Abstract
Background
A multicenter, prospective, blinded study was performed to test the feasibility of using a handheld optical imaging probe for the intraoperative assessment of final surgical margins during breast-conserving surgery (BCS) and to determine the potential impact on patient outcomes.
Methods
Forty-six patients with early-stage breast cancer (one with bilateral disease) undergoing BCS at two study sites, the Johns Hopkins Hospital and Anne Arundel Medical Center, were enrolled in this study. During BCS, cavity-shaved margins were obtained and the final margins were examined ex vivo in the operating room with a probe incorporating optical coherence tomography (OCT) hardware and interferometric synthetic aperture microscopy (ISAM) image processing. Images were interpreted after BCS by three physicians blinded to final pathology-reported margin status. Individual and combined interpretations were assessed. Results were compared to conventional postoperative histopathology.
Results
A total of 2,191 images were collected and interpreted from 229 shave margin specimens. Of the eight patients (17 %) with positive margins (0 mm), which included invasive and in situ diseases, the device identified all positive margins in five (63 %) of them; reoperation could potentially have been avoided in these patients. Among patients with pathologically negative margins (>0 mm), an estimated mean additional tissue volume of 10.7 ml (approximately 1 % of overall breast volume) would have been unnecessarily removed due to false positives.
Conclusions
Intraoperative optical imaging of specimen margins with a handheld probe potentially eliminates the majority of reoperations.
Breast-conserving surgery (BCS) is the standard-of-care surgical intervention for early-stage cancer. Margin status in BCS indicates the completeness of tumor resection and is an important factor for ensuring local control.1 A recent 28,162-patient meta analysis indicated that achieving negative margins is associated with a twofold decrease in ipsilateral tumor recurrence.2
Reoperation is estimated to occur in 23–38 % of BCS patients due to close/positive histologic margins found in postoperative pathology, although significant variability exists3 and some locations have reported lower numbers.3–5 Improved intraoperative assessment may reduce these rates by providing feedback during BCS. Existing intraoperative techniques, such as touch smear/imprinting cytology and frozen-section analysis, have variable accuracy depending on institution, add considerable time (up to 55 min) to the BCS duration, and are not available in some locations.6–9 Intraoperative ultrasound is more time-efficient but poorly detects ductal carcinoma in situ (DCIS).9 Radiofrequency (RF) spectroscopy is a comparatively new technique that analyzes the main BCS specimen using changes in RF impedance spectra and signature matching algorithms to give a “yes–no” determination of cancer presence in a region.10,11 Several additional technologies, including spectroscopic optical, fluorescence, X-ray, and high-frequency ultrasound techniques, are being investigated.8,12
Optical coherence tomography (OCT) with interferometric synthetic aperture microscopy (ISAM) provides high-resolution, cross-sectional optical images of microscopic tissue structure in real time using near-infrared light.13–15 OCT, which is commonly used in ophthalmology, generates images that are similar in appearance to ultrasound but with much higher resolution (<20 μm) and shallower imaging depth (2–3 mm). In a pilot 37-patient trial, OCT images of tumor tissue displayed a high density of small scatterers with irregular morphology relative to normal breast tissue; the sensitivity and specificity of OCT to identify positive margins in a single region of the main BCS specimen were 100 and 82 %, respectively.16
This study is an IRB-approved, multicenter, prospective, blinded study (clinicaltrials.gov NCT10699867) to assess the feasibility of intraoperative margin assessment using a handheld optical imaging probe and to demonstrate the potential impact on reoperation rates and volume of tissue removed.
METHODS
Women older than age 18 years, scheduled to undergo BCS for histologically diagnosed carcinoma of the breast, were recruited from two sites in 2012 and 2013: the Johns Hopkins Hospital (Baltimore, MD) and the Anne Arundel Medical Center (Annapolis, MD). Patients were excluded if they had multicentric disease, T4 tumors, or breast implants. Patients also were excluded if they had undergone neoadjuvant systemic therapy, had previous radiation or open surgical procedures in the same quadrant of the operated breast, were pregnant, or were lactating. Study coordinators screened patients from the surgical calendar and coordinated with surgeons to determine eligibility. All patients completed the informed consent process prior to participation.
