Abstract
Tumor-associated macrophages have been associated with a poor prognosis in most types of tumors. However, tumor-derived signals that activate macrophages have not been well defined. We review our recent finding that tumor-derived lactic acid is necessary and sufficient to polarize tumor-associated macrophages to a tumor-promoting state.
Introduction
Macrophages play critical roles in the maintenance of tissue homeostasis.1 To perform these roles, macrophages must sense the functional states of the parenchymal cells of the tissues in which they exist. Upon detection of deviation from homeostasis, macrophages must provide appropriate support through the production of growth factors or the phagocytosis of damaged cells. Tumors exhibit many features of abnormally developed tissues and organs, including cellular composition and tissue architecture.2 As such, tumor-associated macrophages perform homeostatic functions that facilitate tumor growth.3-5
Tumor-associated Macrophages Express the Majority of VEGF in Tumors
To study the paracrine relationship between tumor-associated macrophages and tumor cells, we first characterized tumor-associated macrophages from 3 different syngeneic tumor xenograft models of Lewis lung carcinoma (LLC), B16-F1 melanoma (B16), and CT-16.WT colon carcinoma (CT16).6 We determined that tumor-associated macrophages (CD11b+ F4/80+) represented a fixed percentage of cells within the tumors depending on tumor type, ranging from approximately 1.5% (B16) to 5.5% (LLC and CT26). When we used fluorescence-activated cell sorting to isolate pure cell populations, we found that macrophages expressed the majority of the vascular endothelial growth factor (VEGF) in the tumors compared to all other cells in the tumor. Landmark studies by Judah Folkman revealed the critical role of neovascularization in the growth of tumors. However, cancer cells have been generally assumed to be the primary source of VEGF. Our findings suggest that, at least in these 3 different tumor models, tumor-associated macrophages are the primary source of VEGF.
HIF1α is Required for Tumor-induced Expression of Vegf by Tumor-associated Macrophages
Hypoxia is well known to induce Vegf and subsequent neovascularization via HIF1α.7 However, some tumors, including lung carcinoma, are well oxygenated and yet still highly vascular. Therefore, we hypothesized that a soluble factor from tumor cells was sufficient to induce Vegf in macrophages under normoxic conditions. Using tumor-conditioned media from LLC, B16, and CT26 cells, as well as more indolent tumor types, we found that media from the most aggressive cultured tumor cells induced Vegf in bone marrow-derived macrophages in normoxia (20% O2) to similar levels as those induced by hypoxia (0.1% O2). As in hyoxia, we found that tumor supernatant stabilized HIF1α protein in bone marrow-derived macrophages under normoxic conditions. To determine whether HIF1α was required for the induction of Vegf by tumor supernatant, we generated mice with macrophages deficient in HIF1α. Induction of Vegf by both hypoxia and tumor supernatant was abrogated in these mice, indicating that this soluble factor signals through stabilization of HIF1α. Therefore, the tumor supernatant can function as a surrogate for hypoxia under normoxic conditions.
Lactic Acid Induces Vegf in Macrophages via HIF1α
Several molecules have been shown to stabilize HIF1α under normoxic conditions.8 To identify the responsible factor in our tumor supernatant, we first performed fractionation by size and determined that the factor was < 3 kDa. There are at least 4 soluble factors that can stabilize HIF1α under normoxic conditions: lactate, pyruvate, adenosine, and acidic pH. We determined that the factor was neither adenosine nor acidic pH and then focused on lactate and pyruvate. Otto Warburg observed that cancer cells preferentially metabolize glucose to lactate via aerobic glycolysis.9 The preferential production and secretion of lactate in many rapidly proliferating cells has been hypothesized to provide a biosynthetic building block for an expanding biomass of proliferating cells. We therefore wondered whether lactate could be the active signaling factor in tumor supernatants. We determined that the most aggressive cancer cell lines produced the highest concentrations of lactate in conditioned media and that this correlated with the induction of Vegf in bone marrow-derived macrophages. Consistent with this observation, stimulation of bone marrow-derived macrophages with lactic acid induced Vegf under normoxic conditions (Fig. 1). Therefore, lactic acid might not only provide a biosynthetic precursor to dividing cells but might also act as a signaling factor to support cells such as macrophages.
Figure 1.
