ABSTRACT
Monitoring functional competence of immune cell populations in clinical routine represents a major challenge. We developed a whole-blood assay to monitor functional competence of peripheral innate immune cells including NK cells, dendritic and monocyte cell subsets through their ability to produce specific cytokines after short-term stimulation, detected through intra-cytoplasmic staining and multi-parametric flow-cytometry. A PMA/ionomycin T cell activation assay complemented this analysis. Comparing cohorts of healthy women and breast cancer (BC) patients at different stages, we identified significant functional alteration of circulating immune cells during BC progression prior to initiation of treatment. Of upmost importance, as early as the localized primary tumor (PT) stage, we observed functional alterations in several innate immune populations and T cells i.e. (i) reduced TNFα production by BDCA-1+ DC and non-classical monocytes in response to Type-I IFN, (ii) a strong drop in IFNγ production by NK cells in response to either Type-I IFN or TLR7/8 ligand, and (iii) a coordinated impairment of cytokine (IL-2, IFNγ, IL-21) production by T cell subpopulations. Overall, these alterations are further accentuated according to the stage of the disease in first-line metastatic patients. Finally, whereas we did not detect functional modification of DC subsets in response to TLR7/8 ligand, we highlighted increased IL-12p40 production by monocytes specifically at first relapse (FR). Our results reinforce the importance of monitoring both innate and adaptive immunity to better evaluate dysfunctions in cancer patients and suggest that our whole-blood assay will be useful to monitor response to treatment, particularly for immunotherapeutic strategies.
KEYWORDS: Breast cancer, immune system, monitoring, multi-parametric flow cytometry, whole blood assay
Introduction
In cancer, the immune system can play a dual role. During the early stages of tumorigenesis, active immune surveillance prevents tumor development, while in more advanced tumor stages immuno-subversion leads to tumor escape. A strong immune signature has been linked with improved patient outcome in BC subtypes, with the highest correlation in the ERneg and Her2-amplified tumors. 1-3 Furthermore, higher levels of infiltrating CD8+ T cells in BC have been associated with better patient survival. 4
During the last 10 years, our group and others have demonstrated a variety of mechanisms favoring primary BC escape from immuno-surveillance. They include altered myeloid dendritic cells (mDC) and plasmacytoid DC (pDC) 5-7 function, regulatory T cell (Treg) recruitment and expansion through ICOS–ICOSL interaction 8-10 and T cell effector neutralization. 11 We have also shown that systemic alterations such as lympho-divpenia (low T cell receptor diversity and reduced lymphocyte number) 12 and more particularly CD4+ lymphopenia 13 strongly influence survival among first-line metastatic BC patients. This strengthens the importance of the integrity of immune system function as measured in periphery to maintain the tumor under control.
Blood T cell alterations such as reduced proliferation capacity in response to mitogens (PHA) 14 and altered cytokine pattern under PMA-Ionomycin (P/I) activation 15-17 have been described in primary and locally advanced BC patients. In particular, the CD4+IL-17+ population is known to contribute to inflammation and autoimmunity, but with a controversial role in cancer. However, the CD4+IL-17+ is significantly reduced in blood of Her2-amplified primary and metastatic BC patients compared to other BC subtypes or healthy donors (HD). 18
In blood, DCs are divided into three main subsets: BDCA-1+ mDCs represent 47.5% of circulating DCs producing inflammatory cytokines and chemokines. 19 BDCA-3+/CD141+ mDCs which represent only 5% of total DCs and are the human homolog of the mouse CD8α+ DC subset producing Type-III IFN (IFNλ) 20,21 are specialized in Ag cross-presentation. 22-25 Finally, pDC (47.5%), natural Type-I IFN-producing cells (IFNα), play a central role in antiviral immune response and are also involved in maintenance of tolerance (for a review, see ref. 26). In BC patients, after LPS-stimulation, blood DCs (Linneg HLADR+) secrete lower levels of IL-12p40 and present reduced activation capacity 27 compared to HD whereas no difference in TNFα and IL-1β secretion 28 are observed.
NK cells are fundamental for host protection against malignancies and today, it is obvious that antitumor functions of NK cells are tightly regulated and expand far beyond the simple killing of cancer cells. 29 Indeed, blood NK cells could modulate DC functions through either release of cytokines or physical interaction with DC. 30,31 Blood NK cells have been also reported to be functionally altered 32 in BC patients.
Monocytes are recruited from the circulation into the tumor, where they accumulate and differentiate into inflammatory and/or tumor-associated macrophages or monocyte-derived DCs with pro or antitumor functions. Therefore, analyzing monocyte functionality in patients' blood may be informative. Recently, transcriptome analysis demonstrated that blood monocytes from renal cell carcinoma patients and HD donors are highly divergent,33 further demonstrating the strong impact of a solid tumor on circulating immune cells. Moreover, BC patient peripheral monocytes are functionally altered as they differentiate into a more suppressive DC phenotype under GM-CSF+IL-4. 34
Due to its easy access, peripheral blood constitutes an interesting source to measure functional competence of immune cell subsets. Whereas T cell function in whole blood (WB) is classically assessed after P/I reactivation, over the last 10 years sparse assays have been setup to evaluate DC subset function without a purification process in different pathologic situations including BC. 35-39 However, as DCs are not the sole innate players in blood for cytokine secretion, evaluation of circulating monocyte subsets has been integrated in such WB assay. 40
Monitoring functional competence of immune cell populations routinely in the clinic represents a major challenge during health and disease. Indeed, remarkable results obtained with anticancer immunotherapy treatments urge us to find relevant biomarkers, and more importantly on understanding precise mechanisms leading to tumor regression. In order to evaluate systemic functional changes, we developed a WB assay aiming at analyzing functional competence of innate immune cells comprising NK, DC and monocyte subsets together in a single tube, as interplay exists between different immune cell populations. We compared different TLR activators and selected IFNα-2b and R848 (TLR7/8 agonist) as complementing activators to evaluate cytokine production through intra-cytoplasmic staining (TNFα, IFNγ, IL-12p40 and IFNα). This assay was combined with a WB T cell polyclonal activation (P/I) to evaluate Th1 (IFNγ, TNFα, IL-2), Th17 (IL-17A) and TFH (IL-21) cytokine production. In the present study, we followed the functional competence of circulating immune cell populations in independent cohorts of BC patients at different stages of tumor progression (PT, FR, second relapse (SR)) compared to HD cohort. This is the first report of an integrated analysis, permitting the identification of altered functional competence of immune cells as early as the stage of localized PT. Furthermore, these alterations are amplified in first-line metastatic patients. Intriguingly, most of these alterations appear to be restored in more advanced metastatic patients.
Results
Based on the capacity of different innate and adaptive immune subsets to secrete selective cytokines, we developed a heparinized WB assay to assess immune cells function after short-term stimulation by flow cytometry. Two distinct activation conditions and associated panels were developed (Table 3).
Table 3.
Staining panels used for flow cytometry analyses.
