ABSTRACT
It is increasingly recognized that trastuzumab, an antibody approved for treating human epidermal growth factor receptor 2 (HER2)-overexpressing breast cancer, exerts some of its antitumor effects through enhanced T cell responses. Full activation of CD8+ T cells requires both expression of major histocompatibility complex class I molecules (HLA-ABC) and expression of the T cell costimulatory molecule CD80 or CD86; however, the impact of trastuzumab treatment on the expression of HLA-ABC and CD80 and CD86 has not been investigated in HER2-overexpressing breast cancer cells. In this study, we found that, while there is no direct correlation between the expression of HER2 and HLA-ABC in breast cancer, knockdown of HER2 or inhibition of HER2 kinase by lapatinib downregulated HLA-ABC expression. Trastuzumab had no meaningful effects on HLA-ABC expression in HER2-overexpressing breast cancer cells in monoculture; however, treatment of such cells with trastuzumab in co-culture with human peripheral blood mononuclear cells (PBMC) significantly upregulated not only HLA-ABC expression but also CD86 expression. We showed that this upregulation was mediated by interferon gamma (IFNγ) secreted from the natural killer (NK) cells in PBMC as a result of engagement of NK cells by trastuzumab. We further confirmed this effect of trastuzumab in vivo using a mouse mammary tumor model transduced to overexpress human HER2. Together, our data provide evidence that trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules in HER2-overexpressing breast cancer cells in the presence of PBMC, which supports the view that T-cell-mediated immune responses are involved in trastuzumab-mediated antitumor effects.
KEYWORDS: HER2, HLA-ABC, IFNγ, MHC-I, PBMC
Abbreviations
- APC
allophycocyanin
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- HER2
human epidermal growth factor receptor 2
- HLA
human leukocyte antigens
- HLA-ABC
HLA-A, HLA-B, sand HLA-C
- IFNγ
interferon gamma
- MFI
mean fluorescence intensity
- MHC-I
major histocompatibility complex class I
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
Introduction
Nearly 20–25% of breast cancers overexpress HER2, which is causally related to breast cancer progression, recurrence, and metastasis.1,2 Trastuzumab, an HER2-targeting antibody approved by the US Food and Drug Administration to treat HER2-overexpressing breast cancer, has significantly improved clinical outcomes of patients with the disease, which is very aggressive and once was very hard to treat.3,4 However, trastuzumab resistance is common in the clinic; further improvement of this treatment is needed.5
Known mechanisms of action of trastuzumab following binding of trastuzumab to the extracellular domain of HER2 include (1) downregulation of HER2 kinase-mediated downstream cell signaling and resulting inhibition of breast cancer cell survival, cell proliferation, and tumor angiogenesis and resulting sensitization of breast cancer cells to chemotherapy and radiotherapy,4-9 and (2) engagement of immune effector cells, mostly NK cells, via the Fc region of trastuzumab to release perforin and granzyme B, which directly kill targeted cells, i.e., antibody-dependent cell-mediated cytotoxicity.10,11 Furthermore, recent animal studies showed that a trastuzumab-like antibody (anti-HER2/neu antibody) lost its antitumor activity if CD8+ T cell immunity was completely abrogated, suggesting that adaptive immune responses are also involved in trastuzumab-mediated antitumor activity.12 Clinical trials have shown that patients with better response to trastuzumab had more tumor-infiltrating lymphocytes and NK cells present in the tumor stroma.13-16 Trastuzumab-treated HER2-overexpressing breast cancer cells were more susceptible to HER2-specific CD8+ cytotoxic T cells in vitro than were HER2-overexpressing breast cancer cells not treated with trastuzumab.17
Expression of major histocompatibility complex class I (MHC-I) molecules, which are known as HLA-A, HLA-B, and HLA-C antigens (HLA-ABC) in humans and H-2 antigens in mice, is necessary and crucial for proper presentation of specific antigens on the cancer cell surface for recognition by cytotoxic CD8+ T cells.18,19 However, cancer cells are known to deploy multiple immunosuppressive mechanisms, including downregulation of MHC-I expression, to evade T cell responses.18 A few early studies reported an inverse correlation between HER2 level and HLA-ABC expression in some breast cancer cell lines.20-22 Also playing a role in T cell activation are the CD80 and CD86 T cell costimulatory molecules, which provide second signals necessary for T cell activation and survival through binding to CD28 on the T cell surface and also binding to CTLA-4 for attenuation of the regulation. Expression of CD80 and CD86 is found not only in antigen-presenting cells but also in some human cancer cell lines.23,24 Whether trastuzumab treatment has any impact on the expression of CD80 and CD86 in HER2-overexpressing breast cancer cells has not been investigated.
In this study, we first examined whether targeting HER2 has an effect on the level of HLA-ABC expression in HER2-overexpressing breast cancer cells by treating such cells with HER2 siRNA, an HER2 kinase inhibitor (lapatinib), and trastuzumab. Next, we tested the impact of trastuzumab treatment on the expression of HLA-ABC and CD80 and CD86 in HER2-overexpressing breast cancer cells in the presence of PBMC and in a mouse mammary tumor model transduced to overexpress human HER2. Our results showed that trastuzumab upregulated the expression of HLA-ABC and CD86 in HER2-overexpressing breast cancer cells in the presence of PMBC and that this upregulation was mediated by IFNγ released from NK cells through engagement of NK cells by trastuzumab.
Results
Lack of significant inverse correlation between HER2 expression level and HLA-ABC expression level across a panel of human breast cancer cell lines
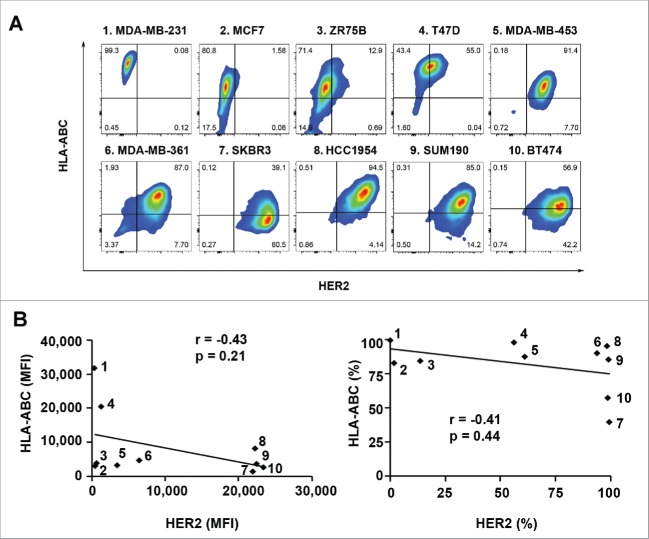
To determine if there is an inverse relationship between HER2 expression level and HLA-ABC expression level across multiple human breast cancer cell lines, we first examined expression of HLA-ABC in a panel of ten breast cancer cell lines with different levels of HER2 expression using flow cytometry analysis after double-staining of the cells with trastuzumab plus fluorescein isothiocyanate (FITC)-conjugated anti-human IgG antibody and allophycocyanin (APC)-conjugated anti-HLA-ABC antibody. As shown in Fig. 1A, in which the ten cell lines are arranged from low to high with respect to mean fluorescence intensity (MFI) value of HER2 expression, cell lines with low or undetectable levels of HER2 expression had MFI values of HLA-ABC expression ranging from very high (in MDA-MB-231 and T47D cells) to very low (in MCF7 and ZR75B cells). Cell lines with high levels of HER2 expression also had MFI values of HLA-ABC expression ranging from high (in MDA-MB-361, SUM190, and HCC1954 cells) to relatively low (in MDA-MB-453, SKBR3, and BT474 cells). Pearson correlation analysis showed no significant inverse correlation between HER2 expression and HLA-ABC expression in the cell line panel in terms of either MFI values (p = 0.21) or percentages of positive cells (p = 0.44) (Fig. 1B). Together, these data suggest that there is no definitive inverse correlation between the levels of HER2 and HLA-ABC expression among these human breast cancer cell lines.
