Abstract
Women are more susceptible than men to develop anxiety disorders, however, the mechanisms involved are still unclear. In this study, we investigated the role of group I metabotropic glutamate receptors (mGluRs), a target for anxiety disorders, and whether estradiol may modulate conflict-based anxiety in female rats by using the Vogel Conflict Test (VCT). We used ovariectomized female rats with high (OVX + EB) and low (OVX) estradiol levels and intact male rats to evaluate sex differences. Infusion of (S)-3,5-Dihydroxyphenylglycine (DHPG), a group I mGluR agonist, into the basolateral amygdala, a region involved in anxiety-responses, statistically increased the number of shocks in OVX, but not OVX + EB female rats at 0.1, nor at 1.0 μM. In contrast, DHPG statistically decreased the number of shocks in male rats at 1.0 μM only. DHPG (0.1 μM) increased the number of recoveries in OVX, but not OVX + EB or male rats. Sex differences were detected for the number of shocks, recoveries and punished licks, where female rats displayed more conflict than male rats. Western blot analyses showed that protein expression of mGluR1, but not mGluR5 was higher in OVX + EB > OVX > male rats in the amygdala, whereas no significant differences were detected in the hippocampus, olfactory bulb and/or the periaqueductal gray. Therefore, DHPG produced paradoxical effects that are sex dependent; producing anxiolytic-like effects in female rats, while anxiogenic-like effects in male rats according to the VCT. These results highlight the importance of including female experimental models to underpin the neural circuitry of anxiety according to sex and for the screening of novel anxiolytic compounds.
Keywords: Vogel Conflict Test, Group I metabotropic glutamate receptos, Estradiol, Basolateral amygdala, Sex differences
1. Introduction
Anxiety disorders affect nearly 40 million American adults annually, as well as contribute to the etiology of depression and drug abuse. It is widely accepted that women have a clear vulnerability to develop anxiety-related disorders [17,24,29]. This vulnerability might be due to differences in hormones levels between women and men [12]. However, other factors such as reproductive, neurodevelopmental, and physiological ones; and in humans, personal and social experiences should be considered as contributors to the high vulnerability of women. Interestingly, most of the available research data have been done and analyzed using male rodents due to the high variability of behavioral responses that females displayed during the estrous cycle. Thus, the scanty information regarding female neurobiological bases of anxiety make imperative to study female anxiety since available treatments might not be equally effective in women than in men.
In this study, we used the Vogel conflict test (VCT), a well-validated conditioned conflict-based anxiety model used for the screening of anxiolytic compounds [34,49]. The VCT is based in the approach-avoidance behaviors where water-deprived animals face the conflict of receiving a mild shock punishment when they drink water from a bottle. Like other conflict-based anxiety models, the VCT is responsive to benzodiazepines, which produces anti-conflict response by increasing the number of punished responses and/or shocks received by the animal in comparison to control animals [25]. In the VCT, intact female rats display more pro-conflict responses (e.g., less punished licks and shocks) than male rats, suggesting that female rats are more anxious [3,16]. These sex differences are independent of the female estrous stage since proestrous, as well as diestrous female rats display more conflict than male rats in the VCT [3]. Sex differences were also found in rodents on a variety of unconditioned anxiety tests. For instance, female rats show greater ambulatory activity [16,40], grooming behavior [36], and open field exploration [16], than male rats. These findings might suggest that in an unconditioned anxiety test, female rats are less anxious in comparison to male rats; whereas, in conditioned conflict-based anxiety models female rats are more anxious than their counterparts.
Metabotropic glutamate receptors (mGluRs) are suggested as a potential target for the treatment of anxiety-related disorders [30,44]. Eight mGluR subtypes have been cloned (mGluR1-mGluR8) and divided in three groups based on pharmacological properties, transduction mechanisms and amino acid sequences [42]. Group I mGluRs (mGluR1 and mGluR5 subtypes) are coupled to phospholipase C (PLC) by Gq11 proteins. Group I mGluRs is expressed in the rat amygdala, a brain structure involved in the anxiolytic effect of different compounds modulating glutamatergic neurotransmission [8,35]. Systemic administration of group I mGluRs antagonists reduce anxiety during the VCT [18,37,39,45,48]. Systemically selective mGluR1 antagonist such as (3,4-dihydro-2H-pyrano[2,3-b]quinolin-7-yl)-(cis-4-methoxycyclo-hexyl)-methanone (JNJ16259685), 6-methoxy-N-(4-methoxyphenyl)-4-quinazo-linamine hydrochloride (LY456236) and (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), produce anxiolytic-like effects in the VCT in male rats [19,46,48]. Antagonist targeting mGluR5 also decreased anxiety in other conditioned conflict-based anxiety tests, including the conditioned emotional response (CER), conditioned lick suppression (CLS) and Geller-Seifter test [2,48]. Similarly, group I mGluRs antagonists decreased anxiety in unconditioned response tests, such as the elevated plus maze (EPM), social interaction test, marble burying and stress-induced hyperthermia in male rats [44]. Notice that most of the work on group I mGluRs has been done using male animal models and systemic and/or intra-cerebroventricular (icv) administration. The only study that assessed the role of group I mGluRs within the amygdala in female rats, showed that (S)-3,5-dihydroxyphenylglycine (DHPG), a group I mGluR agonist, decreases unconditioned conflict-based anxiety in ovariectomized rats with high (OVX + EB), but not low (OVX) estradiol levels and/or male rats [8].
In this study, we tested the hypothesis that activation of group I mGluRs within the BLA, (1) will produce anti- and pro-conflict behaviors according to sex and estradiol levels in female rats when tested in the VCT and (2) sex specific modulation of anxiety-related responses will be dependent on estradiol and sex modulation of group I mGluRs protein expression. In experiment 1, we studied the behavioral effects of intra-BLA infusion of DHPG in OVX + EB and OVX female rats during the VCT. We included intact male rats that resemble low levels of estradiol (e.g., OVX) to analyze sex differences in this conflict-based test. In experiment 2, we conducted western blot analyses to study the expression of mGluR1 and mGluR5 subtypes within the amygdala, hippocampus, olfactory bulb and periaqueductal gray (PAG) of OVX + EB, OVX and male rats.
Taken together, this study highlights the importance of including female experimental models to underpin the neural circuitry of anxiety according to sex for the screening of novel anxiolytic compounds.
2. Methods
2.1. Animals and housing
Naïve adult female and male Sprague-Dawley rats, weighing 220–280 g, were obtained from Charles River Laboratories (Wilmington, MA). The animals were caged in same-sex pairs in acrylic cages (26.7 × 48.3 × 20.3 cm) on a light-controlled room (12:12-h light:dark cycle; lights on at 08:30 h) at a room temperature of 18–26 °C, relative humidity of 30–70%, and with food and water available ad libitum. All experimental procedures were conducted according to the Institutional Animal Care and Use Committee (IACUC) of the University of Puerto Rico Medical Sciences Campus (MSC) and the National Institute of Health Guide for the care and use of laboratory animals. The MSC animal care facility is accredited by the American Association for Laboratory Animal Care (AALAC) and registered at the United States Department of Agriculture.
2.2. Surgical procedures
Surgical procedures were conducted as previously described [8]. Briefly, animals were anesthetized with a combination of ketamine (80 mg/kg) and xylaxine (10 mg/kg, i.p.). We implanted two stainless steel guide cannulas (14 mm long, 23-gauge; Small Parts, Miami FL) two millimeter above the BLA complex using a stereotaxic apparatus (coordinates from bregma of Paxinos and Watson's atlas [33], AP = −2.80 mm, ML = ± 5.0 mm, DV = 6.2 mm; Kopf Instruments, Tujunga CA). Cannulas were fixed to the skull using four stainless steel screws and dental acrylic cement (Stoelting, Il). A 14-mm long stainless steel stylet (14-mm long, 30-gauge, Small Parts, Florida) was used to prevent cannula occlusion and infection.
Immediately after brain cannulation female rats were ovariectomized. Half of the animals received a subcutaneous silastic implant (6 mm;1.47 mm i.d. × 1.96 mm o.d.) containing approximately 4 mg of estradiol (OVX + EB) and the other half received empty implant (OVX) in the posterior neck. This estradiol amount is sufficient to maintain an estradiol concentration three times higher in OVX + EB than in OVX [10,34]. During recovery from anesthesia the animals received 1.0 ml of 0.9% saline (s.c.) to prevent dehydration and buprenorphine (0.05–0.1 mg/kg, i.m.), an analgesic to reduce distress. During the recovery week the animals were daily handled to provide the same pre-testing experience and to reduce animal stress associated to the manual restraint. Periodically, stylets were cleaned and/or replaced by new ones to prevent cannula occlusion and/or infections.
