Abstract
Objective
To examine the association of cardiorespiratory fitness (CRF) with risk of coronary heart disease (CHD) while controlling for an individual’s Framingham Risk Score (FRS)–predicted CHD risk.
Patients and Methods
The study included 29,854 men from the Aerobics Center Longitudinal Study, who received a baseline examination from January 1, 1979, to December 31, 2002. Coronary heart disease events included self-reported myocardial infarction or revascularization or CHD death. Multivariable survival analysis investigated the association between CRF, FRS, and CHD. Cardiorespiratory fitness was analyzed as both a continuous and a categorical variable. The population was stratified by “low” and “moderate or high” risk of CHD to test for differences in the FRS stratified by CRF.
Results
Compared with men without incident CHD, men with incident CHD were older (mean age, 51.6 years vs 44.6 years), had lower average maximally achieved fitness (10.9 metabolic equivalent of tasks vs 12.0 metabolic equivalent of tasks [METs]), and were more likely to have moderate or high 10-year CHD risk (P<.001). Cardiorespiratory fitness, defined as maximal METs, exhibited a 20% lower risk of CHD (hazard ratio, 0.80; 95% CI, 0.77–0.83) for each 1-unit MET increase. Among men in the low CRF strata, individuals with moderate or high 10-year CHD risk, according to the FRS, had a higher CHD risk (hazard ratio, 6.55; 95% CI, 3.64–11.82) than men with low CHD risk according to the FRS.
Conclusion
Clinicians should promote physical activity to improve CRF so as to reduce CHD risk, even to patients with otherwise low CHD risk.
The American Heart Association1 stated that one of its 2020 Impact Goals was to reduce deaths from cardiovascular disease (CVD) by 20%. Most CVD deaths in 2006 and 2007 were caused by coronary heart disease (CHD), which is defined as plaque accumulation in the arteries of the heart, decreasing the supply of oxygen-rich blood.2 Several risk factors have been shown to predict CHD, including smoking,3 diabetes,4 hypertension,5 and hypercholesterolemia.6
Coronary heart disease risk equations, such as the Framingham Risk Score (FRS), have been developed and used to account for these and other risk factors.7 The FRS provides a sex-specific risk score that accounts for age, systolic and diastolic blood pressure, total cholesterol level, high-density lipoprotein cholesterol (HDL-C) level, diabetes diagnosis, and smoking status.8 Previous studies9–11 have modified the FRS with the addition of C-reactive protein,10 deletion of diabetes diagnosis,11 and alterations to blood pressure definitions12; yet none of these modifications involved assessing the association between cardiorespiratory fitness (CRF), FRS, and CHD.
Previous research reports CRF’s significant protective effects on all-cause mortality,13,14 cancer-related mortality,15 diabetes incidence,16 CHD incidence,17 and CHD mortality.8,18,19 Barlow et al20 reported that a 1-unit metabolic equivalent of task (MET) increase in baseline CRF resulted in an 18% decrease in CVD mortality in FRS-classified “low-risk” adults over a 30-year follow-up period. However, this result reflects control for additional factors besides CRF, such as body mass index (calculated as the weight in kilograms divided by the height in meters squared) and family history of early CHD, which are not included in the FRS.
The aim of this study was to examine the association of CRF with 10-year risk of CHD while controlling for an individual’s FRS–predicted CHD risk. Our secondary aim was to investigate whether the relationship between CRF and 10-year risk of CHD differs in “moderate- or high-risk” men.
PATIENTS AND METHODS
Aerobics Center Longitudinal Study
The Aerobics Center Longitudinal Study (ACLS) is a prospective cohort study involving a large group of men and women. The participants were patients of the Cooper Clinic, in which they received a preventive medical examination and counseling on health behaviors during periodic visits. The participants were examined at least once from January 1, 1979, to December 31, 2002, at the Cooper Clinic, Dallas, Texas. The protocol for the ACLS was reviewed annually and approved by the institutional review board of the Cooper Institute. Women were excluded from these analyses because of a small number of CHD events (n=45). Men were included on the basis of the following criteria: (1) age at baseline examination between 30 and 74 years; (2) complete data for outcome and predictor variables; and (3) free of CVD or cancer diagnosis at baseline. A flow diagram of the study population is depicted in Figure 1.
FIGURE 1.
