ABSTRACT
CD137 is expressed on activated T cells and NK cells, among others, and is a potent co-stimulator of antitumor immune responses. CD137 ligand (CD137L) is expressed by antigen presenting cells (APC), and CD137L reverse signaling into APC enhances their activity. CD137–CD137L interactions as main driver of type 1, cell-mediated immune responses explains the puzzling observation that CD137 agonists which enhance antitumor immune responses also ameliorate autoimmune diseases. Upon co-stimulation by CD137, Th1 CD4+ T cells together with Tc1 CD8+ T cells and NK cells inhibit other T cell subsets, thereby promoting antitumor responses and mitigating non-type 1 auto-immune diseases.
KEYWORDS: CD137, T cell polarization, Tc1, Th1
Abbreviations
- AML
Acute myeloid leukemia
- APC
antigen presenting cells
- CAR
chimeric antigen receptor
- CD137L
CD137 ligand
- CLL
chronic lymphocytic leukemia
- DC
dendritic cell
- GVHD
graft versus host disease
- NK
natural killer cell
- Tc cell
cytotoxic T cell
- Th cell
helper T cell.
CD137 agonists enhance type 1, cell-mediated immune responses
CD137 (4-1BB, TNFRSF9) is increasingly recognized as a powerful inducer of antitumor immune responses. The potency of CD137 co-stimulation in enabling the immune system to eliminate tumors has been documented in a wide plethora of murine tumor models including mastocytoma, sarcoma, colon carcinoma, melanoma and lymphoma.1-3 In most studies, agonistic anti-CD137 antibodies were used to initiate CD137 signaling. In addition, a soluble recombinant CD137L protein 4 and a CD137-specific aptamer 5 have also been successfully tested as CD137 agonists in murine tumor models. Similarly, transgenic CD137L expression on lymphoma and Ewing's tumor cells induced the expansion of tumor-specific cytotoxic T cells and limited tumor growth and metastatic spread.6,7 CD137 agonists also work synergistically with cancer vaccines and immune check point inhibitors in boosting anticancer immune responses.8,9
CD137 was first cloned from T cells, on which its expression is activation-dependent.10,11 CD137 is expressed at higher levels on CD8+ T cells than on CD4+ T cells, and it mainly co-stimulates CD8+ T cells.12 Crosslinking of CD137 strongly enhances proliferation, IFNγ secretion and the cytolytic activity of T cells. T cell co-stimulation through CD137 occurs during cognate interaction of T cells with APC. Since only T cells that have been activated through the T cell receptor express CD137, T cell co-stimulation through CD137 is antigen-specific.13 This restricted expression of CD137 on antigen-specific T cells endows CD137 agonists with a high degree of specificity. Thus, the selective expression of CD137 on antigen-specific T cells may possibly allow a prescreening of patients who are likely to respond to a CD137 agonist-based immunotherapy.
The encouraging results in preclinical tumor models soon led to the humanization of existing murine anti-CD137 antibodies, as well as the screening of fully human antibodies, to be used in human cancer patients.14 However, the first clinical trials with agonistic anti-CD137 antibodies were discontinued in phase II after resulting in severe liver toxicities and deaths in several cancer patients.15
A more in-depth understanding of the CD137 biology, especially of the role of CD137 in natural killer (NK) cells has led to a revival of the use of anti-CD137 antibodies in cancer immunotherapy. CD137 can be expressed by NK cells and, similar as in T cells, the expression is activation-dependent.16 CD137 expression on NK cells is induced by Fc receptor signaling, and when stimulated by agonistic anti-CD137 antibodies, NK cells exert enhanced antitumor activity.17-19 Although CD137 expression on NK cells is not antigen-specific per se, the Fc receptor-mediated induction of CD137 expression only occurs at cells and tissues which have been opsonized by a therapeutic antibody, and therefore the specificity of the antibody determines in which tissues CD137 expression on NK cells is induced.
In the new approach, anti-CD137 antibodies are administered subsequent to the administration of antibodies that target antigens on the tumor cell surface (e.g. Her2/neu, epidermal growth factor receptor or CD20). The tumor antigen-targeting antibodies induce CD137 expression in adjacent NK cells, which upon further co-stimulation by anti-CD137 antibodies, exert enhanced tumor killing. Used in such combination therapies, and at lower concentrations, current data with agonistic anti-CD137 antibodies look promising.20
The potency of the CD137 co-stimulatory signal also became evident in the evolution of chimeric antigen receptors (CARs). The inclusion of the cytoplasmic CD137 signaling domain into the second generation CARs greatly enhanced their efficacy in augmenting CAR-mediated T cell responses by reducing exhaustion and enhancing the persistence of CAR T cells.21-23
In addition, the immune-stimulatory activity of agonistic anti-CD137 antibodies is also manifested in their strong enhancement of anti-viral immune responses, as demonstrated for influenza, pox virus, respiratory syncytia virus and human immunodeficiency virus among others.24,25 Even under physiological conditions CD137–CD137L interaction is required to mount a proper anti-viral immune response.26 Furthermore, agonistic anti-CD137 antibodies have been shown to accelerate rejection of cardiac and intestinal allografts as well as of skin transplants.27,28 The potent enhancement of T cell and NK cell activity by CD137 signaling explains the antitumor effects of agonistic anti-CD137 antibodies in clinical applications.
Injection of an agonistic anti-CD137 antibody into otherwise untreated mice led to an increase in the CD8+ T cell number and a reduction in the B cell number. Furthermore, these mice developed liver pathology that was dependent on CD8+ T cells, IFNγ and TNF. This phenotype can be explained by the agonistic anti-CD137 antibody inducing a type 1, cell-mediated response.29
Two studies identified a time dependence in the therapeutic activity of agonistic anti-CD137 antibodies. Administration of anti-CD137 antibody to mice infected with lymphocytic choriomeningitis virus or influenza within the first three days of infection led to a complete collapse of anti-viral immunity, resulting in high mortality. In contrast, antibody administration 3 days post infection gave rise to a robust immune response resulting in an effective elimination of the viral load in all mice. It was found that antibody administration within the first 3 days post infection had caused a deletion of CD4+ and CD8+ T cells.30 Similarly, anti-CD137 antibody administration to mice with lymphoma xenografts within 3 days of tumor cell injection did not cause tumor rejection, while antibody administration at later time points resulted in an effective elimination of the tumors, and increased survival of the mice.31 This time dependence may indicate that a certain degree of T cell activation is required for a successful co-stimulation by CD137, otherwise T cell death may ensue. Alternatively, stimulation of CD137 on certain DC during priming of an immune response may cause T cell death.32 Although, the reasons for this time dependence of CD137 co-stimulation are not yet known, these findings are consistent with the notion that the CD137 signal in T cells promotes a Th1/Tc1 response, since it is type 1, cell-mediated immunity that is required for the clearance of viral infections and the elimination of tumors (Fig. 1).