During standard-of-care BCS, the main lesion was excised with a rim of grossly normal tissue. Cavity shaving of each margin, a routinely used technique, was subsequently performed.9,17,18 Shave specimens, which were employed to reduce pathology disorientation and standardize specimen handling procedures, were evaluated with the handheld imaging probe on the final margin (i.e., the true margin or new margin).19 At least four images per specimen were acquired in regions most suspicious for disease, as identified using the image data. Specimens were marked with sutures or ink to indicate final margin orientation before submission to pathology for standard-of-care gross and histology evaluation. Specimens with fewer than four images were excluded from the final analysis, because the data were deemed insufficient to cover the entire specimen.
The device used in this study (Fig. 1) employed OCT hardware, including a swept-source near-infrared laser and a handheld probe containing custom focusing optics and a microelectromechanical mirror system to scan the light across the tissue (1300 nm center wavelength, <15 μm resolution, 9-mm scan length, >2.3 cm/s scan speed). The device also incorporated ISAM software to provide uniform resolution over the entire image.20
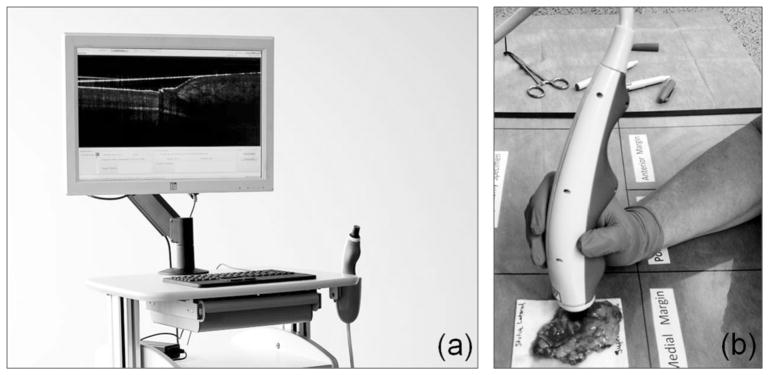
FIG. 1.
Optical coherence tomography imaging system with a handheld probe (a) was used to intraoperatively evaluate excised shave specimens (b)
De-identified images were interpreted after surgery by surgeon, pathologist, and radiologist readers who scored the images based on shadowing, brightness, and texture features previously identified as being indicative of tumor.16 Training was completed using representative images showing normal (15 images from four patients) and tumor-bearing specimens (18 images from 5 patients, including invasive lobular carcinoma, invasive ductal carcinoma, and DCIS), which were available for comparison during image interpretation. Physicians were blinded to results of the pathology specimen examination during image review; preoperative diagnoses were available as they would typically be in a clinical setting. Images were grouped by patient, displayed on a computer monitor (61 cm, 1920 × 1080 pixels, luminance 300 cd/m2) and scored from 0 to 10 for the presence of tumor. The scoring scale, similar to those used in ultrasound studies, was used to classify the images as incomplete (0), containing benign tissue only (1–4), containing suspicious structures (5), or containing tumor (6–10).21,22
For each image, the median of the three reviewer scores (consensus score) was used. For each shave specimen, from which at least four images were acquired, the maximum score among all images (specimen score) was used as a summary measure. The specimen score represents the likelihood of identifying tumor in the specimen. Four total specimen scores were calculated: the pathologist score, surgeon score, radiologist score, and consensus score (Fig. 2).
