Abstract
BACKGROUND
The initiation of coagulation in trauma is thought to originate from exposed tissue factor (TF); recent data have led to the alternative hypothesis that DAMPs may contribute to post-injury coagulation. In acute traumatic coagulopathy (ATC), aberrant coagulation is mediated via the activated protein C (aPC) pathway; the upstream regulators of this process, and its relation to TF, remain uncharacterized. To examine the role of the TF pathway in mediating ATC, we employed specific antibody blockades in an established murine model of traumatic hemorrhagic shock, hypothesizing that both coagulation activation after injury and aPC-mediated coagulopathy are driven by TF via thrombin.
METHODS
Mice underwent an established model of trauma and hemorrhage, and were subjected to either sham (vascular cannulation), or trauma-hemorrhage (cannulation, laparotomy, shock to MAP 35mmHg); they were monitored for 60 min prior to sacrifice. Mice in each group were pre-treated with either targeted anti-TF antibody to block the TF pathway, or hirudin for specific blockade of thrombin. Plasma was assayed for thrombin-antithrombin (TAT) and aPC by ELISA.
RESULTS
Compared to controls, trauma-hemorrhage mice treated with anti-TFAb had significantly reduced levels of TAT (2.3 vs. 5.7 ng/mL, p=0.016), and corresponding decreases in aPC (16.3 vs. 31.6 ng/mL, p=0.034), with reductions to levels seen in sham mice. Direct inhibition of thrombin yielded similar results, with reduction in aPC to levels below those seen in sham mice.
CONCLUSIONS
In this study, blockade of the TF pathway led to attenuation of both thrombin production and aPC activation observed in traumatic shock. Specific thrombin inhibition achieved similar results, indicating that aPC-related coagulopathy is mediated via thrombin activated by the TF pathway. The near-complete blockade of TAT and aPC observed in this model argues for a dominant role of the TF-thrombin pathway in both coagulation activation after injury and traumatic coagulopathy.
STUDY TYPE
Animal study.
Keywords: Tissue factor, activated protein C (aPC), coagulation, coagulopathy
BACKGROUND
Around the world and across geopolitical borders, trauma is the leading cause of mortality in the young; death by trauma exceeds that due to human immunodeficiency virus, tuberculosis, and malaria combined in all age groups (1). While central nervous system injury and hemorrhage are the leading causes of early mortality in trauma, hemorrhage is more amenable to therapeutic intervention (2). As such, efforts to mitigate hemorrhage and optimize coagulation after critical injury have become a major focus of trauma care (3, 4).
The proliferation of interest in traumatic hemorrhage and associated interventions has been spurred by an emerging understanding of characteristic disturbances in hemostasis following severe injury. First recognized over a decade ago, a distinct acute traumatic coagulopathy has been identified in over 25% of critically injured trauma patients upon presentation, independent of potential iatrogenic causes (5, 6). This coagulopathy has been associated with shock and tissue injury, and in clinical studies has been correlated to increased activation of the protein C pathway (7, 8).
Since these early descriptions of endogenous post-traumatic coagulopathy, further clinical work has implicated activated protein C (aPC) as a significant causal mediator (9, 10). These findings have led to the development of a model wherein injury-induced tissue factor prompts thrombin formation, which is diverted to activate zymogenic protein C in the setting of endothelial thrombomodulin; thrombomodulin itself may be upregulated in the setting of shock. Such an increase in aPC would lead to proteolytic degradation of factors Va and VIIIa, effectively ceasing thrombin production (11, 12), while at the same time disinhibiting fibrinolysis (13, 14), leading to an overall hypocoagulable state.
This etiologic framework has been corroborated by a mouse model of acute traumatic hemorrhage, where the combination of trauma and hypotension consistently reproduced a coagulopathy akin to the one seen in human studies (15). This was correlated to increased plasma levels of aPC, and the mechanistic link was confirmed when specific antibody blockade of the aPC anticoagulant domain prevented the development of coagulation abnormalities. The upstream mediators of this process remain uncharacterized.