| Marker | Fluorochrome | Clone | Manufacturer |
|---|---|---|---|
| A : Panel used for T cell functionality assay | |||
| Violet laser | |||
| IFNγ | Brilliant violet 421™ | 4S.B3 | Biolegend |
| CD14 | BD Horizon™ V500 | M5E2 | BD Biosciences |
| CD15 | BD Horizon™ V500 | HI98 | BD Biosciences |
| CD19 | BD Horizon™ V500 | HIB19 | BD Biosciences |
| CD8+ | Brilliant violet 570™ | RPA-T8 | Biolegend |
| Blue laser | |||
| TCRγ9 | FITC | B3 | BD Biosciences |
| IL-17A | PerCP-Cy5.5 | N49-653 | BD Biosciences |
| Yellow laser | |||
| CD4 | PE | SK3 | BD Biosciences |
| CD45RA | ECD | 2H4LDH11LDB9 | Beckman Coulter |
| IL-2 | PE-Cy7 | MQ1-17H12 | BD Biosciences |
| Red laser | |||
| IL-21 | Alexa 647 | 3A3-N2.1 | BD Biosciences |
| TNFα | Alexa 700 | MAb11 | BD Biosciences |
| CD3 |
APC-H7 |
SK7 |
BD Biosciences |
| B: Panel used for “Innate Immunity” assay | |||
| Violet laser | |||
| IFNγ | Brilliant violet 421™ | 4S.B3 | Biolegend |
| CD3 | BD Horizon™ V500 | UCHT1 | BD Biosciences |
| CD14 | BD Horizon™ V500 | M5E2 | BD Biosciences |
| CD15 | BD Horizon™ V500 | HI98 | BD Biosciences |
| CD19 | BD Horizon™ V500 | HIB19 | BD Biosciences |
| CD56 | Brilliant violet 570™ | HCD56 | Biolegend |
| Blue laser | |||
| IFNα | FITC | LT27:295 | Miltenyi Biotec |
| CD11c | PerCP-Cy5.5 | Bu15 | Biolegend |
| Yellow laser | |||
| IL12p40/70 | PE | C11.5 | BD Biosciences |
| CD16 | ECD | 3G8 | Beckman Coulter |
| CD1c | PE-Cy7 | L161 | Biolegend |
| Red laser | |||
| BDCA-2 | APC | AC144 | Miltenyi Biotec |
| BDCA-3 | AD5-14H12 | Miltenyi Biotec | |
| TNFα | Alexa 700 | MAb11 | BD Biosciences |
| HLA-DR | APC-H7 | G46-6 | BD Biosciences |
11-color flow cytometry permits simultaneous functional characterization of T cell subsets in WB
The first condition consisted of short-term reactivation of T cells with P/I in presence of brefeldin A, in the evaluation of Th1 (IFNγ, TNFα, IL-2), Th17 (IL-17A) and TFH (IL-21) cytokines. These T lymphocytes were characterized by the expression of CD3 and exclusion of CD14, CD15 and CD19 markers. We distinguished γδ (CD3+TCRγ9+) from αβ (CD3+TCRγ9neg) T lymphocytes in which we identified respectively CD8+ and CD4+ subsets. Then, based on the expression of CD45RA, we discriminated CD8+CD45RA+ (CD3+TCRγ9negCD8+CD45RA+), memory CD8+ (CD3+TCRγ9negCD8+CD45RAneg), CD4+CD45RA+ (CD3+TCRγ9negCD4+CD45RA+) and memory CD4+ (CD3+TCRγ9negCD4+CD45RAneg). The gating strategy and results obtained for a HD are shown in Fig. 1A. As expected, in response to P/I, almost all γδ T cells produced both IFNγ and TNFα. CD4+ and CD8+ memory T cells produced IFNγ, TNFα and IL-2, and CD4+ memory subpopulation also produced either IL-21 or IL-17A potentially representative of TFH and Th17 subsets. Naive CD4+ T cells produced only IL-2 whereas CD8+CD45RA+ and CD4+CD45RA+ T cells co-producing IFNγ and TNFα represent effector memory T cells (TEMRA) subpopulation as previously described (Figure S1). 42
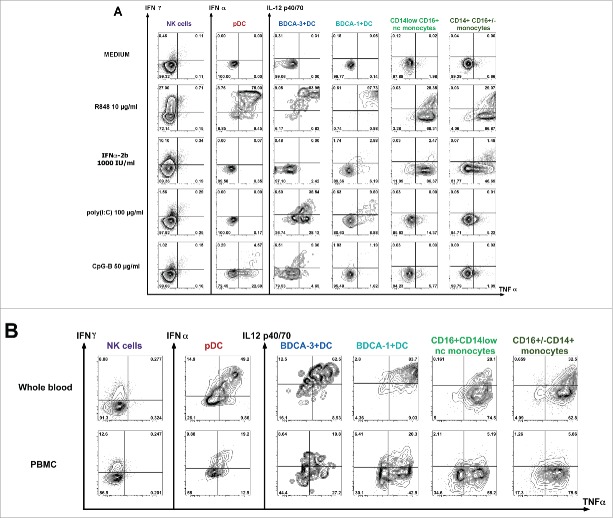
Figure 1.
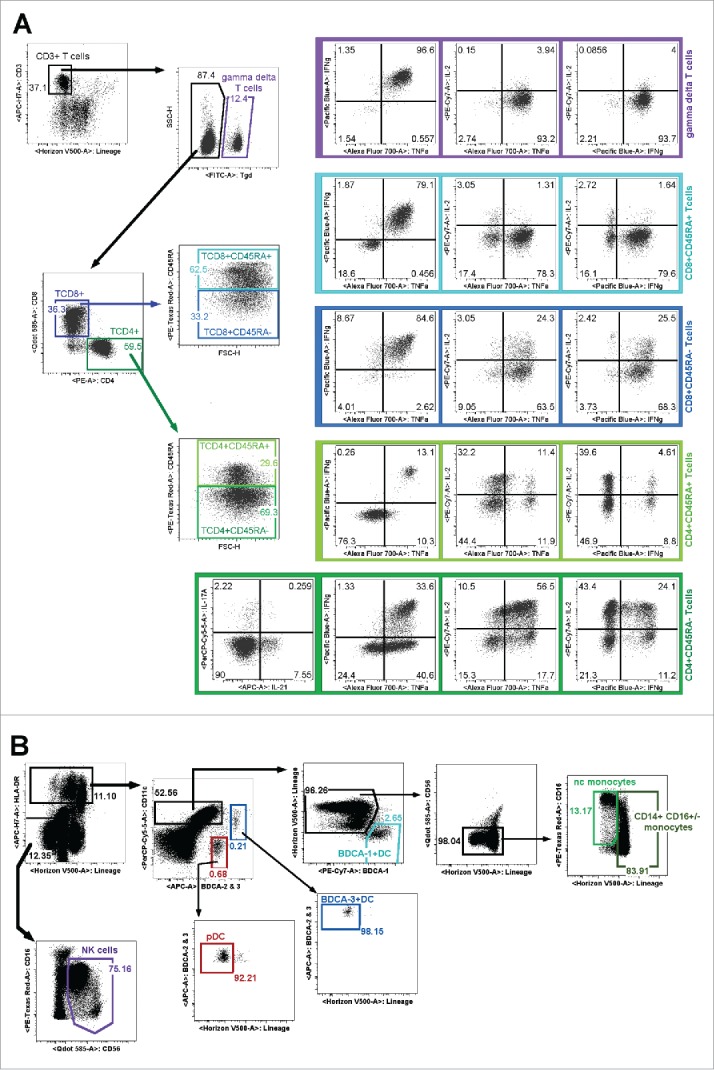
11-color flow cytometry gating strategies to assess circulating immune cell functionality. Dot plots represent results obtained after a healthy donor WB stimulation. (A) After short term P/I activation, we analyzed by multi-parametric flow cytometry the ability of γδ T cells (CD3+TCRγ9+), LTCD8+CD45RA+ (CD3+TCRγ9negCD8+CD45RA+), memory LTCD8+ (CD3+TCRγ9negCD8+CD45RAneg), naive CD4+ (CD3+TCRγ9negCD4+CD45RA+) and memory CD4+ T cells (CD3+TCRγ9negCD4+CD45RAneg) to synthetize IFNγ, IL-2, TNFα, IL-21 or IL-17A. (B) The “Innate Immunity” panel allowed the simultaneous identification of NK cells (LINnegHLA-DRnegCD56+), DC subsets (LINnegHLA-DR+) including pDC (BDCA2+CD11cneg), BDCA-3+ mDC (CD11c+BDCA-3high), BDCA-1+ mDC (CD11c+BDCA-1+), monocytes (HLA-DR+Lin+CD11c+CD56neg) including CD14+CD16+/−monocytes and non-classical (nc-monocytes CD14lowCD16+).
11-color flow cytometry permit simultaneous functional characterization of monocytes, NK and DC subsets in WB
The second panel named “innate immunity” allowed the simultaneous identification of all monocyte and DC subsets along with NK cells (Fig. 1B). NK cells were characterized based on exclusion of HLA-DR and lineage markers (CD3/CD14/CD15/CD19) and CD56 and CD16 expression. All DC subsets were identified by their expression of HLA-DR. pDC were identified based on BDCA-2 expression and lack of CD11c. mDC were discriminated from pDC based on CD11c expression among which we distinguished BDCA-3+ DC (CD11c+BDCA-3high) and BDCA-1+ DC (CD11c+ BDCA-3negBDCA-1+). Contaminating cells were eliminated based on expression of lineage markers. As previously described, monocytes express HLA-DR+ and CD11chigh and CD14 and CD16 expression, allows defining CD14+CD16+/− monocytes and CD14lowCD16+ non-classical monocytes (nc-monocytes). 43
In order to define the optimal experimental conditions inducing simultaneous activation of all innate immune cell subsets, different activators were tested in WB (Fig. 2A, Fig. S2). To favor the cellular cooperation and particularly DC/NK cross talk, brefeldin A was added following 1-h stimulation.41 We particularly focused on activators previously shown to directly or indirectly induce NK cell activation such as poly(I:C),44 R848,45 CpG-B 46 and IFNα-2b. 47 As shown in Fig. 2A, R848 was the only TLR-L able to induce a simultaneous and strong response of all monocytes, DC subsets and NK cells. Indeed, 5-h WB stimulation allowed the production of IFNγ by NK cells, co-production of IFNα and TNFα by pDC and co-production of IL-12p40 and TNFα by mDCs subsets. High TNFα levels and low IL-12p40 levels were produced by both monocyte subsets.
Figure 2.
Evaluation of the functional capacity of innate immune cells upon short-term stimulation by multi-parametric flow cytometry. After 5 h of culture in presence of medium or activators, brefeldin A being added after 1 h, we assessed the functional capacity of different innate immune cells characterized as described in Fig. 1B. Dot plots present cytokine intracytoplasmic staining to evaluate production of IFNγ/TNFα by NK cells or IFNα/TNFα by pDC as well as IL-12p40/TNFα by mDC (BDCA-3+, BDCA-1+) or monocyte (CD14+CD16+/−, CD14lowCD16+) subsets. (A) The efficiency of different activators (TLR7/8 ligand (R848), Type-I IFN (IFNα−2b), TLR3 ligand (poly(I:C)), TLR9 ligand (CpG-B)) was compared to medium condition in whole blood assay. (B) Comparison of results obtained after 5 h of stimulation with R848 (10µg/mL) on whole blood or PBMC from the same donor.
