ABSTRACT
Introduction: Ipilimumab is effective in the treatment of metastatic malignant melanoma, but few biomarkers reliably predict treatment response. Methods: Patients were treated with Ipilimumab for metastatic malignant melanoma. Blood and serum samples were collected before and during treatment. Mononuclear cells in peripheral blood were subjected to immune phenotypic analyses and cytokine levels were measured in serum samples. Results were correlated with clinical data. Results: A total of 40 patients were included in the analyses. Clinical response were associated with an increase after one series of treatment in absolute lymphocyte count (ALC) (p = 0.008), absolute T cell count (p = 0.02) and the absolute number of activated T cells in peripheral blood (p = 0.003). A high frequency of myeloid derived suppressor cells (MDSC) and a higher level of IL6 were associated with treatment failure, though not significantly. Levels of IL6 in serum above the median showed a tendency to associate with reduced survival by the 4th treatment series. Finally, treatment with Ipilimumab led to a decreased frequency of FOXP3+ regulatory T cells (p = 0.009). Conclusion: Ipilimumab leads to increased ALC, T cell count and T cell activation in malignant melanoma patients responding to treatment. A high baseline frequency of myeloid-derived suppressor cells and high levels of IL6 is associated with a reduced chance of responding to therapy.
Keywords: CTLA-4; IL-6; Immunotherapy; Ipilimumab; Malignant Melanoma; Myeloid-derived Suppressor Cells (MDSC); Regulatory T Cells (Tregs)
Introduction
Over the past decade, treatment of metastatic melanoma has been revolutionized by new blocking antibodies targeting check point molecules widely expressed throughout the immune system. Notable examples of these include the anti-Cytotoxic T Lymphocyte Antigen 4 (CTLA-4) antibody Ipilimumab and the anti-Programmed Cell Death 1 (PD-1) receptor antibody Pembrolizumab, both of which are US Food and Drug-approved in the treatment of metastatic malignant melanoma.1 T cell activation during priming of immune responses is tightly linked to the balance between stimulatory and inhibitory inputs to the cell. CTLA-4 is a co-inhibitory receptor that regulates activation of T cells during priming and maintenance of adaptive immune responses.2 It has structural homology with the co-activating receptor CD28 and shares ligand restriction, though with 10–20 times higher affinity. Upon binding by molecules B7.1 and B7.2 (CD80 and CD86 respectively), expressed on antigen presenting cells, CTLA-4 inhibit T cell function and proliferation.3 Early work by Allison, J. P. et al., who pioneered in the use of anti-CTLA-4 antibodies in cancer treatment, have demonstrated, that T cell activation can be dramatically augmented ex vivo upon treatment with anti-CTLA-4 antibody.4
Ipilimumab is a fully human IgG1 antibody specific for CTLA-4, with clinical efficacy against metastatic melanoma proven in a phase III clinical trial.5 The drug may have a modest antibody dependent cellular cytotoxicity effect in CTLA-4-expressing melanoma cell lines in vitro6 but Ipilimumab is generally not believed to have any significant tumoricidal effect per se. Instead the action is believed to be linked to the disinhibiting effect on T cells, promoting T cell mediated killing of tumor cells.7 In support of this theory, prospective studies have reported a dramatic infiltration of T cells in the tumor during treatment, and intratumoral changes in the ratio between CD8+ T cells and regulatory T cells may be associated with extend of tumor necrosis.8,9 Furthermore, an interesting recent report demonstrated a close correlation between the number of tumor-mutations giving rise to neo-antigen-epitopes and clinical response, thereby underscoring the role of cytotoxic T cells in the mechanism of action.10 The exact mechanism by which anti-CTLA-4 antibodies promotes this immune mediated tumor-killing is still not completely understood. Some studies have pointed toward an induction of tumor specific CD8+ T cells,11,12 while other authors have speculated that effect might be conveyed though inhibition of regulatory T cells.13 In addition to that, levels of circulating MDSC have recently been reported to be reduced by Ipilimumab treatment, a phenomenon which reportedly is augmented in clinical responders.14
Treatment of metastatic melanoma with Ipilimumab is associated with an overall response rate approximately 10% and a clinical benefit rate of nearly 30% in a large phase III study5 and subsequent analyses have indicated that responses may be durable and long lasting. As a rare thing in solid cancers Ipilimumab may induce durable complete responses indicating that a cure is possible, even in the metastatic setting. However, there is still a majority of patients not responding to therapy with Ipilimumab and as treatment is associated with potentially deleterious side effects and the drug is overtly costly, biomarkers predicting response to subsequent treatment and methods of improving the effect of treatment are in demand. Several recent reports have focused on identification of biomarkers prior to and during therapy that is correlating with treatment response. Changes in CD4+ T cell expression of inducible T cell co-stimulator (ICOS) in bladder cancer and level of MDSC in melanoma has been identified as on-treatment markers associated with clinical response.14,15
In this study, we scrutinized possible baseline and treatment-related biomarkers in patients treated for metastatic malignant melanoma with Ipilimumab. Prospectively collected peripheral blood mononuclear cell (PBMC) and serum samples were subjected to multicolor flow cytometry and cytokine measurements.
Results
Patient characteristics
A total of 40 patients receiving at least two doses of Ipilimumab were included in the study. Patients were treated at the Herlev Hospital, Copenhagen University (n = 28) and Aarhus University Hospital (n = 12), two of three designated melanoma treatment centers in Denmark. Patients were included between October 2012 and July 2014. Baseline patient characteristics are presented in Table 1.
Table 1.