In the absence of coagulopathy, the initiation of coagulation after injury is traditionally thought to originate from exposed tissue factor, which stimulates activation of the extrinsic pathway and formation of thrombin (16). However, recent data have led to the alternative hypothesis that the intrinsic pathway may have a significant role in mediating coagulation after injury, via the release of pro-inflammatory molecules such as extracellular histones and DNA (17-19). Additionally, tissue factor itself has been implicated in multiple non-canonical pathways and functions, and appears to have far-reaching effects beyond coagulation (20). The importance of these pathways in the setting of trauma are unknown, as are the interactions between tissue factor and aPC, which also plays a major role in inflammation through an independent cytoprotective pathway (21).
To better understand which coagulation pathway predominates in traumatic shock, and to better define the relationship between the pleiotropic tissue factor pathways and acute traumatic coagulopathy, we employed specific antibody and pharmacologic blockades in an established murine model of traumatic hemorrhagic shock. We hypothesized that both coagulation activation after injury and aPC-mediated coagulopathy are primarily driven by tissue factor via thrombin, and that both would be attenuated by blocking the tissue factor pathway.
METHODS
The mouse protocol of traumatic hemorrhage was conducted based on a previously established model (15). The experiments were carried out in accordance with the National Institutes of Health (Bethesda, Maryland) guidelines and were approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Male C57BL/6 mice 8 to 10 weeks old (Jackson Laboratory, Sacramento, California) were used in all experiments. Animals were allowed water and food ad libitum and permitted at least 24 hours to acclimate to the housing facility. Anesthesia was administered with isoflurane 1.2% in air:O2 at 1:0.5 L/min, and mice were then secured with plastic tape on a firm plastic board in supine position. A lubricated rectal temperature probe was inserted for continuous monitoring, and a heat lamp was used to maintain core body temperature between 36-37°C. The right jugular vein and left femoral artery were cannulated with PE-10 tubing, pre-flushed with isotonic sodium chloride solution.
Mice in the trauma-hemorrhage groups were pre-treated with either targeted anti-tissue factor antibody (TFAb21E10, 0.5mg, Scripps Research Institute, La Jolla, California (22)) or recombinant hirudin (Lepirudin, 0.5mg) infused via the right jugular vein catheter. Mice in the sham group were pre-treated with either control antibody (IgG) or sodium chloride infused via the right jugular vein catheter, at a volume matched to the experimental infusions. There were at least six (and up to nine) mice in each group (sham + IgG, trauma-hemorrhage + IgG, trauma-hemorrhage + TF Ab, sham + saline, trauma-hemorrhage + saline, trauma-hemorrhage + hirudin).
The cannulation incision sites were bathed in lidocaine 1% for analgesia. In sham animals, isoflurane was then discontinued to allow emergence from general anesthesia. These animals subsequently underwent 60 minutes of board stress.
In trauma-hemorrhage animals, tissue trauma was induced 10 minutes following pre-treatment (as described above) via sterile 2 cm laparotomy; once the underlying organs were inspected to confirm no damage from the procedure, the incisional wound was closed using 5-0 prolene suture. This incision was then bathed in lidocaine 1% for analgesia, and isoflurane was then discontinued to allow emergence from general anesthesia. Mean arterial pressure (MAP) was monitored using a pressure transducer and amplifier (TSD104A and MP1004-CE, respectively; Biopac Systems, Goleta, California) attached to the left femoral artery catheter. Transducer output was analyzed with AcqKnowledge Software (Biopac Systems) with continuous arterial waveform display. After emergence from general anesthesia, baseline MAP greater than 90 mmHg was confirmed, then hypotensive shock period was initiated. Shock was initiated by removing blood via the left femoral arterial catheter with a pipette; based on the previously established model, at 10 minutes following emergence from anesthesia, 25% of estimated total blood volume (calculated as body weight in grams multiplied by 0.077, in mL) was removed. MAP was monitored closely, and subsequent aliquots of 60-70 μL blood (up to approximately 40% of estimated total blood volume) were removed to maintain target MAP of 35 +/− 5 mmHg over a period of 60 minutes. Temperature, MAP, and respiratory rate were monitored during the shock period, with tachypnea serving as a surrogate marker of successful induction of hypotensive shock and attendant metabolic acidosis. Isotonic sodium chloride solution was used to gently flush catheters to maintain patency, with less than 100 μL of solution infused in each animal.
