancerbinding protein homologous protein (CHOP) and binding of immunoglobulin protein (BIP).6
Our aim was to evaluate the activation and modulation of intrinsic β-cell stress pathways in subjects at T1D diagnosis and during the early honeymoon period. β-cell secretory activity was quantified by measuring serum C-peptide and PI. To provide a functional assessment of β-cell stress and ER function, PI to C-peptide (PI:C) ratios were calculated and total serum HSP90 concentrations measured. Relationships between measures of β-cell stress and variables that might influence stress attenuation or diabetes remission including age, gender, body mass index (BMI), and serum bicarbonate at diagnosis were also defined.
RESEARCH DESIGN AND METHODS
Subjects
This study was approved by the Indiana University Institutional Review Board. Subjects aged 7–18 years with new-onset T1D were sequentially recruited over a 12-month period whereas hospital inpatients within 1–3 days of diagnosis. Informed consent was obtained from parents with assent from children. Subjects were defined as having T1D if they had 1 or more positive autoantibodies with clinical features of T1D (including hyperglycemia, weight loss, normal BMI) or were autoantibody negative but aged <10 years at diagnosis. Exclusion criteria included diabetic ketoacidosis requiring an intensive care unit stay, diabetes other than T1D, history of prior chronic illness known to affect glucose metabolism, use of medications known to affect glucose metabolism, history of smoking, use of statins or angiotensin converting enzyme inhibitors, psychiatric impairment, or current use of antipsychotic medications. Subjects received a $10 gift card for each visit. All T1D subjects had peripheral blood drawn at 2 time points: at diagnosis and at the first return clinic visit 6–10 weeks after diagnosis during the honeymoon initiation period. Random (mostly nonfasting) samples were collected in serum-separator tubes. Serum was isolated by centrifugation and stored at −80°C.
Control sera from nondiabetic healthy children were obtained from a biorepository at Indiana University School of Medicine and matched based on gender, age, and BMI. BMI z scores were calculated using online software (http://stokes.chop.edu/web/zscore/). Of note, 14 subjects did not have a height obtained at diagnosis, and therefore heights from the second assessment time were used for BMI calculations.
Laboratory assays
Autoantibodies to glutamic acid decarboxylase 65, Insulin (mIAA), and Islet Antigen 2 (IA-2) were assayed from peripheral blood at diagnosis or at the first clinic follow-up at Mayo Clinic Laboratories (Rochester, Minnesota). HbA1c levels were measured at diagnosis and at first clinic visit by point-of-care sampling using either the Bayer A1cNow system or the Bayer DCA 2000 (Tarrytown, New York). Values obtained from other facilities before transfer to our hospital at the time of diagnosis were sometimes measured using other assays. For samples with values above the assay upper limit of detection (13.1% [120 mmol/mol]), 13.1% was used for subsequent analyses. Serum C-peptide, PI, and HSP90 were quantified in stored serum samples using capture enzyme linked immunosorbent assays and performed according to the manufacturer instructions. The C-peptide assay (Alpco, Salem, New Hampshire) detected levels in the range of 20–3000 pM with a sensitivity of 2.95 pM. The PI assay (Alpco) detected levels in the range of 2.5–180 pM with a sensitivity of 1.25 pM. Four samples had serum PI levels below the assay lower limit of detection. For these samples, a value of one-half the lower limit of detection was used.17 The HSP90 assay detected levels in the range of 0.78–50 ng/mL with a sensitivity of 0.2 ng/mL (Enzo Life Sciences, Farmingdale, New York).
Animals, islet preparations, and immunoblots
Animals were maintained under protocols approved by the Indiana University Institutional Animal Care and Use Committee, the United States Department of Agriculture Animal Welfare Act (9 Code of Federal Regulations Parts 1, 2, and 3), and the Guide for the Care and Use of Laboratory Animals.18 Female NOD/ShiLTJ (NOD) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine), and control CD1 mice were obtained from Charles River (Wilmington, Massachusetts) at the age of approximately 8 weeks. Mouse cages were kept in a standard light-dark cycle with ad libitum access to food and water. At 10 weeks, islets were isolated from both NOD and control CD1 mice as described previously.19 Immunoblot analysis was performed as described previously using anti-HSP90 (Enzo Life Sciences) and anti-Actin mouse antibodies (MP Biomedical, Santa Ana, California).20 Immunoblots were scanned using an LI-COR Odyssey 1828 scanner and analyzed with LI-COR Image Studio software. Densitometries of scanned images were calculated using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Statistics
Descriptive statistics were calculated for all variables. Means ± standard deviations are reported unless otherwise noted. Two sample t tests were used to compare the T1D group with the control group. Paired t tests were used to compare the T1D samples at the 2 time points (diagnosis and honeymoon initiation). Levene’s test for equality of variances and Pearson correlations were used to find linear relationships between 2 variables. SPSS version 20.0 (SPSS Inc., Chicago, Illinois) was used for all statistical analyses. Where indicated, adjustments were made for age, gender, and diagnosis C-peptide.