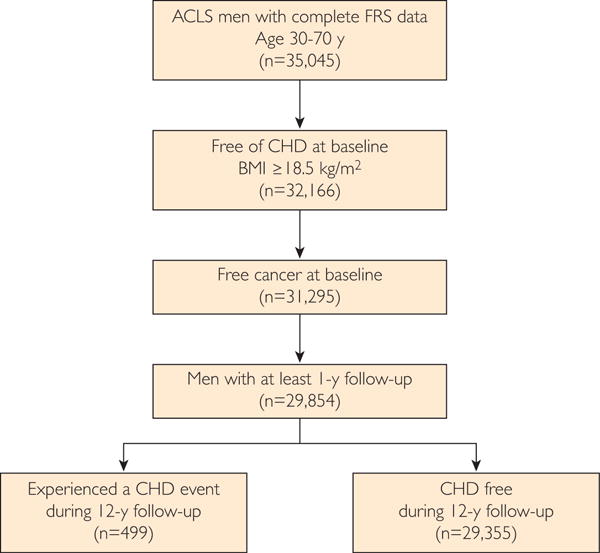
Study flow and Aerobic Center Longitudinal Study (ACLS) inclusion criteria depicting final sample size and coronary heart disease (CHD) event frequency. BMI = body mass index; FRS = Framingham Risk Score.
Clinical Examination
The baseline clinical examination included an electrocardiogram, a 12-hour fasting blood chemistry analyses including cholesterol and glucose measurements, blood pressure assessment, and a maximal exercise test.21–23 A standardized questionnaire was used to assess smoking status and other risk factors.
Outcome Measure
Coronary heart disease was defined through self-report of either revascularization (including bypass, coronary balloon, angioplasty, or stent) or myocardial infarction or CHD- specific mortality. A mail-back questionnaire was administered in 1982, 1986, 1990, 1995, 1999, and 2004, in which participants were asked to report their history of revascularization or myocardial infarction along with the incident date. The National Center for Health Statistic’s National Death Index was used to identify CHD deaths in the ACLS cohort; International Classification of Disease (Ninth and Tenth revisions) codes 410.0 to 414.0 were used to determine CHD as the primary cause of death. In accordance with FRS’s follow-up time definition, the maximal follow-up time for the ACLS study population was 12 years.
Application of the FRS
The FRS was derived from the Framingham Heart Study,7 which is an ongoing observational study initiated in 1948 and primarily recruits residents of Framingham, Massachusetts. In a study published in 1998,8 the main outcome was a CHD event defined as a myocardial infarction, coronary insufficiency, or CHD death. This version of the FRS8 incorporated categorical variables for age, hypertension, total cholesterol level, HDL-C level, smoking status, and diabetes to determine a point value that could be summed and interpreted as an overall 10-year risk of CHD. The definition of the risk factors was kept consistent with the 1998 FRS report,8 and the risk factors were categorized on the basis of the score sheet. Diabetes was diagnosed if the fasting glucose level was higher than 140 mg/dL (to convert to mmol/L, multiply by 0.0259), and smoking status was self-reported.
The FRS was applied using the categorical risk factors and the specified points8 to every individual, and men were stratified on the basis of their level of 10-year CHD risk according to the FRS. Men with 10-year CHD risk lower than 10%20 (ie, point summation, ≤5 points) were classified as “low” risk of CHD, and men with 10-year CHD risk higher than 10% (ie, point summation, >5 points) were classified as “moderate or high” risk of CHD.
Cardiorespiratory Fitness
The Balke maximal exercise treadmill test24 was used to determine CRF, which was analyzed as a categorical variable.
The categorical definition of CRF was based on a participant’s age-specific treadmill time from the entire ACLS cohort and consisted of 3 levels: low (bottom least fit 20%), moderate (next fit 40%), and high (most fit top 40%). The corresponding treadmill time and MET values have been assigned across 20 to 39, 40 to 49, 50 to 59, and 60 years and above on the basis of our previously published articles25 to maintain methodology consistency, because there are no established cut points for CRF to classify different levels of fitness. In the present analysis, the mean treadmill time and MET values across low, moderate, and high CRF groups were 11.5 minutes and 8.6 METs, 16.1 minutes and 10.7 METs, and 22.1 minutes and 13.6 METs, respectively. The following regression formula was used to convert maximal treadmill time to METs26:
Treadmill time converted to METs is analogous to Vo2peak.27
Statistical Analyses
Descriptive statistics were computed for the total ACLS male population and stratified by incidence of CHD. Men with and without incident CHD were compared for mean age; proportion of men with low, moderate, or high CRF; proportion of men with moderate or high 10-year CHD risk according to the FRS; hypertension classification; total cholesterol and HDL-C levels; diabetes diagnosis; and smoking status. We calculated age-adjusted incidence rates per 10,000 person-years for 10-year CHD risk and CRF. To determine each of the aforementioned covariate’s association with CHD events, univariate survival analysis was performed. Cox proportional hazards models adjusted for baseline examination year were fit to determine the association between CRF and CHD events while controlling for 10-year CHD risk. We tested for the interaction between CRF and FRS using survival analysis of a population stratified by low, moderate, and high CRF while adjusting for baseline examination year. Men with low 10-year CHD risk served as the reference group. We constructed a receiver operating characteristic curve for a sensitivity analysis to determine the CRF’s added predictive power of the FRS for 10-year CHD risk. SAS version 9.3 (SAS Institute Inc) was used to perform all analyses.