Figure 1.
Expression of HLA-ABC in a panel of human breast cancer cell lines with and without overexpression of HER2. The indicated breast cancer cells were double-stained with APC-conjugated anti-HLA-ABC antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody and subjected to flow cytometry analysis. (A). Flow cytometric data on HLA-ABC expression versus HER2 expression in each cell line in contour plots. (B). MFI values of HLA-ABC expression vs. MFI values of HER2 expression (left) and percentages of HLA-ABC-positive cells versus percentages of HER2-overexpressing cells (right) in scatter plots. Pearson correlation coefficients (r) and p values are shown. The numbers next to the dots in the scatter plots correspond to the numbers next to the names of breast cancer cell lines in A.
Effect of altering HER2 level or kinase activity on the level of HLA-ABC expression in breast cancer cells
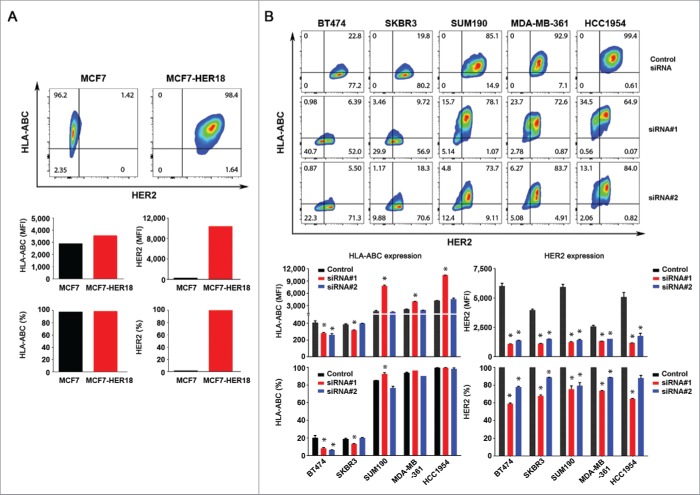
To determine whether there is a causal relationship between HER2 expression and HLA-ABC expression in HER2-overexpressing breast cancer cells within the same genetic context, we first compared the HLA-ABC expression between MCF7 breast cancer cells and MCF7-HER18 cells, a subline of MCF7 cells transfected to overexpress HER2 that effectively activates the signaling pathways downstream of HER2 in the cells.25,26 As shown in Fig. 2A, overexpression of HER2 does not lead to a decrease but rather a moderate increase in the MFI value of HLA-ABC expression in MCF7-HER18 cells compared to MCF7 cells.
Figure 2.
Effect of HER2 overexpression and knockdown on the level of HLA-ABC expression in breast cancer cells. (A). MCF7 and MCF7-HER18 cells were double-stained with APC-conjugated anti-HLA-ABC antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody and subjected to flow cytometry analysis. Upper panel, flow cytometric data on HLA-ABC expression vs. HER2 expression in MCF7 and MCF7-HER18 cells in contour plots. Lower panel, MFI values of HLA-ABC and HER2 expression (left) and the percentages of cells expressing HLA-ABC and HER2 (right) in MCF7 and MCF7-HER18 cells. (B). The indicated HER2-overexpressing human breast cancer cells were transfected with one of two different HER2-specific siRNAs or control siRNA using Lipofectamine for 72 h in culture. The cells were double-stained for flow cytometry analysis of HLA-ABC and HER2 and the data were plotted as described in (A). * p < 0.05 compared with corresponding control.
We next examined HLA-ABC expression in five HER2-overexpressing breast cancer cell lines (BT474, SKBR3, SUM190, MDA-MB-361, and HCC1954) after knockdown of HER2 expression by each of two different siRNAs. As shown in Fig. 2B and in Fig. S1, the impact of HER2 knockdown on HLA-ABC expression differed by HER2-targeting siRNA and cell line. Knockdown of HER2 by siRNA#1 led to a significant increase in HLA-ABC expression (in terms of both MFI values and percentages of HLA-ABC-positive cells) in SUM190, MDA-MB-361, and HCC1954 cells, in which the baseline MFI and the baseline percentage of HLA-ABC-expressing cells were high, but a significant decrease in HLA-ABC expression in BT474 and SKBR3 cells, in which the baseline MFI and the baseline percentage of HLA-ABC-expressing cells were relatively low (Fig. 2B, lower panel). In contrast, siRNA#2, which produced slightly weaker HER2 knockdown than siRNA#1 did, had no significant effect on HLA-ABC expression except in BT474 cells, in which siRNA#2 lowered HLA-ABC expression as measured by both MFI and percentage of HLA-ABC-positive cells.
Thus, we expanded the number of siRNAs to target different regions of HER2 mRNA sequence and further explore the effect of HER2 knockdown on the level of HLA-ABC expression in breast cancer cells. For these experiments, we used SKBR3 cells, which have relatively low HLA-ABC expression, SUM190 cells, which have intermediate to high HLA-ABC expression, and HCC1954 cells, which have high HLA-ABC expression. The results from use of three additional HER2 siRNAs (siRNA#3, siRNA#4, and siRNA#5) were consistent with the findings with siRNA#2 (no significant impact) but not with siRNA#1, suggesting a potential off-target effect of HER2 siRNA#1 that led to an increase in HLA-ABC expression in some cell lines (Fig. S2). Indeed, we found that in the three cell lines in which siRNA#1 led to an increase in HLA-ABC expression (SUM190, MDA-MB-361, and HCC1954; Fig. 2A), siRNA#1 also led to a significant increase in the levels of both total and Y-701-phosphorylated STAT1 (Fig. S1), which is a major transcription regulator of HLA-ABC expression,27 whereas siRNA#2 either had no effect on or led to a decrease in the level of Y-701-phosphorylated STAT1.
