Abstract
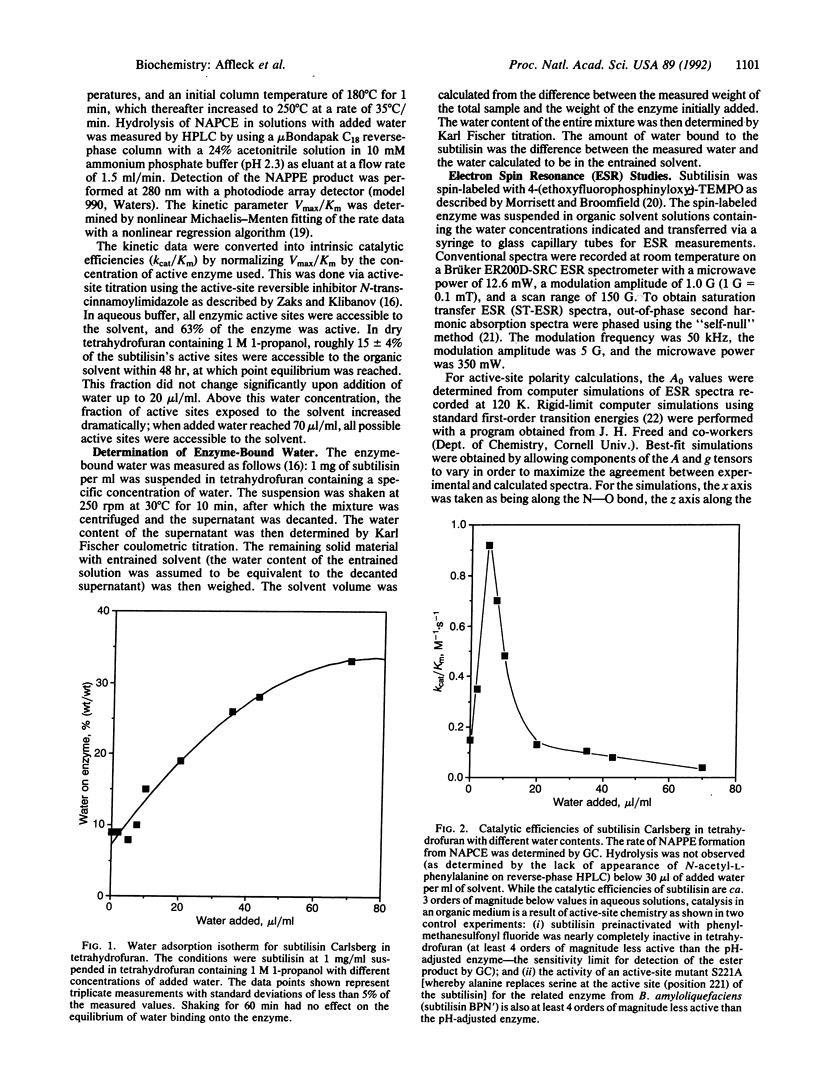
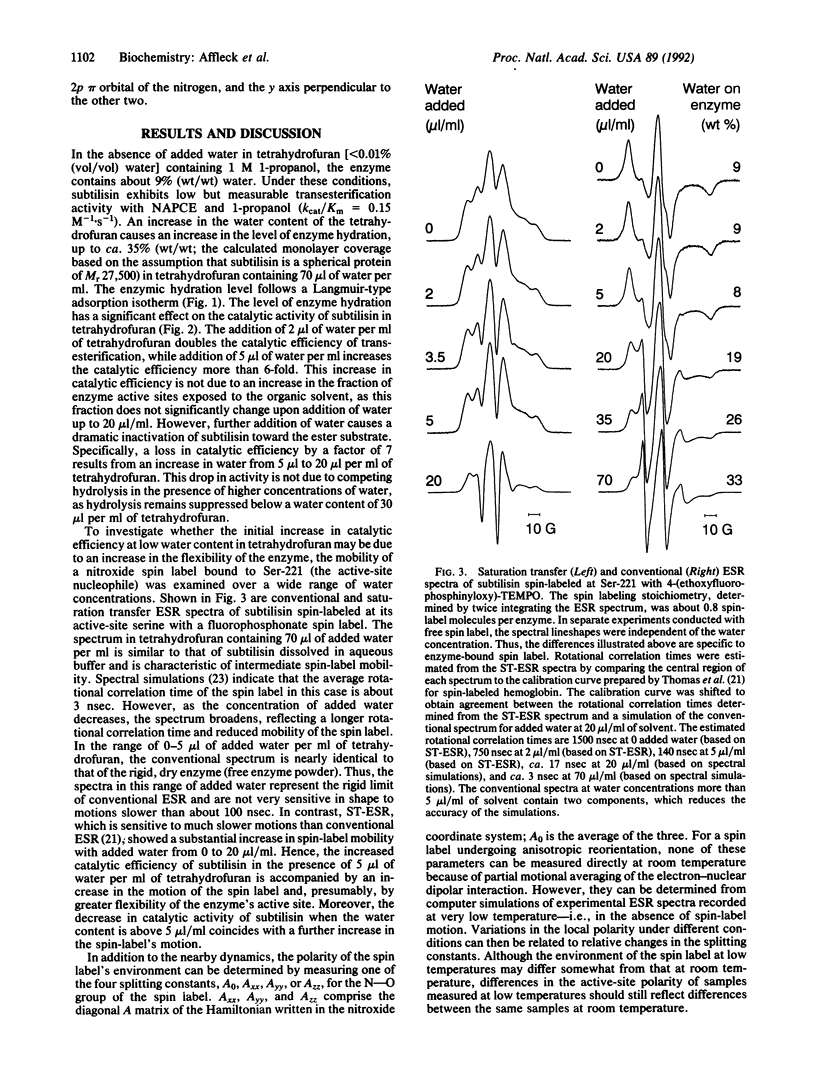
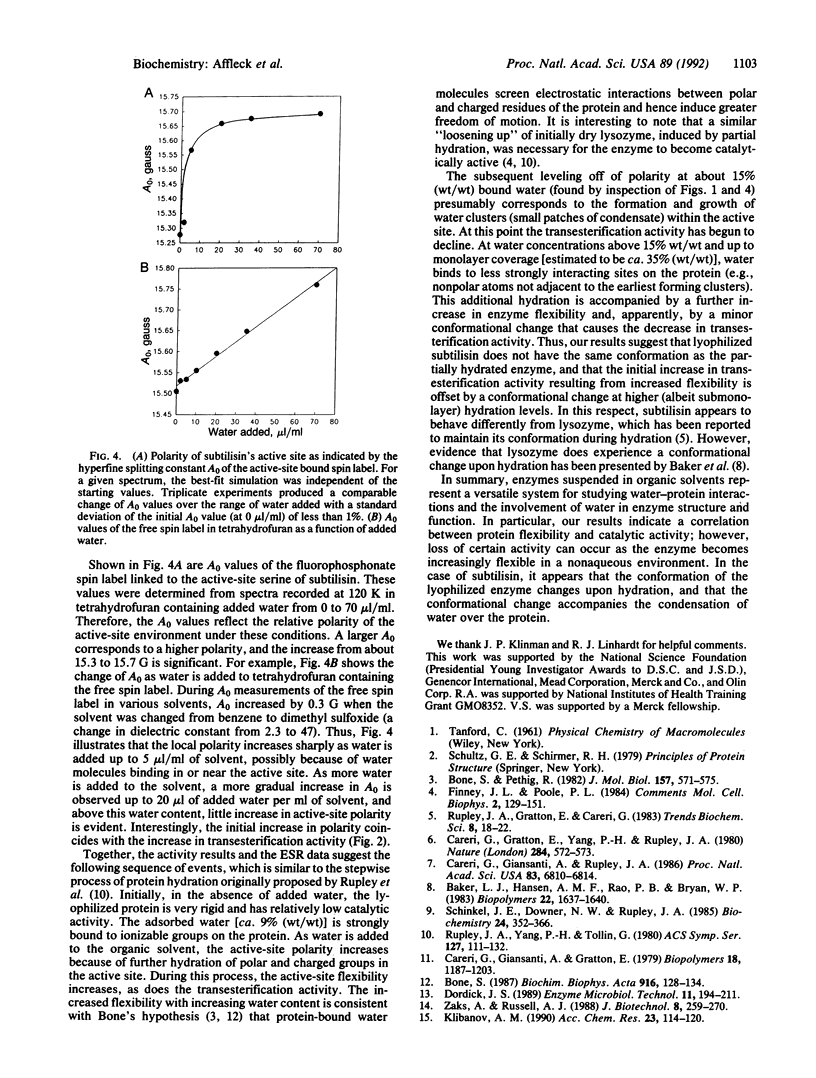
Enzymes suspended in organic solvents represent a versatile system for studying the involvement of water in enzyme structure and function. Addition of less than 1% (vol/vol) water to tetrahydrofuran containing 1 M 1-propanol leads to a substantial increase in the transesterification activity of subtilisin Carlsberg (from Bacillus licheniformis) that correlates with a sharp increase in the active-site polarity and a 90% decrease in the rotational correlation time (i.e., increase in mobility) of a nitroxide spin label within the active site. Water in excess of 1% has little additional effect on active-site polarity and coincides with a further increase in spin-label mobility, yet the transesterification activity decreases dramatically. Thus, transesterification activity increases and then decreases with increasing enzyme hydration and flexibility (which are presumably coupled through dielectric screening), suggesting that the conformation of partially hydrated subtilisin is different from that of the nearly dry enzyme--i.e., enzyme containing less than 9% (wt/wt) water.