Significance
Selective autophagy of damaged mitochondria (mitophagy) requires protein kinases PINK1 and TBK1, ubiquitin ligase Parkin, and autophagy receptors such as OPTN, driving ubiquitin-labeled mitochondria into autophagosomes. Because all proteins have been genetically linked to either Parkinson’s disease (PINK1 and Parkin) or amyotrophic lateral sclerosis and frontotemporal lobar degeneration (TBK1 and OPTN), it is of great interest to understand their physiological functions. By utilizing quantitative proteomics we show that TBK1 phosphorylates four receptors on several autophagy-relevant sites. Constitutive interaction of TBK1 with OPTN and the ability of OPTN to bind to ubiquitin chains are essential for TBK1 recruitment and activation on mitochondria. TBK1-mediated phosphorylation of OPTN creates a signal amplification loop through combining recruitment and retention of OPTN/TBK1 on ubiquitinated mitochondria.
Keywords: mitophagy, TBK1, OPTN, ubiquitin, phosphorylation
Abstract
Selective autophagy of damaged mitochondria requires autophagy receptors optineurin (OPTN), NDP52 (CALCOCO2), TAX1BP1, and p62 (SQSTM1) linking ubiquitinated cargo to autophagic membranes. By using quantitative proteomics, we show that Tank-binding kinase 1 (TBK1) phosphorylates all four receptors on several autophagy-relevant sites, including the ubiquitin- and LC3-binding domains of OPTN and p62/SQSTM1 as well as the SKICH domains of NDP52 and TAX1BP1. Constitutive interaction of TBK1 with OPTN and the ability of OPTN to bind to ubiquitin chains are essential for TBK1 recruitment and kinase activation on mitochondria. TBK1 in turn phosphorylates OPTN’s UBAN domain at S473, thereby expanding the binding capacity of OPTN to diverse Ub chains. In combination with phosphorylation of S177 and S513, this posttranslational modification promotes recruitment and retention of OPTN/TBK1 on ubiquitinated, damaged mitochondria. Moreover, phosphorylation of OPTN on S473 enables binding to pS65 Ub chains and is also implicated in PINK1-driven and Parkin-independent mitophagy. Thus, TBK1-mediated phosphorylation of autophagy receptors creates a signal amplification loop operating in selective autophagy of damaged mitochondria.
As a cell survival pathway, autophagy selectively frees the cytosolic compartment from bulky protein aggregates, invading bacteria or damaged organelles such as mitochondria and peroxisomes (1, 2). In this context, the posttranslational modifier ubiquitin (Ub) has been widely recognized as a selective signal driving autophagy of such cellular components and cargoes (3, 4). Recently, ubiquitin itself has been discovered to be phosphorylated to promote autophagic clearance of damaged mitochondria (mitophagy; reviewed in refs. 5 and 6). Ser/Thr kinase PINK1 phosphorylates S65 of Ub, which is critical for two steps of this process: allosteric activation of the E3 Ub ligase Parkin and recruitment of the autophagic machinery, including autophagy receptors (7–14).
Autophagy receptors function as decoders for the various ubiquitin signals on cargoes, linking cargoes to autophagosomal membranes (4); however, the basis of their individual recruitment to cargo as well as their distinct and cooperative functions in cargo sequestration are still poorly understood. The autophagy receptors optineurin (OPTN) and p62 are first activated by protein kinases to effectively target autophagic membranes or their polyUb cargo (15–17). TANK-binding kinase 1 (TBK1) phosphorylates OPTN on S177, thereby enhancing LC3-binding affinity and autophagic clearance of cytosolic Salmonella (15). Activity and specificity of TBK1 are defined by adaptor proteins; these recruit TBK1 to microdomains on ubiquitinated Salmonella or mitochondria, thereby facilitating its local clustering and activation (18), where it in turn can phosphorylate autophagy receptors (15). It is relevant to stress that a number of mutations in both OPTN and TBK1 have been identified in patients suffering from amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD), which points toward an important role of the OPTN–TBK1 complex in autophagy and neurodegeneration (19–22).
Here, we provide evidence that TBK1 integrates upstream Ub-dependent signaling events by phosphorylating the autophagy receptor OPTN in the Ub-binding domain (UBD) in ABIN proteins and NEMO (UBAN), thus controlling its binding to Ub chains and regulating autophagy of damaged mitochondria. We also show that the ALS-associated mutant TBK1 E696K that is unable to bind to OPTN also fails to translocate to damaged mitochondria, highlighting an important role for OPTN in the regulation of TBK1.
Results
TBK1 Directly Phosphorylates the UBAN Domain of OPTN.
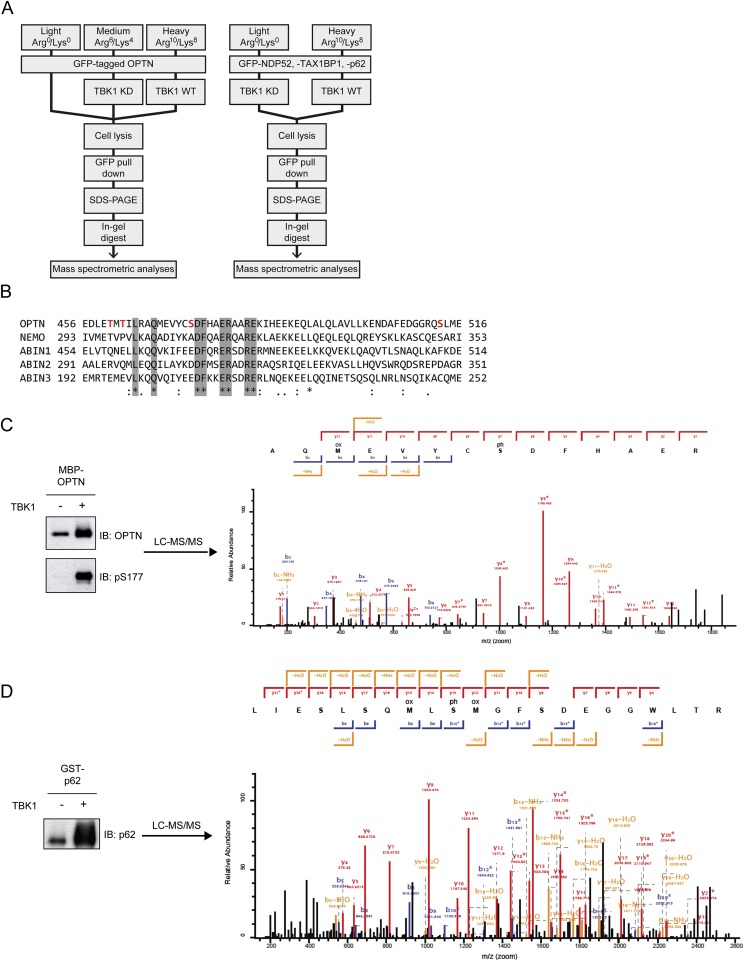
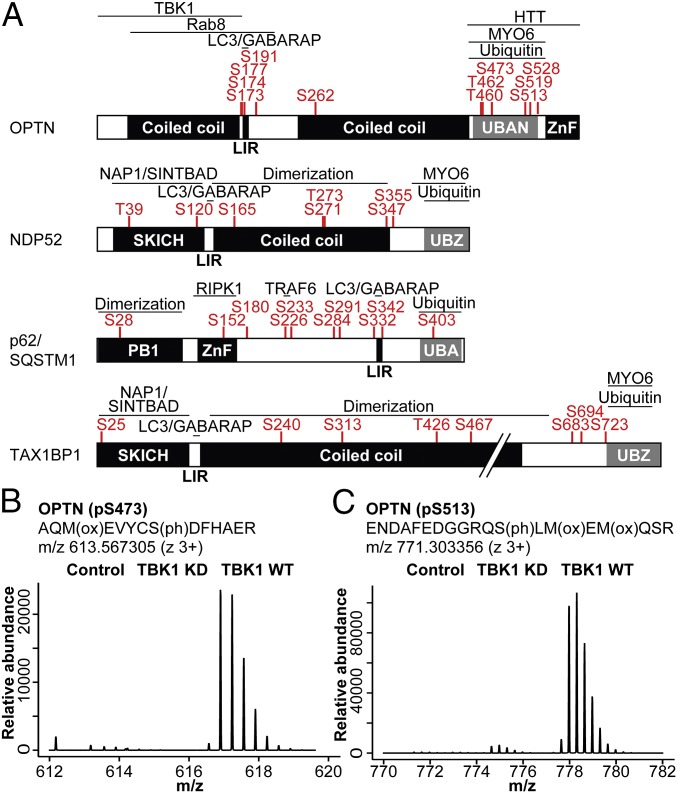
TBK1 has been reported to regulate the autophagy receptors OPTN and p62 during bacterial infection (15, 17) and, more recently, during mitophagy (13, 23). We next used stable isotope labeling with amino acids in cell culture (SILAC)-based quantitative MS analysis to systematically identify TBK1-depedent phosphorylation sites on multiple autophagy receptors. To this end, SILAC-labeled HEK293T cells expressing GFP-tagged OPTN, NDP52, p62, or TAX1BP1 were cotransfected with TBK1 WT or kinase-deficient (KD) mutant (TBK1 K38A). Autophagy receptors were enriched using affinity purification under denaturing conditions followed by MS analysis (Fig. S1A). By determining the relative abundance of phosphopeptides from cells expressing WT or KD TBK1, we were able to identify phosphorylation sites that are dependent on the active kinase. Surprisingly, TBK1 directly or indirectly mediates phosphorylation of all four receptors on multiple, autophagy-relevant sites, including the UBAN domain of OPTN, UBA domain of p62, SKICH domains of NDP52, and TAX1BP1 and LIR domains of OPTN and p62 (Fig. 1A). OPTN showed two prominent clusters of TBK1-regulated phosphosites: multiple phosphoserines adjacent to the LIR, including S177 as described previously (15), as well as multiple phosphosites located within (T460, T462, S473) or adjacent (S513) to the UBAN domain (Fig. 1A). The relative abundance of pS473 and pS513 was significantly increased in cells expressing TBK1 WT compared with KD, with pS513 being ∼4–5× more abundantly phosphorylated than pS473 (Fig. 1 B and C). Sequence analysis revealed that pS473 is in direct proximity to a conserved motif of the coiled-coil UBAN dimer (474DFxxER479; Fig. S1B) that is essential for Ub binding (15, 24–26). p62 phosphorylation sites were distributed over all domains, and in line with previous reports S403 was identified as being regulated by TBK1 (Fig. 1A) (16, 17).
Fig. S1.
TBK1 directly mediates phosphorylation of UBDs. (A) Schematic representation of the experimental workflow for quantitative analysis of TBK1-dependent phosphorylation sites on autophagy receptors. (Left) Triple-SILAC workflow as for OPTN. (Right) Double-SILAC workflow as for NDP52, p62/SQSTM1, and TAX1BP1. (B) Alignment of OPTN, NEMO, and ABIN1-3 UBAN domains with conserved residues (light gray) and detected phosphosites (red letters, only for OPTN) highlighted. Consensus symbols: asterisk (*), identical residues, colon (:), conserved substitution, and period (.), semiconserved substitution. (C and D) In vitro kinase assay followed by mass spectrometric analysis: bacterially purified MBP–OPTN (C) or GST–p62/SQSTM1 (D) was incubated with 50 ng of recombinant TBK1 for 30 min at 30 °C. A sample (5%) of each reaction volume was used for immunoblotting (Left). (Right) Mass spectrometric fragment ion scan of the OPTN pS473 (C) and SQSTM1 pS403 (D) peptides. Identified b and y ions are labeled in blue and red, respectively.