To determine whether there is an association between the level of HER2 kinase activity and HLA-ABC expression in HER2-overexpressing breast cancer cells, we examined whether treatment of HER2-overexpressing breast cancer cells with lapatinib, a HER2 kinase inhibitor, had an effect on HLA-ABC expression in such cells. As shown in Fig. 3A, treatment with lapatinib at 0.5 or 1.0 µM for 24 h inhibited HER2 kinase activity with different degrees in all five HER2-overexpressing breast cancer cell lines tested, as previously reported by others.28 However, we found no increase in HLA-ABC expression in any of the five cell lines; rather, we found decreases in the MFI value, the percentage of cells expressing HLA-ABC, or both, with particularly pronounced decreases in SKBR3 and SUM190 cells (Fig. 3B). Of note, as shown in Fig. 3A, lapatinib, in addition to inhibiting the well-documented pathways downstream of HER2, including phosphorylation of PI3K/Akt and MEK/Erk, inhibited tyrosine phosphorylation of STAT1. This inhibition of activation-specific phosphorylation of STAT1 may be responsible for lapatinib-induced decrease in HLA-ABC expression.
Figure 3.
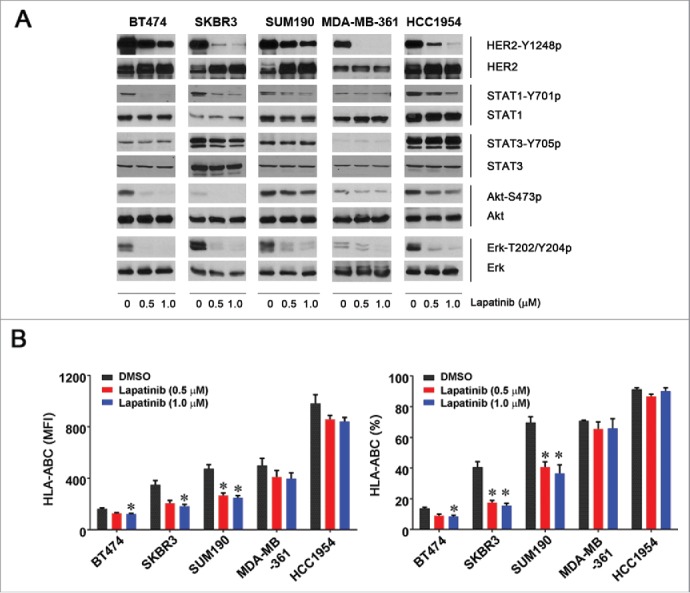
Effect of lapatinib on the level of HLA-ABC expression in HER2-overexpressing breast cancer cells. The indicated HER2-overexpressing human breast cancer cells were treated with vehicle (DMSO) or lapatinib (0.5 or 1.0 µM) for 24 h. After treatment, aliquots of cells were lysed for Western blot analysis with the indicated antibodies (A) or stained with APC-conjugated anti-HLA-ABC antibody for 30 min and subjected to flow cytometry analysis (B). Any cells undergoing apoptosis following treatment (Annexin-V-positive cells) were gated out before analysis of the flow cytometric data. Shown in (B) are the MFI values of HLA-ABC expression (left) and the percentages of HLA-ABC-positive cells in the cell lines treated with vehicle or lapatinib (right). * p < 0.05 compared with corresponding control.
Together, our data show that there is a lack of direct inverse correlation between the levels of HER2 expression and HLA-ABC expression in breast cancer cells. Although knockdown of HER2 expression by one particular siRNA (siRNA#1), which was associated with an increase in the level of STAT1, upregulated HLA-ABC expression in a cell type/context-specific manner, knockdown of HER2 expression by other siRNAs or inhibition of HER2 kinase activity by lapatinib downregulated HLA-ABC expression in the cell line panel, suggesting that inhibition of HER2 signaling alone does not enhance and may even inhibit HLA-ABC expression in HER2-overexpressing breast cancer cells.
Trastuzumab increases HLA-ABC expression in HER2-overexpressing breast cancer cells in the presence of PBMC by engaging NK cells and thereby stimulating IFNγ secretion
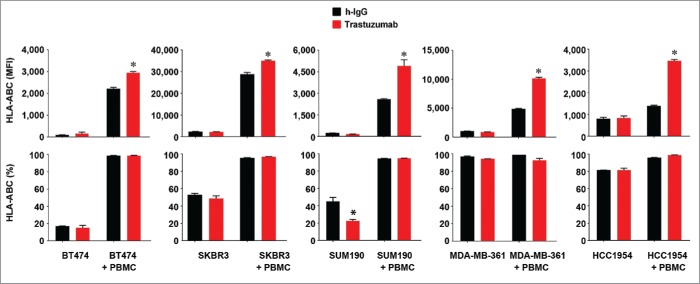
A previous study showed that trastuzumab had no impact on the expression of HLA-ABC or CD80 and CD86 T cell costimulatory molecules in non-breast cancer cells.29 Trastuzumab was recently reported to induce transcriptional inhibition of HER2 expression in HER2-overexpressing breast cancer cells by engaging Fc receptor-expressing immune effector cells to stimulate IFNγ secretion in the presence of PBMC through STAT1 activation.30 Because IFNγ is a potent cytokine that stimulates HLA-ABC expression by activating STAT1 signaling,31 we decided to examine whether the presence of immune effector cells influences any effect of trastuzumab on HLA-ABC expression. We first cultured BT474, SKBR3, SUM190, MDA-MB-361, and HCC1954 cells for 48 h in the presence or absence of trastuzumab in culture medium. As shown in Fig. 4, there were no significant changes in HLA-ABC expression level in these cells, and trastuzumab produced a decrease in the percentage of SUM190 cells expressing HLA-ABC. As expected, when these cells were co-cultured with PBMC, both the MFI value and the percentage of HLA-ABC-positive cells were markedly increased compared to the values in mono-culture. Notably, however, in all five cell lines, when cells were co-cultured with PBMC, the MFI value of HLA-ABC expression (but not the percentage of HLA-ABC-positive cells) was significantly higher in trastuzumab-treated cells than in control antibody-treated cells.
Figure 4.
Increased HLA-ABC expression in HER2-overexpressing breast cancer cells by trastuzumab in the presence of PBMC. The indicated HER2-overexpressing human breast cancer cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL (˜30 nM) trastuzumab or a control humanized IgG (h-IgG; bevacizumab) in mono-culture or co-culture with human PBMC at a ratio of 1:5 (cancer cells versus PBMC) for 48 h. After treatment, any PBMC grown in suspension were removed by washing the cells with PBS. The cells were then stained with APC-conjugated anti-HLA-ABC antibody and subjected to flow cytometry analysis. Any residual PBMC in the co-culture were gated out during flow cytometric analysis on the basis of the size difference between PBMC and the co-cultured breast cancer cells. Shown are the MFI values of HLA-ABC expression (upper) and the percentages of HLA-ABC-positive cells (lower). The data presented are from multiple experiments using PBMC from different donors. * p < 0.05 compared with corresponding control.