RESULTS
Evaluation of β-cell stress markers
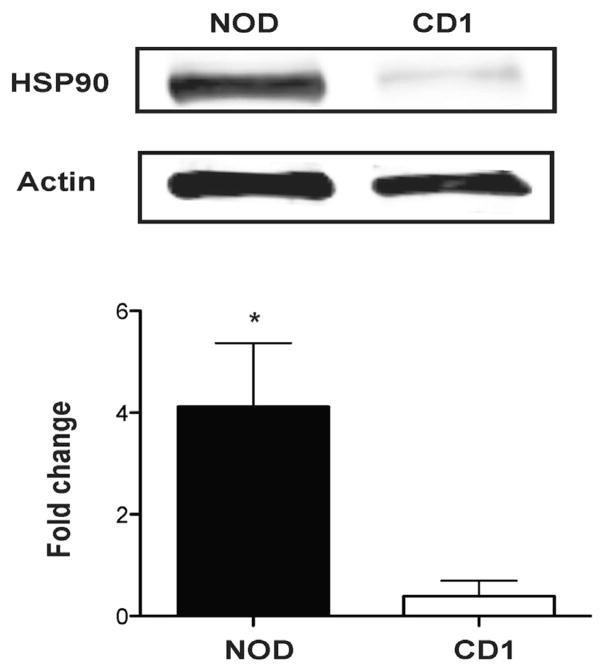
Previous work by our group has demonstrated a nearly 9-fold increase in serum PI:insulin ratios in prediabetic NOD mice, correlating with increased expression of established markers of ER stress signaling including spliced XBP-1, Bip, and Chop.4 To define whether islet expression of the protein chaperone HSP90 was similarly increased, immunoblots were performed. Results revealed a ~4-fold increase in HSP90 levels in islets isolated from NOD mice compared with islets isolated from age- and sex-matched CD1 controls (Fig 1; P < 0.05).
Fig 1.
Expression of HSP90 in islets from CD1 and NOD mice. A representative western blotting of 3 experiments (upper panel) shows that the expression of HSP90 is higher in islets from 10-week-old NOD mice compared with those from the age-matched control (CD1 mice). O.D for HSP90 normalized to the O.D for actin shows that HSP90 increases about 4 times in the islets from NOD relative to those from CD1 (lower panel). *P < 0.05. HSP, heat shock protein; NOD, nonobese diabetic; O.D, optical density.
Although these data support the use of the PI:C ratio and HSP90 as indicators of β-cell stress in a mouse model of T1D, the main goal of this work was to establish their utility in human T1D. We assembled and studied a cohort of 29 children with new-onset T1D. T1D subjects were then matched based on age, gender, and BMI matched to 16 healthy non-T1D control subjects. See Table I for demographic details. For 3 samples, complete matching was not possible, and samples were partially matched based on either subject age or subject BMI.21 Samples from control subjects were analyzed for all biomarkers; however, for T1D samples, analysis of HSP90 levels was carried out using serum from 27 patients.
Table I.
Study demographics
| Characteristic | Non-T1D controls | T1D subjects |
|---|---|---|
| Number of subjects* | 16 | 29 |
| Age (y), (range) | 10.5 ± 3.0 (4–15) | 10.6 ± 3.0 (4–15) |
| Gender (male) | 56% | 55% |
| BMI† (Kg/m2) | 18.15 ± 2.46 | 17.93 ± 3.31 |
| BMI z score†(range) | — | −0.12 ± 1.24 [−2.68 to 2.33] |
| Number of autoantibodies positive‡,§ | — | 0 AutoAb positive: 6.9% |
| 1 AutoAb positive: 17.2% | ||
| 2 AutoAb positive: 65.5% | ||
| 3 AutoAb positive: 10.3% | ||
| Basal insulin requirement prior to hospital discharge (units/kg/d) | — | 0.31 ± 0.09 |
| Basal insulin requirement at first outpatient clinic follow-up (units/kg/d) | — | 0.20 ± 0.1 |
| Time from diagnosis to first blood draw (range) | — | 1.2 d (1–3) |
| Time from diagnosis to first outpatient clinic follow-up (range) | — | 8.2 ± 1.2 wk (6–10) |
| HbA1c at diagnosis (range)|| | — | 11.3 ± 1.7% (7.5–15.7) |
| HbA1c at first outpatient clinic follow-up (range) | — | 7.6 ± 0.9% (6.4–9.7) |
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; T1D, type 1 diabetes.
Values displayed are means ± standard deviations unless otherwise noted.
For HSP90 analysis, number of T1D subjects = 27.
For BMI and z score calculation, 14 subjects did not have diagnosis heights. For calculation of BMI and z score at diagnosis, heights from clinic follow-up were used.
The following 3 diabetes-associated antibodies were tested: GAD, miAA, and IA-2A.
All autoantibody-negative subjects were aged <10 years.
Four subjects at diagnosis had HbA1c recorded as >13.1. For data analysis, a value of 13.1 was used.
