ABSTRACT
Analysis of PDL1 mRNA expression in ∼5,500 breast cancers showed PDL1 upregulation in 38% of basal tumors and 38% of inflammatory breast cancers (IBC). Upregulation, associated with signs of strong cytotoxic local immune response, was associated with a better survival in the basal or triple-negative subtypes, and with a better pathological response to chemotherapy in these subtypes and IBC. Reactivation of dormant tumor-infiltrating lymphocytes (TILs) by PD1/PDL1-inhibitors represents a promising strategy in these aggressive tumors.
KEYWORDS: Breast cancer, chemotherapy, immune response, inflammatory breast cancer, PDL1, survival, triple-negative
Abbreviations
- CTLA4
cytotoxic T-lymphocyte associated antigen 4
- ER
estrogen receptor
- IBC
inflammatory breast cancer
- IFNγ
interferon gamma
- PD1
Programmed cell Death 1
- PDL1
Programmed cell Death Ligand 1
- PDL2
Programmed cell Death Ligand 2
- PR
progesterone receptor
- TILs
tumor-infiltrating lymphocytes
- TNBC
triple-negative breast cancer.
Breast cancer has been regarded as poorly immunogenic and immunotherapy with interleukin 2 or interferons gave disappointing results. In fact, breast cancer is a heterogeneous disease that includes several molecular subtypes, and in some of these subtypes the role of immunity recently emerged. In the triple-negative (ER/PR/ERBB2-negative) and ERBB2-positive subtypes, the presence of TILs1 or signatures of immune response2 are favorable prognostic features.
Over the last decade, great advances were done in the field of cancer immunotherapy, notably with the development of immune checkpoints inhibitors3 such as ipilumumab, inhibitor of cytotoxic T-lymphocyte associated antigen 4 (CTLA4), and more recently inhibitors of the Programmed cell Death 1 (PD1) — Programmed cell Death Ligand 1 (PDL1) axis. PD1, a cell surface membrane protein expressed by various immune cells including T cells, is activated by its ligands PDL1 and PDL2, expressed by antigen-presenting cells such as dendritic cells, macrophages or B cells. After engagement by its ligands, PD1 attenuates lymphocyte activation and promotes T-regulatory cell development and function, allowing the inhibition of the immune response. In cancers, this represents a common mechanism of immune escape used by tumor cells,3 which in different locations express PDL1. Clinical trials of PD1/PDL1 inhibitors showed very promising results with durable responses, notably in melanoma, lung and bladder carcinomas.4 Phase 3 studies are ongoing, but PD1 inhibitors are already marketed in advanced melanoma and lung cancer. Furthermore, correlation between PDL1 expression on tumor and/or immune cells and objective response has been reported.4
PDL1 expression has been scarcely studied in breast cancer, with only two recent prognostic studies, which provided divergent results.5,6 To further characterize PDL1 expression, we analyzed PDL1 mRNA expression in 45 breast cancer cell lines and 5,454 non-IBCs profiled using DNA microarrays.7 This mRNA analysis avoided the limitations of immunohistochemistry and allowed the study of a large series of samples, providing for the first time the opportunities to address clinical issues (survival, response to chemotherapy) and to work on each molecular subtype separately. Basal or mesenchymal cell lines showed higher PDL1 expression level than luminal cell lines. Twenty percent of clinical samples showed PDL1 upregulation, defined by a ratio of tumor/normal breast expression superior or equal to 2. PDL1 upregulation was associated with poor-prognosis features: ductal type, large tumor size, high grade, ER-negativity, PR-negativity, ERBB2-positivity, high proliferation rate and aggressive molecular subtypes (basal and ERBB2-enriched), with PDL1 upregulation in 38% of basal tumors. PDL1 upregulation was independently associated with better five-year metastasis-free survival (63% vs. 44%) and overall survival (82% vs. 68%) in basal breast cancers, as well as in triple-negative breast cancer (TNBC), but not in the whole series, nor in the other molecular subtypes. PDL1 upregulation was associated with a better pathological complete response rate to pre-operative chemotherapy (50% vs.21%). This predictive value was limited to basal and ERBB2-enriched tumors. In another study,8 we analyzed PDL1 mRNA expression in 112 pretherapeutic samples of IBC, a very aggressive form of breast cancer. PDL1 expression was higher in IBC than in non-IBC, with 38% upregulation rate. In IBC, PDL1 upregulation was associated with a better pathological response to chemotherapy in multivariate analysis.
The favorable prognostic and predictive values of PDL1 upregulation seem paradoxical given the immunosuppressive function of PDL1. We previously showed the similarly counterintuitive favorable prognostic value of IDO expression.9 A biological explanation may be that PDL1 expression is rather a marker of strong cytotoxic local immune response, itself a favorable prognostic and predictive feature.1 Indeed, the comparison of gene expression profile of breast tumors with vs. without PDL1 upregulation identified a robust signature in the “PDL1-upregulated” group. This signature was characteristic of a strong cytotoxic response, involving CD8+ T cells, but also other actors of antitumor immunity (γδ-T-cells, NKG2D+ cells, dendritic cells, B cells …), consistent with the reported correlation between PDL1 expression and a TIL infiltrate.6 While associated with other immunosuppressive molecules, such as IDO and CTLA4, this signature suggested an activated profile of differentiated T cells, clearly TH1-biased (IL12 and IFN-induced pathways), endowed with cytotoxic effector functions. The likely biological link is the production by activated TILs of IFNγ, which positively regulates PDL1 expression (Fig. 1).
Figure 1.
