Significance
Invasive species represent a largely unquantified threat to ecosystem services. Although investment in the prevention of species invasions may sustain ecosystem services, these effects of invasions are rarely measured in monetary terms useful to decision makers. We quantify the economic damages of the degradation of an important ecosystem service, water clarity, caused by invasion by the spiny water flea. We find that the costs of restoring this service, US$86.5 million–US$163 million, are comparable with the willingness to pay for the service itself: US$140 million. This finding highlights the severity of invasive species’ impacts when their damages to ecosystem services are considered. Costs of invasive species’ secondary spread aggregated across many invasive species and ecosystem services may be large.
Keywords: invasive species, ecosystem service, eutrophication, Bythotrephes, Daphnia
Abstract
Despite growing recognition of the importance of ecosystem services and the economic and ecological harm caused by invasive species, linkages between invasions, changes in ecosystem functioning, and in turn, provisioning of ecosystem services remain poorly documented and poorly understood. We evaluate the economic impacts of an invasion that cascaded through a food web to cause substantial declines in water clarity, a valued ecosystem service. The predatory zooplankton, the spiny water flea (Bythotrephes longimanus), invaded the Laurentian Great Lakes in the 1980s and has subsequently undergone secondary spread to inland lakes, including Lake Mendota (Wisconsin), in 2009. In Lake Mendota, Bythotrephes has reached unparalleled densities compared with in other lakes, decreasing biomass of the grazer Daphnia pulicaria and causing a decline in water clarity of nearly 1 m. Time series modeling revealed that the loss in water clarity, valued at US$140 million (US$640 per household), could be reversed by a 71% reduction in phosphorus loading. A phosphorus reduction of this magnitude is estimated to cost between US$86.5 million and US$163 million (US$430–US$810 per household). Estimates of the economic effects of Great Lakes invasive species may increase considerably if cases of secondary invasions into inland lakes, such as Lake Mendota, are included. Furthermore, such extreme cases of economic damages call for increased investment in the prevention and control of invasive species to better maximize the economic benefits of such programs. Our results highlight the need to more fully incorporate ecosystem services into our analysis of invasive species impacts, management, and public policy.
Despite growing recognition of the importance of ecosystem services (1) and the harm caused to ecosystems by invasive species (2, 3), linkages between species invasions and ecosystem services are rarely made (4–6). Investments in the prevention of species invasions may sustain ecosystem services. However, the effects of invasions are rarely quantified in monetary terms that assess damages to services alongside the costs and ecological mechanisms of restoration options (6, 7). Invasive species are a major threat to freshwater ecosystems (8) and thereby, endanger several ecosystem services that are essential for human wellbeing.
Freshwater ecosystems are a cornerstone of human society, providing drinking water, fisheries, pollution dilution, recreation, and other goods and services (9). Valuation of these services is critical for public policy (10, 11), but many of the services provided by freshwater ecosystems are not monetized (12, 13), leaving them overlooked and poorly integrated into decision frameworks (1, 3). Water quality of lakes and reservoirs has been degraded by phosphorus (P) pollution, leading to loss of recreation and aesthetic value, decreased lakeshore property values, beach closures, fish kills, harmful blooms of cyanobacteria, and loss of water clarity (14). Daphnia, a genus of freshwater zooplankton, improves water quality by consuming algae (15, 16). Accordingly, lakes are sometimes managed to support large Daphnia populations by reducing the abundance of their predators (15, 17).
The spiny water flea, Bythotrephes longimanus (hereafter Bythotrephes), which is nonnative in North America, is a voracious zooplanktivore that has the capacity to consume more zooplankton than fish and other invertebrate planktivores combined (18). Despite this planktivory and large documented ecological impacts on zooplankton communities (19, 20), Bythotrephes has not been found to have cascading effects on lake primary production and water clarity (21). The lack of cascading effects of Bythotrephes invasion is perhaps because the productive lakes most vulnerable to impaired water clarity are thought to be relatively unsuitable for Bythotrephes establishment (22).
Bythotrephes was detected in the well-studied eutrophic Lake Mendota in the fall of 2009 at some of the highest densities on record (>150 m−3; mean open water density). The invasion was of immediate concern, because a preferred prey of Bythotrephes, Daphnia pulicaria, has been the focal point of Lake Mendota’s food web management, supporting the lake’s fishery (23) and maintaining clear water through grazing algae (24) (Fig. 1). Lake Mendota is located within an agricultural watershed and receives large amounts of P from farm runoff, reducing water quality by stimulating algal growth (25) (Fig. 1). This ecosystem service provided by D. pulicaria has delivered huge economic benefits, providing recreational value to citizens who have been estimated to be willing to pay US$140 million (present-day value) for 1 m of water clarity (1.6- to 2.6-m change in summer clarity) (26, 27).
