Significance
Corticotropin releasing factor binding protein (CRF-BP) belongs to the CRF family that is fundamental in the stress response and in the interaction between stress and addiction. The mechanisms by which CRF-BP regulates the CRF system are not fully understood. Most G protein-coupled receptors (GPCRs) are located mainly intracellularly and depend on specific escort proteins for their trafficking to the cell surface. CRF2αR is also located intracellularly; however, no escort protein regulating its presence on the cell surface has been described. We show that CRF-BP interacts with CRF2αR, acting as an escort protein, increasing the presence of the receptor on the cell surface.
Keywords: accessory protein, CRH, protein interactions, escort protein
Abstract
Corticotropin releasing factor binding protein (CRF-BP) was originally recognized as CRF sequestering protein. However, its differential subcellular localization in different brain nuclei suggests that CRF-BP may have additional functions. There is evidence that CRF-BP potentiates CRF and urocortin 1 actions through CRF type 2 receptors (CRF2R). CRF2R is a G protein-coupled receptor (GPCR) that is found mainly intracellularly as most GPCRs. The access of GPCRs to the cell surface is tightly regulated by escort proteins. We hypothesized that CRF-BP binds to CRF2R, exerting an escort protein role. We analyzed the colocalization of CRF-BP and CRF2R in cultured rat mesencephalic neurons, and the localization and interaction of heterologous expressed CRF-BP and CRF2αR in yeast, human embryonic kidney 293, and rat pheochromocytoma 12 cells. Our results showed that CRF-BP and CRF2R naturally colocalize in the neurites of cultured mesencephalic neurons. Heterologous expression of each protein showed that CRF-BP was localized mainly in secretory granules and CRF2αR in the endoplasmic reticulum. In contrast, CRF-BP and CRF2αR colocalized when both proteins are coexpressed. Here we show that CRF-BP physically interacts with the CRF2αR but not the CRF2βR isoform, increasing CRF2αR on the cell surface. Thus, CRF-BP emerges as a GPCR escort protein increasing the understanding of GPCR trafficking.
The corticotropin releasing factor (CRF) system plays a key role in the response and adaptation to stressful stimuli (1, 2) and in the interaction between stress and addiction (3). The CRF system acts on the hypothalamic–pituitary–adrenal axis (4, 5) and in different brain regions (1, 6). The CRF system includes four peptides, CRF type 1 (CRF1R) and type 2 (CRF2R), G protein-coupled receptors (GPCRs) (6–8), and CRF binding protein (CRF-BP) (9).
CRF-BP was described as a circulating polypeptide in pregnant women (10, 11). CRF-BP binds CRF and urocortin with high affinity (12). Different functions have been proposed for CRF-BP (13). On one hand, CRF-BP exerts an inhibitory role by sequestering CRF peptide (9, 14–16). On other hand, a facilitatory role of CRF-BP on CRF-dependent neuronal plasticity depending on CRF2R in the rat ventral tegmental area (VTA) has been described (17, 18). Recently, it has been shown that CRF-BP and CRF2R are important for ethanol binge drinking behavior (19). The anatomical evidence showing that CRF-BP has different subcellular distribution depending on the neuronal context (20) further supports several roles for CRF-BP.
Three isoforms of CRF2R have been reported, the α isoform being the most expressed in the brain (21). CRF2αR is localized intracellularly in neurons of the rat dorsal raphe nucleus and that exposure to acute (22) and repeated stress (23) increases its presence in the plasma membrane. CRF2αR overexpressed in human embryonic kidney (HEK293T) cells is associated with the endoplasmic reticulum (ER) (24). Schulz et al. (25) showed that CRF2αR is retained in the ER due to the interaction of the CRF2αR with the calnexin/calreticulin chaperone system. Most GPCRs are found as stocks of functional receptors retained mainly in the ER or Golgi apparatus. These GPCRs are mobilized to the cell surface by their interactions with escort proteins (26, 27). For instance, the receptor activity-modifying protein 2 (RAMP2) increases CRF1R (28) and RAMP1 increases calcitonin-receptor-like receptor (CRLR) (29) in the plasma membrane. Protachykinin interacts with δ-opioid receptor mobilizing the receptor to the regulated secretory pathway, increasing its localization in the plasma membrane in a stimulus-dependent manner (30). Rat renal outer cortical tissue slices incubated with atrial natriuretic peptide or neuropeptide Y resulted in an increased plasma membrane amount of dopamine type 1 receptor and α1A-adrenergic receptors, respectively (31).
The aforementioned data and the evidence showing that CRF-BP and CRF2R are coexpressed in lateral hypothalamic area (LHA) neurons and in VTA nerve terminals (32) led us to hypothesize that CRF-BP binds to CRF2R regulating its subcellular localization. Herein, we observed that CRF-BP and CRF2R colocalize in cultured mesencephalic neurons and that CRF-BP physically interacts with CRF2αR exerting an escort protein role increasing the presence of CRF2αR on the cell surface.
Results
CRF-BP and CRF2R Colocalize in Cultured Mesencephalic Neurons.