Evaluation at diagnosis of T1D: glycemic control, islet secretory function, and level of β-cell stress
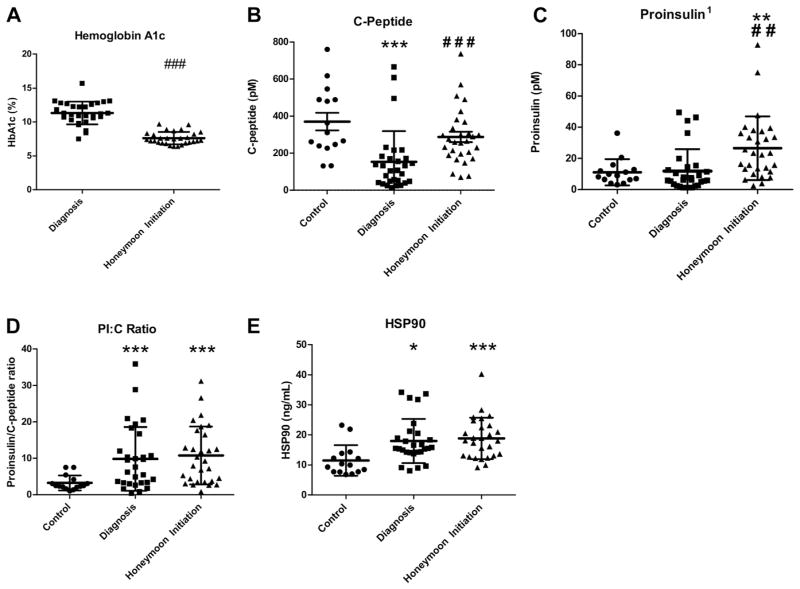
As expected, subjects newly diagnosed with T1D were hyperglycemic (mean HbA1c 11.3 ± 1.7% [100 mmol/mol], range 7.5%–15.7% [58 mmol/mol–148 mmol/mol]), with decreased serum C-peptide levels relative to healthy controls (P < 0.001). Serum PI concentrations of persons with T1D at diagnosis were not different than those of controls (P = 0.86). However, the PI:C ratio and serum HSP90 concentrations were significantly elevated at the time of diagnosis of T1D compared with controls (PI:C, P < 0.001; HSP90, P = 0.02; Fig 2, A–E).
Fig 2.
(A–E): Glycemic control (A), β-cell secretory activity (B, C), and β-cell stress (D, E) quantification. Healthy control and T1D subject sera at diagnosis and honeymoon period were analyzed for C-peptide, PI, and HSP90. (A) Hemoglobin A1c of T1D subjects at diagnosis and at honeymoon. (B) C-peptide levels in control and T1D subjects at diagnosis and at honeymoon. (C) PI levels in control and T1D subjects at diagnosis and at honeymoon. (D) β-cell stress assessed by the ratio of PI to C-peptide (PI:C) in control and T1D subjects at diagnosis and at honeymoon. (E) Stress assessed by serum HSP90 in controls and T1D subjects at diagnosis and at honeymoon. Error bars display standard deviations from the mean. Each circle represents one control subject, each square represents one T1D subject at diagnosis, each triangle represents one T1D subject at honeymoon initiation. *P < 0.05, **P < 0.01, ***P < 0.001 vs control based on independent sample t test. ##P < 0.01, ###P < 0.001 vs T1D subject at time of diagnosis based on paired sample t test. 1For subjects (n = 4) with serum PI below the LLD, a value of one-half the LLD was used. HSP, heat shock protein; LLD, lower limit of detection; PI, proinsulin; T1D, type 1 diabetes.
Evaluation at the initiation of honeymoon period: glycemic control, islet secretory function, and level of β-cell stress
During the honeymoon period, glycemic control significantly improved, with a mean HbA1c of 7.6 ± 0.9% (60 mml/mol) and range 6.4%–9.7% (46 mmol/mol–83 mmol/mol); P < 0.001 vs diagnosis (Fig 2, A). β-cell secretory activity improved, with significantly increased C-peptide concentrations (P < 0.001 vs diagnosis), to levels that were comparable to those of the controls (Fig 2, B). Interestingly, PI measured at this time point was increased relative to levels at the time of diagnosis (P = 0.003), and PI levels were also significantly higher than those observed in the controls (P = 0.001; Fig 2, C).
At the second blood draw 6–10 weeks after T1D diagnosis, the PI:C ratio and HSP90 levels remained significantly elevated compared with controls (PI:C, P < 0.001; HSP90, P < 0.001), suggesting continued β-cell stress (Fig 2, D and E). Compared with diagnosis, both markers trended toward increased concentrations, yet changes were not statistically significant.
Evaluation at the honeymoon initiation period: changes in islet secretory activity with time after diagnosis
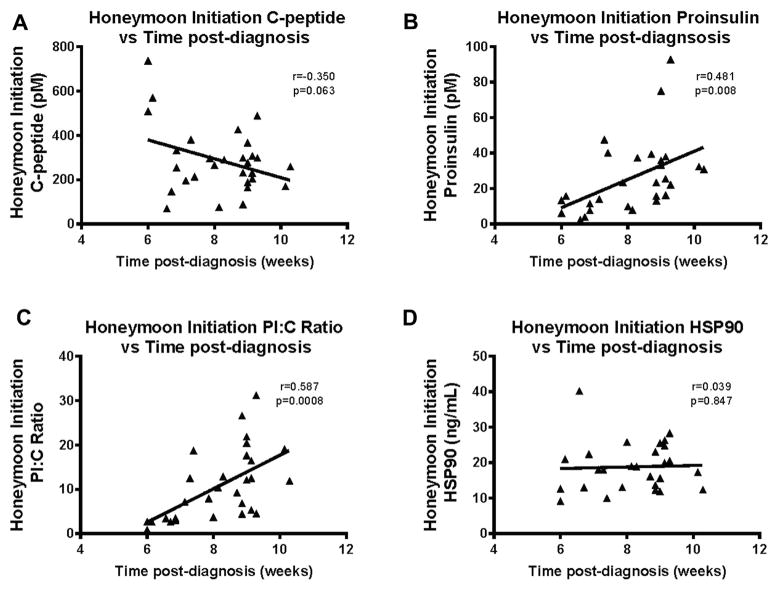
We then compared the relationship between C-peptide and PI and time elapsed between diagnosis and the second blood draw. T1D subjects’ total C-peptide concentrations trended to be lower as more time elapsed since diagnosis (r = −0.35; P = 0.063). However, a significant positive correlation (r = 0.481; P = 0.008) was observed between the PI concentration and the amount of time after diagnosis at second evaluation (Fig 3, A and B). This correlation with time after diagnosis was not attenuated after adjustment for age, gender, or C-peptide concentration at the time of diagnosis.
