Significance
As RNA polymerase (RNAP) translocates along the DNA template for repetitive nucleotide additions, its active site opens and closes for NTP association and catalysis, and a pyrophosphate ion (PPi) is generated after each nucleotide incorporation. Understanding the role of PPi release is important for elucidating the polymerase mechanism. The structures of the σS-containing transcription initiation complexes (σS-TICs) provide insights into the mechanism of σS-dependent selective gene expression. In addition, the highly stressed σS-TICs trap a PPi at the RNAP active site, a previously unobserved but catalytically relevant functional state. Our study also demonstrates that PPi release is not directly related to either translocation or active site opening but causes extensive conformational changes on the periphery of the RNAP secondary channel.
Keywords: transcription initiation, RNA polymerase, σS factor, promoter recognition, pyrophosphate release
Abstract
In bacteria, multiple σ factors compete to associate with the RNA polymerase (RNAP) core enzyme to form a holoenzyme that is required for promoter recognition. During transcription initiation RNAP remains associated with the upstream promoter DNA via sequence-specific interactions between the σ factor and the promoter DNA while moving downstream for RNA synthesis. As RNA polymerase repetitively adds nucleotides to the 3′-end of the RNA, a pyrophosphate ion is generated after each nucleotide incorporation. It is currently unknown how the release of pyrophosphate affects transcription. Here we report the crystal structures of E. coli transcription initiation complexes (TICs) containing the stress-responsive σS factor, a de novo synthesized RNA oligonucleotide, and a complete transcription bubble (σS-TIC) at about 3.9-Å resolution. The structures show the 3D topology of the σS factor and how it recognizes the promoter DNA, including likely specific interactions with the template-strand residues of the −10 element. In addition, σS-TIC structures display a highly stressed pretranslocated initiation complex that traps a pyrophosphate at the active site that remains closed. The position of the pyrophosphate and the unusual phosphodiester linkage between the two terminal RNA residues suggest an unfinished nucleotide-addition reaction that is likely at equilibrium between nucleotide addition and pyrophosphorolysis. Although these σS-TIC crystals are enzymatically active, they are slow in nucleotide addition, as suggested by an NTP soaking experiment. Pyrophosphate release completes the nucleotide addition reaction and is associated with extensive conformational changes around the secondary channel but causes neither active site opening nor transcript translocation.
Cellular organisms transfer genetic information from DNA to RNA using multisubunit RNA polymerases (RNAPs) that are conserved from bacteria to humans (1, 2). In bacteria, a single five-subunit core enzyme of RNA polymerase (α2ββ′ω) is responsible for all RNA synthesis, whereas multiple σ factors compete to associate with the RNAP core enzyme to form a holoenzyme that is required for initiating the process at DNA promoter sites (3, 4). RNAP remains associated with the upstream promoter DNA during transcription initiation and moves downstream for RNA synthesis, causing DNA scrunching to form a stressed and unstable initiation complex (5–8). Processive RNA synthesis happens only after the initiation complex escapes the promoter as transcription progresses from initiation to elongation (9–11).
RNA synthesis in both transcription initiation and elongation involves repetitive cycles of nucleotide addition comprising translocation, NTP binding, catalysis, and pyrophosphate release steps. During this cycling process, the RNAP active site opens for NTP association and closes to align the incoming NTP with the RNA 3′ hydroxyl group for catalysis. Nucleotide addition extends the RNA by 1 nt and generates a pyrophosphate ion (PPi). Previous structural studies of transcription complexes frequently used DNA fragments that form a partial transcription bubble. Most of these complexes display an open active site in the posttranslocation state, and the active site-closed conformation has been observed only with NTP-bound precatalysis complexes in which the nucleotide addition reaction was prevented by using 3′-deoxy RNA or nonhydrolysable NTP analogs (Table S1). A PPi-associated complex has never been observed previously in cellular RNAP structures. How release of PPi affects the opening of the RNAP active site and transcription translocation remains to be established.
Table S1.
Structural survey of transcription complexes
| NAC conformation | PDB ID | Comments | Reference |
| I TL/open | 1I6H | Yeast Pol II complex with upstream DNA and 13–14 nt RNA synthesized with GTP+ATP+CTP and stalled because of a lack of UTP | (29) |
| 3M3Y | Yeast Pol II complex with pyriplatin-dG:C base pair at initiation + 1 (i+1) site; translocation impaired | (49) | |
| 4Q5S | TthRNAP initiation complex; stressed with σ3.2 blocking of 6-nt RNA | (50) | |
| I → II ? TL/open | 2VUM | Yeast Pol II complex with α-amanitin; translocation intermediate | (31) |
| I ↔ II ? TL/open (failed or incomplete translocation?) | 3RZD | Yeast Pol II complex; hybrid tilted* | (51) |
| 3RZO | Yeast Pol II complex; hybrid tilted* | ||
| 3S1R | Yeast Pol II complex with 3′-deoxy RNA and E site GTP; hybrid tilted* | ||
| 4A3B | Yeast Pol II complex; hybrid tilted* | (52) | |
| 4A3C | Yeast Pol II complex; hybrid tilted* | ||
| 4A3D | Yeast Pol II complex; hybrid tilted* | ||
| 4A3K | Yeast Pol II complex; hybrid tilted* | ||
| 4BY7 | Yeast Pol II complex; hybrid tilted* | (53) | |
| II TL/open | 1SFO | Yeast Pol II complex | (54) |
| 1Y1W | Yeast Pol II complex | (55) | |
| 2JA5 | Yeast Pol II complex | (56) | |
| 2JA6 | Yeast Pol II complex | ||
| 2JA7 | Yeast Pol II complex with dT-dT dimer at i and i+1 sites | ||
| 2JA8 | Yeast Pol II complex with dT-dT dimer at i and i+1 sites; unusual downstream DNA shifts (similar to 4A93) | ||
| 2O5I | TthRNAP complex | (57) | |
| 2R7Z | Yeast Pol II complex with cisplatin-crosslinked T-strand dGi+2 and dGi+3 | (58) | |
| 2R92 | Yeast Pol II complex with RNA stem loop | (59) | |
| 2R93 | Yeast Pol II complex with RNA duplex | ||
| 3H3V | Yeast Pol II complex | (60) | |
| 3HOU | Yeast Pol II complex with dT-U mismatch at i site | (61) | |
| 3HOV | Yeast Pol II complex | ||
| 3HOW | Yeast Pol II complex with dT-U mismatch at i site and frayed 3′-U | ||
| 3HOX | Yeast Pol II complex | ||
| 3HOY | Yeast Pol II complex | ||
| 3HOZ | Yeast Pol II complex with dT-U mismatch at i site, frayed 3′-G | ||
| 3I4M | Yeast Pol II complex with 8-oxo-dG:C base pair at i site, frayed 3′-U | (62) | |
| 3I4N | Yeast Pol II complex with 8-oxo-dG:A base pair at i site, frayed 3′-U | ||
| 3M4O | Yeast Pol II complex with pyriplatin-dG at i+1 site | (49) | |
| 3S14 | Yeast Pol II complex | (51) | |
| 3S15 | Yeast Pol II complex | ||
| 3S16 | Yeast Pol II complex | ||
| 3S17 | Yeast Pol II complex | ||
| 3S1M | Yeast Pol II complex | ||
| 3S1N | Yeast Pol II complex | ||
| 3S2D | Yeast Pol II complex | ||
| 3S2H | Yeast Pol II complex | ||
| 4A3G | Yeast Pol II complex | (52) | |
| 4A93 | Yeast Pol II complex with dT-dT dimer at i+1 and i+2 sites, unusual downstream DNA shifts (similar to 2JA8) | (63) | |
| 4BBS | Yeast Pol II initiation complex with TFIIB | (64) | |
| 4G7O | TthRNAP initiation complex | (18) | |
| 4S20 | EcoRNAP complex with RapA | (47) | |
| 4XLN | TaqRNAP initiation complex with a 13-nt bubble | (17) | |
| 4XLR | TaqRNAP initiation complex with a 13-nt bubble and TthCarD | (65) | |
| 5C3E | Yeast Pol II complex with a 15-nt bubble (upstream invisible) | (66) | |
| 5C44 | Yeast Pol II complex with a 15-nt bubble (upstream invisible) | ||
| 5C4A | Yeast Pol II complex with a 12-nt bubble (upstream invisible) | ||
| 5C4J | Yeast Pol II complex with a 15-nt bubble | ||
| 5C4X | Yeast Pol II complex with a 15-nt bubble | ||
| III TL/open | 1R9S | Yeast Pol II complex with 3′-deoxy RNA and A site UTP | (37) |
| 1R9T | Yeast Pol II complex with 3′-deoxy RNA and E site noncognitive ATP (template dA) | ||
| 1Y77 | Yeast Pol II complex with pre-A site GMPcPP | (55) | |