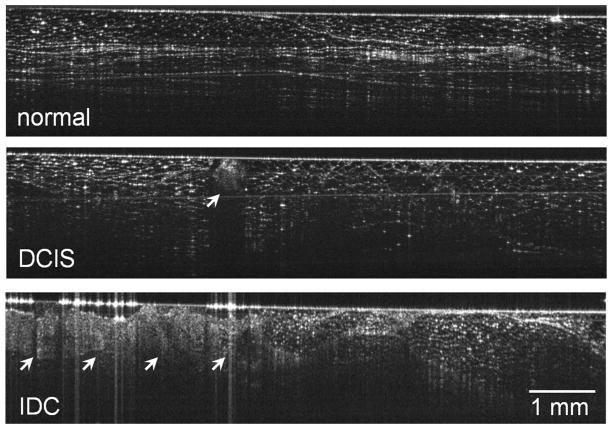
FIG. 2.
Representative images from this study. The top image shows regions of normal fibrofatty breast tissue with well-defined boundaries, linear structures, and regular texture. The middle image of ductal carcinoma in situ shows a small central region (arrow) with irregular texture and significant shadowing. The bottom image of invasive ductal carcinoma (arrows) shows a significant region with irregular texture and poorly defined boundaries
Patient-level analyses were performed to determine the projected impact on patient outcomes had the device results been available during surgery for interventional decision-making. The percentage of patients correctly identified as having positive/close margins was calculated as , where is the number of patients with all positive/close margins correctly identified by the device and N+ is the number of patients with at least one positive/close margin. The mean number of false positive specimens (margin-negative specimens identified as margin positive by the device) was calculated across patients. For analysis purposes, margin status was divided into four categories according to the tumor distance from the surface: 0 mm (positive margin), <1 mm, <2 mm (close margin), or ≥2 mm (negative margin). Analyses were repeated for each category.
Sensitivity and specificity of the device, when compared to pathology, were calculated for all combinations of margin distance and image rating threshold (0–10) to construct receiver operating characteristic (ROC) curves. The area under each ROC curve (AUC) was computed for comparison purposes.23,24 Statistical analyses were performed using Stata 12.1 (StataCorp LP, College Station, TX); lroc and roccomp commands were used to compute and compare AUCs.
To measure the image score rating agreement between the three physicians, intraclass correlation coefficients (ICCs) were calculated using a two-way, mixed-effects model.25 Because there was no “gold standard” rating in this study, the consistency of agreement rather than absolute agreement was analyzed. Average ICCs were reported based on the reviewer rating for each image; this is equivalent to Cronbach’s alpha.26
Finally, a non-randomness analysis was performed to verify that the device results are correlated to the specimen pathology results. A Monte Carlo simulation (N = 1 million) was performed in which image scores for each reader were randomized and the AUC was computed for each permutation. The probability that the AUC output from a random score distribution would equal physician consensus performance was computed.
RESULTS
Forty-seven patients were enrolled in this study. Of these, one patient had bilateral BCS and one patient (6 shave specimens) was excluded due to insufficient images in all specimens (1 image acquired per specimen due to researcher misunderstanding of the protocol). Three additional specimens (1 patient) were excluded due to insufficient images (2–3 images acquired per specimen due to researcher misunderstanding of the protocol). Forty-six patients were included in the final analysis (23 per site; Table 1). Fifteen of 46 BCS procedures (33 %) were performed for a preoperative diagnosis of DCIS alone.
TABLE 1.