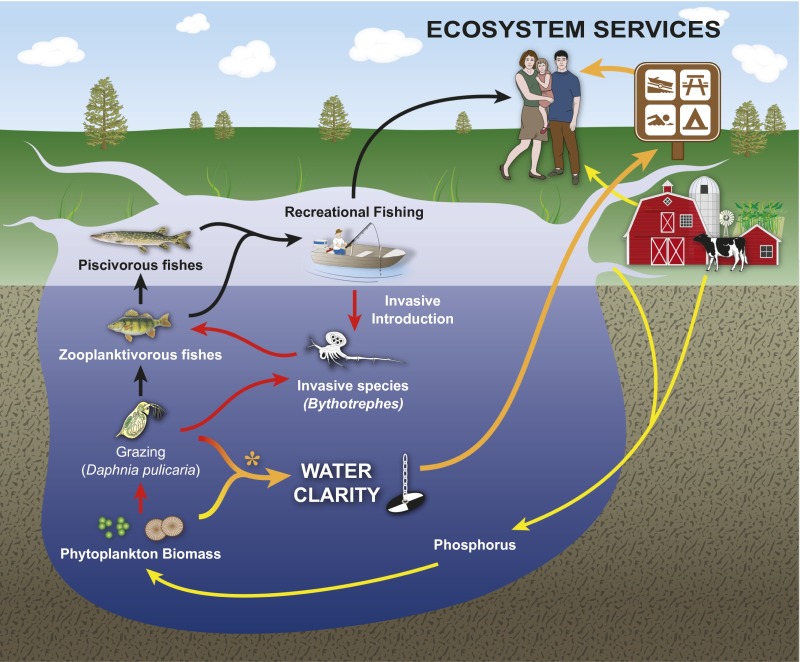
Fig. 1.
Arrows represent connections among major components of the socioecological system: Lake Mendota. The introduction of Bythotrephes (red arrows) is presented here in the context of existing pathways affecting water clarity (orange arrows), a key ecosystem service in the lake, such as agricultural runoff (yellow arrows), and top-down control of the food web (black arrows). *Increasing phytoplankton biomass resulting from increased nutrient input or decreased grazing decreases water clarity; there are no direct options for the control or eradication of Bythotrephes.
Results and Discussion
Since the detection of Bythotrephes in 2009, average water clarity in Lake Mendota has declined by 0.9 m (Fig. 2F) alongside a 60% reduction in D. pulicaria biomass (Fig. 2B). In addition, there was a decrease in total phosphorus (TP) (Fig. 2D), despite no clear change in P loading (Fig. 2E), and an overall increase in total grazing zooplankton biomass (Fig. 2C) (17% overall and 56% increase in non-D. pulicaria grazers). These findings show a cascading impact of Bythotrephes that has not been previously documented in other lakes (21) and that is unusually large for an invertebrate predator (28). Possibly, this effect is related to the feeding mode of Bythotrephes vs. other invertebrate predators (18, 29, 30), and this topic could be addressed by additional research.
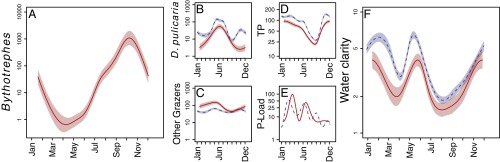
Fig. 2.
Seasonal dynamics pre-Bythotrephes (blue dashed lines; 1995–2007) and post-Bythotrephes (red line; 2009–2014) of (A) Bythotrephes (micrograms meter−3), (B and C) zooplankton grazers (milligrams meter−3), (D and E) P dynamics (micrograms TP liter−1 and kilograms P day−1, respectively), and (F) water clarity (Secchi depth in meters) are plotted as a smoothed generalized additive model function of day of the year. Shaded areas represent 1 SE. Note that all y axes are log scaled.
The strongest effects of Bythotrephes on D. pulicaria are observed in the fall (Fig. 2B), when Bythotrephes is most abundant (Fig. 2A). These effects occur at a time critical to the overwintering success of Daphnia, which may explain declines that linger into the spring (Fig. 2B). Despite a compensatory increase in other zooplankton grazers (e.g., Daphnia mendotae), spring water clarity declined because of an overall decline in algae filtration rates by zooplankton. This decline further reveals the distinct advantage in filtration efficiency of D. pulicaria (31). Notably, Daphnia of all species collapsed from fall of 2014 to spring of 2015, including the less efficient but more predation-resistant D. mendotae. Before 2014, D. mendotae increased with the Bythotrephes invasion in Lake Mendota (Fig. 2C) as reported in other lakes (32, 33). Although not as efficient of a grazer as D. pulicaria, D. mendotae does provide better water clarity relative to smaller, more selective grazers, like copepods (24). If D. mendotae declines in years with high Bythotrephes biomass, such as in the fall of 2014, water clarity could decline in the future.