Figure 1.
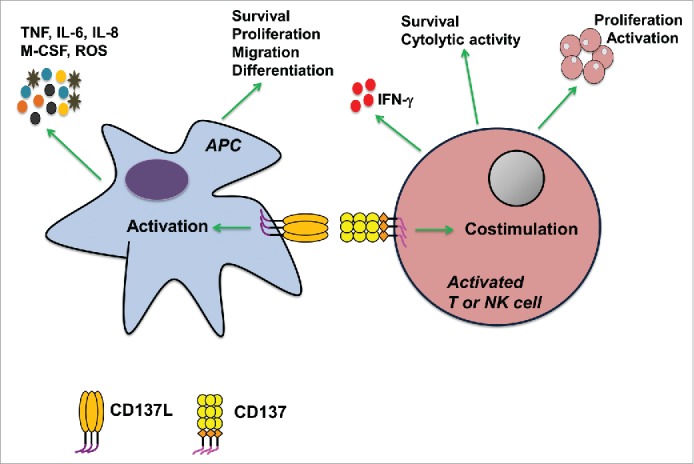
Schematic representation of bidirectional signal transduction for the CD137 receptor/ligand system and its effects on antigen presenting cells (APC), T cells and natural killer (NK) cells.
A time-dependent modulation of CD137–CD137L interaction has also been identified in murine graft vs. host disease (GVHD) models, where the administration of anti-CD137 or anti-CD137L antibody elicits different effects on acute versus chronic GVHD. Neutralization of CD137L by antagonistic anti-CD137L antibodies inhibited acute GVHD by reducing proliferation, IFNγ secretion and cytotoxicity of CD8+ T cells, which reduced mortality in the mice. However, inhibition of CD137L, in chronic GVHD exacerbated pathology. In chronic GVDH, similarly as in acute GVHD, the CD8+ T cell activity was inhibited, but the CD4+ T cell response was increased. This resulted in an increased number of IL-4-expressing CD4+ T cells, and promoted a Th2 cell differentiation and an early production of auto-antibodies.33 In contrast, and as one would expect, stimulation of CD137 by agonistic antibodies increased acute GVHD, resulting in increased mortality,34 whereas CD137 stimulation ameliorated chronic GVHD by eliminating CD4+ T cells via activation-induced cell death.35
The data from the murine GVHD models demonstrate that CD137–CD137L interaction during acute GVHD exacerbate disease activity by promoting type 1 immunity. In contrast, chronic GVHD, which is mediated to a larger extent by a humoral response,36 is ameliorated by the CD137 agonist-induced cellular response. Therefore, the studies on GVHD provide additional evidence that CD137–CD137L interaction drives a type 1, cell-mediated immune response.
Reverse CD137 ligand signaling in APC strengthens type 1, cell-mediated immune responses
The CD137 receptor/ligand system has the ability to signal bidirectionally, a characteristic shared with several other members of the tumor necrosis factor receptor/ligand families (Fig. 1). The molecular basis of bidirectional signaling is that CD137L, just as CD137, is expressed as a transmembrane protein on the cell surface, which allows it to transmit signals into the cells it is expressed on, a process referred to as reverse signaling.37 Such bidirectional interaction allows for a two-way exchange of signals between the receptor- and ligand-bearing cells, and thus a simultaneous fine-tuning and modulation of their activities.
CD137L is expressed by all types of APC, and it delivers a stimulatory signal into APC, referred to as reverse CD137L signaling (Fig. 1). In monocytes, the reverse CD137L signal induces activation and the secretion of pro-inflammatory cytokines such as TNF, IL-6 and IL-8, while it inhibits the anti-inflammatory cytokine IL-10.38 Furthermore, reverse CD137L signaling results in increased survival and proliferation of monocytes 39,40 and stimulates their migration and extravasation.41,42 These activities lead to an increased influx of inflammatory monocytes into tissues.
When monocytes encounter CD137-expressing cells in the tissue, reverse CD137L signaling into monocytes may induce their differentiation to inflammatory dendritic cells (DC).43,44 These CD137L-stimulated DC (in short, CD137L-DC) are strong inducers of Th1/Tc1 immune responses which potently stimulate the proliferation of T cells that produce high levels of IFNγ. CD137L-DC are 2–3 times more potent than GM-CSF + IL-4 derived DC in activating allogeneic T cells 44 and in enhancing the cytolytic activity of autologous T cells in an antigen-specific manner.45 Reverse CD137L signaling also induces the maturation of monocyte-derived DC generated by GM-CSF + IL-4.46 Therefore, monocytes that have migrated to an inflamed tissue under the influence of reverse CD137L signaling, are prone to differentiate to inflammatory DC, also under the influence of reverse CD137L signaling.
The influence of reverse CD137L signaling on myelopoiesis is not restricted to mature cells. In haematopoietic progenitor cells, reverse CD137L signaling induces myelopoiesis,47,48 which occurs especially during infections. This allows for the generation of more inflammatory APC which can assist in the elimination of the pathogens.49 CD137L signaling in DC induces the secretion of IL-12p70 but has no effect on the secretion of IL-5 and IL-10, thus indicating that it promotes polarization toward Tc1.46 These data on reverse CD137L signaling are based on in vitro experiments, and await confirmation in vivo. However, current data suggest that forward signaling through CD137 as well as reverse signaling through CD137L promote and strengthen cell-mediated, type 1 immune responses.