Fig 3.
Pearson correlations of relationship between β-cell secretory activity measures (A and B) and β-cell stress measures (C and D) with time after diagnosis of T1D. (A) A nonsignificant negative correlation is observed with lower C-peptide levels at second time point. (B) Significant positive correlation demonstrating that when second time point was further from diagnosis, C-peptide was higher. (C) Significant positive correlation is observed with subjects returning further from diagnosis having higher levels of PI:C. (D) No correlation was observed between HSP90 and time from diagnosis. Each triangle represents one T1D subject at the time of honeymoon initiation. HSP, heat shock protein; PI, proinsulin; T1D, type 1 diabetes.
Evaluation at the honeymoon initiation period: changes in PI:C and HSP90 with time after diagnosis
PI:C and HSP90 did not change significantly between diagnosis and the honeymoon period. However, we observed a positive correlation between PI:C and the time from diagnosis to the second time point (r = 0.587; P = 0.0008). The correlation between HSP90 and time from diagnosis was not significant (r = 0.03; P = 0.847; Fig 3, C and D).
β-cell secretory activity and β-cell stress with variables: age, gender, and BMI at diagnosis
Because data from other studies suggest that younger children have a shorter honeymoon period compared with older children, 1,2 we next examined how age at diagnosis related to β-cell secretory function and degree of β-cell stress. We divided our population into 2 groups: (1) young children, age <10 years (n = 12; mean age 7.6 ± 1.6 years; 58% male; BMI z score at diagnosis 0.0 ± 1.3; HbA1c at diagnosis 11.2 ± 1.4% [99 mmol/mol]; HbA1C at honeymoon initiation 7.5 ± 0.7% [58 mmol/mol]), and (2) older subjects age ≥10 years (n = 17; mean age 12.8 ± 1.5; 53% male; BMI z score at diagnosis −0.2 ± 1.3; HbA1C at diagnosis 11.4% ± 1.9 [101 mmol/mol]; HbA1C at honeymoon initiation 7.6% ± 1.1 [60 mmol/mol]).
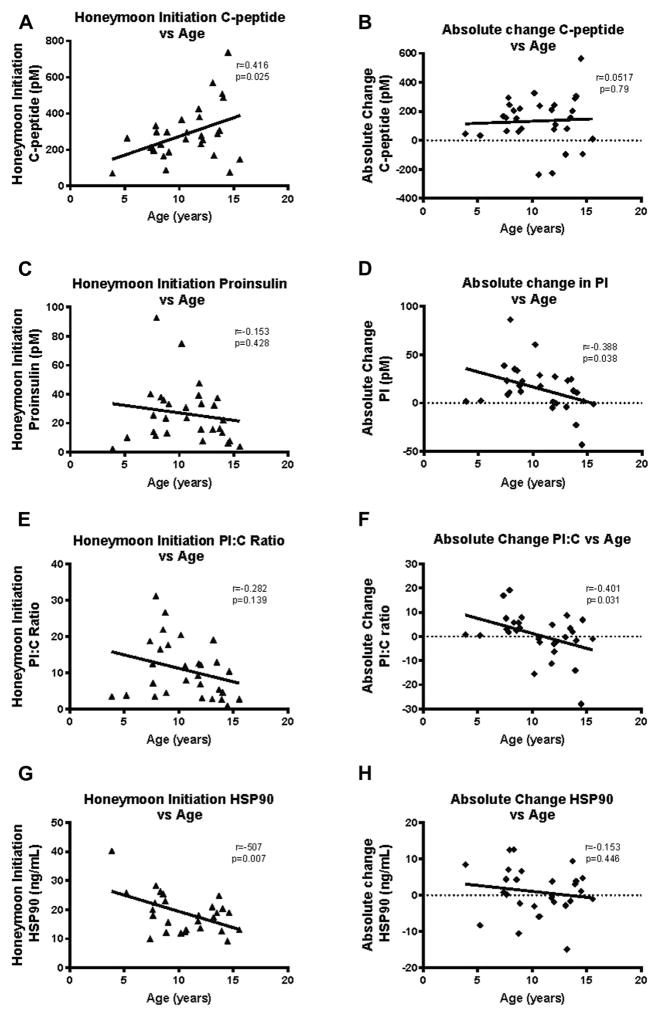
At diagnosis and at the time of the second blood draw, subjects aged ≥10 years had significantly higher C-peptide compared with the younger children (diagnosis: P = 0.008; honeymoon: P = 0.011). We also examined age as a continuous variable and found that at the time of second visit, younger subjects had lower C-peptide (r = 0.416 P = 0.025). The absolute changes in C-peptide concentrations between diagnosis and honeymoon did not vary with subject age (Fig 4, A and B).