2.3. Drug and infusion protocol
We used (s)-3,5-dihydroxyphenylglycine (DHPG; Tocris Cookson, Ellisville MO), a selective agonist for mGluR1 and mGluR5 subtypes. The working concentrations of DHPG (0.1 or 1.0 μM) were chosen based on previous experiments [8]. Briefly, DHPG was diluted using sterile saline (0.9%) in aliquots of 1 mM and stored at −20 °C for up to one month. On the test day, DHPG was the diluted to its final concentration using sterile saline (0.9%). DHPG (0.1 or 1.0 μM) or saline (0.9%) was infused into the BLA complex at a volume of 0.5 μL/site/min using stainless steel needle injectors (16-mm long, 30-gauge, Small Parts, Florida) connected to an infusion pump (Harvard Apparatus, Holliston MA). The needle injectors remained in place for an additional minute and then removed and replaced with stylets. We followed aseptic techniques by using a hot bead sterilizer to clean injectors between animals. After a five minutes period, the VCT was conducted.
2.4. Vogel conflict test (conflict drinking test)
The VCT is a well-established conflict-based anxiety model, in which thirsty animals have a conflict between the appetitive behavior of drinking water and the risk of receiving an aversive shock [25,49]. We conducted the experiments during the dark phase of the light/dark cycle, under red light illumination (20W) as previously described [34]. Testing chambers consisted of acrylic boxes (18.42 cm long; 23.5 cm high; 22.23 cm wide) with a metal grid floor (1.3-cm stainless steel mesh). Briefly, on the day prior to the test the animals were acclimatized to the testing chamber for 30 min and then water deprived for 24 h. On test day, the animals were transferred from the research animal facility to the testing room, habituated to the room for at least one hour and then infused with either vehicle or the drug. After five minutes, animals were individually placed inside each testing chamber. The water bottle was electrically coupled to the metal grid floor and to a current generator controlled by a computer. The test lasted twenty-minutes; in which during the first 30 s the animal could drink water without punishment (warm-up period) followed by alternated and consecutive patterns of free (20 s) and punishment (20 s) periods. During the punishment periods, a mild shock (0.3 mA intensity, 100 ms duration), was randomly delivered to the floor of the cage after 3 consecutive licks. We monitored the animals’ drinking behavior by analyzing the following parameters: total number of shocks, recoveries (number of events characterized by two consecutive punished licks), recovery time (time between two consecutive punished licks), punished licks (number of licks performed during punishment periods), total licks (number of licks performed during free and punishment periods), and total latency (time between licks during free and punishment periods). We also analyzed the number of licks during the warm up period and the latency to lick during the first free period and first punished period as an index of the animals’ water thirst and their aversive perception of the electric shock, respectively.
2.5. Histology
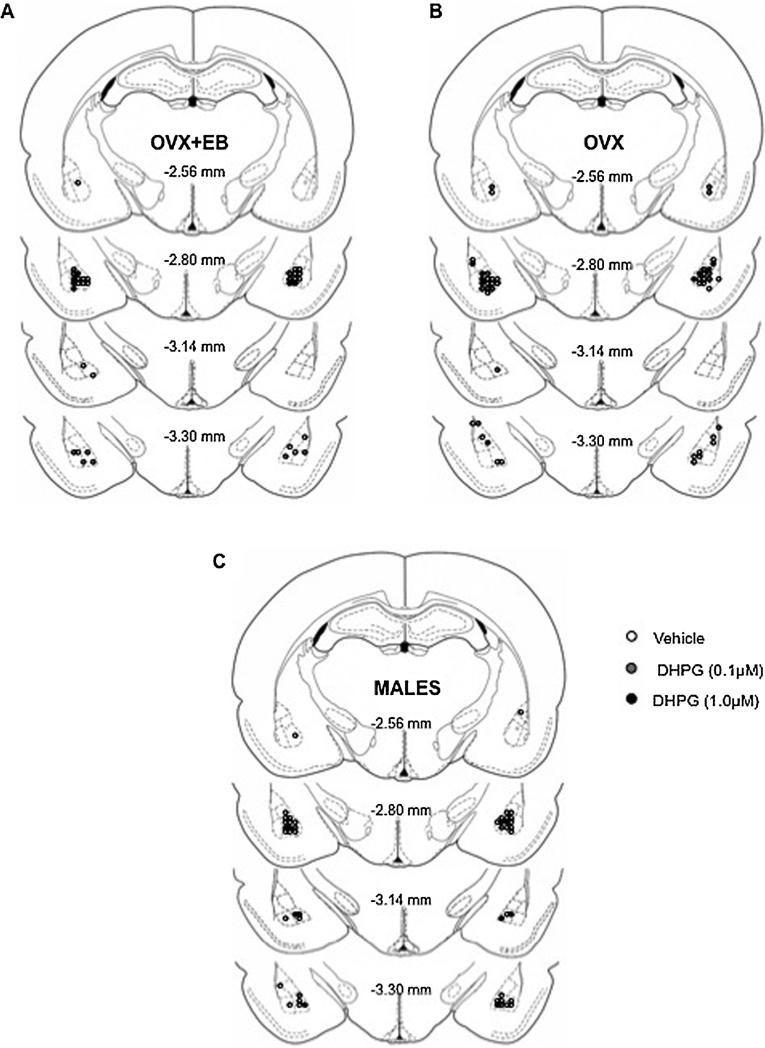
The animals were euthanized by rapid decapitation. The brains were quickly removed, stored at −80 °C and sectioned at 50 μm using a microtome (Leica CM 1900, on, Meyer Instruments, Houston, TX). Coronal sections were stained with cresyl violet and cannula placement was analyzed using a light microscope. Cannula localization (Fig. 1) was identified in diagrams modified from the Paxinos and Watson atlas [33]. The animals with misplaced cannulas were not included in the data analysis.
Fig. 1.
Schematic diagram showing the histological localization of cannula tips in the basolateral amygdala (BLA) of OVX + EB (A), OVX (B) and male rats (C) included in the analysis. White circles = vehicle-treated animals; gray circles = DHPG (0.1 μM); black circles = DHPG (1.0 μM). OVX + EB: vehicle, n = 19; DHPG (0.1 μM), n = 9; DHPG (1.0 μM), n = 12; OVX: vehicle, n = 20; DHPG (0.1 μM), n = 10; DHPG (1.0 μM), n = 17; Male: vehicle, n = 12; DHPG (0.1 μM), n = 8; DHPG (1.0 μM), n = 6.
2.6. Western blots experiments
Frozen brains from naïve OVX + EB, OVX and male rats were sectioned using a 1 mm matrix. Using the [33] Paxinos and Watson atlas as reference, tissues were collected from the amygdala, hippocampus, PAG and olfactory bulb, which was our positive control due to its high protein expression of group I mGluRs [11]. Coordinates from bregma for the amygdala and hippocampus were: − 1.88 to −3.30 mm and for PAG were: −5.50 mm to −6.30 mm. Tissue samples were obtained using a 2 mm micro-punch (OD; Harris micro-punch system). The amygdala samples contain the main nuclei including the LA, BLA, CeA and ITCs. Samples were manually homogenized in Tris–NaCl containing a protease inhibitor cocktail (2X Protease Arrest, Calbiochem, La Jolla, CA), EDTA (1 mM) and EGTA (1 mM) and centrifuged at 900 G for 10 min at 4 °C. The supernatant fraction was kept and stored at −80 °C until protein quantification using the Bradford method (Bio-Rad Protein Assay, Hercules, CA). Denatured samples (about 5–10 μg per lane) with Laemmli Sample Buffer (Bio-Rad Laboratories) containing 5%-2β-mercaptoethanol were separated in 6% polyacrylamide-SDS gel. Proteins transferred to PDVF membranes were blocked in blotto (3–5% non-fat milk dry milk, 20 mM Tris–HCl, 150 mM NaCL and 0.1% Tween-20) for 2 h at room temperature. Blots were incubated overnight in the corresponding primary antibodies for mGluR1 and mGluR5 detection. Primary antibodies (N-16 of Santa Cruz Biotechnology for mGluR1 and AB1596 of Millipore for mGluR5) were diluted in blotto to 1:3000. For the experiment of antibody specificity, primary antibodies were pre-incubated for 24 h with their corresponding control peptides (sc-47131 P for mGluR1 and AG374 for mGluR5). Then, blots were washed and incubated in horseradish peroxidase-conjugated antiserum (donkey-anti-goat and anti-rabbit for mGluR1 and mGluR5, respectively) diluted in blotto solution for two hours. After washes, HPR signal was enhanced with Super Signal West Femto Maximum Sensitivity Substrate (Pierce, IL) and membranes were exposed and developed using the Versadoc™ Imaging System. The immunoreactivity was quantified using Quantity One Software (Bio-Rad, Hercules, CA). The β-actin immunoreactivity was used as the loading control and the expression of mGluR1 or mGluR5 was standardized to the level of this housekeeping gene.