Patient demographics.
| Characteristic Age—median (range) | 67.25 (35–83) |
| Gender—no. (%) male female | 16 (40) 24 (60) |
| Stage of disease (AJCC)*—no. (%) | |
| M1a | 14 (35) |
| M1b | 6(15) |
| M1c | 20 (50) |
| Prior systemic therapy—no. (%) | |
| none | 15 (37.5) |
| IL2 | 16 (40) |
| Temozolomide | 8 (20) |
| BRAF inhibitor | 1 (2.5) |
| Series of Ipilimumab—no. (%) | |
| 2 | 3 (7.5) |
| 3 | 2(5) |
| 4 | 35 (87.5) |
| Line of therapy—no. (%) | |
| 1st | 15 (37.5) |
| 2nd | 24 (60) |
| 3rd | 1 (2.5) |
| Lactate dehydrogenase—no. (%) | |
| sULN | 31 (77.5) |
| > ULN | 9 (22.5) |
| >2x ULN | 1 (2.5) |
| RECIST response—no. (%) | |
| PD | 25 (62.5) |
| SD | 9 (22.5) |
| PR | 5 (12.5) |
| CR | 1 (2.5) |
Absolute lymphocyte count, CD4+ and CD8+ T cells
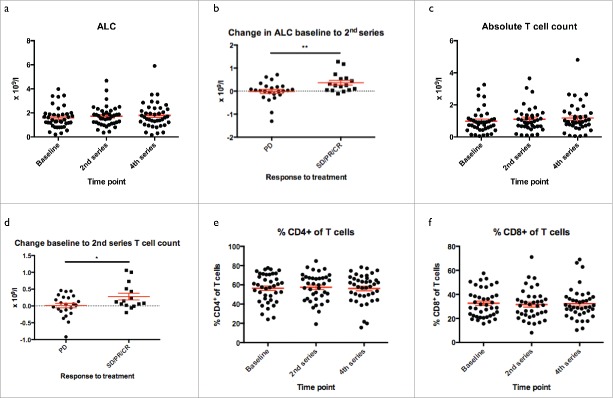
Clinical response to Ipilimumab has previously been linked to an early increase in ALC during therapy.16 In our cohort of patients we observed a small but significant increase in the ALC during treatment (Fig. 1a, repeated measure ANOVA p = 0.05). When dichotomizing into patients with unequivocal progression and patients with stable disease (SD) at 1st evaluation or better, we saw significantly higher increases in patients responding to therapy compared to non-responders, with regard to changes in ALC from baseline to the time of 2nd dose of treatment (Fig. 1b, p = 0.008), thus confirming previous reports.
Figure 1.
Absolute lymphocyte and T cell count. Absolute Lymphocyte Count (ALC). a) ALC increased from baseline to 4th series of treatment. b) Change in ALC from baseline to 2nd series in patients progressing despite treatment and in responding patients (SD, PR and CR). c) Absolute T cell count increased during treatment. d) Change in absolute T cell count from baseline to 2nd series in patients progressing despite treatment and in responding patients (SD, PR and CR). e+f) Proportion of CD4+ and CD8+ cells in T cells throughout treatment, which remained unchanged despite therapy.
As Ipilimumab affects numerous aspects of T cell biology,17,18 we assessed whether this increase could be attributed to increased frequencies of T cells or a more general increase in lymphocytes. As shown in Fig. 1c, at least part of the early increase in ALC in responding patients could be accounted for by an increased absolute T cell count (p = 0.02). In line with this, change in absolute T cell count from baseline to 2nd treatment dose was significantly higher in patients responding to therapy (Fig. 1d, p = 0.03). We observed no changes in the distribution of CD4+/CD8+ T cells (Fig. 1e and f).
Ipilimumab induces a shift from naive to effector T cells
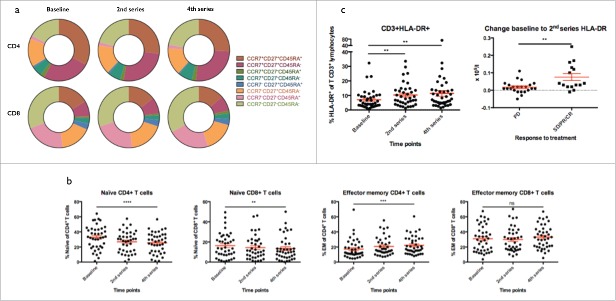
Based on expression of a number of different extracellular markers, several maturation states of T cells are recognized. We used CD45RA, chemokine receptor 7 (CCR7) and CD27 to distinguish between these effector-stages, which has previously been identified as markers of maturation, telomere-length and replicative potential.19
As depicted in Fig. 2a and b, we observed dynamic changes in the distribution of different phenotypic subsets in both CD4+ and CD8+ T cells during treatment. The frequency of triple-positive cells, i.e., CCR7+CD27+CD45RA+, suggesting a naive phenotype, decreased during treatment (Overall p < 0.0005 and p = 0.01 for CD4+ and CD8+ respectively and pairwise: baseline to 4th treatment dose, p < 0.0005 and p = 0.05 for CD4+ and CD8+ respectively). In CD4+ T cells, this was paralleled by a reciprocal increase of triple-negative cells suggesting an intermediate or late effector stage (repeated measure ANOVA p < 0.0005). This change was less pronounced with regard to effector CD8+ T cells, though we did see a tendency toward higher frequency of late effector cells (p = 0.3). Additionally, CD3+ T cells were scrutinized for expression of activation marker HLA-DR (Fig. 2c). As shown in Fig. 2c, we saw a significant increase in frequency of HLA-DR+ cells (p = 0.003). This was evident after one dose of Ipilimumab with an increase from mean 7% at baseline to 10.3% at the time of the 2nd treatment (p = 0.005). By the time of the 4th treatment dose, the proportion of HLA-DR+ cells had increased to 11.4%, representing more than a 50% relative increase (p = 0.007). Additionally, we observed significantly higher change in the absolute number of HLA-DR+ T cells in patients responding to therapy (p = 0.003). Due to the design of the flow-panel, we were not able to distinguish CD4+ and CD8+ T cells in this context.
Figure 2.
T cell phenotype. a) Phenotype of CD4+ and CD8+ T cells according to expression of CCR7, CD27 and CD45RA. b) The proportion of naïve CCR7+CD27+CD45RA+ cells decreased in both CD4+ and CD8+ T cells during treatment (p < 0.0001 and p = 0.009 respectively. The proportion of effector memory CCR7-CD27-CD45RA- cells increased significantly in CD4+ T cells (p = 0.0001) and borderline significant in CD8+ T cells (p = 0.2). c) The proportion of HLA-DR+ T cells out of lymphocytes increased from baseline to 2nd and 4th series (both p = 0.002). The increase from baseline to 2nd series in the absolute number of HLA-DR+ T cells was significantly higher in responding patients (SD, PR and CR) as compared to non-responders (p = 0.003).
