ABSTRACT
The epidermal growth factor receptor 2 (HER-2) oncogene is a major target for the immunotherapy of breast cancer. Following up to the therapeutic success achieved with Her-2-targeting monoclonal antibodies, immune-prophylactic approaches directed against Her-2 have also been investigated taking into account, and trying to overcome, Her-2 self-tolerance. Perhaps due to safety (and efficacy) concerns, the least explored anti-Her-2 active immunization strategy so far has been the one relying on viral-vectored vaccine formulations. Taking advantage of the favorable properties of bovine herpesvirus 4 (BoHV-4) in terms of safety and ease of manipulation as well as its previously documented ability to transduce and confer immunogenicity to heterologous antigens, we tested the ability of different recombinant HER-2-BoHV-4 immunogens to 8break tolerance and elicit a protective, anti-mammary tumor antibody response in HER-2 transgenic BALB-neuT mice. All the tested constructs expressed the HER-2 transgenes at high levels and elicited significant cellular immune responses in BALB/c mice upon administration via either DNA vaccination or viral infection. In BALB-neuT mice, instead, only the viral construct expressing the membrane-bound chimeric form of Her-2 protein (BoHV-4-RHuT-gD) elicited a humoral immune response that was more intense and earlier-appearing than that induced by DNA vaccination. In keeping with this observation, two administrations of BoHV-4-RHuT-gD effectively protected BALB-neuT mice from tumor formation, with 50% of vaccinated animals tumor-free after 30 weeks from immunization compared to 100% of animals exhibiting at least one palpable tumor in the case of animals vaccinated with the other BoHV-4-HER-2 constructs.
KEYWORDS: BoHV-4-based vector, BALB-neuT mice, Her-2 oncogene, HER-2 antibodies, recombinant BoHV-4s, vaccination
Abbreviations
- ATP
adenosine triphosphate
- BoHV-4
bovine herpesvirus 4
- BAC
bacteria artificial chromosome
- BEK
bovine embryo kidney
- BSA
bovine serum albumin
- CFSE
carboxyfluorescein-diacetate-succinimidyl ester
- CPE
cytopathic effect
- DMEM
Dulbecco's modified eagle medium
- ELISPOT
enzyme linked immune-spot
- EMEM
Eagle's minimal essential medium
- Fc
crystallizable fragment
- FBS
fetal bovine serum
- gD
glycoprotein D
- HEK
human embryo kidney
- HER-2
epidermal growth factor receptor 2
- HSV-1
herpes simplex virus 1
- IFNγ
interferon gamma
- IgG
immunoglobulin G
- LB
Luria Bertani's medium
- M.O.I.
multiplicity of infection
- PBS
phosphate buffer saline
- PEI
polyethylenimine
- PCR
polymerase chain reaction
- RPMI
Roswell Park Memorial Institute medium
- SEM
standard error mean
- SFU
spot forming units
- SPC
spot-forming cells
- TCID50
tissue culture infectious dose 50
- TK
tyrosine kinase.
Introduction
Despite the significant therapeutic improvements achieved in the last decades, breast cancer remains the most important, women-affecting, solid neoplasm worldwide.1 Over-expression of the HER-2 oncogene, mainly due to gene amplification-based mechanisms2,3 occurs in ∼15–25% breast cancers, where it has been consistently associated with metastasization propensity, poor prognosis and reduced survival.4
Her-2 is a four transmembrane domain tyrosine kinase (TK) receptor. Although it is structurally and functionally well characterized, its specific ligand is still unknown. Her-2 homo/hetero dimerization induces TK domain phosphorylation, thus triggering the activation of multiple signal transduction pathways.5-7 Among these pathways, those centered on the Ras/Raf mitogen-activated protein kinase and the phosphatidil-inositole-3-kinase are the best characterized. Their deregulated activation is causally involved in cancerous phenotype development and results in altered cellular growth/division, differentiation and adhesion properties.5-7 For these reasons, Her-2 and its associated pathways are major clinical therapeutic targets. At present, two main classes of molecules are employed in the clinic to target Her-2. The first is represented by humanized monoclonal antibodies (Trastuzumab and Pertuzumab), that by targeting the extracellular portion of the receptor, interfere with Her-2 dimerization thus inducing receptor endocytosis and degradation.8,9 Importantly, these antibodies can also activate antibody- and complement-mediated cellular cytotoxicity.10 The second class of therapeutics is composed of synthetic small-molecules that interact with the ATP-binding site of the intracellular TK domain of Her-2 and block receptor phosphorylation/activation, thus preventing downstream signaling events.1,11 Of note, Trastuzumab and Her-2 TK domain inhibitors have been shown to act synergistically thus paving the way to their combined therapeutic use also in association with traditional endocrine, chemo and radiation therapies.1
Prompted by the effectiveness of passive immunization relying on anti-Her-2 antibodies, immune-prophylactic, active immunization approaches directed against Her-2 have also been extensively explored in pre-clinical models of mammary cancer.12,13 However, since Her-2 is a self-tolerated antigen,14 a major hurdle for these approaches has been the breaking of central and peripheral tolerance.14-17 Several ways to overcome this problem have been developed,18-20 including vaccination with hybrid DNA constructs coding for chimeric rat/human Her-2 proteins.21-23
A very little explored, but potentially promising tolerance breaking/immunization strategy relies on the use of viral-vectored vaccine formulations. In fact, viral vectors can deliver the antigen directly into host cells, thus leading to high-level transgene cellular expression. Key properties of an effective, and potentially translatable, viral vector are safety, the ability to properly present the expressed antigen to the immune system and to remain within host cells long enough to stimulate an effective response. A viral vector apparently meeting these criteria, including ease of manipulation (with the possibility to insert up to 30 kb of foreign DNA) and the ability to confer strong immunogenicity to heterologous antigens, is bovine herpesvirus 4 (BoHV-4). Cattle is the natural host of this virus, but BoHV-4 isolates have been retrieved from other animal species as well. In vitro, BoHV-4 is able to replicate in primary cultures and cell lines from a variety of animal species.24-30 Experimental infection of many non-natural hosts (mice,28 rats,31 rabbits,27 sheep,25 swine32 and goats30) as well as ex vivo tissue explants from non-human primates has been documented (personal communication), suggesting that BoHV-4 is most likely also competent for human cell transduction. In infected mice, BoHV-4 behaves as a replication-incompetent virus33 that preferentially localizes to cells of the monocyte/macrophage lineage.34 At variance with other gamma-herpesviruses, no evidence for growth-transformation, nor any virus-associated pathology has been reported for BoHV-4 so far. In fact, recombinant BoHV-4s expressing immune-dominant antigens from different pathogens have been successfully employed to immunize genetically modified mice without any detrimental effect, overt clinical sign or pathology correlated to viral vector inoculation.28 Furthermore, a BoHV-4-based vector armed with a Herpes Simplex virus-1 thymidine kinase (HSV-1-TK) gene displayed enhanced oncolytic properties in immune-competent orthotopic syngenic mouse and rat glioma models.29
In view of all these favorable properties, and good potential for clinical translation, we set out to test BoHV-4 as a HER-2 expression carrier and novel immuno-prophylactic agent against Her-2+ mammary cancer. Since vaccine delivery and cellular localization of vaccine-encoded antigens are key factors in modulating the induced immune responses, we assembled different recombinant HER-2-BoHV-4 viral vectors and tested their immunogenicity as well as cancer prevention capacity. The recombinant vector expressing the membrane-bound form of a hybrid, rat-human Her-2 antigen was found to be the only one capable of eliciting high anti-Her-2 antibody titers in immune-tolerant, rat HER2 transgenic (BALB-neuT) mice and to afford strong protection against autochthonous Her-2+ mammary cancer development in these animals.
Results
Design and expression of different Her-2 chimeric proteins
Before generating BoHV-4-based vectors expressing specific portions of HER-2 oncogene, three optimized ORFs coding for different HER-2 derived chimeric fragments were customized taking into account antigen subcellular localization and recognition by the immune system. RHuT-gD, a cell surface associated form, was assembled by fusing the N-terminal 1–390 aminoacids region of rat HER-2 with 299 amino acids (residues 301–691) derived from the C-terminal region of human HER-2 and gD106, a 33 peptide tag derived from bovine herpesvirus-1 glycoprotein D35 (Fig. S1). RRT-gD, a secreted form lacking the transmembrane domain, was constructed by fusing the N-terminal 1–390 amino acids region of rat HER-2 with the gD106 tag peptide (Fig. S2). An additional secreted form, potentially capable of interacting with Fc receptors and designated RRT-Fc, was generated by substituting the gD106 region of RHuT-gD with a stretch of 240 amino acids derived from the C-terminus of mouse IgG Fc (Fig. S3). RHuT-gD, RRT-gD and RRT-Fc were all placed under the transcriptional control of the CMV promoter and the bovine growth hormone polyadenylation signal to obtain the CMV-RHuT-gD, CMV-RRT-gD and CMV-RRT-Fc expression cassettes. The latter cassettes were excised from the plasmid backbone and sub-cloned into the pINT2 shuttle vector containing two BoHV-4 TK flanking sequences,24 in order to generate the targeting vectors pTK-CMV-RHuT-gD-TK (pINT2-RHuT-gD), pTK-CMV-RRT-gD-TK (pINT2-RRT-gD) and pTK-CMV-RRT-Fc-TK (pINT2-RRT-Fc) (Fig. 1A-C). The resulting constructs were functionally validated in terms of protein expression by transient transfection into HEK 293T cells and immunoblotting with a monoclonal antibody directed against the gD106 tag peptide. All three chimeric proteins were well expressed in transfected cells (Fig. 1D-F) and, as expected, RRT-gD and RRT-Fc were found to be secreted (data not shown).
Figure 1.