Fig 4.
Pearson correlation between β-cell secretory activity (A and B) and β-cell stress (C and D) with age at diagnosis. (A) A significant positive correlation is observed with younger subjects having lower levels of C-peptide at honeymoon. (B) No correlation was observed between the age of subject at diagnosis and the absolute change in PI:C levels between diagnosis and honeymoon. (C) A nonsignificant negative correlation is observed between PI at honeymoon and subject age. (D) A significant negative correlation is observed between absolute change in PI levels from diagnosis to honeymoon and subject age. Older subjects were more likely to have a decrease in PI than younger subjects during the honeymoon initiation time point. (E) There was a nonsignificant negative correlation between β-cell stress measured by PI:C ratios and age at diagnosis. (F) A significant negative correlation was observed between the absolute change in PI:C ratio from diagnosis to honeymoon period and subject age at diagnosis. Older subjects were more likely to have a decrease in PI:C ratios at the honeymoon time point. (G) A significant negative correlation was observed between HSP90 levels at honeymoon initiation time point and subject age. Older subjects had lower HSP90 concentrations at honeymoon. (H) A nonsignificant negative correlation was observed between the absolute change in HSP90 between diagnosis and honeymoon. Each triangle represents one T1D subject at the time of honeymoon initiation; each diamond represents the difference between baseline and honeymoon initiation values in one T1D subject. HSP, heat shock protein; PI, proinsulin.
Similar to C-peptide, PI levels at diagnosis in the ≥10-year age group were significantly elevated compared with those of <10 (P = 0.004). The PI concentrations in the≥10-year age group at the honeymoon initiation were overall lower than in those aged <10 years, but this comparison was not significant (P=0.693; Supplementary Fig 1, B). There was a small nonsignificant negative correlation between PI level at the honeymoon initiation and subject age. Of note, there was a significant negative correlation between subject age and absolute change in PI levels from diagnosis to honeymoon (r=−0.388; P=0.037). It was more common for older subjects to have decreases in PI from time of diagnosis to honeymoon initiation than younger subjects (Fig 4, C and D).
There were no significant differences in PI:C either at diagnosis or at the second blood draw between the 2 age categories (<10 and ≥10 years). Although differences were not significant, the ≥10-year age group had higher PI:C ratios than the younger age group at diagnosis, but at follow-up, the ≥10 years of age category PI:C was lower (Supplementary Fig 1, C). When age was considered as a continuous variable, although older patients again tended to have lower PI:C in the honeymoon period, this trend was not significant. Of note, age at diagnosis and the absolute change in PI:C ratio from diagnosis to follow-up were significantly correlated, with older subjects being more likely to have a decrease in PI:C ratios than younger subjects (Fig 4, E and F).
At diagnosis and at the honeymoon time point, subjects aged ≥10 years had lower HSP90 concentrations than subjects aged <10 years. During the honeymoon period, this trend achieved statistical significance (P = 0.017; Supplementary Fig 1, D). A significant negative correlation was observed between subject age and HSP90 at the honeymoon initiation time point (r = −0.507, P= 0.007). There was a nonsignificant negative correlation between absolute change in HSP90 concentration between diagnosis and honeymoon and subject age at T1D diagnosis (Fig 4, G and F). HSP90 and PI:C ratios were not correlated with HbA1c at either time point, either before or after adjustment for age.
There were no significant differences based on gender for C-peptide, PI, or PI:C at either of the 2 time points. At the honeymoon time point, males had higher HSP90 concentrations (P = .041). However, at diagnosis, the HSP90 increase in males was not significant.
There were no significant correlations between BMI z scores or bicarbonate at diagnosis and C-peptide, PI, PI:C ratio, and HSP90 concentrations at T1D diagnosis or at the honeymoon period.
DISCUSSION
The prediagnostic phase of T1D is marked by clinically silent changes in β-cell function. Insulitis and β-cell injury occur early and are followed by decreased first-phase insulin response and changes in β-cell glucose sensitivity.22 With continued β-cell destruction, dysglycemia ensues and T1D becomes clinically evident.23 Recently, there has been an increasing appreciation for the role of intrinsic β-cell stress pathways in T1D pathophysiology and progression. However, noninvasive β-cell stress measures in vivo are lacking, and little is known about changes in β-cell stress after diagnosis.
The T1D honeymoon represents a period of improved metabolic control clinically characterized by low exogenous insulin requirements and decreased blood glucose lability. This period is associated with improved β-cell secretory function, shown by greater endogenous C-peptide and improved insulin sensitivity.24 As expected, within the studied cohort, we observed improved β-cell secretory function during the honeymoon period. Our primary goal was to provide an assessment of β-cell health at T1D diagnosis and to determine how β-cell stress evolved during the honeymoon period. To this end, we measured the relative proportion of PI to fully processed insulin secretion (assessed by measurement of C-peptide) and levels of the stress chaperone HSP90. As validation that these markers accurately reflected a state of β-cell stress, the same assessments were performed in age, gender, and BMI-matched controls.