Disabling CD137 – CD137L driven type 1 immune responses facilitates the escape of tumors from immune surveillance
An inflammatory environment is known to favor cancer progression. Specifically, it is the chronic inflammation associated with a type 2 immune response that promotes cancer cell survival while type 1 immune responses, which are associated with acute inflammation, are detrimental to cancer.50 A type 1, cell-mediated immune response is the main immune threat for malignant cells. Therefore, blunting a type 1 immune response is essential for a cancer to escape immune surveillance. Since CD137–CD137L interaction is one of the main drivers of type 1 immune responses, it is not surprising that cancers have developed diverse mechanisms to inhibit CD137 and CD137L signaling. In chronic lymphocytic leukemia (CLL) high levels of soluble CD137 are secreted 51 which is antagonistic, as it binds to CD137L, thereby blocking the interaction of CD137L on APC with CD137 on the surface of activated T and NK cells.52 Thus, high levels of soluble CD137 decrease CD137-mediated costimulation of an anti-CLL immune response.
A different mechanism to disable CD137 co-stimulation is found in Hodgkin lymphoma. Hodgkin and Reed Sternberg cells, the malignant cells in Hodgkin lymphoma, express CD137 ectopically, which allows them to transfer CD137 to CD137L-expressing APC by trogocytosis. The CD137-CD137L complex is then internalized and undergoes degradation in the APC, resulting in the downregulation of CD137L, a major type 1 immunity-inducing factor, on the cell surface of APC, and thereby reduces an anti-Hodgkin lymphoma immune response.53 The trogocytic transfer of CD137 and the subsequent removal of T cell co-stimulation is a likely mechanism that regulates the activity of the CD137–CD137L system under physiological conditions, but is usurped by Hodgkin and Reed Sternberg cells in Hodgkin lymphoma to escape immune surveillance.54
Further evidence for a role of CD137 and CD137L in antitumor immunity comes from a study which found that CD137L-deficient mice are prone to develop B cell lymphomas, and concluded that CD137L acts as a tumor suppressor. It identified as underlying mechanism the enhanced expression of growth-promoting genes in germinal center B cells that are normally inhibited by CD137L.55 Whether a reduced type 1, cell-mediated immune response, caused by the lack of CD137L, contributed to the emergence of lymphomas was not investigated in that study.
Reverse CD137L signaling also impacts the development of acute myeloid leukemia (AML). When CD137L on AML cells is cross linked by CD137 on NK cells, reverse CD137L signaling into AML cells induces IL-10 and TNF, which inhibit NK cell activity, and facilitate escape of AML cells from immune surveillance.56 Another study demonstrated reverse CD137L signaling into AML cells induces differentiation, resulting in reduced proliferation, and suggested this approach to be explored as a novel differentiation therapy for AML.57 In summary, the data from the role of CD137 and CD137L in cancer further support the notion of CD137–CD137L interaction promoting type 1, cell-mediated immune responses.
CD137 agonists ameliorate autoimmune disease in murine models
CD137–CD137L interaction as a main driver of type 1, cell-mediated immune responses explains the puzzling observation that the very same agonistic anti-CD137 antibodies which enhance antitumor and anti-pathogen immune responses and transplant rejections, are also able to ameliorate autoimmune diseases in many murine models, including experimental autoimmune encephalomyelitis,58 systemic lupus erythematosus,59,60 collagen-induced arthritis,61,62 uveoretinitis,63 allergic airway inflammation and asthma,64,65 inflammatory bowel disease 66 and chronic graft vs. host disease.35
Several mechanisms for the immune inhibitory effects of agonistic anti-CD137 antibodies in autoimmune disease models have been demonstrated. These include the induction of CD11c+ CD8+ T cells that stimulate indoleamine-pyrrole 2,3-dioxygenase (IDO) in APC,62 the enhancement of regulatory T cell activity 67 and of regulatory DC activity,68 CD4+ T cell inhibition 64,69 or elimination 35 and immune deviation to the novel subset of Eomesodermin+ helper T cells.70 However, the key question still remains unresolved: Under what conditions does an agonistic anti-CD137 antibody induce these immune inhibitory mechanisms instead of activating immune responses that lead to tumor regression, pathogen clearance or transplant rejection? Why are agonistic anti-CD137 antibodies not inducing immune inhibitory mechanisms in a tumor setting, and why are they not enhancing immune activity in autoimmune disease?
The CD137–CD137L working model CD137- and CD137L-mediated T cell polarization
There is consensus in the published literature that CD137 signals promote CD8+ T cell proliferation and activation. In contrast, different and conflicting results of CD137 signaling in CD4+ T cells have been reported, ranging from activation to inhibition and induction of cell death.71 Despite that, little to no distinction has been made between different CD4+ T cell subsets, even though the immune activities of CD4+ T cells can vary profoundly depending on how they are polarized. We propose that it is the Th1-polarized CD4+ T cells that are co-stimulated by CD137 signals which—together with activated Tc1 CD8+ T cells and activated NK cells—inhibit other T cell subsets (Fig. 2). This notion is consistent with the observation that agonistic anti-CD137 antibodies suppressed the induction of Th2-dependent antibodies to sheep red blood cells in mice 72 and to ovalbumin in non-human primates.73
Figure 2.
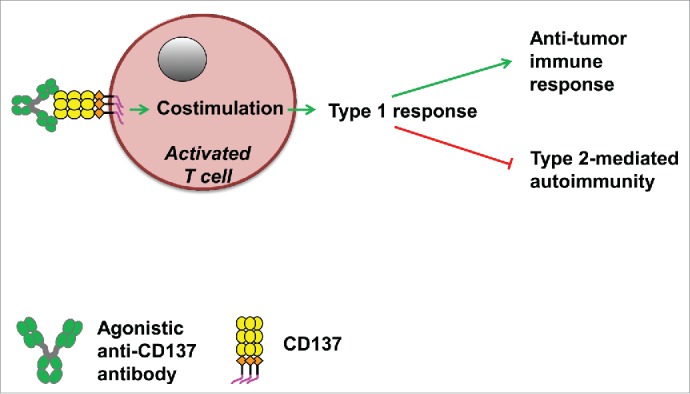
The effects of agonistic anti-CD137 antibodies on type 1 polarization which promotes (green arrows) anticancer immune responses and inhibits (red lines) type 2-mediated autoimmune reactions.