Following 60 minutes of either board stress or trauma-hemorrhage, mice were rapidly euthanized with an overdose of isoflurane. The laparotomy incision was then reopened and blood was drawn via inferior vena cava puncture into a syringe, and was transferred to tubes containing 3.2% citrate (for thrombin-antithrombin (TAT) analysis) or 3.2% citrate with the reversible protease inhibitor benzamidine at 1mM concentration (for aPC analysis). Blood samples were centrifuged at 4,000 rpm for 10 minutes, supernatant was removed, and then centrifuged at 13,000 rpm for 10 minutes to remove cells and platelets. Supernatant was stored at −80°C until the time of further analysis.
Plasma was assayed for overall coagulation activation as measured by thrombin formation: thrombin-antithrombin levels were determined using a TAT enzyme-linked immunosorbent assay (Enzyme Research Laboratories, South Bend, Indiana). Activated protein C levels were measured using an assay specific for the activated form of protein C developed in the laboratory of Charles Esmon. Briefly, a 96-well vinyl plate was coated overnight at 4°C with monoclonal antibody AMGDPC 1591 at 5 μg/mL in coating buffer (15mM Na2CO3, 35 mM NaHCO3 pH 9.6). Plates were then blocked with blocking buffer (0.02 M Tris, 0.1 M NaCl, 0.25% gelatin, pH 7.5). Standard dilutions of mouse aPC and mouse plasma samples were loaded onto the plate and incubated at room temperature for 1 hour on a 150-rpm rocker. Plates were washed with wash buffer (0.02 M Tris, 0.1 M NaCl, 0.05% Tween, pH 7.5). Biotin-labeled monoclonal antibody MPC 1609 diluted in wash buffer at 1μg/ml was added to the plate. After washing, Streptavidin-HRP (1μg/ml) in wash buffer was added and incubated at 37°C for 30 minutes. OPD substrate was then added for 5-10 minutes. The subsequent reaction was stopped with 2.5M H2SO4. Absorbance was measured at 490 nm with sample concentration derived from standard curve.
Results are presented graphically as mean with standard error. Statistical analysis was conducted to assess differences between groups, using Student's t test for normally distributed data and Wilcoxon rank-sum testing for skewed data. An α of 0.05 was considered significant. All statistical analysis was performed by the authors using Stata Version 12 (College Station, Texas).
RESULTS
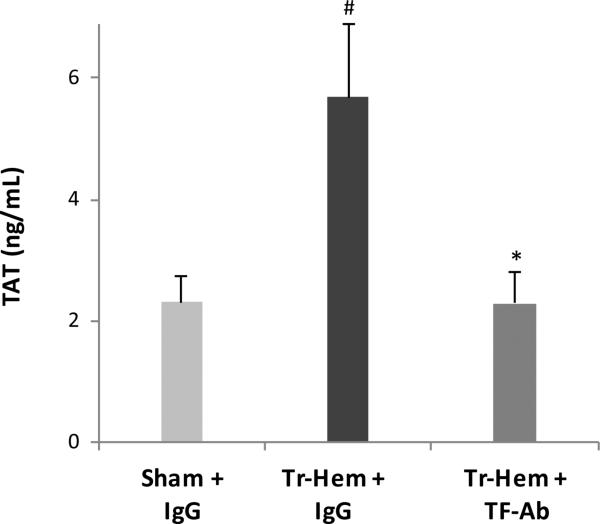
The IgG-treated mice subjected to trauma-hemorrhage had significantly higher mean TAT levels than IgG-treated mice that underwent sham procedures (5.68 ng/mL trauma-hemorrhage vs. 2.30 ng/mL sham, p=0.016, Figure 1). This was in keeping with previous findings and as expected, indicating that the trauma-hemorrhage model did in fact produce a significant surge of thrombin production. The trauma-hemorrhage mice pre-treated with tissue factor antibody, however, had significantly lower TAT levels than their IgG-treated counterparts (2.29 ng/mL TF Ab trauma-hemorrhage vs. 5.68 ng/mL IgG trauma-hemorrhage, p = 0.016, Figure 1). These levels were not significantly different from the TAT found in sham IgG-treated animals (p = 0.631).
Figure 1.
Thrombin production attenuated by TF Ab.
# indicates p<0.05 vs. Sham + IgG; * indicates p<0.05 vs. Tr-Hem + IgG. TF Ab, tissue factor antibody; TAT, thrombin-antithrombin; Tr-Hem, trauma-hemorrhage.