Fig. 1.
TBK1 phosphorylates autophagy receptors on multiple, autophagy-relevant sites. (A) Domain structure and TBK1-dependent phosphorylation sites (log2TBK1 KD/TBK1 WT ≥ 1) of individual autophagy receptors. (B and C) Mass spectrometric parent ion scans of the peptides corresponding to pS473 and pS513 on OPTN with increased relative abundance of phosphorylated peptides in cells expressing WT TBK1.
To determine if recombinant TBK1 directly phosphorylates the UBAN domain of OPTN, we performed in vitro kinase assays. MS analysis revealed that TBK1 can directly phosphorylate multiple sites near the UBAN of OPTN, including S473 and S513 (Fig. S1C) and also as a positive control, S403 in the UBA of p62 (Fig. S1D) (17).
Phosphorylation of OPTN’s UBAN Domain Enhances Binding to Multiple Ubiquitin Chains.
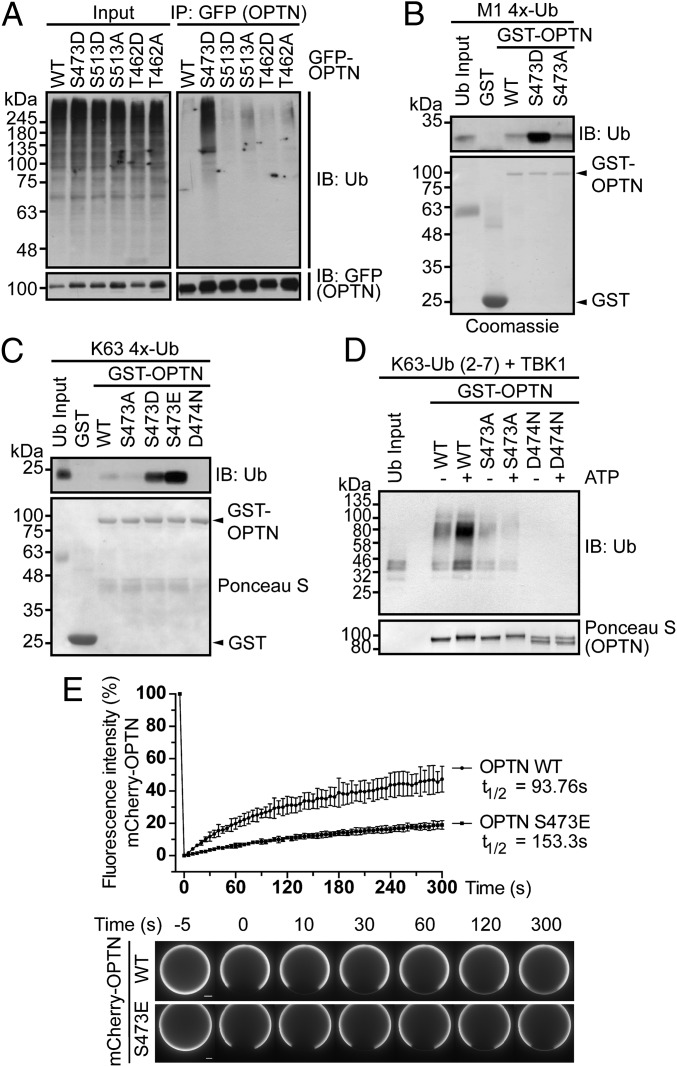
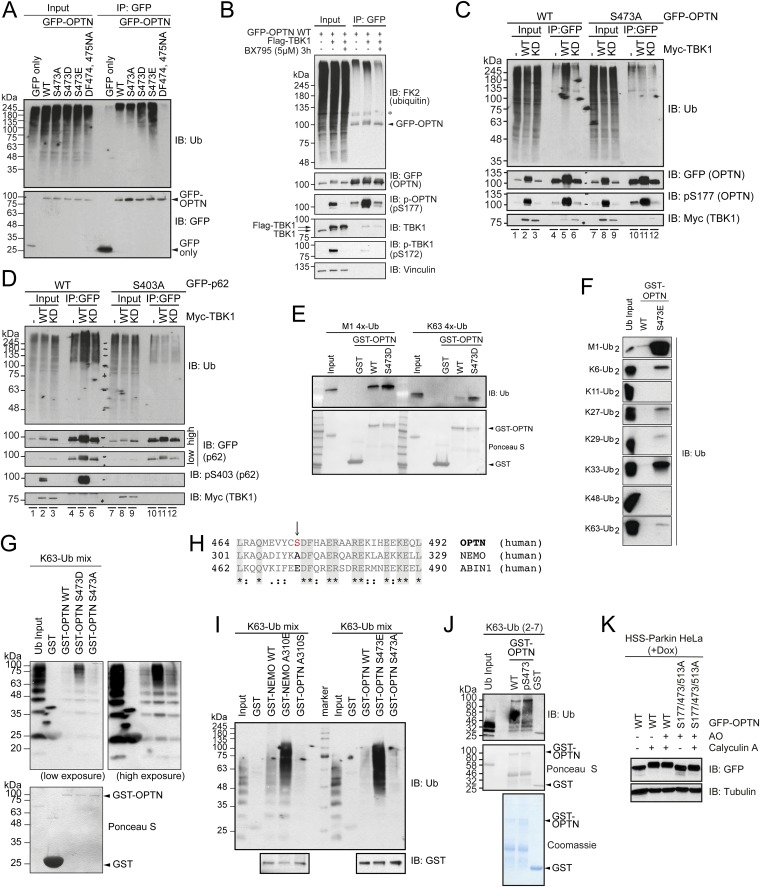
To test if any of the identified OPTN phosphorylation sites affect Ub binding in cells, we immunoprecipitated GFP–OPTN WT or the corresponding phosphomimetic mutants. Only OPTN S473, and none of the other identified sites, showed a detectable increase in affinity toward cellular polyUb proteins (Fig. 2A and Fig. S2A). When coexpressed with Myc-TBK1, GFP–OPTN WT showed increased capacity to coprecipitate polyUb proteins, but not in the presence of a TBK1 inhibitor (BX795; Fig. S2B). This effect indeed relied on phosphorylation of OPTN S473 because the mutant S473A was significantly reduced in its ability to bind polyUb proteins upon TBK1 coexpression (Fig. S2C, lanes 5 and 11) comparable to p62 when S403 is mutated to S403A (Fig. S2D, lanes 5 and 11) (16). Thus, phosphorylation of S473 is sufficient to increase the capacity of OPTN to bind to polyUb chains in cells.
Fig. 2.
Phosphorylated OPTN UBAN domain enhances binding to ubiquitin chains. (A) GFP immunoprecipitation of GFP–OPTN WT and indicated mutants from HEK293T cell lysates. (B and C) Indicated GST proteins were incubated with purified M1-linked (B) or K63-linked (C) tetra-Ub chains. (D) GST–OPTN WT and mutants were phosphorylated in vitro by TBK1 and subsequently incubated with purified Ub chains. Efficient phosphorylation of OPTN is indicated through an electrophoretic mobility shift. (A–D) Samples were subjected to immunoblot. Coomassie or Ponceau S staining show equal GST protein levels. (E) FRAP assay of recombinant mCherry–OPTN WT (●) or S473E (▪) on the surface of diUb-conjugated beads (Upper). Images were taken before and after photobleaching at indicated time points (Lower). (Scale bar, 10 µm.)
Fig. S2.
Phosphorylated OPTN UBAN domain enhances binding to ubiquitin chains. (A) GFP IP of GFP–OPTN WT and indicated UBAN mutants from HEK293T cell lysates. Coprecipitated, endogenous polyUb proteins were analyzed by immunoblotting. (B) HEK293T cells transiently expressing GFP–OPTN alone or GFP–OPTN and Myc–TBK1 WT were either left untreated or treated with a TBK1 inhibitor (BX795) for 3 h prior lysis, followed by GFP IP. Samples were analyzed by immunoblotting with the indicated antibodies. (C) GFP–OPTN WT or S473A or (D) GFP–p62/SQSTM1 WT or S403A were expressed alone or together with Myc–TBK1 WT or K38A (KM) in HEK293T cells, followed by GFP IP and immunoblotting. (E–G) GST pull-down: recombinant, immobilized GST–OPTN WT or GST–OPTN UBAN mutants were incubated with the indicated tetraUb (E), diUb (F), or polyUb (G) chains. Coprecipitated proteins were resolved by SDS/PAGE and immunoblotted with the indicated antibodies. (H) Multiple sequence alignment of OPTN, NEMO, and ABIN1 UBAN domains highlighting the position of OPTN S473, NEMO A310, and ABIN1 E471 close to the DFxxERxxRE UBAN core motif. (I) GST pull-down: recombinant, immobilized GST–NEMO and GST–OPTN WT or GST–NEMO and GST–OPTN UBAN mutants were incubated with K63 polyUb chains. Coprecipitated proteins were resolved by SDS/PAGE and immunoblotted with the indicated antibodies. (J) Phosphorylated GST–OPTN S473 was generated using an orthogonal phosphoserine translation system (27). Immobilized GST–OPTN WT and pS473 were incubated with purified K63-linked Ub chains. Coprecipitated Ub was analyzed by immunoblotting (IB: Ub). Equal GST protein levels were confirmed by Ponceau S and Coomassie staining. (K) HeLa cells stably expressing HA-Strep-Strep (HSS)-Parkin under a tetracycline inducible promoter (HeLa Flp-In T-REx HSS-Parkin cells) were preincubated with doxycyline for 48 h and transfected with GFP–OPTN WT or GFP–OPTN S177/473/513A. Cells were treated with AO and 100 nM calyculin A for 30 min. Extracts were resolved by SDS/PAGE and immunoblotted with the indicated antibodies.
To test Ub binding in vitro, we performed pull-down experiments with recombinant GST–OPTN and Ub chains. Until recently only M1- and K63-linked Ub chains were implicated in binding to OPTN (15, 23, 26). Phosphomimetic OPTN S473D bound significantly more potently to tetraUb chains than WT or S473A (Fig. 2 B and C), and also overcame the weak affinity toward K63-Ub, resulting in binding comparable to M1-linked Ub chains (Fig. S2E).
We then tested the binding of OPTN WT vs. S473E toward all available diUb chains. Whereas OPTN WT bound only weakly to short M1-diUb, we observed increased binding of OPTN S473E to nearly all Lys-linked chains (Fig. S2F). Ub chain length appears also important for binding to OPTN: whereas K63-diUb weakly bound to OPTN WT or S473E (Fig. S2F), longer K63-Ub chains potently bound to the phosphomimetic mutant OPTN S473D (Fig. S2G). Additionally, NEMO, if provided with a negative charge at the corresponding site in its UBAN (NEMO A310E), also more potently binds K63 polyUb chains (Fig. S2 H and I). To demonstrate that OPTN S473D/E functionally resembles pS473, we performed in vitro phosphorylation assays: OPTN WT but not S473A (Fig. 2D) showed an increase in Ub binding similar to OPTN S473D/E (Fig. 2 B and C). Similarly, pS473 OPTN purified from Escherichia coli using an orthogonal phosphoserine translation system (27) showed increased binding to Ub (Fig. S2J). Phosphorylation of OPTN at S177, S473, and S513 in cells was further confirmed, as visualized by an electrophoretic mobility shift of GFP–OPTN upon treatment with the phosphatase inhibitor calyculin A, which was lost if these sites were mutated to alanine (Fig. S2K).