In type 2-dominated autoimmune diseases, such as systemic lupus erythematosus, CD137 signaling into T cells drives a type 1 polarization, with subsequent IFNγ secretion, which in turn inhibits immunoglobulin synthesis and reduces the disease index. This has been demonstrated in two different murine lupus models, the CD95-deficient lymphoproliferative (lpr) mice 59 and the New Zealand Black x New Zealand White F1 mice.74 In collagen-induced arthritis, antibodies against collagen II play an important role in pathogenesis, and agonistic anti-CD137 antibodies inhibited auto-antibody production and reduced clinical scores.61,62 A similar therapeutic effect of agonistic anti-CD137 antibodies has been reported for type 2-driven allergic inflammation of the lung, where CD137 stimulation resulted in a decrease in the levels of the Th2 cytokines IL-4 and IL-5, and of IgE, as well as a reduced T cell and eosinophil infiltration into the lung and airways.64,65 Sun et al., 2006 claim that an agonistic anti-CD137 antibody directly inhibits Th2 CD4+ T cells.64 However, this claim is challenging to prove since Th2 CD4+ T cells cannot readily be separated from Th1 CD4+ T cells.
Inhibition of type 2 immune responses by agonistic anti-CD137 antibodies may occur by several mechanisms, such as through stimulation of existing Th1-polarized CD4+ T cells and subsequent inhibition (via induction of anergy or apoptosis) of Th2-polarized CD4+ T cells by IFNγ and other Th1-promoting factors. Another possible mechanism could be the prevention of Th2 cell polarization by anti-CD137 antibody during priming of naïve CD4+ T cells.
For autoimmune diseases which are mainly driven by a type 1 response, such as type 1 diabetes, it is predicted that the Th1/Tc1-promoting activity of an agonistic anti-CD137 antibody would exacerbate disease. Indeed, CD137 stimulation by an agonistic anti-CD137 single chain fragment worsened disease in non-obese diabetic (NOD) mice,75 whereas soluble CD137 prevented diabetes.76
This type 1 polarization by CD137–CD137L interaction also explains the seemingly contradictory observations in gene-modified mice, such as the exacerbation of lupus in the absence of CD137,77 and the inhibition of experimental auto-immune encephalomyelitis in the absence of CD137L.78 In the lupus-inflicted lpr, CD137−/− mice, the lack of the CD137 signal prevented a type 1-driven counterbalance of the pathogenic type 2 immune response. Conversely, in the CD137L−/− mice, the lack of the CD137 signal restricted the development of a pathogenic type 1 immune response which could have caused experimental autoimmune encephalomyelitis.
Predictions and future directions
A prediction of this model, i.e. CD137–CD137L interaction being a pivotal driver for type 1, cell-mediated immune responses, is that neutralization of CD137L would also ameliorate auto-immune diseases (Fig. 3). This prediction is supported by studies in which an antagonistic anti-CD137L antibody has been shown to reduce collagen-induced arthritis 62 and LPS-induced sepsis 79 in mice. Furthermore, blockade of CD137L by a recombinant CD137-Fc fusion protein inhibited allograft rejection by CD8+ T cells.28 Both, agonistic anti-CD137 antibodies as well as antagonistic anti-CD137L antibodies can reduce inflammatory signals through CD137L since both are able to block binding of CD137 to CD137L. But since agonistic anti-CD137 antibodies induce in addition a type 1 polarization, they should be more potent. This is exactly what has been observed experimentally in collagen-induced arthritis in which an agonistic anti-CD137 antibody proved to be significantly more effective than an antagonistic anti-CD137L antibody in reducing pro-inflammatory cytokine release, proliferation of collagen-specific T cells and clinical scores.62
Figure 3.
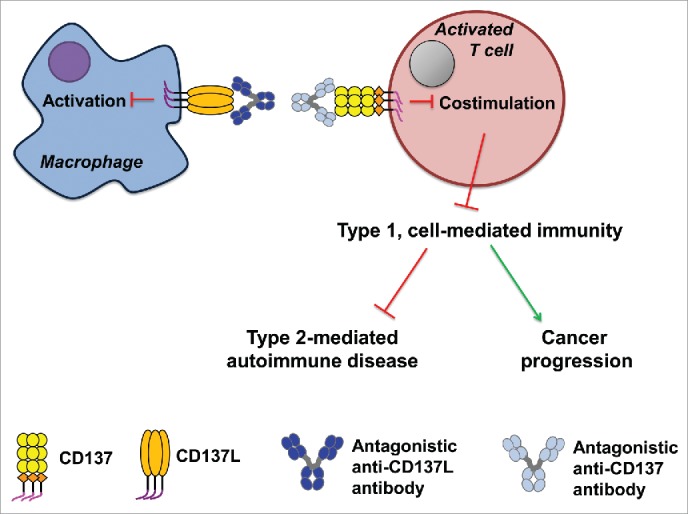
The effects of inhibition of CD137–CD137L interaction by antagonistic anti-CD137 or anti-CD137L antibodies on the bidirectional signal transduction for the CD137 receptor/ligand system is depicted exemplary for the case of an APC–T cell interaction.
An antagonistic anti-CD137 antibody is expected to block CD137–CD137L interaction, and to exert similar effects as an antagonistic anti-CD137L antibody, i.e. be less effective in ameliorating autoimmune diseases that have a strong type 2/B cell involvement than an agonistic anti-CD137 antibody (Fig. 3). However, for type 1-dominated auto-immune diseases, an antagonistic anti-CD137 or anti-CD137L antibody would be the preferred treatment. The same would be applicable for pathogen-induced type 1 immune pathologies, and for the prevention of transplant rejection. In these situations, an agonistic anti-CD137 antibody cannot be used as it would exacerbate the type 1 immunity-driven disease.
There are some observations that do not fit the model proposed here. For example, CD137 stimulation by an agonistic anti-CD137 antibody inhibited trinitrobenzene sulphonic acid (TNBS)—induced colitis in mice.66 Since TNBS-induced colitis is considered to be a type 1-mediated autoimmune disease, a worsening rather than an inhibition of the disease would have been expected by CD137 co-stimulation. Currently, the reason for this discrepancy is not known.