2.7. Statistics
For experiment 1, data is presented as mean ± standard error of the mean (SEM) values. Statistical analysis for female (OVX + EB vs OVX) and male rats were analyzed separately since the experiments were conducted at different time points. OVX + EB and OVX data was analyzed using a two-way analysis of variance (ANOVA) to determine the effects of: (1) drug; (2) estradiol treatment; and (3) the interaction between drug and estradiol treatment. Male data was analyzed using a one-way ANOVA to determine whether DHPG modulates anxiety depending upon drug concentration. Post-hoc multiple comparison analyses were performed using Student–Newman–Keuls tests. One-way analyses of variance (ANOVA), including vehicle infused animals, were conducted to analyze sex differences and whether estradiol potentiated sex differences on baseline responses. For experiment 2, a one-way ANOVA was performed to analyze differences in the expression levels of mGluR1 and mGluR5 subtypes, respectively, in OVX + EB, OVX and male rats. Differences were considered statistically significant when p values were ≤ 0.05.
3. Results
3.1. Experiment 1
3.1.1. Effects of DHPG on conditioned conflict-based anxiety according to estradiol levels in ovariectomized rats and sex
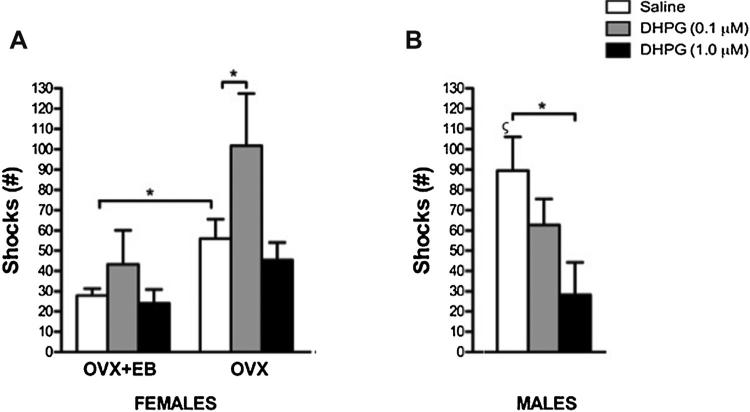
We analyzed the effects of intra-BLA infusion of DHPG on conditioned conflict-based anxiety by using the VCT. In female rats, a two-way ANOVA revealed a significant effect of DHPG (Fig. 2A; F(2,83) = 4.16; p = 0.02) and a significant effect of estradiol treatment (Fig. 2A; F(1,83) = 11.69; p = 0.001) on the number of shocks. The interaction between DHPG and estradiol was not detected (Fig. 2A; F(2,83) = 1.37; p = 0.26). DHPG at 0.1, but not 1.0 μM increased the number of shocks received in OVX (Fig. 2A; p = 0.01, p = 0.46, respectively), but not in OVX + EB (Fig. 2 A; p = 0.65, p = 0.91, respectively). Estradiol by itself decreased the number of shocks received when compared OVX + EB-vehicle and OVX-vehicle (Fig. 2A; p = 0.045). In male rats, a one-way ANOVA revealed a significant effect of DHPG on the number of shocks received (Fig. 2B; F(2,25) = 3.32; p = 0.05). DHPG at 1.0, but not 0.1 μM decreased the number of shocks received (p = 0.045, p = 0.23, respectively).
Fig. 2.
Effects of intra-BLA of DHPG on the number of shocks received during the VCT. (A) DHPG at 0.1 μM but not 1.0 μM increased the number of shocks received in OVX but not OVX + EB. Estradiol by itself decreased the number of shocks received when compared OVX + EB-vehicle and OVX-vehicle. (B) DHPG statistically decreased the number of shocks at 1.0 but not 0.1 μM. Male received more shocks than OVX + EB and OVX. Values represent mean ± SEM. * = p ≤ 0.05; ζ = sex differences when compared male-vehicle with OVX + EB-vehicle and OVX-vehicle. OVX + EB: vehicle, n = 19; DHPG (0.1 μM), n = 9; DHPG (1.0 μM), n = 12; OVX: vehicle, n = 20; DHPG (0.1 μM), n = 10; DHPG (1.0 μM), n = 17; male: vehicle, n = 12; DHPG (0.1 μM), n = 8; DHPG (1.0 μM), n = 6.
In female rats, a two-way ANOVA showed a significant effect of DHPG (F(2,83) = 4.35; p = 0.02) and estradiol treatment (Fig. 3A; F(1,83) = 12.05; p < 0.001) on the number of recoveries. The interaction between DHPG and estradiol was not detected (Fig. 3A; F(2,83) = 1.43; p = 0.25). DHPG at 0.1 μM, but not 1.0 μM increased the number of recoveries in OVX (Fig. 3A; p = 0.01 and p = 0.43, respectively), but not in OVX + EB (Fig. 3A; p = 0.42 and p = 0.97, respectively). Estradiol by itself decreased the number of recoveries when OVX + EB-vehicle and OVX-vehicle were compared (Fig. 3B; p = 0.05). In male rats, a one-way ANOVA revealed no effect of DHPG (0.1 and 1.0 μM) on the number of recoveries (Fig. 3B; F(2,25) = 2.47; p = 0.12).
Fig. 3.
Effects of intra-BLA of DHPG on the number of recoveries during the VCT. (A) DHPG at 0.1 μM but not 1.0 μM increased the number of recoveries in OVX but not OVX + EB rats. Estradiol by itself decreased the number of recoveries when compared OVX + EB-vehicle and OVX-vehicle. (B) DHPG (0.1 and 1.0 μM) did not alter the number of recoveries the number of recoveries in male rats. Male rats displayed significantly more recoveries when compared to OVX + EB Values represent mean ± SEM. * = p ≤ 0.05; ζ = sex differences when compared male-vehicle with OVX + EB-vehicle and OVX-vehicle. OVX + EB: vehicle, n = 19; DHPG (0.1 μM), n = 9; DHPG (1.0 μM), n = 12; OVX: vehicle, n = 20; DHPG (0.1 μM), n = 10; DHPG (1.0 μM), n = 17; male: vehicle, n = 12; DHPG (0.1 μM), n = 8; DHPG (1.0 μM), n = 6.
DHPG (0.1 and 1.0 μM) did not modulate the number of punished licks, total number of licks or the recovery time (Table 1). In female rats, a two-way ANOVA revealed no effect of DHPG on the number of punished licks (F(2,83) = 1.15; p = 0.32), but a significant effect of estradiol treatment (F(1,83) = 8.12; p = 0.006). The interaction between DHPG and estradiol was not detected (F(2,83) = 0.78; p = 0.46). In male rats, a one-way ANOVA revealed no effect of DHPG (0.1 and 1.0 μM) on the number of punished licks (F(2,25) = 1.56; p = 0.23). In female rats, a two-way ANOVA revealed no effect of DHPG on the number of total licks (F(2,83) = 0.83; p = 0.44), but a significant effect of estradiol treatment (F(1,83) = 5.13; p = 0.03). The interaction between DHPG and estradiol was not detected (F(2,83) = 0.28; p = 0.76). In male rats, a one-way ANOVA revealed no effect of DHPG (0.1 and 1.0 μM) on the total number of licks (F(2,25) = 1.53; p = 0.24). In female rats, a two-way ANOVA revealed no effect of DHPG (F(2,83) = 0.93; p = 0.40) and estradiol treatment (F(1,83) = 2.46; p = 0.12) on the recovery time. The interaction between DHPG and estradiol treatment was not detected (F(2,83) = 0.03; p = 0.97). In male rats, a one-way ANOVA revealed no effect of DHPG (0.1 and 1.0 μM) on the recovery time (F(2,25) = 1.48; p = 0.25).
Table 1.