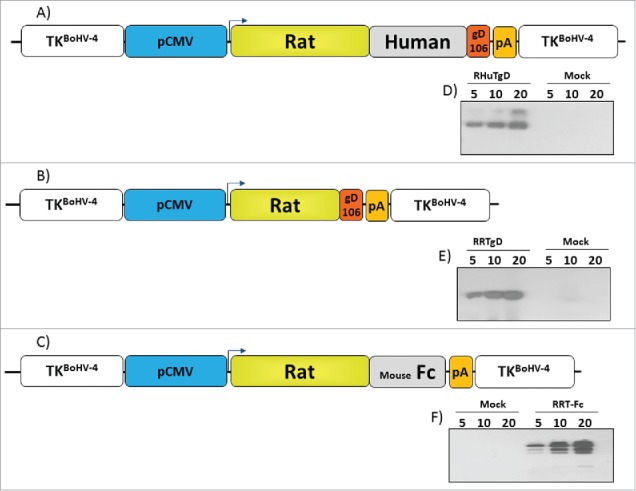
Design and expression of Her-2 chimeric proteins. Diagrams (not to scale) of (A) pTK-CMV-RHuT-gD-TK (pINT2-RHuT-gD), (B) pTK-CMV-RRT-gD-TK (pINT2-RRT-gD) and (C) pTK-CMV-RRT-Fc-TK (pINT2-RRT-Fc) targeting vectors with expression cassettes under the control of the CMV promoter (pCMV, blue) and the bovine growth hormone polyadenylation signal (PA, orange). RHuT-gD (A) and RRT-gD (B) ORFs are tagged with the gD106 peptide (red), while the RRT-Fc ORF (C) was fused to a mouse IgG Fc encoding fragment (gray). All expression cassettes are flanked by BoHV-4 TK homologous sequences (white). The results of immunoblotting analyses conducted with an anti-gD106 antibody on HEK 293Tcells transfected with pINT2-RHuT-gD, pINT2-RRT-gD and pINT2-RRT-Fc are shown in panels D-F, respectively. Individual lanes were loaded with different amounts of total protein cell extract (5, 10 and 20 μg); cells transfected with pEGFP-1 served as negative controls (Mock).
Immunogenicity profiling of the different HER-2 constructs delivered to syngeneic mice by DNA vaccination
Although all three targeting vectors (pINT2-RHuT-gD, pINT2-RRT-gD and pINT2-RRT-Fc) led to high chimeric Her-2 protein levels in HEK 293T cells, we wished to evaluate their immunogenic properties more directly before converting them to the corresponding viral delivery vectors. To this end, 3 groups of BALB/c mice (6 animals/group) were immunized twice, at 2-weeks intervals, with 50 µg of each plasmid and anti-rat Her-2 humoral and cellular immune responses were evaluated 2 weeks after the second vaccination. A targeting vector, pTK-CMV-A29gD-TK (pINT2-A29-gD), carrying an unrelated antigen from Monkeypoxvirus,36 was administered to a fourth group of mice and served as a negative control. Only mice vaccinated with pINT2-RHuT-gD, the plasmid coding for the membrane-bound form of the antigen, yielded a well-detectable anti-rat-Her-2 antibody response (Fig. 2A). However, as revealed by parallel in vivo cytotoxicity assays using lysis of syngeneic splenocytes pulsed with the immune-dominant (H2d) rat Her-2 peptide TYVPANASL as readout, all plasmids elicited a specific anti rat-HerR-2 cellular immune response (Fig. 2B). Similarly, all plasmids triggered IFNγ-producing cells upon TYVPANASL peptide re-stimulation in ELISPOT assays (Fig. 2C).
Figure 2.
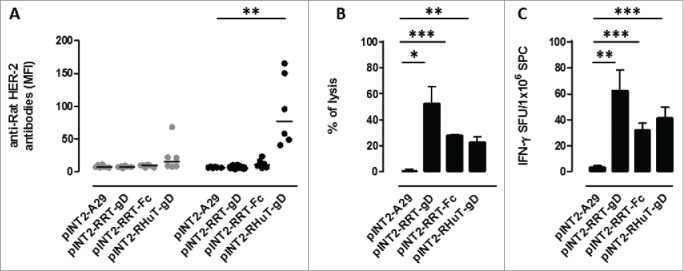
Anti-rat-Her-2 immune responses induced by DNA vaccination with the different pINT2 expression plasmids. (A) Sera from BALB/c mice collected two weeks after the first (gray dots; n=6) and the second (black dots; n=6) vaccination were analyzed (at a dilution of 1:100) for the presence of specific anti-rat-Her-2 antibodies by flow cytometry. Results are expressed as MFI values for each serum. Horizontal lines represent median values (**: p = 0.003, Student's t test). (B) In vivo cytotoxic responses against the H2d dominant, rat Her-2 TYVPANASL peptide measured two weeks after the second vaccination in mice (n=3) immunized with the indicated pINT2 plasmids; data are mean values ± SEM. (*: p = 0.02; **: p = 0.005; ***: p <0.0001; Student's t-test). (C) T-cell responses against the H2d dominant, rat Her-2 TYVPANASL peptide measured in vitro, two weeks after the second vaccination, in mice (n=3) immunized with the indicated pINT2 plasmids, using an IFNγ-based ELISPOT assay. Data, expressed as SFU/1×106 SPC, are presented as mean ± SEM values (**: p = 0.001, ***: p = 0.0008; Student's t-test).
To determine immunogenicity in a mouse model more closely resembling the cancer situation (i.e., Her-2 tolerance), we next evaluated the ability of pINT2-RHuT-gD, pINT2-RRT-gD and pINT2-RRT-Fc to induce anti-rat Her-2 antibodies in BALB-neuT mice.37 These mice display a central tolerance with deletion of rat Her-2 TYVPANASL peptide-reactive CD8+ T cells.14,15 Following vaccination (16-week-old BALB-neuT mice; n=7 per group), only animals receiving the pINT2-RHuT-gD plasmid displayed a significant anti-rat Her-2 humoral immune response (Fig. S4). As expected, given the expression of rat-Her-2 in the thymus of newborn BALB-neuT mice,21 these animals failed to mount any in vivo cytotoxic response against the TYVPANASL peptide and no IFNγ was produced by splenocytes derived from vaccinated BALB-neuT mice upon TYVPANASL re-stimulation (data not shown).
Despite the unique ability of pINT2-RHuT-gD to elicit anti-rat Her-2 antibodies in both mouse strains, all plasmids appeared to be capable to induce cellular immune responses in BALB/c mice. Therefore all three targeting vectors were carried on and used to construct the corresponding recombinant viruses.
Construction of recombinant BoHV-4 viruses containing different HER-2 expression cassettes
The genome molecular clone of a safe BoHV-4 isolate (designated as BoHV-4-A) derived from the milk cell fraction of a clinically healthy cow26 was used to construct the three recombinant HER-2-BoHV-4 vectors (BoHV-4-RHuT-gD, BoHV-4-RRT-gD and BoHV-4-RRT-Fc) plus a control viral vector (BoHV-4-A29-gD) delivering a completely unrelated antigen. To this end, pINT2-RHuT-gD, pINT2-RRT-gD, pINT2-RRT-Fc and the pINT2-A29-gD plasmid vectors were first linearized by a restriction enzyme digestion sparing the BoHV-4 TK flanking regions. Linearized plasmids were then electroporated into SW102 E. coli cells containing the artificial chromosome pBAC-BoHV-4-A-KanaGalKΔTK26,38,39 (Fig. 3A), in order to generate pBAC-BoHV-4-A-CMV-RHuT-gD-ΔTK, pBAC-BoHV-4-A-CMV-RRT-gD-ΔTK, pBAC-BoHV-4-A-CMV-RRT-Fc-ΔTK (Fig. 3B) and pBAC-BoHV-4-A-CMV-A29-gD-ΔTK artificial chromosomes via heat-inducible homologous recombination.40 The TK locus of the BoHV-4 genome is extremely stable even after repeated passages in vitro and in vivo, and it can thus be reliably employed to integrate foreign DNA sequences into the BoHV-4 genome without any transgene or viral replication efficiency loss due to recombination.24-30
Figure 3.
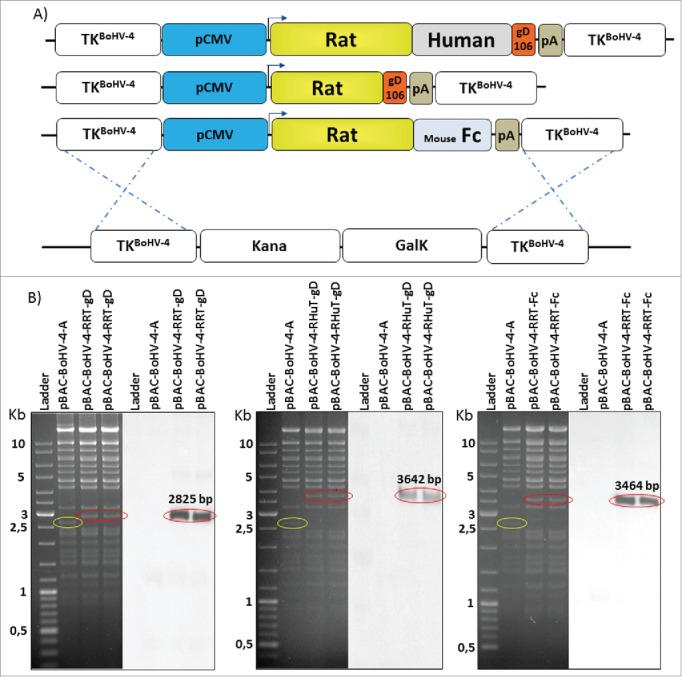
Recombinant BoHV-4 constructs. (A) Diagram (not to scale) illustrating the re-targeting event (i.e., replacement of the Kana/GalK cassette with the CMV-RHuT-gD, CMV-RRT-gD and CMV-RRT-Fc expression cassettes) generated by heat-inducible homologous recombination in SW102 E. coli cells containing pBAC-BoHV-4-A-TK-KanaGalK-TK. (B) Two representative, 2-deoxy-galactose resistant colonies for each recombinant pBAC-BoHV-4 genome, tested by Hind III restriction enzyme analysis and DNA gel blotting performed with a probe targeting the rat HER-2 portion of each chimeric ORF. The 2,650 bp band (circled in yellow) corresponding to the non-retargeted pBAC-BoHV-4-A-TK-KanaGalK-TK control is replaced by 2,825 bp, 3,642 bp and 3,464 bp bands (circled in red) in pBAC-BoHV-4-RRT-gD, pBAC-BoHV-4-RHuT-gD and pBAC-BoHV-4-RRT-Fc, respectively.
Selected SW102 E. coli clones carrying pBAC-BoHV-4 recombinants were analyzed by HindIII restriction enzyme digestion and confirmed by DNA blotting with probes specific for the three chimeric ORFs (Fig. 3B). Stability of the pBAC-BoHV-4 recombinant clones in E. coli cells (i.e., the absence of restriction pattern alterations upon artificial chromosome propagation) was verified by restriction enzyme digestion after multiple (up to 20) serial passages (Fig. S5).