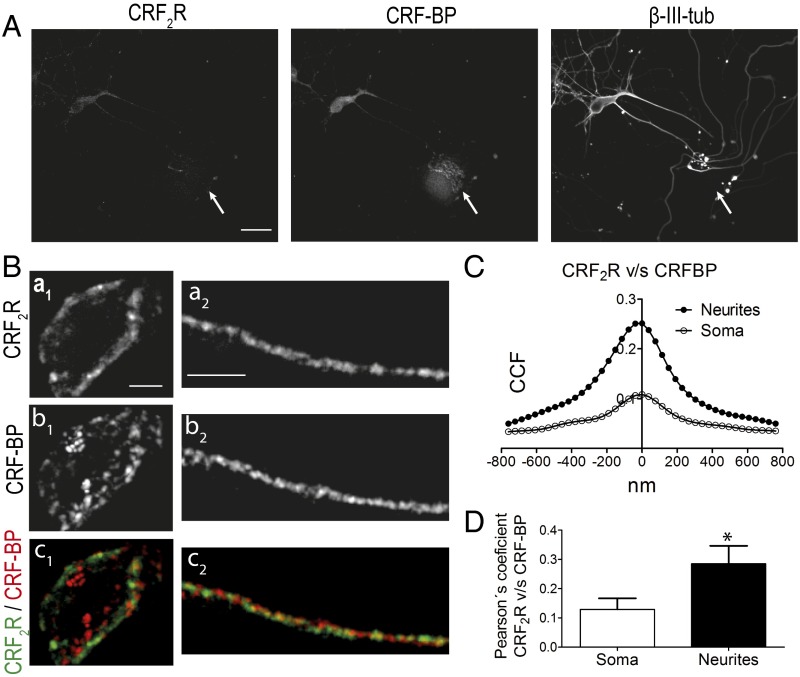
Immunodetection and colocalization analyzes for CRF-BP and CRF2R were done on embryonic day 18. The expression of CRF-BP and CRF2αR was analyzed both in neurons (β-III-tubulin positive) and nonneuronal cells (β-III-tubulin negative; Fig. 1A, arrows). CRF-BP was detected in both neurons and nonneuronal cells, in contrast to CRF2αR, which was detected only in neurons (Fig. 1A). Immunofluorescence detection (Fig. 1B) and Van Steensel's colocalization analyzes (33) showed that CRF-BP colocalizes with CRF2αR preferentially in neurites, as indicated by a more pronounced bell shaped curve with a cross-correlation coefficient (CCF) (at X = 0) of 0.25 ± 0.07 vs. CCF (at X = 0) of 0.12 ± 0.04 in the soma (Fig. 1 C and D).
Fig. 1.
CRF-BP and CRF2R colocalize in cultured mesencephalic neurons. (A) Confocal immunodetection for CRF-BP, CRF2R, and the neuronal marker β-III-tubulin (β-III-tub). Arrows depict stained nonneuronal cells. (Scale bar, 5 µm.) (B) Confocal images for CRF-BP, CRF2R, and merge showing colocalization in soma (a1–c1) and neurites (a2–c2). (Scale bar, 5 µm for soma and 8 µm for neurites.) (C and D) Van Steensel´s analyses (C) and Pearson’s coefficient (D) of CRF-BP and CRF2R colocalization. The soma and two to three neurites from 10 neurons of three independent experiments were analyzed. Data indicate the mean ± SEM (*P < 0.05).
CRF-BP Interacts with CRF2αR in an Isoform-Specific Manner.
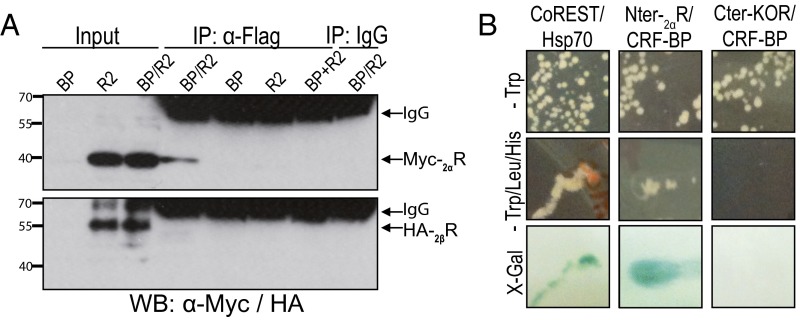
The possibility of physical interaction between CRF-BP and the α and β isoforms of CRF2R was evaluated by coimmunoprecipitation. HEK293T cells were cotransfected with Flag-tagged CRF-BP (CRF-BPFlag) and Myc-tagged CRF2αR (MycCRF2αR) or HA-tagged CRF2βR (HACRF2βR) expression plasmids. The anti-Flag antibody immunoprecipitated (IP) MycCRF2αR (∼42 kDa) but not HACRF2βR (∼55 kDa) (Fig. 2A). No bands were detected in the following negative controls: (i) cell lysates from CRF-BP or each CRF2R isoform cotransfected with the empty expression plasmid pcDNA; (ii) the mixture of the cell lysates from cells expressing only CRF-BP or CRF2R; and (iii) immunoprecipitation using a preimmune mouse IgG. Both MycCRF2αR and HACRF2βR were detected in the corresponding inputs. The bands observed between 55 and 70 kDa in the IP lanes correspond to IgG. These results showed that CRF-BP forms a protein complex with the α but not the β isoform of CRF2R.
Fig. 2.
CRF-BP interacts with CRF2αR. (A) Coimmunoprecipitation of FlagCRF-BP and MycCRF2αR (Myc-2αR) or HACRF2βR (HA-2βR) from HEK293T cell lysates. BP, CRF-BP; R2, CRF2R. (B) Yeast two-hybrid assay of CRF-BP and N-terminal domain of CRF2αR (Nter-2αR).
To confirm that CRF-BP physically interacts with CRF2αR, L40 yeasts were transformed with expression plasmids encoding the N-terminal domain of CRF2αR (Nter-2αR) and CRF-BP. The Nter-2αR was selected because both CRF2R isoforms differ in their N-terminal domains (21). A positive interaction was observed for CRF-BP and Nter-2αR, as colonies grew in the restricted medium (−Trp/Leu/His) (Fig. 2B, Middle), and were positive for β-galactosidase gene (lacZ) expression assay (Fig. 2B, Lower). The specificity was confirmed by the negative interaction of CRF-BP with the C-terminal domain of the κ-opioid receptor (Cter-KOR), another GPCR and the positive interaction between corepressor for RE1 silencing transcription factor (CoREST) and heat shock protein 70 (Hsp70) (34). Transformed yeasts viability was shown by growing them in noninteraction selecting medium (Fig. 2B, Upper). Thus, CRF-BP interacts with the N-terminal domain of CRF2αR.