Due to the bidirectional signaling in the CD137 receptor/ligand system, engaging CD137 may not only enhance T cell and NK cell activity, but it also influences the activities of the CD137L-expressing cells. While enhancing T cell and NK cell activities, an agonistic anti-CD137 antibody can block access of CD137L to CD137, and thereby inhibit reverse signaling of CD137L into CD137L-expressing cells (Fig. 2). This would be predicted to occur based on the bidirectional signaling of the CD137–CD137L system although it has not yet been demonstrated experimentally. Many innate immune cell types are activated by reverse CD137L signaling. Hence, blocking this reverse signaling will reduce inflammation. Among adaptive immune cells, B cells are activated by reverse CD137L signaling which enhances their proliferation and immunoglobulin secretion.80 Immunoglobulins secreted by B cells are important contributors to inflammation, especially in autoimmune disease such as systemic lupus erythematosus and rheumatoid arthritis. Blocking reverse CD137L signaling into B cells is therefore expected to have a therapeutic effect in such diseases.
The data discussed here are based on more than two decades of research since CD137 was first discovered.10,11 They indicate that CD137–CD137L interactions are main drivers of type 1, cell-mediated immune responses which are essential for the elimination of malignant cells and intracellular pathogens. Manipulation of the CD137/CD137L system can also be therapeutically employed for preventing transplant rejection and for ameliorating type 2-driven autoimmune diseases.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grant NMRC/CBRG/0066/2014 from the National Medical Research Council, Singapore.
References
- 1.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med 1997; 3:682-5; PMID:9176498; http://dx.doi.org/ 10.1038/nm0697-682 [DOI] [PubMed] [Google Scholar]
- 2.Vinay DS, Kwon BS. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Reports 2014; 47:122-9; PMID:24499671; http://dx.doi.org/ 10.5483/BMBRep.2014.47.3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Chen L. Immunobiology of cancer therapies targeting CD137 and B7-H1/PD-1 cosignal pathways. Current topics in microbiology and immunology 2011; 344:245-67; PMID:20582531; http://dx.doi.org/ 10.1007/82_2010_81 [DOI] [PubMed] [Google Scholar]
- 4.Srivastava AK, Dinc G, Sharma RK, Yolcu ES, Zhao H, Shirwan H. SA-4-1BBL and monophosphoryl lipid A constitute an efficacious combination adjuvant for cancer vaccines. Cancer Res 2014; 74:6441-51; PMID:25252915; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1768-A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrand B, Berezhnoy A, Brenneman R, Williams A, Levay A, Kong LY, Rao G, Zhou S, Heimberger AB, Gilboa E. Targeting 4-1BB costimulation to the tumor stroma with bispecific aptamer conjugates enhances the therapeutic index of tumor immunotherapy. Cancer Immunol Res 2014; 2:867-77; PMID:24938283; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinn BA, DeBenedette MA, Watts TH, Berinstein NL. 4-1BBL cooperates with B7-1 and B7-2 in converting a B cell lymphoma cell line into a long-lasting antitumor vaccine. J Immunol 1999; 162:5003-10; PMID:10202049 [PubMed] [Google Scholar]
- 7.Zhang H, Merchant MS, Chua KS, Khanna C, Helman LJ, Telford B, Ward Y, Summers J, Toretsky J, Thomas EK et al.. Tumor expression of 4-1BB ligand sustains tumor lytic T cells. Cancer Biology Ther 2003; 2:579-86; PMID:14614331; http://dx.doi.org/22754760 10.4161/cbt.2.5.545 [DOI] [PubMed] [Google Scholar]
- 8.McGray AJ, Bernard D, Hallett R, Kelly R, Jha M, Gregory C, Bassett JD, Hassell JA, Pare G, Wan Y et al.. Combined vaccination and immunostimulatory antibodies provides durable cure of murine melanoma and induces transcriptional changes associated with positive outcome in human melanoma patients. Oncoimmunology 2012; 1:419-31; PMID:22754760; http://dx.doi.org/ 10.4161/onci.19534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houot R, Kohrt H. CD137 stimulation enhances the vaccinal effect of anti-tumor antibodies. Oncoimmunology 2014; 3:e941740; PMID:25610724; http://dx.doi.org/ 10.4161/21624011.2014.941740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. ProcNatlAcadSciUSA 1989; 86:1963-7; PMID:2784565; http://dx.doi.org/8262389 10.1073/pnas.86.6.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz H, Tuckwell J, Lotz M. A receptor induced by lymphocyte activation (ILA): a new member of the human nerve-growth-factor/tumor-necrosis-factor receptor family. Gene 1993; 134:295-8; PMID:8262389; http://dx.doi.org/ 10.1016/0378-1119(93)90110-O [DOI] [PubMed] [Google Scholar]
- 12.Takahashi C, Mittler RS, Vella AT. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J Immunol 1999; 162:5037-40; PMID:10227968 [PubMed] [Google Scholar]
- 13.Ye Q, Song DG, Poussin M, Yamamoto T, Best A, Li C, Coukos G, Powell DJ Jr. CD137 accurately identifies and enriches for naturally occurring tumor-reactive T cells in tumor. Clin Cancer Res 2014; 20:44-55; PMID:24045181; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulatory monoclonal antibodies in cancer therapy. Oncoimmunology 2014; 3:e27297; PMID:24701370; http://dx.doi.org/ 10.4161/onci.27297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Sem Oncol 2010; 37:508-16; PMID:21074066; http://dx.doi.org/9878117 10.1053/j.seminoncol.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 16.Melero I, Johnston JV, Shufford WW, Mittler RS, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol 1998; 190:167-72; PMID:9878117; http://dx.doi.org/ 10.1006/cimm.1998.1396 [DOI] [PubMed] [Google Scholar]