Similar results were obtained after PBMC purification (Fig. 2B). However, the percentage of DC subsets co-producing IL-12p40 and TNFα (mDC subsets and monocytes) or IFNα and TNFα (pDC subset) was from 2- to 5-fold lower in PBMC than in WB according to the subset analyzed (co-producing cells in WB and PBMC respectively (mean ± SD): 51± 2% vs. 26.5 ± 7.5% for pDC; 71.1±9% vs. 46.9 ± 10% for BDCA-3+DC; 88.5 ± 0.7% vs. 17 ± 4 % for BDCA-1+DC; 18.65±1.45 vs. 5.9 ± 0.7 for monocytes and 28.55 ± 3.95 vs. 6.3 ± 0.3 for nc-monocytes). IFNγ production by NK was slightly higher in PBMC than in WB (8.6 ± 0.41% and 13.41 ± 5.22 %).
In our WB assay, stimulation by poly(I:C) or CpG-B induced weak NK response (<2% of IFNγ) (Fig. 2A). Moreover, stimulation by poly(I:C) only induced mDCs response and weak response by monocyte subsets. As previously described 20,25 in response to poly(I:C), we observed higher cytokine levels produced by BDCA-3+ DC than by BDCA-1+ DC (co-producing IL12p40/TNFα (mean ± SD): 21±4.4% for BDCA-3+ vs. 8±2.3% for BDCA-1+ subset). CpG-B, but not CpG-A stimulation (Fig. S2), induced moderate pDC and BDCA-3+ DC response.
WB activation with IFNα-2b (Fig. 2A) induced low IFNγ production by NK cells (10.1%), high TNFα production by monocytes subsets (nc-monocytes=86.4%; CD14+CD16+/− monocytes=46.7%), whereas only a low percentage of BDCA-1+ DC (BDCA-1+ DC =6.2%) producing TNFα were detected. No specific cytokine synthesis was detected in pDC and BDCA-3+ DC (data not shown).
In conclusion, we identified and selected R848 and IFNα-2b as the best and complementary stimulators to monitor monocytes, NK and DC simultaneously.
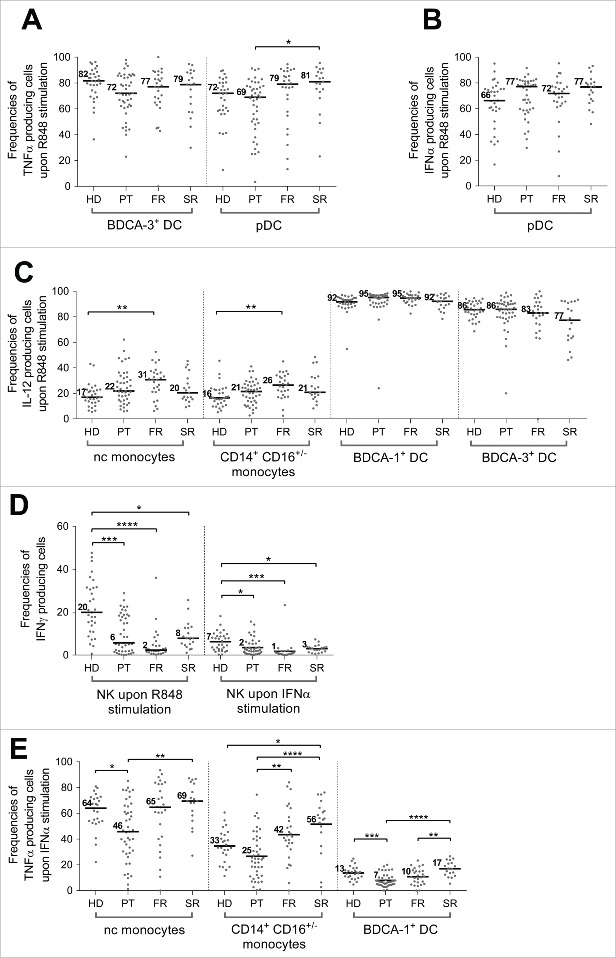
Increased IL-12p40 production by monocytes in response to R848 stimulation in BC patients
We applied this WB assay to independent BC patient cohorts (PT, FR or SR according to the progression of the pathology, see Table 1 for patient characteristics) and results were compared with HD cohort. In response to R848, we did not observe significant modification of TNFα or IFNα production by DC subsets regardless of the stage of the pathology (Fig. 3A, Fig. 3B, Fig. S3). However, focusing on CD14+CD16+/− and nc-monocyte subsets, we observed a gradual increase of median percentage of IL-12p40 produced in BC patients from PT to FR stages (Fig. 3C), with this difference being significant only with the latter compared to HD (nc-monocytes: HD = 17%, PT = 22%, FR = 31% (p value =**); CD14+CD16+/− monocytes: HD = 16%, PT = 21%, FR = 26% (p value = **)). In contrast, IL-12p40 secretion remained stable for BDCA-1+ and BDCA-3+ DC subsets (Fig. 3C). In more advanced patients (SR) median percentage of IL-12p40 secretion by monocytes or DC was not significantly different from HD values.
Table 1.
Patients' characteristics.
| Healthy donors | Primary tumors | First relapse | Second relapse | |
|---|---|---|---|---|
| N | 30 | 46 | 34 | 20 |
| Age [min–max] | 51 years [34–63] | 44 years [29–63] | 55 years [32–77] | 60 years [39–76] |
| Histologic type | n (%) | n (%) | n (%) | |
| Missing data | 1 | 1 | ||
| Lobular carcinoma | 2 (4.4%) | 6 (17.6%) | 3 (15.8%) | |
| Ductal carcinoma | 43 (95.6%) | 28 (82.4%) | 16 (84.2%) | |
| ER/PgR status | ||||
| ER+ | 26 (56.5%) | 27 (79.4%) | 15 (75%) | |
| PgR+ | 23(50%) | 25 (73.5%) | 9 (45%) | |
| HER2 status | ||||
| Positive | 7 (15.2%) | 1 (2.9%) | 2 (10%) | |
| Triple-negative tumors | 17 (36.9%) | 6 (17.64%) | 4 (20%) | |
| SBR status | ||||
| Missing data | 1 | |||
| 1 | 3 (8.8%) | |||
| 2 | 23 (50%) | 16 (47.1%) | 10 (52.6%) | |
| 3 | 23 (50%) | 15 (44.1%) | 9 (47.4%) | |
| Number of metastatic LN | ||||
| missing data | 5 | 5 | ||
| <3 | 37 (80.4%) | 15 (44.1%) | 7 (35%) | |
| ≥3 | 9 (19.6%) | 14 (41.2%) | 13 (65%) | |
| Main metastatic sites | ||||
| Bone | 0 | 15 (44.11%) | 16 (80%) | |
| Liver | 0 | 14 (41.17%) | 13 (65%) | |
| Bone only | 0 | 2 (5.88%) | 1 (5%) |
Figure 3.
(See previous page) Innate immune cell subset functional alterations observed in periphery during breast tumor progression. The functionality of innate immune cells was assessed in WB after TLR7/8 ligand (R848, 10µg/mL) or IFNα2b (1000 IU/mL) stimulation in cohorts of patients with breast cancer at different stage of disease (PT (n = 46), FR (n = 34), SR (n = 20)) and compared to a HD cohort (n = 31) and presented as percentage of cell subset producing a specified cytokine in the different cohorts: (A) percentage of BDCA-3+DC and pDC subsets producing TNFα upon TLR7/8 ligand stimulation, (B) percentage of pDC producing IFNα upon TLR7/8 ligand stimulation, (C) percentage of nc-monocytes, monocytes (CD14+CD16+/−), BDCA-1+DC and BDCA-3+DCs producing IL12p40/70 upon TLR7/8 ligand stimulation, (D) percentage of NK cells producing IFNγ upon TLR7/8 ligand and IFNα-2b stimulations and (E) percentage of nc-monocytes, monocytes (CD14+CD16+/−) and BDCA-1+DC producing TNFα upon IFNα-2b stimulation. *: p value < 0.05, **: p value < 0.01, ***: p value < 0.001.
Alteration of NK cell functionality in all BC patient cohorts in response to R848 and IFNα-2b
Alteration of NK cell function in BC patients has been demonstrated in purified NK in response to a class I negative target stimulation 32 but was never evaluated in WB in the absence of target in response to TLR stimulation, known to depend on crosstalk with DC. Therefore, we monitored NK response to TLR7/8 stimulation without any separation process, in WB in the presence of accessory cells such as monocytes and DC. As shown in Fig. 3D, in response to TLR7/8 and IFNα-2b, NK cells secreted significant levels of IFNγ in HD with a high dispersion (median HD: 20% [0.6–47.6]). Remarkably, IFNγ production drastically dropped in BC patients both at PT (median IFNγ=6% [0–28.8]; p value=***) and FR stages (median IFNγ=2% [0.2–36.0], p value=****) but was partly recovered at SR (median IFNγ =8% [1.1–25.6]; p value=*). Similar results were obtained under IFNα-2b activation, even if global levels were lower (Fig. 3D). In contrast, very low levels of TNFα were detected in HD in response to various stimulators including IFNα-2b (Fig. 2A and Fig. S2) and no variation was detected in patients.
IFNα-2b stimulation highlights major alterations in monocytes and BDCA-1+ DC subsets
In HD, we confirmed the production of TNFα in response to IFNα-2b (Fig. 3E), although at lower levels compared to R848 (Fig. 2A), by monocyte subsets (CD14+CD16+/− and nc-monocyte subsets) and BDCA-1+ DC subset (median TNFα: CD14+CD16+/−=33%; nc-monocytes=64% and BDCA-1+ DC=13%). BDCA-3+ DC and pDC subsets did not produce TNFα in this condition.