T cell PD-1 expression
T cell activation is tightly linked to the balance between signaling through activating and inhibitory receptors. We sought to assess whether treatment with Ipilimumab had an impact on four markers; B- and T-lymphocyte attenuator (BTLA), Lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin mucin 3 (TIM-3) and programmed death receptor 1 (PD-1), all considered to be inhibitory receptors.20-23 The majority of cells did not express any of these markers; mean 86.3% of CD4+ T cells and 82.9% of CD8+ T cells. No significant change in the fraction of cells negative for inhibitory markers was observed during therapy (data not shown). As seen on Fig. 3a, we did observe small dynamic changes within subpopulations defined by combinations of the scrutinized markers. However, due to large inter-patient variability, none of these changes were significant for subpopulations possibly reflecting fluctuations in T cell dynamics. In both CD4+ and CD8+ T cells, the predominant inhibitory receptor positive population was PD-1+LAG-3−TIM-3−BTLA−, but though neither of the subpopulations of PD-1 positive cells changed significantly as a result of treatment, we did observe a modest increase in the level of CD4+ T cells expressing this antigen from a mean of 6.6% at baseline to 8.4% (p = 0.03, Fig. 3b).
Figure 3.
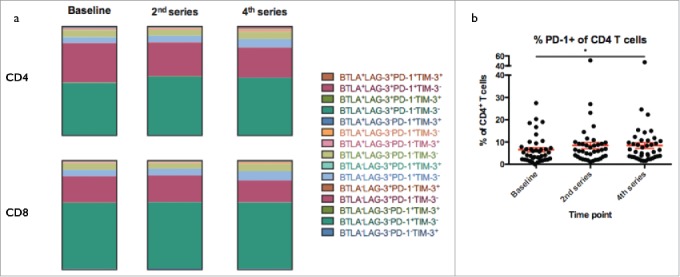
Exhaustion marker expression. a) Expression of combinations of the exhaustion markers PD-1, LAG-3, TIM-3 and BTLA in T cells and b) proportion of CD4+ T cells expressing surface bound PD-1. None of the individual subsets of T cells showed any change in frequency during therapy but a significant increase in the proportion of PD-1+ cells in CD4+ T cells was observed from baseline to 4th series (p = 0.03).
Regulatory T cells decrease during therapy
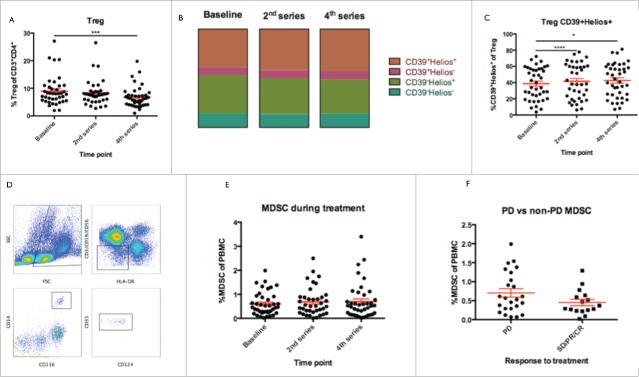
Regulatory T cells have been suggested as a possible target for Ipilimumab-treatment,13 as Tregs reportedly express high levels of CTLA-4.24 We set out to investigate whether the frequency, in peripheral blood, of this particular cell-type was affected by treatment. As illustrated in Fig. 4a, the proportion of CD4+ T cells displaying a Treg phenotype, did decrease significantly during treatment (repeated measures ANOVA, p < 0.0005). This effect was evident already by the 2nd treatment, though not yet significant (p = 0.26), and even more pronounced after three doses of Ipilimumab (p = 0.001). We observed no significant differences in terms of neither frequency of Tregs or change in frequency during therapy between responders and non-responders (data not shown).
Figure 4.
Frequency of regulatory cells. a) Frequency of Tregs decreased during treatment from baseline to 2nd series and 4th series (p = 0.08 and 0.0001 respectively). b) Treg expression of transcription factor Helios and activation marker CD39. c) Tregs were enriched for cells expression both CD39 and Helios, suggesting an activated phenotype of naturally occurring regulatory cells (change from baseline to 2nd and 4th series, p = 0.0001 and 0.03 respectively. d) Gating strategy for MDSC, which were defined as Lin-HLA-DRlow/-CD14+CD11b+CD33+ (Lin defined as CD3, CD19 and CD56) out of PBMC. e) No changes in the frequency of MDSC were observed during treatment. f) A trend towards lower baseline frequency of PBMC were observed in patients attaining subsequent clinical response to treatment (SD, PR and CR) as compared to non-responding patients (p = 0.1).
Additionally, we scrutinized dynamics of subpopulations of regulatory T cells based on expression of activation-marker CD39 and transcription-factor Helios, as illustrated in Fig. 4b. Though we did observe small reciprocal changes in both double-positive and double-negative subsets (not significant), only the double-positive compartment increased significantly (Fig. 4c, repeated measures ANOVA, p = 0.04) (mean values from baseline to the 2nd and to the 4th 38.7% vs. 41.5% vs. 42.6% respectively, p < 0.0005 and p = 0.1 respectively).
Frequencies of MDSC are associated with response but remain unchanged during therapy
MDSC are a diverse group of inhibitory cells of myeloid origin, frequently found to be increased in frequency in peripheral blood of melanoma patients negatively correlating with prognosis.14,25 Furthermore, treatment with Ipilimumab has been reported to reduce the frequency of MDSC. We quantified the frequency of monocytic MDSC only, as the granulocytic counterpart is sensitive to cryopreservation.26 MDSC were defined as Lin−HLA−DRlow/−CD14+CD11b+CD33+ (Lin defined as CD3, CD19 and CD56), and frequency was assessed out of PBMC (a representative example of gating strategy is presented in Fig. 4d). Results are presented in Fig. 4e. As seen, we did not observe any consistent treatment-related changes in the frequency of MDSCs. Interestingly, we observed higher baseline frequency of MDSC in patients with continued PD after Ipilimumab, though this difference was only borderline significant (Fig. 4f, p = 0.1) but no difference between responding and non-responding patients with regard to change in frequency throughout therapy (data not shown). We measured the concentration of interleukin 6 (IL6) in patient sera (see later), and sought to correlate this concentration to the frequency of MDSC before and during treatment. These analyses did not indicate any significant correlations between these parameters (data not shown).
NK cell were not affected by treatment
Though it is generally agreed that the mechanism-of-action of Ipilimumab is a disinhibiting effect on tumor-reactive T cells, the drug has been shown to exert some degree of NK cell mediated antibody-dependent cellular cytotoxicity (ADCC) in vitro.6 Additionally, NK cells may play a role in tumor immune surveillance.27,28 We therefore assessed the frequency of NK cells, defined as CD56bright lymphocytes not positive for CD3 and CD19, in peripheral blood. No consistent changes in the frequency of NK cells were observed during treatment (data not shown).