DHPG (0.1 and 1.0 μM) did not modulate the number of punished licks, total number of licks and the recovery time in OVX+EB and OVX, and male rats.
| Behavioral Response |
||||
|---|---|---|---|---|
| Treatment | Punished licks (#) | Total licks (#) | Recovery time (sec) | Total latency (sec) |
| OVX + EB | ||||
| Vehicle | 192.21 ± 58.13 | 555.32 ± 129.14 | 20.72 ± 4.08 | 84.82 ± 9.80 |
| DHPG (0.1 μM) | 203.56 ± 84.46 | 629.56 ± 187.64 | 19.60 ± 5.93 | 121.27 ± 14.25* |
| DHPG (1.0 μM) | 213.33 ± 84.46 | 517.11 ± 187.64 | 13.36 ± 5.93 | 45.79 ± 14.25* |
| OVX | ||||
| Vehicle | 302.25 ± 56.66 | 743.95 ± 125.87 | 26.20 ± 3.98 | 77.32 ± 9.56 |
| DHPG (0.1 μM) | 317.77 ± 61.45 | 1053.00 ± 178.01 | 25.62 ± 5.63 | 97.52 ± 13.51 |
| DHPG (1.0 μM) | 491.30 ± 80.12 | 791.77 ± 136.53 | 21.24 ± 4.32 | 66.63 ± 10.37 |
| MALE | ||||
| Vehicle | 493.00 ± 104.81ς | 1133.42 ± 228.26 | 31.26 ± 7.87 | 94.21 ± 18.16 |
| DHPG (0.1 μM) | 202.83 ± 119.65 | 794.88 ± 260.95 | 20.55 ± 4.69 | 111.05 ± 12.88 |
| DHPG (1.0 μM) | 333.63 ± 114.66 | 496.33 ± 267.45 | 13.29 ± 7.31 | 99.40 ± 18.69 |
Note: Data was analyzed using a two-way ANOVA for female rats and one-way ANOVA for male rats.
Statistical significant:
drug within group effect
differences between OVX female vs male rats
and ε: estradiol effect.
In female rats, a two-way ANOVA revealed a significant effect of DHPG on latency to lick (Table 1; F(2,83) = 8.14; p = 0.001). Estradiol treatment (F(1,83) = 0.12; p = 0.72) and the interaction between DHPG and estradiol (F(2,83) = 1.53; p = 0.22) were not detected. DHPG (0.1 and 1.0 μM) produced biphasic effects in OVX + EB (p = 0.04 and p = 0.03, respectively), but not in OVX rats (p = 0.23 and p = 0.45, respectively). In OVX + EB, DHPG at 0.1 μM increased while at 1.0 μM decreased the latency to lick. In male rats, a one-way ANOVA revealed no effect of DHPG (0.1 and 1.0 μM) on the latency to lick (F(2,25) = 0.25; p = 0.78).
3.1.2. Sex differences on conditioned conflict-based anxiety tested in the VCT
We analyzed sex differences and whether estradiol potentiates these sex differences on conditioned conflict-based anxiety during the VCT. A one-way ANOVA revealed sex differences for the number of shocks (Fig. 2A,B; F(2,50) = 9.01). Post-hoc comparisons revealed that male rats received more shocks when compared to OVX (p = 0.025) and OVX + EB (p < 0.01). One-way ANOVAs revealed that recovery events and punished licks were higher in males than OVX and OVX + EB female rats (Fig. 3A,B; F(2,50) = 7.06; p = 0.002 and Table 1; F(2,50) = 4.60; p = 0.02, respectively). Post-hoc comparisons revealed that male rats displayed more recovery events and punished licks when compared to OVX + EB (p = 0.002, p = 0.011, respectively) and a strong tendency for OVX female rats (p = 0.058 and p = 0.058, respectively). Sex differences were not detected for the recovery events, recovery time, total number of licks, total latency and latency to lick during punishment periods (Table 1; p > 0.05).
3.1.3. Initial water consumption and the latency to lick during the first free and punished periods of animals tested in the VCT
The number of licks during the warm up period was analyzed as an index of water thirst in animals (Table 2). A two-way ANOVA revealed no effect of DHPG (F(2,83) = 2.89; p = 0.06) and estradiol treatment (F(2,83) = 1.99; p = 0.16) on the number of licks during the warm-up period in female rats. The interaction between DHPG and estradiol treatment was not detected on the number of licks during the warm-up period (F(2,83) = 2.63; p = 0.08). In male rats, a one-way ANOVA revealed no effect of DHPG on the number of licks during the warm-up period (H = 0.60; p = 0.74). To analyze the impact of estradiol treatment and animal sex on the aversive perception of electric shocks, the latency to lick during the first free period was compared with the latency to lick during the first punishment period (Table 2). Paired t-test revealed that OVX + EB-vehicle (p = 0.016), displayed longer latency to lick during the punishment period than during the free period. No differences were detected for OVX or male rats (paired t-test; p > 0.05).
Table 2.
Animal latency during the first free and punished period and the initial water consumption according to sex and estradiol treatment in female rats.
| Latency |
Licks | ||
|---|---|---|---|
| Experimental group | 1st Free period | 1st Punished period | Warm period |
| OVX + EB | |||
| Vehicle | 0.07 ± 0.99ε | 1.72 ± 1.08 | 14.58 ± 7.23 |
| 0.1μM DHPG | 1.03 ± 1.45 | 1.39 ± 1.58 | 43.78 ± 16.91 |
| 1.0μM DHPG | 0.69 ± 1.45 | 4.05 ± 1.58 | 2.78 ± 10.5 |
| OVX | |||
| Vehicle | 3.89 ± 0.97ε | 1.81 ± 1.06 | 18.20 ± 7.05 |
| 0.1μM DHPG | 1.91 ± 1.36 | 0.28 ± 1.50 | 8.50 ± 7.65 |
| 1.0μM DHPG | 2.46 ± 1.06 | 2.56 ± 1.15 | 3.47 ± 7.65 |
| MALE | |||
| Vehicle | 3.89 ± 1.72 | 1.15 ± 1.10 | 16.50 ± 9.23 |
| 0.1μM DHPG | 3.12 ± 2.05 | 3.89 ± 2.58 | 22.12 ± 9.95 |
| 1.0μM DHPG | 0.35 ± 0.02 | 3.31 ± 2.26 | 16.66 ± 10.16 |
Note: Data of 1st unpunished period were compared with data of 1st punished period using a paired t-test.
estradiol effect.
3.2. Experiment 2
3.2.1. Antibody specificity assays
Western blot analyses for mGluR1 and mGluR5 expressions were conducted using separated blots. Analyses consistently revealed immunoreactive bands corresponding to the molecular weight of ~140 kDa for mGluR1 and ~150 kDa for mGluR5. We detected a band with a molecular weight over 250 kDa, probably corresponding to the dimeric form of the proteins [23]. Pre-absorption of the primary antibodies with their corresponding control peptides, attenuated the immunoreactivity of mGluR1 band (Fig. 4) and completely abolished the immunoreactivity for mGluR5 monomer band (Fig. 5), demonstrating the antibody specificity. We focused our analysis in the monomeric form of mGluR1 and mGluR5, since their immunoreactivities were consistently detected throughout the samples.
Fig. 4.
Western blot analysis of proteins extracted from olfactory bulb (OB), amygdala (A), periaqueductal grey (PAG) and hippocampus (HC) in male rats. Lanes 1–4 shows the mGluR1 immunoreactivity (monomer at 140 kDa and apparently the dimmer over 280 kDa) obtained using the anti-mGluR1 antibody (N-16; Santa Cruz Bio). Lanes 5–8 shows the mGluR1 anti-body immunoreactivity attenuated after incubation of N-16 pre-absorbed with the control peptide (sc-47131; Santa Cruz Bio.). Lower panel shows immunoreactivity of actin (lanes 1–8) obtained used as loading control.  Represents the molecular weight size standard in kilo Dalton (KDa).
Represents the molecular weight size standard in kilo Dalton (KDa).
Fig. 5.
Western blot analysis of proteins extracted from olfactory bulb (OB), amygdala (A), periaqueductal grey (PAG) and hippocampus (HC) in male rats. Lanes 1–4 shows the mGluR5 immunoreactivity (monomer at 150 KDa and apparently the dimmer over 250 KDa) obtained using the anti-mGluR5 antibody (AB5675; Millipore). Lanes 5–8 shows the mGluR5 anti-body immunoreactivity attenuated after incubation of AB5675 pre-absorbed with the control peptide (AG374; Millipore). Lower panel shows immunoreactivity of actin (lanes 1–8) obtained used as loading control.  Represents the molecular weight size standard in kilo Dalton (kDa).