| 2E2I | Yeast Pol II complex with 3′-deoxy RNA and A site/E site dGTP | (27) | |
| 2E2J | Yeast Pol II complex with A site GMPcPP | ||
| 2NVQ | Yeast Pol II complex with 3′-deoxy RNA and A site/E site dUTP | ||
| 2NVT | Yeast Pol II complex with A site GMPcPP | ||
| 2NVX | Yeast Pol II complex with 3′-deoxy RNA and A site/E site dUTP | ||
| 2YU9 | Yeast Pol II complex with 3′-deoxy RNA and A site/E site UTP | ||
| 2PPB | TthRNAP complex with A site AMPcPP and streptolydigin | (25) | |
| 4OIO | TthRNAP initiation complex with 2 NTPs (ATP and CMPcPP) | (67) | |
| IV? TH/close† | 4A3E | Yeast Pol II complex with A site AMPcPP | (52) |
| 4A3F | Yeast Pol II complex with A site AMPcPP | ||
| 4A3J | Yeast Pol II complex with A site GMPcPP | ||
| 4A3L | Yeast Pol II complex with A site AMPcPP | ||
| 4A3M | Yeast Pol II complex with A site AMPcPP | ||
| 4BY1 | Yeast Pol II complex with A site AMPcPP | (53) | |
| 4Q4Z | TthRNAP initiation complex with 2 NTPs (ATP and CMPcPP) | (50) | |
| IV TH/close | 2E2H | Yeast Pol II complex with 3′-deoxy RNA and A site GTP | (27) |
| 2NVZ | Yeast Pol II complex with 3′-deoxy RNA and A site UTP | ||
| 2O5J | TthRNAP complex with A site AMPcPP | (25) | |
| 3S1Q | Yeast Pol II complex with 3′-deoxy RNA and A site ATP | (51) | |
| V TH/close | σS-EcoRNAP initiation complex with a 14-nt bubble and Ppi | This work | |
| V → VI TH/close | σS-EcoRNAP initiation complex with a 14-nt bubble | This work | |
| σS-EcoRNAP initiation complex with a 14-nt bubble | |||
| VI TH/close | 4YLN | σ70-EcoRNAP initiation complex with a 14-nt bubble | (5) |
| 4YLO | σ70-EcoRNAP initiation complex with a 14-nt bubble | ||
| 4YLP | σ70-EcoRNAP initiation complex with a 15-nt bubble | ||
| Transcription complexes of other conformations | |||
| Ratcheted TL/blocked‡ | 3AOH | TthRNAP complex with Gfh1; cleft open | (68) |
| 3AOI | TthRNAP complex with Gfh1; cleft open | ||
| Elemental pause TL/blocked‡ | 4GZY | TthRNAP complex; cleft open | (69) |
| 4GZZ | TthRNAP complex; cleft open | ||
| Backtracked TL/blocked§ | 3GTG | Yeast Pol II backtracked complex | (70) |
| 3GTJ | Yeast Pol II backtracked complex | ||
| 3GTK | Yeast Pol II backtracked complex | ||
| 3GTL | Yeast Pol II backtracked complex | ||
| 3GTM | Yeast Pol II backtracked complex with TFIIS | ||
| 3GTO | Yeast Pol II backtracked complex | ||
| 3GTP | Yeast Pol II backtracked complex | ||
| 3GTQ | Yeast Pol II backtracked complex | ||
| 3PO2 | Yeast Pol II backtracked complex; hybrid tilted* | (71) | |
| 3PO3 | Yeast Pol II backtracked complex with TFIIS; hybrid tilted* | ||
All available crystal structures of transcription complexes of cellular RNA polymerases as of September 25, 2015 are categorized in regard to their relative conformations in the nucleotide addition cycle (NAC). NAC conformations: I, pretranslocation state with an unfolded TL and an open active site (TL/open); II, posttranslocation state with TL/open; III, posttranslocation state with a bound NTP and an unfolded TL (TL/open); IV, posttranslocation state with a bound NTP and a active site closed by TH formation (TH/close); V, pretranslocation state immediately after catalysis with a PPi-associated and closed active site (TH/close); VI, pretranslocation state after PPi release with the active site remaining closed by THs (TH/close). AMPcPP, α,β-methyleneadenosine 5′-triphosphate; CMPcPP, α,β-methylenecytosine 5′-triphosphate; Eco, Escherichia coli; GMPcPP, α,β-methyleneguanosine 5′-triphosphate; Pol II, RNA polymerase II; Taq, Thermus aquaticus; Tth, Thermus thermophilus.
“Hybrid tilted” refers to the hybrid conformation in which the RNA nucleotide at register n base pairs with the DNA template nucleotide at register n+1.
These structures display a largely folded N-terminal helix of the TL, and thus the active sites are closed, more like that of complex IV.
Both “ratcheted” and “elemental pause” display a cleft-open conformation with a bent BH that is incompatible with the helical conformation of the TL. This bent BH conformation also might be incompatible with A-site/pre–A-site NTP binding.
Backtracked complexes have backtracked RNA residues that prevent NTP binding and are also incompatible with a folded TL conformation.
In this work we determined the crystal structures of the E. coli transcription initiation complexes (TICs) containing the σS factor, a de novo synthesized RNA oligonucleotide, and a complete transcription bubble (σS-transcription initiation complexes, σS-TICs). The σS factor controls the expression of many genes in response to general stresses, such as nutrient deprivation upon entry into stationary phase. The structures show the specific interactions of the σS factor with the promoter −10 element and provide insights into the mechanism of σS-dependent selective gene expression under stress conditions. In addition, the σS-TIC crystals display a pretranslocated initiation complex with a PPi associated at the active site that remains closed. The position of the PPi and the unusual phosphodiester linkage between the two terminal RNA residues suggest an unfinished nucleotide addition reaction that likely is at equilibrium between nucleotide addition and pyrophosphorolysis. The slow enzymatic activity of the σS-TIC crystals allowed us to observe PPi dissociation without nucleotide addition in an NTP-soaking experiment. PPi release appears to be associated with extensive conformational changes around the secondary channel but causes neither active site opening nor transcript translocation.
Results and Discussion
Overall Structure of the E. coli σS-Based TIC.
By using a synthetic DNA scaffold containing the promoter consensus −35 and −10 sequences, we assembled and crystallized the E. coli TIC that contains the general stress response σS factor. The σS-TIC crystals are enzymatically active for additional nucleotide incorporation. The structures were solved by molecular replacement, and the final models were refined to about 3.6-Å resolution (Table 1). The σS-TIC structures display a closed active site and a well-ordered nascent RNA–DNA hybrid in the pretranslocation register.
Table 1.
Data collection and refinement statistics
| Parameters | σS-TIC1 | σS-TIC2 1-h soaking with CTP only | σS-TIC3 1-h soaking with CTP, UTP, and GTP |
| Data collection | |||
| Space group | P212121 | P212121 | P212121 |
| Cell dimensions, Å | |||
| a | 131.71 | 132.75 | 132.87 |
| b | 152.67 | 151.97 | 152.17 |
| c | 226.70 | 228.06 | 229.12 |
| Resolution, Å | 100–3.60 (3.66–3.60) | 100–4.20 (4.27–4.20) | 100–4.60 (4.68–4.60) |
| Rsym, % | 12.5 (>100) | 10.2 (>100) | 8.5 (>100) |
| I/σI* | 9.87 (0.37) | 8.44 (0.46) | 10.06 (0.55) |
| Completeness, % | 99.6 (99.4) | 99.9 (100) | 99.3 (99.6) |
| Redundancy | 5.1 (4.2) | 7.7 (7.9) | 6.9 (6.8) |
| Refinement | |||
| Resolution, Å | 100–3.60 | 100–4.20 | 100–4.60 |
| No. reflections | 50,952 | 32,692 | 24,772 |
| Rwork/Rfree, % | 24.7/28.7 | 26.7/32.7 | 24.0/33.4 |
| No. atoms | 27,632 | 29,036 | 29,036 |
| Protein | 26,167 | 27,572 | 27,572 |
| DNA/RNA/ions | 1,465 | 1,464 | 1,464 |
| B factors | 129.5 | 177.4 | 183.1 |
| Rmsd | |||
| Bond length, Å | 0.011 | 0.011 | 0.008 |
| Bond angle, ° | 1.448 | 1.422 | 1.179 |
Values in parentheses are for highest-resolution shell. Each dataset was collected from a single crystal.
I/σI = 2.0 at 4.2 Å (σS-TIC1), 5.6 Å (σS-TIC2), 6.2 Å (σS-TIC3); I/σI = 1.0 at 3.9 Å (σS-TIC1), 4.7 Å (σS-TIC2), 5.2 Å (σS-TIC3).
The σS-TIC that initially crystallized contains a 14-nt bubble and an RNA tetranucleotide synthesized de novo from three NTPs and traps a PPi at the active site (Fig. 1). Continuous electron densities are seen for both strands of the ssDNA in the bubble region (Fig. S1A). Much of the nontemplate (NT) strand of the bubble that is downstream of the −10 hexamer could not be resolved unambiguously in the density map, suggesting a slippery feature of this DNA segment that displays limited interactions with the protein. The ssDNA residues on the template (T) strand are much better resolved, although residues in the middle of this section appear disordered. The upstream promoter DNA runs into the downstream DNA duplex of a symmetry-related molecule, causing strand separation and disordering of a significant portion of the upstream DNA (Fig. S1B).
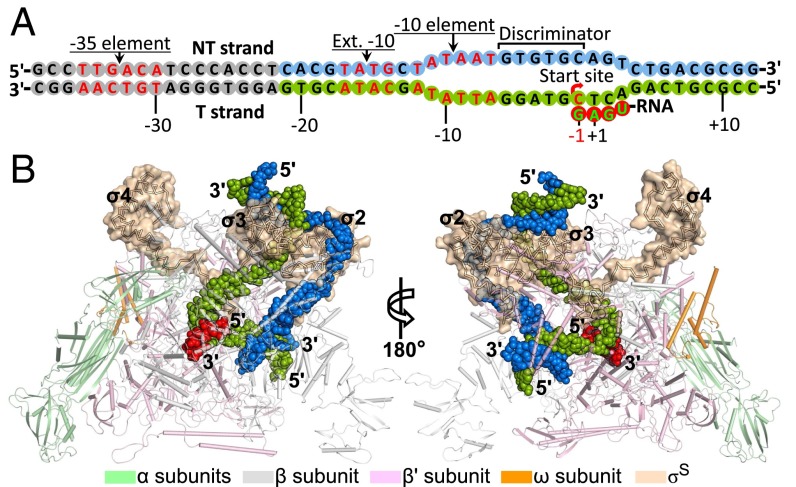
Fig. 1.