On-treatment IL6 levels correlate with survival
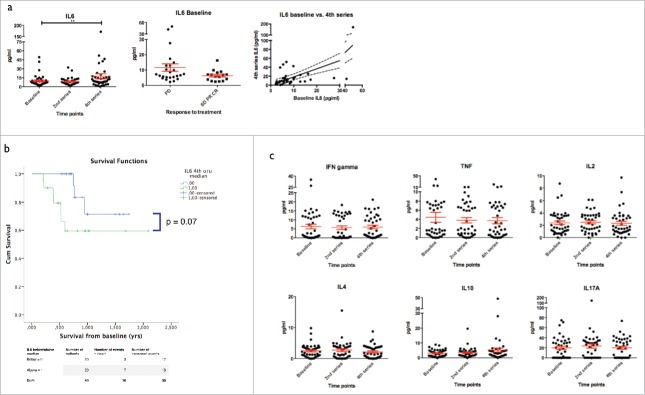
In cancers, including melanoma, tumor cell and stromal production of IL6 may serve as an autocrine/paracrine stimulator of tumor-growth.29 Numerous reports have focused on IL6 as a prognostic marker in metastatic melanoma and two relatively large studies have demonstrated a significant negative prognostic value of cytokine levels.30,31 Other studies, however, have yielded conflicting results.32
We measured serum-levels of IL6 at baseline, after the 1st and 3rd dose of Ipilimumab using a cytokine bead array. We observed a high degree of inter-patient variation in cytokine-levels ranging at baseline from 2.3–49.4 pg/mL, a significant increase in the level of IL6 during treatment (p = 0.046) (Fig. 5a) and a widening of the range of IL6 concentration. In addition to this, there was correlation between baseline-levels and levels by the 4th dose of treatment (R = 0.64, p = 0.001) and patients with subsequent response (SD, PR and CR combined) displayed a trend toward lower levels of IL6 at baseline as compared to non-responding patients (p = 0.09). To test a possible prognostic role of IL6 levels, we dichotomized the serum-levels by the median and compared survival according to IL6 above or below median. Results are plotted in Fig. 5b. Baseline-levels did not significantly predict survival (log rank, p = 0.5, data not shown), whereas higher levels of IL6 by the time of the 4th dose of Ipilimumab was associated with shorter survival though not significantly (log rank, p = 0.07). In order to determine if this might reflect a general unfavorable profile, a possible correlation between the level of lactate dehydrogenase (LDH) and IL6 was scrutinized. No correlation between IL6 and LDH was found either at baseline or by the 4th treatment (R2 = 0.003 and R2 < 0.001 respectively, data not shown), suggesting an independent value of this cytokine.
Figure 5.
Serum concentration of cytokines during treatment. a) left: IL6 levels increased from baseline to 4th series (p = 0.008). Middle: IL6 level at baseline were higher in patients with continued progression despite treatment as compared to clinical responders (SD, PR and CR, p = 0.09). Right: Level of IL6 at baseline correlated to the cytokine level by the 4th series. b) Above median levels of IL6 by the 4th series were associated with reduced survival (log rank, p = 0.07). c) Serum concentration of TNF, IFN-γ, IL2, IL4, IL10 and IL17A at baseline and during treatment.
Changes in levels of cytokines throughout treatment
We measured serum-concentration of six different cytokines: IL2, IL4, IL10, IL17A, interferon (IFN)γ and tumor necrosis factor (TNF) α using cytokine bead array. As shown in Fig. 5c, most of these cytokines were detectable in variable concentrations in the majority of patients. Neither IL2, IL4, IL10, IL17A, IFNγ, nor TNF-α showed any significant changes during therapy. However, IL17A and TNF-α showed great inter-patient variation. The level of these two cytokines did not correlate to the frequency of CD4+ and CD8+ T cells staining positive for IL17A and TNF-α in PMA/ionomycin stimulated PBMC samples (data not shown).
Discussion
Ipilimumab is only effective in a minority of patients, and in addition to that, rather costly. In a pursuit of appropriate biomarkers for a better selection of patients likely to respond to therapy, several have been identified correlating with treatment efficacy, but until recently non useful as predictive biomarkers.
It has previously been shown that early increases in ALC after treatment initiation correlate with treatment response.16 In order to confirm these data, we dichotomized our patient-cohort into patients with unequivocal progression at first evaluation and patients with SD or better and assessed ALC after one cycle of Ipilimumab. We were able to demonstrate similar ALC increases in patients with clinical response, thereby confirming previous findings. Furthermore, we saw that at least part of this increase could be accounted for by increases in the absolute T cell count. As Ipilimumab is directed at a broadly expressed target, CTLA-4, treatment is likely to have on-target effects on non-tumor-specific T cells, and these data could represent proliferation or mobilization of both T cells specific for viral or other pathogens in addition to tumor associated antigens (TAA). Previous studies have demonstrated that Ipilimumab induces proliferation of peripheral T cells,33 and data suggest a propensity in responding patients to react to treatment with proliferation of T cells, possibly reflecting a responsive state of the immune system less pronounced in non-responders. Furthermore, we found a general increase in the proportion of activated HLA-DR+ T cells as previously shown.7 Interestingly, this translated into generally higher increases in the absolute number of HLA-DR+ T cells after the first series of Ipilimumab in responding patients, further pointing to higher responsiveness of T cells in patients obtaining treatment response.
When looking further into the differentiation stage of the T cells a highly significant reduction in the frequency of naive triple-positive T cells in both CD4+ and CD8+ T cells during treatment was demonstrated. This was paralleled by increased frequency of triple-negative cells, i.e., cells with an intermediate or late effector state, partly confirming previous findings.7 This could either be due to a differentiation of naive cells into effector-cells or proliferation of cells in the latter compartment. It has previously been shown that treatment with anti-CTLA-4 antibodies induces a broadening of the T cell repertoire, and that the majority of the clones displaying altered abundance are derived from the non-naive compartment of cells.17 Though the mentioned study only assessed the relative frequency of clones in the upper quartile with regard to abundance, it indicate that the increased frequency of experienced cells evident in our patient cohort is likely due to proliferation in this compartment rather than cell-death in the naive compartment.