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L. J., Hansen A. M., Rao P. B., Bryan W. P. Effects of the presence of water on lysozyme conformation. Biopolymers. 1983 Jul;22(7):1637–1640. doi: 10.1002/bip.360220703. [DOI] [PubMed] [Google Scholar]
- Bone S., Pethig R. Dielectric studies of the binding of water to lysozyme. J Mol Biol. 1982 May 25;157(3):571–575. doi: 10.1016/0022-2836(82)90477-6. [DOI] [PubMed] [Google Scholar]
- Bone S. Time-domain reflectrometry studies of water binding and structural flexibility in chymotrypsin. Biochim Biophys Acta. 1987 Nov 5;916(1):128–134. doi: 10.1016/0167-4838(87)90219-6. [DOI] [PubMed] [Google Scholar]
- Bott R., Ultsch M., Kossiakoff A., Graycar T., Katz B., Power S. The three-dimensional structure of Bacillus amyloliquefaciens subtilisin at 1.8 A and an analysis of the structural consequences of peroxide inactivation. J Biol Chem. 1988 Jun 5;263(16):7895–7906. [PubMed] [Google Scholar]
- Careri G., Giansanti A., Gratton E. Lysozyme film hydration events: an ir and gravimetric study. Biopolymers. 1979 May;18(5):1187–1203. doi: 10.1002/bip.1979.360180512. [DOI] [PubMed] [Google Scholar]
- Careri G., Giansanti A., Rupley J. A. Proton percolation on hydrated lysozyme powders. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6810–6814. doi: 10.1073/pnas.83.18.6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careri G., Gratton E., Yang P. H., Rupley J. A. Correlation of IR spectroscopic, heat capacity, diamagnetic susceptibility and enzymatic measurements on lysozyme powder. Nature. 1980 Apr 10;284(5756):572–573. doi: 10.1038/284572a0. [DOI] [PubMed] [Google Scholar]
- Morrisett J. D., Broomfield C. A. A comparative study of spin-labeled serine enzymes: acetylcholinesterase, trypsin, -chymotrypsin, elastase, and subtilisin. J Biol Chem. 1972 Nov 25;247(22):7224–7231. [PubMed] [Google Scholar]
- Schinkel J. E., Downer N. W., Rupley J. A. Hydrogen exchange of lysozyme powders. Hydration dependence of internal motions. Biochemistry. 1985 Jan 15;24(2):352–366. doi: 10.1021/bi00323a018. [DOI] [PubMed] [Google Scholar]
- Zaks A., Klibanov A. M. Enzymatic catalysis in nonaqueous solvents. J Biol Chem. 1988 Mar 5;263(7):3194–3201. [PubMed] [Google Scholar]