Overall structure of the E. coli σS-TIC. (A) Schematic representation of the synthetic promoter DNA and a de novo synthesized RNA transcript in the σS-TIC crystals. RNA synthesis starts from the −1 position with a GTP residue as observed previously with related promoters in the σ70-TICs (5). The disordered upstream DNA residues are shown as gray cycles. (B) Structure of the σS-TIC. The E. coli RNAP core enzyme is shown in a tube-and-arrow cartoon representation. The σS factor is shown as a Cα trace within a surface representation (wheat). The promoter DNA (NT strand, blue; T strand, green) and the nascent RNA (red) are shown as filled spheres.
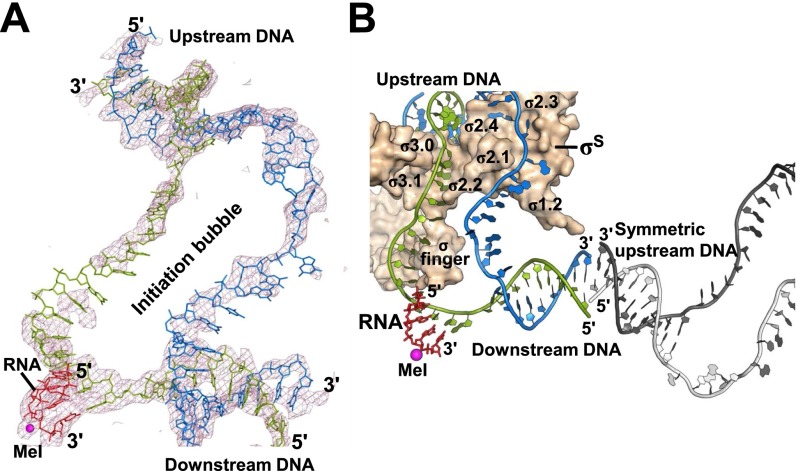
Fig. S1.
Nucleic acid in the σS-TIC. (A) An overall view of the entire transcription bubble in the σS-TIC. The Fo-Fc electron density map (mesh, contoured at 2.0 σ) was calculated using the phase from the protein-only model. (B) Crystal packing involving the dsDNA. The downstream DNA from one complex stacks noncoaxially with the upstream DNA from a symmetry mate. The RNAP core enzyme is omitted for clarity. The DNA from the symmetry mate is colored gray. σS is shown in a surface representation. A metal ion (MeI) is shown as a magenta sphere to mark the active center.
E. coli σS Factor and σS-RNAP Core Interactions.
Like the primary σ factors, the E. coli σS factor contains a highly negatively charged N-terminal domain (σ1.1) that we have not been able to trace in the σS-TIC structures. Other than a nonconserved region (NCR) that is commonly present in primary σ factors, σS displays very high sequence identity with the primary σ factors (Fig. S2A) (10). Consistent with their sequence similarity, the σS factor in the σS-TIC forms essentially the same fold as the σ70 factor throughout the conserved regions, from region 1.2 to the very C terminus, including the long σ3.2 loop that inserts deep into the RNAP active site chamber (Fig. 2A).
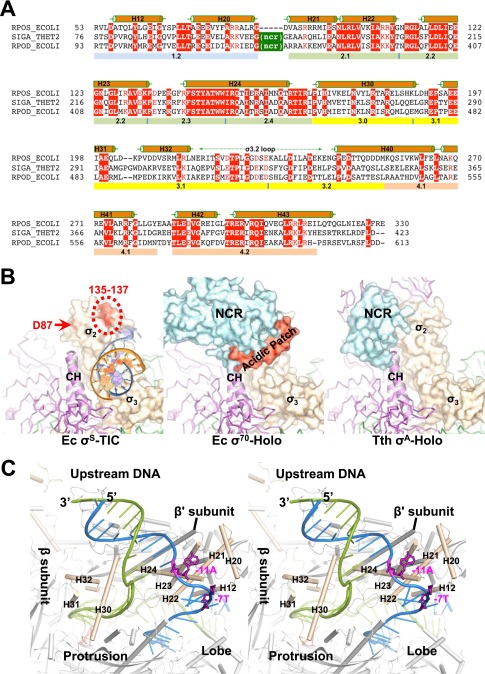
Fig. S2.
E. coli σS factor. (A) A sequence alignment of the E. coli σS factor (RPOS_ECOLI, GenBank accession no. gi:1710676) with the Thermus thermophilus σA factor (SIGA_THET2, gi:81699349) and the E. coli σ70 factor (RPOD_ECOLI, gi:1710680). Sequences are presented with the one-letter amino acid code; numbers at the beginning and end of each line indicate amino acid positions relative to the start of each protein sequence. Secondary structures (α helices) of the E. coli σS factor are indicated above the sequences, and evolutionarily conserved regions are labeled below the sequences. NCR marks the nonconserved region of the primary σ factors. (B) A comparison of the interactions of the σ2 domains of different σ factors with the RNAP core enzyme. Structures of the E. coli σS-TIC (Left), E. coli σ70-RNAP holoenzyme (Ec σ70-Holo, PDB ID code 4JKR) (Center), and the RNAP holoenzyme in the T. thermophilus initiation complex (Tth σA-Holo, PDB ID code 4G7H) (Right) are superimposed using the clamp helices (E. coli β′ residues 263–308; T. thermophilus β′ residues 538–583) and are shown separately in the same orientation. The σ factors are shown in surface representation; clamp helices are shown in ribbons; and the rest of the core enzymes are shown as Cα traces and are colored as in Fig. 1B. The σS residues D87, D135, P136, and E137 that interact with Crl are marked in red. The σ70 factor acidic patch (residues 188–209) is modeled and shown in red. A fragment of the upstream DNA in the σS-TIC (NT strand, blue; T strand, orange) that interacts with the σ2 and σ3 domains is shown for reference. (C) Stereoview of the upstream fork region of the σS-TIC. The promoter DNA is shown as ladders (NT strand, blue; T strand, green); the NT-strand residues −11A and −7T are shown as magenta sticks.
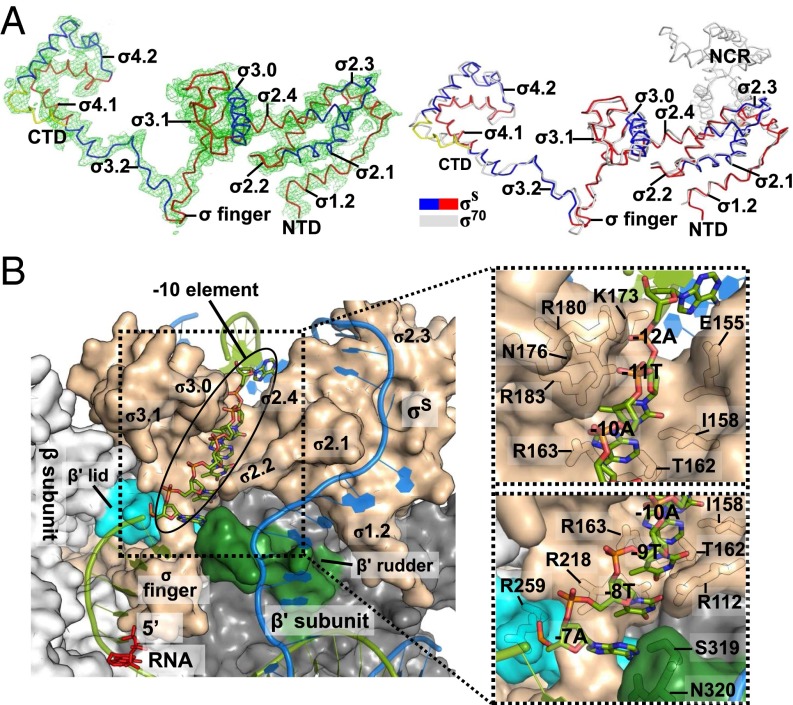
Fig. 2.
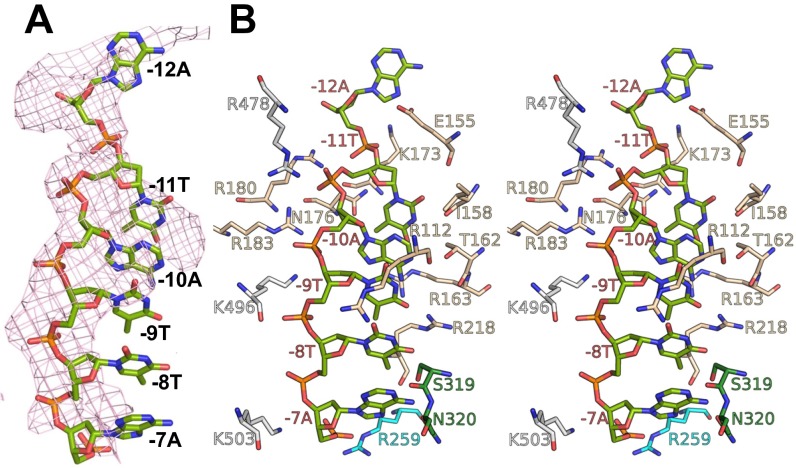
E. coli σS factor. (A) Overall structure of the E. coli σS factor in the σS-TIC. The orientation of σS here is the same as in Fig. 1. (Left) The Fo-Fc electron density map (mesh, contoured at 2.0 σ) was calculated using the phases from the RNAP core-only model. (Right) The E. coli σS factor in the σS-TIC (colored) and the σ70 factor in the σ70-holoenzyme (PDB ID code4JKR) (gray) are superimposed. (B) Recognition of the promoter −10 element in the σS-TIC. The N-terminal half of the β subunit (residues 2–667) is omitted for clarity. The nascent RNA (red) and the −10 hexamer residues of the T strand (green) are shown as sticks. The rest of the nucleic acid is shown in cartoon style (T strand, green; NT strand, marine). The RNA polymerase is shown in surface representation: σS, wheat; β subunit, light gray; β′ lid, cyan; β′ rudder, forest green; the rest of the β′ subunit is shown in dark gray. A few residues that interact directly with the −10 T-strand residues are labeled in the insets.