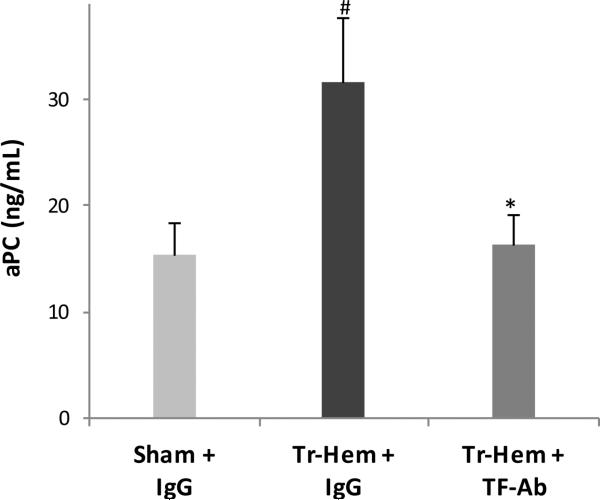
Similarly, aPC was significantly higher in IgG-treated trauma-hemorrhage mice than it was in sham IgG-treated mice (31.60 ng/mL trauma-hemorrhage vs. 15.29 ng/mL sham, p = 0.037, Figure 2), consistent with prior findings and as expected. This increase of aPC in trauma-hemorrhage mice was significantly inhibited by pre-treatment with tissue factor antibody, as seen in Figure 2 (16.28 ng/mL TF Ab trauma-hemorrhage vs. 31.60 ng/mL IgG trauma-hemorrhage, p = 0.034). As in TAT, tissue factor antibody pre-treatment resulted in aPC levels in trauma-hemorrhage mice that were statistically indistinguishable from those seen in non-traumatized sham IgG-treated mice (p = 0.906).
Figure 2.
Protein C activation attenuated by TF Ab.
# indicates p<0.05 vs. Sham + IgG; * indicates p<0.05 vs. Tr-Hem + IgG. TF Ab, tissue factor antibody; aPC, activated protein C; Tr-Hem, trauma-hemorrhage.
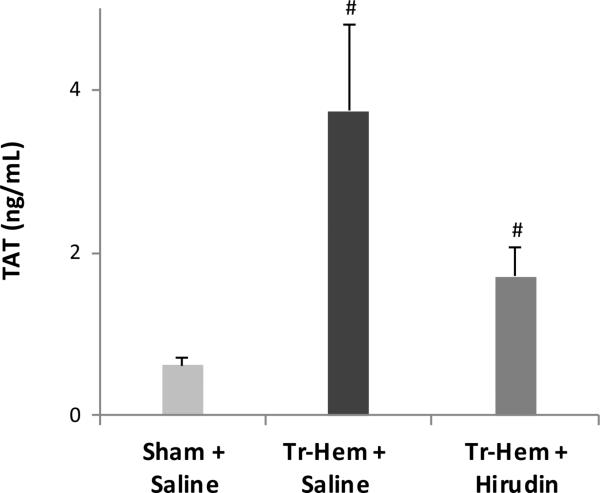
In the hirudin-based experiments, again trauma-hemorrhage resulted in higher levels of TAT in saline-treated animals (3.74 ng/mL trauma-hemorrhage vs. 0.62 ng/mL sham, p = 0.003, Figure 3). Pre-treatment with recombinant hirudin reduced the levels of TAT produced in traumatic hemorrhage, though this did not quite reach statistical significance (1.71 ng/mL hirudin trauma-hemorrhage vs. 3.74 ng/mL saline trauma-hemorrhage, p = 0.088, Figure 3).
Figure 3.
Thrombin production attenuated by hirudin.