Compared to HD, TNFα production in PT cohort was decreased in nc-monocytes (median TNFα: PT=46%, p value=*) and BDCA-1+ DC (median TNFα: PT=7%, p value=***) subsets. This altered TNFα production was specific of PT stage, as the percentage of TNFα production remained either identical (nc-monocytes and BDCA-1+ DC) or higher (CD14+CD16+/− monocytes) to HD ones at stage or relapsed patients.
This TNFα alteration observed in nc-monocytes was strongly correlated with TNFα produced by CD14+CD16+/− monocytes in IFNα-2b (R = 0.791, p <10−4) and BDCA-1+ DC either in IFNα-2b (R = 0.471, p = 0.001) or R848 stimulation (R=0.469, p =0.001) (Fig. S4, Fig. S5A–C). This reveals a common alteration for these three cell subsets regardless of the activator used. Of interest, this TNFα alteration observed in nc-monocytes, was also correlated to decreased TNFα production by pDC in the R848 condition (R = 0.654, p <10−4) whereas it did not correlate with their IFNα secretion (R = 0.246, p =0.112) (Fig. S4, Fig. S5D) further demonstrating global altered TNFα production by innate immune cells analyzed at PT stage independently of the activation pathway.
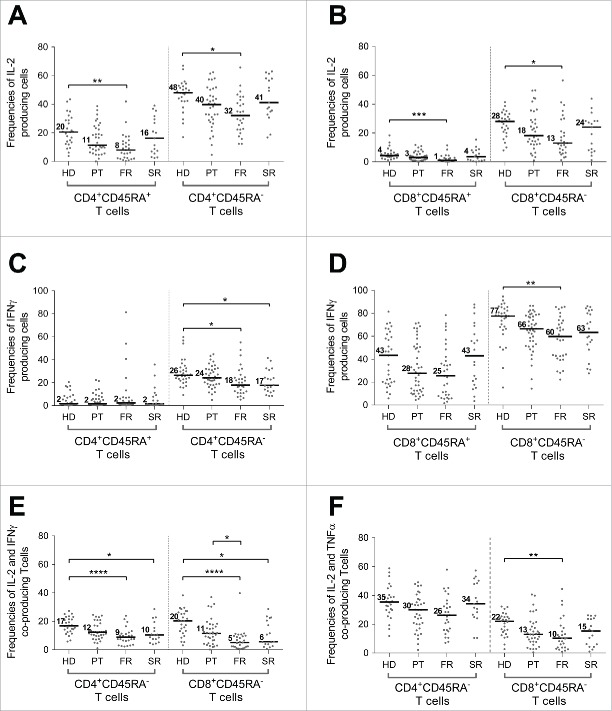
Altered IL-2 production by CD4+ and CD8+ T cells during BC progression
Alteration of IL-2 production capacity was observed in both CD4+ and CD8+ T cells (Fig. S6B) in all BC cohorts either at primary or metastatic stages (FR and SR) although this difference reached statistical significance only in the FR cohort (p value=*). While the production of IL-2 was lower in CD4+CD45RA+ subset (HD = 20%; PT = 11%; FR = 8%, SR = 16%) than in memory CD4+ T cells (HD = 48%; PT = 40%; FR = 32%, SR = 41%), this difference was significant in both populations (p value=*) (Fig. 4A). For CD8+ subsets, IL-2 secretion was mainly produced by memory T cell subset and also significantly altered at the FR stage (HD=28%; PT=18%; FR=13% (p value=*), SR = 24%) (Fig. 4B).
Figure 4.
T cell subset functional alterations observed in periphery during breast tumor progression. The functionality of T cell subsets was assessed on WB after short-term polyclonal stimulation (P/I) in presence of brefeldin A in cohorts of patients with breast cancer at different stages of disease (PT (n = 46), FR (n = 34), SR (n = 20)) and compared to a HD cohort (=31) and presented as percentage of cell subsets producing a specified cytokine in the different cohorts: percentage of IL-2 production by CD4+ (A) and CD8+ (B) T cell subsets (CD45RA+ and CD45RAneg), percentage of IFNγ production by CD4+ (C) and CD8+ (D) T cell subsets (CD45RA+ and CD45RAneg) and (E) percentage of IL2 and IFNγ and IL2 and TNFα (F) co-production by CD45RAneg memory CD4+ and CD8+ T cells. *: p value <0.05, **: p value <0.01, ***: p value <0.001, ****: p value <10−4.
IFNγ secretion by T cells are altered in blood from BC cohorts compared to HD cohort
Interestingly, we pointed out a decrease in CD8+ T cell IFNγ secretion capacity at primary and metastatic stages (Fig. S6C). This reduction was observed either in the memory CD45RAneg population with a statistical significance comparing FR to HD (HD = 77%; PT=66%; FR=60% (p value=*); SR = 63%) and CD45RA+ subset although it remains not significant (HD = 43%; PT = 28%; FR = 25%; SR = 43%) (Fig. 4D). Whereas CD4+ T cells produced normal TNFα levels (Fig. S6A), IFNγ (Fig. S6C) was produced at lower levels. Focusing on memory CD4+ T cells we observed, as for CD8+ subpopulation, a significant reduced percentage of IFNγ producing cells at FR stage compared to HD (HD = 26%; PT = 24%; FR = 18% (p value=*); SR = 17%) (Fig. 4C).
IFNγ/IL-2 co-production is highly and significantly altered in FR patients
As shown in Fig. 1A within CD4+ and CD8+ memory subsets, we noticed a subpopulation producing IFNγ together with IL-2 that represents 16.8 ± 5.8 % of the CD4+CD45RAnegIL-2+ subpopulation and 20.3 ± 8.8% of the CD8+CD45RAnegIL-2+ population. When comparing the percentage of memory T cells coproducing IL-2 and IFNγ (Fig. 4E), among the different cohorts we observed a highly significant decrease in FR patients as compared to both cytokines alone (HD=17% vs. FR=9% for CD4+; HD=20% vs. FR=5% for CD8+; p value=****), that remained significant at SR stage (10% for CD4+ and 6% for CD8+; p value=*).
Similar observations was done for TNFα/IL-2 co-production (HD=35% vs. FR=26% for CD4+; HD=22% vs. FR=10% for CD8+ p value=*) even if this difference was statistically significant only for the memory CD8+ subset (Fig. 4F). In contrast, the percentage of cells coproducing IFNγ/TNF was not significantly modulated among cohorts (not shown). Finally, no modulation was observed for cells co-producing all three cytokines (not shown).
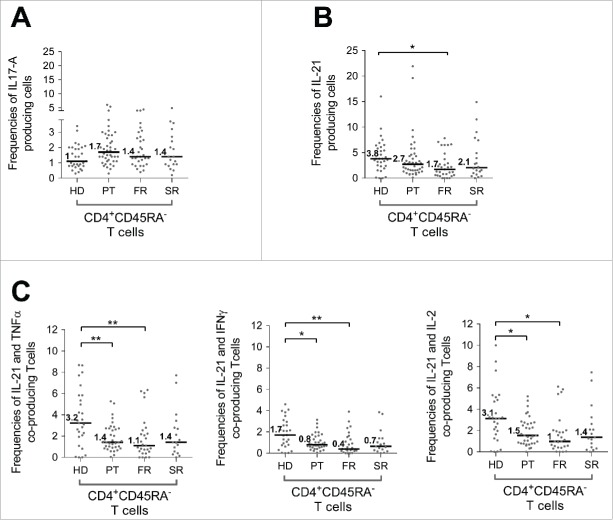
IL-17A and IL-21 modulation observed in BC patients cohorts
The evaluation of IL-17A and IL-21 secretion by CD4+ subsets demonstrated a small subset of CD4+ memory T cells able to secrete IL-17A (Fig. 1A) that, although not statistically significant, was the only T cell cytokine that was increased in the PT cohort (Fig. 5A). In contrast, even if the percentage of CD4+ memory T cells secreting IL-21 remained low (Fig. S7, Fig. 5B), a 2-fold decrease was detected in metastatic patients (FR and SR) that was significantly different comparing FR to HD (3.75% vs. 1.74% respectively, p value=*).
Figure 5.
Modulation of IL-17A and IL-21 production by CD4+ CD45RAneg T cells detected in periphery during breast tumor progression. The capacity of CD4+ CD45RAneg T cells to produce IL-17A or IL-21 was assessed in WB after short-term polyclonal stimulation (P/I) in presence of brefeldin A on cohorts of patients with breast cancers at different stage of disease (PT (n = 46), FR (n = 34), SR (n = 20)) and compared to a HD cohort (n = 31) Results are presented as percentage of CD4+ CD45RAneg T cells producing IL-17A (A) or IL-21 (B) or co-producing IL-21 and TNFα, IL-21 and IFNγ or IL-21 and IL-2 (C). * p value< 0.05, **: p value < 0.01.
Of importance, when focusing on poly-functional CD4+ memory subpopulation co-producing IL-21 with either IL-2, IFNγ or TNFα, the alteration observed at PT stage appeared significant (IL-21+TNFα+ p-value=**; IL-21+IFNγ+ p value=*; IL-21+IL-2+ p value=*) and increased the significance observed at FR stage (IL-21+TNFα+ p value=**; IL-21+IFNγ+ p value=**; IL-21+IL-2+ p value=*) (Figs. 5C–E).