Both HSP90 levels and the PI:C ratio were significantly higher in new-onset subjects compared with healthy, nondiabetic controls. Interestingly, despite amelioration of hyperglycemia and parallel increases in serum PI and C-peptide, there were no differences in the PI:C ratio or HSP90 levels between values obtained at T1D diagnosis and those obtained on average 8 weeks later. These findings indicate that although secretory function of the β cell increased, overall β-cell health or level of β-cell stress may not change substantially in the honeymoon period.
Increased PI:C ratios have been shown previously in autoantibody-positive first-degree relatives of persons with T1D with and without decreased first-phase insulin response.13,25,26 In additon, elevated PI:C ratios have been described in individuals with new-onset T1D before insulin initiation.27 Our findings do contradict 1 previous study demonstrating significantly increased PI:C levels after T1D diagnosis.28 However, we looked at β-cell stress levels immediately after diagnosis and during the very initial honeymoon period, whereas the prior study evaluated β-cell stress levels at longer time periods after diagnosis. It is plausible that levels of stress change with more prolonged disease states. Longitudinal studies capturing additional time points after diagnosis would be needed to address this.
We selected serum HSP90 as a second potential marker of β-cell stress. HSP90 is a cytoplasmic chaperone that plays an active role in mitigating the UPR and ER stress by binding and stabilizing 2 ER stress sensors, PERK and IRE1α, to attenuate cellular damage, including in β-cell lines.29,30 PERK in β cells is a key modulator of insulin synthesis during stress.31 In addition, studies indicate that oxidative stress can promote measurable cellular release of HSP90.32 Notably, alterations in autoantibody responses to HSP90 have been reported in patients with T1D.33 Given the association with ER and oxidative stress, HSP90 is therefore a plausible candidate for a serum marker of β-cell stress.34,35 In the NOD mouse model at 10 weeks, before the onset of overt hyperglycemia, there is an associated 4-fold increase in HSP90. To our knowledge, this is the first assessment of HSP90 as a potential T1D biomarker.
It is noteworthy that the improvement in hyperglycemia due to insulin therapy appeared to have no effect on the level of β-cell stress. Hyperglycemia is known to be toxic to pancreatic β cells and has been shown in vitro to activate ER and oxidative stress pathways.36,37 In addition, preclinical and clinical literature have revealed a role for insulin signaling in maintenance of β-cell health, survival, and proliferation.38 However, the continued high PI:C and HSP90 during the honeymoon period suggests that restoration of insulin signaling with exogenous insulin may play a minor role, if any, in reducing β-cell stress. This finding is consistent with data showing that T1D patients treated with intensive closed-loop insulin therapy at the time of diagnosis did not experience improved preservation of β-cell function measured by C-peptide retention relative to subjects who did not receive closed-loop therapy at diagnosis.39 These data support the continued effect of autoimmunity on β-cell health and also suggest that β-cell pathways activated during the progression of T1D are not easily extinguished despite improvements in the overall metabolic status. The results also suggest that β-cell “rest” in T1D may not dramatically improve overall β-cell health.
Of the variables, age, gender, BMI and bicarbonate at diagnosis, we only observed a significant relationship between the age at diagnosis, β-cell secretory function, and β-cell stress. Our data concur with previous clinical findings that younger individuals have shorter honeymoon periods than those of older T1D patients.1,2 The directly inverse relationship indicates the possibilities of an immature β cell more rapidly succumbing with loss of metabolic stability in this period or of a less mature immune system launching more aggressive immune assault. Younger subjects’ lower PI levels at diagnosis and increase in PI levels after diagnosis indicate that this subset of individuals have a much more rapid loss of β-cell ER functionality. Our findings indicate that younger age is associated with decreased β-cell adaptation at the time of honeymoon initiation. Of note, we did not observe any effect based on gender, BMI, or acidosis. The lack of changes seen with BMI may be attributed to unstable and unpredictable weight changes in the period preceding and after T1D diagnosis. We may not have seen changes with acidosis because we excluded subjects who required an ICU stay at diagnosis.
Our study has several limitations. Although we believe that the observed PI elevations indicate continued secretion of incompletely processed insulin and β-cell protein processing disruption, we acknowledge this could reflect altered PI systemic clearance. Elevated circulating serum PI might also reflect greater immature β-cell granule releasewith a higher content of intact PI.40,41 Furthermore, this was a pilot study with a relatively small sample size. In addition, we assessed random measures of C-peptide, PI, and HSP90. These measures may be affected by the degree of fasting, which was not taken into account with study design because we recruited patients during breaks in inpatient education and did follow-ups during regularly scheduled clinic visits. Future study would benefit from characterization of C-peptide and PI secretion using provocative stimulatory tests such as a mixed-meal tolerance test. Finally, although HSP90 is present in β cells, it is not exclusive to β cells. Stress and inflammation likely exert tissue-specific effects on HSP90 release as serum levels of this chaperone were increased in lupus erythematosus patients, although decreased serum HSP90 was detected in patients with autoimmune bullous pemphigoid.42,43
CONCLUSIONS
The diagnosis of T1D reflects a failure of maintenance of adequate insulin production and is associated with increases in circulating serum PI and HSP90. Despite significant improvements in metabolic control during the honeymoon period, sustained β-cell stress is observed. We observed that young T1D subjects demonstrate greater evidence of β-cell dysfunction and stress, characterized by lower C-peptide and higher PI than older children.