To produce viable, replication-competent recombinant viral particles, pBAC-BoHV-4-A-CMV-RHuT-gD-ΔTK, pBAC-BoHV-4-A-CMV-RRT-gD-ΔTK and pBAC-BoHV-4-A-CMV-RRT-Fc-ΔTK DNA constructs were electroporated into standard or cre recombinase-expressing BEK cells. The latter cells stably express the phage D1 cre recombinase26 and allow for the site-specific removal of the floxed, GFP cassette-containing BAC sequence from the BAC-BoHV-4 genome. As a consequence of this removal and new cassette insertion, viral plaques generated on a BEKcre cell monolayer lost the characteristic GFP fluorescence compared to parallel plaques seeded onto a standard BEK cell monolayer (Fig. 4A, D and G). Although viable BoHV-4-RHuT-gD, BoHV-4-RRT-gD and BoHV-4-RRT-Fc virus particles were successfully reconstituted in BEK or BEKcre cells, as demonstrated by the cytopathic effect (CPE) observed in the cell monolayer, it was of interest to determine their replication properties with respect to the parental BoHV-4-A virus. As apparent in Fig. 4B, E and H, BoHV-4-RHuT-gD, BoHV-4-RRT-gD and BoHV-4-RRT-Fc, all displayed a slower replication rate compared to the reference BoHV-4-A type. Furthermore, as revealed by immunoblotting analysis of infected cell extracts, they all expressed the corresponding HER-2 transgenes (Fig. 4C, F and I) and as expected, BoHV-4-RHuT-gD targeted transgene expression to the cell membrane (Fig. S6).
Figure 4.
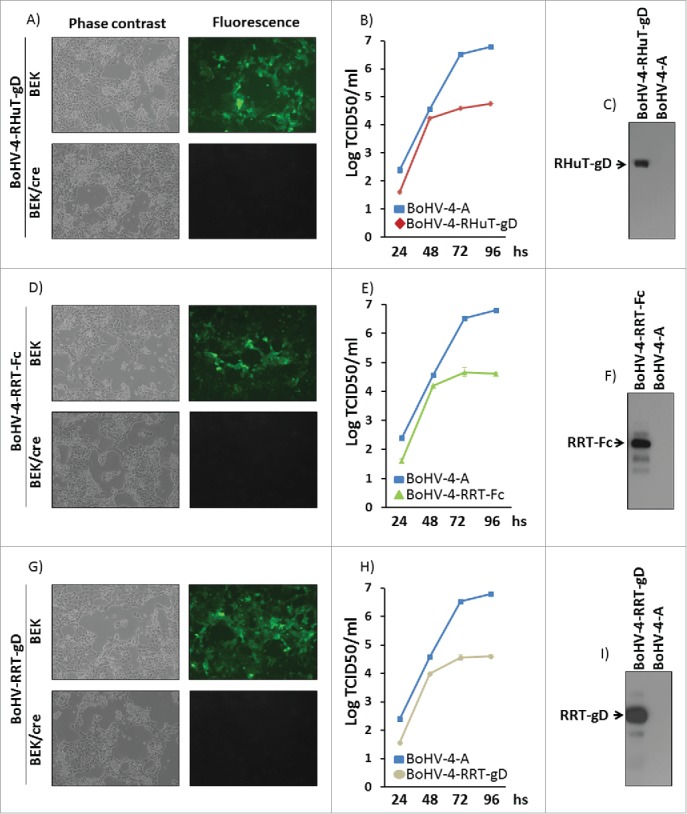
Reconstitution and characterization of recombinant viruses. Representative phase contrast and fluorescent microscopy images of the plaques formed by viable, reconstituted recombinant BoHV-4-RHuT-gD (A), BoHV-4-RRT-Fc (D) and BoHV-4-RRT-gD (G) after electroporation of the corresponding BAC DNA clones into BEK or BEKcre cells (magnification, ×10). Replication rates of BoHV-4-RHuT-gD, BoHV-4-RRT-Fc and BoHV-4-RRT-gD grown in BEK cells are shown in panels (B), (E) and (H), respectively, and compared with those of the parental BoHV-4-A isolate. The data mean ± standard error of triplicate measurements (p >0.05 for all time-points; Student's t-test). The results of immunoblotting analyses conducted on extracts from cells infected with BoHV-4-RHuT-gD, BoHV-4-RRT-Fc and BoHV-4-RRT-gD are shown in panels (C), (F) and (I), respectively; BoHV-4-A infected cells served as negative controls.
Higher immunogenicity of HER-2 antigens delivered as BoHV-4 recombinant viral particles compared to DNA immunization
To test the immunogenicity of the different recombinant virus particles, 4 groups of BALB/c mice (7 animals/group) were vaccinated twice intraperitoneally (i.p.), at two weeks intervals, with BoHV-4-RHuT-gD, BoHV-4-RRT-gD and BoHV-4-RRT-Fc, plus the unrelated BoHV-4-A29-gD control. Two weeks after the second immunization, sera were collected and tested for the presence of anti-rat-Her-2 antibodies. No specific anti-rat-Her-2 humoral immune response was detected in mice vaccinated with either BoHV-4-RRT-gD or BoHV-4-RRT-Fc. In contrast, BoHV-4-RHuT-gD viral particles elicited a sustained anti-rat-Her-2 antibody response (Fig. 5A), significantly higher (˜2.5-fold) than the one previously detected in BALB/c mice immunized with the pINT2-RHuT-gD plasmid (p = 0.02) (Fig. 2A). Also, while BoHV-4-A29-gD viral particles did not induce any appreciable cytotoxic response against TYVPANASL-pulsed syngeneic splenocytes, all HER-2 containing recombinant BoHV-4 viral particles induced a strong cytotoxicity (Fig. 5B), significantly higher (˜3-fold) than that induced by the corresponding pINT2 plasmids delivered through DNA vaccination (p = 0.009 for BoHV-4-RHuT-gD vs. pINT2-RHuT-gD; p = 0.03 for BoHV-4-RRT-gD vs. pINT2-RRT-gD; p < 0.0001 for BoHV-4-RRT-Fc vs. pINT2-RRT-Fc). Similarly, IFNγ–producing cells were induced at high frequency by TYVPANASL peptide restimulation of BoHV-4-HER-2- vaccinated mice (Fig. 5C), with no statistically significant difference between the three experimental groups.
Figure 5.
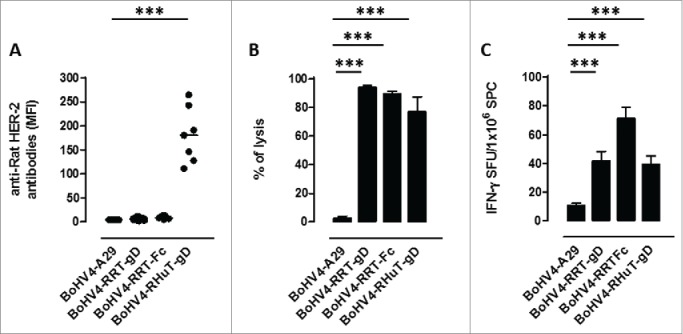
Anti-Her-2-specific immune responses induced by intraperitoneal vaccination with the different recombinant BoHV-4 viral particles. (A) Sera from BALB/c mice (n = 7), collected two weeks after the second vaccination, analyzed (at a 1:100 dilution) for the presence of specific anti-rat-Her-2 antibodies by flow cytometry. Results are expressed as MFI values for each serum; horizontal lines represent median values (***: p < 0.0001; Student's t-test). (B) In vivo cytotoxic responses against the H2d dominant, rat Her-2 peptide TYVPANASL measured in mice immunized with the indicated BoHV-4 viral particles (n = 3) two weeks after the second vaccination; data are mean ± SEM values (***: p = 0.0004; Student's t-test. (C) T-cell responses against the H2d dominant, rat Her-2 TYVPANASL peptide determined two weeks after the second vaccination by an in vitro IFNγ-based ELISPOT assay. IFNγ-producing cells from mice immunized with the indicated BoHV-4 viral particles (n = 3) are expressed as SFU/1×106 SPC; data are mean ± SEM values (***: p < 0.0001; Student's t-test).
BoHV-4-RHuT-gD affords a significant protection against rat-HER-2-driven mammary carcinogenesis in BALB-neuT mice
In HER-2-tolerant BALB-neuT mice, similarly to what we observed after vaccination with pINT2 plasmid DNA (Fig. S4), only BoHV-4-RHuT-gD (i.e., BoHV-4-A containing the RHuT-gD expression cassette) effectively induced anti-rat-Her-2 antibodies, at levels considerably higher (˜3-folds) than those elicited by pINT2 (Fig. 6A). The superior immunogenicity of BoVH-4-RHuT-gD is also supported by the earlier appearance of anti-rat Her-2 antibodies, which were already well detectable after the first vaccination and increased thereafter, reaching titers significantly higher than those elicited by pINT2-RHuT-gD (p <0.0001). Most importantly, the presence of anti-rat-Her-2 antibodies in sera from BALB-neuT mice vaccinated with BoHV-4-RHuT-gD correlated with a significant delay of mammary tumor appearance. In fact, 50% of BoHV-4-RHuT-gD-vaccinated BALB-neuT mice were completely tumor-free at week 30, when 100% of BoHV-4-RRT-gD- and BoHV-4-RRT-Fc-vaccinated animals already displayed at least one palpable tumor (Fig. 6B).
Figure 6.
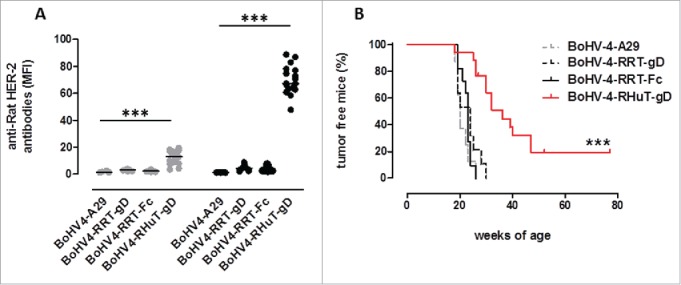
Anti-rat-Her-2 antibody production and delayed mammary tumor appearance induced by vaccination with BoHV-4-RHuT-gD viral particles. (A) Sera from BALB-neuT mice, collected two weeks after the first (gray dots; n = 8 to 17 animals/group) and the second (black dots; n = 7-17 animals/group) i.p. immunization with the indicated BoHV-4 particles, were analyzed by flow cytometry (at a 1: 100 dilution) for the presence of specific anti-rat-Her-2 antibodies. Results are expressed as MFI values for each serum; horizontal lines represent median values (***: p < 0.0001; Student's t-test). (B) Mammary tumor incidence in control BoHV-4-A29-gD (dotted gray line, n = 8), BoHV-4-RRT-gD, (dotted black line, n = 11), BoHV-4-RRT-Fc (solid black line, n = 11) and BoHV-4-RHuT-gD (solid red line, n = 17) BALB-neuT mice vaccinated with the indicated BoHV-4 viral particles (***: p <0.0001; Mantel–Haenszel Log-rank test).