Some controversy has gathered on the mechanism-of-action of Ipilimumab. Two theories dominate: one including a direct disinhibiting effect on tumor-reactive T cells while the other point at inhibition or killing of Tregs in the tumor-microenvironment. The two mechanisms are difficult to distinguish experimentally, and it is not unlikely that the action is dependent on both mechanisms. Adding to this, a recent study by Romano et al demonstrated the presence in peripheral blood of a CD16+ monocyte population exerting ADCC on CTLA-4 positive Tregs in Ipilimumab treated patients.34
We demonstrated a highly significant decline in the frequency of Tregs identified as CD4+CD25highCD127−Foxp3+, and further showed a slight enrichment of CD39+Helios+ Tregs, i.e., activated naturally occurring Tregs.35-37 The enrichment of CD39+Helios+ Tregs may suggest that the overall decrease in the frequency of Tregs is primarily derived from one or more of the other non- CD39+Helios+ Tregs subsets, though none of these showed any significant decrease. Different studies have reported conflicting results regarding how Ipilimumab affects Tregs in peripheral blood. Tarhini et al.8 report increased frequencies correlating with improved progression-free survival. Notably, these authors identified Tregs with a similar panel of markers as used in the current study, but patients were treated with a higher dose (10 mg/kg) of Ipilimumab, which could explain the discrepancy. A different study by Sarnaik et al.,38 where patients were treated with either 3 or 10 mg/kg, found no impact on Tregs,38 whereas a third study, treating patients with 3 mg/kg as used in our cohort, found reduced frequencies.39 Though the mechanism is unclear, it seems that Ipilimumab might have opposite effects on the frequency of Tregs when administered in low and high doses. Furthermore, timing of blood sampling in relation to the course of treatment might have an impact on the findings; in a small-scale study by de Coaña et al,40 where blood samples were acquired at identical time points as in the current study, and found a similar decrease in Treg frequencies. In the current study, a small decrease in the frequency of Tregs was found after one dose of treatment and significant reduction by the 4th Ipilimumab dose. In consequence, this effect of Ipilimumab might have been missed if samples obtained late in the treatment course had not been included. A different subset of inhibitory cells, that have received a lot of attention in relation to Ipilimumab, is MDSC, which is a diverse group of cells implicated in the prognosis of several different cancers including melanoma.14,41 A number of more or less well-defined subtypes have been identified, including a monocytic CD14+ and a granulocytic CD15+ type, both present in peripheral blood. We chose to focus only on the monocytic counterpart, as the granulocytic is highly cryo-sensitive.26 In contrast to previous findings,8 we found no indication that the frequency of MDSC were affected by treatment in neither responders or non-responders. We did, however, find a trend toward higher frequencies of MDSC at baseline in patients not responding to treatment, which is in accordance with previous findings.14 In a patient cohort diagnosed with various gastro intestinal cancers, a correlation between CD15+ MDSC and IL6 concentration in patient serum has been reported, but a similar correlation did not seem to apply to the CD14+ monocytic MDSC.42 In esophagus cancer, however, significant correlation between IL6 and CD14+ MDSC has been reported.43 In the present study, we did not observe any significant correlation between the frequency of MDSC and IL6 at any of the assessed time points.
To this end, different gating-strategies may yield very different results. We decided to assess the MDSC frequency out of live singlet PBMC, which, in our hands, give the most reproducible results, as MDSC do not fall in a well-circumscribed gate in a forward/side scatter plot. However, this approach is inevitably sensitive to fluctuations in other PBMC subsets. Furthermore, MDSC is not an easily gated population, partly because the difference between HLA-DR-negative, low and positive is somewhat fluent, as at least in some patients, the level of expression seems to be a continuum. This leaves room for technical error biased by e.g., monocyte count.
In line with the presumed mechanism of action, most studies have focused on cellular markers in relation to treatment with Ipilimumab. We performed analyses of seven different cytokines in serum obtained at baseline and during therapy. IL6 levels showed interesting correlations to clinical data. During treatment, the level of this cytokine increased significantly in a subset of patients introducing a widening of the range of concentration. IL6 has previously been linked to an adverse prognosis in malignant melanoma,30,31 and in accordance with this, we found a non-significant reduced survival in patients with the highest IL6 levels by the 4th treatment. In order to determine whether the concentrations of this cytokine might just reflect a general unfavorable prognostic profile in patients with above median IL6 level, we scrutinized a possible correlation between the concentration of IL6 and LDH. The latter is a known negative prognostic factor correlating with aggressiveness of disease and negatively correlating with chance of responding to Ipilimumab therapy.44,45 We found no indication of a correlation, possibly indicating that the underlying adverse mechanism inducing an increased level of IL6 is different from the mechanism leading to increased LDH, thereby suggesting IL6 as an independent prognostic marker.
In conclusion, we show that response to Ipilimumab is associated to inductions in the absolute levels of circulating T cells, the level of activation of T cells and that Ipilimumab treatment may reduce the frequency of regulatory T cells. Additionally, we saw higher frequencies of MDSC and IL6 in non-responding patients.
Patients and methods
Patients
Patients were treated for American Joint Committee on Cancer (AJCC) stage IIIc or stage IV malignant melanoma with Ipilimumab as part of standard-of-care at either of two centers: Herlev Hospital, Copenhagen University, Denmark or Aarhus University Hospital, Aarhus, Denmark. Patients were eligible for the study if > 18 y of age and fulfilling standard criteria for treatment with Ipilimumab including histologically verified locally advanced and metastatic skin, ocular or mucosal malignant melanoma, Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2, and acceptable blood counts, liver and renal function tests. Patients were not eligible for treatment if prior medical history was notable of significant autoimmune diseases, known infection with human immunodeficiency virus, hepatitis B or C virus and current medical need for high-dose immunosuppressant(s). Treatment with Ipilimumab was given as a total of four treatments 3 weeks apart, at a fixed dose of 3 mg/kg body weight. Participation in the study was strictly voluntary and patients signed an informed consent form prior to inclusion. A total of three blood samples were acquired from each patient. Samples were obtained at baseline (before initiation of treatment), before the 2nd and 4th dose of treatment, simultaneously with routine blood samples and a total of 108 mL was obtained per sampling event. The protocol was approved by the Ethics Committee for The Capital Region of Denmark (H-2–2012–058). The study was conducted in accordance with the Helsinki Declaration of 1975.