Patient demographic and clinicopathological features
| Age (year) | |
| 27–81 (mean 62, median 61; 39 % ≥65) | |
| Race | |
| White | 37 (80 %) |
| Black or African American | 8 (17 %) |
| Asian | 1 (2 %) |
| Ethnicity | |
| Hispanic or Latino | 1 (2 %) |
| Not Hispanic or Latino | 45 (98 %) |
| Body mass index | |
| 19–46 (mean 30, median 29) | |
| Cup sizea | |
| A | 1 (2 %) |
| B | 8 (17 %) |
| C | 18 (39 %) |
| D | 10 (22 %) |
| E (2D) | 4 (9 %) |
| F (3D) | 1 (2 %) |
| G (4D) | 1 (2 %) |
| Breast density (BIRADS mammographic density)b | |
| Density 1 | 3 (7 %) |
| Density 2 | 22 (48 %) |
| Density 3 | 19 (41 %) |
| Density 4 | 1 (2 %) |
| Preoperative diagnosisc | |
| Invasive ductal carcinoma | 20 (43 %) |
| Ductal carcinoma in situ | 15 (32 %) |
| Ductal carcinoma in situ and invasive ductal carcinoma | 6 (13 %) |
| Invasive ductal carcinoma and invasive lobular carcinoma | 3 (6 %) |
| Invasive lobular carcinoma | 2 (4 %) |
| Invasive tubular carcinoma | 1 (2 %) |
Three unknown
One unknown
Both diagnoses are included from the patient with bilateral disease
Projected Patient Outcomes
Eight (17 %) patients had at least one positive margin, and in five (63 %) of these, all positive margins were correctly identified by the device; reoperation could potentially have been avoided in these patients. In three (38 %) of the positive-margin patients, at least one positive margin was not identified by the device.
Among 35 (74 %) patients with all negative margins (≥2 mm), 63 % had at least one false-positive margin identified by the device. On average, each of these patients would potentially have received 1.32 additional shave excisions during the BCS. Had these sites been excised, an estimated average volume of 10.7 mL would have been removed (approximately 1 % of overall average breast volume), as estimated from bra cup size (Table 1) and re-excision specimen volume in previous study data (Table 2).27,28 Patient results used a consensus specimen score threshold of six.
TABLE 2.
Projected impact on patient outcomes
| Tumor distance to surface | 0 mm | <1 mm | <2 mm |
|---|---|---|---|
| Detection | |||
| Patients with all positive/close margins correctly identified by the device | 5/8 (63 %) | 8/11 (73 %) | 7/12 (58 %) |
| False positivity | |||
| Number of margin-negative patientsa | 39 | 36 | 35 |
| Average number of projected additional excisions per patientb | 1.49 | 1.34 | 1.32 |
Threshold score of 6, consensus score
All final margins were pathologically negative (>0, ≥1, ≥2 mm)
Additional excisions projected to be performed on pathologically negative margins due to a positive device reading
Margin Analysis
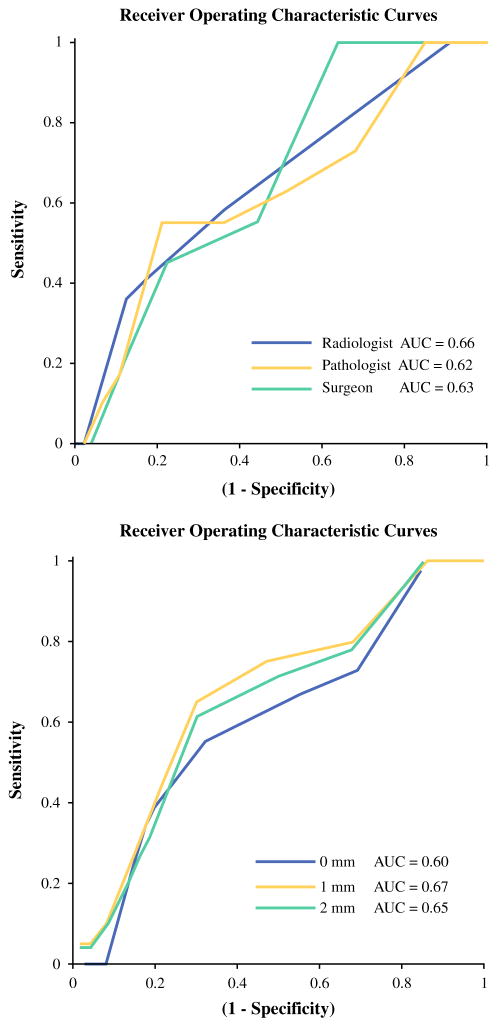
A total of 2,191 images were collected and interpreted from 229 shave margin specimens. Among the 11 (5 %) specimens with positive margins, 7 (64 %) contained DCIS alone, 3 (27 %) contained invasive disease alone, and 1 (9 %) contained both. Of the 23 (10 %) specimens with close margins, 20 (87 %) contained DCIS and 4 (17 %) contained invasive disease. The AUC was 0.62–0.70 for all image interpreters and margin distances (Fig. 3).