The Localization of CRF-BP Changes When Coexpressed with CRF2αR.
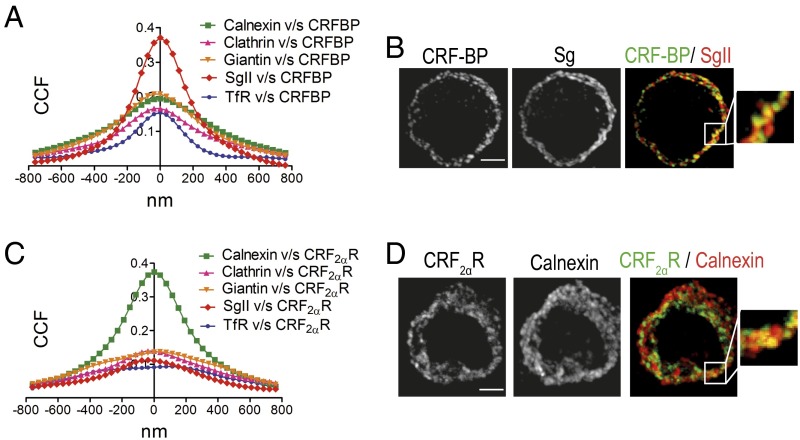
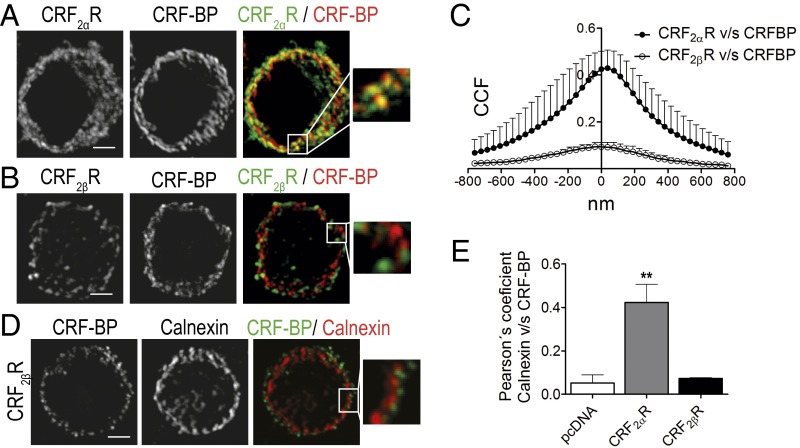
CRF-BP localizes in secretory granules (35) whereas CRF2αR localizes in the ER (24). We performed subcellular localization analysis of each protein overexpressed alone or coexpressed to investigate where in the cells the interaction between both proteins occurs. Immunostainings were carried out with antibodies against Flag or CRF2αR plus markers of ER (calnexin), endocytic and exocytic vesicles (clathrin), Golgi apparatus (giantin), secretory vesicles (SgII), and recycling vesicles (TfR). CRF-BP presented a positive to random CCF with calnexin, clathrin, giantin, and TfR in the range of 0.15–0.21. An approximately twofold higher CCF was obtained with SgII (0.37 ± 0.03) (Fig. 3 A and B). CRF2αR presented a positive to random CCF with clathrin, giantin, SgII, and TfR in the range of 0.09–0.14. An approximately fourfold higher CCF was obtained with calnexin (0.4 ± 0.03) (Fig. 3 C and D). Thus, CRF-BP is mainly localized in secretory granules, whereas CRF2αR is localized in the ER.
Fig. 3.
CRF-BP is localized mainly in secretory granules and CRF2αR in the endoplasmic reticulum. (A and C) Van Steensel´s colocalization analysis of CRF-BPFlag (A) and HACRF2αR (C) with the subcellular markers: calnexin, clathrin, giantin, SgII, and TfR. Data were obtained from 30 cells of three independent experiments. (B and D) Confocal immunodetection for CRF-BP plus SgII (B) or CRF2αR plus calnexin (D). Magnifications of the region in the Insets are shown. (Scale bar, 5 µm.)
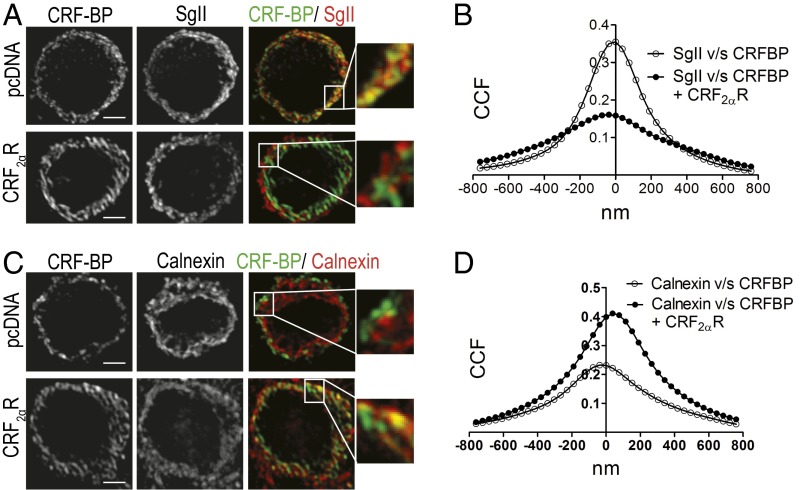
Coexpression of CRF-BP with CRF2αR (Fig. 4 A and B) significantly decreased the colocalization of CRF-BP with SgII, from a CCF (at X = 0) of 0.35 ± 0.03–0.16 ± 0.01 (P < 0.001). In contrast, the colocalization of CRF-BP with calnexin (Fig. 4 C and D) significantly increased from a CCF (at X = 0) of 0.23 ± 0.03–0.46 ± 0.06 (P < 0.001).