Represents the molecular weight size standard in kilo Dalton (kDa).
3.2.2. Protein levels of group I mGluRs (mGluR1 and mGluR5 subtypes) in the amygdala, hippocampus, olfactory bulb and PAG
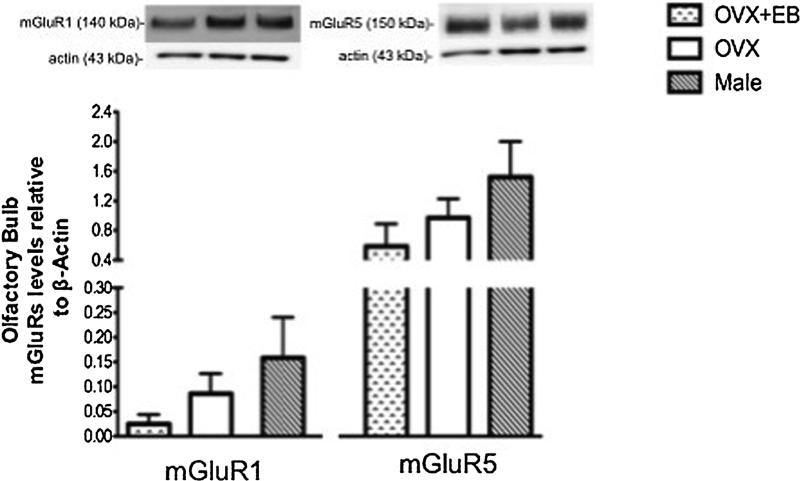
Western blot analyses were conducted in the amygdala, hippocampus, olfactory bulb and PAG regions to analyze differences in group I mGluRs protein levels due to estradiol treatment in female rats and/or animal sex. In the amygdala, a one-way ANOVA revealed significant differences in mGluR1 protein expression levels (Fig. 6; F(2,17) = 13.17; p < 0.001). OVX + EB showed an up-regulation mGluR1 protein levels in comparison to OVX female rats (Fig. 6; p = 0.04). Comparison of mGluR1 protein levels of OVX female rats with male rats revealed sex differences (Fig. 6; p = 0.01), where OVX expressed higher protein levels than male rats. One-way ANOVA revealed no differences in mGluR5 protein expression levels in OVX + EB, OVX and male rats (Fig. 6; F(2,16) = 0.26; p = 0.78). In the hippocampus, a one-way ANOVA revealed no differences in mGluR1 (Fig. 7; F(2,17) = 0.50; p = 0.62) and mGluR5 protein expression levels (Fig. 7; F(2,17) = 0.06; p = 0.94). In the olfactory bulb, a one-way ANOVA revealed no differences in mGluR1 (Fig. 8; F(2,11) = 1.54; p = 0.27) and mGluR5 protein levels (Fig. 8; F(2,8) = 1.72; p = 0.26). Similarly, in the PAG, one-way ANOVAs revealed no differences in mGluR1 (Fig. 9; F(2,17) = 1.44; p = 0.27) and mGluR5 protein levels (Fig. 9; F(2,17) = 0.41; p = 0.67).
Fig. 6.
Expression of group I mGluR subtypes in the amygdala in ovariectomized female with high (OVX + EB), low (OVX) estradiol levels, and male rats. Representative immunoblots of mGluR1 (left panel) and mGluR5 (right panel) with its corresponding loading controls are shown in the upper panel. Histogram graphs of mGluR1 and mGluR5 protein levels normalized to actin are shown in the lower panel. OVX + EB rats showed higher mGluR1/actin ratio when compared to OVX and male rats. OVX showed higher mGluR1/actin ratio than male rats (OVX + EB, n = 6; OVX, n = 6; Male, n = 6). No differences were detected for mGluR5/actin ratio between the experimental groups (OVX + EB, n = 5; OVX, n = 6; male, n = 6). Values represent mean ± SEM. * = p ≤ 0.05.
Fig. 7.
Expression of group I mGluR subtypes in the hippocampus in ovariectomized female with high (OVX + EB), low (OVX) estradiol levels, and male rats. Representative immunoblots of mGluR1 (left panel) and mGluR5 (right panel) with its corresponding loading controls are shown in the upper panel. Histogram graphs of mGluR1 and mGluR5 protein levels normalized to actin are shown in the lower panel. No differences were detected between OVX + EB, OVX and male rats for mGluR1/actin ratio (OVX + EB n = 6; OVX n = 6; Male n = 6) and mGluR5/actin ratio (OVX + EB n = 6; OVX n = 6; Male n = 6). Values represent mean ± SEM.
Fig. 8.
Expression of group I mGluR subtypes in the olfactory bulb in ovariectomized female with high (OVX + EB), low (OVX) estradiol levels, and male rats. Representative immunoblots of mGluR1 (left panel) and mGluR5 (right panel) with its corresponding loading controls are shown in the upper panel. Histogram graphs of mGluR1 and mGluR5 protein levels normalized to actin are shown in the lower panel. No differences were detected between OVX + EB, OVX and male rats for mGluR1/actin ratio (OVX + EB, n = 4; OVX, n = 4; Male, n = 4) and mGluR5/actin ratio (OVX + EB, n = 3; OVX, n = 3; male, n = 3). Values represent mean ± SEM.
Fig. 9.
Expression of group I mGluR subtypes in the periaqueductal grey matter (PAG) in ovariectomized female with high (OVX + EB), low (OVX) estradiol levels, and male rats. Representative immunoblots of mGluR1 (left panel) and mGluR5 (right panel) with its corresponding loading controls are shown in the upper panel. Histogram graphs of mGluR1 and mGluR5 protein levels normalized to actin are shown in the lower panel. No differences were detected between OVX + EB, OVX and male rats for mGluR1/actin ratio (OVX + EB, n = 6; OVX, n = 6; Male, n = 6) and mGluR5/actin ratio (OVX + EB, n = 6; OVX, n = 6; Male, n = 6). Values represent mean ± SEM.
4. Discussion
In the present study we showed for the first time that intra-BLA infusion of DHPG in the VCT produces paradoxical effects according to sex. In ovariectomized female rats, DHPG produces anxiolytic-like effects while in male rats produces anxiogenic-like effects. Sex differences were found in which female showed higher levels of anxiety-related behaviors when compared to male rats. In addition, estradiol by itself increases anxiety in ovariectomized rats during the test. These results support our hypothesis that intra-BLA infusion of DHPG will produce anti- and pro-conflict effects according to sex and estradiol levels in female rats.
DHPG is the most selective group I mGluR agonist, with similar potencies at mGluR1 and mGluR5 subtypes [28]. To the best of our knowledge, we are the first to demonstrate the effects of intra-BLA infusion of DHPG during a conditioned conflict-based anxiety model, producing anti- and pro-conflict effects during the VCT in a sex-specific manner. In male rats, systemic administration of group I mGluRs antagonist, decreases conflict-based anxiety in the VCT (anti-conflict effect) [37,39,45,46,48]. Likewise, intrahippocampal infusion of 7-hydroxyiminocyclopropan [b]chromen-1a-carboxylic acid ethyl ester (CPCCOEt) and (S)-4-carboxy-3-hydroxyphenylglycine (S-4C3H-PG), two selective group I antagonists produce anti-conflict effects during the VCT [6,47]. These studies have consistently evidenced that blocking group I mGluRs signaling, at least in male models, increases the number of shocks received during the test. Potentially, blockage of group I mGluRs could reduce glutamatergic transmission and thus, decreases conditioned conflict-based anxiety. A reduction in glutamatergic neurotransmission may produce similar effects to an increase in GABAergic neurotransmission, which has been widely documented for the benzodiazepines (BZDs), the first-line of medication for generalized anxiety. In rodents, intra-BLA infusion of Ro 15-1788, a BZDs antagonist, attenuates the anti-conflict effect of BZDs systemically demonstrating the implication of BLA excitability during conflict-based anxiety [14]. Therefore, we would expect that an enhancement of BLA glutamatergic transmission by DHPG increases anxiety-related behaviors (pro-conflict effects) during the VCT. We found that DHPG produces pro-conflict effects in male rats, suggesting anxiogenic-like effects. On the other hand, in OVX female rats, DHPG produced the opposite effect, decreasing conflict in the test and thus, suggesting an anxiolytic-like effect. Unfortunately, the role of group I mGluRs during conditioned conflict responses have not been studied in female models, making it difficult for us to compare the effects of DHPG on the basis of existing literature. However, our findings strongly suggest a sexually dimorphic mechanism of BLA glutamatergic neural substrate in conflict-based anxiety. This finding is supported by previous study where Intra-BLA infusion of DHPG decreases anxiety in the elevated plus maze (EPM), an unconditioned conflict-based model, in estradiol treated female rats, but not in male rats [8].