FIG. 3.
Receiver operating characteristic curves showing the positive-margin performance of three individual image interpreters (top) and consensus performance by tumor depth (bottom)
With a threshold consensus score of six, the device identified 76 (33.2 %) shaved margins as positive. The sensitivity and specificity were 55–65 and 68–70 %, respectively, depending on margin criteria. The positive predictive value and negative predictive value were 8–18 and 94–97 %, respectively. For patients with a preoperative diagnosis of DCIS alone, positive margins were detected with 80 % sensitivity and 69 % specificity.
Image Interpretations
The pathologist-surgeon, pathologist-radiologist, and surgeon-radiologist ICCs were 0.364, 0.363, and 0.858, respectively. The three-physician ICC was 0.728. AUC values for all physicians were 0.62–0.70 depending on margin distance and varied by no more than ΔAUC = 0.08 (12 %) between any two physicians for a given tumor distance (Fig. 3). Finally, the non-randomness analysis demonstrated (p <0.02) that the device results were not due to a random distribution of image scores.
DISCUSSION
A joint consensus by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology states that a 0 mm (“tumor on ink”) criteria is appropriate for BCS patients with invasive disease as it is associated with reduced ipsilateral recurrence.2 For patients with in situ disease alone, the appropriate distance is more controversial. A systematic review of 4660 patients, for example, found significant evidence supporting a 2-mm distance for DCIS patients.29 In addition, a survey of 351 surgeons showed that 34 and 52 % of them used 1 and 2 mm, respectively, as the minimal acceptable DCIS distance. The results of the present study suggest that optical margin assessment can be performed in patients with invasive and in situ disease no matter what standard (0–2 mm) is used, because the statistical performance is similar across margin definitions and disease. Furthermore, the device performed well on patients with DCIS alone, demonstrating effective margin status analysis in patients most at risk of reoperation.
The results of this study also demonstrate similar image interpretation outcomes from physicians with varying specialties with minimal image interpretation training. The fact that the ICC for all reviewers was considerably higher than the ICCs based on absolute agreement and that the AUCs for all reviewers were similar suggests that the three readers identified the same phenomena.
Although the patient cohort in this study was relatively small, the performance results compare well to other emerging margin assessment technologies. For example, a commercial RF spectroscopy device approved by the U.S. Food and Drug Administration as an adjunctive tool to identify tumor within 1 mm of the main ex vivo BCS specimen surface detects the electromagnetic radiation reflected from a 7-mm diameter region of tissue and applies a proprietary algorithm to determine tumor presence.10 In a multicenter, prospective study of 596 patients randomized into device and control arms, investigators reported a margin-level device sensitivity of 75 % and specificity of 46 % when evaluating tumor <1 mm from the specimen surface (34 % sensitivity and 83 % specificity for the control arm).11 As a result of device-indicated re-excisions performed during surgery, 23 % fewer patients underwent reoperation due to positive margins. False-positive device readings resulted in 2.68 unnecessary re-excisions per patient on average (21.7 mL breast tissue volume).27 Performance in patients with pure DCIS was not reported. While this RF spectroscopy study is a larger and more direct evaluation of surgical outcomes than the investigation presented in this paper, the statistical performance of the handheld optical imaging device studied here is similar or superior to that of the RF spectroscopy device. A larger optical imaging study is required to compare more conclusively the two devices.