- 17.Kohrt HE, Houot R, Goldstein MJ, Weiskopf K, Alizadeh AA, Brody J, Müller A, Pachynski R, Czerwinski D, Coutre S et al.. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood 2011; 117:2423-32; PMID:21193697; http://dx.doi.org/ 10.1182/blood-2010-08-301945 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, Mueller A, Sagiv-Barfi I, Marabelle A, Lira R et al.. Targeting CD137 enhances the efficacy of cetuximab. J Clin Inv 2014; 124:2668-82; PMID:24837434; http://dx.doi.org/25949907 10.1172/JCI73014 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Kobayashi T, Doff BL, Rearden RC, Leggatt GR, Mattarollo SR. NKT cell-targeted vaccination plus anti-4-1BB antibody generates persistent CD8 T cell immunity against B cell lymphoma. Oncoimmunology 2015; 4:e990793; PMID:25949907; http://dx.doi.org/ 10.4161/2162402X.2014.990793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin Cancer Res 2015; 21:3113-20; PMID:25908780; http://dx.doi.org/ 10.1158/1078-0432.CCR-15-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, Campana D. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004; 18:676-84; PMID:14961035; http://dx.doi.org/ 10.1038/sj.leu.2403302 [DOI] [PubMed] [Google Scholar]
- 22.Campana D, Schwarz H, Imai C. 4-1BB chimeric antigen receptors. Cancer J 2014; 20:134-40; PMID:24667959; http://dx.doi.org/ 10.1097/PPO.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 23.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR et al.. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015; 21:581-90; PMID:25939063; http://dx.doi.org/ 10.1038/nm.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mbanwi AN, Watts TH. Costimulatory TNFR family members in control of viral infection: outstanding questions. Sem Immunol 2014; 26:210-9; PMID:24910294; http://dx.doi.org/12021777 10.1016/j.smim.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 25.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8+ T cell responses. Nat Immunol 2002; 3:536-41; PMID:12021777; http://dx.doi.org/ 10.1038/ni798 [DOI] [PubMed] [Google Scholar]
- 26.Lin GH, Sedgmen BJ, Moraes TJ, Snell LM, Topham DJ, Watts TH. Endogenous 4-1BB ligand plays a critical role in protection from influenza-induced disease. J Immunol 2009; 182:934-47; PMID:19124736; http://dx.doi.org/ 10.4049/jimmunol.182.2.934 [DOI] [PubMed] [Google Scholar]
- 27.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP et al.. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 1997; 186:47-55; PMID:9206996; http://dx.doi.org/ 10.1084/jem.186.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Guo Z, Dong Y, Kim O, Hart J, Adams A, Larsen CP, Mittler RS, Newell KA. Role of 4-1BB in allograft rejection mediated by CD8+ T cells. Am J Transplant 2003; 3:543-51; PMID:12752310; http://dx.doi.org/ 10.1034/j.1600-6143.2003.00088.x [DOI] [PubMed] [Google Scholar]
- 29.Niu L, Strahotin S, Hewes B, Zhang B, Zhang Y, Archer D, Spencer T, Dillehay D, Kwon B, Chen L et al.. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J Immunol 2007; 178:4194-213; PMID:17371976; http://dx.doi.org/ 10.4049/jimmunol.178.7.4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Maris CH, Foell J, Whitmire J, Niu L, Song J, Kwon BS, Vella AT, Ahmed R, Jacob J et al.. Immune suppression or enhancement by CD137 T cell costimulation during acute viral infection is time dependent. J Clin Invest 2007; 117:3029-41; PMID:17853940; http://dx.doi.org/ 10.1172/JCI32426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Houot R, Goldstein MJ, Kohrt HE, Myklebust JH, Alizadeh AA, Lin JT, Irish JM, Torchia JA, Kolstad A, Chen L et al.. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood 2009; 114:3431-8; PMID:19641184; http://dx.doi.org/ 10.1182/blood-2009-05-223958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Zhang Y, Niu L, Vella AT, Mittler RS. Dendritic cells and Stat3 are essential for CD137-induced CD8 T cell activation-induced cell death. J Immunol 2010; 184:4770-8; PMID:20351189; http://dx.doi.org/ 10.4049/jimmunol.0902713 [DOI] [PubMed] [Google Scholar]
- 33.Nozawa K, Ohata J, Sakurai J, Hashimoto H, Miyajima H, Yagita H, Okumura K, Azuma M. Preferential blockade of CD8(+) T cell responses by administration of anti-CD137 ligand monoclonal antibody results in differential effect on development of murine acute and chronic graft-versus-host diseases. J Immunol 2001; 167:4981-6; PMID:11673505; http://dx.doi.org/ 10.4049/jimmunol.167.9.4981 [DOI] [PubMed] [Google Scholar]
- 34.Kim W, Kim J, Jung D, Kim H, Choi HJ, Cho HR, Kwon B. Induction of lethal graft-versus-host disease by anti-CD137 monoclonal antibody in mice prone to chronic graft-versus-host disease. Biol Blood Marrow Transplant 2009; 15:306-14; PMID:19203721; http://dx.doi.org/ 10.1016/j.bbmt.2008.11.035 [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Choi WS, La S, Suh JH, Kim BS, Cho HR, Kwon BS, Kwon B. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood 2005; 105:2206-13; PMID:15522958; http://dx.doi.org/ 10.1182/blood-2004-06-2080 [DOI] [PubMed] [Google Scholar]
- 36.Kansu E. The pathophysiology of chronic graft-versus-host disease. Int J Hematol 2004; 79:209-15; PMID:15168586; http://dx.doi.org/ 10.1532/IJH97.04015 [DOI] [PubMed] [Google Scholar]
- 37.Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 2011; 89:21-9; PMID:20643812; http://dx.doi.org/ 10.1189/jlb.0510315 [DOI] [PubMed] [Google Scholar]
- 38.Langstein J, Michel J, Fritsche J, Kreutz M, Andreesen R, Schwarz H. CD137 (ILA/4-1BB), a member of the TNF receptor family, induces monocyte activation via bidirectional signaling. J Immunol 1998; 160:2488-94; PMID:9498794 [PubMed] [Google Scholar]
- 39.Langstein J, Schwarz H. Identification of CD137 as a potent monocyte survival factor. J Leukoc Biol 1999; 65:829-33; PMID:10380906 [DOI] [PubMed] [Google Scholar]
