ABSTRACT
With checkpoint inhibitors, patients with advanced melanoma display durable responses suggesting cure of disease. However, the immune system has dual roles in cancer; while the immune system may eradicate a tumor, a subtotal elimination may selectively destroy immunogenic cells driving the proliferation of non-immunogenic tumors. Here, we performed a retrospective analysis of results obtained in a controlled trial of patients with melanoma treated with adjuvant, multisubtype interferon-α. The survival curves displayed a late divergence for treated patients and controls resulting in substantially higher estimates of overall (OS) and relapse-free survival (RFS) rates among treated patients after 9 y of follow up. Interestingly, succumbing patients in the treatment group displayed reduced time between relapse and death, suggesting therapy-induced acceleration of disease progression. These findings suggest that effective immunotherapy that induces durable, curative responses in some patients, may potentially accelerate disease progression in others, highlighting the importance of developing advanced strategies to identify patients who are likely to benefit from immunotherapy.
Keywords: Immunotherapy, Type I interferon, immunoediting, treatment outcome
Introduction
The idea that the immune system has a role in preventing and combatting cancer was first described by Burnet in 1957.1 After decades of intense debate, it is now generally accepted that the immune system has a role in cancer,2 and that it may act as a double-edged sword on a tumor.3 Thus, on the one hand, cytotoxic effector cells eliminate malignant cells with an aberrant phenotype; on the other hand, by selectively killing off immunogenic cells, the immune system contributes to the development of immunoevasive cancer cell phenotypes that may increase disease progression. These processes were coined cancer immunoediting by Dunn et al.4 The immunoediting processes have been thoroughly studied in rodents, but few, if any, human studies have addressed this concept in clinical studies. In rodents, mechanistic studies using genetically engineered mice have demonstrated that Type I interferons plays important roles in antitumor immunity, both by editing the afferent antitumor immune response via effects on dendritic cells and by stimulating efferent immune responses via actions on various cytotoxic cells.5,6 In humans it has been found that activation of afferent immunity is stimulated by the IFN-α subtype IFN-α1,7 whereas efferent immunity to a major part is stimulated by IFN-α2.7,8 In a study by Stadler et al patients with melanoma were treated with adjuvant, native, multisubtype IFN-α comprising both IFN-α1 and -α2.9
A long-term follow-up of the patients in the original study by Stadler and coworkers showed that the therapeutic regimen employed predominantly prevented late relapses and deaths.10 In the present study, we retrospectively reevaluated the results obtained in a homogeneous subgroup of patients (stage 3b) of this study, focusing on the patients who succumbed to melanoma. In agreement with the imunoediting theory we found that IFN-α immunotherapy substantially increased overall and RFS, but also that treated patients who relapsed and died displayed a higher rate of disease progression than control patients.
Results and Discussion
Relapse-free survival (RFS) and overall survival (OS) in treated patients and controls
In the original, prospective, randomized, multicentre study by Stadler et al,9 patients with totally resected cutaneous melanoma in various stages were either treated with dacarbazine (DTIC) followed by a 6-mo treatment with highly purified natural interferon-α, or received no adjuvant treatment. The results of the original trial comprising 252 individuals have been described previously.9 In brief, the OS curves of the two treatments arms initially overlapped and the treatment effect did not become apparent until several years after the end of treatment. Beneficial effect of the treatment was observed in all the three separately randomized strata of the trial. The effects in stage two patients were principally observed in stage 2b patients and stage 3a patients were too few to provide a basis for a meaningful analysis. By contrast, stage 3b patients comprised a homogeneous group of a sufficient number of individuals (n = 106; 54 treated and 52 controls) to justify a detailed retrospective analysis of the results.