We used enzyme-linked immunosorbent assay (ELISA) to measure the level of IFNγ in conditioned media from the co-culture experiment of BT474, SUM190, and HCC1954 cells with PBMC shown in Fig. 4. We found that the level of IFNγ was significantly higher in the conditioned media from trastuzumab-treated cell cultures than in the conditioned media from control antibody-treated cell cultures in the presence of PBMC, with particularly pronounced increases in BT474 and SUM190 cells (Fig. 5A). Because PBMC can secrete many cytokines, to confirm that the increase in IFNγ was the cause of the trastuzumab-induced upregulation of HLA-ABC expression in breast cancer cells shown in Fig. 4, we co-cultured BT474 and SUM190 cells with PBMC with and without an IFNγ-neutralizing antibody. We found that the presence of an IFNγ-neutralizing antibody abolished the trastuzumab-induced increase in the MFI of HLA-ABC in the co-cultures (Fig. 5B). This finding strongly indicates that IFNγ secreted as a result of trastuzumab engagement with immune effector cells in PBMC plays a critical causal role in trastuzumab-mediated increase in HLA-ABC expression.
Figure 5.
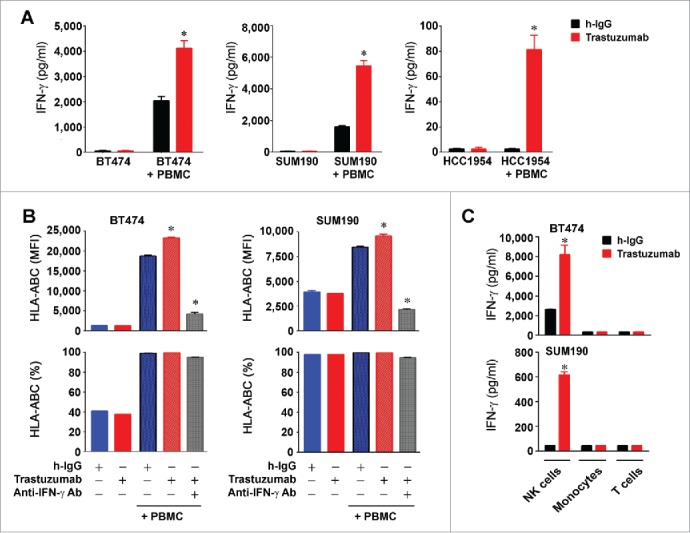
Increased HLA-ABC expression in HER2-overexpressing breast cancer cells by trastuzumab through engagement of NK cells and stimulation of IFNγ secretion. (A). BT474, SUM190, and HCC1954 cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL (˜30 nM) trastuzumab or a control humanized IgG (h-IgG; bevacizumab) in mono-culture or co-culture with human 1.5×106 PBMC (at a ratio of 1:5, cancer cells vs. PBMC) for 48 h. Cell-free conditioned medium from each culture was used for detecting the presence of human IFNγ with an ELISA kit. * p < 0.05 compared with corresponding control. (B). BT474 and SUM190 cells were treated with the conditioned medium from the monoculture and co-culture described in (A) or with the conditioned medium plus an IFNγ-neutralizing antibody as indicated for 48 h. The cells were then stained with APC-conjugated anti-HLA-ABC antibody and subjected to flow cytometry analysis. Shown are the MFI values of HLA-ABC expression (upper) and the percentages of HLA-ABC-positive cells (lower). * p < 0.05 compared with corresponding control or compared with the group treated with an IFNγ-neutralizing antibody. (C). BT474 and SUM190 cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL (˜30 nM) trastuzumab or a control humanized IgG (h-IgG; bevacizumab) for 24 h in co-culture at a ratio of 1:5 (cancer cells versus immune effector cells) with 1.5×106 NK cells (CD56+), monocytes (CD14+), or T cells (CD3+). The immune effector cells were sorted by flow cytometry from PBMC after staining of PBMC with PE-Cy7-conjugated anti-CD56 antibody, APC-conjugated anti-CD14 antibody, and FITC-conjugated anti-CD3 antibody. Conditioned medium from each culture was used for detecting the presence of human IFNγ with an ELISA kit. * p < 0.05 compared with corresponding control.
To identify the type of immune effector cell with the greatest contribution to the IFNγ production in the conditioned media from the co-culture experiments, we sorted the NK cells (CD56+), monocytes/macrophages (CD14+), and T cells (CD3+) from the PBMC and subjected BT474 and SUM190 cells to co-culture with the individually sorted cells in the absence or presence of trastuzumab or a control antibody. The results (Fig. 5C) showed that NK cells were the main contributors to the increase in IFNγ production in HER2-overexpressing breast cancer cells after trastuzumab treatment.
Trastuzumab increases the expression of CD86 costimulatory molecule in HER2-overexpressing breast cancer cells in the presence of PBMC
We also determined whether trastuzumab increased the expression of CD80 and CD86 costimulatory molecules. We first examined whether CD80 and CD86 are expressed in the breast cancer cell lines used in our study. We confirmed that HEK293 cells do not express CD80 or CD86 (Fig. 6A), as previously reported.32 Using these HEK293 cells as a negative control, we found that CD86 but not CD80 was expressed in BT474 and SUM190 cells (Fig. 6A). As shown in Fig. 6B, both the MFI value and the percentage of CD86+ cells were higher in BT474 cells co-cultured with PBMC than in BT474 cells in mono-culture. While trastuzumab marginally decreased CD86 expression in BT474 cells in mono-culture, trastuzumab significantly increased the MFI value of CD86 in co-culture. A similar pattern of results was found in SUM190 cells, although the results were less remarkable than in BT474 cells (Fig. 6B), which may be due to differences in genetic contexts between the cells.
Figure 6.
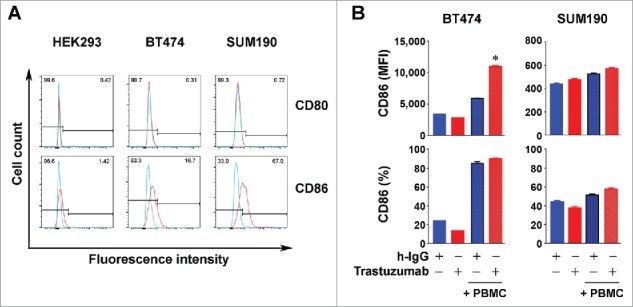
Increased expression of CD86 in HER2-overexpressing breast cancer cells by trastuzumab in the presence of PBMC. (A). HEK293, BT474, and SUM190 cells were stained with PE-conjugated anti-human CD80 antibody and PE-Cy7-conjugated anti-human CD86 antibody and subjected to flow cytometry analysis. (B). BT474 and SUM190 cells were cultured in medium supplemented with 10% fetal bovine serum and were treated with 5 µg/mL trastuzumab (˜30 nM) or a control humanized IgG (h-IgG; bevacizumab) in mono-culture or co-culture with human PBMC at a ratio of 1:5 (cancer cells vs. PBMC) for 48 h. The cells were then stained with PE-Cy7-conjugated anti-human CD86 antibody and subjected to flow cytometry analysis. Shown are the MFI values of CD86 expression (upper) and the percentages of CD86-positive cells (lower). * p < 0.05 compared with corresponding control.