Discussion
The aim of this study was to investigate the potential of BoHV-4 as a safe, potent and large-capacity vaccine vector able to deliver HER-2-derived engineered antigens and to protect HER-2 transgenic, BALB-neuT mice from autochthonous mammary cancer. BALB-neuT mice were used as a model system because they share a number of features with human breast cancer. In fact, following multi-step progression,14 each BALB-neuT mammary gland spontaneously develops an independent rat-Her-2+ tumor,12,41 which becomes invasive and metastasizes to the bone marrow first and subsequently to the lungs.42 Moreover, similarly to patients with Her-2+ carcinomas, BALB-neuT mice lack high-affinity, Her-2 peptide-recognizing cytotoxic T lymphocytes,15 with an expansion of T regulatory 14 and myeloid immature suppressor cells43 during carcinogenesis progression. For all these reasons, BALB-neuT mice represent an excellent model system to set up and test novel therapeutic and immune-prophylactic approaches to control breast cancer.37
Initially, CMV-RHuT-gD, CMV-RRT-gD and CMV-RRT-Fc expression cassettes were successfully tested in terms of immune response by DNA vaccination. While all plasmids were able to induce a significant anti-rat Her-2 cellular immune response, only mice vaccinated with pINT-2-RHuT-gD displayed a significant production of anti-rat Her-2 antibodies. As expected and already seen in other rat-HER-2 transgenic (CB6F1-neuT) mice vaccinated with a DNA plasmid coding for RHuT, the antibody titer measured in BALB-neuT mice was significantly lower than that of BALB/c mice, being the former deeply tolerant to rat-Her-2 protein.21
The fact that mice vaccinated with the plasmids coding for the secreted forms of rat Her-2 did not produce anti-rat Her-2 antibodies was somehow surprising considering that we have previously demonstrated that vaccination with the soluble extracellular domain (ECD) of the rat Her-2, administered either as DNA or as protein, is able to induce serum anti-rat Her-2 antibodies.41,44 However, in the present work the Her-2 soluble portion encoded by the pINT-2-RTT-gD and pINT-2-RTT-Fc plasmids contains only the first 390 amino acids of the rat protein. The majority of antibody-recognized epitopes is conformational and is made up of discontinuous segments of a peptide that are brought together during the protein folding process.45 In the case of the rat Her-2 protein it has been demonstrated that a stable conformation of the ECD is achieved thanks to a large interface between domains I and III.46 It is thus conceivable that the lack of part of domain III (from amino acid 391 to amino acid 488)47 in the soluble rat Her-2 protein encoded by our plasmids prevents the classical folding, leading to the exposition of epitopes not expressed or inaccessible in the naïve protein.
We then constructed the three corresponding BoHV-4 vectors and evaluated their replication capacity by comparison with the parental BoHV-4-A strain. HER-2-containing recombinant viruses displayed a slower replication, likely attributable to a toxic effect caused by transgene over-expression. Despite their lower replication rate, however, all recombinant viruses abundantly expressed HER-2-derived transgenes in infected cells and triggered sustained T cell immune responses in BALB/c mice. In contrast, BoHV-4-RHuT-gD only was found to be capable of inducing a strong humoral anti-rat Her-2 response in BALB/c and BALB-neuT mice. In both mouse strains, the intensity of this response was significantly higher than that observed in mice vaccinated with the RHuT-encoding pINT-2 plasmid and an earlier anti-rat Her-2 antibody response, already detectable after the first vaccination, was observed in BALB-neuT mice. This sustained antibody production likely explains the striking delay in mammary cancer appearance brought about by BoHV-4-RHuT-gD vaccination. In fact, anti-rat Her-2 antibodies have previously been shown to cause a marked downregulation and cytoplasmic confinement of rat Her-2 both in vitro13,41 and in vivo,12,21 with a concomitant impairment of Her-2 mediated PI3K/Akt signaling.48 In addition, anti-Her-2 antibodies may activate complement-mediated lysis and antibody-dependent cytotoxicity.12,21
BoHV-4-delivered, membrane-bound rat-human Her-2 proved to be superior to the same antigen delivered through DNA vaccination with regard to tolerance breaking and humoral immune response induction. This suggests that BoHV-4-based Her-2 vaccines might be superior to pINT2-based Her2 vaccines also in preventing mammary tumor formation. It should be noted that BoHV-4-RHuT-gD vaccine protects 20% of BALB-neuT mice for all their life, a result never obtained after two immunizations against Her-2 using other vaccination strategies, performed at the same stage of tumor progression as in the present work.21,49-51 Optimization of dosage, administration route and boosting schedule of BoHV-4-RHuT-gD vaccine, would result in a even more sustained antitumor efficacy. Further experiments are warranted to demonstrate this issue.
Various anti-Her-2 vaccine formulations have been tested in recent years, both in pre-clinical cancer models and in the clinic. These include allogenic Her-2+ tumor cells,52-55 Her-2 peptide-presenting autologous dendritic cells,55,56 Her-2 protein/peptide immunogens,57,58 Her-2-based DNA vaccines,12,21, 23,41, 59 virus-like particles carrying Her-250,60 and even a chimeric recombinant Her-2 antigen expressed by an attenuated strain of Listeria monocytogenes.61 Viral delivery vectors, instead, have received much less attention, recombinant vaccinia62,63 and adenovirus based vectors are the only viral vectors tested, with quite encouraging results, so far.51,64 This likely reflects potential concerns with safety and anti-vector immunity. Since risk associated with virus-mediated delivery represents a major issue in viral vector development, attenuation is usually regarded as a highly desirable feature and many efforts are directed toward the development of highly attenuated viral strains with decreased virulence. In this study, we took advantage of the natural non-pathogenicity of BoHV-4, which was previously proved in both standard and genetically modified mouse strains.28,33 Further to this point, we also previously inoculated high BoHV-4 doses intracerebrally with no apparent negative side-effect, and found that the virus effectively transduced brain cells in the area of inoculation, leading to high-level expression of the GFP transgene.65 Therefore, BoHV-4 naturally behaves as a replication-incompetent viral vector that does not require further attenuation. One other major advantages of BoHV-4 is its natural inability to induce serum neutralizing antibody responses. This alleviates most concerns regarding the occurrence of pre-existing, host anti-vector antibodies (as it is the case for adenovirus-based vectors) and allows for multiple immunizations, if required.
In conclusion, our study highlights the favorable properties and potential advantages of BoHV-4 as a highly effective viral vector for cellular Her-2 delivery in order to achieve mammary cancer prophylaxis through a potential one-shot active immunization. Given the previous demonstration of the oncolytic properties of BoHV-4,29 future work will address the feasibility (and efficacy) of combined prophylactic and therapeutic approaches based on the use of this particular viral vector.
Materials and methods
Cell lines
Bovine embryo kidney [(BEK) from Dr. M. Ferrari, Istituto Zooprofilattico Sperimentale, Brescia, Italy; (BS CL-94)], BEK expressing cre recombinase (BEK cre),26 Human Embryo Kidney 293T [(HEK 293T) ATCC: CRL-11268], Mus musculus mammary gland [(NMuMG) ATCC: CRL-1636] and NIH3T3 murine fibroblasts expressing rat-Her-2 protein (3T3/NKB cells)66 cell lines were cultured in Eagle's minimal essential medium (EMEM, Lonza) containing 10% fetal bovine serum (FBS), 2 mM of L-glutamine (SIGMA), 100 IU/mL of penicillin (SIGMA), 100 μg/mL of streptomycin (SIGMA) and 2.5 μg/mL of Amphotericin B (SIGMA) and incubated at 37°C, 5% CO2.
PCR
The 2,067 bp rat-human transmembrane protein (RHuT) and the 1,250 bp rat ECD of rat Her-2 protein (RRT) were ampl-ified from pVAX RHuT plasmid67 with NheI-RHuT sense (5′-CCCGCTAGCCCACCATGATCATCATGGAGCTGGCG-GCC-3′) and SalI-RHuT antisense (5′-CCCCGAGTCGACCTTCCGGATCTTCTGCTGCCGTCG-3′) and with NheI-RRT sense (5′-CCCGCTAGCCCACCATGATCATCATGGAGCTG-3′) and SalI-RRT antisense (5′-CCCCGAGTCGACTGTGATCTCCTCCAGGGTTTCGAAC-ACTTGGAG3′) primer pairs, respectively.
The PCR amplification reactions were carried out in a final volume of 50 μL, containing 10 mM Tris–hydrochloride pH 8.3, 0.2mM deoxynucleotide triphosphates, 3 mM MgCl2, 50 mM KCl and 0.25 μM of each primer. One hundred nanograms of DNA was amplified over 35 cycles, each cycle consisting of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min and chain elongation with 1U of Pfu DNA polymerase (Fermentas) at 72°C for 150 sec, in the case of RHuT, and at 72°C for 90 sec, in the case of RRT. The so generated 2,067 bp and 1,250 bp amplicons were then checked in 1% agarose gel and visualized after ethidium bromide staining in 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA).
Constructs generation
The NheI-RHut-SalI amplified fragment (2,067 bp) was firstly sub-cloned inside the previously NheI/SalI digested pIgkE2gD106, an eukaryotic expression vector containing a gD106 epitope of Bovine herpesvirus 1 glycoprotein D, successfully used as a tag during the cloning.35 The obtained pIgkRHuTgD106 was subsequently digested with NheI/BamHI to insert the 2,169 bp fragment RHuTgD106 into pEGFP-C1 vector (Addgene), digested with the same enzymes, to remove EGFP gene and to generate pCMVRHuTgD106. Finally, the 2,421 bp Nhe/MluI-blunt ended fragment, containing RHuTgD106 with Simian Virus 40 poly A, was excised and inserted inside pINT2EGFPTK shuttle vector,24 cut with NheI/SmaI restriction enzymes, to obtain pINT2-RHuT-gD.
The amplified NheI-RRT-SalI (1,250 bp) fragment was sub-cloned into NheI/SalI previously digested pIgkE2gD10635 and pIgkE2Fc, an eukaryotic expression vector expressing the crystallizable fragment (Fc) of mouse Immunoglobulin, used as a tag and soluble secreted fragment. RRT-gD106 (1,352 bp) and RRTFc (1,992 bp) were excised with the double digestion NheI/BamHI and inserted into NheI/BamHI digested pEGFP-C1 (Addgene), to remove EGFP gene and generate pCMV-RRT-gD106 and pCMV-RRT-Fc. NheI/MluI-blunt ended fragments containing RRT-gD106 (1,604 bp) or RRT-Fc (2,244 bp), containing the Simian Virus 40 poly A, were excised and inserted inside pINT2EGFPTK shuttle vector,24 cut with NheI/SmaI restriction enzymes, to obtain pINT2-RRT-gD and pINT2-RRT-Fc.