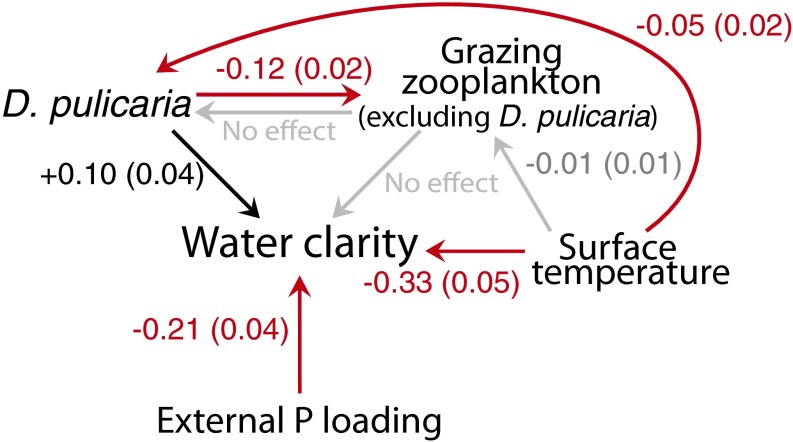
Multivariate autoregressive state space modeling (MARSS) (34) revealed that higher external P loading and seasonal surface temperatures accompany lower water clarity, whereas D. pulicaria biomass is associated with higher water clarity over the past two decades (1995–2014) in Lake Mendota (Fig. 3). These results are consistent with previous evaluations of the lake’s food web (24) and suggest that reducing external P loading into the lake can offset the negative impact of Bythotrephes on D. pulicaria and thus, water clarity.
Fig. 3.
MARSS estimates of ecological interactions are shown with arrows, and the strengths of the interactions are shown as estimates (SEs). Red arrows are significant negative effects, black arrows are significant positive effects, and gray arrows are nonsignificant effects.
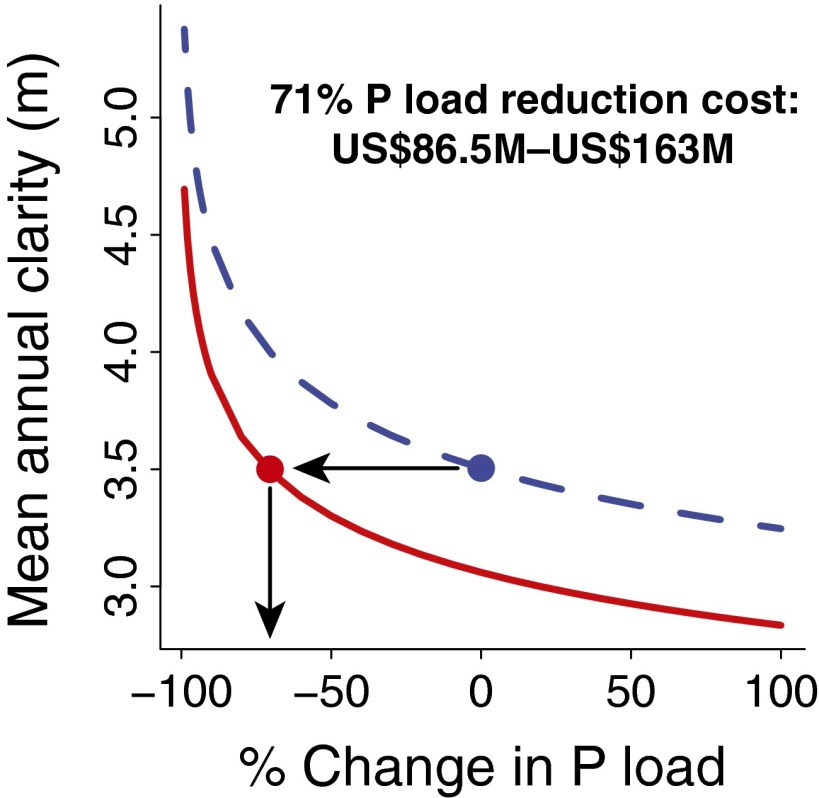
To quantify the P loading reduction required to offset the impact of Bythotrephes, we predicted water clarity under high (pre-2009) and low (post-2009) D. pulicaria biomass under varying P loading scenarios (−99% to +100%) using the fitted MARSS model. External P load reduction of 71% is needed to offset the decline in D. pulicaria (i.e., obtain pre-2009 water clarity under post-2009 D. pulicaria biomass) (Fig. 4).
Fig. 4.
The cost of offsetting Bythotrephes impact through P loading reductions is revealed through predicting water clarity under high (dashed blue line; pre-2009) and low (red line; post-2009) grazing under a range of P loading conditions. This restoration cost is calculated as the cost of the P load reduction necessary to return the lake to pre-2009 clarity (blue circle) under post-2009 grazing (red circle). Here, cost is the estimated total present-day cost over a 20-y project.
Recent estimates of the cost of P diversion from Lake Mendota indicate that a P load reduction of 71% will cost between US$86.5 million and US$163 million (US$430–US$810 per household in Dane County) (35). This conservative estimate is drawn from a detailed, itemized, and expert-elicited review investigating this very question in Lake Mendota. Investing in a 71% reduction would return the lake to preinvasion clarity, and any additional improvements to water clarity would have to be made on top of this investment. These costs illustrate the challenges associated with mitigating invasive species impacts.