A major contribution to the σ-RNAP core association comes from interactions between the σ2 domain and a helix-turn-helix formation, termed a “clamp helices,” of the clamp domain of the β′ subunit. Compared with σ70, which displays strong affinity for the RNAP core enzyme (Kd ∼0.26 nM), σS binds relatively weakly to the RNAP core in the absence of nucleic acids (Kd ∼4.26 nM) (12). This weaker affinity of σS might be attributed partly to its lack of an NCR domain that provides additional interactions with the clamp helices of the RNAP core enzyme (Fig. S2B).
In E. coli and many γ-proteobacteria, a small protein called “Crl” was found to stimulate σS-dependent transcription by promoting the formation of the σS–RNAP holoenzyme (13, 14). It was shown previously that the σS–Crl interaction involves a general area on the surface of σS including residues of Asp87, Asp135, Pro136, and Glu137 (15). This general area corresponds to the attachment face of the NCR to the primary σ factors (Fig. S2B), suggesting that Crl might function like the NCR of a primary σ factor to help encompass the clamp helices and enhance the interactions of σS with the core enzyme.
Promoter Recognition by E. coli σS Factor.
The E. coli σS factor was shown to recognize the same consensus −35 and −10 hexamers as the σ70 factor in an in vitro selection experiment (16). The σS-TIC structures show that σS recognizes the –10 hexamer of the NT strand with specific interactions with the bases of the −7T and −11A residues in the same manner as the primary σ factors (Fig. S2C) (5, 17–19). It was suggested recently that the −10 T strand passes through a cleft between the σ2 and σ3 domains in an initiation complex (5, 17), but it remained unclear how the −10 T strand interacts with the RNAP. The σS-TIC structures suggest that the single-stranded T-strand residues −7 to −11 stack on each other inside the narrow tunnel formed by the σ2–σ3 cleft and the β1 domain (Fig. 2B). The DNA backbone appears to make extensive interactions with positively charged side chains from the β1 domain and the σ2 and σ3 domains, and the bases may form several hydrogen-bonding interactions with the σS factor (Fig. S3), raising the possibility that the T-strand residues may contribute to the specific recognition of the −10 hexamer by the σ70 family factors. Additional evidence is required to determine whether the −10 template region contributes to the promoter recognition.
Fig. S3.
Local contacts at the −10 element of the T strand. (A) The pink Fo-Fc electron density map (mesh, contoured at 2.0 σ) was calculated using the phase from the protein-only model. (B) Stereoview of the local contacts at the −10 element of the T strand. Colors are as in Fig. 2. Most residues involved in the contacts, including R112, I158, R163, N176, R180, and R183, are conserved between σ70 and σS.
The few differences between σS and σ70 in their interactions with the extended −10 region include residues Ile169 and Lys173 in σS and the corresponding residues Val454 and Glu458 in σ70. It was reported that Lys173 is responsible for a preference of cytidine by σS at the promoter −13 site (20). Lys173 potentially could interact with the −13 base of the T strand, but a specific recognition could not be established at the current resolution.
In the σS-TIC crystals shown here, the helix-turn-helix of the σS C-terminal domain (σ4) that potentially interacts with the promoter −35 hexamer, including helix H42 and the N-terminal half of helix H43, is involved in crystal packing, and thus there is no space to accommodate the −35 region of the promoter DNA duplex. Although σS interactions with the promoter −35 element are not observed in the σS-TIC crystals, the sequence and structural conservation and the shared recognition of promoter sequences suggest that the σS and σ70 factors would interact with the DNA promoters in a very similar manner.
Selective Gene Expression Under Stress Conditions.
An intriguing question is how σS achieves selective gene expression, given that it recognizes consensus −35 and −10 sequences essentially identical to those recognized by σ70. The σS-dependent promoters display higher sequence deviations from the consensus −35 hexamer (21); these deviations, again, might be related to the absence of an NCR domain in the σS factor. Although the NCR domain in σ70 might make it interact better with the RNAP core enzyme, a stretch of acidic residues (18 of the E. coli σ70 residues 188–209) (Fig. S2B) is expected to inhibit promoter loading directly through interactions of the σ70-holoenzyme with the −10 element (22), thus making interactions with the −35 element important for recruiting the σ70-holoenzyme to the promoter. In contrast, the smaller σ2 domain of the σS factor would allow it to interact directly with the promoter −10/extended −10 region, thus making the σS-holoenzyme less dependent on the −35 element for promoter loading.
The protein concentration of the σS factor in E. coli is tightly regulated at the levels of transcription, translation, and protein stability (23, 24). During exponential growth in rich medium, the σS protein level in E. coli is negligible, but when E. coli enters the stationary phase or under certain stress conditions the σS protein level might increase by a thousand-fold and reach a level comparable to that of σ70. Easier access not only would provide the σS-holoenzyme an advantage in competing for promoters in the heavily packed DNA during the stationary phase but also would justify the requirement of tight regulation of the σS protein concentration under normal growth conditions.
Stressed TICs.
The σ70-TICs that we reported recently contain a complete transcription bubble and display a well-ordered nascent RNA–DNA hybrid lying at the pretranslocation position; we suggested that this pretranslocated hybrid may be a manifestation of the stressed feature of an initiation complex (5). Similar to the σ70-TICs, the σS-TICs we report here also contain a complete transcription bubble and an RNA oligonucleotide synthesized de novo from NTPs, and the σS-TIC structures display a pretranslocated RNA–DNA hybrid as well (Fig. 3). Moreover, the active site of the σS-TICs is fully closed by the folding of the trigger loop (TL) into helices (TH). Although the helical conformation of the TL in a pretranslocated transcription complex also might exist in our low-resolution σ70-TIC structures (5), it has never been observed previously in any other transcription complexes.
Fig. 3.
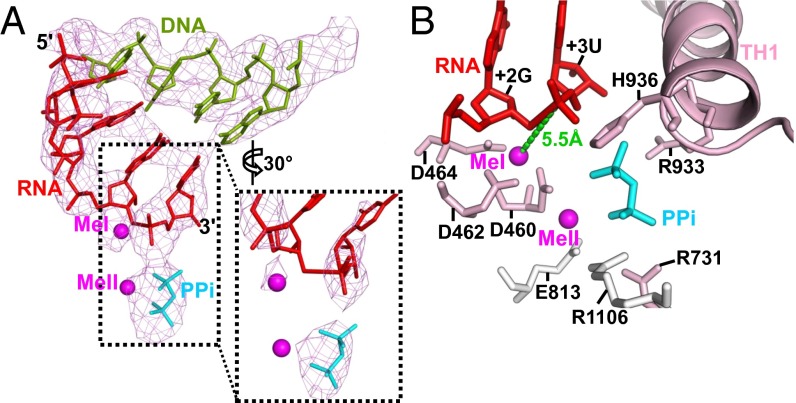
σS-TIC active site. (A) A close-up view of the DNA–RNA hybrid and PPi at the active site in the structure before NTP soaking. The Fo-Fc electron density map (mesh, contoured at 3 σ) was calculated using the phases calculated from the protein-only model. The inset shows a zoomed-in view of the electron density (contoured at 5 σ) around the catalytic site and the RNA 3′-terminal residue. (B) A close-up view of the active site showing the major interactions involved in stabilizing the PPi. The metal ions (MeI and MeII) are shown as magenta spheres.
It has been shown that conformational changes of a flexible TL and the bridge helix (BH), which traverses across the active site cleft, remodel the active site of cellular RNA polymerases (1, 2, 25–27). An unfolded TL leaves the active site open to the secondary channel and thus allows NTP binding to the active site, whereas the folding of the TL into two α-helices (THs) closes the active site and helps align the incoming NTP with the RNA 3′-hydroxyl group and with a conserved histidine residue (β′His936 in E. coli RNAP) that serves as a general acid for catalysis (25, 27). The BH stacks against the RNA–DNA hybrid and could bend or bulge toward the template base, which forms the basis of the BH-controlled transcription translocation model (28, 29). The TL folding has a profound effect on the rate of transcription (30) and is affected by the BH conformation (25, 27). It was also suggested that transcription translocation might require an unfolded TL (31).