In our experiments, animal water consumption was not affected by DHPG since it did not alter the total number of licks during the warm-up period and the total lick test session. Paired analyses of the latency to lick during the first- free and punishment periods did not reveal any effect of the drug infusion in female and male rats. These results suggest that DHPG modulation of VCT parameters was by changes in anxiety levels and not by confounding effects such as water intake and/or shock threshold pain perception. Our interpretation is also supported by an experiment where intra-BLA infusions of group-I subtype specific antagonists by itself did not modulate the pain threshold perception to acute mechanical aversive stimulation of the hind rat's paws [21]. In addition, systemic administration of JNJ16259685, an mGluR1 antagonist, did not modulate acute pain sensitivity during tail withdrawal or tail pinch tests [46]. Similarly, systemic administration of group I mGluR antagonists, AIDA (for mGluR1) and/or MPEP (for mGluR5), did not modulate the response to shock threshold [45]. In addition, we reduced a possible interference of the pain perception in the interpretation of our data using VCT protocol with moderate shock intensity (0.3 mA). Interestingly, Basso and colleagues (2011) showed that acute injection of the analgesic morphine did not change the punished response during the VCT parameters. These results support our interpretation that DHPG modulation during of VCT parameters are by changes in anxiety levels opposed to any alterations in pain perception. However, further experiments need to be done to study specifically the role of group I mGluRs within the BLA during acute pain, which are beyond the scope of the present study.
Conflict behavioral responses during VCT are sexually dimorphic. Cycling female rats performed less punished licks in comparison to male rats [3,4,16]. Scrutiny of punished lick frequency throughout the estrous cycle demonstrated no significant effect of the estrous stage on punished lick behavior [3]. Despite these sex differences, few experiments evaluate high and low estradiol levels in ovariectomized female rats (OVX + EB and OVX, respectively) during the VCT in comparison to male rats [34]. We found that female rats displayed less punished licks, shocks and recoveries than male rats. These differences are potentiated by estradiol treatment, suggesting that sex differences on VCT performance are mediated by organizational sex differences and activational effects by estradiol. These results are in agreement with previous experiments where ovariectomized female rats received less shocks than male rats and in female rats, estradiol by itself, decreased the number of shocks received [34]. On the other hand, systemic administration of acute estradiol in aged female mice, a model for menopause, one hour prior to the test did not modulate VCT behavior [51]. Thus, our results strongly suggest that female rats displayed more conflict-based anxiety in comparison to male rats and these differences are potentiated by estradiol treatment. In addition, female performance during the VCT seems to be modulated by differences in timing of estradiol depletion and/or the estradiol replacement regimen.
Paired analysis indicates that the latency to lick increased during the first punishment period in OVX + EB, but not in OVX or male rats. This result could be interpreted as an index of increased shock sensitivity by estradiol treatment. In fact, cycling female rats displayed less resistance for given shock levels in comparison to male rats [3,4,16]. The fact that OVX + EB female rats are engaged in drinking behavior faster (less latency during the first free period) than OVX female or male rats without differences in water consumption suggests that estradiol increases female's approach behavior toward a novel stimuli and/or environment during the VCT. This is supported by the fact that female rats during estrous cycle displayed more approach behavior than diestrous and male rats [13]. In addition, it has been shown that estradiol treatment increased the percentage time exploring a novel object in ovariectomized female rats [52].
Sex differences on VCT performance and sex-dependent DHPG effects might be underlined by organizational and activational differences on BLA mGluRs protein levels. To evaluate whether the results obtained in the behavioral experiments were due to differences in group I mGluRs protein expression in regions related to the anxiety-brain circuitry, we conducted western blot experiments. To date, the protein expression of group I mGluRs has been studied only in male rats [20]. Thus, to the best of our knowledge, we are the first to study the expression of group I mGluRs in naïve ovariectomized rats with and without estradiol treatment. We showed that mGluRs are differentially expressed in emotional-related regions. The protein expression of mGluR1, but not mGluR5 was sensitive to estradiol and sex factors in a region-specific manner. In samples from the amygdala, OVX + EB expressed higher mGluR1 protein levels than OVX and male rats (OVX + EB > OVX > male). We also found sex differences where OVX expressed higher mGluR1 protein levels than male rats. In contrast, the protein expression of mGluR5 remained akin between OVX + EB, OVX and male rats. We did not expect to find differences in both subtypes of group I mGluRs since the behavioral observations in a previous work in our laboratory suggested subtype specific roles for mGluR1 and mGluR5 on generalized anxiety-related behaviors depending upon sex and estradiol levels in female rats [8]. However, some antidepressant drugs used to treat anxiety disorders like imipramine, up-regulates the expression of mGluR1 and mGluR5 subtypes within the hippocampus [1,43]. While chronic corticosterone treatment and chronic mild stress, down-regulates the expression of mGluR5 subtype in the same region [53]. It appears that the expression of group I mGluRs vary according to different anxiety-related factors including the sex and animal hormonal levels. Note that in the present study, we analyzed the total pool of proteins that included the receptors expressed in the membrane surface and those present in the intracellular pools. Membrane trafficking and sequestration of group I mGluRs, is highly regulated by scaffold proteins including homer 1a and 1b, and by kinases proteins [54]. Thus, estradiol- and/or sex-differences on membrane mGluRs expression and functionality must be further studied to elucidate whether sex specific effects of DHPG are underlaid by differences in mGluRs membrane expression and function in the BLA projecting neurons.
We used olfactory bulb (OB) as our positive control due to its high mGluR protein expression [11]. Interestingly, in whole OB samples, we found a subtle down-regulation of mGluR1, but not mGluR5 expression in OVX + EB in comparison to OVX and male rats. Contrary to what we found in the amygdala, there is a tendency of OVX + EB to express lower mGluR1 protein levels than OVX and male rats (OVX + EB < OVX < male). Lower levels of mGluR1 in OVX + EB than OVX and male rats may imply differences in OB depolarization and signal transduction and it must be addressed by future experiments. In the hippocampus, which relays context-related afferent information to the amygdala, as well as in the PAG, which is an amygdala effector region, the expression of mGluR1 and mGluR5 protein levels were not significantly different between OVX + EB, OVX and male rats. These results did not rule out the possibility of: (1) changes in another neurotransmitter system within these regions and/or (2) changes in the expression of group I mGluRs in other brain regions involved in anxiety according to sex and estradiol levels. However, further experiments are needed and these experiments were beyond of our scope.
The amygdala and mGluRs are estradiol sensitive. Estradiol produces transcriptional as well as structural effects within the amygdala. For instance, estradiol increases the expression of proteins related to CREB signaling cascade [9,15] and promotes synaptogenesis, increasing the number of dendritic shaft and spines [27,41] within the amygdala. Antidepressant drugs used for anxiety disorders similar to estradiol induce synaptogenesis, which has been conceptualized as their molecular mechanism of action [30]. According to our experiments, estradiol up-regulates the expression of mGluR1, but not mGluR5 in OVX + EB in the amygdala. Therefore, amygdalar synaptogenesis might indirectly entail mGluR1 up-regulation. Sensitivity of mGluR1 expression to estradiol treatment is region-specific not affecting at all the hippocampus and PAG. These results are supported by previous experiments where estradiol treatment in OVX rats did not modulate mGluR1 protein expression in the arcuate nucleus of the hypothalamus [22]. Hence, we suggest that selective up-regulation of amygdalar mGluR1 protein might underlay molecular mechanism to modulate anxiety in female rats.