To study the kinetics of pS473 OPTN to Ub binding, we performed time-lapse microscopy imaging in combination with fluorescence recovery after photobleaching (FRAP) using bacterially purified mCherry–OPTN WT or S473E and diUb-conjugated beads. Images were taken before and after photobleaching (Fig. 2E, Lower). The slower and reduced recovery of OPTN S473E after photobleaching compared with OPTN WT indicates that a negative charge at S473 leads to a more stable interaction with a lower molecular exchange rate. Indeed, the half-time of recovery for OPTN WT and S473E was 93.76 and 153.3 s, respectively, with a recovery rate of ∼45% and ∼20% (Fig. 2E, Upper). Thus, ∼80% of OPTN S473E remained immobile compared with ∼55% of OPTN WT, suggesting an increased residence time of OPTN S473E on diUb.
TBK1 Is Recruited to Mitochondria and Activated via OPTN Binding.
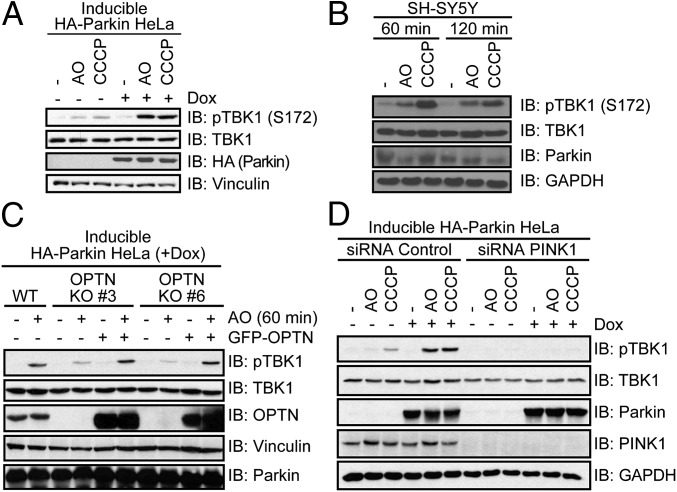
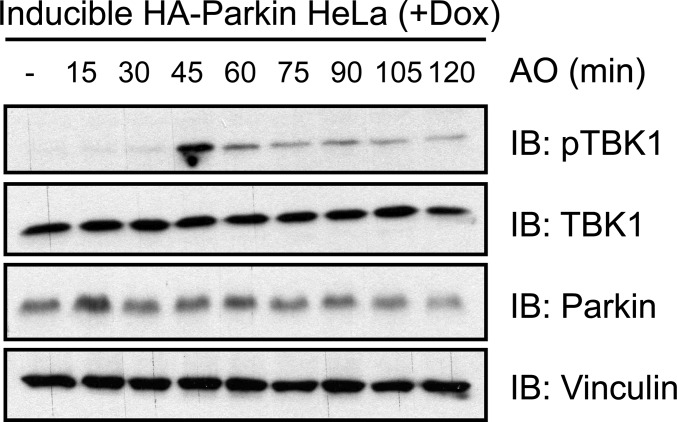
TBK1 and OPTN act together downstream of the PINK1–Parkin pathway to facilitate the autophagic removal of damaged mitochondria (mitophagy) (13, 23). WT OPTN, but not an ALS-associated, Ub-binding deficient mutant (OPTN E478G) colocalized to and facilitated clearance of damaged mitochondria independent of p62 function (13, 28). In accordance, we demonstrate that endogenous TBK1 is activated (autophosphorylation of S172) upon treatment with antimycin A and oligomycin (AO) or carbonyl cyanide m-chlorophenylhydrazone (CCCP) in SH-SY5Y and HeLa cells after 45 min (Fig. 3 A and B and Fig. S3). Robust TBK1 activation relied on inducible expression of E3 Ub ligase Parkin in HeLa cells (Fig. 3A), indicating the existence of two distinct TBK1 activation pathways, Parkin dependent and Parkin independent. Increased activation of TBK1 upon Parkin overexpression is presumably connected to its interplay with OPTN, because stable recruitment of OPTN to mitochondria also depends on the presence of Parkin (28). Consistent with this hypothesis, overexpression of Parkin in OPTN KO HeLa cells did not lead to further activation of TBK1 (Fig. 3C). In contrast, knockdown of the protein kinase PINK1 completely abolished activation of TBK1, indicating an upstream role of this kinase in TBK1 activation, which is consistent with recent reports (Fig. 3D) (13, 23).
Fig. 3.
TBK1 activation through mitophagy induction. (A, C, and D) Doxycycline (Dox)-inducible HA-Parkin HeLa cells were preincubated with Dox for 48 h before AO/CCCP treatment for (A and D) 80 min or (C) 60 min. (B) SH-SY5Y cells were treated with AO or CCCP for the indicated time. (C) WT or OPTN KO HeLa cells (nos. 3 and 6) stably expressing HA-Parkin were reconstituted with empty vector or GFP–OPTN for 30 h. (D) Cells were transfected with PINK1 or control siRNA for 48 h before treatment and lysis. (A–D) Cell lysates were subjected to immunoblot analysis with indicated antibodies. Active TBK1 was detected with a phosphospecific antibody (pTBK1 = pS172).
Fig. S3.
TBK1 activation through mitophagy induction. HeLa cells stably expressing HSS-Parkin under a tetracycline-inducible promoter (HeLa Flp-In T-REx HSS-Parkin cells) were preincubated with doxycyline for 48 h and cells were treated with AO for the indicated time points. Extracts were resolved by SDS/PAGE and immunoblotted with the indicated antibodies.
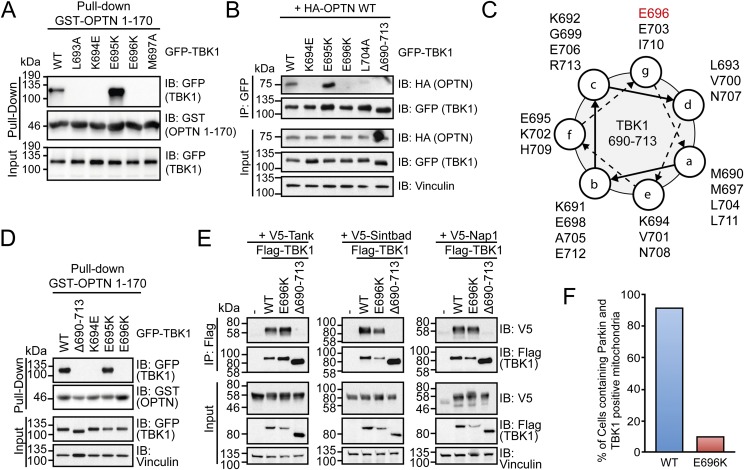
The N terminus of OPTN (amino acids 1–179) directly binds to the C-terminal adaptor-binding domain of TBK1 to form a stable complex in cells (29, 30). Mutagenesis of this domain indicates that OPTN binding may depend on the coiled-coil structure of TBK1 because several point mutations, including ALS-related missense mutation E696K, disrupt OPTN binding (Fig. S4 A–C). In addition to OPTN, TBK1 is able to bind Tank, Nap1, and Sintbad in a mutually exclusive manner (29, 30). Notably, OPTN is the only adaptor protein directly linking TBK1 to autophagy pathways. Whereas deletion of the complete adaptor-binding domain (∆690–713) disrupts the interaction with all four adaptors, TBK1 E696K only inhibited binding to OPTN (Fig. S4 D and E).
Fig. S4.
OPTN–TBK1 complex formation is blocked in ALS-associated human mutations. (A) HEK293T cells expressing GFP–TBK1 WT and indicated mutants were incubated with immobilized GST–OPTN 1–170. Binding of TBK1 variants was analyzed via immunoblot (IB). (B) Coimmunoprecipitation (co-IP) of GFP–TBK1 (WT and indicated mutants) and HA–OPTN WT from HEK293T cell lysates. (C) Helical wheel projection of the C-terminal coiled-coil and adapter-binding domain (amino acids 690–713) of TBK1 (using MARCOIL: toolkit.tuebingen.mpg.de/marcoil). (D) GST–OPTN 1–170 pull-down as in A, including TBK1 mutant delta690-713. (E) Co-IP of Flag–TBK1 (WT and indicated mutants) and V5-tagged Tank, Sintbad, and Nap1 from HEK293T cell lysates. Coprecipitated proteins were analyzed by immunoblotting with the indicated antibodies. (F) HeLa cells stably expressing HA-Parkin were transfected with TBK1 WT or E696K for 48 hours and treated with CCCP for 105 min. Quantification of cells containing Parkin- and TBK1-positive mitochondria from each condition. For representative images see Fig. 4.
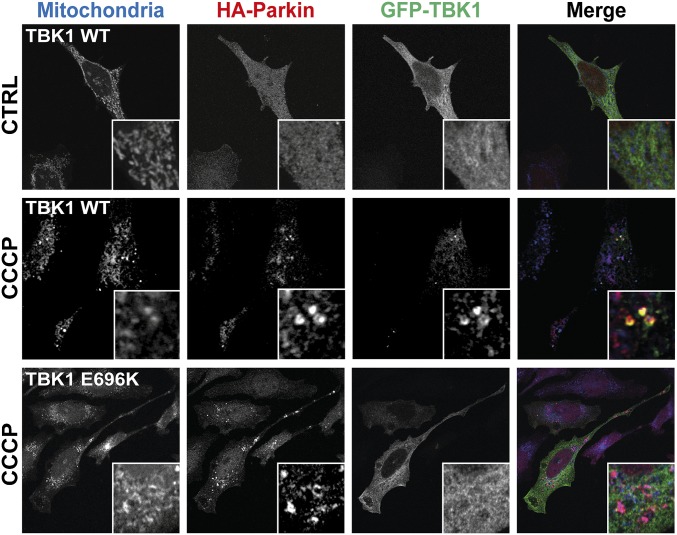
To further analyze the importance of OPTN binding to TBK1 in mitophagy, we performed immunofluorescence experiments with TBK1 WT and E696K mutant (Fig. 4). Stable translocation of TBK1 to damaged, Parkin-positive mitochondria in HeLa cells was in most instances dependent on OPTN: whereas upon CCCP treatment, TBK1 WT was seen to border Parkin-positive mitochondria in over 90% of cells, this was true for less than 10% in case of TBK1 E696K (Fig. 4 and Fig. S4F). These findings point to a possible pathogenic role of defective mitophagy in ALS pathogenesis.
Fig. 4.
OPTN–TBK1 complex formation is blocked by ALS-associated human mutations. Immunofluorescence of HeLa cells stably expressing HA-Parkin. Cells were transfected with GFP–TBK1 WT or E696K for 48 h and treated with CCCP for 105 min. Individual and merged images show mitochondria (blue), Parkin (red), and TBK1 (green).
Phosphorylation of Ub on S65 Affects OPTN Binding.