Transient transfection
Confluent HEK 293T cells were seeded into six well plates (3 × 105 cells/well) and incubated at 37 °C with 5% CO2; when the cells were sub-confluent the culture medium was removed and the cells were transfected with pINT2-RHuT-gD, pINT2-RRT-gD and pINT2-RRT-Fc, using Polyethylenimine (PEI) transfection reagent (Polysciences, Inc.). Briefly, 3 μg of DNA were mixed with 7,5 μg PEI (1mg/mL) (ratio 1:2.5 DNA-PEI) in 200 μL of Dulbecco's modified essential medium (DMEM) at high glucose percentage (Euroclone) without serum. After 15 min at RT, 800 μL of medium without serum were added and the transfection solution was transferred to the cells and left on the cells for 6 h at 37°C with 5% CO2 in air, in a humidified incubator. The transfection mixture was then replaced with fresh medium (EMEM, with 10% FBS, 50 IU/mL of penicillin, 100 μg/mL of streptomycin and 2.5 μg/mL of Amphotericin B) and incubated for 24 h at 37°C with 5% CO2.
Viruses
BoHV-4-RHuT-gD, BoHV-4-RRT-gD, BoHV-4-RRT-Fc and BoHV-4-A were propagated by infecting confluent monolayers of BEK cells at a multiplicity of infection (M.O.I.) of 0.5 50% tissue culture infectious doses (TCID50) per cell and maintained in medium with only 2% FBS for 2 h. The medium was then removed and replaced with fresh EMEM containing 10% FBS. When the CPE interested the majority of the cell monolayer (∼72 h post infection), the virus was prepared by freezing and thawing cells three times and pelleting the virions through a 30% sucrose cushion, as described previously.65 Virus pellets were then resuspended in cold EMEM without FBS. TCID50 were determined with BEK cells by limiting dilution.
Western immunoblotting
Protein cell extracts were obtained from a six-well confluent plate of HEK 293T transfected with pINT2-RHuT-gD, pINT2-RRT-gD and pINT2-RRT-Fc and from 25-cm2 confluent flasks of BEK infected with BoHV-4-RHuT-gD, BoHV-4-RRT-gD, BoHV-4-RRT-Fc by adding 100 μL of cell extraction buffer (50 mM Tris–HCl, 150 mM NaCl, and 1% NP-40; pH 8). A 10% SDS–PAGE gel electrophoresis was used to analyze cell extracts containing 50 μg of total protein, after protein transfer in nylon membranes by electroblotting, the membranes were incubated with primary bovine anti BoHV-1 glycoprotein D monoclonal antibody (clone 1B8-F11; VRMD, Inc.., Pullman, WA), diluted 1:15.000, and then with a secondary antibody probed with horseradish peroxidase-labeled anti-mouse immunoglobulin (A 9044; Sigma), diluted 1:10.000, to be visualized by enhanced chemiluminescence (ECL Kit; Pierce). pINT2-RRT-Fc and BoHV-4-RRT-Fc protein extracts were directly incubated with the secondary antibody probed with horseradish peroxidase-labeled anti-mouse immunoglobulin (A 9044; Sigma), recognizing the Fc tag.
BAC recombineering and selection
Recombineering was performed as previously described40 with some modifications. Five hundred microliters of a 32°C overnight culture of SW102 containing BAC-BoHV-4-A-Kana-GalKΔTK, were diluted in 25 ml Luria–Bertani (LB) medium with or without chloramphenicol (SIGMA) selection (12.5 mg/mL) in a 50 mL baffled conical flask and grown at 32°C in a shaking water bath to an OD600 of 0.6. Then, 10 mL were transferred to another baffled 50 mL conical flask and heat-shocked at 42°C for exactly 15 min in a shaking water bath. The remaining culture was left at 32°C as the un-induced control. After 15 min the two samples, induced and un-induced, were briefly cooled in ice/water bath slurry and then transferred to two 15 mL Falcon tubes and pelleted using 5,000 r.p.m. (eppendorf centrifuge) at 0°C for 5 min. The supernatant was poured off and the pellet was resuspended in 1mL ice-cold ddH2O by gently swirling the tubes in ice/water bath slurry. Subsequently, 9 mL ice-cold ddH2O were added and the samples pelleted again. This step was repeated once more, the supernatant was removed and the pellet (50 μL each) was kept on ice until electroporated with gel-purified PvuI (Fermentas) linearized pINT2-RHuT-gD, pINT2-RRT-gD and pINT2-RRT-Fc. An aliquot of 25 μL was used for each electroporation in a 0.1 cm cuvette at 25 μF, 2.5 kV and 201Ω. After electroporation, for the counter selection step, the bacteria were recovered in 10 mL LB in a 50 mL baffled conical flask and incubated for 4.5 h in a 32°C shaking water bath. Bacteria serial dilutions were plated on M63 minimal medium plates containing 15 g/L agar, 0.2% glycerol, 1mg/L D-biotin, 45 mg/L L-leucine, 0.2% 2- deoxy-galactose and 12.5 mg/mL chloramphenicol. All the complements for M63 medium were purchased from SIGMA.
Plates were incubated 3–5 d at 32°C; then several selected colonies were picked up, streaked on McConkey agar indicator plates (DIFCO, BD Biosciences) containing 12.5 mg/mL of chloramphenicol and incubated at 32°C for 3 d until white colonies appeared. White colonies were grown in duplicate for 5–8 h in 1mL of LB containing 50 mg/mL of kanamycin (SIGMA) or LB containing 12.5 mg/mL of chloramphenicol. Only those colonies that were kanamycin negative and chloramphenicol positive were kept and grown overnight in 5 mL of LB containing 12.5 mg/mL of chloramphenicol. BAC DNA was purified and analyzed through HindIII restriction enzyme digestion. DNA was separated by electrophoresis overnight in a 1% agarose gel, stained with ethidium bromide, and visualized to UV light.
Original detailed protocols for recombineering can also be found at the recombineering website (http://recombineering.ncifcrf).
Non isotopic Southern blotting
DNA from 1% agarose gel was capillary transferred to a positively charged nylon membrane (Roche), and cross-linked by UV irradiation by standard procedures.26
The membrane was pre-hybridized in 50 mL of hybridization solution (7% SDS, 0.5 M phosphate, pH 7.2) for 1 h at 65°C in a rotating hybridization oven (Techna instruments). The 1,250 bp amplicon for RRT digoxigenin-labeled probe was generated by PCR with the primers NheI-RRT sense and SalI-RRT antisense, as previously described.27 PCR amplification was carried out in a final volume of 50 μL of 10 mM Tris–HCl, pH 8.3, containing 0.2 mM deoxynucleotide triphosphates, 0.02 mM alkaline labile digoxigenin-dUTP (Roche), 3 mM MgCl2, 50 mM KCl, and 0.25 μM of each primer over 35 cycles, each cycle consisting of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min, and chain elongation with 1 U of Taq polymerase (Thermo Scientific) at 72°C for 90 sec. A parallel reaction omitting digoxigenin dUTP was performed, because digoxigenin incorporation into the amplicon can be checked through the size shift of the amplicon by gel electrophoresis. Five microliters of the probe were added to 500 μL of ddH2O into a screw-cap tube, denatured in boiling water for 5 min, and cooled down on ice for another 2 min. Denatured probe was added to 50 mL of pre-heated 65°C hybridization solution (7% SDS, 0.5 M phosphate, pH 7.2 and 1 mM EDTA) to the pre-hybridized membrane and hybridized overnight at 65°C in a rotating hybridization oven (Techna Instruments). Following hybridization, the membrane was washed twice for 30 min with 100 mL of washing solution I (0.5 × SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate] and 0.1% SDS) and twice for 30 min with 100 mL of washing solution II (40 mM phosphate, pH 7.2, 0.05% SDS) at 65°C. On a freshly washed dish, the membrane was incubated for 30 min at room temperature in 100 mL of blocking solution (100 mM maleic acid, pH 7.5, 150 mM NaCl, 1% blocking reagent [Roche]). Anti-digoxigenin Fab fragment (150 U/200 μL [Roche]), diluted 1:15.000 in 50 mL of blocking solution, was applied to the membrane for 30 min under gentle shaking at room temperature and washed twice for 15 min with 100 mL of washing solution (100 mM maleic acid, pH 7.5, 150 mM NaCl, 0.3% Tween 20). Detection was performed following equilibration of the membrane in detection buffer (100 mM Tris–HCl, pH 9.5, 1 mM EDTA) for 2 min at room temperature. Chemiluminescent substrate (CSPD, Roche) was added by scattering the drops over the surface of the membrane after placement of the membrane between two plastic sheets, and any bubbles present under the sheet were eliminated with a damp lab tissue to create a liquid seal around the membrane. Signal detection was obtained, exposing the membrane to X-ray film. The exposure time was adjusted with the intensity of the signal.
Cell culture electroporation and recombinant virus reconstitution
BEK or BEKcre cells were maintained as a monolayer with complete EMEM growth medium with 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin and 100 µg/mL streptomycin.
When cells were sub-confluent (70–90%) they were split to a fresh culture vessel (i.e., every 3–5 d) and were incubated at 37°C in a humidified atmosphere of 95% air–5% CO2.
BAC DNA (5 μg) was electroporated in 600 μL DMEM without serum (Equibio apparatus, 270 V, 960 mF, 4-mm gap cuvettes) into BEK and/or BEKcre cells from a confluent 25–cm2 flask. Electroporated cells were returned to the flask, after 24 h the medium was replaced with fresh medium, and cells were split 1:2 when they reached confluence at 2 d post-electroporation. Cells were left to grow until the appearance of CPE. Recombinant viruses were propagated by infecting confluent monolayers of BEK cells at a M.O.I. of 0.5 TCID50 per cell and maintaining them in MEM with 10% FBS for 2 h.
Viral growth curves
BEK cells were infected with BoHV-4-A, BoHV-4-RHuT-gD, BoHV-4-RRT-gD, BoHV-4-RRT-Fc at a M.O.I. of 0,1 TCID50/cell and incubated at 37°C for 4 h. Infected cells were washed with serum-free EMEM and then overlaid with EMEM containing 10% FBS, 2 mM L-glutamine, 100 IU/mL penicillin, 100 µg/mL streptomycin and 2.5 µg/mL Amphotericin B. The supernatants of infected cultures were harvested after 24, 48, 72 and 96 h, and the amount of infectious virus was determined by limiting dilution on BEK cells.
Mice
BALB/c (Charles River) and BALB-neuT (Ariano Irpino, Italy)68 mice were bred under specific pathogen-free conditions (Allentown Caging Equipment, Allentown, NJ, USA) at the Molecular Biotechnology Centre (Torino, Italy) and treated according to the European Guidelines and policies, as approved by the University of Torino Ethical Committee. To assess mammary tumor incidence BALB-neuT females were inspected weekly by palpation, and progressively growing masses with a mean diameter of >1 mm were regarded as tumors. Each tumor mass was measured with a calliper in the two perpendicular diameters. Growth was monitored until all 10 mammary glands displayed a tumor or until a tumor exceeded a mean diameter of 10 mm, at which time mice were sacrificed for humane reasons.