Fig. 4.
CRF2αR retained CRF-BP in the endoplasmic reticulum when coexpressed. (A and C) Confocal immunodetection for CRF-BP and SgII (A) or calnexin (C). Magnifications of the region in the Insets are shown. (Scale bar, 5 µm.) (B and D) Van Steensel´s colocalization analysis of CRF-BP with SgII (B) or calnexin (D). Data were obtained from 10 cells of three independent experiments.
The Change in CRF-BP Subcellular Localization Is Specifically Induced by the α Isoform of CRF2R.
We evaluated whether the change in CRF-BP subcellular localization was specifically induced by the α isoform of CRF2R. Confocal images showed that CRF-BP colocalizes with HACRF2αR (Fig. 5A) but not with HACRF2βR (Fig. 5B). Van Steensel's colocalization analyzes (Fig. 5C) confirmed that CRF-BPFlag colocalizes with HACRF2αR but not with HACRF2βR, as reflected by a positive (bell shaped curve) and a null (no appreciable peak) overlap, respectively. In addition, confocal images showed different subcellular distribution between CRF-BP and calnexin when CRF-BPFlag was coexpressed with HACRF2βR (Fig. 5D) than with HACRF2αR (Fig. 4). The obtained Pearson's correlation coefficient for CRF-BPFlag with calnexin from pheochromocytoma 12 (PC12) cells cotransfected with CRF-BPFlag plus pcDNA, HACRF2αR, or HACRF2βR showed that only HACRF2αR generated a significant increase in the colocalization of CRF-BP with calnexin (Fig. 5E).
Fig. 5.
CRF-BP is retained in the ER when coexpressed with CRF2αR but not with CRF2βR. (A and B) Confocal immunodetection for CRF-BP and HA epitope. The detection of HACRF2αR is shown in A and HACRF2βR in B. Magnifications of the region in the Insets are shown. (Scale bar, 5 µm.) (C) Van Steensel's colocalization analysis of CRF-BPFlag with HACRF2αR or HACRF2βR. Data were obtained from 10 cells of four independent experiments. (D) Confocal immunodetection for CRF-BP and calnexin in cells coexpressing CRF-BPflag and HACRF2βR. Magnification of the region in the Inset is shown. (Scale bar, 5 µm.) (E) Pearson's correlation coefficient of CRF-BP with calnexin obtained from images of PC12 cells transfected with CRF-BPflag alone (pcDNA) or cotransfected with HACRF2αR or HACRF2βR. Data were obtained from 10 cells of three independent experiments. Data indicate the mean ± SEM (**P < 0.01).
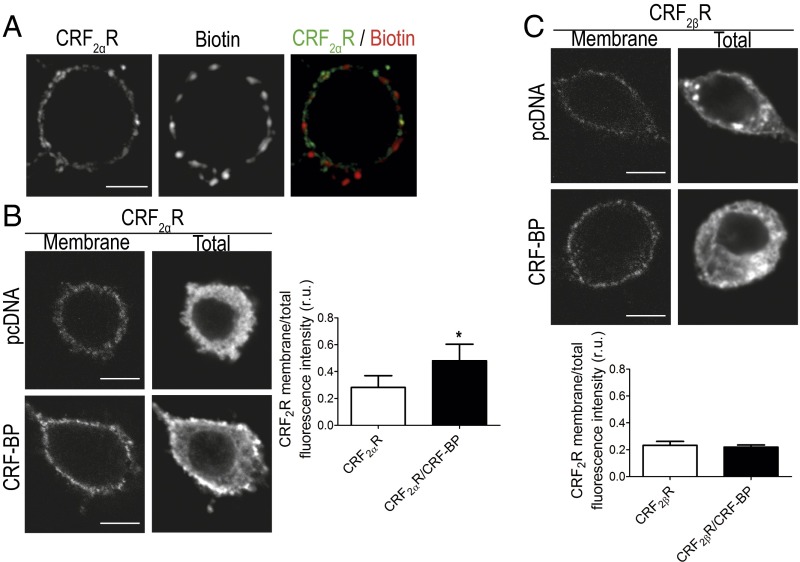
CRF-BP Acts Like an Escort Protein Increasing the Presence of CRF2αR on the Cell Surface.
To determine the possible role of CRF-BP as escort protein, we studied the amount of CRF2αR present in the plasma membrane in cells expressing CRF2αR alone or with CRF-BP. To evaluate the specificity of the membrane staining for HACRF2αR, nonpermeabilized PC12 cells were incubated with the antibody against HA and biotin. All of the staining detected for HACRF2αR in nonpermeabilized cells was found in the plasma membrane, as its localization was similar to biotin staining (Fig. 6A). The HACRF2αR membrane/total fluorescence intensity ratio significantly increased from 0.29 ± 0.09–0.48 ± 0.1 relative fluorescence units (r.u.) (Fig. 6B), when HACRF2αR was coexpressed with CRF-BP compared with when it was expressed alone. To proof the specificity of the escort protein role of CRF-BP with CRF2αR, the HACRF2βR membrane/total fluorescence intensity ratio was evaluated in the presence and absence of CRF-BP. No changes were observed (0.23 ± 0.03–0.22 ± 0.02 r.u.) (Fig. 6C).
Fig. 6.