An additional perspective on this economic impact is offered by the estimate that citizens in the region (Dane County, WI) are willing to pay US$140 million present-day value (US$640 per household) for 1 m of water clarity gained by managing the lake (26)—roughly equivalent in magnitude to the loss of 0.9 m caused by the Bythotrephes invasion. The similarly large costs of restoring clarity suggest that managers should consider new strategies to directly mitigate the Bythotrephes effect on D. pulicaria [e.g., by managing the fishery (Fig. 1) or limiting the production and hatching of Bythotrephes resting eggs]. Direct control of populations is thought to be an inefficient use of management funds (36); however, the high cost of Bythotrephes impacts makes investment in research and development of control options more attractive. Additionally, Bythotrephes amplifies the effect of cultural eutrophication and its many negative implications for freshwater ecosystem services. Therefore, managers should focus on limiting additional spread of this nonnative species among agriculturally impacted lakes.
Although only representing a single aspect of Bythotrephes impact on ecosystem services, these damage and cost estimates illustrate the potential harm caused by a single invasive species in a single lake affecting a single ecosystem service. Additional impacts of Bythotrephes, such as fouling fishing lines and disrupting the base of lake food webs (19), were not considered here but could increase these costs. Although we do not have the data to put a price tag on current and future economic damages of Bythotrephes or other invasive species at the landscape scale, our work highlights the importance of considering the aggregate impact of chains of secondary invasions as inland lakes in the region are colonized. We also note that, in just the US states bordering the Great Lakes, inland lakes have 36 times more shoreline than do the Great Lakes (37). Because lake ecosystem services are often delivered at this critical ecotone, the importance of inland lakes cannot be ignored (38).
Accounting for the many possible effects of secondary invasions on a diverse range of ecosystems and their services will require more research, but this accounting is critical in accurately weighing the costs and benefits of invasive species management options. Furthermore, this accounting must consider economic damages, while taking into account potential positive effects of invasions. Our study shows substantial economic damages from a secondary invasion, suggesting that investments in large-scale prevention or research on control and eradication tools may yield net economic benefits.
Furthermore, the economic damages quantified here have broader public policy implications regarding transatlantic shipping and the Laurentian Great Lakes St. Lawrence Seaway. Although allowing transatlantic ships to enter the Great Lakes provides US$55 million of annual savings relative to alternative transport options (39), these ships are also responsible for the majority of the nonnative species that have established in the Great Lakes in recent decades. Damage estimates for these invasions have been confined to the Great Lakes only and have not considered costs of secondary invasions, such as that of Bythotrephes in Lake Mendota (40). Benefit–cost assessments of transatlantic shipping in the Great Lakes may indicate significantly larger costs if secondary invasions are included in these assessments.
Linkages of ecological processes with economic or social benefits are needed to apply ecosystem service concepts in public policy development, implementation, and evaluation (1, 10, 12). Understanding impacts of nonnative species requires field measurements of ecosystem consequences in terms useful to managers (41). We have shown how an invasive species altered a lake food web, amplifying the harmful effects of cultural eutrophication and impairing water clarity, thereby reducing the benefits that humans derive from lakes. Water quality targets for the lake are more difficult and expensive to achieve as a result of the invasion. These economic damages followed a trophic cascade triggered by a voracious predator that originated in Eurasia and invaded through the Great Lakes and overland to Lake Mendota. Ecological mechanisms as well as economic ones must be analyzed together to bring ecosystem services into decision processes regarding species invasion (12).
Methods Summary
Lake Mendota.
Lake Mendota is a 39.6-km2 dimictic (mixes in spring and fall) and culturally eutrophic lake located adjacent to Madison, Wisconsin (25). Maximum and mean depths are 25.3 and 12.7 m, respectively, and the lake has a mean water residence time of roughly 4 y. A large portion of the land use within total drainage area in the watershed (602 km2) is agricultural and urban. Lake Mendota is the largest, deepest, and most upstream lake in the Madison Chain of Lakes connected by the Yahara River. Therefore, P dynamics in Lake Mendota have important implications for waters downstream, like the southern chain lakes—Lake Monona, Lake Waubesa, and Lake Kegonsa—as well as the Rock River, which flows into the Mississippi River.
Time Series.
We obtained time series data of Lake Mendota’s water clarity (Secchi depth), zooplankton community (species abundance and mean length), TP concentrations in the surface waters, and surface temperature from the North Temperate Lakes Long-Term Ecological Research program database (https://lter.limnology.wisc.edu/). Samples are taken on a monthly basis in the early spring and late fall, at least once during ice cover (all variables are sampled or observed through the ice), and fortnightly during the open season (roughly May to October). Data are available from 1995 to 2014 (2013 in the case of TP in Fig. 2D). Zooplankton abundance was converted to biomass using mean lengths and length to dry weight equations (42). Daily P loading measurements are available through the US Geological Survey (usgs.gov). Rather than calculate the TP load into the lake directly, we use the Yahara River at the Windsor Site as a proxy for loading into the lake (total load = 4.5 × Yahara River at Windsor load; R2 = 0.97). We summed daily P loading over fortnightly time steps. Clarity, zooplankton biomass, and P loading were log-transformed, and all variables were converted to fortnightly means and then, z scored. To visualize seasonal dynamics of pre- and post-Bythotrephes invasion time series, we fit cyclic cubic regression splines of day of the year to the log-transformed data for time periods both before (1995–2007) and after (2010–2014) the year of Bythotrephes' detection (2009) using generalized additive models with the package mgcv in R (43). We exclude 2008 and 2009 as transition years. All statistical analyses were conducted in R (44).