Mice immunization
Recombinant pINT2 plasmids were purified by large scale preparation using the EndoFree Plasmid Giga kits (Qiagen, Inc., CA, USA). Ten week-old BALB/c and BALB-neuT mice were anesthetized by intramuscular injection (i.m.) of 40 μL of a solution containing 5.7 μL of Zoletil 100 (Vibrac, Milano, Italia), 3.5 μL of Rompum (Bayer, Milano, Italia) and 37.5 μL of Phosphate Buffer Saline (PBS) (GIBCO, Grand Island, NY, USA). Anesthetized mice were injected in the quadriceps muscle with 50 μg of plasmid DNA diluted in 20 μL of saline solution. Immediately after injection, two 25-ms trans-cutaneous electric low voltage pulses with amplitude of 150 V and a 300 ms interval were administered at the injection site via a multiple needle electrode connected to the Cliniporator™ (IGEA s.r.l., Carpi, Italy). The DNA vaccination course consisted of two i.m. injections of plasmid followed by electroporation repeated with an interval of 14 d.
Ten6 TCID50 recombinant BoHV-4 viral particles were diluted in 200 μL of Dubecco's Modified Eagle Medium (DMEM; Gibco, Rockville, MD) and injected intraperitoneal (i.p.) twice at two weeks interval in groups of 10 week-old BALB/c or BALB-neuT females.
Anti-rat-Her-2 antibody response
Two weeks after the second immunization, mice were bled and their sera were tested by flow cytometry for their ability to bind 3T3/NKB cells.66 Briefly, sera diluted 1:100 in PBS were incubated for 30 min at 4°C with 2 × 105 3T3/NKB cells pre-treated with Fc receptor blocker (CD16/CD32; PharMingen, St. Diego, CA) for 5 min at 4°C. The Ab4 mAb (Calbiochem, San Diego, CA), was used as positive control for rat Her-2 positivity. After washes with PBS containing 2% bovine serum albumin (BSA, Sigma-Aldrich, Milano, Italy) and 0.1% NaN3 (Sigma-Aldrich) (wash solution) cells were incubated with 1:50 dilution of a FITC-conjugate anti-mouse immunoglobulin G (IgG) Fc antibody (DakoCytomation, Milano, Italy) for 30 min at 4°C. Washed cells were then acquired and analyzed on the CyAn ADP using Summit 4.3 software (DakoCytomation, Heverlee, Belgium). The results were expressed as Mean Fluorescence Intensity (MFI).
Anti-rat-Her-2 cellular immune response
To prepare target cells for in vivo cytotoxicity detection, spleens from BALB/c and BALB-neuT mice were mechanically dissociated and the erythrocytes were removed from the cells suspension by osmotic lysis. Cells were then washed and labeled with two different CFSE (carboxyfluorescein-diacetate-succinimidyl ester) (Molecular Probes Invitrogen, Carlsbad, CA) concentration (5 and 0.5 μM). Cells labeled with 5 μM CFSE (CFSEhigh cells) were also pulsed with the rat-Her-2 H2d dominant TYVPANASL peptide (INBIOS Srl) at a concentration of 15 μg/mL for 1 h at 37°C; those labeled with 0.5 μM CFSE (CFSElow cells) were left unpulsed. 10 × 106 CFSEhigh cells plus 10 × 106 CFSElow cells were injected in the tail vein of vaccinated mice. Forty eight hours after spleen cells injection, mice were sacrificed and the presence of CFSEhigh and CFSElow in the spleen was measured by using a CyAn ADP Flow Cytometer (DakoCytomation). The percentage of the low peaks was normalized on control untreated low peaks and consequently the specific cytolytic activity was calculated as percentage of lysis as previously described.69
To measure the number of rat-Her-2 specific IFNγ releasing T lymphocytes a mouse IFNγ ELISPOT assay kit purchased from BD Biosciences (San Jose, CA, USA) was used. Briefly, two weeks after the vaccination course, 0.5 × 106 spleen cells were added to the wells of 96-well HTS IP plates (Millipore, Billerica, MA) pre-coated with 5 μg/mL of rat anti-mouse IFNγ (clone R4–6A2, BD Biosciences, San Jose, CA, USA). Spleen cells were stimulated with 15 μg/mL of TYVPANASL peptide (INBIOS Srl, Napoli, Italy) for 24 h at 37°C in a humidified 5% CO2 atmosphere. Concanavalin A (Sigma-Aldrich) at the concentration of 2 µg/mL and RPMI-1640 medium (Sigma-Aldrich) alone were used as positive and negative control, respectively. IFNγ spots were scanned and counted using an ImmunoSpot Image Analyzer software (Aelvis, Germany). Results were plotted as median of spot values among triplicates.
Immunofluorescence assay
For rat Her-2 detection, 4 × 105 Nmug cells were plated on glass coverslips and left to adhere overnight at 37 °C in a 5% CO2 incubator. The next day, cells were infected for 24 h with 0.5 TCID50/cell of BoHV-4-RHuT-gD or with the same TCID50/cell of BoHV-4-RHuT-gD inactivated at 70°C for 30 min. After infection, cells were fixed with 4% formalin (Sigma-Aldrich) solution in PBS for 5 min at room temperature, washed twice with PBS and blocked with 10% bovine serum albumin (BSA, Sigma-Aldrich) in PBS for 40 min at room temperature. Her-2 was detected incubating coverslips with an anti rat/Her-2 monoclonal antibody (1:20, clone number, Calbiochem, San Diego, CA) for 1 h at room temperature in PBS containing 1% BSA. Cells were rinsed twice with PBS and then incubated with AlexaFluor488 goat anti-mouse (1:1000, clone A11017, Invitrogen) in PBS containing 1% BSA. Cells were rinsed three times with PBS and nuclei were counterstained with DAPI (Invitrogen). Coverslips were air dried and mounted with Fluoromount mounting medium (Sigma-Aldrich) and visualized with Apotome fluorescence microscope (Leica). Photographs were taken using a digital CCD camera and images were processed using the AxioVision software (Zeiss, V. 4.4).
FACS analysis
For rat Her-2 detection, 4 × 105 Nmug cells were plated and left to adhere overnight at 37°C in a 5% CO2 incubator. The next day, cells were infected for 24 h with 0.5 TCID50/cell of BoHV-4-RHuT-gD or with the same TCID50/cell of BoHV-4-RHuT-gD inactivated at 70°C for 30 min. After infection, cells were detached with tripsin 1X (Invitrogen o Sigma), incubated with Fc receptor blocker (CD16/CD32; PharMingen, St. Diego, CA) for 5 min at 4°C to block aspecific site. Her-2 was detected incubating cells with an anti rat/Her-2 monoclonal antibody (1:25, clone number, Calbiochem, San Diego, CA) for 30 min at 4°C. Then cells were washed with PBS containing 2% BSA (Sigma-Aldrich, Milano, Italy) and 0.1% NaN3 (Sigma-Aldrich) (wash solution) and incubated with 1:50 dilution of a FITC-conjugate anti-mouse immunoglobulin G (IgG) Fc antibody (DakoCytomation, Milano, Italy) for 30 min at 4°C. Washed cells were then acquired and analyzed on the CyAn ADP using Summit 4.3 software (DakoCytomation, Heverlee, Belgium).
Statistical analysis
Statistical differences were evaluated using GraphPad software 5.0 (GraphPad Inc..). The Mantel-Cox log-rank test was used to evaluate the differences in the tumor incidence between different experimental groups. The two-tailed unpaired Student's t test was used to evaluate differences in the antibody titer, % of lysis and number of IFNγ secreting T cells between different experimental groups.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Acknowledgments
This work was supported by grants from the Italian Association for Cancer Research (AIRC, grant IG 12956 and IG 11675), the Compagnia di San Paolo (Progetti di Ricerca Ateneo/CSP, TO_call02_2012_0026), the University of Torino (Italy), the Italian Ministry for Education, University and Research (MIUR), Grant # 2010LLXR94_004 and University of Parma (Italy).