# indicates p<0.05 vs. Sham + Saline. TAT, thrombin-antithrombin; Tr-Hem, trauma-hemorrhage.
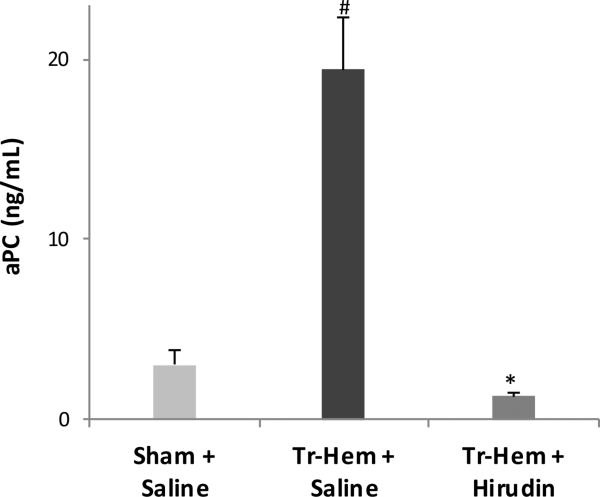
Hirudin had a more profound effect on protein C activation. Again, as expected, traumatic shock produced higher aPC levels than sham board stress in saline-treated animals (19.44 ng/mL trauma hemorrhage vs 3.04 ng/mL sham, p = 0.004, Figure 4). This increase was completely abrogated following pre-treatment with hirudin, with levels significantly decreased to those of sham or below (19.44 ng/mL saline trauma-hemorrhage vs. 1.27 ng/mL hirudin trauma-hemorrhage), as demonstrated in Figure 4.
Figure 4.
Protein C activation attenuated by hirudin.
# indicates p<0.05 vs. Sham + Saline; * indicates p<0.05 vs. Tr-Hem + Saline. aPC, activated protein C; Tr-Hem, trauma-hemorrhage.
DISCUSSION
The burden of trauma mortality and morbidity represents a critical public health concern worldwide, and hemorrhage causes the majority of deaths within the first hour of trauma center care (23). The control of traumatic hemorrhage and coagulation disorders is thus a key priority in preventing early trauma mortality; this awareness has driven a marked recent interest in the principles of hemostasis following trauma (24). Advances in hematology, ongoing resuscitation needs in active combat zones, and increased point-of-care testing have invigorated such interest over the past two decades (25).
Accompanying this intensified focus on hemorrhage after trauma is the awareness that not all trauma patients bleed equally. Since 2003, multiple reports have shown that one in four severe trauma patients will present with disordered coagulation, or acute traumatic coagulopathy (5-7). Clinical studies have identified the activated protein C pathway as a driving force in this coagulopathy, with compelling results from single-center and multi-center trials (10, 26). The mechanistic link of aPC to coagulopathy has been established in a murine model of traumatic shock (15).
While protein C is known to be activated by thrombin, the upstream drivers of thrombin production in trauma have not been clearly delineated. The central role of tissue factor and the extrinsic pathway in hemostasis have been long established (16, 27), but recent data have given rise to the possibility that certain inflammatory mediators may play a role in driving coagulation changes after trauma. Mitochondrial damage-associated molecular pattern (DAMP) particles and extracellular DNA are released following injury (17, 28), and these types of mediators have been shown to activate coagulation through the intrinsic pathway (19, 29). Which pathway drives the activation of coagulation after trauma, and how it relates to pathological coagulopathy, remains uncharacterized.
In this study, blockade of the tissue factor pathway led to significant attenuation of overall coagulation in traumatic shock, as measured by thrombin production; this suggests that tissue factor and the extrinsic pathway are the primary drivers of coagulation after injury. Tissue factor pathway blockade also attenuated the activation of protein C found in traumatic shock. Specific thrombin inhibition achieved similar results, indicating that aPC-related coagulopathy is mediated via thrombin activated by the tissue factor pathway. This corresponds to the known enzymatic role of thrombin in complexing with endothelial thrombomodulin to activate protein C, and argues against possible activation of protein C via multiple recently delineated alternative tissue factor-modulated pathways. The near-complete attenuation of TAT and aPC observed in this model argues for a dominant role of the tissue factor-thrombin axis in both coagulation activation after injury and traumatic coagulopathy.
The clinical implications of these findings are numerous. An improved understanding of such critical pathways will enable future attempts to modulate acute traumatic coagulopathy and develop potential therapeutic approaches. The ability to reduce thrombin's role in coagulopathy is intriguing, but also fraught with difficulty given the absolute necessity of thrombin in forming sufficient clot for hemostasis. It may be that intervening at specific time points after traumatic insult might prove fruitful. In our prior murine investigations, we blocked aPC and found that 100% of treated mice died, usually within 45 minutes – this is consistent with aPC's key cytoprotective role in the response to inflammation and the maintenance of endothelial integrity. However, a human version of the targeted antibody used in that study, blocking aPC's serine protease anticoagulant function but leaving its cytoprotective function intact, could be a powerful therapeutic agent in traumatic coagulopathy. Such therapeutics are currently under development by multiple investigators, primarily for the treatment of sepsis (30). Understanding how much blockade to employ, and especially at what point it would be most effective following traumatic injury, represent major questions for future study.