RESULTS
During a 12-year follow-up period (248,890 person-years of exposure), there were 499 incident CHD events. The FRS was applied to approximately 30,000 men in the ACLS cohort (Table 1). The incidence of CHD in men with moderate or high 10-year CHD risk was 37.6 per 10,000 person-years as compared with 19.7 for men with low 10-year CHD risk. Age-adjusted CHD incidence in men with low, moderate, or high CRF was 26.5, 23.6, and 16.5 per 10,000 person-years, respectively. Compared with men without incident CHD, men with incident CHD were older, had higher prevalence of stage I hypertension, had a lower HDL-C level (<35 mg/dL), and were more likely to have moderate or high 10-year CHD risk (P<.0001 for all stated comparisons).
TABLE 1.
Comparison of Demographic Characteristics Between Men (n=29,854) With Incident CHD and Those Without Incident CHD in the Aerobics Center Longitudinal Study Prospective Cohorta,b
| Risk factor | Total population (N=29,854) | Men with incident CHD (n=499) | Men without incident CHD (n=29,355) | Pc |
|---|---|---|---|---|
| Age range (y) | 30–74 | 31–73 | 30–74 | |
|
| ||||
| Mean age (y) | 44.7 | 51.6 | 44.6 | <.001 |
|
| ||||
| Mean fitness, maximally achieved MET | 12.0 | 10.9 | 12.0 | <.001 |
|
| ||||
| Cardiorespiratory fitness | ||||
| Low | 11.6 | 13.4 | 11.5 | .19 |
| Moderate | 38.5 | 43.3 | 38.4 | .03 |
| High | 50.0 | 43.3 | 50.1 | .0029 |
|
| ||||
| Mean FRS (points) | 3.5 | 6.2 | 3.5 | <.001 |
|
| ||||
| Moderate or high 10-y CHD risk | 2.1 | 10.4 | 1.9 | <.0001 |
|
| ||||
| Blood pressure (mm Hg) | ||||
| Optimal (SBP<120, DBP<80) | 28.8 | 19.4 | 29.0 | <.0001 |
| Normal (SBP<130, DBP<85) | 31.8 | 30.3 | 31.9 | .45 |
| High normal (SBP<140, DBP<90) | 16.0 | 19.6 | 15.9 | .02 |
| Stage I HTN (SBP<160, DBP<100) | 18.7 | 25.5 | 18.6 | <.001 |
| Stage II–IV HTN (SBP≥160, DBP≥100) | 4.7 | 5.2 | 4.7 | .56 |
|
| ||||
| Total cholesterol level (mg/dL) | ||||
| <160 | 9.2 | 3.8 | 9.3 | <.001 |
| 160–199 | 34.1 | 19.2 | 34.4 | <.001 |
| 200–239 | 36.9 | 40.5 | 36.8 | .09 |
| 240–279 | 15.3 | 27.3 | 15.1 | <.001 |
| ≥280 | 4.5 | 9.2 | 4.5 | <.001 |
|
| ||||
| HDL-C level (mg/dL) | ||||
| <35 | 15.6 | 25.3 | 15.4 | <.001 |
| 35–44 | 34.1 | 38.7 | 34.1 | .03 |
| 45–49 | 15.6 | 13.0 | 15.6 | .11 |
| 50–59 | 21.4 | 15.8 | 21.5 | .021 |
| ≥60 | 13.3 | 7.2 | 13.4 | <.001 |
|
| ||||
| Diabetes | 1.4 | 4.4 | 1.3 | <.001 |
|
| ||||
| Current smoking | 16.6 | 21.8 | 16.5 | .001 |
CHD = coronary heart disease; DBP = diastolic blood pressure; FRS = Framingham Risk Score; HDL-C = high-density lipoprotein cholesterol; HR = hazard ratio; HTN = hypertension; MET = metabolic equivalent of task; SBP = systolic blood pressure.