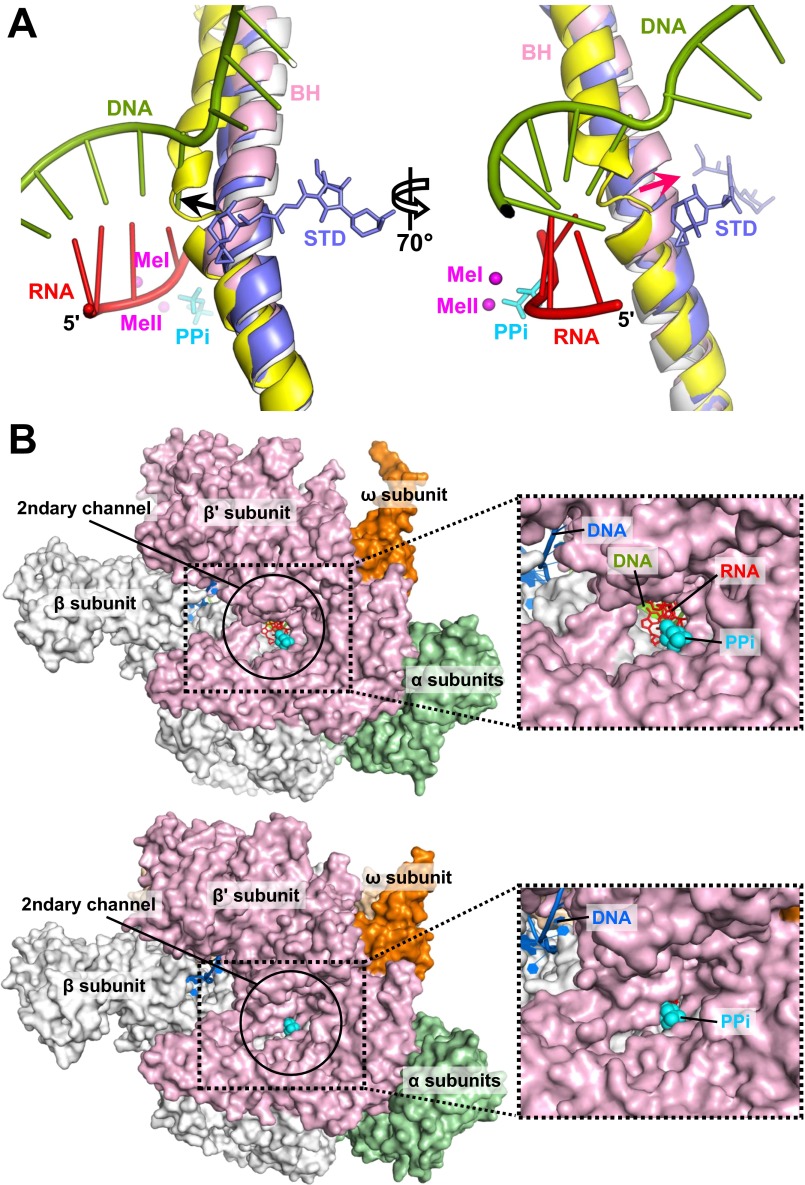
In the stressed σS-TIC, the BH displays a slight bending perpendicular to the translocation direction with a shift of about 3 Å around β′ residues 785–789 (Fig. S4A). This small shift in the BH appears to create enough space to allow the TL to fold into helices. TH formation significantly reduces the dimensions of the secondary channel entrance to the active site, from about 15 × 12 Å in the RNAP apo holoenzyme to about 8 × 6 Å in the σS-TIC (Fig. S4B), and thus even the dissociation of PPi might involve a concerted effort by the protein, involving more than amino acid side chain movements. Intriguingly, the phosphate of the RNA 3′-terminal residue appears not to interact directly with the metal ion (MeI) coordinated by the conserved carboxyl triad at the active center (Fig. 3A). The electron density contoured at higher levels displays some discontinuity between the two terminal residues, and the linkage of the terminal phosphate (+3U) to the penultimate RNA residue (+2G) also appears to be significantly distorted at the sugar ring of the penultimate residue. This finding suggests that the σS-TIC crystal might contain a mixture of initiation complexes, likely at equilibrium between the forward nucleotide addition reaction and presumably its reverse reaction, pyrophosphorolysis (32–34).
Fig. S4.
Active site conformations. (A) BH bending in the stressed TIC. Structures of the apo E. coli holoenzyme (PDB ID code 4LJZ) (gray), T. thermophilus elemental pause complex (PDB ID code 4GZY) (yellow), and T. thermophilus elongation complex with AMPcPP (APC) and streptolydigin (STD) (PDB ID code 2PPB) (blue) are superimposed using the RNAP β subunit. Streptolydigin and PPi (cyan) are presented as sticks. The nascent RNA and the T-strand DNA in the σS-TIC are shown as ladders. The black arrow marks the direction of BH bending related to transcription translocation; the red arrow marks the direction of BH bending observed in the TICs. Both directions of BH bending are based on a comparison with the RNAP apo holoenzyme. (B, Upper) An open active site. A view down to the secondary channel of the RNAP apo holoenzyme (PDB ID code 4LJZ) is presented. Nucleic acids and a PPi were modeled by superimposing the σS-TIC structure onto the RNAP apo holoenzyme using the β subunit. (Lower) A closed active site. A view down to the secondary channel of the σS-TIC (before NTP soaking) is displayed. The insets on the right are zoomed-in views of the secondary channel.
Unreleased Pyrophosphate in the σS-TIC Crystals.
Because RNA polymerase adds nucleotides repetitively to the RNA 3′ end, one pyrophosphate ion is generated after each addition reaction. During this process, it is generally believed that the active site closes after NTP association and opens immediately after or concurrently with the dissociation of the PPi. For T7 RNA polymerase (T7RNAP), a PPi-associated complex has been obtained by supplementing the solution for crystallization with PPi; the PPi-bound and unbound states of T7RNAP were found to associate with the pretranslocation and posttranslocation states, which correspond to the closed and open conformations of the active site, respectively (35). However, a PPi-associated structure has never been observed for the cellular RNA polymerases (Table S1). It was shown previously that translocation occurs shortly after or concurrently with PPi release (36). However, how PPi release affects the opening of the RNAP active site and the translocation of the enzyme along the DNA template remains obscure.
The intrinsic abortive feature of transcription initiation would cause many rounds of oligonucleotide synthesis during the process of complex assembly and crystallization, and these multiple rounds of synthesis could lead to a significant accumulation of PPi in the crystallization drops that reaches a level comparable to or even exceeding the levels of NTPs. On the other hand, because of DNA scrunching, TICs are stressed and tend to rest at the pretranslocation state, a conformation clearly more favorable than others for PPi association. Not surprisingly, a well-ordered PPi remains associated with the σS-TIC active site.
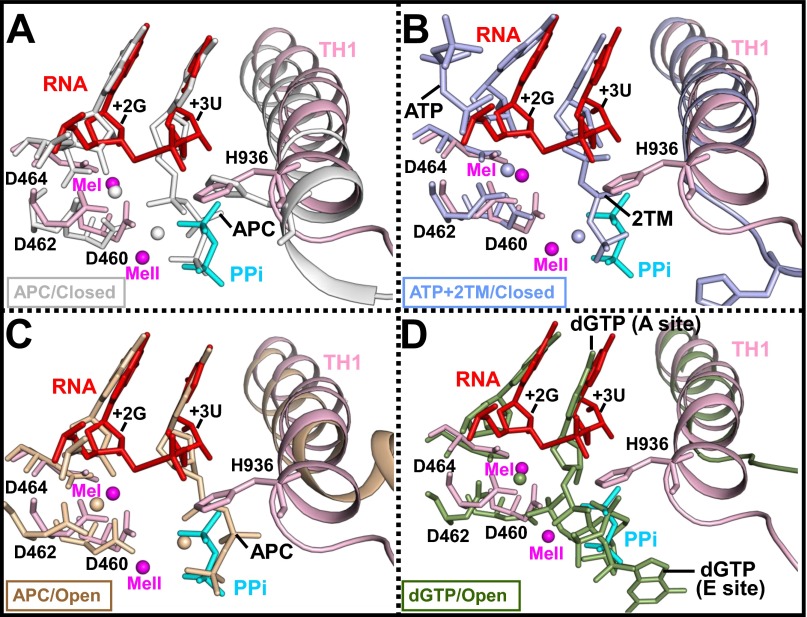
The pyrophosphate in the σS-TIC interacts via a metal ion (MeII) with the carboxyl group of β′Asp460, one of the conserved carboxyl triad of the active center, with the side chains of the TL residues β′Arg933 and β′His936, and with the side chains of βArg1106 and β′Arg731 that line as the secondary channel (Fig. 3B). The observation that both the PPi and the phosphate of the RNA 3′-terminal residue interact with the side chain of β′His936 is consistent with the proposal that this conserved TL histidine residue is involved in both nucleotide addition and pyrophosphorolysis (25, 27). It is not clear whether the position and the network of PPi interactions we observed here also represent those of the β- and γ-phosphates of an incoming NTP before nucleotide incorporation, although it is likely that they do (Fig. S5).
Fig. S5.
PPi and NTP binding. A comparison of the active site of the σS-TIC before NTP soaking with that of the T. thermophilus transcription elongation complex with the NTP analog AMPcPP (APC) (PDB ID code 2O5J) (gray) (A); the T. thermophilus TIC with ATP and CMPcPP (2TM) (PDB ID code 4Q4Z) (light blue) (B); the T. thermophilus transcription elongation complex with APC and streptolydigin (PDB ID code 2PPB) (wheat) (C); and yeast RNAP II elongation complex with 2′-deoxyguanosine-5′-triphosphate (dGTP) (PDB ID code 2E2I) (green) (D). The superimposition was performed using the β subunit of bacterial RNAP or the second-largest chain of the yeast RNA polymerase II.
Dissociation of PPi and RNAP Conformational Changes.
To test the ability of the σS-TIC crystals to incorporate nucleotides, we soaked the crystals in solutions containing CTP that was omitted in the original complex assembly. Unlike the σ70-TIC crystals that readily extend the RNA by 1 nt after soaking (5), the σS-TIC crystals appear to be much less efficient in RNA synthesis. Soaking σS-TIC crystals with CTP only (for a 1-nt extension) or with three NTPs (CTP, UTP, and GTP, for RNA extension of up to 3 nt) for 1 h did not result in noticeable nucleotide addition. RNA extension was observed only after NTP soaking for 2 h or longer. It remains unclear why the σS-TIC crystals display such a low reactivity, but it is worth mentioning that the concentration of free Mg2+ might be low during the soaking of NTP into the crystals.
Although soaking of the σS-TIC crystals in solutions containing NTPs for 1 h did not lead to nucleotide incorporation, the two NTP soaking experiments led to similar, significant conformational changes in both the protein and the nucleic acid in the σS-TIC crystals (Fig. 4). Because the soaking solution contains no PPi, soaking leads to the release of the associated PPi as expected, and coordinately, the phosphate group of the RNA 3′-terminal residue shifts toward MeI and makes a more normal phosphodiester linkage with the penultimate RNA residue, suggesting that completion of the nucleotide addition reaction might require PPi release. Interestingly, it appears that there is no noticeable NTP binding to the NTP “entry site” that would overlap with the PPi-binding site (27, 37).