Trastuzumab increases the expression of MHC-I molecules and CD80/CD86 in HER2-overexpressing breast cancer cells in vivo
Although co-culture of breast cancer cells with PBMC mimics the interaction of breast cancer cells with stromal cells in the tumor microenvironment following trastuzumab treatment, co-culture does not completely recapitulate that interaction in vivo. Thus, we further determined whether trastuzumab increased the expression of MHC-I molecules and CD80/CD86 in vivo in a mouse model. Because murine IFNγ could not activate interferon receptor signaling in human breast cancer cells (as shown in SUM190 cells in Fig. 7A), whereas trastuzumab recognizes only human HER2, for the in vivo study we used 4T1 transplantable murine mammary tumor cells that were transduced to overexpress human HER2. We found that over 75% of 4T1 cells were positive for murine MHC-I (H-2Kd) expression; overexpression of human HER2 in the 4T1 cells did not have significant effect on the expression level of MHC-I (H-2Kd) (data not shown). When the 4T1/HER2 cells had grown into palpable tumors after transplantation in Swiss female nude mice, which are negative for H-2Kd expression, we treated the mice with trastuzumab or a control anti-human IgG. Analysis of H-2Kd expression in the tumors by flow cytometry showed a significant increase in the percentage of H-2Kd-expressing cells in the tumors from trastuzumab-treated mice compared with the tumors from control antibody-treated mice (Fig. 7B). Similarly, the percentages of CD80- and CD86-expressing cells were higher in the tumors from trastuzumab-treated mice than in the tumors from control antibody-treated mice (Fig. 7C). This experiment provides important in vivo evidence further supporting a role of trastuzumab in increasing expression of HLA-ABC/MHC-I and CD80/CD86 molecules in HER2-overexpressing breast cancer cells.
Figure 7.
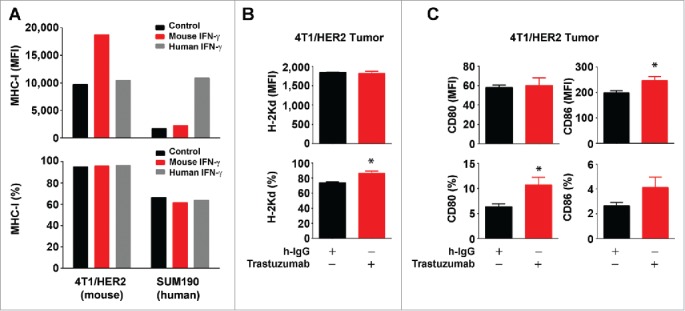
Increased expression of MHC-I and CD80/CD86 molecules by trastuzumab in HER2-overexpressing breast cancer cells in vivo. (A). 4T1 mouse mammary tumor cells transduced to overexpress HER2 (4T1/HER2) and SUM190 human breast cancer cells were cultured in medium supplemented with 10% fetal bovine serum in the presence or absence of mouse IFNγ (300 units/mL) or human IFNγ (300 units/mL) for 24 h. The cells were then stained with APC-conjugated anti-H-2Kd (for 4T1/HER2) or APC-conjugated anti-HLA-ABC antibody (for SUM190) and subjected to flow cytometry analysis. Shown are the MFI values of MHC-I expression (upper) and the percentages of MHC-I-positive cells (lower). (B). 4T1/HER2 cells (2×106 cells) were implanted in the mammary fat pad of female Swiss nude mice (4–6 weeks old). Two weeks after the tumors were palpable, the mice were treated once with 200 µg of trastuzumab or a control humanized IgG (rituximab) intraperitoneally. At 48 h after treatment, the mice were euthanized, the tumors were removed, and single cell suspensions were prepared and double-stained with APC-conjugated anti-H-2Kd antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody. Cells were then subjected to flow cytometry analysis. Shown are the MFI values of H-2Kd expression (upper) and the percentages of H-2Kd positive cells (lower) gated for HER2-positive cells. *p < 0.05 compared with corresponding control. (C). Single cell suspensions from 4T1/HER2 tumors described in (B) were triple-stained with APC-conjugated anti-mouse CD80 antibody, PE-conjugated anti-mouse CD86 antibody and trastuzumab plus FITC-conjugated anti-human IgG antibody, and subjected to flow cytometry analysis as described in (A). Shown are the MFI values of CD80 and CD86 expression (upper) and the percentages of CD80- and CD86-positive cells (lower) gated for HER2-positive cells. *p < 0.05 compared with corresponding control.
Discussion
Our data confirm our hypothesis and support the model depicted in Fig. 8, wherein the binding of trastuzumab to HER2 engages NK cells, resulting in secretion of IFNγ, which upregulates HLA-ABC and CD80/CD86 expression. These activities of trastuzumab may contribute to the overall antitumor activity of trastuzumab through eliciting a cytotoxic T-cell-mediated response to targeted cancer cells. This effect would be in addition to trastuzumab's well-documented inhibition of HER2 downstream cell signaling and induction of antibody-dependent cell-mediated cytotoxicity.
Figure 8.
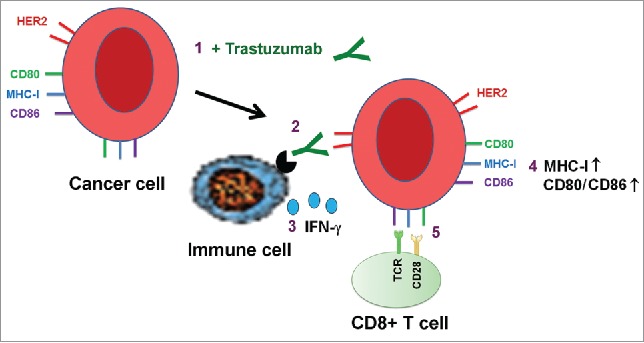
Proposed model of trastuzumab-induced upregulation of expression of HLA-ABC and CD80/CD86 T cell costimulatory molecules in cancer cells. Binding of trastuzumab to HER2 on breast cancer cell surface engages immune cells through interaction with the antibody's Fc fragment, leading to secretion of IFNγ, which upregulates the expression of MHC-I and CD80/CD86 molecules on targeted cells. The numbers show the sequence of events in the proposed model.