CRF-BP increases CRF2αR in the plasma membrane. (A) Confocal immunodetection for HA and biotin in nonpermeabilized cells. (Scale bar, 5 µm.) (B and C) Confocal immunodetection for membrane and total HA and quantification of the HACRF2R membrane/total fluorescence intensity ratio. Data were obtained from three images per cell, from 10 cells of three independent experiments. Data indicate the mean ± SEM (*P < 0.05). (Scale bar, 5 µm.)
Discussion
In the present study, we reveal a previously unidentified function of CRF-BP acting as CRF2αR escort protein facilitating its presence in the plasma membrane. We showed that CRF-BP physically interacts with CRF2αR in an isoform-specific manner, because no interaction was detected between CRF-BP and CRF2βR. CRF-BP and CRF2αR, when expressed alone, have distinct subcellular localization, CRF-BP is localized mainly in secretory granules, and CRF2αR in the ER. Interestingly, high levels of colocalization between CRF-BP and CRF2R were observed in mesencephalic neurons and when both proteins were coexpressed in cell lines. Furthermore, the interaction between CRF-BP and CRF2αR changed the subcellular localization of both proteins. CRF-BP and CRF2αR coexpression retained CRF-BP in the ER and increased the presence of CRF2αR in the plasma membrane. Thus, our results evidenced new regulatory mechanisms within CRF family members. In addition, CRF-BP emerges as a new GPCR escort protein.
Most GPCRs are present in the ER and Golgi as stock of functional receptors (36). As stated by Roux and Cottrell (27), most studies have addressed the regulation of the desensitization and down-regulation process of GPCRs, but only few studies have focused on their trafficking and access to the cell surface. GPCRs interaction with ER proteins results in their intracellular retention and interaction with so-called escort proteins facilitates or determines their access to the cell surface (27, 36). Our results show that CRF2αR is mainly located intracellularly and that its presence in the cell surface is facilitated by CRF-BP. Thus, CRF-BP has functional consequences acting as a CRF2αR escort protein. In addition, CRF-BP increases the repertoire of escort proteins that participate in the trafficking of GPCRs.
GPCRs located in intracellular compartments, most probably correspond to nonmature forms of the receptors that undergo premature proteosomal degradation (36–39). CRF2αR is not exempted from this observation, because it has been shown that about 70% of the intracellular stock of CRF2αR is in a high mannose and nonglycosylated form (25). Furthermore, Schulz et al. (25) showed that the lack of CRF2αR maturation is due to the presence of a noncleavable pseudosignal peptide present in its N-terminal region. Interestingly, some escort proteins are responsible for increasing the cell surface localization of GPCRs by increasing the mature form of the receptors. For example, the glycosylation state of CRLR varies depending on its interaction with different RAMPs (29), GEC1 increases the mature form and the localization of the κ-opioid receptor in the plasma membrane (40), and MRAP2 increases the cell surface presence and maturation of the melanocortin-2 receptor (41). It has also been shown that the pseudosignal peptide of CRF2αR inhibits the trafficking of the receptor to the plasma membrane by allowing its interaction with calnexin (25, 42). PRAF2, another ER-resident protein such as calnexin, retains the GB2 subunit of GABAB receptor in the ER. The presence of GB2 in the plasma membrane depends on its interaction with the GB1 subunit of GABAB receptor that acts as an escort protein releasing GB2 from PRAF2 (43). Our results show that CRF-BP specifically interacts through the CRF2αR N-terminal that contains the pseudosignal peptide, increasing its presence in the cell surface. Further studies should address whether CRF-BP is increasing CRF2αR in the cell surface due to: (i) increasing the level of glycosylation of CRF2αR, (ii) decreasing the association of CRF2αR with calnexin, and/or (iii) escorting CRF2αR through the secretory pathway to the cell surface.
The pseudosignal peptide of CRF2αR also plays a role in the signaling of the receptor. CRF2αR signals only through Gs but when the pseudosignal peptide is replaced by the CRF1R signal peptide, its signaling is biphasic through Gs and Gi (25). Interestingly, Ungless et al. (17) have shown that CRF2R in VTA signals through Gq when activated by ligands with affinity to CRF-BP. We have observed that CRF-BP specifically interacts with CRF2αR and not CRF2βR. The α and β CRF2R isoforms differ only in their N-terminal sequence (21) resulting in a cleavable signal peptide in CRF2βR in contrast to a noncleavable pseudosignal peptide in CRF2αR (43). Thus, further studies should analyze the signaling properties of CRF2αR and CRF2βR coexpressed with CRF-BP to determine whether the isoform-specific interaction of CRF-BP with CRF2αR has another functional consequence in addition to increase its presence in the cell surface.
We observed that CRF-BP is detected in both neuronal and nonneuronal cells, whereas CRF2αR was only detected in neurons. CRF-BP and CRF2R colocalize mainly in the neurites of mesencephalic neurons. Furthermore, we have previously shown that CRF-BP and CRF2R are coexpressed in cells of the LHA and colocalize in LHA nerve terminals arriving to VTA (32). Interestingly, CRF-BP has a distinct subcellular localization depending on the brain region studied (20). Altogether, the previous anatomical data and our present results sustain the possibility that CRF-BP exerts multiple specific functions depending on the brain region, inhibiting CRF actions in some cases and potentiating them in a CRF2αR-specific manner in other cases. It is tempting to suggest that the interaction of CRF-BP with CRF2αR determines the differential subcellular localization and function of CRF-BP.
In summary, our results show that CRF-BP interacts in an isoform-specific manner with CRF2αR increasing its presence on the cell surface. Further studies should determine if this interaction plays a role in the facilitatory role of CRF-BP/CRF2R observed in the rat VTA after acute stress (17), repeated cocaine autoadministration (18), and in ethanol binge drinking behavior (19).