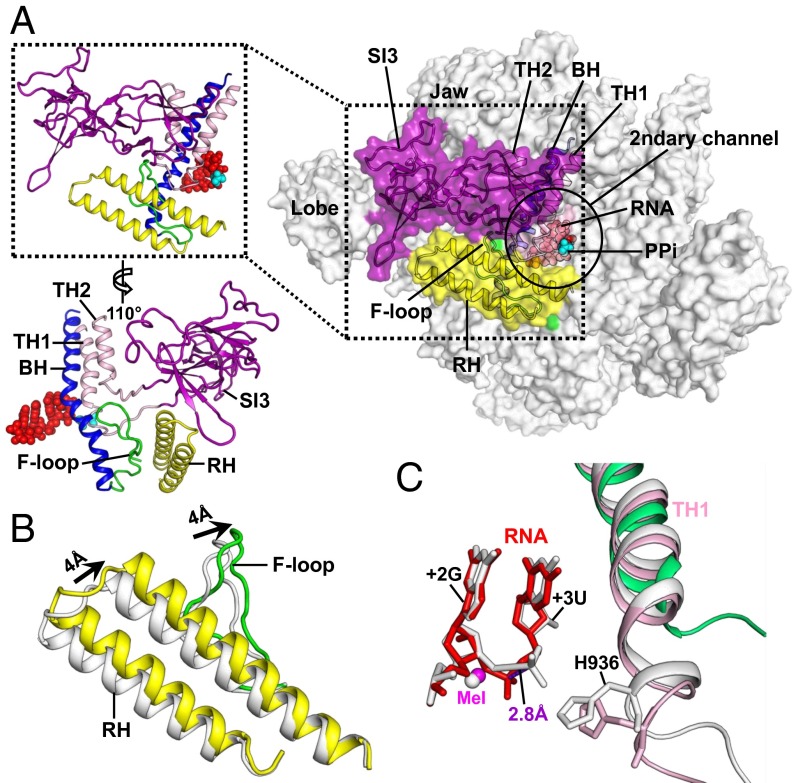
Fig. 4.
Conformational changes after PPi release. (A) The secondary channel of the σS-TIC after the crystals were soaked in NTP-containing solutions. RNAP is shown in surface representation. The TL insertion domain (SI3) (purple), F-loop (green), RHs (yellow), BH (blue), and TH1 and TH2 (pink) are also presented in cartoon representations. The insets on the left are close-up views of the flexible structural elements. The nascent RNA (red) and Ppi (cyan) are shown as filled spheres. The PPi was modeled in as a reference. (B) A close-up view of the F-loop and RHs in the σS-TICs before (gray) and after (yellow) NTP soaking and PPi release. (C) A close-up view of the active site of the σS-TICs before (gray) and after (pink and red) PPi release. The corresponding region of the E. coli RNAP apo-holoenzyme (PDB ID code 4LJZ) (green) is shown for reference. The structures are superimposed on the β subunits.
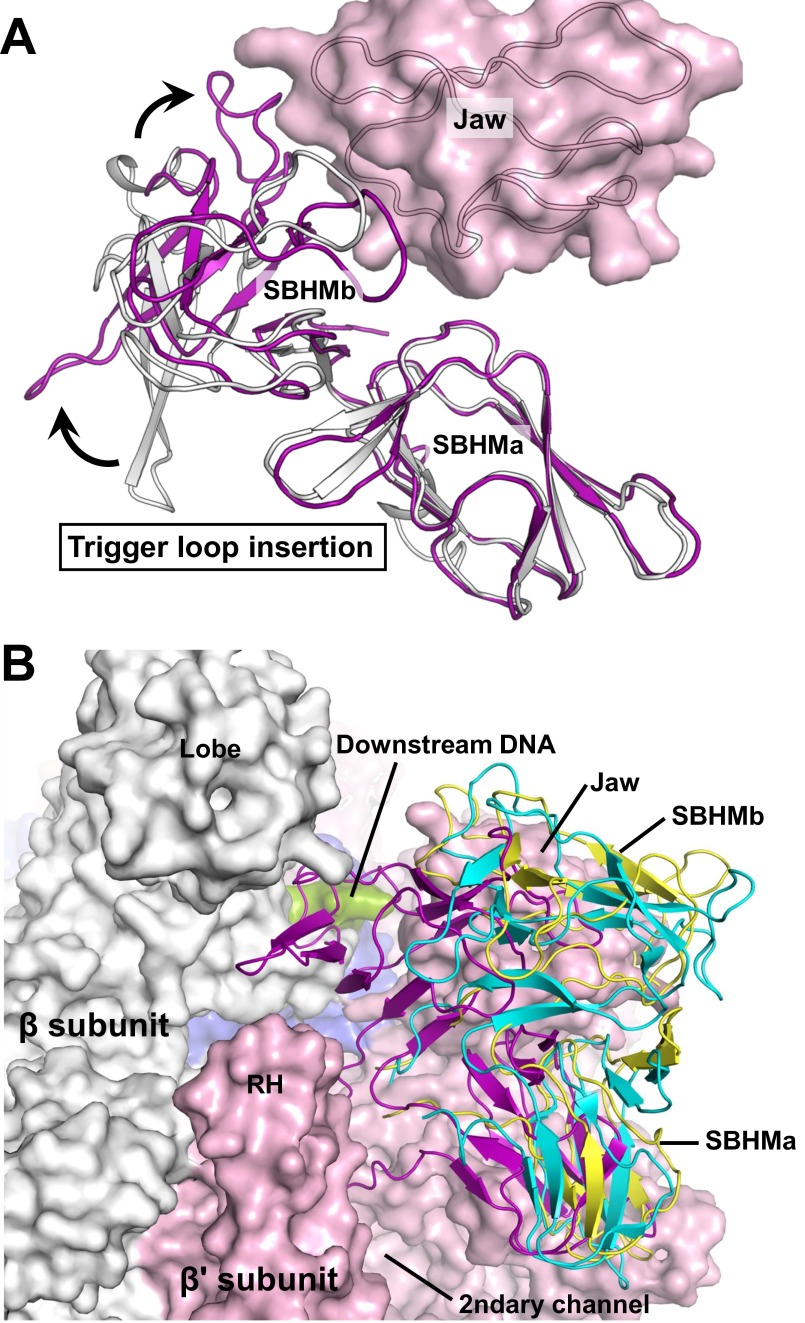
E. coli RNA polymerase carries a large lineage-specific insertion, termed “sequence insertion 3” (SI3), in the middle of the TL (38). This TL insertion of 188 amino acids could make a large shift around the secondary channel (39) and has been shown to affect E. coli transcription because it disfavors TH formation and stimulates pausing (40). In all of the three σS-TIC crystals described here, SI3 is expected to make similar contacts with symmetry-related molecules and contributes negligibly to crystal packing. However, a long hairpin loop in the C-terminal domain of SI3 projects into an intermolecular space and likely prevents SI3 from making large movements. The single most prominent conformational change on the σS-TIC after NTP soaking appears to be that the normally disordered SI3 becomes ordered and visible in the electron density map. In this ordered conformation SI3 fills the gap between the lobe and jaw domains of E. coli RNAP and forms part of the wall that separates the primary downstream DNA channel and the secondary channel (Fig. 4A).
In addition to SI3 becoming localized and ordered, the rim helices (β′ residues 650–703) on the edge of the secondary channel are rotated by about 7° toward the SI3–TH linkers (a shift of about 4 Å at the far end). At the same time, the F-loop (β′ residues 742–762) that interacts with both BH and TH makes a similar rotation to press on the SI3–TH linkers (Fig. 4B). The conformational changes of the rim helices and the F-loop, which are likely induced by the release of PPi, appear to function together to tighten the SI3–TH linkers and to lock SI3 against the jaw domain with its cleft between two β-folds (Fig. S6).
Fig. S6.
The E. coli RNAP TL insertion domain SI3. (A) The structure of the free SI3 protein fragment (PDB ID code 2AUK) (gray) is superimposed on the SI3 in the σS-TIC after soaking (purple) using the N-terminal β-fold of SI3 (SBHMa). The β' jaw domain in the σS-TIC (pink) is shown as a reference. (B) Superimposition of SI3 on this structure (purple), E. coli RNAP model (yellow) (48), and σ54-RNAP holoenzyme (PDB ID code 5BYH) (cyan) (39). The superimposition was performed using the β subunit of bacterial RNAP. RNAP and downstream DNA are shown using surface representations. Colors are as in Fig. S4.
Because NTP soaking caused very limited changes to the crystal packing, it is likely that the significant conformational changes of SI3, the rim helices, and the F-loop are directly related to the release of PPi. After PPi dissociation, there appears to be a very minor shift (∼1–2 Å) at the C terminus of the first trigger helix (TH1) (Fig. 4C), and the σS-TIC remains at the pretranslocated position with a closed active site.
Concluding Remarks.
The thermodynamic (Brownian ratchet) model of transcription translocation suggests that RNA polymerase oscillates between pre- and posttranslocation states with the forward movement biased by NTP binding. Interestingly, structures of cellular RNA polymerase complexes are overwhelmingly at the posttranslocation state with an open active site (Table S1). Although structural studies could not be used as an evidence for thermodynamic analysis, the structural preference suggests that the posttranslocation state with an open active site might be the thermodynamically more favored state. However, in both the σ70-TIC and σS-TIC crystals that contain a complete bubble, all the hybrids that we observed lie at the pretranslocation position, and the active site remains closed. It is likely that the structural preference seen in previous structural studies is a biased representation directly related to the substrate designs, which frequently involve DNA fragments lacking the upstream portion of the transcription bubble. Apparently, transcription complexes containing a complete transcription bubble to showcase the thermodynamic barriers for translocation would be of more biological significance, as we show here with the σS-TICs.
Intriguing questions are how fast PPi release happens and how PPi release is related to active site opening and transcription translocation. For T7 RNA polymerase, it was suggested that PPi release is directly coupled with active site opening and translocation (35). For cellular RNA polymerases, PPi release was suggested to be a thermodynamically controlled quick process coupled to the RNAP conformational change that is associated with the binding of the next cognate nucleotide (32). The NTP soaking experiment presented here displays release of PPi by the σS-TIC crystals and shows that the active site of the σS-TIC remains closed after PPi release. Although PPi release appears to be associated with a small shift at the C terminus of TH1 (Fig. 4C), a movement that likely occurs during the unfolding of TH to TL, it causes neither active site opening nor transcript translocation. However, it is possible that a reversal of the observed conformational changes on the periphery of the secondary channel could be coupled to TL unfolding and active site opening.