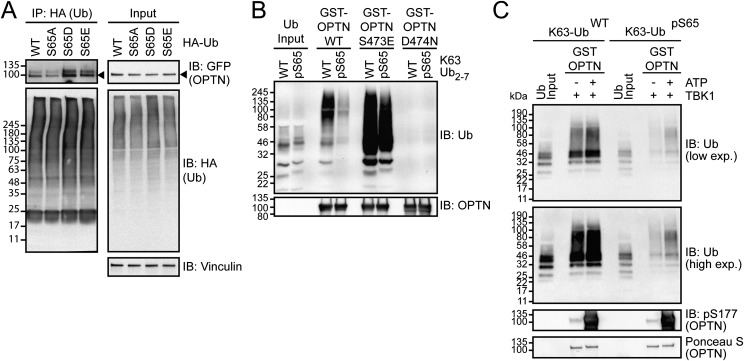
The protein kinase PINK1 phosphorylates S65 on Ub, which is critical for Parkin activation, and for recruitment of autophagy receptors OPTN and NDP52 to ubiquitinated mitochondria following their depolarization (7–14, 23). To investigate the binding behavior of OPTN to these phosphoUb species, we first expressed WT and phosphomimicking S65 Ub (S65E and S65D) together with OPTN in cells. Expression of phosphomimetic Ub led to an increase in coprecipitation with GFP–OPTN compared with WT or S65A mutant Ub (Fig. S5A) (13). However, the situation in vitro was different because phosphorylation of K63-linked Ub chains by PINK1 (stoichiometry of S65 phosphorylation of 0.5) reduced binding to GST–OPTN (Fig. S5B), which is consistent with previous findings when direct interactions were tested (31). Notably, phosphomimetic OPTN S473E and TBK1-phosphorylated OPTN could rescue the interaction and enabled moderate binding to pS65 Ub (Fig. S5 B and C). Although the reasons for the observed discrepancy in binding of OPTN to phosphorylated Ub are yet to be discovered, our findings indicate that phosphorylation of OPTN alone or in complex with other proteins may act as a mechanism to overcome the precluding effects of UBD binding to phosphorylated Ub in vivo.
Fig. S5.
Phosphorylation of Ub on Ser65 affects OPTN binding. (A) HEK293 cells were cotransfected with GFP–OPTN and HA–Ub WT or S65A, S65D, and S65E 30 h before lysis. After IP, with HA antibodies, samples were subjected to immunoblot (IB). (B and C) Pull-down of unphosphorylated or TcPINK1-phosphorylated recombinant K63-linked Ub chains and GST–OPTN variants. (C) GST–OPTN was preincubated with recombinant TBK1 for 30 min. Phosphorylation of OPTN S177 was confirmed by IB.
Functional Characterization of OPTN Phosphorylation in Mitophagy.
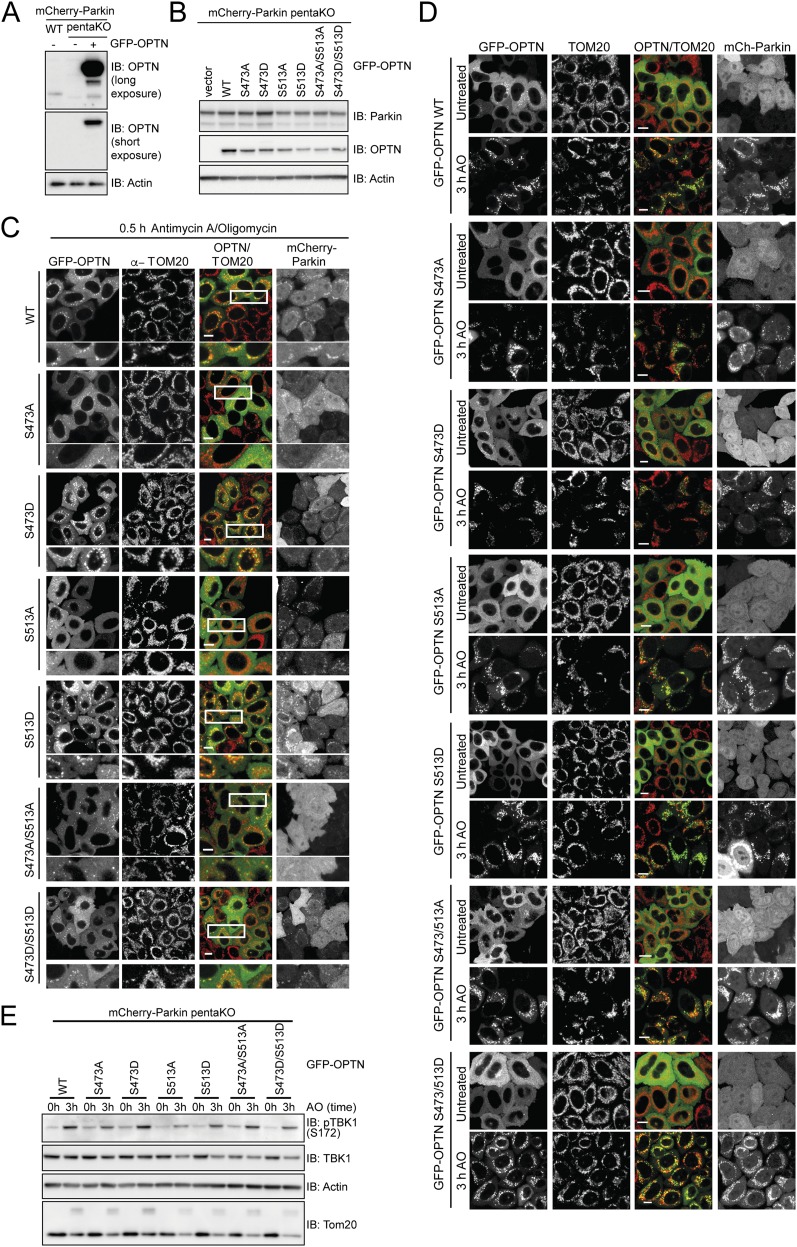
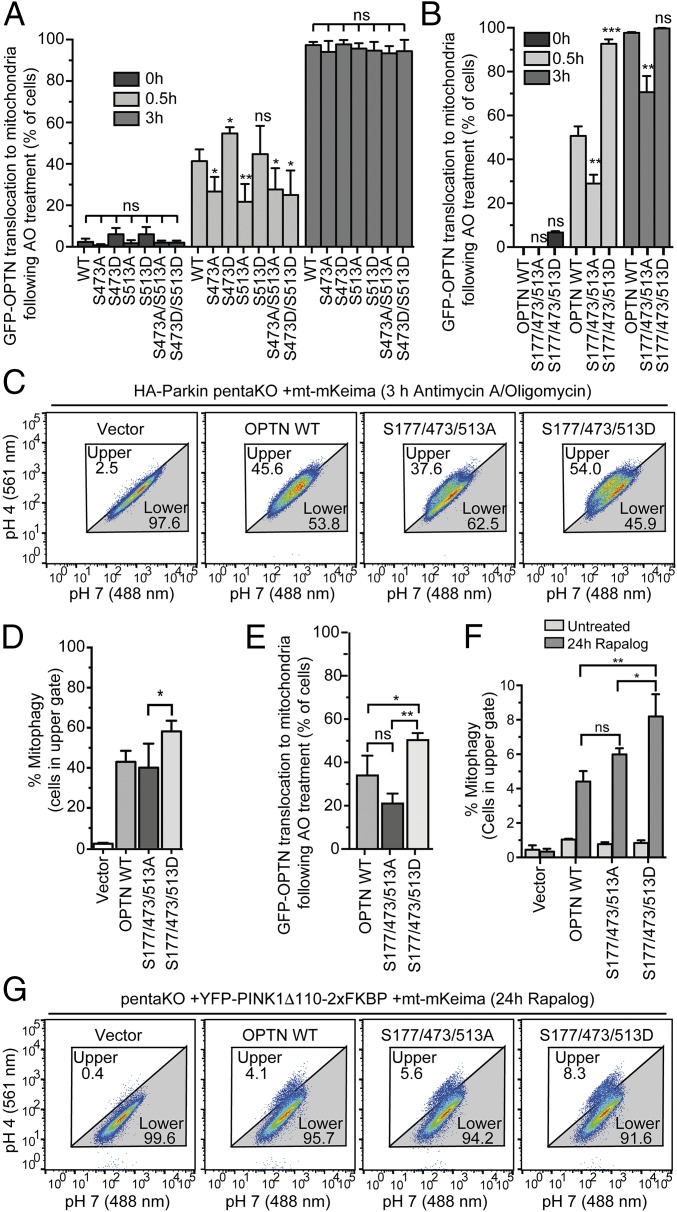
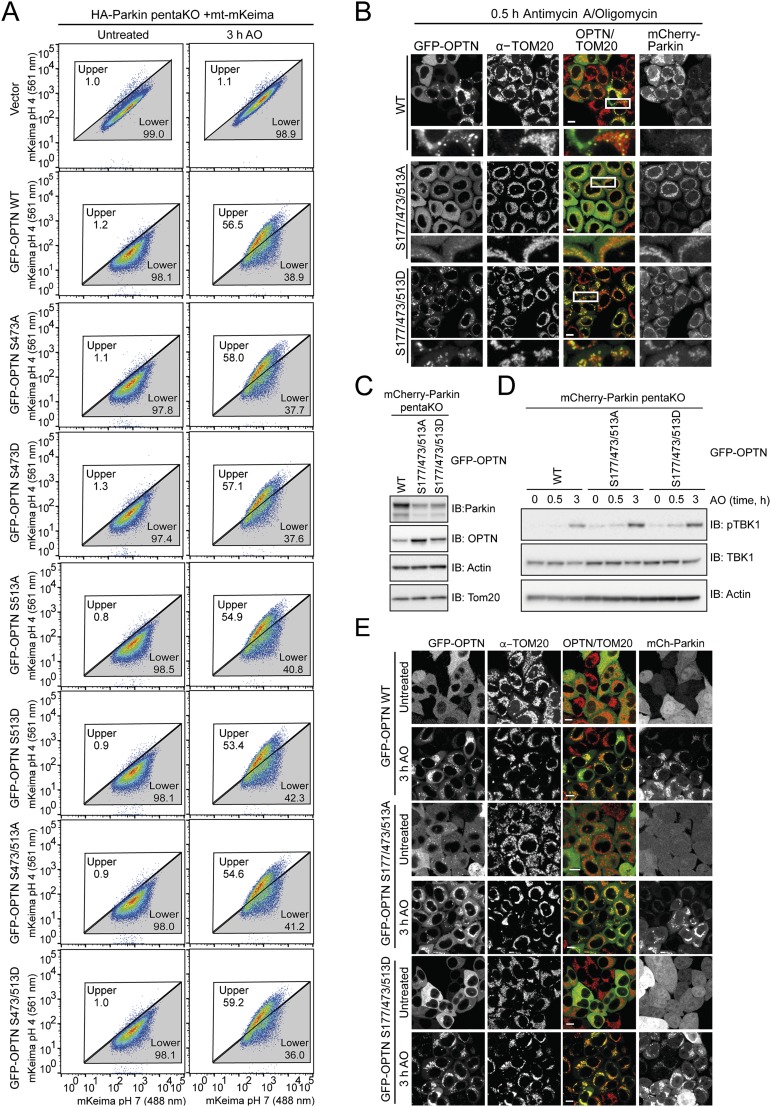
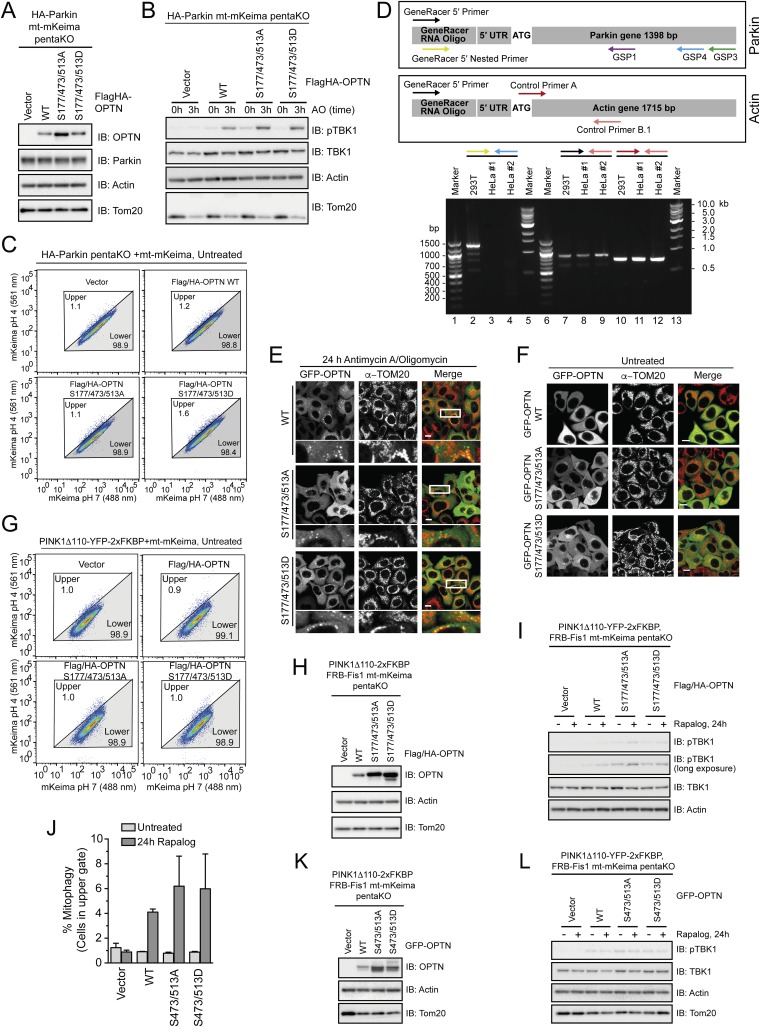
To test the functional consequence of TBK1-mediated phosphorylation of OPTN in mitophagy, pentaKO cells (HeLa cells engineered by CRISPR lacking NDP52, OPTN, TAX1BP1, NBR1, and p62) (13) were rescued with GFP–OPTN WT or mutants S473A, S513A, S473/S513A or phosphomimetics S473D, S513D, S473/S513D (Fig. S6 A and B). Following 0.5-h AO treatment, GFP–OPTN S473D translocated moderately better than WT, S513D translocated as well as WT, and the remaining mutants, GFP–OPTN S473A, S513A, S473/513A, and S473/513D translocated to mitochondria significantly slower than WT (Fig. 5A and Fig. S6C). After prolonged mitochondrial depolarization (3 h AO), WT and all mutants translocated to mitochondria, suggesting that UBAN phosphorylation may play important roles in early stages of mitophagy or in settings were ubiquitin is limited (Fig. 5A and Fig. S6D). TBK1 was activated to the same degree in these cells following AO treatment (Fig. S6E). We examined mitophagy using FACS analysis of mitochondrially targeted mKeima (mt-mKeima) (13, 32). The excitation spectrum of mKeima shifts when it is exposed to low pH, allowing measurement of the recruitment of mitochondria into lysosomes as a measure of mitophagy. Consistent with the lack of translocation defects at a later time point (Fig. 5A and Fig. S6D), all OPTN mutants could induce mitophagy to the same degree as OPTN WT (Fig. S7A).