γδ T cell functional defects
A recent publication suggests that peripheral blood γδ T cell IFNγ production capacity, under polyclonal stimulation, is reduced in newly diagnosed untreated primary breast tumors patients. 48 In contrast, we demonstrated in our WB assay that γδ T cell capacity to secrete IFNγ was significantly altered in FR patients (58% [1.6–95.4], p value=**) compared to HD or PT patients that remained similar (HD=88.5% [5–98.4]; PT=87. 7% [2.6–96.5]) (Fig. 6) whereas TNFα secretion capacity in the different cohorts remained unchanged compared to HD.
Figure 6.
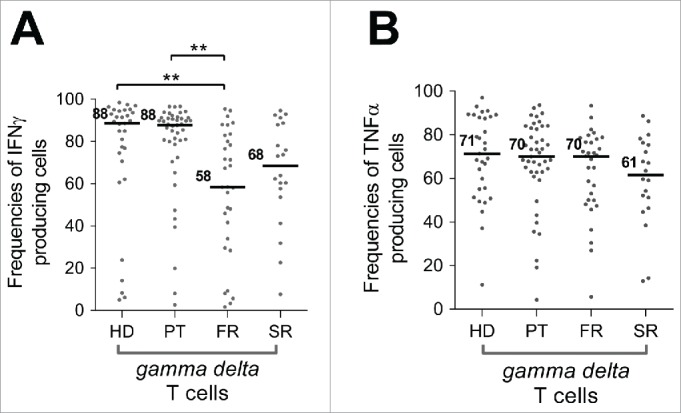
Characterization of γδ T cell functional alterations in periphery during breast tumor progression. The capacity of γδ T cells to produce IFNγ and TNFα was assessed in WB after short-term polyclonal stimulation (P/I) in presence of brefeldin A in cohorts of patients with breast cancer at different stages of disease (PT (n = 46), FR (n = 34), SR (n = 20)) and compared to a HD cohort (n = 31) and results are presented as percentage of γδ T cells producing IFNγ or TNFα. **:p value < 0.01.
Absence of correlation between innate and adaptive immune alteration and tumor characteristics
Integration of tumor patient characteristics (age, hormone receptor expression, SBR grade, lymph node involvement, molecular subtypes) did not show correlation with the peripheral immune cell alterations observed in the different BC cohorts (data not shown). Reaching the median clinical follow-up will allow us to assess the clinical impact of these innate and adaptive immune alterations on time to progression and overall survival.
Discussion
In this study, we developed a new WB flow cytometry assay to address the alteration of major innate immune cell subsets (monocytes and DC subsets together with NK cells) during BC progression. R848 was selected for its ability to favor cytokine production by all innate immune subsets whereas IFNα-2b that stimulates another pathway was selected to characterize complementary alterations. When combined with WB P/I activation to assess T cell subset functional alterations, this WB innate immunity assay allowed us to identify functional immune cell alteration during BC progression, including at the stage of localized PT.
WB and PBMC assay comparisons demonstrate the importance of cellular cross talk to favor innate immune cell cytokine production
Over the past 10 years, flow cytometry has allowed the functional evaluation of innate immune cells (DC, monocytes or NK cells) in WB or PBMC assays. However, no study to date has reported the simultaneous functional analysis of all DC and monocytes subsets together with NK cells. Herein, we show that TLR7/8 ligation induced full activation of the different DC subsets that may depend on additional indirect cytokine mediated effects in WB. Of importance, this response is strongly decreased when freshly isolated PBMC are used, possibly due to the elimination of populations that can respond to TLR7/8 ligand (polynuclear cells, platelets) but also soluble mediators in plasma. Moreover, among the TLR ligands tested (Fig. 2A and Fig. S2), R848 is the most efficient to trigger IL-12p40 in BDCA-3+ DC and IFNα production in pDC. Importantly, IFNγ production by NK cells in response to R848 in WB assay in HD donors required a 1-h delayed addition of inhibitor of secretion following activators (not shown), demonstrating the need for cell cooperation via secreted mediators. This is in line with previous data demonstrating that within PBMC, NK cells secrete IFNγ in response to R848 through indirect pathways involving IL-18 and IL-12 secretion 44,45 but not IFNα.30,49
BDCA-3+ DC subset are responsive to CPG-B stimulation in WB assay despite their lack of TLR9
We also observed IL-12p40 and TNFα production by BDCA-3+ DC under activation with CpG-B, but not CpG-A (Fig. S2), confirming a previous report. 37 Indeed, whereas BDCA-3+ DC are known to express TLR3 and TLR4 leading to the production of IL-12p40 and TNFα 24,25,50 their response to CpG-B is surprising as, in contrast to pDC, TLR9 expression has not been reported on BDCA-3+ subset. 25,37 This may rely on indirect effects resulting from cytokine cascade after the activation of B lymphocytes, pDC or neutrophils expressing high TLR9 levels 51,52 as this CpG-B response is lost on purified BDCA-3+ DC (data not shown).
IL-12p40 production by monocytes and DC subsets in BC patients
Monitoring independent cohorts of BC patients at different stages of progression compared to an HD cohort, we observed no statistical differences in cytokine production capacity (TNFα, IL-12p40, IFNα) by DC subsets (BDCA-1+ DC, BDCA-3+, pDC) under R848 activation. This contrasts with a previous report 27 demonstrating in BC patients with PT in such WB assay, an alteration of IL-12p40 secretion capacity by DC subsets after LPS stimulation that was associated with reduced capacity of cells to be phenotypically activated. In our hands, LPS stimulation induced only low IL-12p40 production compared to R848 (Fig. S2).
In contrast, our results point out an increased IL-12p40 production by monocyte subsets after R848 WB stimulation in BC patients at FR stage that appeared significantly different from HD. IL-12p40 could be associated with IL-23p19 to form a functional IL-23 that favor IL-17A secretion by T cells.53 Of interest, although not reaching statistical value, IL-17A cytokine appears as the only T cell mediator being increased during BC disease progression (Fig. 5A).
Altered TNFα secretion by CD14lowCD16+ nc-monocytes and BDCA-1+ DC in response to IFNα-2b stimulation
When evaluating BC patient cohorts, we observed, at PT stage, a decline in TNFα production by monocyte subsets as well as by BDCA-1+ DC subset under IFNα-2b stimulation whereas no difference was detected after R848 or R848+IFNα-2b (data not shown) stimulation. In this PT cohort, a subset of patients presented a coordinated TNFα default in CD14+CD16+/− and nc-monocyte subsets as well as BDCA-1+ DC under IFNα-2b stimulation (Fig. S4, Fig. S5A-C). However, this same subset of patients showed a reduced capacity of pDC to produce TNFα in response to R848 whereas IFNα levels remained unaffected (Fig. S5D, Fig. S4). In contrast, TNFα produced by T cell subsets after P/I reactivation was not affected (Fig. S6A) suggesting this relates to an alteration of innate but not adaptive immunity in this PT patient population, or to the signaling pathway leading to TNFα production. This may rely on regulatory mechanisms of JAK-STAT pathways involved in IFNα response. 54 Finally, this default did not correlate with tumor characteristics (SBR grade, size, hormone receptor expression).
In the PT cohort, innate immune cells of certain patients produced lower level of TNFα in R848. Although this does not significantly change the median, individual values correlate with those observed in the IFNα-2b condition (Fig. S5D, Fig. S4). Differences between the two activations might rely on the capacity of IFNα-2b to activate only the JAK/STAT-1/IRF pathway whereas R848 activates TLR7/8 and mobilizes many downstream activation pathways (NFKB, MAP kinase, IRF) that could counteract the reduced response to Type-1 IFN. Moreover, the reduced response to IFNα-2b could also reflect a reduced Type-1 IFNR expression in monocyte and BDCA-1+ subsets resulting from previous stimulation by other TLRL inducing desensitization.55 It could also rely on systemic alterations of BC patients' monocytes we recently reported to fail to differentiate into functional MoDC 34 as well as M1 macrophages (Ramos RN in preparation). Such functional alteration has previously been described for peripheral blood monocytes from lung cancer patients that presented a default in TNFα secretion capacity in response to LPS stimulation. 56 Such TNFα alteration of innate immune cells (monocytes, pDC, BDCA-1+ DC) may influence tumor progression and relapse but due to the reduced clinical follow-up (less than 3 years) for this PT cohort, this could not be addressed.
An altered response of innate immune cells to IFNα-2b might underscore a reduced capacity to mount an efficient antitumor immune response as Type-1 IFN participates in the cross talk between pDC and mDC or nc-monocytes to favor antigen-specific antitumor immune response. 57
Functional alterations of NK cells in BC cohorts
Our functional WB “innate immunity” assay was designed to assess NK cell functionality and the crosstalk between NK and DC. The analysis of both primary and metastatic BC patient cohorts demonstrated a significant drop in the IFNγ secretion capacity of NK cells observed with both R848 and IFNα-2b signals either at PT or metastatic stages (FR and SR stages) that was partly restored in SR cohort. This is in accordance with a previous report demonstrating a reduced capacity of blood NK cells to secrete IFNγ following purification and co-culture with K562 target cells among primary and metastatic BC patients, which is restored upon remission. 32 IFNγ secretion by NK cells within PBMC in response to R848 requires crosstalk with monocyte and DC subsets through IL-18 and IL-12. 44,45 This suggests an alteration of crosstalk between NK and DC or monocytes that could influence the capacity of patients to mount an antitumor immune response. Specifically as NK/DC crosstalk, through IFNγ and TNFα, play important role in the cross-presentation process. 23 However, from our analysis, monocyte and DC subsets retain their capacity to produce TNFα, IFNα and IL-12p40 in response to R848, pointing to other defects. This might include lower production of IL-18, as well as other IL-1 members, but IL-12p70 cannot be excluded. This NK defect might also relate to an intrinsic NK defect.