Experimental Procedures
Primary Culture of Rat Mesencephalic Neurons.
Pregnant Sprague-Dawley rats (n = 3) obtained from Pontificia Universidad Católica de Chile were used. The Bioethical Committee of the Faculty of Biological Sciences of Pontificia Universidad Católica de Chile and the Bioethical Committee of Consejo Nacional de Ciencia y Tecnología approved the experimental procedures. At 18 d of gestation, pregnant rats were decapitated with a guillotine. Embryos were removed from the uterus and placed in cold Hank’s medium. Five to six embryos were decapitated with scissors and placed in cold Hank’s medium. The embryo head was located in a stereomicroscope so as to have a sagittal view. One horizontal cut was performed in the isthmus area, allowing separation of the hindbrain region from the rest of the embryo head. A vertical cut with a rostral inclination was performed to separate the midbrain region of the diencephalon. Then, the ventral region where dopamine neurons are located was dissected and placed in 10 mL of Hank's medium and centrifuged for 30 s at 150.9 × g. Media were replaced with Hank's medium containing 4.5 mL of 0.075% papain plus 200 μL of 20× DNase. The tissue was gently mixed and incubated at 37 °C for 20 min and centrifuged for 30 s at 1,000 rpm. Disaggregated cells were incubated in 4 mL of warmed FBS and incubated for 2 min at room temperature. Finally, 5 mL of adhesion medium was added and tissue was disintegrated using a Pasteur pipette. Then, cells were left to stand for 5 min at room temperature, centrifuged for 30 s at 150.9 × g and the supernatant containing the cells was transferred to a well with complete neurobasal medium with 2 µM AraC to inhibit glia growth.
Cell Culture.
Cell lines were grown in 100-mm plates at 37 °C in a 5% CO2 humidified atmosphere. HEK293T cells were grown with DMEM (Gibco) supplemented with 10% (vol/vol) FBS (HyClone Labs), 1% (vol/vol) penicillin/streptomycin 100× (Gibco), and 2 mM GlutaMax (Gibco). PC12 cells were grown with DMEM (Gibco) supplemented with 5% (vol/vol) FBS (HyClone) and 10% horse serum (Gibco), 1% (vol/vol) penicillin/streptomycin 100× (Gibco), and 2 mM GlutaMax (Gibco).
Expression Vectors.
The expression vectors for MycCRF2αR and HACRF2βR were obtained from GeneCopoeia. The construct pcDNA3.1/CRF-BP-Flag was a donation from Wylie Vale´s laboratory, Clayton Foundation Laboratories for Peptide Biology, Salk Institute for Biological Studies, La Jolla, CA. HACRF2αR was generated adding the HA epitope to the plasmid vector obtained previously in our laboratory (24).
Protein Extraction and Immunoprecipitation.
HEK293T cells were seeded at a density of 8 × 106 cells on a 100-mm plate. The cells were transiently transfected with a total of 8 µg DNA using Lipofectamine 2000 reagent (Invitrogen). The mixtures for DNA transfections were as follows: mycCRF2αR with CRF-BPFlag and HACRF2βR with CRF-BPFlag, mycCRF2αR, HACRF2βR, and CRF-BPFlag. All transfections were done using an equimolar mixture of vectors and total amounts of DNA were adjusted by adding empty vector pcDNA. Protein extracts and immunoprecipitation were done as previously described (24). Samples were incubated with 2 µg mouse anti-Flag M2 antibody (Stratagene) or 1 µg mouse IgG (Santa Cruz Biotechnology). Proteins transferred to PVDF membranes were immunoblotted with mouse anti-Myc (sc-40 Santa Cruz Biotechnology) or rabbit ant-HA antibody (Covance), followed by horseradish peroxidase-conjugated donkey anti-rabbit IgG (dilution 1:5,000; Jackson Immnuno Research Laboratories).
Yeast Two-Hybrid Assay.
L40 yeasts were grown in YPDA media [2% (wt/vol) Difco peptone, 1% yeast extract, and 0.02% glucose] at 30 °C over night. L40 were grown until an OD600: 0.7–1.0 and cotransformed with a total of 1 µg DNA in a solution 50% (wt/vol) PEG, 0.1 M LiAc, and 2 mg/mL boiled SS-carrier DNA. The mixtures of DNA used were as follows: the expression plasmids pGAD encoding N-terminal domain of CRF2αR(1-118) (Nter-2αR) fused to the GAL4 DNA binding domain and the expression plasmids pSTT91 encoding CRF-BP(full length) fused to the GAL4 activation domain (Nter-2αR/CRF-BP), CoREST(187-429)/Hsp70(188-646), and N-terminal domain of the κ-opioid receptor(334-380) (Nter-KOR)/CRF-BP. Every vector used for the yeast transformation has a selectable marker; pSTT91 has TRP and pGAD has LEU. L40 yeasts were grown for 3 d at 30 °C before the evaluation of protein interaction. L40 yeast has two reporter genes to evaluate the interaction between the proteins of interest, the genes HIS3, and LacZ. Thus, the interaction was determined by the capacity of the L40 yeast to grow in a Trp-, Leu-, His-free medium, and by a positive β-galactosidase gene (lacZ) expression assay, using the β-galactosidase substrate 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal).
Immunofluorescence.
PC12 cells were seeded at a density of 7 × 106 cells per well on 24-well plates on coverslips coated with poly-l-lysine (Sigma). Cells were transiently transfected with a total of 0.5 µg DNA using Lipofectamine 2000 reagent (Invitrogen). The mixtures for DNA transfections were as follows: HACRF2αR with CRF-BPFlag and HACRF2βR with CRF-BPFlag, HACRF2αR, HACRF2βR, and CRF-BPFlag. Transfections were done using an equimolar mixture of vectors and total amounts of DNA were adjusted by adding the empty vector pcDNA. PC12 cells were fixed 48 h posttransfection and mesencephalic neurons were fixed at days in vitro 7–9, with 4% (wt/vol) p-formaldehyde (PFA) plus 4% (wt/vol) sucrose in PBS for 20 min.