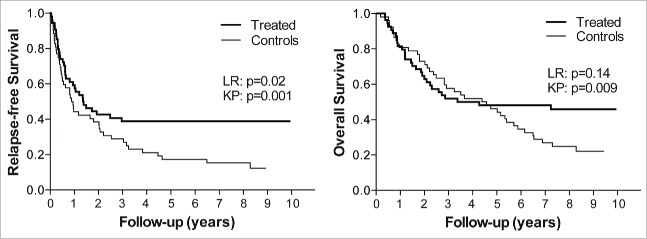
An ITT analysis of the RFS in treated stage 3b patients and controls indicated that the treatment caused an early inhibition of the progression of the disease, resulting in a prolongation of the time to relapse in the patients (Fig. 1A). The rate of relapses was lower among treated patients than in the controls throughout the study. Remarkably, in sharp contrast to the controls, there were no relapses at all among the treated patients after 3 y, that is during a period of 2 to 7.7 y following completion of therapy. Analysis of the OS curves for the two arms of the study (Fig. 1B) showed essentially overlapping curves during the first year and thereafter, for about 2 y, a tendency to accelerated progression in the treatment arm. Finally, after crossover of the curves, there was a clear late divergence of the survival curves. The most prominent finding in the ITT analysis was thus that both RFS and OS curves showed a clear late divergence of treated patients and controls resulting in survival figures at long-term follow-up that were remarkably higher in the treated patients than in the controls (Fig. 1A, B). The late divergence of survival curves is a typical finding in immunotherapy as opposed to chemotherapy, and has commonly been misinterpreted as a sign of a delayed effect of immunotherapy.11-16 However, as we have described earlier, late divergence of survival curves is in fact more likely to reflect early elimination of tumor cells.17 The explanation for this is essentially that late divergence results from the presence of late events only in the control arm of the study. An event that appears late indicates that the disease is slowly progressive, and thus late curve divergence is a consequence of prevention of late events, which means that the immune system preferentially inhibits a slowly progressing tumor. The elimination/control of the tumor in a treated patient may have occurred at any time point preceding the prevented event observed in the control arm, but is arguably more likely to occur in the early phase when the tumor load is the lowest. Therefore, seemingly paradoxically, late divergence of survival curves, which is a typical feature in the present study, is indicative of early tumor cell elimination.
Figure 1.
RFS and OS in patients receiving multisubtype IFN-α The panels show Kaplan–Meier plots of RFS (A) and OS (B) for stage 3b patients treated with native, multisubtype IFN-α (n = 54) or corresponding controls (n = 52). The statistical analysis of RFS (A) using log rank (LR) test showed a p-value of 0.02, while a direct comparison of RFS rates ± standard error at 8.7 y using the Kalbfleisch Prentice (KP) method showed a pronounced and statistically significant (p = 0.001) difference between treated patients and controls (38.9 ± 6.6% vs. 12.3 ± 4.9%). In panel B, as reported previously (17), the analysis of the OS showed no statistical significance using the log rank test (p = 0.14), but a direct comparison of the OS rates after 9 y showed statistically significant differences (p = 0.009) between the OS rates ± standard error of the treated patients and controls (45.9 ± 6.9% vs. 22.1 ± 6.0%).17
As shown in Fig. 1, survival analyses were performed using log rank test as defined in the original study protocol, but also by simple comparison of survival frequencies using a method described by Kalbfleisch and Prentice.18 It is important to note that initially overlapping and later diverging survival curves mean non-proportional hazards over time. As we and others have observed,17,19,20 statistical tests based on the Cox model, such as log rank test or Cox proportional hazards test, for analyzing efficacy in immunotherapy trials are inappropriate in cases where hazard ratios are not constant. In fact, the results obtained using the commonly employed log rank tests were, in the present study, clearly misleading, in particular with respect to the OS results.
Time from relapse to death in treated patients and controls
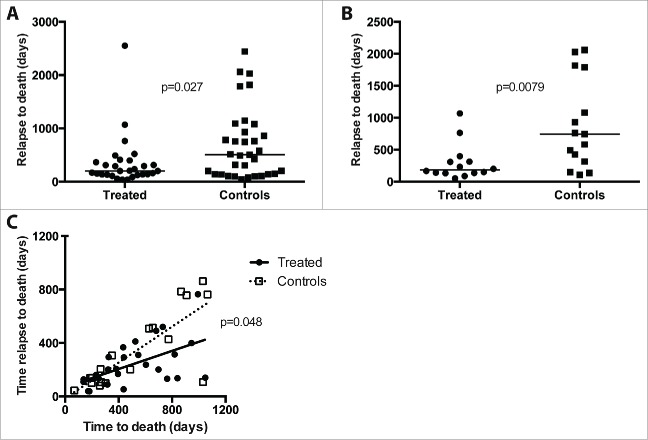
Comparison of the RFS and OS curves shown above suggested that the treatment might have a dual effect on progression of the disease. Therefore, we evaluated the time from relapse to death in all succumbing patients. In the total group of 252 patients who participated in the original study, a comparison of the time between relapse and death showed that this time was shorter in treated patients than in controls (data not shown, p = 0.024). In the homogeneous group of stage 3b patients (n = 106), data regarding relapse and death were available from all treated patients, while seven patients in the control group were lost to follow up regarding date of relapse. As shown in Fig. 2A, an analysis of all succumbing patients (n = 29) in the treatment arm compared to the 33 succumbing control individuals revealed a significantly reduced relapse-death time in the former as compared to the latter group (p= 0.027). Notably, only half of the treated patients who finally succumbed remained relapse-free long enough to have a chance to receive the full treatment schedule of 225 d. A landmark analysis, excluding treated patients and controls who relapsed before 225 d (Fig. 2B) showed a more pronounced difference between treated patients and controls (p = 0.008).