Functional alterations of T cell subsets in BC cohorts
Regardless of the stage of BC patients analyzed, we did not observe any alteration of TNFα production by T cell subsets. In contrast, compared to either HD or PT cohorts, γδ T cells produced significantly less IFNγ at metastatic stages, being significant only at the FR stage. In line with this, Gaafar et al. 48 previously reported the absence of γδ T cell functional alterations at primary BC stage but they did not evaluate metastatic patients. For CD4+ and CD8+ T cell subsets, we detected important alterations of IL-2 and IFNγ production that affected mainly memory subsets at primary and metastatic stages, although only significant at the metastatic stage. Importantly, the evaluation of IL-2 and IFNγ co-production by CD4+ and CD8+ memory T cell subsets identified an alteration, not detectable with each cytokine alone, in a subset of patients at PT stage, although not statistically significant in the global cohort. Furthermore, analysis of IL-2 and IFNγ co-production strengthened the alteration observed at metastatic stage. This demonstrates that the evaluation of each cytokine, but also their combination, is important to better assess patient's immune status. However, these alterations do not correlate with innate immune alterations (Fig. S4B) demonstrating that analyses of both innate and adaptive immune function in blood samples from BC patients at different stages of the disease are complementary.
Several publications report defects in IFNγ and IL-2 secretion, but not TNFα by CD4+ and CD8+ blood T cells in patients with primary and metastatic breast or lung cancer or melanoma. 28,58 However, the reasons of these selective alterations remain unclear. TNFα secretion favors T cell proliferation and survival 59,60 whereas IL-2 and IFNγ secretion reflect the T cell activation status (CD4+ and CD8+) toward a Th1 immune response.
Interestingly within the different BC cohorts, patients with a reduced IFNγ or TNFα production by CD45RAneg but not CD45RA+ T cells (CD4+ or CD8+) or global reduced IL-2 T cell capacity also showed reduced IL-21 secretion by CD4+ memory T cells suggesting a coordinated alteration of the T cell response (Fig. S7, Fig. S4). Moreover, a reduction of IL-21 production by CD4+ memory T cells could affect humoral response,61 CTL activity 62 or NK cell cytotoxic function 63 thus altering antitumor response. In line with this, analyzing coproduction of IL-21 and IFNγ, TNFα or IL-2 allowed us to highlight decreased functionality of T cells at the PT stage that further drops at the FR stage. This reinforces the importance of combined T cell subsets cytokine analysis to better evaluate dysfunctions in patients. While none of the T cell subsets Th1 and CD8+ functional alterations observed correlated to patient's clinical characteristics, it might influence antitumor immune response and time to relapse that will be followed in these cohorts.
As all patients were enrolled before any chemotherapy (for PT) or at distance of any treatment for metastatic stages (FR and SR), we can state that this altered IFNγ, IL-2 and IL-21 secretion represents intrinsic characteristics of patient immune status or tumor immuno-suppressive context (Treg, suppressive cytokines) rather than a consequence of chemotherapy. Moreover, we did not find any correlation between percentages of IFNγ+ or IL-2+ producing cells and T lymphocyte absolute counts (data not shown).
In conclusion, using flow cytometry WB assays, we highlighted alterations on innate and adaptive immune cells that are detectable as early as diagnosis of PT. PT stage is associated with a strong and coordinated alteration of TNFα production by BDCA-1+ DC and monocyte subsets in response to IFN-α2b stimulation and a drop in NK cell capacity to produce IFNγ in response to either IFN-α2b or R848 when no defect in immune cell numbers can be detected. In particular, as shown in Fig. S8, both TNFα production and monocyte numbers are highly dispersed in particular in the PT cohort, and no significant correlation between TNFα frequency and absolute cell count can be detected for any monocyte subsets. It could reflect the critical importance of innate immune subsets alteration by tumor early in the tumor development to escape the immune control. This functional NK cell alteration is also detected at FR stage. Moreover, coordinated cytokine alterations are detected in T cell subpopulations after polyclonal stimulation from the stage of PTs and are amplified at FR stage. These results reinforce the importance of combined analyses of innate and adaptive immunity to better evaluate dysfunctions in BC patients.
The defects observed are highly heterogeneous within a given stage cohort, and whether the defects could be linked to tumor progression or response to treatment will need further investigation.
Materials and methods
Subjects
Heparinized blood samples obtained anonymously from the French national blood transfusion service (Etablissement Français du Sang, Lyon, France) were collected from 31 healthy women (median age 51 years, range 34 to 63 years). BC patient blood samples, collected before new line of treatment, were obtained from different prospective clinical trials developed at the Center Léon Bérard after written informed consent: 46 patients at the diagnosis of PT who will undergo neo-adjuvant chemotherapy (median age 45 years, [27–69]), 34 patients at the diagnosis of FR before the initiation of chemotherapy treatment (median age 54 years, [32–77]) and 20 patients at the diagnosis of SR (median age 60 years [39–76]). The clinical characteristics of these cohorts are described in Table 1.
Activating reagents
Origin and concentrations of TLR ligands and IFNα-2b are shown in Table 2. PMA (50ng/mL) and ionomycin (1µg/mL) were obtained from Sigma Aldrich.
Table 2.
Panel of activators used to develop the whole blood assays.
| Target | Activator | Final concentration | Source |
|---|---|---|---|
| T cell polyclonal activation | PMA | 50 ng/mL | Sigma Aldrich |
| Ionomycin | 1 µg/mL | ||
| TLR1/2 | LTA (B Subtilis) | 100 µg/mL | Invivogen |
| TLR2 | Zymosan | 100 µg/mL | Molecular Probe |
| TLR2/6 | PGN (S. Aureus) | 100 µg/mL | Sigma Aldrich |
| TLR3 and RLR | Poly(I:C) HMW | 100 µg/mL | Invivogen |
| TLR4 | Ultrapure LPS (E Coli 0111:B4 strain) | 1 µg/mL | |
| TLR7/8 | Imiquimod (R837) | 100 µg/mL | |
| Resiquimod (R848) | 10 µg/mL | ||
| CL075 | 10 µg/mL | ||
| TLR9 | CpG-A (ODN 2336) | 50 µg/mL | |
| CpG-B (ODN 2006) | 50 µg/mL | ||
| TLR7 and Helicases | Inactivated Influenza M1 virus | 1000 HAU/mL | Sanofi-Pasteur |
| Type-I IFN | IFNα-2b | 1000 IU/mL | Roferon® |
Whole blood and PBMC stimulation
Within 3 h after blood collection, 900 µL of heparinized WB were incubated at 37°C in a 5% CO2 humidified atmosphere for 5 h, with or without various activators as indicated in Table 2. The protein transport inhibitor, brefeldin A (GolgiPlug, 10 µg/mL, BD biosciences), was added concomitantly with P/I combination or after 1 h for IFNα−2b or TLR stimulation as previously described 41 (Table 2).
PBMC were isolated by Ficoll density gradient centrifugation (Eurobio) and were resuspended at 5 × 106 cells/900 µL in complete RPMI. WB and PBMC stimulation by R848 (10 µg/mL) were performed in parallel, as described above.
Intra-cytoplasmic cytokine staining and multi-parametric flow cytometry
At the end of the 5-h stimulation, erythrocytes were lysed at room temperature with BD Pharmlyse® (Becton Dickinson). White blood cells were washed in staining buffer (PBS 2% FBS 1mM EDTA (Sigma-Aldrich)) and stained with the corresponding surface antibodies panel shown in Table 3A for T cells and Table 3B for “innate immune cell” activation. After washing in PBS, cells were fixed with Formaldehyde (2%, Sigma-Aldrich) for 20 min at 4°C, then washed twice in staining buffer and stored overnight at 4°C. After permeabilization in staining buffer supplemented with 0.5% saponine, cells were stained for 20 min at 4°C with the corresponding intra-cytoplasmic anti-cytokine antibodies (Table 3). Cells were resuspended in 600 µL of staining buffer and all events acquired on a LSRII Fortessa, Becton Dickinson fitted with four lasers (violet, blue, yellow and red).
Results were analyzed using the FlowJo software v9.6.4 (TreeStar, Inc.), and cytokine secretion by different cell subsets defined by the gating strategy was evaluated by creation of Boolean gates.
Statistical analysis
The statistical differences between the different cohorts (HD, PT, FR and SR) were assessed using a one-way ANOVA parametric analysis with a Tukey's correction for multiple comparisons, or using a Kruskal–Wallis non parametric analysis with a Dunn's multiple comparisons test. Results were considered as statistically significant when p value was < 0.05 (*: p value < 0.05; **: p value < 0.01; ***: p value < 0.001).
Correlations between different cytokines within “innate immune” and “T cell” subsets were performed using Spearman tests and results were presented using the rho correlation coefficient and the associated p value.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank D.G. Cox for the correction of the manuscript.