Statistical Analysis: MARSS–Model Fitting.
We used an MARSS to analyze the dynamics of water clarity in Lake Mendota using the MARSS package in R (45). The model takes the following form:
Observations (shown in the lower equation) comprise interacting system variables, such as Secchi depth, D. pulicaria biomass, and biomass of other (non-D. pulicaria) grazers, in vector ys and covariates, such as P loading and surface temperature, in vector yc (observations from 1995 to 2014). All observations are transformed to z scores. The observation vector estimates a partitioned vector of true values of system variable xs and covariate xc, with error v having covariances given by Rs and Rv, respectively. System dynamics (shown in the upper equation) involve a square transition matrix, with partitions for system interactions Bs, covariate effects on system variables C, and covariate changes over time Bc. System error w has covariances given by Qs and Qv corresponding to system variates and covariates, respectively. MARSS models are fit with maximum likelihood using a combination of the Kalman filter and an expectation maximization algorithm.
Model fits estimated all elements of Bs and Qs (interactions among system variables and their variances and covariances) and diagonal elements of Bc and Qv (autoregressive coefficients of covariates and their variances). We allowed the model to estimate terms along the R matrix diagonal (observation variances). The final model structure was selected using Akaike Information Criterion and previously published ecological interactions among variables. Here, we allow interactions between zooplankton grazers and known drivers of water clarity, like P loading, zooplankton, and surface temperature (i.e., arrows in Fig. 3). Temperature was allowed to affect all variates. Model selection (Table S1) and residual analysis (Figs. S1 and S2) can be found in Additional MARSS Modeling Information.
Table S1.
Model selection for varying MARSS combinations as described in Additional MARSS Modeling Information, MARSS: Model Performance and Selection
| MARSS model formulation | Log likelihood | AIC | Convergence (iterations) |
| Selected model | −1,733 | 3,515 | Yes (35) |
| Remove temperature effect on clarity | −1,762 | 3,571 | No |
| Remove P load effect on clarity | −1,742 | 3,530 | Yes (41) |
| Remove D. pulicaria effect on clarity | −1,736 | 3,518 | Yes (35) |
| Add other grazing zooplankton effect on clarity | −1,733 | 3,517 | Yes (35) |
Models are selected using AIC and previously published ecological interactions.
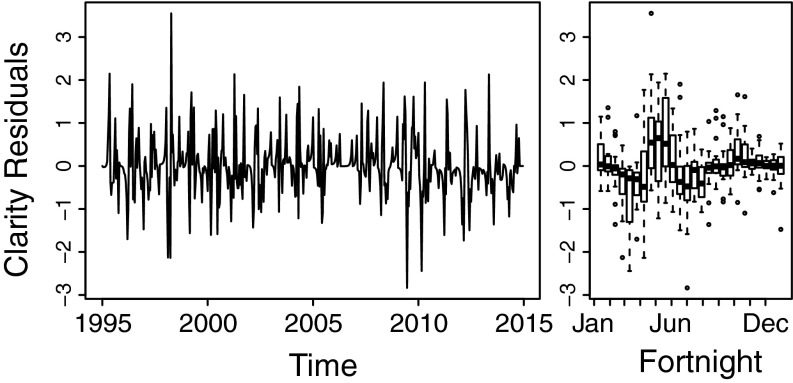
Fig. S1.
Clarity residuals plotted through time (long-term residuals in Left and seasonal residuals in Right).
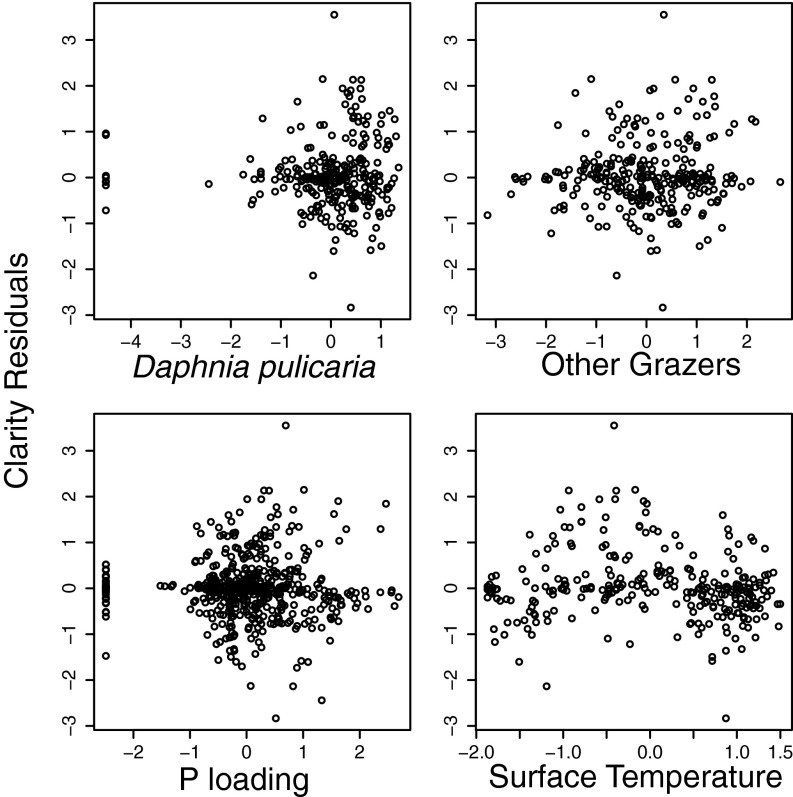
Fig. S2.
