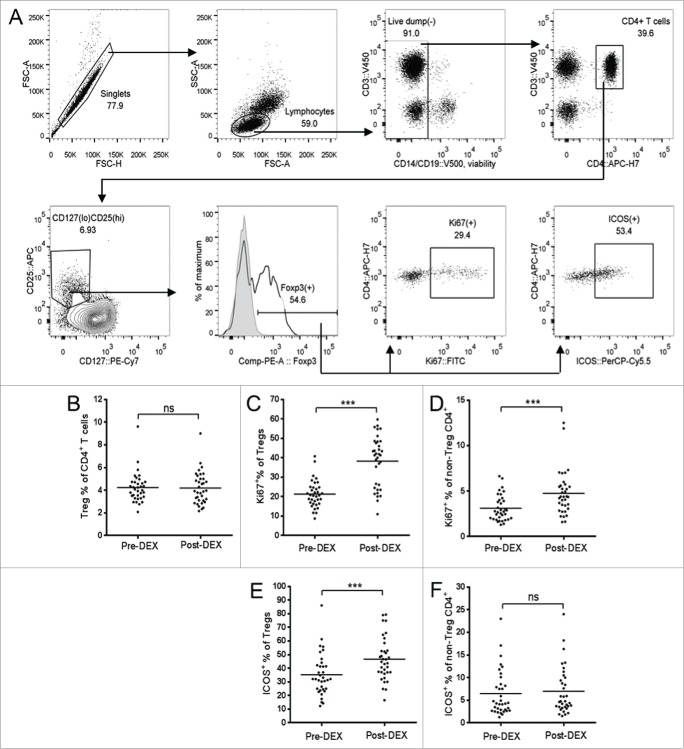
Figure 3.
Dex treatment increases the proliferation and activation state of Tregs. (A) Representative flow cytometry data, demonstrating the gating strategy used for Treg identification and analysis. Forward scatter (FSC) area vs. FSC-height was used for doublet discrimination, and lymphocytes subsequently selected by FSC vs. side scatter. A “dump” channel was used to gate out dead cells (LIVE/DEAD fixable aqua viability stain), CD14+, CD56+, and CD19+ cells. CD4+ T cells were subsequently selected on the basis of CD4 vs. CD3 staining, followed by the identification of Tregs as CD25hi, CD127lo, and Foxp3+. Tregs, or non-Treg CD4+ T cells, were further gated for expression of Ki67 and ICOS. (B–F) Analysis of Tregs in patient PBMC samples collected before (pre-Dex) and after (post-Dex) administration of Dex. (B) Percentage of Tregs (CD25+CD127loFoxp3+) as a proportion of total CD4+ T cells in PBMC samples. (C–D) Proportion of Tregs (C) and non-Treg CD4+ lymphocytes (D) expressing the proliferation marker Ki67. (E–F) Changes in proportional expression of the activation marker ICOS, in Treg (E) and non-Treg CD4+ lymphocytes (F). Each dot represents an individual patient; significant difference between pre-Dex and post-Dex values: ***p < 0.0001, paired students t-test.