SI conversion factor: To convert mg/dL values to mmol/L, multiply by 0.0259.
The Student t test was used to calculate the difference between incident CHD and no incident CHD groups for age, mean fitness level, and mean FRS points. The χ2 test was used to determine the statistically significant difference for remaining categorical variables.
Table 2 presents the univariate associations between the FRS risk factors and the risk of CHD. Men with optimal blood pressure were 33% less likely to experience a CHD event than were men with normal blood pressure (hazard ratio [HR], 0.67; 95% CI, 0.52–0.87), whereas men with stage I hypertension were at a significantly higher risk of CHD (HR, 1.55; 95% CI, 1.23–1.97). Men with an HDL-C level of 60 mg/dL or higher were at a significantly lower risk of CHD than were men with an HDL-C level of 45 to 49 mg/dL. Men diagnosed with diabetes and who reported being smokers were at a significantly higher risk of CHD than were nondiabetic individuals and nonsmokers. Table 3 presents the univariate and multivariable associations between CRF categories, moderate or high 10-year CHD risk, and CHD events. Men with moderate or high 10-year CHD risk had an almost 6-fold (HR, 5.66; 95% CI, 4.25–7.55) higher risk of CHD than did men with low 10-year CHD risk. A univariate analysis revealed an inverse association between CRF and CHD. Cardiorespiratory fitness, categorized into low, moderate, and high, exhibited that men with high CRF had 33% (HR, 0.67; 95% CI, 0.51–0.88) lower risk of CHD than did men who had low CRF (see Table 3). Model 2 evaluates a similar association, but defines CRF as a categorical variable and shows that men with high CRF have 26% lower CHD risk while controlling for moderate or high 10-year CHD risk.
TABLE 2.
Univariate Survival Associations Between the Framingham Risk Score Risk Factors and 10-Y Coronary Heart Disease Riska,b
| Risk factor | Model 1: Univariate model
|
|
|---|---|---|
| HR | 95% CI | |
| Age | 1.09 | 1.08–1.10 |
|
| ||
| Blood pressure (mm Hg) | ||
| Optimal (SBP<120, DBP<80) | 0.67 | 0.51–0.88 |
| Normal (SBP<130, D<85) | 1.00 | – |
| High normal (SBP<140, DBP<90) | 1.36 | 1.06–1.76 |
| Stage I HTN (SBP<160, DBP<100) | 1.55 | 1.23–1.97 |
| Stage II–IV HTN (SBP≥160, DBP≥100) | 1.34 | 0.89–2.04 |
|
| ||
| Total cholesterol level (mg/dL) | ||
| <160 | 0.77 | 0.47–1.26 |
| 160–199 | 1.00 | – |
| 200–239 | 1.87 | 1.47–2.38 |
| 240–279 | 2.89 | 2.22–3.75 |
| ≥280 | 3.03 | 2.14–4.31 |
|
| ||
| HDL-C level (mg/dL) | ||
| <35 | 1.82 | 1.35–2.46 |
| 35–44 | 1.33 | 1.01–1.76 |
| 45–49 | 1.00 | – |
| 50–59 | 0.89 | 0.64–1.23 |
| ≥60 | 0.64 | 0.43–0.96 |
|
| ||
| Diabetes | 3.54 | 2.31–5.42 |
|
| ||
| Current smoking | 1.51 | 1.22–1.87 |
DBP = diastolic blood pressure; HDL-C = high-density lipoprotein cholesterol; HR ¼ hazard ratio; HTN = hypertension; SBP = systolic blood pressure.
SI conversion factor: To convert mg/dL values to mmol/L, multiply by 0.0259.
TABLE 3.
Crude and Adjusted Associations Between CRF, FRS, and CHDa
| Risk factor | Model 1: Univariate model
|
Model 2b: Multivariable model
|
||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| 10-y CHD riskc | ||||
| Low | 1.00 | – | 1.00 | – |
| Moderate or high | 5.66 | 4.25–7.55 | 5.38 | 4.03–7.19 |
|
| ||||
| CRF | ||||
| Low 1.00 | – | 1.00 | – | |
| Moderate | 0.93 | 0.71–1.22 | 0.98 | 0.75–1.30 |
| High | 0.67 | 0.51–0.88 | 0.74 | 0.56–0.98 |
CHD = coronary heart disease; CRF = cardiorespiratory fitness; FRS = Framingham Risk Score; HR = hazard ratio.
