Abstract
Our recent study reported an interesting finding that tumor cells can actively manipulate host immune function to facilitate tumor immune escape through the secretion of microRNAs (miRNAs). As an extension of this finding, we showed that blockage of the function of tumor-secreted miRNAs represents an effective therapeutic approach for cancer treatment.
Keywords: microvesicle, PTEN, regulatory T cell, secreted microRNA, tumor immune escape
The intensive studies of the past several years have documented the importance of miRNAs as an essential cornerstone of the genetic system.1 Although RNA is usually considered an unstable molecule because of the ubiquitous RNase, miRNAs are now known to circulate in the bloodstream and other body fluids in a stable, cell-free form.2,3 Importantly, we and other groups have demonstrated that a large proportion of extracellular miRNAs are actively secreted from host cells through a pathway based on small membranous vesicles known as microvesicles.4,5 Under the protection of microvesicles, secreted miRNAs can be delivered to recipient cells, where they block the translation of their target genes and regulate recipient cell function.4,5 Thus, secreted miRNAs can serve as a novel class of signaling molecules in mediating intercellular communication.
In a newest study,6 we tested the hypothesis, if tumor cells can actively secrete miRNAs to exert a suppressive effect on immune cells, particularly regulatory T cells (Tregs), a subset of CD4+ T cells that plays an important role in maintaining self-tolerance and modulating immune responses. We first identified miR-214 as a major oncogenic miRNA secreted by various types of cancers through microvesicles. Secreted miR-214 then enters recipient CD4+ T cells, in which the exogenous miR-214 decreases the expression of its target gene, phosphatase and tensin homolog (PTEN). Since PTEN is a negative modulator of Treg homeostasis in vivo and expansion ex vivo,7 downregulation of PTEN by tumor-secreted miR-214 results in an expansion of Tregs. Finally, as Tregs are increased during tumorigenesis and efficiently induce tumor immune evasion,8 Treg expansion mediated by secreted miR-214 leads to host immune tolerance, therefore causing rapid growth of tumor cells. Taken together, this study reveals a novel mechanism through which tumor cell actively modulate the host immune system via the secretion of miRNAs and induction of Treg expansion (Fig. 1).
Figure 1.
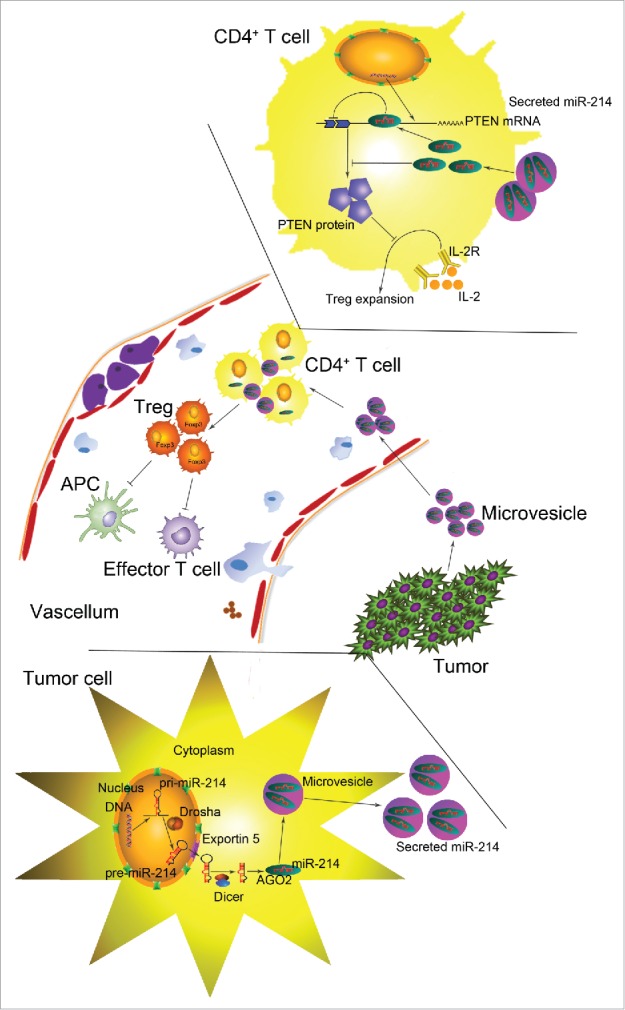
Working model for the role of tumor-secreted miR-214 in inducing tumor immune escape. After being processed into mature miRNA, tumor-specific miR-214 is packaged into microvesicles and secreted to the extracellular environment by tumor cells. Secreted miR-214 is then delivered by microvesicles to the recipient CD4+ T cells, in which it decreases PTEN protein expression and facilitates Treg expansion. Increased Treg population, in turn, leads to host immune suppression and tumor growth.