Once the loss of metabolic control has occurred and individuals leave the honeymoon phase, there is greater difficulty reaching glycemic targets. Thus, the honeymoon period’s stabilized metabolic control and increased β-cell secretion of insulin represents a transient golden period for the T1D patient. Still as shown here, even during the honeymoon period, β-cell stress is sustained pointing toward eventual loss of function. Better characterization of the β cell during this period marks a first step toward defining the optimal window for β-cell–specific therapies.
Supplementary Material
AT A GLANCE COMMENTARY.
Watkins RA, et al
Background
Type 1 diabetes (T1D) is attributed to autoimmune-mediated β-cell destruction. Emerging data suggest that endoplasmic reticulum and oxidative stress pathways are triggered early within the β cell during the evolution of T1D and may initiate and accelerate autoimmune-mediated β-cell destruction.
Translational Significance
Our article describes changes in β-cell C-peptide secretion and biomarkers of β-cell stress in young persons with recent-onset T1D. In particular, we examine 2 biomarkers: proinsulin/C-peptide ratios and heat shock protein 90 concentrations. These data mark initial steps toward a long-term goal of establishing treatments aimed at sustaining β-cell secretory function by alleviating β-cell stress after diagnosis.
Acknowledgments
This work was supported by the Wells Center for Pediatric Research; JDRF grant numbers 47-2012-744, 47-2013-523, and 3-SRA-2014-41-Q-R; NIH National Center for Advancing Translational Sciences Clinical and Translational Sciences Award grant number UL1 TR000006 U01 and RO1 grants RO1 AI079065 (to J. S. Blum) and R01 DK093954 (to C. Evans-Molina); and VA Merit Award 1I01BX001733 (to C. Evans-Molina).
Abbreviations
- BiP
binding of immunoglobulin protein
- BMI
body mass index
- CHOP
CCAAT/enhancer-binding protein homologous protein
- C-peptide
insulin connecting peptide
- ER
endoplasmic reticulum
- FPIR
first phase insulin response
- GAD65
glutamic acid decarboxylase 65
- HbA1c
hemoglobin A1c
- HSP
heat shock protein
- mIAA
insulin autoantibody
- IA-2
islet antigen 2
- LLD
lower limit of detection
- nPOD
Network of Pancreatic Organ Donors with Diabetes
- NOD
nonobese diabetic
- PI
proinsulin
- TID
type 1 diabetes
- UPR
unfolded protein response
Footnotes
Conflicts of Interest: No authors on this article have a conflict of interest that is relevant to the subject matter or materials included in this work. No authors on this article have financial or personal relationships to disclose with organizations that could potentially be perceived as influencing the described research. All authors have read the journal’s policy on conflicts of interest and the journal’s authorship agreement.
References
- 1.Chase HP, MacKenzie TA, Burdick J, et al. Redefining the clinical remission period in children with type 1 diabetes. Pediatr Diabetes. 2004;5:16–9. doi: 10.1111/j.1399-543X.2004.00034.x. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Rasoul M, Habib H, Al-Khouly M. ‘The honeymoon phase’ in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101–7. doi: 10.1111/j.1399-543X.2006.00155.x. [DOI] [PubMed] [Google Scholar]
- 3.Mortensen HB, Hougaard P, Swift P, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32:1384–90. doi: 10.2337/dc08-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–27. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engin F, Yermalovich A, Nguyen T, et al. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Transl Med. 2013;5:211ra156. doi: 10.1126/scitranslmed.3006534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–20. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- 7.Oslowski CM, Urano F. The binary switch between life and death of endoplasmic reticulum-stressed beta cells. Curr Opin Endocrinol Diabetes Obes. 2010;17:107–12. doi: 10.1097/MED.0b013e3283372843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papa FR. Endoplasmic reticulum stress, pancreatic beta-cell degeneration, and diabetes. Cold Spring Harb Perspect Med. 2012;2:a007666. doi: 10.1101/cshperspect.a007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eizirik DL, Cnop M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci Signal. 2010;3:pe7. doi: 10.1126/scisignal.3110pe7. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca SG, Gromada J, Urano F. Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab. 2011;22:266–74. doi: 10.1016/j.tem.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–94. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 12.Lee AH, Heidtman K, Hotamisligil GS, Glimcher LH. Dual and opposing roles of the unfolded protein response regulated by IRE1alpha and XBP1 in proinsulin processing and insulin secretion. Proc Natl Acad Sci U S A. 2011;108:8885–90. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roder ME, Knip M, Hartling SG, Karjalainen J, Akerblom HK, Binder C. Disproportionately elevated proinsulin levels precede the onset of insulin-dependent diabetes mellitus in siblings with low first phase insulin responses. The Childhood Diabetes in Finland Study Group. J Clin Endocrinol Metab. 1994;79:1570–5. doi: 10.1210/jcem.79.6.7989457. [DOI] [PubMed] [Google Scholar]
- 14.Hostens K, Pavlovic D, Zambre Y, et al. Exposure of human islets to cytokines can result in disproportionately elevated proinsulin release. J Clin Invest. 1999;104:67. doi: 10.1172/JCI6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joly AL, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2:238–47. doi: 10.1159/000296508. [DOI] [PubMed] [Google Scholar]