Model 2 investigates the association between CRF (categorized into low, moderate, and high) and CHD events while controlling for moderate or high 10-y CHD risk and baseline examination year.
Low or moderate or high 10-y CHD risk is a comparative risk calculated from the summation of FRS points. Moderate or high risk is defined as a sum of >5 points.
Figure 2 shows the association between CRF, FRS, and 10-year CHD risk through stratification of the population by low, moderate, and high CRF.
FIGURE 2.
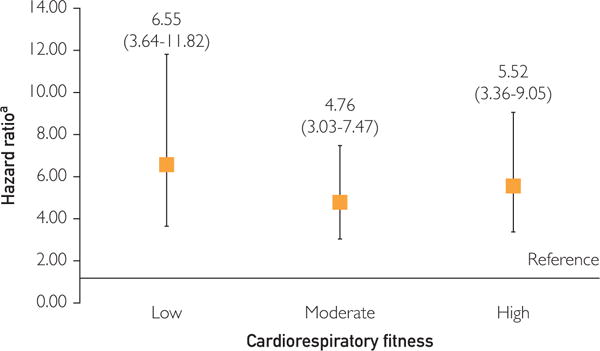
Adjusted hazard ratios (95% CIs) for the relationship between moderate or high 10-year coronary heart disease risk (as classified by the Framingham Risk Score) and coronary heart disease, stratified by low, moderate, and high cardiorespiratory fitness. Low 10-year coronary heart disease risk served as the reference group.
aAdjusted for baseline examination year
Among men with low CRF, individuals with moderate or high 10-year CHD risk were 6.5 times (HR, 6.55; 95% CI, 0.64–11.82) more likely to develop CHD than those with low CHD risk. This association was similar but attenuated in men with moderate and high CRF (HR, 4.76; 95% CI, 3.03–7.47 and HR, 5.52; 95% CI, 3.36–9.05, respectively). Our sensitivity analysis revealed that CRF (area under the curve, 0.80) did not add to the predictive power of the FRS for 10-year CHD risk (area under the curve, 0.80) (P=.97).
DISCUSSION
Both FRS and CRF were strong independent predictors of CHD. Cardiorespiratory fitness had a significant protective effect on CHD in men after controlling for 10-year CHD risk according to the FRS point summation. When men were stratified by low, moderate, and high CRF, CRF’s protective effect was still apparent but overshadowed by the strong association between FRS-predicted CHD risk and CHD events. To our knowledge, this is the first study to investigate the association between CRF and CHD in men with low and moderate or high 10-year CHD risks.
The FRS is composed of CHD risk factors such as hypertension, cholesterol levels, diabetes diagnosis, and smoking status.8 Various versions8,28–30 that have included these risk factors have repeatedly shown the predictive power of the FRS.31 Myocardial ischemia is common in patients with hypertension4,5,32; although a recent study reported a 1.4-mm Hg decrease in mean systolic blood pressure from 1994 to 2005 that could be associated with a 20% reduction in CHD deaths.33 Diabetes diagnosis also has been shown to significantly increase a person’s risk of CHD.34,35 Diabetes can cause impairment in the cardiac muscle that may lead to cardiomyopathy, congestive heart failure, or ischemic heart disease and can increase the 5-year mortality rate after myocardial infarction.4 Doyle et al36 published one of the first studies examining the association between smoking and CHD. They concluded that although problems with blood pressure and cholesterol were absent, participants who reported being smokers were at a significantly higher risk of CHD mortality than were nonsmokers.36
Our univariate analysis revealed that the FRS was a significant predictor of 10-year CHD risk for men in the ACLS cohort. Our previous research reported the categorical FRS risk factors for men in the ACLS cohort, and the HRs31 were similar to those reported in the original FRS report.24 The FRS applied to the ACLS cohort reported a Hosmer-Lemeshow c statistic of 0.78 (95% CI, 0.75–0.79).31
We also found that CRF has a significant protective effect on CHD, similar to findings previously reported in the literature.13,16,18,37,38 Ekelund et al19 investigated the relationship between CRF and CHD in asymptomatic men and found that during a 9-year follow-up the more fit men had the least CHD risk compared with the least fit individuals. Lee et al18 built on these findings by analyzing CRF’s association with CVD while controlling for body composition. They reported that lean unfit men had 3 times higher risk of dying from CVD (relative risk, 3.16; 95% CI, 1.12–8.92) than did lean fit men. Improved CRF may reduce CHD risk through improved muscle mass39,40 and increase in arterial oxygen content.39–41 Research has shown that CRF can increase the double product threshold for ischemic ST-segment depression,42,43 a decrease in the magnitude of ST-segment depression, and diminished maximal ST-segment depression.42 Cardiorespiratory fitness may also have a positive effect on coagulation44,45 and may protect against thrombosis.19