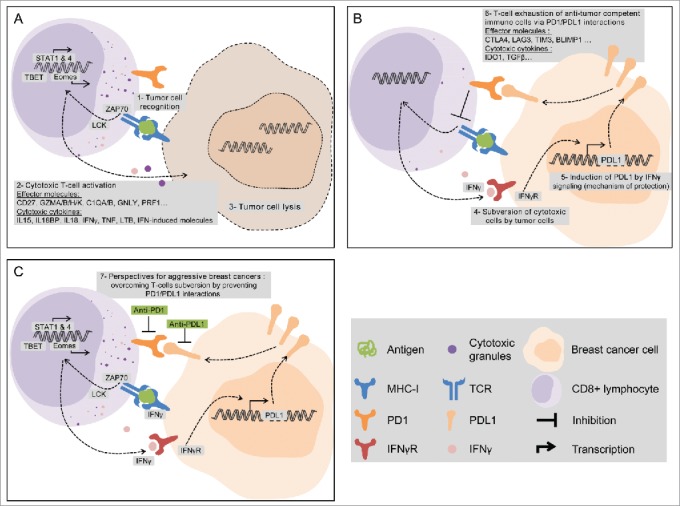
PDL1 expression is associated with favorable clinical outcome (survival and response to chemotherapy) in aggressive breast cancers. PDL1 expression should be viewed as a surrogate marker of an initially strong cytotoxic antitumor immune response, favorable prognostic and predictive feature. Some genes overexpressed in PDL1-upregulated breast cancers are indicated (A–B). The PD1/PDL1 axis inhibition could protect activated T cells or reactivate inhibited T cells and improve the therapeutic response (C).
The therapeutic blockade of the PD1/PDL1 axis should reactivate inhibited T cells and increase the antitumor immune response. This was confirmed by the preliminary results of the Keynote-012 trial,10 a phase 1b trial of single agent pembrolizumab (anti-PD1) in patients with recurrent/metastatic TNBC. PDL1 protein expression in tumor or stroma was required for study entry. Thirty-two heavily pretreated patients were enrolled, ∼90% had received chemotherapy in the early-stage setting and ∼50% three or more lines of therapy in the advanced-stage setting. Among 27 evaluable patients, five responded to treatment (18.5%), including one complete response (3.7%) and four partial responses (14.8%), and seven 7 (25.9%) had stable disease, for an overall clinical benefit rate of 44%. The median duration of response was not yet reached after a 10-mo median follow-up, with many responses lasting longer than 40 weeks. This is the first proof-of-principle study showing a signal of activity to immunotherapy in a group of patients with refractory, metastatic breast cancer. Like in several other studies in other cancers, a small proportion of patients respond, and some of them tend to be long-term responders with durability not seen with other therapies. Indeed, one major cause of failure of classical therapies (hormone therapy, chemotherapy, targeted therapies) that directly target the tumor cells is the development of secondary resistance due to the high mutagenic and adaptable capacity of cancer cells, making the tumor responses temporary. Thanks to the adaptability of the immune response, cancer immunotherapy can theoretically address this issue. Clinical trials with PD1 inhibitors are planned in patients with aggressive breast cancers such as TNBC and IBC, to determine the activity of single agent and in combination and to define molecular predictors for activity. Results are urgently awaited for these patients with few therapeutic resources.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1. Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014; 25:1544-50; PMID:24608200; http://dx.doi.org/ 10.1093/annonc/mdu112 [DOI] [PubMed] [Google Scholar]
- 2. Sabatier R, Finetti P, Mamessier E, Raynaud S, Cervera N, Lambaudie E, Jacquemier J, Viens P, Birnbaum D, Bertucci F. Kinome expression profiling and prognosis of basal breast cancers. Mol Cancer 2011; 10:86; PMID:21777462; http://dx.doi.org/ 10.1186/1476-4598-10-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buque A, Bloy N, Aranda F, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Marabelle A, et al. Trial Watch: Immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology 2015; 4:e1008814; PMID:26137403; http://dx.doi.org/ 10.1080/2162402X.2015.1008814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54; PMID:22658127; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014; 146:15-24; PMID:24842267; http://dx.doi.org/ 10.1007/s10549-014-2988-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin Cancer Res 2014; 20:2773-82; PMID:24647569; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2702 [DOI] [PubMed] [Google Scholar]
- 7. Sabatier R, Finetti P, Mamessier E, Adelaide J, Chaffanet M, Ali HR, Viens P, Caldas C, Birnbaum D, Bertucci F. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015; 6:5449-64; PMID:25669979; http://dx.doi.org/ 10.18632/oncotarget.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bertucci F, Finetti P, Colpaert C, Mamessier E, Parizel M, Dirix L, Viens P, Birnbaum D, van Laere S. PDL1 expression in inflammatory breast cancer is frequent and predicts for the pathological response to chemotherapy. Oncotarget 2015; 6:13506-19; PMID:25940795; http://dx.doi.org/ 10.18632/oncotarget.3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, Houvenaeghel G, Van den Eynde B, Birnbaum D, Olive D, et al. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer 2012; 130:96-104; PMID:21328335; http://dx.doi.org/ 10.1002/ijc.25979 [DOI] [PubMed] [Google Scholar]
- 10. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Dolled-Filhart M, Emancipator K, Gonzalez EJ, et al. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. San Antonio Breast Cancer Symposium Proceedings 2015; 75:S1-09; http://dx.doi.org/ 10.1158/1538-7445.SABCS14-S1-09. [DOI] [Google Scholar]