Figure 2.
Rate of disease progression in treated patients and controls as reflected by time between relapse and death. Panel A shows time between relapse and death in control patients (filled squares; n = 33) and IFN-α-treated patients (filled circles; n = 29). Panel B shows the corresponding results in a landmark analysis (controls, filled squares, n = 15; treated, filled circles, n = 14) excluding patients with relapse dates before day 225 of the study, corresponding to the end of the treatment schedule. Panel C shows time between relapse and death vs. time to death for controls (open squares, dotted line) and treated patients (filled circles, solid line). The slopes of the regression lines were significantly different (p = 0.048).
We have previously reported that immunotherapy with native multi-subtype IFN-α preferentially prevents late relapses and deaths.17 Thus, patients with late relapses, who by definition display a slow disease progression, were predominantly found in succumbing control group patients. To correct for this difference between the two groups, we plotted time between relapse and death versus time to death and excluded all relapses and deaths after 3 y. As shown in Fig. 2C, treated patients who were destined to succumb still displayed a more rapid progression after relapse as illustrated by the less steep regression line.
Taken together, the data obtained suggest that the treatment accelerated disease progression in patients who failed to eliminate the tumor or to establish long-term control of the tumor progression. An aggravated course of disease is in line with the third phase of the cancer immunoediting theory, i.e. the phase of tumor escape. It should be noted, however, that the comparison between rates of progression in treated patients and controls included those patients who had very slow progression, and these patients were predominantly found among the controls. However, also when excluding patients with late relapses and deaths, tumors among succumbing patients in the treatment group seemed to progress more rapidly than in corresponding patients in the control group. Thus, the observed accelerated disease progression in treated patients may at least partly be a consequence of a enhanced elimination of immunogenic tumor cells, allowing for an escape of less immunogenic tumor cells that drive an accelerated progression of disease.
When summarizing the results presented in this investigation, one should be aware that the study is retrospective and of exploratory nature. Results concerning efficacy should therefore be interpreted with caution. However, the results do suggest that the treatment employed will cause early, complete elimination of tumor cells, which as an end result may lead to cure of the disease. In the present study the RFS and OS rates at final follow-up after nearly 9 y showed a substantial increase among treated patients. Remarkably, 39% of the patients who had been assigned to the treatment arm were alive without having experienced any relapses during this long follow-up period compared to only 12% in the control group. Considering the fact that these results were obtained by ITT analyses of a randomized population of patients of which only about half received a full treatment schedule, the results make the possibility of cure in some of these patients likely. At the same time, the study suggests that the treatment in parallel triggered accelerated disease progression and death in another fraction of patients. Although more studies are warranted to confirm these opposing findings in other immunotherapeutic settings, they highlight the importance of developing sophisticated strategies to identify patients who are likely to benefit from immunotherapy.
Patients and methods
The patients in the study were from a controlled trial evaluating treatment with native, multisubtype, IFN-α, preceded by dacarbazine, in patients in various stages of cutaneous melanoma after resection of the primary tumor.9 Prior to treatment the 252 patients in the trial were stratified into 3 strata corresponding to stage 2 (no metastases), 3a (local or in-transit metastases) and 3b (regional lymph node metastases), respectively, and then randomized into either of 2 arms, 1 involving treatment with 2 injections of dacarbazine, 1 mo apart, followed by 24 weeks of treatment with multisubtype IFN-α (Multiferon, 3 million units, 3 times/week), and the second, control, arm given no antitumor treatment. For the present study we included all stage 3b patients. These 106 patients all had signs of regional lymph node involvement at diagnosis, and they were randomized into either a treatment (n=54) or a control (n=52) arm. The mean age of the 54 patient in the treated patients was 49.4 y and in the 52 control patients 52.5 y. The male to female ratio was 1.8:1 among treated patients and 1.7:1 among the controls. The thickness of the primary tumor varied between 0.2 and 40 mm with no statistical significant difference between the two groups of treated patients and controls (median values, 1.8 and 2.2 mm, respectively). Data regarding dates for relapse and death were available from all treated patients, relapse data from seven patients in the control group were not available. Treatment was incomplete (duration of treatment less than 225 d) in 13 patients since it was consistently discontinued as soon as any relapse occurred. In fact, six of the patients in the treatment arm did not receive any interferon-α at all. If not stated otherwise statistical tests used in the study were based on ITT populations.
Survival analyses were performed using log rank test as defined in the original study protocol. However, as reported previously,17 the survival curves for treated and non-treated patients obtained in the original study showed a late divergence resulting in non-proportional hazards, meaning that this type of test was an unsuitable analytical tool. Therefore, in the retrospective analysis described below, we also performed simple comparison of survival frequencies using a method described by Kalbfleisch and Prentice.18 Group comparisons were made using Mann–Whitney U-tests.