Overall, in our molecular experiments, we showed that mGluR1, but not mGluR5 is up-regulated in OVX + EB in comparison with OVX; and in OVX in comparison with male rats in the amygdala. To delineate potential mechanisms by which DHPG modulates anxiety in OVX and male rats, it is of high importance to pinpoint the anatomical localization of up-regulated mGluR1 within the amygdala. The expression of group I mGluR in neurons within the amygdaloid complex has been documented [20]. The basolateral complex acts as an interphase between afferent information and the efferent output of the medial CeA (mCeA). BLA may drive anxiety-related behaviors via direct glutamatergic projections to mCeA and attenuate anxiety-related behaviors through indirect disynaptic routes involving the GABAergic neurons in the intercalated cell masses (ITCs) and in the lateral part of the CeA (LCeA), that project to the mCeA [31,32,38]. In male rats, DHPG could activate glutamatergic-projecting neurons to mediate direct and indirect activation of mCeA increasing conflict-based anxiety. Since activation of medial ITC has been conceptualized as a mechanism for extinction of conditioned fear-like anxiety responses [32]; we hypothesize that mGluR1 is up-regulated on glutamatergic-projecting neurons to medial ITCs, providing a feed-forward inhibition pathway to decrease mCeA activation. In this scenario, infusion of DHPG can meditate anxiolysis in female, but not male rats. The lack of DHPG effects in OVX + EB was unexpected since we found that intra-BLA infusion of DHPG decreased non-conditioned anxiety during the EPM by an mGluR1 subtype dependent mechanism [8]. In addition, since mGluR1 is more expressed in OVX + EB than OVX, we expected to find anxiolytic-like effects by DHPG in OVX + EB. However, it has been widely documented that BLA processes conditioned and unconditioned stimulus differentially [7,50]. In addition, estradiol by itself increased conditioned conflict-based anxiety with no effect in unconditioned conflict-based anxiety [8,34]. Whether estradiol mediates the anxiogenic-like effects is uncertain. Nevertheless, Boulware et al. [5] demonstrated that estradiol can inhibit signal transduction by ERs activation of group II mGluRs at membrane level. Group II mGluRs are presynaptic receptors that can potentially inhibit glutamate release of BLA projecting neurons to medial ITC. Thus, although mGluR1 is hypothetically up-regulated in BLA projecting neurons to medial ITC, ERs activation of group II mGluRs on the terminals of these cells can potentially abolish DHPG initiated excitability at the dendrites and soma of mGluR1 expressing neurons. In addition, estradiol enhances neurotransmission of serotonin, dopamine, and norepinephrine, as well as of neuropeptides such as corticotropin releasing hormone (CRF) [26]. These estrogenic effects undoubtedly provide additional mechanisms for the influence of estradiol on BLA activity and response to DHPG.
In conclusion, our study demonstrates for the first time ever, that activation of group I mGluR within the BLA mediates sex-specific effects, decreasing conflict in OVX, but increasing conflict in male rats. In addition, it argues that the effects of DHPG could be underlined by organizational sex differences on conflict-based anxiety, where OVX are more anxious than male rats and perhaps by activational effects due to the fact that estradiol treatment enhanced sex differences in conflict-based anxiety. We propose that such behavioral effects can be due, in part, to differences in the protein expression levels of mGluRs within the amygdala.
Therefore, it is of pivotal importance for pre-clinical studies to include female models of anxiety while screening for the potential therapeutic effects of novel pharmacological agents, especially while assessing conflict-based anxiety.
HIGHLIGHTS.
We studied the role of group I mGluRs within the BLA according to sex and estradiol levels in females rats using conditioned conflict-based anxiety model.
DHPG produced sex specific effects: anti-conflict effects in OVX female rats and pro-conflict effects male rats.
Sex differences were detected, displaying OVX female rats more conflict than male rats.
Behavioral sex differences were enhanced by estradiol in OVX + EB female rats.
Western Blot experiments detected that mGlu1 but not mGlu5 receptors protein was differentially expressed in the amygdala of OVX + EB, OVX and male rats.
Acknowledgements
This study was funded, in part, by NIH-MRISP (MH048192), the Research Centers in Minority Institutions (RCMI) Program at UPR-MSC (G12RR03051) and the National Institute of Child Health & Human Development (NICHD; NIH-1G11H046326) to Nivia L. Pérez-Acevedo. María I. De Jesús-Burgos was supported by the National Institute of General Medical Sciences-Research Initiative for Scientific Enhancement (NIGMS-RISE) Program at the University of Puerto Rico Medical Sciences Campus (UPR-MSC) grant R25-GM061838. Stephanie González-García and Yanira Cruz-Santana were supported by the NSF Undergraduate Research Mentoring (URM) program in Neural Networks and Behavior, NSF DBI-0932955. We are grateful to our past and present laboratory team (Joan Ballista, Waldemar Feliciano, Alberto Grana, Kelvin Quiñones-Laracuente, Ivette Ortiz, Jennifer Ríos-Pilier and Gabriela Zabala) for assisting with data collection. We also thank Mr. Luis R. Soto and Dr. Juan C. Jorge for their editorial work.
References
- 1.Bajkowska M, Branski P, Smialowska M, Pilc A. Effect of chronic antidepressant or electroconvulsive shock treatment on mGLuR1a immunoreactivity expression in the rat hippocampus. Pol. J. Pharmacol. 1999;51:539–541. [PubMed] [Google Scholar]
- 2.Ballard T, Woolley M, Prinssen E, Huwyler J, Porter R, Spooren W. The effect of the mGlu5 receptor antagonist MPEP in rodent tests of anxiety and cognition: a comparison. Psychopharmacology (Berl.) 2005 Apr;179(1):218–229. doi: 10.1007/s00213-005-2211-9. [DOI] [PubMed] [Google Scholar]
- 3.Basso A, Gallagher K, Mikusa J, Rueter L. Vogel conflict test: sex differences and pharmacological validation of the model. Behav. Brain Res. 2011 Mar;218(1):174–183. doi: 10.1016/j.bbr.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Beatty W, Beatty P. Hormonal determinants of sex differences in avoidance behavior and reactivity to electric shock in the rat. J. Comp. Physiol. Psychol. 1970 Dec;73(3):446–455. doi: 10.1037/h0030216. [DOI] [PubMed] [Google Scholar]
- 5.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group and ii metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J. Neurosc. 2005;25(20):5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chojnacka-Wójcik E, Tatarczyńska E, Pilc A. The anxiolytic-like effect of metabotropic glutamate receptor antagonists after intrahippocampal injection in rats. Eur. J. Pharmacol. 1997 Jan;319(2–3):153–156. doi: 10.1016/s0014-2999(96)00941-7. [DOI] [PubMed] [Google Scholar]
- 7.Davis M, Shi C. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Annu. N. Y. Acad. Sci. 1999 Jun;877:281–291. doi: 10.1111/j.1749-6632.1999.tb09273.x. [DOI] [PubMed] [Google Scholar]
- 8.De Jesús-Burgos M, Torres-Llenza V, Pérez-Acevedo N. Activation of amygdalar metabotropic glutamate receptors modulates anxiety, and risk assessment behaviors in ovariectomized estradiol-treated female rats. Pharmacol. Biochem. Behav. 2012 May;101(3):369–378. doi: 10.1016/j.pbb.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan L, Hanbury R, Pandey S, Cohen R. Dose and time effects of estrogen on expression of neuron-specific protein and cyclic amp response element-binding protein and brain region volume in the medial amygdala of ovariectomized rats. Neuroendocrinology. 2008;88(2):111–126. doi: 10.1159/000129498. [DOI] [PubMed] [Google Scholar]
- 10.Febo M, Jimenez-Rivera C, Segarra A. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943:51–61. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- 11.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326(2):483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 12.Frye C. Steroids reproductive endocrine function, and affect. A review. Minerva Ginecol. 2009;61(6):541–562. [PubMed] [Google Scholar]
- 13.Frye CA, Lacey EH. Progesting influence performance on cognitive task-independent of changes in affective behavior. Psychobiology. 2000;28:550–563. [Google Scholar]
- 14.Hodges H, Green S, Glenn B. Evidence that the amygdala is involved in benzodiazepine and serotonergic effects on punished responding but not on discrimination. Psychopharmacology (Berl.) 1987;92(4):491–504. doi: 10.1007/BF00176484. [DOI] [PubMed] [Google Scholar]
- 15.Jasnow A, Mong J, Romeo R, Pfaff D. Estrogenic regulation of gene and protein expression within the amygdala of female mice. Endocrine. 2008;32(3):271–279. doi: 10.1007/s12020-008-9043-4. [DOI] [PubMed] [Google Scholar]