Blood processing
PBMCs were purified from heparinized blood using lymphoprep™ (StemCell Technologies) density gradient centrifugation in LeucoSep™ tubes (Greiner Bio-One). After processing, cells were frozen in Nunc® 1.8 mL CryoTube (thermo Scientific) and stored at −150°C in 90% human AB serum (Sigma Aldrich) and 10% dimethyl sulphoxide (Herlev Hospital Pharmacy). Patient-to-processing time was sought kept as low as possible and processing was in general initiated within 4 h. Blood for collection of serum was collected in a 8 mL Vacuette® gel-tube containing clot activator (Greiner Bio-One). Serum was aliquoted in Nunc® 1.8 mL CryoTube (thermo Scientific) and stored at −80°C until analyses.
Assessment of clinical response
Clinical response was assessed using positron emission tomography and computed axial tomography (PET-CT) or CT alone evaluated according to Response Evaluation Criteria In Solid Tumors (RECIST v. One.1.). Patients were evaluated within 4 weeks before the first treatment and every 3 mo thereafter until progression. SD had to be confirmed on two independent evaluations. At the treating physician's discretion, patients with apparent increased tumor-volume at first evaluation could be evaluated for pseudo-progression usually with a 8-week treatment-free interval.
Flow cytometry
PBMC samples were thawed in 37°C RPMI 1640 medium (Lonza) supplemented with 2.5 mL DNAse-containing Pulmozyme (Roche) and 0.26 mmol MgCl (Herlev Hospital Pharmacy) per 100 mL buffer. All stainings were done in phosphate buffered saline (PBS) (Lonza) containing 0.5% bovine serum albumin (Sigma-Aldrich). The following antibodies were used: CD80-PerCP (Abcam), FoxP3-PE, IL9-PE, HLA-DR-HV500, CD3-PE-Cy7, CD19-PE-Cy7, CTLA-4-PE, PD-1-PE-Cy7, CD4-HV500, CD11b-APC, CD3-APC (purchased from BD Bioscience), CD33-FITC, CD56-PE, CD124-PE (purchased from BD Pharmigen), CCR7-PE-Cy7, BTLA-PE, CD27-PerCP, HELIOS-PerCP-Cy5.5, TNF-α-PE-Cy7, CD14-BV421, CD86-BV421, CD8-BV421, CD25-BV421 (all purchased from Biolegend), CD56-PE-Cy7, CD16-FITC, CD45RA-FITC, CD127-FITC, IFNγ-FITC, IL17A-PerCP-Cy5.5, CD39-PE-Cy7 (purchased from eBioscience), LAG-3-FITC (LifeSpan Biosciences) and TIM-3-PerCP (R&D Systems). Additionally, all samples were stained with the LIVE/DEAD® Fixable Near-IR Dead Cell Stain Kit (life technologies). Intracellular cytokine staining, cells were fixated and permeabilized using BD Cytofix/Cytoperm Kit according to the manufacturer's instructions. Intracellular staining of transcription factors was carried out using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer's instructions. Cells were acquired on a FACSCanto II using FACSDiva Software version 6.1.3 (BD Biosciences). Analysis of flow data was done using FlowJo software version 10 OSX (TreeStar, Inc., Ashland, OR). Data-analysis was done after filtering of dead cells and doublets.
In vitro activation
For intracellular cytokine staining, cells were thawed day zero and rested overnight in RPMI 1640 medium (Lonza) supplemented with 5% human AB serum (Sigma Aldrich) in 24-well plates at 37°C, 5% CO2 in a humidified atmosphere. Day 1, 2 µL/mL cell suspension Leukocyte Activation Cocktail (Phorbol 12-Myristate 13-Acetate and Ionomycin) with BD GolgiPlug™(Brefeldin A) was added or as a control, 1 µL/mL cell suspension of GolgiPlug™ (both purchased from BD Biosciences). Cells were stimulated for a total of 5 h before staining and acquisition.
Cytokine bead array
Concentrations of IL2, IL4, IL6, IL10, IL17A, TNF and IFNγ in serum from patients before and during treatment was measured using the BD™ Human Th1/Th2/Th17 CBA Kit (BD Biosciences) following the manufacturer's instructions. Cytokine concentrations were measured in thawed undiluted serum-samples. Concentration of cytokines was calculated using the FCAP Array™ software version 3.0 (BD SoftFlow).
Statistical analysis
All statistical calculations were done in GraphPad Prism (GraphPad Software, La Jolla, CA) and SPSS software (Version 22, IBM Corporation). Scatter plots are presented with mean (horizontal line) and standard error of the mean (error bars). All tests were performed two-sided and a p value ≤ 0.05 was considered significant. Between group comparisons were performed without correction for multiple comparisons in this exploratory study. p values for within group assessments were calculated using Bonferroni correction. When applicable, differences between groups and differences over time were assessed using students t-test. Overall differences within groups assessed on multiple time points were assessed using repeated measures ANOVA with the Greenhouse–Geisser Correction. If data did not meet model assumptions for parametric testing, Wilcoxon matched-pairs signed rank test was used to assess differences over time. A difference in survival was assessed using the log-rank test and survival estimates were calculated using Kaplan–Meier's method. Linear correlation was carried out using Spearman's method. Regression-plots are presented with the best fitted line and 95% confidence intervals of the fitted line.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Robert C, Schachter J, Long G V, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M et al.. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 2015; 372:2521-32; http://www.ncbi.nlm.nih.gov/pubmed/25891173;PMID:25891173; http://dx.doi.org/1714933 10.1056/NEJMoa1503093 [DOI] [PubMed] [Google Scholar]
- 2.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone J a. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994; 1:405-13; PMID:7882171; http://dx.doi.org/1714933 10.1016/1074-7613(94)90071-X [DOI] [PubMed] [Google Scholar]
- 3.Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174:561-9; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2118936&tool=pmcentrez&rendertype=abstract; PMID:1714933; http://dx.doi.org/ 10.1084/jem.174.3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krummel M, Allison J. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995; 182:459-65; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2192127&tool=pmcentrez&rendertype=abstract; PMID:7543139; http://dx.doi.org/ 10.1084/jem.182.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; http://www.ncbi.nlm.nih.gov/pubmed/20525992; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laurent S, Queirolo P, Boero S, Salvi S, Piccioli P, Boccardo S, Minghelli S, Morabito A, Fontana V, Pietra G et al.. The engagement of CTLA-4 on primary melanoma cell lines induces antibody-dependent cellular cytotoxicity and TNF-α production. J Transl Med 2013; 11:108; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3663700&tool=pmcentrez&rendertype=abstract; PMID:23634660; http://dx.doi.org/ 10.1186/1479-5876-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 2012; 35:89-97; http://www.ncbi.nlm.nih.gov/pubmed/22130166; PMID:22130166; http://dx.doi.org/ 10.1097/CJI.0b013e31823aa41c [DOI] [PubMed] [Google Scholar]