Native IFN-α
The preparation of native IFN-α used for the therapy was made by Bionative AB (Umeå, Sweden). The preparation contained seven different subtypes of IFN-α, primarily substantial amounts of IFN-α1 and α2, but also, to a lesser degree, IFN-α 8, 10, 14, 21 and trace amounts of IFN α 17.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Burnet M. Cancer - a biological approach. 3. Viruses associated with neoplastic conditions. Br Med J 1957; 1:841–6; PMID:13413231; http://dx.doi.org/ 10.1136/bmj.1.5023.841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74; PMID:21376230; http://dx.doi.org/ 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836–48; PMID:17063185; http://dx.doi.org/ 10.1038/nri1961 [DOI] [PubMed] [Google Scholar]
- 4.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991–8; PMID:12407406; http://dx.doi.org/ 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 5.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, Lee H, Arthur CD, White JM, Kalinke U et al.. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 2011; 208:1989–2003; PMID:21930769; http://dx.doi.org/ 10.1084/jem.20101158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, Diamond MS, Koebel CM, Arthur C, White JM et al.. A critical function for type I interferons in cancer immunoediting. Nat Immunol 2005; 6:722–9; PMID:15951814; http://dx.doi.org/ 10.1038/ni1213 [DOI] [PubMed] [Google Scholar]
- 7.Rhodes J, Ivanyi J, Cozens P. Antigen presentation by human monocytes: effects of modifying major histocompatibility complex class II antigen expression and interleukin 1 production by using recombinant interferons and corticosteroids. Eur J Immunol 1986; 16:370–5; PMID:2422040; http://dx.doi.org/ 10.1002/eji.1830160410 [DOI] [PubMed] [Google Scholar]
- 8.Finter NB. Why are there so many subtypes of α interferons? J Interfer Res 1991:185–94 [Google Scholar]
- 9.Stadler R, Luger T, Bieber T, Kohler U, Linse R, Technau K, Schubert R, Schroth K, Vakilzadeh F, Volkenandt M et al.. Long-term survival benefit after adjuvant treatment of cutaneous melanoma with dacarbazine and low dose natural interferon α: A controlled, randomised multicentre trial. Acta Oncol 2006; 45:389–99; PMID:16760174; http://dx.doi.org/ 10.1080/02841860600630954 [DOI] [PubMed] [Google Scholar]
- 10.Thoren FB, Strannegard O. Adjuvant interferon: extended follow-up times needed? Lancet Oncol 2011; 12:419; PMID:21536217; http://dx.doi.org/ 10.1016/S1470-2045(11)70107-3 [DOI] [PubMed] [Google Scholar]
- 11.Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies–outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC). Vaccine 2007; 25 Suppl 2:B97–B109; PMID:17916465; http://dx.doi.org/ 10.1016/j.vaccine.2007.06.067 [DOI] [PubMed] [Google Scholar]
- 12.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, Macrae S, Nelson M, Canning C, Lowy I et al.. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008; 105:3005–10; PMID:18287062; http://dx.doi.org/ 10.1073/pnas.0712237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C. Four-year survival metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol 2013; 24(8):2174–80; PMID:25210016; http://dx.doi.org/11956267 10.1093/annonc/mdu441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosman JA, Unger JM, Liu PY, Flaherty LE, Park MS, Kempf RA, Thompson JA, Terasaki PI, Sondak VK. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: impact of HLA class I antigen expression on outcome. J Clin Oncol 2002; 20:2067–75; PMID:11956267; http://dx.doi.org/ 10.1200/JCO.2002.08.072 [DOI] [PubMed] [Google Scholar]
- 15.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J et al.. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010; 28:1099–105; PMID:20100959; http://dx.doi.org/ 10.1200/JCO.2009.25.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24:3089–94; PMID:16809734; http://dx.doi.org/ 10.1200/JCO.2005.04.5252 [DOI] [PubMed] [Google Scholar]
- 17.Thoren FB, Anderson H, Strannegard O. Late divergence of survival curves in cancer immunotherapy trials: interpretation and implications. Cancer Immunol Immunother 2013; 62:1547–51; PMID:23979447; http://dx.doi.org/ 10.1007/s00262-013-1458-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalbfleisch J, Prentice R. The statistical analysis of failure time data. New York: Wiley, 2002 [Google Scholar]
- 19.Guidance for industry: Clinical considerations for therapeutic cancer vaccines. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research, 2011 [Google Scholar]
- 20.Paul J. Targeted survival improvements in clinical trials: are you an absolutist or relativist? Cancer 2015; 121:335–8; PMID:25278175; http://dx.doi.org/ 10.1002/cncr.29031 [DOI] [PMC free article] [PubMed] [Google Scholar]