- 16.Marzec M, Eletto D, Argon Y. GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta. 2012;1823:774–87. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenbaum CJ, Beam CA, Boulware D, et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–73. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Research Council. Guide for the care and use of laboratory animals. 8. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 19.Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437. doi: 10.1097/00007890-198510000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JS, Kono T, Tong X, et al. Pancreatic and duodenal homeobox protein 1 (Pdx-1) maintains endoplasmic reticulum calcium levels through transcriptional regulation of sarcoendoplasmic reticulum calcium ATPase 2b (SERCA2b) in the islet β cell. J Biol Chem. 2014;289:32798–810. doi: 10.1074/jbc.M114.575191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics. 1985;41:103–16. [PubMed] [Google Scholar]
- 22.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes. 2010;59:679–85. doi: 10.2337/db09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–26. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 24.Hramiak IM, Dupre J, Finegood DT. Determinants of clinical remission in recent-onset IDDM. Diabetes Care. 1993;16:125–32. doi: 10.2337/diacare.16.1.125. [DOI] [PubMed] [Google Scholar]
- 25.Truyen I, De Pauw P, Jørgensen P, et al. Proinsulin levels and the proinsulin: c-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia. 2005;48:2322–9. doi: 10.1007/s00125-005-1959-0. [DOI] [PubMed] [Google Scholar]
- 26.Heaton DA, Millward BA, Gray IP, et al. Increased proinsulin levels as an early indicator of B-cell dysfunction in non-diabetic twins of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1988;31:182–4. doi: 10.1007/BF00276853. [DOI] [PubMed] [Google Scholar]
- 27.Ludvigsson J, Heding L. Abnormal proinsulin/C-peptide ratio in juvenile diabetes. Acta Diabetol Lat. 1982;19:351–8. doi: 10.1007/BF02629258. [DOI] [PubMed] [Google Scholar]
- 28.Snorgaard O, Hartling S, Binder C. Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:36–42. doi: 10.1007/BF00586459. [DOI] [PubMed] [Google Scholar]
- 29.Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506–13. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ota A, Wang Y. Cdc37/Hsp90 protein-mediated regulation of IRE1α protein activity in endoplasmic reticulum stress response and insulin synthesis in INS-1 cells. J Biol Chem. 2012;287:6266–74. doi: 10.1074/jbc.M111.331264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamani L, Latreille M, Larose L. Interaction of Nck1 and PERK phosphorylated at Y561 negatively modulates PERK activity and PERK regulation of pancreatic β-cell proinsulin content. Mol Biol Cell. 2014;25:702–11. doi: 10.1091/mbc.E13-09-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang H, Tian E, Liu C, Wang Q, Deng H. Oxidative stress induces monocyte necrosis with enrichment of cell-bound albumin and overexpression of endoplasmic reticulum and mitochondrial chaperones. PLoS One. 2013;8(3):e59610. doi: 10.1371/journal.pone.0059610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin HY, Mahon JL, Atkinson MA, Chaturvedi P, Lee-Chan E, Singh B. Type 1 diabetes alters anti-hsp90 autoantibody isotype. J Autoimmun. 2003;20:237–45. doi: 10.1016/s0896-8411(03)00035-0. [DOI] [PubMed] [Google Scholar]
- 34.Chen JS, Hsu YM, Chen CC, Chen LL, Lee CC, Huang TS. Secreted heat shock protein 90alpha induces colorectal cancer cell invasion through CD91/LRP-1 and NF-kappaBmediated integrin alphaV expression. J Biol Chem. 2010;285:25458–66. doi: 10.1074/jbc.M110.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hacker S, Lambers C, Hoetzenecker K, et al. Elevated HSP27, HSP70 and HSP90 alpha in chronic obstructive pulmonary disease: markers for immune activation and tissue destruction. Clin Lab. 2009;55:31–40. [PubMed] [Google Scholar]
- 36.Robertson RP. Chronic oxidative stress as a central mechanismfor glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–4. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 37.Eizirik DL, Korbutt GS, Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992;90:1263. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhodes CJ, White MF, Leahy JL, Kahn SE. Direct autocrine action of insulin on β-cells: does it make physiological sense? Diabetes. 2013;62:2157–63. doi: 10.2337/db13-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckingham B, Beck RW, Ruedy KJ, et al. Effectiveness of early intensive therapy on β-cell preservation in type 1 diabetes. Diabetes Care. 2013;36:4030–5. doi: 10.2337/dc13-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahn SE, McCulloch DK, Schwartz MW, Palmer JP, Porte D., Jr Effect of insulin resistance and hyperglycemia on proinsulin release in a primate model of diabetes mellitus. J Clin Endocrinol Metab. 1992;74:192–7. doi: 10.1210/jcem.74.1.1727820. [DOI] [PubMed] [Google Scholar]
- 41.Ward WK, LaCava EC, Paquette TL, Beard JC, Wallum BJ, Porte D., Jr Disproportionate elevation of immunoreactive proinsulin in type 2 (non-insulin-dependent) diabetes mellitus and in experimental insulin resistance. Diabetologia. 1987;30:698–702. doi: 10.1007/BF00296991. [DOI] [PubMed] [Google Scholar]
- 42.Shukla HD, Pitha PM. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis. 2012;2012:728605. doi: 10.1155/2012/728605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tukaj S, Kleszczyński K, Vafia K, et al. Aberrant expression and secretion of heat shock protein 90 in patients with bullous pemphigoid. PLoS One. 2013;8:e70496. doi: 10.1371/journal.pone.0070496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.