- 8.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, Sander C, Yin Y, Holtzman M, Johnson J et al.. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014; 9:e87705; http://dx.plos.org/ 10.1371/journal.pone.0087705; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodi FS, Butler M, Oble D a, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I et al.. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105:3005-10; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2268575&tool=pmcentrez&rendertype=abstract; PMID:18287062; http://dx.doi.org/ 10.1073/pnas.0712237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS et al.. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med 2014; 141119140020009; http://www.nejm.org/doi/abs/ 10.1056/NEJMoa1406498; PMID:25409260; http://dx.doi.org/19318477 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein O, Ebert LM, Nicholaou T, Browning J, Russell SE, Zuber M, Jackson HM, Dimopoulos N, Tan BS, Hoos A et al.. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin Cancer Res 2009; 15:2507-13; http://www.ncbi.nlm.nih.gov/pubmed/19318477; PMID:19318477; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-2424 [DOI] [PubMed] [Google Scholar]
- 12.Van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJA, Behjati S, Hilkmann H, El Atmioui D et al.. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31:e439-42; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3836220&tool=pmcentrez&rendertype=abstract; PMID:24043743; http://dx.doi.org/ 10.1200/JCO.2012.47.7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Mahony D, Morris JC, Quinn C, Gao W, Wilson WH, Gause B, Pittaluga S, Neelapu S, Brown M, Fleisher T a et al.. A pilot study of CTLA-4 blockade after cancer vaccine failure in patients with advanced malignancy. Clin Cancer Res 2007. 13:958-64; http://www.ncbi.nlm.nih.gov/pubmed/17289891; PMID:17289891; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1974 [DOI] [PubMed] [Google Scholar]
- 14.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014. 63:247-57; http://www.ncbi.nlm.nih.gov/pubmed/24357148; PMID:24357148; http://dx.doi.org/ 10.1007/s00262-013-1508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng Tang D, Shen Y, Sun J, Wen S, Wolchok JD, Yuan J, Allison JP, Sharma P. Increased Frequency of ICOS+ CD4 T Cells as a Pharmacodynamic Biomarker for Anti-CTLA-4 Therapy. Cancer Immunol Res 2013. 1:229-34; http://www.ncbi.nlm.nih.gov/pubmed/24777852; PMID:24777852; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berman DM, Wolchok J, Weber J, Hamid O, O'Day S, Chasalow SD, Bristol-Myers Squibb, Princeton NJ. Memorial Sloan-Kettering Cancer Center, New York, NY; H. Lee Moffitt Cancer Center& Research Institute, Tampa, FL; The Angeles Clinic and Researc N. Association of peripheral blood absolute lymphocyte count (ALC) and clinical activity in patients (pts) with advanced melanoma treated with ipilimumab. J Clin Oncol 2009; 27:Page suppl; abstr 3020; PMID:19470921; http://dx.doi.org/ 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L. Improved Survival with T Cell Clonotype Stability After Anti-CTLA-4 Treatment in Cancer Patients. Sci Transl Med 2014. 6:238ra70; http://www.ncbi.nlm.nih.gov/pubmed/24871131; PMID:24871131; http://dx.doi.org/ 10.1126/scitranslmed.3008211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert L, Tsoi J, Wang X, Emerson R, Homet B, Chodon T, Mok S, Huang RR, Cochran AJ, Comin-Anduix B et al.. CTLA4 blockade broadens the peripheral T-cell receptor repertoire. Clin Cancer Res 2014. 20:2424-32; http://www.ncbi.nlm.nih.gov/pubmed/24583799; PMID:24583799; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gattinoni L, Klebanoff C a, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg S a, Restifo NP. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 2005; 115:1616-26; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1137001&tool=pmcentrez&rendertype=abstract; PMID:15931392; http://dx.doi.org/ 10.1172/JCI24480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA et al.. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002. 415:536-41; http://www.ncbi.nlm.nih.gov/pubmed/11823861; PMID:11823861; http://dx.doi.org/ 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- 21.Iouzalen N, Andreae S, Hannier S, Triebel F. LAP, a lymphocyte activation gene-3 (LAG-3)-associated protein that binds to a repeated EP motif in the intracellular region of LAG-3, may participate in the down-regulation of the CD3/TCR activation pathway. Eur J Immunol 2001. 31:2885-91; http://www.ncbi.nlm.nih.gov/pubmed/11592063; PMID:11592063; http://dx.doi.org/ 10.1002/1521-4141(2001010)31:10%3c2885::AID-IMMU2885%3e3.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 22.Woo S, Turnis M, Goldberg M, Bankoti J. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012. 72:917-27; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3288154&tool=pmcentrez&rendertype=abstract; PMID:22186141; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derré L, Rivals J-P, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest 2010. 120:157-67; http://www.jci.org/articles/view/40070/version/2; http://dx.doi.org/ 10.1172/JCI40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang AL, Teijaro JR, Njau MN, Chandran SS, Azimzadeh A, Nadler SG, Rothstein DM, Farber DL. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol 2008; 20:1601-1609; 181:1806-13; http://www.ncbi.nlm.nih.gov/pubmed/2683757; PMID:18641318; http://dx.doi.org/ 10.4049/jimmunol.181.3.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D et al.. Myeloid-derived suppressor cells predict survival of advanced melanoma patients: comparison with regulatory T cells and NY-ESO-1- or Melan-A-specific T cells. Clin Cancer Res 2013. http://www.ncbi.nlm.nih.gov/pubmed/24323899; PMID:24323899; http://dx.doi.org/ 10.1200/JCO.2011.40.2271 [DOI] [PubMed] [Google Scholar]