Loss or downregulation of HLA class I antigens in tumor cells has been observed in a variety of human malignancies and represents an important mechanism by which cancer escapes the immune system. A few earlier studies showed that HER2 regulates the components of the MHC class I antigen processing machinery and showed that there was an inverse correlation between HER2 and HLA-ABC expression following siRNA-mediated knockdown of HER2 expression in some breast cancer cell lines (SKBR3 and MCF7-HER2) and some non-breast cancer cell lines.20-22,33,34 In our study, we examined the effect of HER2 knockdown in five HER2-overexpressing cell lines using five different siRNAs. We did not find a significant inverse correlation between the levels of expression of HER2 and HLA-ABC except with one particular siRNA. Possible reasons for the difference in results between our study and previous studies could be differences in the cell types and the HER2 sequences targeted by siRNA.20-22 In our study, we found that treatment of the cells with siRNA#1, which knocked down HER2 and increased expression of HLA-ABC, also upregulated expression of STAT1, a known major transcription factor regulating HLA-ABC expression.27 However, we did not find STAT1 upregulation following treatment with any of the other four siRNAs, suggesting a possible off-target effect of siRNA#1. Further, our finding that treatment with the HER2 kinase inhibitor lapatinib alone did not increase but in fact decreased HLA-ABC expression supports lack of an inverse correlation between HER2 kinase activity and HLA-ABC expression. Whereas inhibition of the MAPK pathway, which is downstream of HER2, may increase HLA-ABC expression, as reported by others,35 treatment of cells with lapatinib can inhibit multiple pathways downstream of HER2 in addition to the MAPK pathway, one of which is the STAT1 pathway. Indeed, we found that the decrease in HLA-ABC expression induced by lapatinib was accompanied by a decrease in the level of phosphorylated STAT1.
One recent study showed that trastuzumab downregulates HER2 in HER2-overexpressing breast cancer cells co-cultured with PBMC, and the effect was mediated through engagement of PBMC by the antibody to secrete increased amounts of IFNγ, which activated STAT1, leading to transcription inhibition of HER2 expression.30 Our current study expanded this previous work by demonstrating that antibody-mediated increase in IFNγ production upregulated HLA-ABC expression. Because our data from lapatinib and HER2 siRNA treatment of the cell panel indicate that inhibition of HER2 kinase activity or knockdown of HER2 expression did not increase HLA-ABC expression, it is unlikely that the increase in HLA-ABC expression by trastuzumab in the presence of PBMC directly resulted from downregulation of HER2. However, additional separate studies are needed to determine whether the two events are somewhat linked.
Another novel finding in the current study is that the expression of T cell costimulatory molecules, mainly CD86, was also regulated by trastuzumab when breast cancer cells were co-cultured with PBMC. CD80 was not detectable in the cell lines used in the current study (BT474 and HCC1954); however, we did find increases in the levels of both murine CD80 and murine CD86 when nude mice bearing human HER2-overexpressing mammary tumors were treated with trastuzumab.
Recent studies have shown that activation of the PD-1/PD-L1 immune-checkpoint pathway plays an important role in tumor-induced immunosuppression. It is noteworthy that IFNγ is known to upregulate PD-L1.36 Trastuzumab-mediated induction of IFNγ in the presence of PBMC could therefore also upregulate PD-L1 level that may partially offset the pro-antitumor effect of upregulation of HLA-ABC and CD86 expression by trastuzumab. Indeed, an earlier animal study showed that the combination of anti-neu antibody with anti-PD-1 antibody enhanced antitumor activity in immunocompetent mice.37 We are currently investigating these issues.
In summary, our current work provides evidence that trastuzumab upregulates expression of HLA-ABC and T cell costimulatory molecules in HER2-overexpressing breast cancer cells in the presence of PBMC, which supports the view that T-cell-mediated immune responses are involved in trastuzumab-mediated antitumor effects. It has been reported that patients whose tumors have a better response to trastuzumab have a higher level of tumor-infiltrating lymphocytes, supporting a role of T-cell-mediated response in contributing to the overall response to trastuzumab.13,14 The lack of proper response to trastuzumab seen in treatment-resistant patients may be due in part to suppression of T cell responses. Novel approaches aiming to enhance T-cell-mediated adaptive immune response may therefore improve the outcome of patients treated with trastuzumab.
Materials and methods
Reagents
Trastuzumab, bevacizumab, and rituximab (Genentech) were obtained from the ambulatory pharmacy of The University of Texas MD Anderson Cancer Center.
Cell lines and cell culture
Human breast cancer cell lines (BT474, HCC1954, MDA-MB-231, MDA-MB-361, MDA-MB-453, MCF7, MCF7-HER18, SKBR3, SUM190, T47D, and ZR75B) and HEK293 cells were maintained in Dulbecco's modified Eagle's medium/F12 medium (50/50, v/v) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin in a 5% CO2 atmosphere at 37°C as described previously.38,39
Western blot analysis
Cells were lysed in a lysis buffer containing 50 mM TrisHCl (pH 7.4), 150 mM NaCl, 0.5% Igepal CA-630, 50 mM NaF, 1 mM Na3VO4, 1 mM EGTA, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail (Sigma-Aldrich) and kept on ice for 15 min. The lysates were cleared by centrifugation, and the supernatants were collected. Equal amounts of protein lysate, as determined using the Pierce Coomassie Plus colorimetric protein assay (Thermo Fisher Scientific), were separated by SDS-polyacrylamide gel electro-phoresis, blotted onto nitrocellulose, and probed with primary antibodies against HER2 (Calbiochem) and tyrosine 1248-phosphorylated HER2 (Cell Signaling Technology); STAT1 and Y701-phosphorylated STAT1 (Cell Signaling Technology); STAT3 and Y705-phosphorylated STAT3 (Cell Signaling Technology); Akt and S473-phosphorylated Akt (Cell Signaling Technology); Erk (Santa Cruz Biotechnology) and T202/Y204-phosphorylated Erk (Cell Signaling Technology); and β-actin (Sigma-Aldrich). The signals were visualized using the enhanced chemiluminescence detection kit (GE Healthcare).
Cell preparation for fluorescence-activated cell sorting
Breast cancer cells were detached from culture dishes by incubation with a non-enzymatic citric acid-based buffer (0.135 M KCl, 0.15 M sodium citrate, 0.6 mM EDTA) or TrypLE reagent (Life Technologies) at 37°C until cells detached. Cells from mouse tumor tissues were prepared by mincing tumor tissues into small pieces and passing the samples into a fresh conical tube through a 70-µm mesh cell strainer. Single cell suspensions (0.5–1×106) were prepared in 100 µL of fluorescence-activated cell sorting buffer (0.5% BSA in PBS) and stained with fluorescently conjugated primary antibodies or secondary antibodies (5–20 µL in 100 µL) for 30 min at 4°C. An isotype-matched antibody was used as a control. Excess antibodies were washed away twice with fluorescence-activated cell sorting buffer prior to analysis under a BD Biosciences Canto II analyzer. Flow cytometric detection of HER2 in human breast cancer cells was performed by incubation of the cells with trastuzumab and FITC-conjugated anti-human IgG antibody (1–2 µL in 100 µL) for 30 min at 4°C. The fluorescently conjugated primary antibodies used in this study and the respective vendors were as follows: APC-conjugated anti-HLA-ABC antibody (clone W6/32, BioLegend); APC-conjugated anti-mouse H-2Kd antibody (clone SF1-1.1.1, eBioscience); phycoerythrin-Cy7 (PE-Cy7)-conjugated anti-CD56 (clone MY31), APC-conjugated anti-CD14 (clone 61D3), and FITC-conjugated anti-CD3 antibodies (clone SK7, Tonbo Biosciences); PE-conjugated anti-human CD80 (clone 2D10) and PE-Cy7-conjugated anti-human CD86 (clone IT2.2) antibodies (BioLegend); APC-conjugated anti-mouse CD80 (clone 16-10A1) and PE-conjugated anti-mouse CD86 (clone GL-1) antibodies (Tonbo Biosciences); and IFNγ-neutralizing antibody (clone NIB42, eBioscience). FITC- or PE-labeled Annexin-V antibody was obtained from BD Biosciences PharMingen.