The handheld OCT probe used in this investigation offers several advantages over other technologies. For example, it is difficult to quickly analyze a full margin region with point analysis techniques, whereas OCT generates real-time images, enabling rapid scanning of the entire final margin of excised specimens.30 OCT also allows the surgeon to quantify the margin width, allowing for adherence to re-excision guidelines, whereas other techniques indicate tumor presence without specifying distance. Finally, OCT can be used to analyze the final BCS margins, whether on the primary BCS specimen or shave specimen.16
Importantly, the BCS surgical cavity may be analyzed directly with OCT to assess tissue in the patient. Although the present study was limited to excised specimens, ongoing studies have shown that intraoperative OCT is effective when used to identify tumor in the surgical cavity.31
While the results of this feasibility study are promising, several limitations were noted. Physician training, for instance, was minimal, and the use of a larger image reference database may improve identification of positive margin features. Additionally, image interpretation was performed after surgery, removing important clinical context and intraoperative visual and tactile cues from the decision-making.32 Also, pathology results were reported on a margin-by-margin basis and did not correlate directly with the device analysis locations on the specimen; device imaging and histology sectioning may have been performed at different locations on the specimen, which may result in underreporting of the device performance. Because the device results were not acted on in this study, projected patient outcomes could not be directly verified via surgical results. Addressing these limitations will likely improve the patient outcomes by reducing false positives, which can cause unnecessary tissue excision, and reducing false negatives, which may not eliminate a repeat surgery.
Data presented provide significant support for the use of OCT with ISAM as an intraoperative method for the analysis of BCS margin status. Use of a handheld imaging probe provides surgeons with information that is likely to significantly reduce the number of reoperations by guiding re-excision of positive margins during the primary BCS procedure.
Acknowledgments
This research was funded by the U.S. National Cancer Institute (R44CA165436). We acknowledge Dr. Daniel McCormick for technical contributions and Drs. Robert Buras, Melissa Camp, Mehran Habibi, Clarissa Hammer, Julie Lange, and Wen Liang for study participation and recruiting. This study was funded by the US National Cancer Institute and sponsored by Diagnostic Photonics, Inc. AMZ and AJC are employees of Diagnostic Photonics, Inc. SAB and PSC are co-founders of Diagnostic Photonics, Inc.
References
- 1.Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002;184(5):383–93. doi: 10.1016/s0002-9610(02)01012-7. [DOI] [PubMed] [Google Scholar]
- 2.Moran MS, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Ann Surg Oncol. 2014;21(3):704–16. doi: 10.1245/s10434-014-3481-4. [DOI] [PubMed] [Google Scholar]
- 3.McCahill LE, et al. Variability in reexcision following breast conservation surgery. J Am Med Assoc. 2012;307(5):467–75. doi: 10.1001/jama.2012.43. [DOI] [PubMed] [Google Scholar]
- 4.Morrow M, et al. Surgeon recommendations and receipt of mastectomy for treatment of breast cancer. J Am Med Assoc. 2009;302(14):1551–6. doi: 10.1001/jama.2009.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullen R, et al. Involved anterior margins after breast conserving surgery: is re-excision required? Eur J Surg Oncol. 2012;38(4):302–6. doi: 10.1016/j.ejso.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Fukamachi K, et al. Total-circumference intraoperative frozen section analysis reduces margin-positive rate in breast-conservation surgery. Jpn J Clin Oncol. 2010;40(6):513–20. doi: 10.1093/jjco/hyq006. [DOI] [PubMed] [Google Scholar]
- 7.Osborn JB, et al. Cost-effectiveness analysis of routine frozen-section analysis of breast margins compared with reoperation for positive margins. Ann Surg Oncol. 2011;18(11):3204–9. doi: 10.1245/s10434-011-1956-0. [DOI] [PubMed] [Google Scholar]