An obvious limitation of this study is its context in a controlled murine system, and thus the results herein may not translate directly to the setting of human disease. Still, many key hematologic and inflammatory pathways are conserved across species; also, the utility of a mouse model is that it allows for ready experimental manipulation, including the use of genetically modified organisms. Also, recognizing that aPC is likely one of several drivers of acute traumatic coagulopathy, this investigation must be understood as one targeted assessment of a complex and multi-faceted pathologic process (31). Additionally, while we would have liked to correlate our findings to functional coagulation tests, we were limited by the small amount of blood volume available, especially in trauma-hemorrhage mice. However, this model has already been correlated to distinct coagulopathic changes in both standard coagulation tests (15) and viscoelastic assays such as thromboelastometry (unpublished data).
The disparity in TAT and aPC levels in the control (non-blockade) groups in this study indicates that the effects of antibody vehicle (IgG) are likely not inert; these mechanisms remain unknown at this time. The dosage of recombinant hirudin certainly has an effect on overall TAT level, and was chosen to optimize blockade while maintaining mouse survival, based on prior experiments; this particular dosing regimen may explain the lack of complete TAT attenuation seen in Figure 3. Additionally, one mouse in the hirudin-treated trauma-hemorrhage group did not have enough plasma for TAT analysis, reducing then in that group (for TAT) to 5 and potentially abrogating what may be a statistically significant effect. Nonetheless, the key finding remains that even in the absence of complete thrombin blockade, aPC was reduced to below-sham levels; this underscores the dependency of aPC activation on upstream thrombin production, and by extension on the tissue factor pathway.
The actual source of tissue factor activated in these mice remains undefined, and is the subject of ongoing investigation in our laboratory. To better understand the providence of tissue factor active in this model, and whether it comes from peri-vascular tissue or circulating sources, we are utilizing genetically modified mice with down-regulated myeloid cell tissue factor (32, 33). Concurrent experiments are being conducted to better understand the effects of tissue factor and aPC on inflammation after trauma; these include measurements of lung water and endothelial permeability by radioactive assay following traumatic hemorrhage. Incorporating resuscitation into the trauma-hemorrhage protocol represents another area of active ongoing study.
In sum, we have demonstrated here that in an established murine model of traumatic shock, the activation of coagulation after trauma is driven by the tissue factor pathway via thrombin; this pathway also drives the pathological aPC activation observed in acute traumatic coagulopathy. Improved understanding of these processes will enable future efforts to develop therapeutic agents in the ongoing endeavor to prevent hemorrhagic death after injury.
Acknowledgments
Funding: NIH 1 UM1HL120877 (MJC), NIH 1-UM-1-HL120877 (WR)
Footnotes
This study was presented at the 45th annual meeting of the Western Trauma Association Meeting, March 1–6, 2015, in Telluride, Colorado.
Conflict of Interest Statement: The authors declare no conflicts of interest related to this manuscript.
AUTHOR CONTRIBUTIONS
BMH, BYM, WR, and MJC contributed to study design, data collection, data analysis, data interpretation, writing, and critical revision.
WD, WJC, RFV contributed to study design, data collection, and critical revision.