References
- 1.Whenham N, D'Hondt V, Piccart MJ. HER2-positive breast cancer: from trastuzumab to innovatory anti-HER2 strategies. Clin Breast Cancer 2008; 8:38-49; PMID:18501058; http://dx.doi.org/ 10.3816/CBC.2008.n.002 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM. Amplification of c-erbB-2 and aggressive human breast tumors? Science 1988; 240:1795-8; PMID:3289120; http://dx.doi.org/ 10.1126/science.3289120 [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244:707-12; PMID:2470152; http://dx.doi.org/ 10.1126/science.2470152 [DOI] [PubMed] [Google Scholar]
- 4.Gusterson BA, Gelber RD, Goldhirsch A, Price KN, Save-Soderborgh J, Anbazhagan R, Styles J, Rudenstam CM, Golouh R, Reed R. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol 1992; 10:1049-56; PMID:1351538 [DOI] [PubMed] [Google Scholar]
- 5.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127-37; PMID:11252954; http://dx.doi.org/ 10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- 6.Atalay G, Cardoso F, Awada A, Piccart MJ. Novel therapeutic strategies targeting the epidermal growth factor receptor (EGFR) family and its downstream effectors in breast cancer. Ann Oncol 2003; 14:1346-63; PMID:12954573; http://dx.doi.org/ 10.1093/annonc/mdg365 [DOI] [PubMed] [Google Scholar]
- 7.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene 2000; 19:6102-14; PMID:11156523; http://dx.doi.org/ 10.1038/sj.onc.1203973 [DOI] [PubMed] [Google Scholar]
- 8.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 2007; 357:39-51; PMID:17611206; http://dx.doi.org/ 10.1056/NEJMra043186 [DOI] [PubMed] [Google Scholar]
- 9.McArthur HL, Hudis CA. Breast cancer chemotherapy. Cancer J 2007; 13:141-7; PMID:17620762; http://dx.doi.org/ 10.1097/PPO.0b013e318074dc6f [DOI] [PubMed] [Google Scholar]
- 10.El-Sahwi K, Bellone S, Cocco E, Cargnelutti M, Casagrande F, Bellone M, Abu-Khalaf M, Buza N, Tavassoli FA, Hui P et al.. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer 2010; 102:134-43; PMID:19920829; http://dx.doi.org/ 10.1038/sj.bjc.6605448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higa GM, Abraham J. Lapatinib in the treatment of breast cancer. Expert Rev Anticancer Ther 2007; 7:1183-92; PMID:17892419; http://dx.doi.org/ 10.1586/14737140.7.9.1183 [DOI] [PubMed] [Google Scholar]
- 12.Quaglino E, Iezzi M, Mastini C, Amici A, Pericle F, Di Carlo E, Pupa SM, De Giovanni C, Spadaro M, Curcio C et al.. Electroporated DNA vaccine clears away multifocal mammary carcinomas in her-2/neu transgenic mice. Cancer Res 2004; 64:2858-64; PMID:15087404; http://dx.doi.org/ 10.1158/0008-5472.CAN-03-2962 [DOI] [PubMed] [Google Scholar]
- 13.Quaglino E, Rolla S, Iezzi M, Spadaro M, Musiani P, De Giovanni C, Lollini PL, Lanzardo S, Forni G, Sanges R et al.. Concordant morphologic and gene expression data show that a vaccine halts HER-2/neu preneoplastic lesions. J Clin Invest 2004; 113:709-17; PMID:14991069; http://dx.doi.org/ 10.1172/JCI19850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, Wei WZ, Cavallo F. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res 2006; 66:7734-40; PMID:16885376; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1432 [DOI] [PubMed] [Google Scholar]
- 15.Rolla S, Nicolo C, Malinarich S, Orsini M, Forni G, Cavallo F, Ria F. Distinct and non-overlapping T cell receptor repertoires expanded by DNA vaccination in wild-type and HER-2 transgenic BALB/c mice. J Immunol 2006; 177:7626-33; PMID:17114432; http://dx.doi.org/ 10.4049/jimmunol.177.11.7626 [DOI] [PubMed] [Google Scholar]
- 16.Holmgren L, Ambrosino E, Birot O, Tullus C, Veitonmaki N, Levchenko T, Carlson LM, Musiani P, Iezzi M, Curcio C et al.. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc Natl Acad Sci U S A 2006; 103:9208-13; PMID:16754857; http://dx.doi.org/ 10.1073/pnas.0603110103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pannellini T, Spadaro M, Di Carlo E, Ambrosino E, Iezzi M, Amici A, Lollini PL, Forni G, Cavallo F, Musiani P. Timely DNA vaccine combined with systemic IL-12 prevents parotid carcinomas before a dominant-negative p53 makes their growth independent of HER-2/neu expression. J Immunol 2006; 176:7695-703; PMID:16751417; http://dx.doi.org/ 10.4049/jimmunol.176.12.7695 [DOI] [PubMed] [Google Scholar]
- 18.Rizzuto GA, Merghoub T, Hirschhorn-Cymerman D, Liu C, Lesokhin AM, Sahawneh D, Zhong H, Panageas KS, Perales MA, Altan-Bonnet G et al.. Self-antigen-specific CD8+ T cell precursor frequency determines the quality of the antitumor immune response. J Exp Med 2009; 206:849-66; PMID:19332877; http://dx.doi.org/ 10.1084/jem.20081382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelhorn ME, Guevara-Patino JA, Noffz G, Hooper AT, Lou O, Gold JS, Kappel BJ, Houghton AN. Autoimmunity and tumor immunity induced by immune responses to mutations in self. Nat Med 2006; 12:198-206; PMID:16444264; http://dx.doi.org/ 10.1038/nm1363 [DOI] [PubMed] [Google Scholar]
- 20.Luo W, Hsu JC, Kieber-Emmons T, Wang X, Ferrone S. Human tumor associated antigen mimicry by xenoantigens, anti-idiotypic antibodies and peptide mimics: implications for immunotherapy of malignant diseases. Cancer Chemother Biol Response Modif 2005; 22:769-87; PMID:16110640; http://dx.doi.org/ 10.1016/S0921-4410(04)22036-1 [DOI] [PubMed] [Google Scholar]
- 21.Quaglino E, Mastini C, Amici A, Marchini C, Iezzi M, Lanzardo S, De Giovanni C, Montani M, Lollini PL, Masucci G et al.. A better immune reaction to Erbb-2 tumors is elicited in mice by DNA vaccines encoding rat/human chimeric proteins. Cancer Res 2010; 70:2604-12; PMID:20332241; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2548 [DOI] [PubMed] [Google Scholar]
- 22.Bolli E, Quaglino E, Arigoni M, Lollini PL, Calogero R, Forni G, Cavallo F. Oncoantigens for an immune prevention of cancer. Am J Cancer Res 2011; 1:255-64; PMID:21969087 [PMC free article] [PubMed] [Google Scholar]
- 23.Jacob JB, Quaglino E, Radkevich-Brown O, Jones RF, Piechocki MP, Reyes JD, Weise A, Amici A, Wei WZ. Combining human and rat sequences in her-2 DNA vaccines blunts immune tolerance and drives antitumor immunity. Cancer Res 2010; 70:119-28; PMID:20048073; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donofrio G, Cavirani S, Simone T, van Santen VL. Potential of bovine herpesvirus 4 as a gene delivery vector. J Virol Methods 2002; 101:49-61; PMID:11849683; http://dx.doi.org/ 10.1016/S0166-0934(01)00419-0 [DOI] [PubMed] [Google Scholar]
- 25.Donofrio G, Sartori C, Ravanetti L, Cavirani S, Gillet L, Vanderplasschen A, Taddei S, Flammini CF. Establishment of a bovine herpesvirus 4 based vector expressing a secreted form of the bovine viral diarrhoea virus structural glycoprotein E2 for immunization purposes. BMC Biotechnol 2007; 7:68; PMID:17945009; http://dx.doi.org/ 10.1186/1472-6750-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donofrio G, Sartori C, Franceschi V, Capocefalo A, Cavirani S, Taddei S, Flammini CF. Double immunization strategy with a BoHV-4-vectorialized secreted chimeric peptide BVDV-E2/BoHV-1-gD. Vaccine 2008; 26:6031-42; PMID:18812200; http://dx.doi.org/ 10.1016/j.vaccine.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 27.Donofrio G, Franceschi V, Capocefalo A, Taddei S, Sartori C, Bonomini S, Cavirani S, Cabassi CS, Flammini CF. Cellular targeting of engineered heterologous antigens is a determinant factor for bovine herpesvirus 4-based vaccine vector development. Clin Vaccine Immunol 2009; 16:1675-86; PMID:19793901; http://dx.doi.org/ 10.1128/CVI.00224-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franceschi V, Capocefalo A, Calvo-Pinilla E, Redaelli M, Mucignat-Caretta C, Mertens P, Ortego J, Donofrio G. Immunization of knock-out α/β interferon receptor mice against lethal bluetongue infection with a BoHV-4-based vector expressing BTV-8 VP2 antigen. Vaccine 2011; 29:3074-82; PMID:21320537; http://dx.doi.org/ 10.1016/j.vaccine.2011.01.075 [DOI] [PubMed] [Google Scholar]
- 29.Redaelli M, Franceschi V, Capocefalo A, D'Avella D, Denaro L, Cavirani S, Mucignat-Caretta C, Donofrio G. Herpes simplex virus type 1 thymidine kinase-armed bovine herpesvirus type 4-based vector displays enhanced oncolytic properties in immunocompetent orthotopic syngenic mouse and rat glioma models. Neuro-oncology 2012; 14:288-301; PMID:22228853; http://dx.doi.org/ 10.1093/neuonc/nor219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donofrio G, Franceschi V, Lovero A, Capocefalo A, Camero M, Losurdo M, Cavirani S, Marinaro M, Grandolfo E, Buonavoglia C et al.. Clinical protection of goats against CpHV-1 induced genital disease with a BoHV-4-based vector expressing CpHV-1 gD. PloS one 2013; 8:e52758; PMID:23300989; http://dx.doi.org/ 10.1371/journal.pone.0052758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donofrio G, Martignani E, Poli E, Lange C, Martini FM, Cavirani S, Cabassi CS, Taddei S, Flammini CF. Bovine herpesvirus 4 based vector interaction with liver cells in vitro and in vivo. J Virol Methods 2006; 136:126-36; PMID:16712963; http://dx.doi.org/ 10.1016/j.jviromet.2006.04.008 [DOI] [PubMed] [Google Scholar]
- 32.Donofrio G, Taddei S, Franceschi V, Capocefalo A, Cavirani S, Martinelli N, Ottonello S, Ferrari M. Swine adipose stromal cells loaded with recombinant bovine herpesvirus 4 virions expressing a foreign antigen induce potent humoral immune responses in pigs. Vaccine 2011; 29:867-72; PMID:21115049; http://dx.doi.org/ 10.1016/j.vaccine.2010.11.048 [DOI] [PubMed] [Google Scholar]
- 33.Franceschi V, Stellari FF, Mangia C, Jacca S, Lavrentiadou S, Cavirani S, Heikenwalder M, Donofrio G. In vivo image analysis of BoHV-4-based vector in mice. PloS one 2014; 9:e95779; PMID:24752229; http://dx.doi.org/ 10.1371/journal.pone.0095779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osorio FA, Reed DE. Experimental inoculation of cattle with bovine herpesvirus-4: evidence for a lymphoid-associated persistent infection. Am J Vet Res 1983; 44:975-80; PMID:6307096 [PubMed] [Google Scholar]