- 16.Johnston A, File S. Sex differences in animal tests of anxiety. Physiol. Behav. 1991;49(2):245–550. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS, Thornton LM, Prescott C. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am. J. Psychiatry. 2001;158:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 18.Klodzinska A, Tatarczyńska E, Chojnacka-Wójcik E, Nowak G, Cosford N, Pilc A. Anxiolytic-like effects of MTEP, a potent and selective mGlu5 receptor agonist does not involve GABA(A) signaling. Neuropharmacology. 2004 Sep;47(3):342–350. doi: 10.1016/j.neuropharm.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Klodzinska A, Tatarczynska E, Stachowicz K, Chojnacka-Wojcik E. The anxiolytic-like activity of aida (1-aminoindan-1,5-dicarboxylic acid), an mGlu 1 receptor antagonist. J. Physiol. Pharmacol. 2004;55:113–126. [PubMed] [Google Scholar]
- 20.Lavreysen H, Pereira SN, Leysen JE, Langlois X, Lesage AS. Metabotropic glutamate 1 receptor distribution and occupancy in the rat brain: a quantitative autoradiographic study using [3H]R214127. Neuropharmacology. 2004 Apr;46(5):609–619. doi: 10.1016/j.neuropharm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Luongo L, Novellis V, Gatta L, Palanzzo E, Vita D, Guida F, Giordano C, Siniscalco D, Marabese I, De Chiaro M, Boccella S, Rossi F, Maione S. Role of metabotropic glutamate receptor I in the basolateral amygdala-driven prefrontal cortical deactivation in inflammatory pain in the rat. Neuropharmacology. 2013;66:317–329. doi: 10.1016/j.neuropharm.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Mahavongtrakul M, Kanjiya MP, Maciel M, Kanjiya S, Sinchak K. Estradiol dose-dependent regulation of membrane estrogen receptor-α, metabotropic glutamate receptor-1a, and their complexes in the arcuate nucleus of the hypothalamus in female rats. Endocrinology. 2013 Sep;154(9):3251–3260. doi: 10.1210/en.2013-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matosi N, Fernandez-Enright F, Lum JS, Andrews JL, Engel M, Huang X, Newell KA. Metabotropic glutamate receptor 5, and its trafficking molecules Norbin and Tamalin, are increased in the CA1 hippocampal region of subjects with Schizophrenia. Schizophr. Res. 2015;166:212–218. doi: 10.1016/j.schres.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 24.McLean C, Anderson E. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin. Psychol. Rev. 2009 Aug;29(6):496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Millan M, Brocco M. The vogel conflict test: Procedural aspects, gamma-aminobutyric acid, glutamate and monoamines. Eur. J. Pharmacol. 2003;463(1–3):67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- 26.Morgan M, Schulkin J, Pfaff D. Estrogens and non-reproductive behaviors related to activity and fear. Neurosci. Biobehav. Rev. 2004 Mar;28(1):55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Nishizuka M, Arai Y. Synapse formation in response to estrogen in the medial amygdala developing in the eye. Proc. Natl. Acad. Sci. U. S. A. 1982 Nov;79(22):7024–7026. doi: 10.1073/pnas.79.22.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niswender C, Conn P. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palanza P. Animal models of anxiety and depression: How are females different? Neurosci. Biobehav. Rev. 2001;25(3):219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 30.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol. Ther. 2007;115(1):116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Pape H, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pare D, Quirk G, Ledoux J. New vistas on amygdala networks in conditioned fear. J. Neurophysiol. 2004;92(1):1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 33.Paxino G, Watson C. The rat brain in stereotaxic coordinates. 2nd ed Academic Press; New York: 1998. [Google Scholar]
- 34.Pérez-Acevedo N, Lathroum L, Jorge J. The neurosteroid 3-alpha DIOL modulates place preference when infused in the basolateral amygdala according to sex. Behav. Neurosci. 2006;120:632–640. doi: 10.1037/0735-7044.120.3.632. [DOI] [PubMed] [Google Scholar]
- 35.Pérez de la Mora M, Lara-Garcia D, Jacobsen K, Vazquez-Garcia M, Crespo-Ramirez M, Flores-Gracia C, Escamilla-Marvan E, Fuxe K. Anxiolytic-like effects of the selective metabotropic glutamate receptor 5 antagonist MPEP after its intra-amygdaloid microinjection in three different non-conditioned rat models of anxiety. Eur. J. Neurosci. 2006;23:2749–2759. doi: 10.1111/j.1460-9568.2006.04798.x. [DOI] [PubMed] [Google Scholar]
- 36.Perrot-Sinal T, Gregus A, Boudreau D, Kalynchuk L. Sex and repeated restraint stress interact to affect cat odor-induced defensive behavior in adult rats. Brain Res. 2004 Nov;1027(1–2):161–172. doi: 10.1016/j.brainres.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 37.Pilc A, Kłodzińska A, Brański P, Nowak G, Pałucha A, Szewczyk B, Tatarczyńska E, Chojnacka-Wójcik E, Wierońska JM. M.P.E.P. Multiple, administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology. 2002 Aug;43(2):181–187. doi: 10.1016/s0028-3908(02)00082-5. [DOI] [PubMed] [Google Scholar]
- 38.Pitkanen A, Savander V, LeDoux J. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- 39.Porter R, Jaeschke G, Spooren W, Ballard T, Büttelmann B, Kolczewski S, Peters J, Prinssen E, Wichmann J, Vieira E, Mühlemann A, Gatti S, Mutel V, Malherbe P. Fenobam: a clinically validated nonbenzodiazepine anxiolytic is a potent, selective, and noncompetitive mGlu5 receptor antagonist with inverse agonist activity. J. Pharmacol. Exp. Ther. 2005 Nov;315(2):711–721. doi: 10.1124/jpet.105.089839. [DOI] [PubMed] [Google Scholar]
- 40.Ramos A, Berton O, Mormède P, Chaouloff F. A multiple-test study of anxiety-related behaviours in six inbred rat strains. Behav. Brain Res. 1997 Apr;85(1):57–69. doi: 10.1016/s0166-4328(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 41.Rasia-Filho A, Fabian C, Rigoti K, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126(4):839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Schoepp D. Metabotropic glutamate receptors. Pharmacol. Biochem. Behav. 2002;73(2):285–286. doi: 10.1016/s0091-3057(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 43.Smialowska M, Szewczyk B, Branski P, Wieronska J, Palucha A, Bajkowska M. Effects of chronic imipramine or electrocon- vulsive shock on the expression of mGluR1a and mGluR5a immunoreac- tivity in rat brain hippocampus. Neuropharmacology. 2002;42:1016–1023. doi: 10.1016/s0028-3908(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 44.Spooren W, Lesage A, Lavreysen H, Gasparini F, Steckler T. Metabotropic glutamate receptors: their therapeutic potential in anxiety. Curr. Top. Behav. Neurosci. 2010;2:391–413. doi: 10.1007/7854_2010_36. [DOI] [PubMed] [Google Scholar]
- 45.Stachowicz K, Gołembiowska K, Sowa M, Nowak G, Chojnacka-Wójcik E, Pilc A. Anxiolytic-like action of MTEP expressed in the conflict drinking Vogel test in rats is serotonin dependent. Neuropharmacology. 2007 Nov;53(6):741–748. doi: 10.1016/j.neuropharm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Steckler T, Lavreysen H, Oliveira A, Aerts N, Van Craenendonck H, Prickaerts J, Megens A, Lesage A. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology (Berl.) 2005 Apr;179(1):198–206. doi: 10.1007/s00213-004-2056-7. [DOI] [PubMed] [Google Scholar]
- 47.Tatarczyńska E, Kłodzińska A, Kroczka B, Chojnacka-Wójcik E, Pilc A. The antianxiety-like effects of antagonists of group I and agonists of group II and III metabotropic glutamate receptors after intrahippocampal administration. Psychopharmacology (Berl.) 2001 Octtober;158(1):94–99. doi: 10.1007/s002130100798. [DOI] [PubMed] [Google Scholar]
- 48.Varty G, Grilli M, Forlani A, Fredduzzi S, Grzelak M, Guthrie D, Hodgson R, Lu S, Nicolussi E, Pond A, Parker E, Hunter J, Higgins G, Reggiani A, Bertorelli R. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl.) 2005 Apr;179(1):207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- 49.Vogel J, Beer B, Clody DE. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia. 1971;21(1):1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- 50.Walker D, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in light- enhanced versus fear-potentiated startle. J. Neurosci. 1997;17:9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol. Behav. 2010 Feb;99(2):169–174. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol. Learn. Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wierońska J, Brański P, Szewczyk B, Pałucha A, Papp M, Gruca P, Moryl E, Pilc A. Changes in the expression of metabotropic glutamate receptor 5 (mGluR5) in the rat hippocampus in an animal model of depression. Pol. J. Pharmacol. 2001 Nov-Dec;53(6):659–662. [PubMed] [Google Scholar]
- 54.Xiao B, Tu J, Worley P. Homer: a link between neural activity and glutamate receptor function. Curr. Opin. Neurobiol. 2000 Jun;10(3):370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]