Knockdown of HER2 gene expression by siRNA
HER2-targeted siRNA (target DNA sequence #1, GGGAAACCTGGAACTCACC; #2, CATCAAAGTGTTGAGGGAA; #3, GGACATCTTCCACAAGAAC; #4, GCAGTTACCAGTGCCAATA; and #5, CAGAATGGCTCAGTGACCT) and control siRNA were purchased from Sigma-Aldrich. The siRNA (200 pmol) and Lipofectamine 2000 (5 μL, Life Technologies) were mixed in 100 μL of minimal essential medium (Opti-MEM, Life Technologies) for 15 min, and the siRNA-Lipofectamine 2000 mixture was added into the culture medium of cells seeded at 3×105 cells/well in a 12-well plate. Six hours later, the medium was replaced with regular medium, and the cells were cultured for an additional 72 h before detection of HER2 expression knockdown by flow cytometry.
Co-culture experiments with PBMC
PBMC from healthy donors were isolated by Ficoll-Paque PLUS (GE Healthcare Life Sciences) gradient centrifugation as described previously.40 Cancer cells were incubated with PBMC at a ratio of 1:10 or 1:5 (cancer cells:PBMC) for 48 h. At the end of co-culture experiments, conditioned medium was collected, any PBMC in suspension were washed away with PBS, and the cancer cells from the co-culture were harvested, stained with the desired fluorescently conjugated primary antibody, and subjected to flow cytometry analysis.
ELISA
IFNγ in conditioned medium from the co-culture experiments was analyzed with a human IFNγ ELISA kit from BioLegend. The procedure was performed according to the protocol provided by the vendor. In brief, anti-IFNγ capture antibodies were coated to the wells in a 96-well plate in carbonate buffer and incubated overnight at 4°C. The wells were blocked with PBS containing 10% fetal bovine serum for 1 h at room temperature, and then 100 µL of conditioned medium (diluted if necessary) was added and the wells were incubated for an additional 2 h at room temperature. IFNγ detection antibody was then added. Beginning 1 h later, the wells were incubated for 30 min with HRP-conjugated biotin, and then TMB (3,3′,5,5′-tetramethylbenzidine) substrate was added. The wells were washed three to five times between each step with PBS containing 0.05% Tween-20. The 96-well plates were read at 450 nm for optical density using an Omega microplate reader.
In vivo experiments
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at MD Anderson Cancer Center. Swiss female nude mice (5–6 weeks old) were obtained from the colony facility maintained by the Department of Experimental Radiation Oncology at MD Anderson Cancer Center. 4T1 mouse mammary tumor cells were infected with a pLEX-based recombinant lentivirus containing human HER2. Stable 4T1 cells expressing human HER2 (4T1/HER2, 2×106 cells/mouse) were implanted in the mammary fat pad of Swiss nude mice.
Statistical analysis
Each experiment was repeated at least three times, and the mean values with standard error of the mean are presented. A two-tailed unpaired Student's t test was used to compare two groups of independent samples. For correlation analysis, Pearson's correlation coefficients were computed. p < 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the staff of the Flow Cytometry and Cellular Imaging Core Facility for technical assistance and thank Stephanie Deming of the Department of Scientific Publications at MD Anderson Cancer Center for editorial assistance.
Funding
This work was supported in part by the Breast Cancer Research Foundation and by US National Institutes of Health (NIH) R01 awards CA179015 and CA129036. The work was also supported in part by the NIH through MD Anderson's Cancer Center Support Grant, CA016672.
References
- 1.Slamon DJ. Proto-oncogenes and human cancers ; editorial. N Engl J Med 1987; 317:955-7; PMID:3627214; http://dx.doi.org/ 10.1056/NEJM198710083171509 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235:177-82; PMID:3798106; http://dx.doi.org/ 10.1126/science.3798106 [DOI] [PubMed] [Google Scholar]
- 3.Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med 2005; 353:1734-6; PMID:16236745; http://dx.doi.org/ 10.1056/NEJMe058196 [DOI] [PubMed] [Google Scholar]
- 4.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 2007; 357:39-51; PMID:17611206; http://dx.doi.org/ 10.1056/NEJMra043186 [DOI] [PubMed] [Google Scholar]
- 5.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 2006; 3:269-80; PMID:16683005; http://dx.doi.org/ 10.1038/ncponc0509 [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58:2825-31; PMID:9661897 [PubMed] [Google Scholar]
- 7.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, Paton V, Shak S, Lieberman G et al.. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol 1999; 17:2639-48; PMID:10561337 [DOI] [PubMed] [Google Scholar]
- 8.Liang K, Lu Y, Jin W, Ang KK, Milas L, Fan Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol Cancer Ther 2003; 2:1113-20; PMID:14617784 [PubMed] [Google Scholar]
- 9.Jacob J, Belin L, Pierga JY, Gobillion A, Vincent-Salomon A, Dendale R, Beuzeboc P, Campana F, Fourquet A, Kirova YM. Concurrent administration of trastuzumab with locoregional breast radiotherapy: long-term results of a prospective study. Breast Cancer Res Treat 2014; 148:345-53; PMID:25318926; http://dx.doi.org/ 10.1007/s10549-014-3166-5 [DOI] [PubMed] [Google Scholar]
- 10.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:10742152; http://dx.doi.org/ 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 11.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, Cabaret V, Fermeaux V, Bertheau P, Garnier J et al.. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 2006; 94:259-67; PMID:16404427; http://dx.doi.org/ 10.1038/sj.bjc.6602930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M et al.. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18:160-70; PMID:20708157; http://dx.doi.org/ 10.1016/j.ccr.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology 2013; 2:e24720; PMID:24073365; http://dx.doi.org/ 10.4161/onci.24720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savas P, Loi S. Investigating the positive relationship between tumor-infiltrating lymphocytes and trastuzumab therapy. Immunotherapy 2014; 6:803-5; PMID:25290412; http://dx.doi.org/ 10.2217/imt.14.60 [DOI] [PubMed] [Google Scholar]