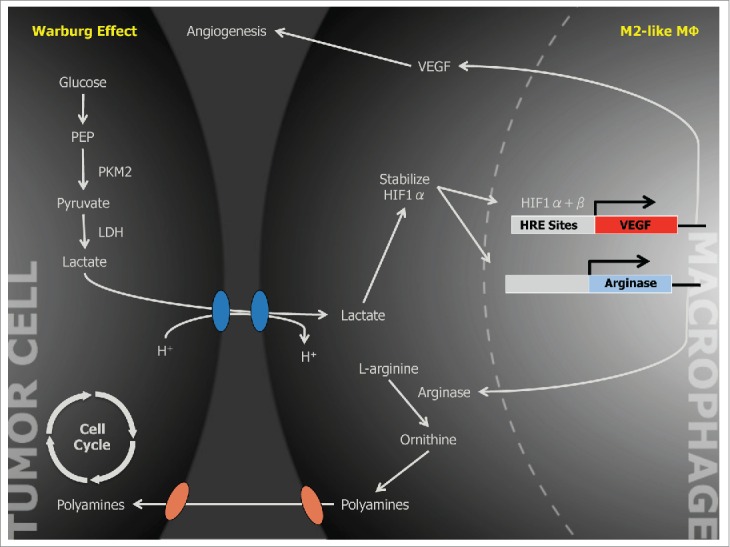
Schematic of a Proposed Tumor-promoting Paracrine Relationship Between Neoplastic Cell and Tumor-associated Macrophages.
Lactic Acid is Sufficient to Polarize Macrophages to an M2-like Tumor-promoting State
To identify the soluble factor responsible for macrophage activation, we focused on Vegf as a key index gene. However, the expression of numerous genes, most of which have unknown functional significance in tumor biology, has been associated with tumor-associated macrophages. We determined that, in addition to Vegf, lactic acid was sufficient to induce a broader set of genes associated with tumor-associated macrophages in stimulated bone marrow-derived macrophages, including Arg1, Fizz1, Mgl1, and Mgl2. Furthermore, in the absence of HIF1α, Arg1, Fizz1, and Mgl2 were expressed at a lower level compared to wild-type mice.
The cytokines IL-4 and IL-13 are well known to induce an M2 phenotype in macrophages.10 Many, but not all, genes associated with M2 macrophages are also expressed by tumor-associated macrophages, therefore tumor-associated macrophages have been described as being “M2-like.” To determine whether IL-4 or IL-13 signaling played a crucial role in tumor-associated macrophage gene induction, CT26 cells were injected into mice deficient in the IL-4 receptor α chain, which abrogates both IL-4 and IL-13 signaling. The tumor-associated macrophages from these tumors revealed no reduction in the canonical M2-associated gene, Arg1, and only a slight reduction in Fizz1 compared to wild-type mice, indicating that neither IL-4 nor IL-13 signaling was necessary for our phenotype. Conversely, macrophages deficient in HIF1α showed reduced induction of Arg1, Fizz1 and Mgl2 upon IL-4 stimulation. Taken together, these findings indicate that IL-4 and IL13 signaling is dispensable for the tumor-associated macrophage phenotype. However, HIF1α is required for the M2-like macrophage phenotype, whether induced by lactic acid or IL-4.
Although the function of VEGF in tumor biology has been well established, the function of ARG1 was unknown. ARG1 catalyzes key steps in the urea cycle and in polyamine synthesis. To determine the functional significance of ARG1, we generated mice with macrophages deficient in Arg1 and found that the tumors were approximately half the mass of tumors in WT mice at 3 weeks. To determine whether lactic acid was required for macrophage polarization in vivo, we generated LLC cells with knockdown of the Pkm2 splice isoform of pyruvate kinase associated with aerobic glycolysis, and thus with lactic acid production. Pkm2 knockdown tumors produced less lactic acid, had lower macrophage expression of Arg1, and were half the size of Pkm2 wild-type tumors. These in vivo findings demonstrated that lactic acid induction of macrophage Arg1 is critical for tumor growth.
Tumor-associated macrophages are known to be important in tumor progression. The significance of our findings is that lactic acid, a by-product of cancer cell metabolism via aerobic glycolysis, also functions as a critical signaling factor to macrophages, indicating the presence of either proliferating or hypoxic tissue. In either case, macrophages attempt to restore tissue homeostasis by expressing growth factors and enzymes, which in turn lead to tumor growth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
ORC is supported by the National Cancer Institute (1K08CA172580-01).
References
- 1.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol 2009; 9:259–70; PMID:19282852; http://dx.doi.org/ 10.1038/nri2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 2010; 18:884–901; PMID:20627072; http://dx.doi.org/ 10.1016/j.devcel.2010.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436–44; PMID:18650914; http://dx.doi.org/ 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883–99; PMID:20303878; http://dx.doi.org/ 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39–51; PMID:20371344; http://dx.doi.org/ 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al.. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513:559–63; PMID:25043024; http://dx.doi.org/ 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992; 359:843–5; PMID:1279431; http://dx.doi.org/ 10.1038/359843a0 [DOI] [PubMed] [Google Scholar]
- 8.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 2002; 277:23111–5; PMID:11943784; http://dx.doi.org/ 10.1074/jbc.M202487200 [DOI] [PubMed] [Google Scholar]
- 9.Warburg O. On the origin of cancer cells. Science 1956; 123:309–14; PMID:13298683; http://dx.doi.org/ 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- 10.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity 2010; 32:593–604; PMID:20510870; http://dx.doi.org/ 10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]