Fig. S6.
OPTN single- and double-Ser mutations have limited effect on translocation and mitophagy. (A) Western blot of WT or PentaKO HeLa cells with or without stable expression of GFP–OPTN for OPTN expression levels. (B) Western blot of mCherry–Parkin-expressing pentaKO cells reconstituted with GFP–OPTN WT and indicated mutants as used in C and D and Fig. 5A. Western blot shows equal expression levels. (C and D) Representative images of mCherry–Parkin pentaKO cells expressing OPTN WT or OPTN mutants as indicated were untreated or treated with antimycin A or oligomycin (AO) for 0.5 h (C) and 3 h (D) and immunostained for TOM20. (E) Cells from B–D were treated with AO for the indicated times and lysates immunoblotted for pTBK1.
Fig. 5.
OPTN translocation to mitochondria and mitophagy are enhanced by OPTN phosphomimetic mutations. (A and B) mCherry–Parkin pentaKO cells expressing GFP–OPTN WT or mutants were treated with AO for 0.5 or 3 h and immunostained for TOM20. Quantification of cells with GFP–OPTN colocalized with TOM20. For representative images, see Figs. S6 C and D and S7 B and E. (C and D) HA-Parkin pentaKO cells expressing mt-mKeima and vector, Flag/HA–OPTN WT or mutants, as indicated, were treated with AO for 3 h and analyzed by FACS for lysosomal-positive mt-mKeima. (D) Graph depicting the average percent of cells in the upper gate from FACS analysis in C (n = 3). (E) PentaKO cells expressing GFP–OPTN WT or mutants were treated with AO for 24 h. For representative images, see Fig. S8 E and F. (F) Graph depicting the average percent of cells in the upper gate from FACS analysis of pentaKO cells expressing PINK1Δ110-YFP-2xFKBP, mt-mKeima and vector, Flag/HA–OPTN WT, or mutants, following treatment with rapalog for 24 h (n = 3). (G) Representative FACS data from rapalog-treated conditions from F are shown. For A, B, and E, 100 cells per condition were counted for n = 3 experiments. For A, B, D, E, and F, data are presented as mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.005; ns, not significant. For the untreated conditions from C and G, see Fig. S8 C and G, respectively.
Fig. S7.
Parkin-dependent OPTN translocation and mitophagy. (A) Representative FACS data from pentaKO cells stably expressing HA-Parkin, mt-mKeima, and GFP–OPTN WT or GFP–OPTN mutants as indicated were untreated or treated with AO for 3 h (n = 3 experiments). (B and E) Representative images of pentaKO cells expressing mCherry–Parkin and GFP–OPTN WT or mutants were untreated or treated with AO for 0.5 h (B) and 3 h (E) and immunostained with TOM20. (C) Western blot of mCherry–Parkin-expressing pentaKO cells reconstituted with GFP–OPTN WT and indicated mutants as used in B and E. (D) Cells as used in B, C, and E were treated with AO for times indicated then immunoblotted for pTBK1.
A third and highly abundant TBK1-dependent phosphorylation site on OPTN, pS177, was recently shown to be also important for mitophagy (13). OPTN S177A localized poorly to mitochondria and only weakly restored mitophagy in pentaKO cells (13), indicating that pS177 may stabilize OPTN on ubiquitinated mitochondria. In pentaKO cells, GFP–OPTN S177/473/513D translocated significantly faster to mitochondria following 0.5-h AO treatment compared with WT, whereas translocation of GFP–OPTN S177/473/513A was significantly reduced (Fig. 5B and Fig. S7 B–D). After 3-h AO treatment, GFP–OPTN WT and S177/473/513D translocated to mitochondria in nearly every cell, but GFP–OPTN S177/473/513A translocation was decreased (Fig. 5B and Fig. S7 C–E). Mitophagy in HA–Parkin-expressing pentaKO cells was also significantly enhanced by expression of Flag/HA–OPTN S177/473/513D (Fig. 5 C and D and Fig. S8 A–C). Taken together, these data support a stabilizing role for TBK1 phosphorylation at S177 and a recruitment function for phosphorylation at S473 and S513.
Fig. S8.
Parkin-dependent and -independent OPTN translocation and mitophagy. (A) Western blot of PentaKO cells expressing HA–Parkin, mt-mKeima, and vector or Flag/HA–OPTN WT or mutants for OPTN expression levels. (B) Cells as in A were treated with AO for times indicated, then immunoblotted for pTBK1. (C) Representative FACS data from untreated pentaKO cells expressing HA–Parkin, mt-mKeima, and vector or Flag/HA–OPTN WT or mutants. (D) Identification of 5′ cDNA ends of the Parkin gene by RLM-RACE using random hexamer primers and total RNA from both 293T (as positive control for Parkin expression; lanes 2, 7, and 10) and HeLa #1 [used in this study and authenticated by Johns Hopkins Genetic Resources Core Facility (GRCF) fragment analysis facility using short tandem repeat (STR) profiling; lanes 3, 8, and 11] and HeLa #2 cells (independent source; lanes 4, 9, and 12). Specific 5′ RACE PCR product using GeneRacer 5′ primer and Actin control primer B.1 (lanes 7–9 and Lower box) or internal primers for Actin (control primers A and B.1; lanes 10–12 and Lower box). GeneRacer 5′ primer and Parkin gene-specific primers (GSP1 or GSP3; Upper box) produce only faint smears without specific PCR products (data not shown). Specific 5′ RACE PCR product using GeneRacer 5′ nested primer and nested Parkin gene specific primer (GSP4; Upper box) from 293T (lane 2) but not HeLa cells (lanes 3 and 4). Multiple bands from 293T cells (lane 2) reflect splicing variants of Parkin. (E and F) Representative images from PentaKO cells expressing GFP-OPTN WT or mutants as indicated were (E) treated with AO for 24 h or (F) left untreated. Lower panels indicate an amplification of the regions contained within the white box. Scale bars, 10 μm. (G) Representative FACS data from untreated pentaKO cells expressing PINK1Δ110-YFP-2xFKBP, mt-mKeima, and vector or Flag/HA-OPTN WT or mutants as indicated. (H) Western blot of PentaKO cells expressing PINK1Δ110-YFP-2xFKBP, mt-mKeima, and vector or Flag/HA-OPTN WT or mutants for OPTN expression levels. (I) Cells as in G and Fig. 5 F and G were treated with Rapalog for 24 h then immunoblotted for pTBK1. (J) Graph depicting the average percent of cells in the upper gate from FACS analysis of pentaKO cells expressing PINK1Δ110-YFP-2xFKBP, mt mKeima, and vector, GFP-OPTN WT or mutants, following treatment with Rapalog for 24 h for n = 2 experiments. (K) Western blot of cells from J for OPTN expression levels. (L) Cells as in J and K were treated with Rapalog for 24 h then immunoblotted for pTBK1.
To test if phosphomimetic OPTN is interacting with phosphorylated ubiquitin on mitochondria and not just unmodified ubiquitin added via Parkin activity, we studied OPTN translocation in cells lacking Parkin expression. A previous study has shown that HeLa cells produce a truncated Parkin transcript lacking the 5′-end (exons 1–6) (33). We investigated this issue in more detail by identifying 5′ cDNA ends of the Parkin gene in HeLa cells using RLM-RACE. Specific PCR products of expected sizes were produced from 293T cDNA but not two HeLa cDNA samples (Fig. S8D), demonstrating that HeLa cells are truly null for Parkin at the transcriptional level. Thus, GFP–OPTN WT translocated to mitochondria in 32% of pentaKO HeLa cells following 24-h AO (Fig. 5E and Fig. S8 E and F), in strong contrast to Parkin-expressing cells, where GFP–OPTN translocates in nearly 100% of cells after 3-h AO (Fig. 5B and Fig. S7E), consistent with the model that PINK1 can directly recruit OPTN to mitochondria and that Parkin amplifies this pathway via ubiquitination. GFP–OPTN S177/473/513D translocated significantly more than both WT and S177/473/513A (Fig. 5E and Fig. S8 E and F), suggesting that phosphoUb preferentially recruits phosphomimetic OPTN relative to OPTN WT or nonphosphorylatable OPTN.