This study reveals some thought-provoking points. Previously, it is believed that tumor cells utilize two mechanisms to evade the immune response: (a) modification of the tumor cells themselves to decrease their sensitivity to attacks by functional immune cells, and (b) direct modulation of the host immune cells by the secretion of cytokines. This study raises a novel standpoint that tumor cells can actively manipulate the antitumor activities of immune cells by delivering tumor-specific miRNAs to immune cells. This study also suggests that: (a) although the levels of secreted miRNAs in extracellular space is quite low, they have the full capacity to exert essential biological functions; (b) secreted miRNAs may represent a new defining hallmark of the tumor microenvironment; (c) secreted miRNAs may play a pivotal role in tumor metastasis.
This finding may also have far-reaching clinical applications. Therapeutics targeting tumor immune escape is a promising approach for cancer treatment. Because secretion of miRNAs likely serves as a common mechanism for various cancer cells to create a tolerant immune environment, blocking the function of tumor-secreted miRNAs is, in principle, a feasible strategy to reverse tumor-induced immune tolerance. By employing cell-derived microvesicles to deliver miR-214 antisense (a strand of oligonucleotide complementary to miR-214) into peripheral CD4+ T cells, we successfully blocked Treg expansion and tumor growth induced by tumor cell-secreted miR-214.6 This revolutionary approach may fill the void left by current methods for systemic gene therapy. Currently, we are making great efforts to improve and validate the effectiveness of this approach in cancer therapy.
The exciting research field of miRNA secretion is still in its infancy stage. In the present study, we reported a new signaling pathway between tumor and immune cells. Interestingly, some recent studies have also shown the active participation of tumor-secreted miRNAs in cancer progression and metastatic spread.9,10 These studies reveal the importance of tumor-secreted miRNAs as a novel and critical cancer hallmark. However, some outstanding questions remain to be addressed. For example, extensive investigation is required to determine how oncogenic miRNAs are specifically targeted for secretion and are recognized for uptake. It is also largely unknown regarding the value of tumor-secreted miRNAs in cancer treatment. We anticipate that future studies in this area will revolutionize the way we perceive how tumor cells communicate with immune cells and open up new avenues for wide-spread and efficient treatments against cancer.
References
- 1. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116:281-97; PMID:14744438; http://dx.doi.org/ 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 2. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008; 18:997-1006; PMID:18766170; http://dx.doi.org/ 10.1038/cr.2008.282 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. P Natl Acad Sci 2008; 105:10513-8; PMID:18663219; http://dx.doi.org/20603081 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang YJ, Liu DQ, Chen X, Li J, Li LM, Bian Z, Sun F, Lu J, Yin Y, Cai X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010; 39:133-44; PMID:20603081; http://dx.doi.org/ 10.1016/j.molcel.2010.06.010 [DOI] [PubMed] [Google Scholar]
- 5. Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010; 285:17442-52; PMID:20353945; http://dx.doi.org/ 10.1074/jbc.M110.107821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J, Wang Z, Chen X, Zhang W, Yokoyama S, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res 2014; 24:1164-80; PMID:25223704; http://dx.doi.org/ 10.1038/cr.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. PTEN inhibits IL-2 receptor-mediated expansion of CD4C CD25C Tregs. J Clin Invest 2006; 116:2521-31; PMID:16917540; http://dx.doi.org/22999714 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Savage PA, Malchow S, Leventhal DS. Basic principles of tumor-associated regulatory T cell biology. Trends Immunol 2013; 34:33-40; PMID:22999714; http://dx.doi.org/ 10.1016/j.it.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou WY, Fong MY, Min YF, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O'Connor ST, Chin AR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014; 25:501-15; PMID:24735924; http://dx.doi.org/ 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. He WA, Calore F, Londhe P, Canella A, Guttridge DC, Croce CM. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. P Natl Acad Sci 2014; 111:4525-9; PMID:24616506; http://dx.doi.org/ 10.1073/pnas.1402714111 [DOI] [PMC free article] [PubMed] [Google Scholar]