- 26.Trellakis S, Bruderek K, Hütte J, Elian M, Hoffmann TK, Lang S, Brandau S. Granulocytic myeloid-derived suppressor cells are cryosensitive and their frequency does not correlate with serum concentrations of colony-stimulating factors in head and neck cancer. Innate Immun 2013. 19:328-36; http://www.ncbi.nlm.nih.gov/pubmed/23160385; PMID:23160385; http://dx.doi.org/ 10.1177/1753425912463618 [DOI] [PubMed] [Google Scholar]
- 27.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000. 356:1795-9; http://www.ncbi.nlm.nih.gov/pubmed/11117911; PMID:11117911; http://dx.doi.org/ 10.1016/S0140-6736(00)03231-1 [DOI] [PubMed] [Google Scholar]
- 28.Hayashi T, Imai K, Morishita Y, Hayashi I, Kusunoki Y, Nakachi K. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res 2006. 66:563-70; http://www.ncbi.nlm.nih.gov/pubmed/16397273; PMID:16397273; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2776 [DOI] [PubMed] [Google Scholar]
- 29.Lu C, Kerbel RS. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol 1993; 120:1281-8; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2119719&tool=pmcentrez&rendertype=abstract; PMID:8436594; http://dx.doi.org/ 10.1083/jcb.120.5.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soubrane C, Rixe O, Meric J-B, Khayat D, Mouawad R. Pretreatment serum interleukin-6 concentration as a prognostic factor of overall survival in metastatic malignant melanoma patients treated with biochemotherapy: a retrospective study. Melanoma Res 2005. 15:199-204; http://www.ncbi.nlm.nih.gov/pubmed/15917702; PMID:15917702; http://dx.doi.org/ 10.1097/00008390-200506000-00009 [DOI] [PubMed] [Google Scholar]
- 31.Hoejberg L, Bastholt L, Johansen JS, Christensen IJ, Gehl J, Schmidt H. Serum interleukin-6 as a prognostic biomarker in patients with metastatic melanoma. Melanoma Res 2012. 22:287-93; http://www.ncbi.nlm.nih.gov/pubmed/22617301; PMID:22617301; http://dx.doi.org/ 10.1097/CMR.0b013e3283550aa5 [DOI] [PubMed] [Google Scholar]
- 32.Tartour E, Blay JY, Dorval T, Escudier B, Mosseri V, Douillard JY, Deneux L, Gorin I, Negrier S, Mathiot C et al.. Predictors of clinical response to interleukin-2–based immunotherapy in melanoma patients: a French multiinstitutional study. J Clin Oncol 1996. 14:1697-703; http://www.ncbi.nlm.nih.gov/pubmed/8622090; PMID:8622090; http://dx.doi.org/ 10.1200/JCO.2011.40.2271 [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber JS. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med 2012. 10:146; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3527361&tool=pmcentrez&rendertype=abstract; PMID:22788688; http://dx.doi.org/ 10.1186/1479-5876-10-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, Michielin O, Weide B, Romero P, Speiser DE. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci 2015;112:6140–6145; PMID:25918390; http://dx.doi.org/23359504 10.1073/pnas.1417320112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Himmel ME, MacDonald KG, Garcia R V, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol 2013. 190:2001-8; http://www.ncbi.nlm.nih.gov/pubmed/23359504; PMID:23359504; http://dx.doi.org/ 10.4049/jimmunol.1201379 [DOI] [PubMed] [Google Scholar]
- 36.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen J-F, Enjyoji K, Linden J, Oukka M et al.. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007. 204:1257-65; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2118603&tool=pmcentrez&rendertype=abstract; PMID:17502665; http://dx.doi.org/ 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornton AM, Korty PE, Tran DQ, Wohlfert E a, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol 2010. 184:3433-41; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3725574&tool=pmcentrez&rendertype=abstract; PMID:20181882; http://dx.doi.org/ 10.4049/jimmunol.0904028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarnaik AA, Yu B, Yu D, Morelli D, Hall M, Bogle D, Yan L, Targan S, Solomon J, Nichol G et al.. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res 2011. 17:896-906; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3041838&tool=pmcentrez&rendertype=abstract; PMID:21106722; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Carac∫ C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E et al.. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014. 63:675-83; http://www.ncbi.nlm.nih.gov/pubmed/24695951; PMID:24695951; http://dx.doi.org/ 10.1007/s00262-014-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pico de Coaña Y, Wolodarski M, Gentilcore G, Yoshimoto Y, Poschke I, Hansson J, Masucci G V, Kiessling R. Ipilimumab treatment decreases circulating Tregs and GrMDSC while enhancing CD4+ T cell activation. J Transl Med 2015; 13:O7; http://www.translational-medicine.com/content/13/S1/O7; http://dx.doi.org/ 10.1186/1479-5876-13-S1-O7 [DOI] [Google Scholar]
- 41.Idorn M, Køllgaard T, Kongsted P, Sengeløv L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother 2014; 63:1177-1187; http://www.ncbi.nlm.nih.gov/pubmed/25085000; PMID:25085000; http://dx.doi.org/ 10.1007/s00262-014-1591-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mundy-Bosse BL, Young GS, Bauer T, Binkley E, Bloomston M, Bill MA, Bekaii-Saab T, Carson WE, Lesinski GB. Distinct myeloid suppressor cell subsets correlate with plasma IL-6 and IL-10 and reduced interferon-α signaling in CD4 + T cells from patients with GI malignancy. Cancer Immunol Immunother 2011; 60:1269-79; PMID:21604071; http://dx.doi.org/ 10.1007/s00262-011-1029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen M-F, Kuan F-C, Yen T-C, Lu M-S, Lin P-Y, Chung Y-H, Chen W-C, Lee K-D. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget 2014; 5:8716-28; http://www.ncbi.nlm.nih.gov/pubmed/25238263; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC4226716; PMID:25238263; http://dx.doi.org/ 10.18632/oncotarget.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwala SS, Keilholz U, Gilles E, Bedikian AY, Wu J, Kay R, Stein CA, Itri LM, Suciu S, Eggermont AMM. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur J Cancer 2009. 45:1807-14; http://www.ncbi.nlm.nih.gov/pubmed/19419855; PMID:19419855; http://dx.doi.org/ 10.1016/j.ejca.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 45.Kelderman S, Heemskerk B, van Tinteren H, van den Brom RRH, Hospers GAP, van den Eertwegh AJM, Kapiteijn EW, de Groot JWB, Soetekouw P, Jansen RL et al.. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother 2014; 63:449-58; http://www.ncbi.nlm.nih.gov/pubmed/24609989; PMID:24609989 [DOI] [PMC free article] [PubMed] [Google Scholar]