Immunoflorescence was performed as previously described (32). Primary antibodies used were as follows: goat anti-CRH2R (dilution 1:200; sc-1826, Santa Cruz Biotechnology), rabbit anti–CRF-BP (dilution 1:500; sc-20630, Santa Cruz Biotechnology), mouse anti-Flag M2 (dilution 1:1,000; Stratagene), mouse anti-HA (dilution 1:1,000; Covance), rabbit anti-calnexin (dilution 1:200; Sigma), rabbit anti-giantin (dilution 1:200; Abcam), goat anti-clathrin (dilution 1:200; sc-6580, Santa Cruz Biotechnology), rabbit anti-transferrin receptor (TfR) (dilution 1:500; Zymed), and rabbit anti-secretogranin II (SgII) (dilution 1:200; Abcam).
Cell Surface Detection of CRF2αR.
Cells were incubated for 10 min at 4 °C with mouse anti-HA antibody (Covance). After three washes with PBS containing 100 mM glycine, cells were fixed with 4% PFA plus 4% sucrose in PBS for 20 min, blocked with PBS containing 1% BSA for 30 min at room temperature and incubated for 1 h with a dilution 1:500 of donkey anti-mouse AlexaFluor488 secondary antibody. Then, the cells were permeabilized with 0.2% Triton X-100 for 5 min at room temperature, blocked again, and incubated with mouse anti-HA antibody plus rabbit anti–CRF-BP (Santa Cruz Biotechnology). Finally, cells were incubated for 1 h with a dilution 1:500 of donkey anti-mouse AlexaFluor647 and Cy3 donkey ant-rabbit secondary antibodies. To control that the staining of CRF2αR in nonpermeabilized cells corresponded to cell surface detection, cells transfected with the receptor were incubated for 30 min with EZ-Link Sulfo-NHS-Biotin (Thermo Scientific) at 4 °C after a 10-min incubation with the anti-HA antibody.
Confocal Microscopy and Image Analyses.
Fluorescence images were captured with a confocal microscope (Fluoview 1000, Olympus) and Fluoview v6.0 software. Images were digitally obtained with a 100× objective (N.A. 1.4 oil) and using a sequential mode of laser scanning. Staking images were obtained with a Z step of 200 nm per cell. Captured images were processed with ImageJ software (rsb.info.nih.gov/ij). The images were deconvolved using the “Iterative Deconvolve 3D” plugin within ImageJ. Colocalization analyses were done as described (33, 35). Sixteen z-plane images per PC12 cell were processed for each dataset (10 cells per experiment). In the case of neurons, 4–8 z-plane images were analyzed from somas and 2–4 neurite segments per neuron (10 cells per experiment).
The CRF2R membrane/total fluorescence intensity ratio was determined with ImageJ software. The cell area to analyze was selected from each image with the drawing/selection tool. Three regions outside the cell were considered as background. The membrane and total fluorescence was calculated with the following equation: fluorescence = integrated density of cell − (area of selected cell × mean fluorescence of background).
Statistical Analyses.
Statistical analyses were performed using the statistical software Prism 5 (GraphPad Software). Values are expressed as the mean ± SEM. Statistical significance of differences were assessed with one-way ANOVA with Bonferroni posttest for the results in Figs. 4 and 5, with paired t test for the data in Fig. 1, and with paired t test for the logarithm of the ratio for the results in Fig. 6.
Acknowledgments
This work was supported by el Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) (Grants 1110390 and 1150244) and Millennium Science Initiative (Grants P06/008-F and P10/063-F). P.G.S. and L.A.P. were recipients of doctoral fellowships from la Comisión Nacional de Investigación Científica y Tecnológica, Chile, and Millenium Science Initiative (Grant P10/063-F).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15(2):71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 2.Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herman JP, et al. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40(5):573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 6.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: Yet more partners discovered. Trends Pharmacol Sci. 2002;23(2):71–77. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 7.Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13(10):436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- 8.Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: New molecular targets. CNS Neurol Disord Drug Targets. 2006;5(4):453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potter E, et al. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349(6308):423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- 10.Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: Clinical implications. Ann Intern Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- 11.McLean M, Smith R. Corticotropin-releasing hormone in human pregnancy and parturition. Trends Endocrinol Metab. 1999;10(5):174–178. doi: 10.1016/s1043-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 12.Kemp CF, Woods RJ, Lowry PJ. The corticotrophin-releasing factor-binding protein: An act of several parts. Peptides. 1998;19(6):1119–1128. doi: 10.1016/s0196-9781(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 13.Westphal NJ, Seasholtz AF. CRH-BP: The regulation and function of a phylogenetically conserved binding protein. Front Biosci. 2006;11:1878–1891. doi: 10.2741/1931. [DOI] [PubMed] [Google Scholar]
- 14.Linton EA, Behan DP, Saphier PW, Lowry PJ. Corticotropin-releasing hormone (CRH)-binding protein: Reduction in the adrenocorticotropin-releasing activity of placental but not hypothalamic CRH. J Clin Endocrinol Metab. 1990;70(6):1574–1580. doi: 10.1210/jcem-70-6-1574. [DOI] [PubMed] [Google Scholar]
- 15.Linton EA, Wolfe CD, Behan DP, Lowry PJ. A specific carrier substance for human corticotrophin releasing factor in late gestational maternal plasma which could mask the ACTH-releasing activity. Clin Endocrinol (Oxf) 1988;28(3):315–324. doi: 10.1111/j.1365-2265.1988.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 16.Saphier PW, et al. A comparison of the clearance of ovine and human corticotrophin-releasing hormone (CRH) in man and sheep: A possible role for CRH-binding protein. J Endocrinol. 1992;133(3):487–495. doi: 10.1677/joe.0.1330487. [DOI] [PubMed] [Google Scholar]