To study the effect of phosphomimetic OPTN on PINK1-driven, Parkin-independent mitophagy, we used an inducible PINK1 dimerization system. Here, PINK1 lacking its N-terminal membrane-targeting domain is fused to YFP and two tandem FKBP domains (PINK1-Δ110-YFP-2XFKBP). By the addition of rapalog, PINK1 is targeted to mitochondria that are stably expressing FKBP12/rapamycin-binding (FRB) fused to Fis1 (FRB–Fis1) (34); this allows PINK1, in the absence of Parkin and mitochondrial membrane depolarization, to phosphorylate mitochondrial ubiquitin to recruit autophagy receptors (13). Following rapalog treatment for 24 h, Flag-HA–OPTN WT induced mitophagy in 4.1% of cells, 10-fold more than without OPTN (vector, 0.4% of cells) and fivefold more than untreated conditions (0.9% of cells), consistent with previous reports (Fig. 5 F and G and Fig. S8 G–I) (13). Flag-HA OPTN S177/473/513A could rescue similar to OPTN WT with 5.6% of cells undergoing mitophagy, likely because TBK1 is not activated by rapalog to mediate phosphorylation of OPTN (Fig. S8I). Rescue with Flag-HA–OPTN S177/473/513D significantly increased mitophagy, to 8.3%, nearly doubling the mitophagy induced by OPTN WT (Fig. 5 F and G). GFP–OPTN S473/S513A and S473/513D rescued mitophagy similar to OPTN WT (Fig. S8 J–L), which again points toward a role for S473 and S513 phosphorylation at early steps in the process and highlights an important stabilizing function for S177.
Discussion
In the present study we have systematically analyzed the activity of TBK1 toward four major autophagy receptors. We found that TBK1 directly phosphorylates several autophagy-relevant sites in all four receptors, highlighting that TBK1 functions as a key player in the control of diverse selective autophagy pathways (5).
In line with two recent reports (13, 23), we confirmed that TBK1 is activated upon depolarization of mitochondria, an effect that requires the upstream kinase PINK1 and is further facilitated by the expression of Parkin. In addition, activation and mitochondrial localization of TBK1 depends on its interaction with OPTN and the ability of OPTN to bind to Ub on mitochondria (23). In turn, OPTN’s function during mitophagy depends on its phosphorylation by activated TBK1, as demonstrated by a reduction in mitochondrial localization and mitophagy upon expression of nonphosphorylatable OPTN mutants. Both of these steps, TBK1 activation as well as OPTN recruitment and phosphorylation, seem to have a functional consequence for clearance of damaged mitochondria and cytosolic bacteria via feed-backward and feed-forward mechanisms (5).
Unphosphorylated OPTN was shown to interact primarily with M1-linked, and weaker with K63-linked, Ub chains (15, 26). In contrast TBK1-mediated phosphorylation of OPTN S473 or phosphomimetic S473D/E mutants resulted in increased binding and promiscuous interactions with multiple Lys-linked chain types (Fig. 2). Such a change in specificity can contribute to multivalent interaction and avidity-based increase in the residence time at the surface of ubiquitinated cargoes (Fig. 2E), indicative of an amplification loop rather than an on/off mechanism; this is also implicated in distinct stages of mitophagy, e.g., phosphorylation of OPTN appears to be critical in early steps of mitophagy, whereas in later steps OPTN phosphorylation is dispensable (Fig. 5). Moreover, only a subpopulation of OPTN can be modified on a specific site by locally enriched TBK1. Consistent with previous findings (23), we also show that the abundance of phosphorylated OPTN S473 is low if compared with S177 and S513 (Fig. 1), suggesting that its local enrichment in specific microdomains on mitochondria (23, 28) can determine the functional significance in vivo. Taken together, we propose that TBK1-mediated phosphorylation of OPTN is locally restricted to mitochondria and results in diversification of the binding spectrum of its UBAN domain toward distinct Ub chains, which in turn regulates its resident time on mitochondria and its function as an autophagy receptor (5).
In response to mitochondrial depolarization, PINK1 and Parkin establish a positive feedback loop that results in the robust decoration of damaged mitochondria with Ub chains that contain pS65 Ub (7–14). All tested autophagy receptors have been shown to translocate to damaged mitochondria; however, only OPTN and NDP52 seem to be required for efficient mitophagy in HeLa cells. Notably, whereas PINK1-mediated phosphorylation of Ub S65 increases the recruitment of autophagy receptors to damaged mitochondria in cells (Fig. 5) (13, 23), in vitro binding assays have shown opposite effects (Fig. S5) (23, 31). This obvious discrepancy can have several reasons. It could be that in vitro phosphorylated Ub chains show a different pattern of phosphorylation that does not match the in vivo situation. In fact, up to 20% of mitochondrial Ub is phosphorylated on S65 upon mitochondrial damage (7) and it is likely that Ub chains exposed to autophagy receptors consist of a mixture of pS65 Ub and unphosphorylated Ub. In this respect, if the effects seen in vitro apply to the situation in vivo, UBDs would favor unmodified Ub instead of pS65 Ub, and thereby preventing a competition with Parkin for pS65 Ub binding. However, TBK1 activation can result in phosphorylation of the UBAN domain and enhanced binding of OPTN to available S65 phosphorylated and unphosphorylated Ub chains that when coupled to TBK1-mediated phosphorylation of the LIR domain of OPTN supports early steps in mitophagy by stabilizing autophagic membranes on ubiquitinated cargo.
Previous studies identified mutations of OPTN, TBK1, and p62/SQSTM1 in patients with ALS–FTLD, an aggressive neurodegeneration characterized by the loss of upper and lower motor neurons, leading to rapid muscle weakness, paralysis, and death (19–22, 35). However, the spectrum of in vivo targets of autophagy in motor neurons remains unclear. Among possible targets are mutated superoxide dismutase 1 (SOD1) (36), the RNA-processing TAR DNA-binding protein 43 (TDP-43), fused in sarcoma (FUS), and mitochondria (13, 23). In a SOD1 mutant ALS mouse model, morphological abnormalities of mitochondria appeared before the onset of neurodegenerative symptoms, indicating a role for mitochondria in disease initiation (37). In cultured cells, we and others have shown that ALS-associated mutations in either TBK1 (E696K, abolishes OPTN binding) or OPTN (E478G, abolishes Ub binding) blocked mitochondrial translocation and activation of TBK1 resulting in impaired mitophagy (13, 23, 28). More studies are needed to dissect the contribution of selective autophagy pathways in the pathogenesis of ALS and other neurodegenerative diseases like Parkinson’s disease (PD) where mutations in PINK1 and Parkin are causative to the development of disease. It remains intriguing that mutations in the same selective mitophagy pathway (PINK1-Parkin-OPTN-TBK1) have been genetically segregated to ALS (mutations in OPTN and TBK1) and to PD (PINK1 and Parkin), yet they share common principles of signal transduction, whereby kinases PINK1 and TBK1 initiate two independent amplification loops amplifying mitophagy by phosphorylating Ub and Ub receptors, respectively.
Materials and Methods
SILAC-IP and Phosphopeptide Identification.
Lysates of SILAC-labeled HEK293T cells expressing GFP-tagged receptors were combined and incubated with GFP-Trap beads (ChromoTek) for 1 h, followed by washes under denaturing conditions (8 M Urea, 1% SDS in 1× PBS). Proteins were subjected to gradient SDS/PAGE gels followed by tryptic In-Gel digest. Extracted peptides were desalted and finally analyzed on a Hybrid Quadrupole-Orbitrap mass spectrometer. For details, see SI Materials and Methods.
Protein Expression and Purification.
GST-fusion proteins were generated as described (15) with modifications. pSer473 OPTN was purified as described (27) with modifications. TBK1 was obtained from Merck Millipore. For details, see SI Materials and Methods.
FRAP Assay.
GST–diUb bound to Glutathione Sepharose beads was incubated with purified mCherry–OPTN for 1 h. Subsequently, beads and proteins were dispensed in a 96-well plate for imaging with a spinning disk microscope. Three FRAP curves were generated for each experiment (n = 2) and sample. For details, see SI Materials and Methods.
Cell Culture, Cell Line Generation, and Knockdown.
Inducible HA-Parkin HeLa cells were generated as described (38). OPTN KO and PentaKO HeLa cells were generated using CRISPR/Cas9 as described in refs. 39 and 13, respectively. For siRNA treatments, cells were reverse transfected with siRNAs against PINK1 or control using Lipofectamine RNAiMAX before treatment with AO. For details, see SI Materials and Methods.
Immunoprecipitation, Pull-Down, and Immunoblotting.
Immunoprecipitation, pull-down, and immunoblotting were carried out as described (15) with modifications. For details and a listing of all antibodies, see SI Materials and Methods.
Mitochondrial Translocation Assays.
GFP–OPTN translocation to mitochondria in pentaKO cells was assayed by immunofluorescence as described (13). PentaKO cells were treated with AO for times indicated and immunostained for TOM20. For details, see SI Materials and Methods.
Mt-mKeima Mitophagy Assay.
Parkin-dependent and -independent mt-mKeima assays were performed by using FACS analysis as described in refs. 13 and 34, respectively. For details, see SI Materials and Methods.
SI Materials and Methods
Cell Culture and Stable Cell Line Generation.
HEK293T and HeLa cells were obtained from ATCC. SH-SY5Y cells were kindly provided by J. H. Weishaupt, Neurology Department, Ulm University, Ulm, Germany, and the HeLa FRT/TO cells for the generation of stable cell lines using the Flp-In T-REx System (Invitrogen) were generously provided by S. Taylor, Faculty of Life Sciences, University of Manchester, Manchester, United Kingdom, and maintained as described (39). PentaKO HeLa cells were generated as described in ref. 13. All cells were cultured in DMEM supplemented with 10% (vol/vol) FBS, penicillin, and streptomycin. For SILAC labeling, cells were maintained in custom-made SILAC DMEM (heavy/medium/light) for 4–5 passages or 10 d, and incorporation of labeled amino acids to more the 95% was verified. All cells were cultured at 37 °C in a humidified incubator containing 5% CO2. Hygromycin B was purchased from Enzo Life Sciences (ALX-380-306-G001) and Blasticidin S from Thermo Fisher Scientific (R210-01). For transient overexpression, DNA plasmid transfections were performed with GeneJuice (Merck Millipore) following the manufacturer instructions. siRNAs were transfected with Lipofectamine RNAiMAX (Invitrogen) following the manufacturer instructions. For the generation of inducible Parkin cells, HA-Strep-Strep (HSS)–Parkin was cloned into the pcDNA5/FRT/TO vector (Invitrogen) and cotransfected with Flp-recombinase pOG44 into tetracycline transactivator HeLa cells. Hygromycin B-resistant colonies were pooled and expanded. Parkin expression was induced by 1 μg/mL Dox (D9891; Sigma-Aldrich). Protein phosphatases were inhibited by addition of 100 nM calyculin A (no. 9902; Cell Signaling Technology) to the cell culture media for 0.5 h if indicated.
SILAC-IPs.
GFP-tagged OPTN, NDP52, p62, and TAX1BP1 were transfected in HEK293T cells. At 24 h posttransfection, cells were lysed in lysis buffer (50 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 0.5% Triton) supplemented with protease (P5726, P0044; Sigma-Aldrich) and phosphatase inhibitors (cOmplete, EDTA-free; Roche Diagnostics). The lysates from different SILAC states were combined before the pull-down. A total of 20 µL of pre-equilibrated GFP-Trap_A beads (ChromoTek GmbH) were added to the cleared lysate and incubated for 1 h at 4 °C on a rotation wheel. The beads were washed once with buffer containing 10 mM Tris⋅HCl, pH 7.5, 150 mM NaCl, 0.5 mM EDTA, protease, and phosphatase inhibitors, followed by 3× washing with buffer containing 8 M Urea, 1% SDS in 1× PBS, and once with 1% SDS in 1× PBS . Bound proteins were eluted in NuPAGE LDS Sample Buffer (Life Technologies) supplemented with 1 mM DTT, boiled at 70 °C for 10 min, alkylated by addition of 5.5 mM chloroacetamide for 30 min, and loaded onto 4–12% gradient SDS/PAGE gels. Proteins were stained using the Colloidal Blue Staining Kit (Life Technologies) and digested in-gel using trypsin. Peptides were extracted from gel and desalted on reversed phase C18 StageTips.