Clarity residuals plotted against z-scored model variates.
Estimating Economic Costs.
To estimate the effect of P loading on water clarity, we made predictions using our MARSS model under varying grazing (pre- and post-Bythotrephes; i.e., high and low grazing from D. pulicaria) and P loading (−99% to +100%) scenarios under post-2009 surface temperature conditions and long-term average P loading conditions. We chose to investigate improving water clarity through P loading reduction as opposed to other methods (e.g., chemical treatment or biological control) because of existing efforts to reduce P loading into Lake Mendota (26, 27) and additional benefits to water quality of lakes downstream of Lake Mendota (25).
The costs of P load reductions are estimated using a report by Strand Associates, Inc. from 2013 (35) (pdf available). The goal of the Yahara CLEAN Engineering Report was to develop a list of action items that would result in a 50% P load reduction into Lake Mendota in addition to the costs of those items. The report takes into account not only the efficiency of each action item (in US dollars per 1 lb P reduced) but also, nonmonetary factors that will influence the prioritization of action items, like implementability, social acceptance, benefits visible to the public, water management, maintaining functional farmland and farming culture, nutrient distribution, reliability of the action item or technology, and ancillary benefits. P loading reduction costs are estimated as present-day value over a 20-y project period. The Yahara CLEAN Engineering Report also details the necessary investment for a maximum implementation plan or 86% reduction (97% reduction of direct drainage sources) in P loading. The cost of a 50% reduction was estimated to be US$70 million over a 20-y period, and the cost of an 86% reduction was estimated to be US$177 million over a 20-y period. We estimated the economic costs of offsetting Bythotrephes' impact using table 4.01–2 in ref. 35, which details the costs, efficiency, and P load reduction of each action item. We bound the estimate by summing the most and least cost-efficient (in US dollars per 1 lb P reduced) items that would achieve the 71% P load reduction.
Updating Willingness to Pay Estimates from the Work by Stumborg et al. (26).
Stumborg et al. (26) estimate Madison’s willingness to pay for 1 m of water clarity in Lake Mendota at US$353.53 per household. We adjust this number to present value or buying power, US$645.49, using the Consumer Price Index Inflation Calculator (www.bls.gov/data/). We also updated the number of households in Dane County from the 1990 census of 155,200 households to the 2014 estimate by the US Census Bureau of 217,100 households (census.gov).
Additional MARSS Modeling Information
MARSS: Model Performance and Selection.
Base model formations were based on expected ecological interactions to help constrain the many possible B-matrix compositions for a matrix of five interacting variables. We selected the best model that included all five variables of interest using Akaike Information Criterion (AIC) (selected model AIC in Table S1).
Here, we limit comparisons of different model configurations to observing performance without each variable’s effect on water clarity. This constraint assumes that the B matrix described in Methods Summary creates a model that is both parsimonious and ecologically sound. It should be noted that adding ecologically reasonable effects (e.g., other zooplankton effects on Daphnia pulicaria) does not significantly change the B-matrix interactions or AIC of the model unless the number of interactions that the model is forced to estimate becomes so high that it can no longer converge on an estimate for all model parameters.
By removing each variable effect on water clarity, we see that temperature is the most important variable (AIC increases the most with its removal) followed by P loading, D. pulicaria, and other grazing zooplankton (Table S1) (note that adding the other grazing zooplankton effect made model fit worse). Each variable is highly seasonal. Therefore, including surface temperature as a seasonal surrogate should be a very important component of the model. Interestingly, P loading was more important to the model than D. pulicaria biomass. The important role of external P loading in driving water clarity may have implications for the efficiency of P load reductions in the new low-D. pulicaria grazing regime.
MARSS: Residual Analysis of Clarity Predictions.
We plotted the residuals of water clarity in our best fit model through time (Fig. S1) and against z-scored model variates (Fig. S2). We found that the model fit the data well, with a small seasonal signal possibly linked to surface temperature as a seasonal surrogate capturing indirectly related seasonal variation (e.g., low temperatures driving an overestimate of water clarity in spring months in Fig. S1) or a nonlinear response in clarity to D. pulicaria biomass (e.g., slight bell shape of clarity residuals plotted against z-scored D. pulicaria biomass in Fig. S2, which may describe seasonal discrepancies in Fig. S1). However, with little to no shift in model residuals with the detection of Bythotrephes in 2009 (Fig. S1), it is unlikely that these affect the overall conclusions of the restoration cost estimate (i.e., obtaining preinvasion clarity under postinvasion grazing).