- 8.Butler-Henderson K, et al. Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast. 2014;23(2):112–9. doi: 10.1016/j.breast.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Tengher-Barna I, et al. Cavity margins examination in breast-conserving therapy. Diagn Histopathol. 2011;17(5):232–7. [Google Scholar]
- 10.Karni T, et al. A device for real-time, intraoperative margin assessment in breast-conservation surgery. Am J Surg. 2007;194(4):467–73. doi: 10.1016/j.amjsurg.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Schnabel F, et al. A randomized prospective study of lumpectomy margin assessment with use of MarginProbe in patients with nonpalpable breast malignancies. Ann Surg Oncol. 2014;21(5):1589–95. doi: 10.1245/s10434-014-3602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thill M, Baumann K, Barinoff J. Intraoperative assessment of margins in breast conservative surgery: still in use? J Surg Oncol. 2014;110(1):15–20. doi: 10.1002/jso.23634. [DOI] [PubMed] [Google Scholar]
- 13.Boppart SA, et al. In vivo cellular optical coherence tomography imaging. Nat Med. 1998;4(7):861–5. doi: 10.1038/nm0798-861. [DOI] [PubMed] [Google Scholar]
- 14.Huang D, et al. Optical coherence tomography. Science. 1991;254(5035):1178–81. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ralston TS, et al. Interferometric synthetic aperture microscopy. Nat Phys. 2007;3(2):129–34. doi: 10.1038/nphys514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen FT, et al. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009;69(22):8790–6. doi: 10.1158/0008-5472.CAN-08-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parvez E, et al. Survey of american and canadian general surgeons’ perceptions of margin status and practice patterns for breast conserving surgery. Breast J. 2014;20(5):481–8. doi: 10.1111/tbj.12299. [DOI] [PubMed] [Google Scholar]
- 18.Hewes JC, et al. Importance of routine cavity sampling in breast conservation surgery. Br J Surg. 2009;96(1):47–53. doi: 10.1002/bjs.6435. [DOI] [PubMed] [Google Scholar]
- 19.Molina MA, et al. Breast specimen orientation. Ann Surg Oncol. 2009;16(2):285–8. doi: 10.1245/s10434-008-0245-z. [DOI] [PubMed] [Google Scholar]
- 20.Ralston TS, et al. Cross-validation of interferometric synthetic aperture microscopy and optical coherence tomography. Opt Lett. 2010;35(10):1683–5. doi: 10.1364/OL.35.001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsen JF, et al. Strain elastography ultrasound: an overview with emphasis on breast cancer diagnosis. Diagnostics. 2013;3(1):117–25. doi: 10.3390/diagnostics3010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qvistgaard E, et al. Reproducibility and inter-reader agreement of a scoring system for ultrasound evaluation of hip osteoarthritis. Ann Rheum Dis. 2006;65(12):1613–9. doi: 10.1136/ard.2005.050690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 25.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 26.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. [Google Scholar]
- 27.United States Food and Drug Administration Executive Summary Prepared for the June 21, 2012 Meeting of the General and Plastic Surgery Devices Panel (P110014) Dune Medical Devices, Inc. MarginProbeTM System.
- 28.Huang SY, et al. The characterization of breast anatomical metrics using dedicated breast CT. Med Phys. 2011;38(4):2180–91. doi: 10.1118/1.3567147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunne C, et al. Effect of margin status on local recurrence after breast conservation and radiation therapy for ductal carcinoma in situ. J Clin Oncol. 2009;27(10):1615–20. doi: 10.1200/JCO.2008.17.5182. [DOI] [PubMed] [Google Scholar]
- 30.Allweis TM, et al. A prospective, randomized, controlled, multicenter study of a real-time, intraoperative probe for positive margin detection in breast-conserving surgery. Am J Surg. 2008;196(4):483–9. doi: 10.1016/j.amjsurg.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Erickson-Bhatt SJ, et al. In vivo assessment of the surgical cavity during breast-conserving surgery with a handheld optical imaging probe. Cancer Res. in press. [Google Scholar]
- 32.Hooley RJ, Scoutt LM, Philpotts LE. Breast ultrasonography: state of the art. Radiology. 2013;268(3):642–59. doi: 10.1148/radiol.13121606. [DOI] [PubMed] [Google Scholar]