Our findings regarding the association between CRF, FRS, and risk of CHD are consistent with recent findings.46,47 Barlow et al20 investigated the association between CRF and CVD mortality in men and women who were at low risk of CHD events. They concluded that a 1-unit MET increase in CRF resulted in an 18% decrease in CVD mortality during a 30-year follow-up period.20 Gupta et al48 used the ACLS cohort with data ranging from 1970 through 2006 and used a traditional CHD risk factor model that adjusts for age, systolic blood pressure, diabetes diagnosis, total cholesterol level, and smoking status and reported that CRF-augmented CHD risk factor model correctly reclassified participants with CHD death on the basis of their 10-year risk48 as compared with the traditional FRS model.
The present study builds on the aforementioned research by applying the FRS to a large, single-center, longitudinal cohort with the same level of precision as that of the Framingham Heart Study that generated the FRS. The American College of Cardiology and the American Heart Association recently developed the Pooled Cohort Equation for estimating artherosclerotic CVD49 that encompasses risk factors similar to those of the FRS but offers risk estimates for myocardial infarction, CHD death, stroke, and stroke death and does not include fitness.50 This project focused on previously defined CHD that includes angioplasty and revascularization while excluding stroke and stroke death. Future research should investigate the potential effect the Pooled Cohort Equation may have on artherosclerotic CVD with the addition of CRF.
To our knowledge, this is the first prospective cohort to investigate CRF’s association with 10-year risk of CHD while controlling for the FRS8 in its entirety. A possible limitation to the present study is the homogeneity of the ACLS population. At the time of enrollment, the ACLS consisted of mostly men (mean age, 42 years) and was predominantly non-Hispanic whites (>95%). However, a comparison study between the ACLS and 2 large population-based cohorts found that the results of the ACLS were similar to those obtained from those 2 cohorts.51 In addition, ACLS’ homogeneity improves internal validity by controlling potential confounders such as socioeconomic status and education, although generalizations from this study should be made cautiously.
CONCLUSION
Our study found that CRF and FRS are both significant predictors of CHD events. Men with moderate and high CRF have a lower risk of CHD than do men with low CRF; this association remains in men with moderate or high 10-year CHD risk when stratified into low, moderate, and high CRF. Cardiorespiratory fitness is a modifiable predictor of CHD, and improved CRF may lead to an improvement in the FRS and 10-year CHD risk, as well as an improvement in the ability to predict long-term CHD risk. Clinicians should vigorously promote exercise therapy and increase in physical activity to their patients in an effort to increase CRF in the long-term prevention of CHD. Researchers should consider developing a randomized clinical trial to determine the overall effect that CRF changes may have on an individual’s FRS–predicted CHD risk, the individual components of the risk score, and ultimately the effect on 10-year risk of CHD.
Acknowledgments
We thank physicians and technicians of the Cooper Clinic for collecting baseline data as well as staff at the Cooper Institute for data entry and data management.
Grant Support: This work was supported by the National Institutes of Health (grant nos. AG06945, HL62508, and DK088195). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Hébert was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute (award no. K05 CA136975).