MS Analysis.
Peptide fractions were analyzed on a quadrupole-Orbitrap mass spectrometer (Q Exactive Plus; Thermo Scientific) equipped with a ultrahigh-performance liquid chromatography (UHPLC) system (EASY-nLC 1000; Thermo Scientific) as described (41). Peptide samples were loaded onto C18 reversed-phase columns (15 cm length, 75 µm inner diameter, 1.9 µm bead size) and eluted with a linear gradient from 8% to 40% acetonitrile containing 0.1% formic acid in 2 h. The mass spectrometer was operated in data-dependent mode, automatically switching between MS and MS2 acquisition. Survey full-scan MS spectra (m/z 300–1,700) were acquired in the Orbitrap. The 10 most intense ions were sequentially isolated and fragmented by higher-energy C-trap dissociation (HCD). An ion selection threshold of 5,000 was used. Peptides with unassigned charge states, as well as with charge states less than +2, were excluded from fragmentation. Fragment spectra were acquired in the Orbitrap mass analyzer.
Peptide Identification.
Raw data files were analyzed using MaxQuant (version 1.5.2.8) (42). Parent ion and MS2 spectra were searched against a database containing 88,473 human protein sequences human protein sequences obtained from the UniProtKB released in December 2013 using Andromeda search engine (43). Spectra were searched with a mass tolerance of 6 ppm in MS mode, 20 ppm in HCD MS2 mode, strict trypsin specificity and allowing up to three miscleavages. Cysteine carbamidomethylation was searched as a fixed modification, and protein N-terminal acetylation, methionine oxidation, phosphorylation of serines, threonines, and tyrosines as variable modifications. Site localization probabilities were determined by MaxQuant using the PTM scoring algorithm as described previously. The dataset was filtered based on posterior error probability to arrive at a false discovery rate of below 1% estimated using a target-decoy approach.
Immunoblotting and Antibodies.
For immunoblotting, proteins were resolved by SDS/PAGE and transferred to NitroBind 0.45-μM nitrocellulose membrane (1215471; Maine Manufacturing, LCC). Blocking and primary antibody incubations were carried out in 5% (wt/vol) BSA (Roth) in TBS (150 mM NaCl, 20 mM Tris, pH 8.0) or 5% (wt/vol) low-fat milk (Roth) in TBS-Tween (150 mM NaCl, 20 mM Tris, pH 8.0, and 0.1% Tween-20) and washings in TBS-Tween. Secondary antibody incubations were carried out in 5% (wt/vol) low-fat milk (Roth) in TBS-Tween. Blots were developed using Western Blotting Luminol Reagent (sc-2048; Santa Cruz). The following antibodies were used in this study: anti-ubiquitin FK2 (BML-PW8810; Enzo Life Science), anti-ubiquitin P4D1 (sc-8017; Santa Cruz), anti-ubiquitin Ubi-1 (13-1600; Invitrogen), anti-FlagM2 (F3165; Sigma-Aldrich), anti-HA (MMS-101P; Covance), anti-Myc 9E10 (sc-40; Santa Cruz), anti-GFP (Living Colors 632592; Clontech), anti-GST (sc-138; Santa Cruz), anti-V5 (46-0705; Invitrogen), anti-Parkin (sc-32282; Santa Cruz), anti-PINK1 (no. 9646; Cell Signaling Technology), anti-TBK1 (no. 3013; Cell Signaling Technology), anti-pTBK1 (pS172; no. 5483; Cell Signaling Technology) and anti-TOM20 (sc-11415; Santa Cruz). The pS177 OPTN antibody was generated by immunoGlobe GmbH as described previously (15). Secondary HRP conjugated antibodies, goat anti-mouse (sc-2031; Santa Cruz), and goat anti-rabbit (sc-2030; Santa Cruz) IgGs were used for immunoblotting. Donkey anti-mouse Cy3- and Cy5-conjugated secondary antibody (Jackson ImmunoResearch) or Alexa Fluor 633 goat anti-rabbit (A21071; Life Technologies) were used for immunofluorescence studies.
Complementary DNAs, siRNAs, and Guide RNAs.
Site-directed mutagenesis was performed by PCR to introduce desired amino acid substitutions and mutations into the cDNA constructs. DNA sequences were sequenced by Eurofins Genomics GmbH and Seqlab. V5-tagged Tank, Nap1 and Sintbad expression plasmids were kindly provided by G. Superti-Furga, Research Center for Molecular Medicine of the Austrian Academy of Sciences (CeMM), Vienna, Austria, and generated as described (30). siRNAs targeting human PINK1 (siPINK1-1 5′-GCCAUCUUGAACACAAUGA-3′ and siPINK1-2 5′-CCUCGUUAUGAAGAACUAU-3′) were purchased from Eurofins Genomics. For siRNA treatments, inducible HSS-Parkin HeLa cells in six-well plates were transfected with 20 nM of each siRNA for 48 h before treatment with AO. Penta knockout (PentaKO) HeLa cells were generated as described (13). OPTN knockout HeLa FRT/TO cells were generated using the CRISPR/Cas9 nickase (Cas9 D10A) system as previously described (40): two guide RNAs (TGCTCTGTATCTCAAAAAAC and AGAAGCAAAAGAGCGTCTAA) targeting exon 5 (ensemble exon ID: ENSE00003722976) were each cloned into plasmid PX462 (Addgene 48141; pSpCas9n(BB)-2A-Puro) and cotransfected into HeLa FRT/TO cells. At 24 h after transfection, transfected cells were selected with 1 μg/mL puromycin for 2–3 d before seeding into 96-well plates. Single colonies were expanded and initially screened by immunoblotting. Next, genomic DNA was isolated and the targeted region was amplified using PCR (forward primer: 5′-ACCACTTCGTCTTTTTGCTGC-3′, reverse primer: 5′-ACTTCTTCCAAGACCAGGCAA-3′). CRISPR/Cas9-mediated insertion and deletion mutations were identified using a PAGE-based genotyping method as previously described (43).
Protein Expression, Purification, and Phosphorylation.
GST and MBP fusion proteins were cloned into pGEX-4T-1 (GE Life Sciences) and pMAL-c2x (New England BioLabs), respectively and expressed in E. coli strain BL21 (DE3). Bacteria were cultured in LB medium supplemented with 100 µg/mL ampicillin and 0.25 mM ZnSO4 at 37 °C in a shaking incubator (150 rpm) until OD600 ∼0.5–0.6. For the expression of MBP fusions, 0.2% (wt/vol) glucose was supplemented. Expression was induced by the addition of 0.2 mM IPTG and cells were incubated at 16 °C for additional 20–24 h. Bacteria were harvested by centrifugation (4.000 × g) and lysed by sonication in GST lysis buffer (20 mM Tris⋅HCl, pH 7.5, 10 mM EDTA, pH 8.0, 5 mM EGTA, 150 mM NaCl, 0,1% β-mercaptoethanol, and 1 mM PMSF). Lysates were cleared by centrifugation (10.000 × g for 20 min) and immediately applied to glutathione Sepharose 4B beads (GE Life Sciences) or amylose resin (New England BioLabs). After several washes in GST wash buffer (20 mM Tris⋅HCl, pH 7.5, 10 mM EDTA, pH 8.0, 150 mM NaCl, 0,5% Triton X-100, 0,1% β-mercaptoethanol, and 1 mM PMSF), immobilized proteins were reconstituted in GST storage buffer (20 mM Tris⋅HCl, pH 7.5, 0.1% NaN3 and 0.1% β-mercaptoethanol). K63-linked ubiquitin chains were produced as described (25). Recombinant MBP-TcPink, Ser65 phosphorylated K63-linked and M1-linked Ub chains were a gift from Wade Harper, Department of Cell Biology, Harvard Medical School, Boston, and generated as described previously (23). Recombinant phospho-Ser473 OPTN was purified as described with modifications (27): First, amber stop codon (TAG) was introduced at the position of phosphoserine (Sep) incorporation in GST–OPTN (pGEX4T1). Plasmids pGEX4T1-GST-OPTN and B40 OTS (pKD-SepRS-EFSep-5xtRNASep; Addgene 52054) were transformed simultaneously into E. coli strain EcAR7 (Addgene 52055). Transformed bacteria were cultured in LB medium supplemented with 100 µg/mL ampicillin and 25 µg/mL kanamycin, 0.25 mM ZnSO4, 0,08% glucose, and 2 mM phosphoserine (P0878; Sigma-Aldrich) at 30 °C in a shaking incubator (150 rpm) until OD600 ∼0.5–0.6. Sep incorporation and protein expression was induced with 1 mM IPTG, and incubation was continued at 30 °C overnight. Bacteria were harvested by centrifugation (4.000 × g) and GST–OPTN purified as described above.
GST Pull-Down.
For GST pull-down assays using HEK293T cell lysates, cells were transiently transfected with plasmids encoding the indicated proteins using GeneJuice (Merck Millipore) transfection reagent. At 24–48 h after transfection, cells were harvested, washed with ice-cold 1× PBS, and lysed in lysis buffer (50 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 25 mM NaF) supplemented with protease inhibitors (PMSF, aprotinin, leupeptin), and cleared by centrifugation. For GST pull-down assays using recombinant ubiquitin chains, GST fusions and 0.5 µg ubiquitin chains were combined in 500-µL pull-down buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 0.1% Nonidet P-40, supplemented with 5 mM DTT and 0.25 mg/mL BSA). Lysates or recombinant proteins were incubated for at least 5–6 h with immobilized GST fusion proteins. Following four washes with buffer, proteins were resolved by SDS/PAGE and analyzed by immunoblotting with the indicated antibodies.
In Vitro Phosphorylation Assay.
Immobilized GST fusion proteins were washed with kinase buffer (20 mM Hepes, pH 7.5, 50 mM NaCl, 10 mM MgCl2) prior to incubation with 50 ng of recombinant TBK1 (14-628; Merck Millipore) in 30 µL kinase buffer supplemented with 20 mM β-glycerophosphate, 1 mM DTT, and 0.1 mM NaVO3 in the presence or absence of 200 µM ATP for 30 min at 30 °C. The reaction was stopped with washes in pull-down buffer (to remove free ATP). Phosphorylated GST fusions were immediately used for GST pull-down assays with recombinant ubiquitin. Phosphorylation of GST–OPTN was verified by immunoblotting using the indicated phosphospecific antibodies.
Immunoprecipitation.
HEK293T or HeLa cells transiently expressing bait proteins were lysed in lysis buffer supplemented with protease and phosphatase inhibitors. Lysates were cleared by centrifugation and incubated with pre-equilibrated GFP-Trap_A agarose (ChromoTek GmbH) or anti-FlagM2 (IgG) affinity gel (A2220; Sigma-Aldrich) for 1 h under rotation at 4 °C. Precipitated proteins were washed with lysis buffer and eluted with Laemmli buffer and resolved by SDS/PAGE and detected by immunoblotting using chemiluminescence-based detection.
AO and CCCP Treatments.
For the induction of Pink1-dependent mitophagy, cells were either left untreated or treated for the indicated time points with 10 μM Antimycin A (A8674; Sigma-Aldrich) and 5 μM oligomycin A (75351; Sigma-Aldrich) and/or 40 μM CCCP (Sigma-Aldrich). Before treatment of inducible HSS-Parkin HeLa cells with AO and/or CCCP, Parkin expression was induced by 1 μg/mL doxycycline for 12–24 h. At the indicated time points, cells were washed with ice-cold 1× PBS and lysed with lysis buffer supplemented with protease and phosphatase inhibitors. Lysates were cleared by centrifugation, and proteins were eluted with Laemmli buffer and resolved by SDS/PAGE and finally analyzed by immunoblotting using chemiluminescence-based detection.