Funding
This work was financially supported by the Canceropole Lyon Auvergne Rhône-Alpes (CLARA, Grant LYMPHOS'1), FUI AAP-08 PLATINE (Grant no. 0801420601), FUI AAP-10 DIVRESCUE (OSEO F1105032Z and Région Rhône-Alpes no. 1101030301), INCa translational programs (Grants 2009-113 and 2011-052) and the SIRIC project (LYRIC, grant no. INCa_4664). This work was performed within the framework of the LABEX DEVWECAN (ANR-10-LABX-0061) of Université de Lyon, within the program “Investissements d'Avenir” (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR).
References
- 1.Alexe G, Dalgin GS, Scanfeld D, Tamayo P, Mesirov JP, DeLisi C, Harris L, Barnard N, Martel M, Levine AJ et al.. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res 2007; 67:10669-76; PMID:18006808; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-0539 [DOI] [PubMed] [Google Scholar]
- 2.Ascierto ML, Idowu MO, Zhao Y, Khalak H, Payne KK, Wang XY, Dumur CI, Bedognetti D, Tomei S, Ascierto PA et al.. Molecular signatures mostly associated with NK cells are predictive of relapse free survival in breast cancer patients. J Transl Med 2013; 11:145; PMID:23758773; http://dx.doi.org/ 10.1186/1479-5876-11-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol 2007; 8:R157; PMID:17683518; http://dx.doi.org/ 10.1186/gb-2007-8-8-r157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949-55; PMID:21483002; http://dx.doi.org/ 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 5.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J et al.. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res 2013; 73:4629-40; PMID:23722543; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-3058 [DOI] [PubMed] [Google Scholar]
- 6.Sisirak V, Faget J, Gobert M, Goutagny N, Vey N, Treilleux I, Renaudineau S, Poyet G, Labidi-Galy SI, Goddard-Leon S et al.. Impaired IFN-alpha Production by Plasmacytoid Dendritic Cells Favors Regulatory T-cell Expansion That May Contribute to Breast Cancer Progression. Cancer Res 2012; 72:5188-97; PMID:22836755; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-3468 [DOI] [PubMed] [Google Scholar]
- 7.Sisirak V, Vey N, Goutagny N, Renaudineau S, Malfroy M, Thys S, Treilleux I, Labidi-Galy SI, Bachelot T, Dezutter-Dambuyant C et al.. Breast cancer-derived transforming growth factor-beta and tumor necrosis factor-alpha compromise interferon-alpha production by tumor-associated plasmacytoid dendritic cells. Int J Cancer 2013; 133:771-8; PMID:23389942; http://dx.doi.org/ 10.1002/ijc.28072 [DOI] [PubMed] [Google Scholar]
- 8.Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, Durand I, Léon-Goddard S, Blay JY, Caux C et al.. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res 2011; 71:6143-52; PMID:21852386; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0573 [DOI] [PubMed] [Google Scholar]
- 9.Faget J, Bendriss-Vermare N, Gobert M, Durand I, Olive D, Biota C, Bachelot T, Treilleux I, Goddard-Leon S, Lavergne E et al.. ICOS-Ligand Expression on Plasmacytoid Dendritic Cells Supports Breast Cancer Progression by Promoting the Accumulation of Immunosuppressive CD4+ T Cells. Cancer Res 2012; 72:6130-41; PMID:23026134; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2409 [DOI] [PubMed] [Google Scholar]
- 10.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D et al.. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 2009; 69:2000-9; PMID:19244125; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2360 [DOI] [PubMed] [Google Scholar]
- 11.Ferrari S, Malugani F, Rovati B, Porta C, Riccardi A, Danova M. Flow cytometric analysis of circulating dendritic cell subsets and intracellular cytokine production in advanced breast cancer patients. Oncol Rep 2005; 14:113-20; PMID:15944777 [PubMed] [Google Scholar]
- 12.Manuel M, Tredan O, Bachelot T, Clapisson G, Courtier A, Parmentier G, Rabeony T, Grives A, Perez S, Mouret JF et al.. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology 2012; 1:432-40; PMID:22754761; http://dx.doi.org/ 10.4161/onci.19545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tredan O, Manuel M, Clapisson G, Bachelot T, Chabaud S, Bardin-Dit-Courageot C, Rigal C, Biota C, Bajard A, Pasqual N et al.. Patients with metastatic breast cancer leading to CD4(+) T cell lymphopaenia have poor outcome. Eur J Cancer 2013; 49:1673-82; PMID:23265706; http://dx.doi.org/ 10.1016/j.ejca.2012.11.028 [DOI] [PubMed] [Google Scholar]
- 14.Head JF, Elliott RL, McCoy JL. Evaluation of lymphocyte immunity in breast cancer patients. Breast Cancer Res Treat 1993; 26:77-88; PMID:8400326; http://dx.doi.org/ 10.1007/BF00682702 [DOI] [PubMed] [Google Scholar]
- 15.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat 2005; 91:163-71; PMID:15868444; http://dx.doi.org/ 10.1007/s10549-004-7048-0 [DOI] [PubMed] [Google Scholar]
- 16.Goto S, Sato M, Kaneko R, Itoh M, Sato S, Takeuchi S. Analysis of Th1 and Th2 cytokine production by peripheral blood mononuclear cells as a parameter of immunological dysfunction in advanced cancer patients. Cancer Immunol Immunother 1999; 48:435-42; PMID:10550548; http://dx.doi.org/ 10.1007/s002620050620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verma C, Eremin JM, Robins A, Bennett AJ, Cowley GP, El-Sheemy MA, Jibril JA, Eremin O. Abnormal T regulatory cells (Tregs: FOXP3+, CTLA-4+), myeloid-derived suppressor cells (MDSCs: monocytic, granulocytic) and polarised T helper cell profiles (Th1, Th2, Th17) in women with large and locally advanced breast cancers undergoing neoadjuvant chemotherapy (NAC) and surgery: failure of abolition of abnormal treg profile with treatment and correlation of treg levels with pathological response to NAC. J Transl Med 2013; 11:16; PMID:23320561; http://dx.doi.org/ 10.1186/1479-5876-11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br J Cancer 2009; 100:1061-7; PMID:19277040; http://dx.doi.org/ 10.1038/sj.bjc.6604963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccioli D, Tavarini S, Borgogni E, Steri V, Nuti S, Sammicheli C, Bardelli M, Montagna D, Locatelli F, Wack A. Functional specialization of human circulating CD16 and CD1c myeloid dendritic-cell subsets. Blood 2007; 109:5371-9; PMID:17332250; http://dx.doi.org/ 10.1182/blood-2006-08-038422 [DOI] [PubMed] [Google Scholar]
- 20.Balan S, Ollion V, Colletti N, Chelbi R, Montanana-Sanchis F, Liu H, Vu Manh TP, Sanchez C, Savoret J, Perrot I et al.. Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J Immunol 2014; 193:1622-35; PMID:25009205; http://dx.doi.org/ 10.4049/jimmunol.1401243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A et al.. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med 2010; 207:2703-17; PMID:20975040; http://dx.doi.org/ 10.1084/jem.20092720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW et al.. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 2010; 207:1273-81; PMID:20479115; http://dx.doi.org/ 10.1084/jem.20100348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deauvieau F, Ollion V, Doffin AC, Achard C, Fonteneau JF, Verronese E, Durand I, Ghittoni R, Marvel J, Dezutter-Dambuyant C et al.. Human natural killer cells promote cross-presentation of tumor cell-derived antigens by dendritic cells. Int J Cancer 2015; 136:1085-94; PMID:25046660; http://dx.doi.org/ 10.1002/ijc.29087 [DOI] [PubMed] [Google Scholar]
- 24.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V et al.. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med 2010; 207:1247-60; PMID:20479116; http://dx.doi.org/ 10.1084/jem.20092140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E et al.. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med 2010; 207:1261-71; PMID:20479117; http://dx.doi.org/ 10.1084/jem.20092618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol 2011; 29:163-83; PMID:21219184; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della BS, Gennaro M, Vaccari M, Ferraris C, Nicola S, Riva A, Clerici M, Greco M, Villa ML et al.. Altered maturation of peripheral blood dendritic cells in patients with breast cancer. Br J Cancer 2003; 89:1463-72; PMID:14562018; http://dx.doi.org/ 10.1038/sj.bjc.6601243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caras I, Grigorescu A, Stavaru C, Radu DL, Mogos I, Szegli G, Salageanu A. Evidence for immune defects in breast and lung cancer patients. Cancer Immunol Immunother 2004; 53:1146-52; PMID:15185014; http://dx.doi.org/ 10.1007/s00262-004-0556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo SR, Corrales L, Gajewski TF. Innate immune recognition of cancer. Annu Rev Immunol 2015; 33:445-74; PMID:25622193; http://dx.doi.org/ 10.1146/annurev-immunol-032414-112043 [DOI] [PubMed] [Google Scholar]
- 30.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195:327-33; PMID:11828007; http://dx.doi.org/ 10.1084/jem.20010938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretta L, Ferlazzo G, Bottino C, Vitale M, Pende D, Mingari MC, Moretta A. Effector and regulatory events during natural killer-dendritic cell interactions. Immunol Rev 2006; 214:219-28; PMID:17100887; http://dx.doi.org/ 10.1111/j.1600-065X.2006.00450.x [DOI] [PubMed] [Google Scholar]