- 35.Capocefalo A, Franceschi V, Mertens PP, Castillo-Olivares J, Cavirani S, Di Lonardo E, Leni Z, Donofrio G. Expression and secretion of Bluetongue virus serotype 8 (BTV-8)VP2 outer capsid protein by mammalian cells. J Virol Methods 2010; 169:420-4; PMID:20705105; http://dx.doi.org/ 10.1016/j.jviromet.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 36.Franceschi V, Parker S, Jacca S, Crump RW, Doronin K, Hembrador E, Pompilio D, Tebaldi G, Estep RD, Wong SW et al.. BoHV-4-Based Vector Single Heterologous Antigen Delivery Protects STAT1(-/-) Mice from Monkeypoxvirus Lethal Challenge. PLoS Negl Trop Dis 2015; 9:e0003850; PMID:26086739; http://dx.doi.org/ 10.1371/journal.pntd.0003850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quaglino E, Mastini C, Forni G, Cavallo F. ErbB2 transgenic mice: a tool for investigation of the immune prevention and treatment of mammary carcinomas. Curr Protoc Immunol 2008; Chapter 20:Unit 200 9 1- 9-10; PMID:18729063; http://dx.doi.org/ 10.1002/0471142735.im2009s82 [DOI] [PubMed] [Google Scholar]
- 38.Franceschi V, Capocefalo A, Cavirani S, Donofrio G. Bovine herpesvirus 4 glycoprotein B is indispensable for lytic replication and irreplaceable by VSVg. BMC Vet Res 2013; 9:6; PMID:23302472; http://dx.doi.org/ 10.1186/1746-6148-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capocefalo A, Mangia C, Franceschi V, Jacca S, van Santen VL, Donofrio G. Efficient heterologous antigen gene delivery and expression by a replication-attenuated BoHV-4-based vaccine vector. Vaccine 2013; 31:3906-14; PMID:23830977; http://dx.doi.org/ 10.1016/j.vaccine.2013.06.052 [DOI] [PubMed] [Google Scholar]
- 40.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 2005; 33:e36; PMID:15731329; http://dx.doi.org/ 10.1093/nar/gni035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rovero S, Amici A, Di Carlo E, Bei R, Nanni P, Quaglino E, Porcedda P, Boggio K, Smorlesi A, Lollini PL et al.. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol 2000; 165:5133-42; PMID:11046045; http://dx.doi.org/ 10.4049/jimmunol.165.9.5133 [DOI] [PubMed] [Google Scholar]
- 42.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G et al.. Systemic spread is an early step in breast cancer. Cancer Cell 2008; 13:58-68; PMID:18167340; http://dx.doi.org/ 10.1016/j.ccr.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 43.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 2003; 102:2138-45; PMID:12750171; http://dx.doi.org/ 10.1182/blood-2003-01-0190 [DOI] [PubMed] [Google Scholar]
- 44.Matic S, Quaglino E, Arata L, Riccardo F, Pegoraro M, Vallino M, Cavallo F, Noris E. The rat ErbB2 tyrosine kinase receptor produced in plants is immunogenic in mice and confers protective immunity against ErbB2 mammary cancer. Plant biotechnology journal 2015; PMID:25865255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gershoni JM, Roitburd-Berman A, Siman-Tov DD, Tarnovitski Freund N, Weiss Y. Epitope mapping: the first step in developing epitope-based vaccines. BioDrugs 2007; 21:145-56; PMID:17516710; http://dx.doi.org/ 10.2165/00063030-200721030-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW Jr., Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003; 421:756-60; PMID:12610629; http://dx.doi.org/ 10.1038/nature01392 [DOI] [PubMed] [Google Scholar]
- 47.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 2004; 5:317-28; PMID:15093539; http://dx.doi.org/ 10.1016/S1535-6108(04)00083-2 [DOI] [PubMed] [Google Scholar]
- 48.Porzia A, Lanzardo S, Citti A, Cavallo F, Forni G, Santoni A, Galandrini R, Paolini R. Attenuation of PI3K/Akt-mediated tumorigenic signals through PTEN activation by DNA vaccine-induced anti-ErbB2 antibodies. J Immunol 2010; 184:4170-7; PMID:20220087; http://dx.doi.org/ 10.4049/jimmunol.0903375 [DOI] [PubMed] [Google Scholar]
- 49.Park JM, Terabe M, Sakai Y, Munasinghe J, Forni G, Morris JC, Berzofsky JA. Early role of CD4+ Th1 cells and antibodies in HER-2 adenovirus vaccine protection against autochthonous mammary carcinomas. J Immunol 2005; 174:4228-36; PMID:15778385; http://dx.doi.org/ 10.4049/jimmunol.174.7.4228 [DOI] [PubMed] [Google Scholar]
- 50.Tegerstedt K, Lindencrona JA, Curcio C, Andreasson K, Tullus C, Forni G, Dalianis T, Kiessling R, Ramqvist T. A single vaccination with polyomavirus VP1/VP2Her2 virus-like particles prevents outgrowth of HER-2/neu-expressing tumors. Cancer Res 2005; 65:5953-7; PMID:15994974; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-0335 [DOI] [PubMed] [Google Scholar]
- 51.Gallo P, Dharmapuri S, Nuzzo M, Maldini D, Cipriani B, Forni G, Monaci P. Adenovirus vaccination against neu oncogene exerts long-term protection from tumorigenesis in BALB/neuT transgenic mice. Int J Cancer 2007; 120:574-84; PMID:17096348; http://dx.doi.org/ 10.1002/ijc.22274 [DOI] [PubMed] [Google Scholar]
- 52.Dols A, Meijer SL, Smith JW 2nd, Fox BA, Urba WJ. Allogeneic breast cancer cell vaccines. Clin Breast Cancer 2003; 3 Suppl 4:S173-80; PMID:12620156; http://dx.doi.org/ 10.3816/CBC.2003.s.008 [DOI] [PubMed] [Google Scholar]
- 53.Dols A, Smith JW 2nd, Meijer SL, Fox BA, Hu HM, Walker E, Rosenheim S, Moudgil T, Doran T, Wood W et al.. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther 2003; 14:1117-23; PMID:12885350; http://dx.doi.org/ 10.1089/104303403322124828 [DOI] [PubMed] [Google Scholar]
- 54.Dols A, Meijer SL, Hu HM, Goodell V, Disis ML, Von Mensdorff-Pouilly S, Verheijen R, Alvord WG, Smith JW 2nd, Urba WJ et al.. Identification of tumor-specific antibodies in patients with breast cancer vaccinated with gene-modified allogeneic tumor cells. J Immunother 2003; 26:163-70; PMID:12616108; http://dx.doi.org/ 10.1097/00002371-200303000-00009 [DOI] [PubMed] [Google Scholar]
- 55.Park JW, Melisko ME, Esserman LJ, Jones LA, Wollan JB, Sims R. Treatment with autologous antigen-presenting cells activated with the HER-2 based antigen Lapuleucel-T: results of a phase I study in immunologic and clinical activity in HER-2 overexpressing breast cancer. J Clin Oncol 2007; 25:3680-7; PMID:17704416; http://dx.doi.org/ 10.1200/JCO.2006.10.5718 [DOI] [PubMed] [Google Scholar]
- 56.Occhipinti S, Sponton L, Rolla S, Caorsi C, Novarino A, Donadio M, Bustreo S, Satolli MA, Pecchioni C, Marchini C et al.. Chimeric rat/human HER2 efficiently circumvents HER2 tolerance in cancer patients. Clin Cancer Res 2014; 20:2910-21; PMID:24668647; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2663 [DOI] [PubMed] [Google Scholar]
- 57.Kitano S, Kageyama S, Nagata Y, Miyahara Y, Hiasa A, Naota H, Okumura S, Imai H, Shiraishi T, Masuya M et al.. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res 2006; 12:7397-405; PMID:17189412; http://dx.doi.org/ 10.1158/1078-0432.CCR-06-1546 [DOI] [PubMed] [Google Scholar]
- 58.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother 2008; 57:1511-21; PMID:18536917; http://dx.doi.org/ 10.1007/s00262-008-0540-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aurisicchio L, Peruzzi D, Conforti A, Dharmapuri S, Biondo A, Giampaoli S, Fridman A, Bagchi A, Winkelmann CT, Gibson R et al.. Treatment of mammary carcinomas in HER-2 transgenic mice through combination of genetic vaccine and an agonist of Toll-like receptor 9. Clin Cancer Res 2009; 15:1575-84; PMID:19240169; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-2628 [DOI] [PubMed] [Google Scholar]
- 60.Andreasson K, Tegerstedt K, Eriksson M, Curcio C, Cavallo F, Forni G, Dalianis T, Ramqvist T. Murine pneumotropic virus chimeric Her2/neu virus-like particles as prophylactic and therapeutic vaccines against Her2/neu expressing tumors. Int J Cancer 2009; 124:150-6; PMID:18839427; http://dx.doi.org/ 10.1002/ijc.23920 [DOI] [PubMed] [Google Scholar]
- 61.Seavey MM, Pan ZK, Maciag PC, Wallecha A, Rivera S, Paterson Y, Shahabi V. A novel human Her-2/neu chimeric molecule expressed by Listeria monocytogenes can elicit potent HLA-A2 restricted CD8-positive T cell responses and impact the growth and spread of Her-2/neu-positive breast tumors. Clin Cancer Res 2009; 15:924-32; PMID:19188163; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-2283 [DOI] [PubMed] [Google Scholar]
- 62.Masuelli L, Marzocchella L, Focaccetti C, Lista F, Nardi A, Scardino A, Mattei M, Turriziani M, Modesti M, Forni G et al.. Local delivery of recombinant vaccinia virus encoding for neu counteracts growth of mammary tumors more efficiently than systemic delivery in neu transgenic mice. Cancer Immunol Immunother 2010; 59:1247-58; PMID:20364378; http://dx.doi.org/ 10.1007/s00262-010-0850-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masuelli L, Fantini M, Benvenuto M, Sacchetti P, Giganti MG, Tresoldi I, Lido P, Lista F, Cavallo F, Nanni P et al.. Intratumoral delivery of recombinant vaccinia virus encoding for ErbB2/Neu inhibits the growth of salivary gland carcinoma cells. J Transl Med 2014; 12:122; PMID:24886178; http://dx.doi.org/ 10.1186/1479-5876-12-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park JM, Terabe M, Steel JC, Forni G, Sakai Y, Morris JC, Berzofsky JA. Therapy of advanced established murine breast cancer with a recombinant adenoviral ErbB-2/neu vaccine. Cancer Res 2008; 68:1979-87; PMID:18339880; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-5688 [DOI] [PubMed] [Google Scholar]
- 65.Donofrio G, Cavaggioni A, Bondi M, Cavirani S, Flammini CF, Mucignat-Caretta C. Outcome of bovine herpesvirus 4 infection following direct viral injection in the lateral ventricle of the mouse brain. Microbes Infection 2006; 8:898-904; PMID:16503181; http://dx.doi.org/ 10.1016/j.micinf.2005.10.016 [DOI] [PubMed] [Google Scholar]
- 66.Jacob J, Radkevich O, Forni G, Zielinski J, Shim D, Jones RF, Wei WZ. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cell Immunol 2006; 240:96-106; PMID:16930573; http://dx.doi.org/ 10.1016/j.cellimm.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 67.Marchini C, Kalogris C, Garulli C, Pietrella L, Gabrielli F, Curcio C, Quaglino E, Cavallo F, Amici A. Tailoring DNA Vaccines: Designing Strategies Against HER2-Positive Cancers. Front Oncol 2013; 3:122; PMID:23675574; http://dx.doi.org/ 10.3389/fonc.2013.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boggio K, Nicoletti G, Di Carlo E, Cavallo F, Landuzzi L, Melani C, Giovarelli M, Rossi I, Nanni P, De Giovanni C et al.. Interleukin 12-mediated prevention of spontaneous mammary adenocarcinomas in two lines of Her-2/neu transgenic mice. J Exp Med 1998; 188:589-96; PMID:9687535; http://dx.doi.org/ 10.1084/jem.188.3.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barutello G, Curcio C, Spadaro M, Arigoni M, Trovato R, Bolli E, Zheng Y, Ria F, Quaglino E, Iezzi M et al.. Antitumor immunization of mothers delays tumor development in cancer-prone offspring. Oncoimmunology 2015; 4:e1005500; PMID:26155401; http://dx.doi.org/ 10.1080/2162402X.2015.1005500 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