Acknowledgments
We thank Kirsten Rhude, Ted Bier, Elizabeth Runde, and Emily Stanley for North Temperate Lakes Long Term Ecological Research program data collection; Bill Provencher and Dan Phaneuf for economic advice; Naomi Walsh and Carol Dizack (University of Wisconsin Media Solutions) for assistance with graphics and editing; and Tony Ives, Randy Jackson, Alex Latzka, Alison Mikulyuk, and Scott Van Egeren for comments. This work was funded by the Wisconsin Department of Natural Resources, NSF North Temperate Long-Term Ecological Research Program Grants DEB-0217533 and DEB-1440297, and NSF Water Sustainability and Climate Program Grant DEB-1038759.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600366113/-/DCSupplemental.
References
- 1.Guerry AD, et al. Natural capital and ecosystem services informing decisions: From promise to practice. Proc Natl Acad Sci USA. 2015;112(24):7348–7355. doi: 10.1073/pnas.1503751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehrenfeld JG. Ecosystem consequences of biological invasions. Annu Rev Ecol Evol Syst. 2010;41(1):59–80. [Google Scholar]
- 3.Sidle RC, Benson WH, Carriger JF, Kamai T. Broader perspective on ecosystem sustainability: Consequences for decision making. Proc Natl Acad Sci USA. 2013;110(23):9201–9208. doi: 10.1073/pnas.1302328110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pejchar L, Mooney HA. Invasive species, ecosystem services and human well-being. Trends Ecol Evol. 2009;24(9):497–504. doi: 10.1016/j.tree.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Charles H, Dukes JS. Impacts of invasive species on ecosystem services. In: Nentwig W, editor. Ecological Studies—Biological Invasions. Springer; Berlin: 2007. pp. 217–237. [Google Scholar]
- 6.Keller RP, Lodge DM, Lewis MA, Shogren JF. Bioeconomics of Invasive Species: Integrating Ecology, Economics, Policy, and Management. Oxford Univ Press; New York: 2009. [Google Scholar]
- 7.Vander Zanden MJ, Olden JD. A management framework for preventing the secondary spread of aquatic invasive species. Can J Fish Aquat Sci. 2008;65(7):1512–1522. [Google Scholar]
- 8.Strayer DL. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw Biol. 2010;55(1):152–174. [Google Scholar]
- 9.Ecosystems and Human Well-being: Biodiversity Synthesis. World Resources Institute; Washington, DC: Millennium Ecosystem Assessment (2005) [Google Scholar]
- 10.Schaefer M, Goldman E, Bartuska AM, Sutton-Grier A, Lubchenco J. Nature as capital: Advancing and incorporating ecosystem services in United States federal policies and programs. Proc Natl Acad Sci USA. 2015;112(24):7383–7389. doi: 10.1073/pnas.1420500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keeler BL, et al. Linking water quality and well-being for improved assessment and valuation of ecosystem services. Proc Natl Acad Sci USA. 2012;109(45):18619–18624. doi: 10.1073/pnas.1215991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polasky S, Tallis H, Reyers B. Setting the bar: Standards for ecosystem services. Proc Natl Acad Sci USA. 2015;112(24):7356–7361. doi: 10.1073/pnas.1406490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson MA, Carpenter SR. Economic valuation of freshwater ecosystem services in the United States: 1971-1997. Ecol Appl. 1999;9(3):772–783. [Google Scholar]
- 14.Carpenter SR, Stanley EH, Vander Zanden MJ. State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu Rev Environ Resour. 2011;36(1):75–99. [Google Scholar]
- 15.Hansson L-A, et al. Biomanipulation as an application of food-chain theory: Constraints, synthesis, and recommendations for temperate lakes. Ecosystems. 1998;1(6):558–574. [Google Scholar]
- 16.Carpenter SR, Kitchell JF. Consumer control of lake productivity. Bioscience. 1988;38(11):764–769. [Google Scholar]
- 17.Carpenter SR, et al. Biological control of eutrophication in lakes. Environ Sci Technol. 1995;29(3):784–786. [Google Scholar]
- 18.Bunnell DB, Davis BM, Warner DM, Chriscinske MA, Roseman EF. Planktivory in the changing Lake Huron zooplankton community: Bythotrephes consumption exceeds that of Mysis and fish. Freshw Biol. 2011;56(7):1281–1296. [Google Scholar]
- 19.Yan ND, Leung B, Lewis MA, Peacor SD. The spread, establishment and impacts of the spiny water flea, Bythotrephes longimanus, in temperate North America: A synopsis of the special issue. Biol Invasions. 2011;13(11):2423–2432. [Google Scholar]
- 20.Yan N, Girard R, Boudreau S. An introduced invertebrate predator (Bythotrephes) reduces zooplankton species richness. Ecol Lett. 2002;5(4):481–485. [Google Scholar]
- 21.Strecker AL, Arnott SE. Invasive predator, Bythotrephes, has varied effects on ecosystem function in freshwater lakes. Ecosystems. 2008;11(3):490–503. [Google Scholar]