- 32.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Gonçalves A, André P, Romagné F, Thibault G et al.. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:21841316; http://dx.doi.org/ 10.1172/JCI45816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chittezhath M, Dhillon MK, Lim JY, Laoui D, Shalova IN, Teo YL, Chen J, Kamaraj R, Raman L, Lum J et al.. Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity 2014; 41:815-29; PMID:25453823; http://dx.doi.org/ 10.1016/j.immuni.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 34.Ramos RN, Chin LS, Dos Santos AP, Bergami-Santos PC, Laginha F, Barbuto JA. Monocyte-derived dendritic cells from breast cancer patients are biased to induce CD4+CD25+Foxp3+ regulatory T cells. J Leukoc Biol 2012; 92:673-82; PMID:22636320; http://dx.doi.org/ 10.1189/jlb.0112048 [DOI] [PubMed] [Google Scholar]
- 35.Della BS, Giannelli S, Taddeo A, Presicce P, Villa ML. Application of six-color flow cytometry for the assessment of dendritic cell responses in whole blood assays. J Immunol Methods 2008; 339:153-64; PMID:18835394; http://dx.doi.org/ 10.1016/j.jim.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 36.Duffy D, Rouilly V, Libri V, Hasan M, Beitz B, David M, Urrutia A, Bisiaux A, Labrie ST, Dubois A et al.. Functional analysis via standardized whole-blood stimulation systems defines the boundaries of a healthy immune response to complex stimuli. Immunity 2014; 40:436-50; PMID:24656047; http://dx.doi.org/ 10.1016/j.immuni.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 37.Hemont C, Neel A, Heslan M, Braudeau C, Josien R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J Leukoc Biol 2013; 93:599-609; PMID:23341538; http://dx.doi.org/ 10.1189/jlb.0912452 [DOI] [PubMed] [Google Scholar]
- 38.Ida JA, Shrestha N, Desai S, Pahwa S, Hanekom WA, Haslett PA. A whole blood assay to assess peripheral blood dendritic cell function in response to Toll-like receptor stimulation. J Immunol Methods 2006; 310:86-99; PMID:16455104; http://dx.doi.org/ 10.1016/j.jim.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 39.Mueller SC, Marz R, Schmolz M, Drewelow B. Intraindividual long term stability and response corridors of cytokines in healthy volunteers detected by a standardized whole-blood culture system for bed-side application. BMC Med Res Methodol 2012; 12:112; PMID:22853196; http://dx.doi.org/ 10.1186/1471-2288-12-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shey MS, Hughes EJ, de KM, Barnard C, Stone L, Kollmann TR, Hanekom WA, Scriba TJ. Optimization of a whole blood intracellular cytokine assay for measuring innate cell responses to mycobacteria. J Immunol Methods 2012; 376:79-88; PMID:22155193; http://dx.doi.org/ 10.1016/j.jim.2011.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jansen K, Blimkie D, Furlong J, Hajjar A, Rein-Weston A, Crabtree J, Reikie B, Wilson C, Kollmann T. Polychromatic flow cytometric high-throughput assay to analyze the innate immune response to Toll-like receptor stimulation. J Immunol Methods 2008; 336:183-92; PMID:18565537; http://dx.doi.org/ 10.1016/j.jim.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A 2008; 73:975-83; PMID:18785267; http://dx.doi.org/ 10.1002/cyto.a.20643 [DOI] [PubMed] [Google Scholar]
- 43.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ et al.. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116:e74-e80; PMID:20628149; http://dx.doi.org/ 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 44.Perrot I, Deauvieau F, Massacrier C, Hughes N, Garrone P, Durand I, Demaria O, Viaud N, Gauthier L, Blery M et al.. TLR3 and Rig-like receptor on myeloid dendritic cells and Rig-like receptor on human NK cells are both mandatory for production of IFN-gamma in response to double-stranded RNA. J Immunol 2010; 185:2080-8; PMID:20639488; http://dx.doi.org/ 10.4049/jimmunol.1000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorski KS, Waller EL, Bjornton-Severson J, Hanten JA, Riter CL, Kieper WC, Gorden KB, Miller JS, Vasilakos JP, Tomai MA et al.. Distinct indirect pathways govern human NK-cell activation by TLR-7 and TLR-8 agonists. Int Immunol 2006; 18:1115-26; PMID:16728430; http://dx.doi.org/ 10.1093/intimm/dxl046 [DOI] [PubMed] [Google Scholar]
- 46.Marshall JD, Heeke DS, Abbate C, Yee P, Van NG. Induction of interferon-gamma from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-alpha and tumour necrosis factor-alpha. Immunology 2006; 117:38-46; PMID:16423039; http://dx.doi.org/ 10.1111/j.1365-2567.2005.02261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Huang X, Yang Y. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol Ther 2008; 16:1300-7; PMID:18443600; http://dx.doi.org/ 10.1038/mt.2008.88 [DOI] [PubMed] [Google Scholar]
- 48.Gaafar A, Aljurf MD, Al-Sulaiman A, Iqniebi A, Manogaran PS, Mohamed GE, Al-Sayed A, Alzahrani H, Alsharif F, Mohareb F et al.. Defective gammadelta T-cell function and granzyme B gene polymorphism in a cohort of newly diagnosed breast cancer patients. Exp Hematol 2009; 37:838-48; PMID:19446661; http://dx.doi.org/ 10.1016/j.exphem.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 49.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol 2005; 174:727-34; PMID:15634892; http://dx.doi.org/ 10.4049/jimmunol.174.2.727 [DOI] [PubMed] [Google Scholar]
- 50.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B et al.. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012; 37:60-73; PMID:22795876; http://dx.doi.org/ 10.1016/j.immuni.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood 2003; 101:4500-4; PMID:12560217; http://dx.doi.org/ 10.1182/blood-2002-11-3569 [DOI] [PubMed] [Google Scholar]
- 52.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM et al.. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol 2001; 31:3026-37; PMID:11592079; http://dx.doi.org/ 10.1002/1521-4141(2001010)31:10%3c3026::AID-IMMU3026%3e3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- 53.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W et al.. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006; 116:1310-6; PMID:16670770; http://dx.doi.org/ 10.1172/JCI21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol 2000; 18:143-64; PMID:10837055; http://dx.doi.org/ 10.1146/annurev.immunol.18.1.143 [DOI] [PubMed] [Google Scholar]
- 55.Severa M, Remoli ME, Giacomini E, Ragimbeau J, Lande R, Uze G, Pellegrini S, Coccia EM. Differential responsiveness to IFN-alpha and IFN-beta of human mature DC through modulation of IFNAR expression. J Leukoc Biol 2006; 79:1286-94; PMID:16624932; http://dx.doi.org/ 10.1189/jlb.1205742 [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Gonzalez JS, Avila-Moreno F, Prado-Garcia H, Aguilar-Cazares D, Mandoki JJ, Meneses-Flores M. Lung carcinomas decrease the number of monocytes/macrophages (CD14+ cells) that produce TNF-alpha. Clin Immunol 2007; 122:323-9; PMID:17175197; http://dx.doi.org/ 10.1016/j.clim.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 57.Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol 2007; 178:1534-41; PMID:17237402; http://dx.doi.org/ 10.4049/jimmunol.178.3.1534 [DOI] [PubMed] [Google Scholar]
- 58.Botella-Estrada R, Escudero M, O'Connor JE, Nagore E, Fenollosa B, Sanmartin O, Requena C, Guillén C. Cytokine production by peripheral lymphocytes in melanoma. Eur Cytokine Netw 2005; 16:47-55; PMID:15809206 [PubMed] [Google Scholar]
- 59.Chatzidakis I, Mamalaki C. T cells as sources and targets of TNF: implications for immunity and autoimmunity. Curr Dir Autoimmun 2010; 11:105-18; PMID:20173390; http://dx.doi.org/ 10.1159/000289200 [DOI] [PubMed] [Google Scholar]
- 60.Shi M, Ye Z, Umeshappa KS, Moyana T, Xiang J. Alpha tumor necrosis factor contributes to CD8(+) T cell survival in the transition phase. Biochem Biophys Res Commun 2007; 360:702-7; PMID:17618911; http://dx.doi.org/ 10.1016/j.bbrc.2007.06.126 [DOI] [PubMed] [Google Scholar]
- 61.Nakano H, Kishida T, Asada H, Shin-Ya M, Shinomiya T, Imanishi J, Shimada T, Nakai S, Takeuchi M, Hisa Y et al.. Interleukin-21 triggers both cellular and humoral immune responses leading to therapeutic antitumor effects against head and neck squamous cell carcinoma. J Gene Med 2006; 8:90-9; PMID:16097036; http://dx.doi.org/ 10.1002/jgm.817 [DOI] [PubMed] [Google Scholar]
- 62.Sutherland AP, Joller N, Michaud M, Liu SM, Kuchroo VK, Grusby MJ. IL-21 promotes CD8+ CTL activity via the transcription factor T-bet. J Immunol 2013; 190:3977-84; PMID:23479229; http://dx.doi.org/ 10.4049/jimmunol.1201730 [DOI] [PubMed] [Google Scholar]
- 63.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE III. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol 2006; 177:120-9; PMID:16785506; http://dx.doi.org/ 10.4049/jimmunol.177.1.120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.