Abbreviations and Acronyms
- ACLS
Aerobics Center Longitudinal Study
- CHD
coronary heart disease
- CRF
cardiorespiratory fitness
- CVD
cardiovascular disease
- FRS
Framingham Risk Score
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- MET
metabolic equivalent of task
References
- 1.Lloyd-Jones DM, Hong Y, Labarthe D, et al. American Heart Association Strategic Planning Task Force and Statistics Committee Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2013 update: a report from the American Heart Association [published corrections appear in Circulation. 2013;127(23):e841 and Circulation. 2013;127(1):doi:10. 1161/CIR.0b013e31828124ad] Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheidt S. Changing mortality from coronary heart disease among smokers and nonsmokers over a 20-year interval. Prev Med. 1997;26(4):441–446. doi: 10.1006/pmed.1997.0185. [DOI] [PubMed] [Google Scholar]
- 4.Grossman E, Messerli FH. Diabetic and hypertensive heart disease. Ann Intern Med. 1996;125(4):304–310. doi: 10.7326/0003-4819-125-4-199608150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Strauer BE. Myocardial oxygen consumption in chronic heart disease: role of wall stress, hypertrophy and coronary reserve. Am J Cardiol. 1979;44(4):730–740. doi: 10.1016/0002-9149(79)90295-9. [DOI] [PubMed] [Google Scholar]
- 6.Wijeysundera HC, Machado M, Farahati F, et al. Association of temporal trends in risk factors and treatment uptake with coronary heart disease mortality, 1994–2005. JAMA. 2010;303(18):1841–1847. doi: 10.1001/jama.2010.580. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38(1):46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 8.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 9.Tzoulaki I, Liberopoulos G, Ioannidis JP. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA. 2009;302(21):2345–2352. doi: 10.1001/jama.2009.1757. [DOI] [PubMed] [Google Scholar]
- 10.Pischon T, Möhlig M, Hoffmann K, et al. Comparison of relative and attributable risk of myocardial infarction and stroke according to C-reactive protein and low-density lipoprotein cholesterol levels. Eur J Epidemiol. 2007;22(7):429–438. doi: 10.1007/s10654-007-9141-2. [DOI] [PubMed] [Google Scholar]
- 11.Gallo WT, Teng HM, Falba TA, Kasl SV, Krumholz HM, Bradley EH. The impact of late career job loss on myocardial infarction and stroke: a 10 year follow up using the health and retirement survey. Occup Environ Med. 2006;63(10):683–687. doi: 10.1136/oem.2006.026823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson PW, Bozeman SR, Burton TM, Hoaglin DC, Ben-Joseph R, Pashos CL. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118(2):124–130. doi: 10.1161/CIRCULATIONAHA.108.772962. [DOI] [PubMed] [Google Scholar]
- 13.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 14.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605–611. doi: 10.7326/0003-4819-132-8-200004180-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lee IM, Paffenbarger RS., Jr Physical activity and its relation to cancer risk: a prospective study of college alumni. Med Sci Sports Exerc. 1994;26(7):831–837. [PubMed] [Google Scholar]
- 16.Sui X, Hooker SP, Lee IM, et al. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31(3):550–555. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oja P, Teräslinna P, Partanen T, Kärävä R. Feasibility of an 18 months’ physical training program for middle-aged men and its effect on physical fitness. Am J Public Health. 1974;64(5):459–465. doi: 10.2105/ajph.64.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69(3):373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 19.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. N Engl J Med. 1988;319(21):1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 20.Barlow CE, Defina LF, Radford NB, et al. Cardiorespiratory fitness and long-term survival in “low-risk” adults. J Am Heart Assoc. 2012;1(4):e001354. doi: 10.1161/JAHA.112.001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair SN, Kannel WB, Kohl HW, Goodyear N, Wilson PW. Surrogate measures of physical activity and physical fitness: evidence for sedentary traits of resting tachycardia, obesity, and low vital capacity. Am J Epidemiol. 1989;129(6):1145–1156. doi: 10.1093/oxfordjournals.aje.a115236. [DOI] [PubMed] [Google Scholar]
- 22.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–1098. [PubMed] [Google Scholar]
- 23.Blair SN, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 24.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959;10(6):675–688. [PubMed] [Google Scholar]
- 25.Sui X, LaMonte MJ, Blair SN. Cardiorespiratory fitness as a predictor of nonfatal cardiovascular events in asymptomatic women and men. American J Epidemiol. 2007;165:1413–1423. doi: 10.1093/aje/kwm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper KH, Pollock ML, Martin RP, White SR, Linnerud AC, Jackson A. Physical fitness levels vs selected coronary risk factors: a cross-sectional study. JAMA. 1976;236(2):166–169. [PubMed] [Google Scholar]