- 15.Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, Spadi R, Donadio M, Ciuffreda L, Matera L. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med 2008; 6:25; PMID:18485193; http://dx.doi.org/ 10.1186/1479-5876-6-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladoire S, Arnould L, Mignot G, Apetoh L, Rébé C, Martin F, Fumoleau P, Coudert B, Ghiringhelli F. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer 2011; 105:366-71; PMID:21750556; http://dx.doi.org/ 10.1038/bjc.2011.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer zum Buschenfelde C, Hermann C, Schmidt B, Peschel C, Bernhard H. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res 2002; 62:2244-7; PMID:11956077 [PubMed] [Google Scholar]
- 18.Cabrera T, Stern P, Garrido F. HLA and cancer In Ochoa AC. (ed.), Mechanisms of tumor escape from the immune response, 1st ed, London: Taylor & Francis, 2003: 1-17. [Google Scholar]
- 19.Fischer LK. On naming H2 haplotypes: functional significance of MHC class Ib alleles. Immunogenetics 1997; 46:53-62; PMID:9148789; http://dx.doi.org/ 10.1007/s002510050242 [DOI] [PubMed] [Google Scholar]
- 20.Choudhury A, Charo J, Parapuram SK, Hunt RC, Hunt DM, Seliger B, Kiessling R. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer 2004; 108:71-7; PMID:14618618; http://dx.doi.org/ 10.1002/ijc.11497 [DOI] [PubMed] [Google Scholar]
- 21.Herrmann F, Lehr HA, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B. HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res 2004; 64:215-20; PMID:14729627; http://dx.doi.org/ 10.1158/0008-5472.CAN-2522-2 [DOI] [PubMed] [Google Scholar]
- 22.Inoue M, Mimura K, Izawa S, Shiraishi K, Inoue A, Shiba S, Watanabe M, Maruyama T, Kawaguchi Y, Inoue S et al.. Expression of MHC Class I on breast cancer cells correlates inversely with HER2 expression. Oncoimmunology 2012; 1:1104-10; PMID:23170258; http://dx.doi.org/ 10.4161/onci.21056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Yang Y, Inoue H, Mori M, Akiyoshi T. The expression of costimulatory molecules CD80 and CD86 in human carcinoma cell lines: its regulation by interferon gamma and interleukin-10. Cancer Immunol Immunother 1996; 43:213-9; PMID:9003466; http://dx.doi.org/ 10.1007/s002620050324 [DOI] [PubMed] [Google Scholar]
- 24.Zheng Z, Takahashi M, Aoki S, Toba K, Liu A, Osman Y, Takahashi H, Tsukada N, Suzuki N, Nikkuni K et al.. Expression patterns of costimulatory molecules on cells derived from human hematological malignancies. J Exp Clin Cancer Res 1998; 17:251-8; PMID:9894758 [PubMed] [Google Scholar]
- 25.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat 1992; 24:85-95; PMID:8095168; http://dx.doi.org/ 10.1007/BF01961241 [DOI] [PubMed] [Google Scholar]
- 26.Liang K, Esteva FJ, Albarracin C, Stemke-Hale K, Lu Y, Bianchini G, Yang CY, Li Y, Li X, Chen CT et al.. Recombinant human erythropoietin antagonizes trastuzumab treatment of breast cancer cells via Jak2-mediated Src activation and PTEN inactivation. Cancer Cell 2010; 18:423-35; PMID:21075308; http://dx.doi.org/ 10.1016/j.ccr.2010.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min W, Pober JS, Johnson DR. Kinetically coordinated induction of TAP1 and HLA class I by IFN-gamma: the rapid induction of TAP1 by IFN-gamma is mediated by Stat1 alpha. J Immunol 1996; 156:3174-83; PMID:8617938 [PubMed] [Google Scholar]
- 28.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J et al.. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009; 28:803-14; PMID:19060928; http://dx.doi.org/ 10.1038/onc.2008.432 [DOI] [PubMed] [Google Scholar]
- 29.Kono K, Sato E, Naganuma H, Takahashi A, Mimura K, Nukui H, Fujii H. Trastuzumab (Herceptin) enhances class I-restricted antigen presentation recognized by HER-2/neu-specific T cytotoxic lymphocytes. Clin Cancer Res 2004; 10:2538-44; PMID:15073134; http://dx.doi.org/ 10.1158/1078-0432.CCR-03-0424 [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Fan X, Meng W, Deng H, Zhang N, An Z. Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res 2014; 16:R33; PMID:24693969; http://dx.doi.org/ 10.1186/bcr3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrel S, Schmidt-Kessen A, Giuffre L. Recombinant interferon-gamma can induce the expression of HLA-DR and -DC on DR-negative melanoma cells and enhance the expression of HLA-ABC and tumor-associated antigens. Eur J Immunol 1985; 15:118-23; PMID:3918869; http://dx.doi.org/ 10.1002/eji.1830150204 [DOI] [PubMed] [Google Scholar]
- 32.Giguere JF, Bounou S, Paquette JS, Madrenas J, Tremblay MJ. Insertion of host-derived costimulatory molecules CD80 (B7.1) and CD86 (B7.2) into human immunodeficiency virus type 1 affects the virus life cycle. J Virol 2004; 78:6222-32; PMID:15163715; http://dx.doi.org/ 10.1128/JVI.78.12.6222-6232.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maruyama T, Mimura K, Sato E, Watanabe M, Mizukami Y, Kawaguchi Y, Ando T, Kinouchi H, Fujii H, Kono K. Inverse correlation of HER2 with MHC class I expression on oesophageal squamous cell carcinoma. Br J Cancer 2010; 103:552-9; PMID:20628381; http://dx.doi.org/ 10.1038/sj.bjc.6605772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N et al.. T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer 2011; 128:390-401; PMID:20715101; http://dx.doi.org/ 10.1002/ijc.25613 [DOI] [PubMed] [Google Scholar]
- 35.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, Yong WP, Fujii H, Seliger B, Kiessling R et al.. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol 2013; 191:6261-72; PMID:24244023; http://dx.doi.org/ 10.4049/jimmunol.1301597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muhlbauer M, Fleck M, Schutz C, Weiss T, Froh M, Blank C, Schölmerich J, Hellerbrand C. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J Hepatol 2006; 45:520-8; PMID:16876901; http://dx.doi.org/ 10.1016/j.jhep.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 37.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 2011; 108:7142-7; PMID:21482773; http://dx.doi.org/ 10.1073/pnas.1016569108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu H, Liang K, Lu Y, Fan Z. The anti-EGFR antibody cetuximab sensitizes human head and neck squamous cell carcinoma cells to radiation in part through inhibiting radiation-induced upregulation of HIF-1alpha. Cancer Lett 2012; 322:78-85; PMID:22348829; http://dx.doi.org/ 10.1016/j.canlet.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, Sturgis EM, Ow TJ, Lotan R, Carey TE et al.. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clin Cancer Res 2011; 17:7248-64; PMID:21868764; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck A, Wurch T, Bailly C, Corvaia N. Strategies and challenges for the next generation of therapeutic antibodies. Nat Rev Immunol 2010; 10:345-52; PMID:20414207; http://dx.doi.org/ 10.1038/nri2747 [DOI] [PubMed] [Google Scholar]