- 22.Potapov A, Muirhead J, Yan N, Lele S, Lewis M. Models of lake invasibility by Bythotrephes longimanus, a non-indigenous zooplankton. Biol Invasions. 2011;13(11):2459–2476. [Google Scholar]
- 23.Johnson T, Kitchell J. Long-term changes in zooplanktivorous fish community composition: Implications for food webs. Can J Fish Aquat Sci. 1996;53(12):2792–2803. [Google Scholar]
- 24.Lathrop R, et al. Stocking piscivores to improve fishing and water clarity: A synthesis of the Lake Mendota biomanipulation project. Freshw Biol. 2002;47(12):2410–2424. [Google Scholar]
- 25.Lathrop RC, Carpenter SR. Water quality implications from three decades of phosphorus loads and trophic dynamics in the Yahara chain of lakes. Inland Waters. 2014;4(1):1–14. [Google Scholar]
- 26.Stumborg BE, Baerenklau KA, Bishop RC. Nonpoint source pollution and present values: A contingent valuation study of Lake Mendota. Rev Agric Econ. 2001;23(1):120–132. [Google Scholar]
- 27.Lathrop RC, Carpenter SR, Stow CA, Soranno PA, Panuska JC. Phosphorus loading reductions needed to control blue-green algal blooms in Lake Mendota. Can J Fish Aquat Sci. 1998;55(5):1169–1178. [Google Scholar]
- 28.Terborgh J, Estes JA. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Island Press; Washington, DC: 2010. [Google Scholar]
- 29.Branstrator D. Contrasting life histories of the predatory cladocerans Leptodora kindtii and Bythotrephes longimanus. J Plankton Res. 2005;27(6):569–585. [Google Scholar]
- 30.Yurista PM, Vanderploeg HA, Liebig JR, Cavaletto JF. Lake Michigan Bythotrephes prey consumption estimates for 1994-2003 using a temperature and size corrected bioenergetic model. J Great Lakes Res. 2010;36(3):74–82. [Google Scholar]
- 31.Kasprzak P, Lathrop RC, Carpenter SR. Influence of different sized Daphnia species on chlorophyll concentration and summer phytoplankton community structure in eutrophic Wisconsin lakes. J Plankton Res. 1999;21(11):2161–2174. [Google Scholar]
- 32.Boudreau S, Yan N. The differing crustacean zooplankton communities of Canadian Shield lakes with and without the nonindigenous zooplanktivore Bythotrephes longimanus. Can J Fish Aquat Sci. 2003;60(11):1307–1313. [Google Scholar]
- 33.Yan N, Pawson T. Changes in the crustacean zooplankton community of Harp Lake, Canada, following invasion by Bythotrephes cederstroemi. Freshw Biol. 1997;37(2):409–425. [Google Scholar]
- 34.Holmes EE, Ward EJ, Wills K. MARSS: Multivariate autoregressive state-space models for analyzing time-series data. R J. 2012;4(1):11–19. [Google Scholar]
- 35.Strand Associates, Inc. 2013 Yahara CLEAN Engineering Report. Available at www.madsewer.org/Portals/0/ProgramInitiatives/YaharaWINs/Resources/Documents/Yahara%20CLEAN%20Engineering%20Report.FINAL.March%202013.pdf. Accessed October 17, 2013.
- 36.Leung B, et al. An ounce of prevention or a pound of cure: bioeconomic risk analysis of invasive species. Proc R Soc Lond B Biol Sci. 2002;269(1508):2407–2413. doi: 10.1098/rspb.2002.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winslow LA, Read JS, Hanson PC, Stanley EH. Lake shoreline in the contiguous United States: quantity, distribution and sensitivity to observation resolution. Freshw Biol. 2014;59(2):213–223. [Google Scholar]
- 38.Strayer DL, Findlay SEG. Ecology of freshwater shore zones. Aquat Sci. 2010;72(2):127–163. [Google Scholar]
- 39.Taylor JC, Roach JL. Ocean shipping in the Great Lakes: An analysis of industry transportation cost savings. Transp J. 2009;48(1):53–67. [Google Scholar]
- 40.Rothlisberger JD, Finnoff DC, Cooke RM, Lodge DM. Ship-borne nonindigenous species diminish Great Lakes ecosystem services. Ecosystems. 2012;15(3):1–15. [Google Scholar]
- 41.Strayer DL. Eight questions about invasions and ecosystem functioning. Ecol Lett. 2012;15(10):1199–1210. doi: 10.1111/j.1461-0248.2012.01817.x. [DOI] [PubMed] [Google Scholar]
- 42.McCauley E. The estimation of the abundance and biomass of zooplankton in samples. In: Edmonson WT, editor. A Manual on Methods for the Assessment of Secondary Productivity in Fresh Waters. Blackwell; Oxford: 1984. pp. 228–265. [Google Scholar]
- 43.Wood S. 2015 mgcv: Mixed GAM Computation Vehicle with GCV/AIC/REML Smoothness Estimation. Available at https://cran.r-project.org/web/packages/mgcv/index.html. Accessed August 5, 2015.
- 44.R Core Team 2014 R: A Language and Environment for Statistical Computing. Available at www.R-project.org/. Accessed June 16, 2014.
- 45.Holmes E, et al. 2014 MARSS: Multivariate Autoregressive State-Space Modeling. Available at https://cran.r-project.org/web/packages/MARSS/index.html. Accessed August 5, 2015.