REFERENCES
- 1.Norton R, Kobusingye O. Injuries. The New England journal of medicine. 2013;368(18):1723–30. doi: 10.1056/NEJMra1109343. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. The Journal of trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Hess JR, Holcomb JB, Hoyt DB. Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion. 2006;46(5):685–6. doi: 10.1111/j.1537-2995.2006.00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Jenkins D, Rhee P, Johannigman J, Mahoney P, Mehta S, Cox ED, Gehrke MJ, Beilman GJ, Schreiber M, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. The Journal of trauma. 2007;62(2):307–10. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. The Journal of trauma. 2003;54(6):1127–30. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 6.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. The Journal of trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 7.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Annals of surgery. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, Pittet JF. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. The Journal of trauma. 2008;64(5):1211–7. doi: 10.1097/TA.0b013e318169cd3c. discussion 7. [DOI] [PubMed] [Google Scholar]
- 9.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Current opinion in critical care. 2007;13(6):680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 10.Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, Pittet JF. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Annals of surgery. 2012;255(2):379–85. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esmon CT. The protein C pathway. Chest. 2003;124(3 Suppl):26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 12.Stavenuiter F, Bouwens EA, Mosnier LO. Down-regulation of the clotting cascade by the protein C pathway. Hematology education / Congress of the European Hematology Association European Hematology Association Congress Education Program. 2013;7(1):365–74. [PMC free article] [PubMed] [Google Scholar]
- 13.Bajzar L, Manuel R, Nesheim ME. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. The Journal of biological chemistry. 1995;270(24):14477–84. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- 14.Rezaie AR. Vitronectin functions as a cofactor for rapid inhibition of activated protein C by plasminogen activator inhibitor-1. Implications for the mechanism of profibrinolytic action of activated protein C. The Journal of biological chemistry. 2001;276(19):15567–70. doi: 10.1074/jbc.C100123200. [DOI] [PubMed] [Google Scholar]
- 15.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet JF, Cohen MJ. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock. 2009;32(6):659–65. doi: 10.1097/SHK.0b013e3181a5a632. WTA-2015-129R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(8):1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nature medicine. 2009;15(11):1318–21. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(9):1977–84. doi: 10.1161/ATVBAHA.114.304114. [DOI] [PubMed] [Google Scholar]
- 20.Aberg M, Siegbahn A. Tissue factor non-coagulant signaling - molecular mechanisms and biological consequences with a focus on cell migration and apoptosis. Journal of thrombosis and haemostasis : JTH. 2013;11(5):817–25. doi: 10.1111/jth.12156. [DOI] [PubMed] [Google Scholar]
- 21.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109(8):3161–72. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 22.Furlan-Freguia C, Marchese P, Gruber A, Ruggeri ZM, Ruf W. P2X7 receptor signaling contributes to tissue factor-dependent thrombosis in mice. The Journal of clinical investigation. 2011;121(7):2932–44. doi: 10.1172/JCI46129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. Journal of the American College of Surgeons. 1998;186(5):528–33. doi: 10.1016/s1072-7515(98)00082-9. [DOI] [PubMed] [Google Scholar]
- 24.Johansson PI, Ostrowski SR, Secher NH. Management of major blood loss: an update. Acta anaesthesiologica Scandinavica. 2010;54(9):1039–49. doi: 10.1111/j.1399-6576.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohen MJ. Towards hemostatic resuscitation: the changing understanding of acute traumatic biology, massive bleeding, and damage-control resuscitation. The Surgical clinics of North America. 2012;92(4):877–91, viii. doi: 10.1016/j.suc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Cohen MJ, Kutcher M, Redick B, Nelson M, Call M, Knudson MM, Schreiber MA, Bulger EM, Muskat P, Alarcon LH, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. The journal of trauma and acute care surgery. 2013;75(1 Suppl 1):S40–7. doi: 10.1097/TA.0b013e31828fa43d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pawlinski R, Pedersen B, Erlich J, Mackman N. Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thrombosis and haemostasis. 2004;92(3):444–50. doi: 10.1160/TH04-05-0309. [DOI] [PubMed] [Google Scholar]
- 28.Lo YM, Rainer TH, Chan LY, Hjelm NM, Cocks RA. Plasma DNA as a prognostic marker in trauma patients. Clinical chemistry. 2000;46(3):319–23. [PubMed] [Google Scholar]
- 29.Swystun LL, Mukherjee S, Liaw PC. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. Journal of thrombosis and haemostasis : JTH. 2011;9(11):2313–21. doi: 10.1111/j.1538-7836.2011.04465.x. [DOI] [PubMed] [Google Scholar]
- 30.Bae JS, Yang L, Manithody C, Rezaie AR. Engineering a disulfide bond to stabilize the calcium-binding loop of activated protein C eliminates its anticoagulant but not its protective signaling properties. The Journal of biological chemistry. 2007;282(12):9251–9. doi: 10.1074/jbc.M610547200. [DOI] [PubMed] [Google Scholar]
- 31.Maegele M, Schochl H, Cohen MJ. An update on the coagulopathy of trauma. Shock. 2014;41(Suppl 1):21–5. doi: 10.1097/SHK.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 32.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research. 1999;8(4):265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 33.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116(5):806–14. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]