- 40.Langstein J, Michel J, Schwarz H. CD137 induces proliferation and endomitosis in monocytes. Blood 1999; 94:3161-8; PMID:10556203 [PubMed] [Google Scholar]
- 41.Drenkard D, Becke FM, Langstein J, Spruss T, Kunz-Schughart LA, Tan TE, Lim YC, Schwarz H. CD137 is expressed on blood vessel walls at sites of inflammation and enhances monocyte migratory activity. FASEB J 2007; 21:456-63; PMID:17167064; http://dx.doi.org/ 10.1096/fj.05-4739com [DOI] [PubMed] [Google Scholar]
- 42.Quek BZ, Lim YC, Lin JH, Tan TE, Chan J, Biswas A, Schwarz H. CD137 enhances monocyte-ICAM-1 interactions in an E-selectin-dependent manner under flow conditions. Mol Immunol 2010; 47:1839-47; PMID:20347151; http://dx.doi.org/ 10.1016/j.molimm.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 43.Ju S, Ge Y, Qiu H, Lu B, Qiu Y, Fu J, Liu G, Wang Q, Hu Y, Shu Y et al.. A novel approach to induce human DCs from monocytes by triggering 4-1BBL reverse signaling. Int Immunol 2009; 21:1135-44; PMID:19684160; http://dx.doi.org/ 10.1093/intimm/dxp077 [DOI] [PubMed] [Google Scholar]
- 44.Kwajah MMS, Schwarz H. CD137 ligand signaling induces human monocyte to dendritic cell differentiation. Eur J Immunol 2010; 40:1938-49; PMID:20432236; http://dx.doi.org/ 10.1002/eji.200940105 [DOI] [PubMed] [Google Scholar]
- 45.Harfuddin Z, Kwajah S, Chong Nyi Sim A, Macary PA, Schwarz H. CD137L-stimulated dendritic cells are more potent than conventional dendritic cells at eliciting cytotoxic T-cell responses. Oncoimmunology 2013; 2:e26859; PMID:24482752; http://dx.doi.org/ 10.4161/onci.26859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lippert U, Zachmann K, Ferrari DM, Schwarz H, Brunner E, Latif AH, Neumann C, Soruri A. CD137 ligand reverse signaling has multiple functions in human dendritic cells during an adaptive immune response. Eur J Immunol 2008; 38:1024-32; PMID:18395851; http://dx.doi.org/ 10.1002/eji.200737800 [DOI] [PubMed] [Google Scholar]
- 47.Jiang D, Chen Y, Schwarz H. CD137 induces proliferation of murine hematopoietic progenitor cells and differentiation to macrophages. J Immunol 2008; 181:3923-32; PMID:18768847; http://dx.doi.org/ 10.4049/jimmunol.181.6.3923 [DOI] [PubMed] [Google Scholar]
- 48.Jiang D, Yue PS, Drenkard D, Schwarz H. Induction of proliferation and monocytic differentiation of human CD34+ cells by CD137 ligand signaling. Stem Cells 2008; 26:2372-81; PMID:18566330; http://dx.doi.org/ 10.1634/stemcells.2008-0158 [DOI] [PubMed] [Google Scholar]
- 49.Tang Q, Jiang D, Alonso S, Pant A, Martinez Gomez JM, Kemeny DM, Chen L, Schwarz H. CD137 ligand signaling enhances myelopoiesis during infections. Eur J Immunol 2013; 43:1555-67; PMID:23519951; http://dx.doi.org/ 10.1002/eji.201243071 [DOI] [PubMed] [Google Scholar]
- 50.Palucka K, Coussens LM, O'Shaughnessy J. Dendritic cells, inflammation, and breast cancer. Cancer J 2013; 19:511-6; PMID:24270350; http://dx.doi.org/ 10.1097/PPO.0000000000000007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Furtner M, Straub RH, Kruger S, Schwarz H. Levels of soluble CD137 are enhanced in sera of leukemia and lymphoma patients and are strongly associated with chronic lymphocytic leukemia. Leukemia 2005; 19:883-5; PMID:15744355; http://dx.doi.org/ 10.1038/sj.leu.2403675 [DOI] [PubMed] [Google Scholar]
- 52.Shao Z, Sun F, Koh DR, Schwarz H. Characterisation of soluble murine CD137 and its association with systemic lupus. Mol Immunol 2008; 45:3990-9; PMID:18640726; http://dx.doi.org/ 10.1016/j.molimm.2008.05.028 [DOI] [PubMed] [Google Scholar]
- 53.Ho WT, Pang WL, Chong SM, Castella A, Al-Salam S, Tan TE, Moh MC, Koh LK, Gan SU, Cheng CK et al.. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res 2013; 73:652-61; PMID:23204227; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3849 [DOI] [PubMed] [Google Scholar]
- 54.Shao Z, Harfuddin Z, Pang WL, Nickles E, Koh LK, Schwarz H. Trogocytic CD137 transfer causes an internalization of CD137 ligand on murine APCs leading to reduced T cell costimulation. J Leukoc Biol 2015; PMID:25765680 [DOI] [PubMed] [Google Scholar]
- 55.Middendorp S, Xiao Y, Song JY, Peperzak V, Krijger PH, Jacobs H, Borst J. Mice deficient for CD137 ligand are predisposed to develop germinal center-derived B-cell lymphoma. Blood 2009; 114:2280-9; PMID:19608748; http://dx.doi.org/ 10.1182/blood-2009-03-208215 [DOI] [PubMed] [Google Scholar]
- 56.Baessler T, Charton JE, Schmiedel BJ, Grunebach F, Krusch M, Wacker A, Rammensee HG, Salih HR. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood 2010; 115:3058-69; PMID:20008791; http://dx.doi.org/ 10.1182/blood-2009-06-227934 [DOI] [PubMed] [Google Scholar]
- 57.Cheng K, Cheng Wong S, Ching Linn Y, Pock Ho L, Joo Chng W, Schwarz H. CD137 ligand signalling induces differentiation of primary acute myeloid leukaemia cells. Br J Haematol 2014; 165(1):134-44; PMID: 24428589; http://dx.doi.org/ 10.1111/bjh.12732 [DOI] [PubMed] [Google Scholar]
- 58.Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, Fu YX. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol 2002; 168:1457-65; PMID:11801689; http://dx.doi.org/ 10.4049/jimmunol.168.3.1457 [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Chen HM, Subudhi SK, Chen J, Koka R, Chen L, Fu YX. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nat Med 2002; 8:1405-13; PMID:12426559; http://dx.doi.org/ 10.1038/nm1202-796 [DOI] [PubMed] [Google Scholar]
- 60.Foell J, McCausland M, Burch J, Corriazzi N, Yan XJ, Suwyn C, O'Neil SP, Hoffmann MK, Mittler RS. CD137-mediated T cell co-stimulation terminates existing autoimmune disease in SLE-prone NZB/NZW F1 mice. Ann NY Acad Sci 2003; 987:230-5; PMID:12727643; http://dx.doi.org/ 10.1111/j.1749-6632.2003.tb06052.x [DOI] [PubMed] [Google Scholar]