- 17.Ungless MA, et al. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39(3):401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: Roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193(2):283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 19.Albrechet-Souza L, et al. Corticotropin releasing factor binding protein and CRF receptors in the ventral tegmental area: Modulation of ethanol binge drinking in C57BL/6J mice. Alcohol Clin Exp Res. 2015;39(9):1609–1618. doi: 10.1111/acer.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peto CA, Arias C, Vale WW, Sawchenko PE. Ultrastructural localization of the corticotropin-releasing factor-binding protein in rat brain and pituitary. J Comp Neurol. 1999;413(2):241–254. [PubMed] [Google Scholar]
- 21.Grigoriadis DE, Lovenberg TW, Chalmers DT, Liaw C, De Souze EB. Characterization of corticotropin-releasing factor receptor subtypes. Ann N Y Acad Sci. 1996;780:60–80. doi: 10.1111/j.1749-6632.1996.tb15112.x. [DOI] [PubMed] [Google Scholar]
- 22.Waselus M, Nazzaro C, Valentino RJ, Van Bockstaele EJ. Stress-induced redistribution of corticotropin-releasing factor receptor subtypes in the dorsal raphe nucleus. Biol Psychiatry. 2009;66(1):76–83. doi: 10.1016/j.biopsych.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood SK, et al. Cellular adaptations of dorsal raphe serotonin neurons associated with the development of active coping in response to social stress. Biol Psychiatry. 2013;73(11):1087–1094. doi: 10.1016/j.biopsych.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuenzalida J, et al. Dopamine D1 and corticotrophin-releasing hormone type-2α receptors assemble into functionally interacting complexes in living cells. Br J Pharmacol. 2014;171(24):5650–5664. doi: 10.1111/bph.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulz K, et al. The pseudo signal peptide of the corticotropin-releasing factor receptor type 2a decreases receptor expression and prevents Gi-mediated inhibition of adenylyl cyclase activity. J Biol Chem. 2010;285(43):32878–32887. doi: 10.1074/jbc.M110.129627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achour L, Labbé-Jullié C, Scott MG, Marullo S. An escort for GPCRs: Implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci. 2008;29(10):528–535. doi: 10.1016/j.tips.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Roux BT, Cottrell GS. G protein-coupled receptors: What a difference a ‘partner’ makes. Int J Mol Sci. 2014;15(1):1112–1142. doi: 10.3390/ijms15011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wootten D, et al. Receptor activity modifying proteins (RAMPs) interact with the VPAC2 receptor and CRF1 receptors and modulate their function. Br J Pharmacol. 2013;168(4):822–834. doi: 10.1111/j.1476-5381.2012.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLatchie LM, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 30.Guan JS, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122(4):619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Holtbäck U, et al. Receptor recruitment: A mechanism for interactions between G protein-coupled receptors. Proc Natl Acad Sci USA. 1999;96(13):7271–7275. doi: 10.1073/pnas.96.13.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slater PG, Noches V, Gysling K. Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur J Neurosci. 2016;43(2):220–229. doi: 10.1111/ejn.13113. [DOI] [PubMed] [Google Scholar]
- 33.van Steensel B, et al. Partial colocalization of glucocorticoid and mineralocorticoid receptors in discrete compartments in nuclei of rat hippocampus neurons. J Cell Sci. 1996;109(Pt 4):787–792. doi: 10.1242/jcs.109.4.787. [DOI] [PubMed] [Google Scholar]
- 34.Gómez AV, et al. CoREST represses the heat shock response mediated by HSF1. Mol Cell. 2008;31(2):222–231. doi: 10.1016/j.molcel.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Blanco EH, Zúñiga JP, Andrés ME, Alvarez AR, Gysling K. Corticotropin-releasing factor binding protein enters the regulated secretory pathway in neuroendocrine cells and cortical neurons. Neuropeptides. 2011;45(4):273–279. doi: 10.1016/j.npep.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Doly S, Marullo S. Gatekeepers controlling GPCR export and function. Trends Pharmacol Sci. 2015;36(10):636–644. doi: 10.1016/j.tips.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Petaja-Repo UE, et al. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276(6):4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- 38.Lu M, Echeverri F, Moyer BD. Endoplasmic reticulum retention, degradation, and aggregation of olfactory G-protein coupled receptors. Traffic. 2003;4(6):416–433. doi: 10.1034/j.1600-0854.2003.00097.x. [DOI] [PubMed] [Google Scholar]
- 39.Pietilä EM, et al. Inefficient maturation of the rat luteinizing hormone receptor. A putative way to regulate receptor numbers at the cell surface. J Biol Chem. 2005;280(28):26622–26629. doi: 10.1074/jbc.M413815200. [DOI] [PubMed] [Google Scholar]
- 40.Chen C, et al. GEC1 interacts with the kappa opioid receptor and enhances expression of the receptor. J Biol Chem. 2006;281(12):7983–7993. doi: 10.1074/jbc.M509805200. [DOI] [PubMed] [Google Scholar]
- 41.Chan LF, et al. MRAP and MRAP2 are bidirectional regulators of the melanocortin receptor family. Proc Natl Acad Sci USA. 2009;106(15):6146–6151. doi: 10.1073/pnas.0809918106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutz C, et al. The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem. 2006;281(34):24910–24921. doi: 10.1074/jbc.M601554200. [DOI] [PubMed] [Google Scholar]
- 43.Doly S, et al. GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]