Materials and Methods
Preparation and Crystallization of E. coli σS-TIC.
To form the σS-TIC, we used a synthetic DNA scaffold corresponding to the promoter region between positions −38 and +12 relative to the expected transcription start site (Fig. 1A). The synthetic promoter, which contains the consensus −35 and −10 hexamers and the extended −10 motif, was prepared by annealing the NT strand to an equal molar amount of the T-strand DNA that is complementary to the NT strand except for a 6-nt discriminator region (Fig. 1A). The σS-TIC was assembled by directly incubating the σS-RNAP holoenzyme with a twofold molar excess of the preformed DNA promoter in buffer A [20 mM Tris (pH 7.5), 50 mM NaCl, 0.1 mM EDTA, 5 mM MgCl2] at 37 °C for 20 min in the presence of ATP, GTP, and UTP (2 mM each). This mixture then was used for crystallization at room temperature by vapor diffusion with a reservoir containing 18% (wt/vol) PEG 3350, 0.1 M NaCl, and 0.1 M Hepes (pH 7.8). After the crystals grew to full size for (for about 1 wk), they were cryo-protected in the mother liquor containing 15% (wt/vol) ethylene glycol before flash-freezing in liquid nitrogen. The σS-TIC crystallizes in the orthorhombic P212121 space group with one copy of the complex per asymmetric unit different from that of the σ70-TICs (5).
NTP Soaking for RNA Synthesis in the Crystal.
The initially obtained σS-TIC crystals containing an RNA tetranucleotide were soaked in the reservoir solution supplemented with either CTP only (for a 1-nt extension) or three NTPs (CTP, UTP, and GTP, for an RNA extension of up to 3 nt) (2 mM each) at room temperature for various time periods. The crystals then were cryo-protected and flash-frozen in the same manner as aforementioned.
Data Collection, Processing, and Structure Determination.
X-ray diffraction data were collected at 100 K at the beamlines 24-ID-C and 24-ID-E at Argonne National Laboratory, Chicago, IL. All data were integrated and scaled with HKL2000 (41). The structures were solved by molecular replacement with PHASER (42) using a structure of the E. coli σ70-TIC (5) as the starting model. The molecular replacement solution was subjected to rigid body refinement with Refmac5 (43) using multiple rigid groups, and the phases were improved by density modification. The maps were improved further by temperature factor sharpening that allowed building the σS factor and the nucleic acid models into the density using COOT (44). After model building in Coot, 10 cycles of TLS (translation libration screw-motion) and restrained refinement were performed using Refmac5 (43) in the CCP4 suite (45). Data collection and structural refinement statistics are summarized in Table 1. All figures were created using PyMOL (46).
SI Materials and Methods
Cloning, Expression, and Purification of E. coli σS Factor.
A DNA fragment encoding E. coli σS factor was amplified from genomic DNA of E. coli K12 and cloned into a pET21d expression vector between the NcoI and XhoI sites. The primers we used were 5′-AAACCATGGGTCAGAATACGCTGAAAGTTCATG-3′ and 5′-AAACTCGAGGAACAGCGCTTCGATATTCAG-3′. Two point mutations (S2G and R329L) were introduced for cloning purpose, and a 6xHis tag was engineered into the C terminus of the σS gene to facilitate protein purification. After the expression vector was transformed into E. coli Rosetta2(DE3)pLysS cells, overexpression of the C-terminal his-tagged σS protein (σS-his) was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside at the late log phase. Cells were harvested after overnight induction at 30 °C and were lysed using a continuous-flow French press. The σS-his protein was first purified by a Ni2+-affinity column, followed by monoQ anion exchange and size-exclusion chromatography. The purified σS-his protein was used directly for assembling the E. coli σS-RNAP holoenzyme.
Preparation of E. coli σS-RNAP Holoenzyme.
The E. coli RNA polymerase core enzyme that lacks the C-terminal 94 amino acids of the α subunit (Δα236–329, or ΔαCTD) was prepared as described previously (5, 47). The ΔαCTD RNAP holoenzyme containing the σS factor (σS-RNAP) was prepared by mixing the purified mutant core enzyme with purified σS-his protein (about 1:3 molar ratio) at room temperature for 15 min followed by size-exclusion chromatography to remove the extra σS-his protein. The holoenzyme was concentrated to about 30 mg/mL in buffer A [20 mM Tris (pH 7.5), 50 mM NaCl, 0.1 mM EDTA, 5 mM MgCl2] and stored in small aliquots at −80 °C after flash freezing in liquid nitrogen.
Acknowledgments
We thank the staff of Argonne National Laboratory beamlines 24-ID-C and 24-ID-E for help during data collection, the Center for Structural Biology Facility at Yale University for computational support, and Dr. Jimin Wang for contributions to our data processing and structural refinement. This work was supported by NIH Grant GM22778 (to T.A.S.). T.A.S. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The structure(s) reported in this paper have been deposited in the Protein Data Bank (PDB) database (PDB ID codes 5IPL, 5IPM, and 5IPN).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520555113/-/DCSupplemental.
References
- 1.Murakami KS, Darst SA. Bacterial RNA polymerases: The wholo story. Curr Opin Struct Biol. 2003;13(1):31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 2.Cramer P. Multisubunit RNA polymerases. Curr Opin Struct Biol. 2002;12(1):89–97. doi: 10.1016/s0959-440x(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 3.Saecker RM, Record MT, Jr, Dehaseth PL. Mechanism of bacterial transcription initiation: RNA polymerase - promoter binding, isomerization to initiation-competent open complexes, and initiation of RNA synthesis. J Mol Biol. 2011;412(5):754–771. doi: 10.1016/j.jmb.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgess RR, Travers AA, Dunn JJ, Bautz EK. Factor stimulating transcription by RNA polymerase. Nature. 1969;221(5175):43–46. doi: 10.1038/221043a0. [DOI] [PubMed] [Google Scholar]
- 5.Zuo Y, Steitz TA. Crystal structures of the E. coli transcription initiation complexes with a complete bubble. Mol Cell. 2015;58(3):534–540. doi: 10.1016/j.molcel.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revyakin A, Liu C, Ebright RH, Strick TR. Abortive initiation and productive initiation by RNA polymerase involve DNA scrunching. Science. 2006;314(5802):1139–1143. doi: 10.1126/science.1131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapanidis AN, et al. Initial transcription by RNA polymerase proceeds through a DNA-scrunching mechanism. Science. 2006;314(5802):1144–1147. doi: 10.1126/science.1131399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straney DC, Crothers DM. A stressed intermediate in the formation of stably initiated RNA chains at the Escherichia coli lac UV5 promoter. J Mol Biol. 1987;193(2):267–278. doi: 10.1016/0022-2836(87)90218-x. [DOI] [PubMed] [Google Scholar]
- 9.Goldman SR, Ebright RH, Nickels BE. Direct detection of abortive RNA transcripts in vivo. Science. 2009;324(5929):927–928. doi: 10.1126/science.1169237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 11.Hansen UM, McClure WR. A noncycling activity assay for the omega subunit of Escherichia coli RNA polymerase. J Biol Chem. 1979;254(13):5713–5717. [PubMed] [Google Scholar]
- 12.Maeda H, Fujita N, Ishihama A. Competition among seven Escherichia coli sigma subunits: Relative binding affinities to the core RNA polymerase. Nucleic Acids Res. 2000;28(18):3497–3503. doi: 10.1093/nar/28.18.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaal T, Mandel MJ, Silhavy TJ, Gourse RL. Crl facilitates RNA polymerase holoenzyme formation. J Bacteriol. 2006;188(22):7966–7970. doi: 10.1128/JB.01266-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratt LA, Silhavy TJ. Crl stimulates RpoS activity during stationary phase. Mol Microbiol. 1998;29(5):1225–1236. doi: 10.1046/j.1365-2958.1998.01007.x. [DOI] [PubMed] [Google Scholar]
- 15.Banta AB, et al. Key features of σS required for specific recognition by Crl, a transcription factor promoting assembly of RNA polymerase holoenzyme. Proc Natl Acad Sci USA. 2013;110(40):15955–15960. doi: 10.1073/pnas.1311642110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaal T, et al. Promoter recognition and discrimination by EsigmaS RNA polymerase. Mol Microbiol. 2001;42(4):939–954. doi: 10.1046/j.1365-2958.2001.02703.x. [DOI] [PubMed] [Google Scholar]
- 17.Bae B, Feklistov A, Lass-Napiorkowska A, Landick R, Darst SA. Structure of a bacterial RNA polymerase holoenzyme open promoter complex. eLife. 2015;4:e08504. doi: 10.7554/eLife.08504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, et al. Structural basis of transcription initiation. Science. 2012;338(6110):1076–1080. doi: 10.1126/science.1227786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feklistov A, Darst SA. Structural basis for promoter-10 element recognition by the bacterial RNA polymerase σ subunit. Cell. 2011;147(6):1257–1269. doi: 10.1016/j.cell.2011.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker G, Hengge-Aronis R. What makes an Escherichia coli promoter sigma(S) dependent? Role of the -13/-14 nucleotide promoter positions and region 2.5 of sigma(S) Mol Microbiol. 2001;39(5):1153–1165. doi: 10.1111/j.1365-2958.2001.02313.x. [DOI] [PubMed] [Google Scholar]
- 21.Wise A, Brems R, Ramakrishnan V, Villarejo M. Sequences in the -35 region of Escherichia coli rpoS-dependent genes promote transcription by E sigma S. J Bacteriol. 1996;178(10):2785–2793. doi: 10.1128/jb.178.10.2785-2793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra A, Severinova E, Darst SA. Crystal structure of a sigma 70 subunit fragment from E. coli RNA polymerase. Cell. 1996;87(1):127–136. doi: 10.1016/s0092-8674(00)81329-x. [DOI] [PubMed] [Google Scholar]