- 61.Foell JL, ez-Mendiondo BI, Diez OH, Holzer U, Ruck P, Bapat AS, Hoffmann MK, Mittler RS, Dannecker GE. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunol 2004; 113:89-98; PMID:15312139; http://dx.doi.org/15448685 10.1111/j.1365-2567.2004.01952.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat Med 2004; 10:1088-94; PMID:15448685; http://dx.doi.org/ 10.1038/nm1107 [DOI] [PubMed] [Google Scholar]
- 63.Choi BK, Asai T, Vinay DS, Kim YH, Kwon BS. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. Cytokine 2006; 34:233-42; PMID:16899371; http://dx.doi.org/ 10.1016/j.cyto.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 64.Sun Y, Blink SE, Liu W, Lee Y, Chen B, Solway J, Weinstock J, Chen L, Fu YX. Inhibition of Th2-mediated allergic airway inflammatory disease by CD137 costimulation. J Immunol 2006; 177:814-21; PMID:16818735; http://dx.doi.org/ 10.4049/jimmunol.177.2.814 [DOI] [PubMed] [Google Scholar]
- 65.Polte T, Foell J, Werner C, Hoymann HG, Braun A, Burdach S, Mittler RS, Hansen G. CD137-mediated immunotherapy for allergic asthma. J Clin Invest 2006; 116:1025-36; PMID:16528411; http://dx.doi.org/ 10.1172/JCI23792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee J, Lee EN, Kim EY, Park HJ, Chang CY, Jung DY, Choi SY, Lee SK, Lee KW, Kwon GY et al.. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol Lett 2005; 101:210-6; PMID:16026855; http://dx.doi.org/ 10.1016/j.imlet.2005.06.001 [DOI] [PubMed] [Google Scholar]
- 67.Palma C, Vendetti S, Cassone A. Role of 4-1BB receptor in the control played by CD8(+) T cells on IFN-gamma production by Mycobacterium tuberculosis antigen-specific CD4(+) T Cells. PloS One 2010; 5:e11019; PMID:20544034; http://dx.doi.org/ 10.1371/journal.pone.0011019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SW, Park Y, Eun SY, Madireddi S, Cheroutre H, Croft M. Cutting edge: 4-1BB controls regulatory activity in dendritic cells through promoting optimal expression of retinal dehydrogenase. J Immunol 2012; 189:2697-701; PMID:22896640; http://dx.doi.org/ 10.4049/jimmunol.1201248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myers L, Takahashi C, Mittler RS, Rossi RJ, Vella AT. Effector CD8 T cells possess suppressor function after 4-1BB and Toll-like receptor triggering. Proc Nat Acad Sci USA 2003; 100:5348-53; PMID:12695569; http://dx.doi.org/ 10.1073/pnas.0837611100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Curran MA, Geiger TL, Montalvo W, Kim M, Reiner SL, Al-Shamkhani A, Sun JC, Allison JP. Systemic 4-1BB activation induces a novel T cell phenotype driven by high expression of Eomesodermin. J Exp Med 2013; 210:743-55; PMID:23547098; http://dx.doi.org/ 10.1084/jem.20121190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev 2009; 229:192-215; PMID:19426223; http://dx.doi.org/ 10.1111/j.1600-065X.2009.00765.x [DOI] [PubMed] [Google Scholar]
- 72.Mittler RS, Bailey TS, Klussman K, Trailsmith MD, Hoffmann MK. Anti-4-1BB monoclonal antibodies abrogate T cell-dependent humoral immune responses in vivo through the induction of helper T cell anergy. J Exp Med 1999; 190:1535-40; PMID:10562327; http://dx.doi.org/ 10.1084/jem.190.10.1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hong HJ, Lee JW, Park SS, Kang YJ, Chang SY, Kim KM, Kim JO, Murthy KK, Payne JS, Yoon SK et al.. A humanized anti–4-1BB monoclonal antibody suppresses antigen-induced humoral immune response in nonhuman primates. J Immunother 2000; 23:613-21; PMID:11186149; http://dx.doi.org/ 10.1097/00002371-200011000-00002 [DOI] [PubMed] [Google Scholar]
- 74.Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, Chander PN, Bapat AS, Yan XJ, Chiorazzi N et al.. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest 2003; 111:1505-18; PMID:12750400; http://dx.doi.org/ 10.1172/JCI200317662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sytwu HK, Lin WD, Roffler SR, Hung JT, Sung HS, Wang CH, Cheng TL, Tsou SC, Hsi SC, Shen KL. Anti-4-1BB-based immunotherapy for autoimmune diabetes: lessons from a transgenic non-obese diabetic (NOD) model. J Autoimmun 2003; 21:247-54; PMID:14599849; http://dx.doi.org/ 10.1016/S0896-8411(03)00112-4 [DOI] [PubMed] [Google Scholar]
- 76.Kachapati K, Bednar KJ, Adams DE, Wu Y, Mittler RS, Jordan MB, Hinerman JM, Herr AB, Ridgway WM. Recombinant soluble CD137 prevents type one diabetes in nonobese diabetic mice. J Autoimmun 2013; 47:94-103; PMID:24145149; http://dx.doi.org/ 10.1016/j.jaut.2013.09.002 [DOI] [PubMed] [Google Scholar]
- 77.Vinay DS, Choi JH, Kim JD, Choi BK, Kwon BS. Role of endogenous 4-1BB in the development of systemic lupus erythematosus. Immunology 2007; 122:394-400; PMID:17608689; http://dx.doi.org/ 10.1111/j.1365-2567.2007.02653.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinez Gomez JM, Croxford JL, Yeo KP, Angeli V, Schwarz H, Gasser S. Development of experimental autoimmune encephalomyelitis critically depends on CD137 ligand signaling. J Neurosci 2012; 32:18246-52; PMID:23238738; http://dx.doi.org/ 10.1523/JNEUROSCI.2473-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bang BR, Kim SJ, Yagita H, Croft M, Kang YJ. Inhibition of 4-1BBL-regulated TLR response in macrophages ameliorates endotoxin-induced sepsis in mice. Eur J Immunol 2015; 45:886-92; PMID:25501291; http://dx.doi.org/ 10.1002/eji.201445174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pauly S, Broll K, Wittmann M, Giegerich G, Schwarz H. CD137 is expressed by follicular dendritic cells and costimulates B lymphocyte activation in germinal centers. J Leukoc Biol 2002; 72:35-42; PMID:12101260 [PubMed] [Google Scholar]