- 27.Pollock ML, Foster C, Schmidt D, Hellman C, Linnerud AC, Ward A. Comparative analysis of physiologic responses to three different maximal graded exercise test protocols in healthy women. Am Heart J. 1982;103(3):363–373. doi: 10.1016/0002-8703(82)90275-7. [DOI] [PubMed] [Google Scholar]
- 28.Kagan A, Gordon T, Rhoads GG, Schiffman JC. Some factors related to coronary heart disease incidence in Honolulu Japanese men: the Honolulu Heart Study. Int J Epidemiol. 1975;4(4):271–279. doi: 10.1093/ije/4.4.271. [DOI] [PubMed] [Google Scholar]
- 29.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile: a statement for health professionals. Circulation. 1991;83(1):356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 30.D’Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, et al. General cardiovascular risk profile for use in primary care: the Framingham Risk Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 31.Gander J, Sui X, Hazlett LJ, Cai B, Hébert JR, Blair SN. Factors related to coronary heart disease risk among men: validation of the Framingham Risk Score. Prev Chronic Dis. 2014;11:E140. doi: 10.5888/pcd11.140045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman JI. A critical view of coronary reserve. Circulation. 1987;75(1 Pt 2):I6–I11. [PubMed] [Google Scholar]
- 33.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356(23):2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 34.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB, McGee D. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2(2):120–126. doi: 10.2337/diacare.2.2.120. [DOI] [PubMed] [Google Scholar]
- 36.Doyle JT, Dawber TR, Kannel WB, Kinch SH, Kahn HA. The relationship of cigarette smoking to coronary heart disease: the second report of the combined experience of the Albany, NY. and Framingham, Mass. studies. JAMA. 1964;190(10):886–890. [PubMed] [Google Scholar]
- 37.Farrell SW, Kampert JB, Kohl HW, III, et al. Influences of cardiorespiratory fitness levels and other predictors on cardiovascular disease mortality in men. Med Sci Sports Exerc. 1998;30(6):899–905. doi: 10.1097/00005768-199806000-00019. [DOI] [PubMed] [Google Scholar]
- 38.Abudiab M, Aijaz B, Konecny T, et al. Use of functional aerobic capacity based on stress testing to predict outcomes in normal, overweight, and obese patients. Mayo Clin Proc. 2013;88(12):1427–1434. doi: 10.1016/j.mayocp.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1977;39:221–251. doi: 10.1146/annurev.ph.39.030177.001253. [DOI] [PubMed] [Google Scholar]
- 40.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 41.Herrlich HC, Raab W, Gigee W. Influence of muscular training and of catecholamines on cardiac acetylcholine and cholinesterase. Arch Int Pharmacodyn Ther. 1960;129:201–215. [PubMed] [Google Scholar]
- 42.Ehsani AA, Heath GW, Hagberg JM, Sobel BE, Holloszy JO. Effects of 12 months of intense exercise training on ischemic ST-segment depression in patients with coronary artery disease. Circulation. 1981;64(6):1116–1124. doi: 10.1161/01.cir.64.6.1116. [DOI] [PubMed] [Google Scholar]
- 43.Ehsani AA, Biello D, Seals DR, Austin MB, Schultz J. The effect of left ventricular systolic function on maximal aerobic exercise capacity in asymptomatic patients with coronary artery disease. Circulation. 1984;70(4):552–560. doi: 10.1161/01.cir.70.4.552. [DOI] [PubMed] [Google Scholar]
- 44.Kopitsky RG, Switzer ME, Williams RS, McKee PA. The basis for the increase in factor VIII procoagulant activity during exercise. Thromb Haemost. 1983;49(1):53–57. [PubMed] [Google Scholar]
- 45.Williams RS, Logue EE, Lewis JG, et al. Physical conditioning augments the fibrinolytic response to venous occlusion in healthy adults. N Engl J Med. 1980;302(18):987–991. doi: 10.1056/NEJM198005013021802. [DOI] [PubMed] [Google Scholar]
- 46.Vuori IM, Lavie CJ, Blair SN. Physical activity promotion in the health care system. Mayo Clin Proc. 2013;88(12):1446–1461. doi: 10.1016/j.mayocp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 47.Swift DL, Lavie CJ, Johannsen NM, et al. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77(2):281–292. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality: clinical perspective. Circulation. 2011;123(13):1377–1383. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in J Am Coll Cardiol. 2014;63(25 Pt B):3026] J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Myers J. New American Heart Association/American College of Cardiology guidelines on cardiovascular risk: when will fitness get the recognition it deserves? Mayo Clin Proc. 2014;89(6):722–726. doi: 10.1016/j.mayocp.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Sui X. Longitudinal Analyses of Physical Activity and Cardiorespiratory Fitness on Adiposity and Glucose Levels. ProQuest Dissertations and Theses. 2012;126 [Google Scholar]