Microscope Imaging.
For visualization of TBK1 recruitment to mitochondria, HSS-Parkin HeLa FRT/TO cells were plated on glass coverslips. Cells were transfected with 1 µg of DNA 48 h before treatment, and Parkin expression was induced by adding 1 μg/mL doxycyclin to the media. For induction of mitophagy, cells were treated with 40 μM CCCP for 105 min followed by fixation in 4% (wt/vol) paraformaldehyde for 5 min. At 40 min before fixation, MitoTracker far red (Life Technologies) was added to the media. Coverslips were mounted on 10 µL of Prolonged Diamond mountant containing DAPI (Molecular Probes) and placed on a glass holder. Images were acquired by the TCP SP8 laser-scanning microscope (Leica) using a 63× oil-immersion lens. Image analysis was performed using ImageJ.
For translocation of GFP–OPTN WT or mutants, pentaKO cells stably expressing OPTN and/or Parkin as indicated were plated on two-well chamber slides (Lab-Tek) then treated with 10 μM antimycin A, 10 mg/mL oligomycin, and 20 μM Q-VD for 0.5, 3, or 24 h. After treatment, cells were fixed in 4% (wt/vol) paraformaldehyde for 20 min. Cells were permeabilized and blocked with 0.1% Triton ×100, 3% (vol/vol) goat serum in PBS for 45 min. Anti-TOM20 was diluted in 3% (vol/vol) goat serum and incubated for 1 h at room temperature. Cells were rinsed with PBS and then incubated with Alexa Fluor 633-conjugated secondary antibody. Cells were counted manually in a blinded fashion for colocalization of OPTN puncta with TOM20 immunostaining. Cells were binned as positive for translocation if any OPTN was colocalized to TOM20, and 100 cells per sample were counted for three independent experiments.
Mt-Keima Mitophagy Assay.
pentaKO HeLa cells stably expressing mt-mKeima [a gift from A. Miyawaki, Laboratory for Cell Function and Dynamics, Brain Science Institute (BSI), RIKEN, Japan], Pink1Δ110-YFP-2XFKBP, FRB-Fis1 (in the Parkin-independent mitophagy assay only), and either GFP- and Flag/HA–OPTN WT or mutants were generated as described in ref. 13. For mitophagy, cells were treated with 10 μM antimycin A, 10 mg/mL oligomycin, and 20 μM Q-VD for 3 h or 0.5 mM rapalog for 24 h. Cells were collected in cell-sorting media (145 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 10 mM Hepes, 10 mM glucose, 0.1% BSA) containing 10 mg/mL DAPI. Mitophagy analysis was performed on a Beckman Coulter MoFlo Astrios cell sorter using Summit software (v6.2.6.16198). Measurements of lysosomal mt-mKeima were made using dual-excitation ratiometric pH measurements at 488 (pH 7)- and 561 (pH 4)-nm lasers with 620/29-nm and 614/20-nm emission filters, respectively. For each sample, 50,000 events were collected and subsequently gated for YFP/mt-mKeima double-positive cells that were DAPI negative. Data were analyzed using FlowJo (v10; Tree Star).
FRAP Assay.
For each sample, 20 µL dry Glutathione Sepharose beads 4B (GE Healthcare) were equilibrated twice with SEC buffer (25 mM Hepes, pH 7.5, 150 mM NaCl, 1 mM DTT). Beads were incubated with 30 µg of purified GST-2xUb for 30 min at 4 °C, washed once with buffer, and incubated with 100 µL of purified mCherry–OPTN WT or S473E at 2 µM in SEC buffer for 1 h at 4 °C. Immediately before imaging, 7.5-µL beads suspension were diluted in 50 µL of respective OPTN solution and dispensed in a 96-well plate well for imaging. Imaging was performed with a spinning disk microscope (Visitron), using a 63× oil-immersion lens.
RLM-RACE.
Total RNAs from 293T and HeLa cells were isolated with Qiagen’s RNeasy Plus mini kit that removes genomic DNA contamination. Rapid amplification of cDNA ends (5′ RACE) was conducted with Invitrogen’s GeneRacer Kit with SuperScript III RT (cat# L150201). Total RNA from 293T and HeLa cells (each 5 μg) and total RNA from HeLa cells provided by the kit (1 μg) were used for cDNA synthesis. After checking actin levels in these cDNA samples, various amount of cDNA were used for 5′ RACE PCR to achieve equal actin level and the adjusted amount of cDNAs were then used for Parkin 5′ RACE PCR. The following primers were used for Parkin 5′ RACE PCR: Parkin-GSP1 (GGCTCCTGACGTCTGTGCACGTAAT, 715–739, inside exon 6), Parkin-GSP3 (CTTCATGTGCATGCAGCCTCCATTT, 1281–1305, inside exon 12), Parkin-GSP4 (CGGCGGCTCTTTCATCGACTCTGTA, 1171–1195, inside exon 12).
Acknowledgments
We thank Simin Rahighi and Masato Akutsu for helpful discussions; Evgenij Fiskin for the generation of HA-Parkin cells; Wade J. Harper and Alban Ordureau for purified pS65 Ub chains; Patricia Boya for mitophagy assays in mouse embryonic fibroblasts (MEFs); Giulio Superti-Furga for complementary DNA constructs; Jochen H. Weishaupt and Stephen Taylor for cells; and Dragan Maric and the National Institute of Neurological Disorders and Stroke (NINDS) Flow Cytometry Core for FACS. This work was supported by Deutsche Forschungsgemeinschaft Grant DI 931/3-1 (to I.D.); the Cluster of Excellence “Macromolecular Complexes” of the Goethe University Frankfurt (EXC115; to I.D.); LOEWE Grant Ub-Net (to I.D.) and LOEWE Centrum for Gene and Cell Therapy Frankfurt (I.D. and B.R.); the NIH-NINDS intramural program (R.J.Y., D.A.S., and C.W.); and an European Molecular Biology Organization (EMBO) long-term postdoctoral fellowship (to L.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1523926113/-/DCSupplemental.
References
- 1.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34(3):259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16(6):495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 5.Herhaus L, Dikic I. Expanding the ubiquitin code through post-translational modification. EMBO Rep. 2015;16(9):1071–1083. doi: 10.15252/embr.201540891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ordureau A, et al. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014;56(3):360–375. doi: 10.1016/j.molcel.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kane LA, et al. PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J Cell Biol. 2014;205(2):143–153. doi: 10.1083/jcb.201402104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kazlauskaite A, et al. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014;460(1):127–139. doi: 10.1042/BJ20140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koyano F, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510(7503):162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- 11.Wauer T, et al. Ubiquitin Ser65 phosphorylation affects ubiquitin structure, chain assembly and hydrolysis. EMBO J. 2015;34(3):307–325. doi: 10.15252/embj.201489847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swaney DL, Rodríguez-Mias RA, Villén J. Phosphorylation of ubiquitin at Ser65 affects its polymerization, targets, and proteome-wide turnover. EMBO Rep. 2015;16(9):1131–1144. doi: 10.15252/embr.201540298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarou M, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazlauskaite A, et al. Binding to serine 65-phosphorylated ubiquitin primes Parkin for optimal PINK1-dependent phosphorylation and activation. EMBO Rep. 2015;16(8):939–954. doi: 10.15252/embr.201540352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild P, et al. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333(6039):228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol Cell. 2011;44(2):279–289. doi: 10.1016/j.molcel.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 17.Pilli M, et al. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity. 2012;37(2):223–234. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helgason E, Phung QT, Dueber EC. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: The emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett. 2013;587(8):1230–1237. doi: 10.1016/j.febslet.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Freischmidt A, et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat Neurosci. 2015;18(5):631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 21.Pottier C, et al. Whole-genome sequencing reveals important role for TBK1 and OPTN mutations in frontotemporal lobar degeneration without motor neuron disease. Acta Neuropathol. 2015;130(1):77–92. doi: 10.1007/s00401-015-1436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cirulli ET, et al. FALS Sequencing Consortium Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347(6229):1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo JM, Ordureau A, Paulo JA, Rinehart J, Harper JW. The PINK1-PARKIN mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol Cell. 2015;60(1):7–20. doi: 10.1016/j.molcel.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner S, et al. Ubiquitin binding mediates the NF-kappaB inhibitory potential of ABIN proteins. Oncogene. 2008;27(26):3739–3745. doi: 10.1038/sj.onc.1211042. [DOI] [PubMed] [Google Scholar]
- 25.Rahighi S, et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-kappaB activation. Cell. 2009;136(6):1098–1109. doi: 10.1016/j.cell.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Gleason CE, Ordureau A, Gourlay R, Arthur JS, Cohen P. Polyubiquitin binding to optineurin is required for optimal activation of TANK-binding kinase 1 and production of interferon β. J Biol Chem. 2011;286(41):35663–35674. doi: 10.1074/jbc.M111.267567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinemann IU, et al. Enhanced phosphoserine insertion during Escherichia coli protein synthesis via partial UAG codon reassignment and release factor 1 deletion. FEBS Lett. 2012;586(20):3716–3722. doi: 10.1016/j.febslet.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA. 2014;111(42):E4439–E4448. doi: 10.1073/pnas.1405752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morton S, Hesson L, Peggie M, Cohen P. Enhanced binding of TBK1 by an optineurin mutant that causes a familial form of primary open angle glaucoma. FEBS Lett. 2008;582(6):997–1002. doi: 10.1016/j.febslet.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 30.Goncalves A, et al. Functional dissection of the TBK1 molecular network. PLoS One. 2011;6(9):e23971. doi: 10.1371/journal.pone.0023971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ordureau A, et al. Defining roles of PARKIN and ubiquitin phosphorylation by PINK1 in mitochondrial quality control using a ubiquitin replacement strategy. Proc Natl Acad Sci USA. 2015;112(21):6637–6642. doi: 10.1073/pnas.1506593112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bingol B, et al. The mitochondrial deubiquitinase USP30 opposes parkin-mediated mitophagy. Nature. 2014;510(7505):370–375. doi: 10.1038/nature13418. [DOI] [PubMed] [Google Scholar]
- 33.Denison SR, et al. Alterations in the common fragile site gene Parkin in ovarian and other cancers. Oncogene. 2003;22(51):8370–8378. doi: 10.1038/sj.onc.1207072. [DOI] [PubMed] [Google Scholar]
- 34.Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell. 2012;22(2):320–333. doi: 10.1016/j.devcel.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fecto F, et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol. 2011;68(11):1440–1446. doi: 10.1001/archneurol.2011.250. [DOI] [PubMed] [Google Scholar]
- 36.Korac J, et al. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J Cell Sci. 2013;126(Pt 2):580–592. doi: 10.1242/jcs.114926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18(9):3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tighe A, Staples O, Taylor S. Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J Cell Biol. 2008;181(6):893–901. doi: 10.1083/jcb.200712028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ran FA, et al. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8(11):2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalski A, et al. Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol Cell Proteomics. 2011;10(9):M111.011015. doi: 10.1074/mcp.M111.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 42.Cox J, et al. Andromeda: A peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10(4):1794–1805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, et al. An efficient genotyping method for genome-modified animals and human cells generated with CRISPR/Cas9 system. Sci Rep. 2014;4:6420. doi: 10.1038/srep06420. [DOI] [PMC free article] [PubMed] [Google Scholar]