BIOCHEMISTRY Correction for “Redox-coupled proton transfer mechanism in nitrite reductase revealed by femtosecond crystallography,” by Yohta Fukuda, Ka Man Tse, Takanori Nakane, Toru Nakatsu, Mamoru Suzuki, Michihiro Sugahara, Shigeyuki Inoue, Tetsuya Masuda, Fumiaki Yumoto, Naohiro Matsugaki, Eriko Nango, Kensuke Tono, Yasumasa Joti, Takashi Kameshima, Changyong Song, Takaki Hatsui, Makina Yabashi, Osamu Nureki, Michael E. P. Murphy, Tsuyoshi Inoue, So Iwata, and Eiichi Mizohata, which appeared in issue 11, March 15, 2016, of Proc Natl Acad Sci USA (113:2928–2933; first published February 29, 2016; 10.1073/pnas.1517770113).
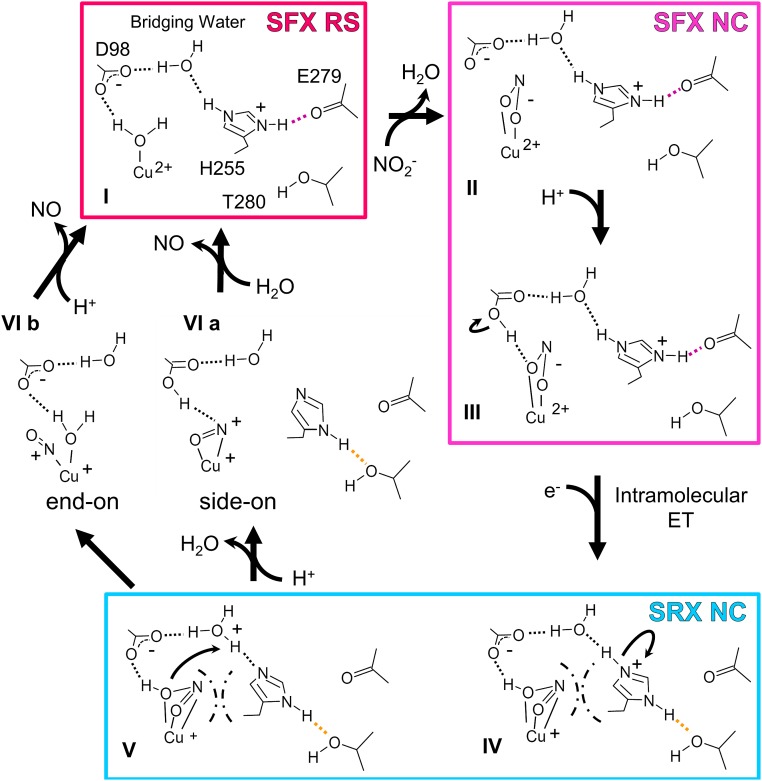
The authors note that Fig. 4 appeared incorrectly. The corrected figure and its legend appear below.
Fig. 4.
Updated reaction mechanism of nitrite reduction. Dashed lines represent H-bonds. Strong and weak H-bonds involved in PCET are colored as in Fig. 2B. Chain lines mean steric hindrance between the near face-on substrate and His255.