- 23.Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol. 2011;65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hengge R. Proteolysis of sigmaS (RpoS) and the general stress response in Escherichia coli. Res Microbiol. 2009;160(9):667–676. doi: 10.1016/j.resmic.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Vassylyev DG, et al. Structural basis for substrate loading in bacterial RNA polymerase. Nature. 2007;448(7150):163–168. doi: 10.1038/nature05931. [DOI] [PubMed] [Google Scholar]
- 26.Toulokhonov I, Zhang J, Palangat M, Landick R. A central role of the RNA polymerase trigger loop in active-site rearrangement during transcriptional pausing. Mol Cell. 2007;27(3):406–419. doi: 10.1016/j.molcel.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: Role of the trigger loop in substrate specificity and catalysis. Cell. 2006;127(5):941–954. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassylyev DG, et al. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 A resolution. Nature. 2002;417(6890):712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 29.Gnatt AL, Cramer P, Fu J, Bushnell DA, Kornberg RD. Structural basis of transcription: An RNA polymerase II elongation complex at 3.3 A resolution. Science. 2001;292(5523):1876–1882. doi: 10.1126/science.1059495. [DOI] [PubMed] [Google Scholar]
- 30.Mejia YX, Nudler E, Bustamante C. Trigger loop folding determines transcription rate of Escherichia coli’s RNA polymerase. Proc Natl Acad Sci USA. 2015;112(3):743–748. doi: 10.1073/pnas.1421067112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brueckner F, Cramer P. Structural basis of transcription inhibition by alpha-amanitin and implications for RNA polymerase II translocation. Nat Struct Mol Biol. 2008;15(8):811–818. doi: 10.1038/nsmb.1458. [DOI] [PubMed] [Google Scholar]
- 32.Johnson RS, Strausbauch M, Carraway JK. Rapid pyrophosphate release from transcriptional elongation complexes appears to be coupled to a nucleotide-induced conformational change in E. coli core polymerase. J Mol Biol. 2011;412(5):849–861. doi: 10.1016/j.jmb.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Rozovskaya TA, Chenchik AA, Beabealashvilli RSh. Processive pyrophosphorolysis of RNA by Escherichia coli RNA polymerase. FEBS Lett. 1982;137(1):100–104. doi: 10.1016/0014-5793(82)80323-2. [DOI] [PubMed] [Google Scholar]
- 34.Maitra U, Hurwitz J. The role of deoxyribonucleic acid in ribonucleic acid synthesis. 13. Modified purification procedure and additional properties of ribonucleic acid polymerase from Escherichia coli W. J Biol Chem. 1967;242(21):4897–4907. [PubMed] [Google Scholar]
- 35.Yin YW, Steitz TA. The structural mechanism of translocation and helicase activity in T7 RNA polymerase. Cell. 2004;116(3):393–404. doi: 10.1016/s0092-8674(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 36.Malinen AM, et al. Active site opening and closure control translocation of multisubunit RNA polymerase. Nucleic Acids Res. 2012;40(15):7442–7451. doi: 10.1093/nar/gks383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westover KD, Bushnell DA, Kornberg RD. Structural basis of transcription: Nucleotide selection by rotation in the RNA polymerase II active center. Cell. 2004;119(4):481–489. doi: 10.1016/j.cell.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Chlenov M, et al. Structure and function of lineage-specific sequence insertions in the bacterial RNA polymerase beta’ subunit. J Mol Biol. 2005;353(1):138–154. doi: 10.1016/j.jmb.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, et al. TRANSCRIPTION. Structures of the RNA polymerase-σ54 reveal new and conserved regulatory strategies. Science. 2015;349(6250):882–885. doi: 10.1126/science.aab1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Windgassen TA, et al. Trigger-helix folding pathway and SI3 mediate catalysis and hairpin-stabilized pausing by Escherichia coli RNA polymerase. Nucleic Acids Res. 2014;42(20):12707–12721. doi: 10.1093/nar/gku997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otwinowski Z, Minor W. In: Methods Enzymol. Carter CW, Sweet RM, editors. Academic; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 42.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 45.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; San Carlos, CA: 2002. [Google Scholar]
- 47.Liu B, Zuo Y, Steitz TA. Structural basis for transcription reactivation by RapA. Proc Natl Acad Sci USA. 2015;112(7):2006–2010. doi: 10.1073/pnas.1417152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Opalka N, et al. Complete structural model of Escherichia coli RNA polymerase from a hybrid approach. PLoS Biol. 2010;8(9):e1000483. doi: 10.1371/journal.pbio.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang D, Zhu G, Huang X, Lippard SJ. X-ray structure and mechanism of RNA polymerase II stalled at an antineoplastic monofunctional platinum-DNA adduct. Proc Natl Acad Sci USA. 2010;107(21):9584–9589. doi: 10.1073/pnas.1002565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Basu RS, et al. Structural basis of transcription initiation by bacterial RNA polymerase holoenzyme. J Biol Chem. 2014;289(35):24549–24559. doi: 10.1074/jbc.M114.584037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Bushnell DA, Silva DA, Huang X, Kornberg RD. Initiation complex structure and promoter proofreading. Science. 2011;333(6042):633–637. doi: 10.1126/science.1206629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheung AC, Sainsbury S, Cramer P. Structural basis of initial RNA polymerase II transcription. EMBO J. 2011;30(23):4755–4763. doi: 10.1038/emboj.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kinkelin K, et al. Structures of RNA polymerase II complexes with Bye1, a chromatin-binding PHF3/DIDO homologue. Proc Natl Acad Sci USA. 2013;110(38):15277–15282. doi: 10.1073/pnas.1311010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Westover KD, Bushnell DA, Kornberg RD. Structural basis of transcription: Separation of RNA from DNA by RNA polymerase II. Science. 2004;303(5660):1014–1016. doi: 10.1126/science.1090839. [DOI] [PubMed] [Google Scholar]
- 55.Kettenberger H, Armache KJ, Cramer P. Complete RNA polymerase II elongation complex structure and its interactions with NTP and TFIIS. Mol Cell. 2004;16(6):955–965. doi: 10.1016/j.molcel.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 56.Brueckner F, Hennecke U, Carell T, Cramer P. CPD damage recognition by transcribing RNA polymerase II. Science. 2007;315(5813):859–862. doi: 10.1126/science.1135400. [DOI] [PubMed] [Google Scholar]
- 57.Vassylyev DG, Vassylyeva MN, Perederina A, Tahirov TH, Artsimovitch I. Structural basis for transcription elongation by bacterial RNA polymerase. Nature. 2007;448(7150):157–162. doi: 10.1038/nature05932. [DOI] [PubMed] [Google Scholar]
- 58.Damsma GE, Alt A, Brueckner F, Carell T, Cramer P. Mechanism of transcriptional stalling at cisplatin-damaged DNA. Nat Struct Mol Biol. 2007;14(12):1127–1133. doi: 10.1038/nsmb1314. [DOI] [PubMed] [Google Scholar]
- 59.Lehmann E, Brueckner F, Cramer P. Molecular basis of RNA-dependent RNA polymerase II activity. Nature. 2007;450(7168):445–449. doi: 10.1038/nature06290. [DOI] [PubMed] [Google Scholar]
- 60.Dengl S, Cramer P. Torpedo nuclease Rat1 is insufficient to terminate RNA polymerase II in vitro. J Biol Chem. 2009;284(32):21270–21279. doi: 10.1074/jbc.M109.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sydow JF, et al. Structural basis of transcription: Mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA. Mol Cell. 2009;34(6):710–721. doi: 10.1016/j.molcel.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 62.Damsma GE, Cramer P. Molecular basis of transcriptional mutagenesis at 8-oxoguanine. J Biol Chem. 2009;284(46):31658–31663. doi: 10.1074/jbc.M109.022764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walmacq C, et al. Mechanism of translesion transcription by RNA polymerase II and its role in cellular resistance to DNA damage. Mol Cell. 2012;46(1):18–29. doi: 10.1016/j.molcel.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sainsbury S, Niesser J, Cramer P. Structure and function of the initially transcribing RNA polymerase II-TFIIB complex. Nature. 2013;493(7432):437–440. doi: 10.1038/nature11715. [DOI] [PubMed] [Google Scholar]
- 65.Bae B, et al. CarD uses a minor groove wedge mechanism to stabilize the RNA polymerase open promoter complex. eLife. 2015;4:e08505. doi: 10.7554/eLife.08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barnes CO, et al. Crystal Structure of a Transcribing RNA Polymerase II Complex Reveals a Complete Transcription Bubble. Mol Cell. 2015;59(2):258–269. doi: 10.1016/j.molcel.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, et al. GE23077 binds to the RNA polymerase ‘i’ and ‘i+1’ sites and prevents the binding of initiating nucleotides. eLife. 2014;3:e02450. doi: 10.7554/eLife.02450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tagami S, et al. Crystal structure of bacterial RNA polymerase bound with a transcription inhibitor protein. Nature. 2010;468(7326):978–982. doi: 10.1038/nature09573. [DOI] [PubMed] [Google Scholar]
- 69.Weixlbaumer A, Leon K, Landick R, Darst SA. Structural basis of transcriptional pausing in bacteria. Cell. 2013;152(3):431–441. doi: 10.1016/j.cell.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang D, et al. Structural basis of transcription: Backtracked RNA polymerase II at 3.4 angstrom resolution. Science. 2009;324(5931):1203–1206. doi: 10.1126/science.1168729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheung AC, Cramer P. Structural basis of RNA polymerase II backtracking, arrest and reactivation. Nature. 2011;471(7337):249–253. doi: 10.1038/nature09785. [DOI] [PubMed] [Google Scholar]