Significance
Defining targets and functional impacts of systemic treatment with antiangiogenic drugs on tumors and healthy tissues are crucial for understanding their therapeutic efficacy, drug resistance, adverse effects, and timeline of therapy. In the present study, we used both mouse and human tumor models to recapitulate clinical situations in which antiangiogenic drugs were systemically delivered to mice. Antiangiogenic drugs at low dosages produced broad effects on vessel regression in several endocrine organs. Prolonged treatment with the nonantitumor low dose of antiangiogenic drugs causes endocrine dysfunction in thyroid and adrenal gland. In fact, clinically manifested adverse effects, including hypertension, hypothyroidism, and glucocorticoid dysfunction, have been reproduced in our preclinical models by a low dose of an anti-VEGF drug.
Keywords: tumor, angiogenesis, VEGF, antiangiogenic therapy, adverse effects
Abstract
Anti-VEGF–based antiangiogenic drugs are designed to block tumor angiogenesis for treatment of cancer patients. However, anti-VEGF drugs produce off-tumor target effects on multiple tissues and organs and cause broad adverse effects. Here, we show that vasculatures in endocrine organs were more sensitive to anti-VEGF treatment than tumor vasculatures. In thyroid, adrenal glands, and pancreatic islets, systemic treatment with low doses of an anti-VEGF neutralizing antibody caused marked vascular regression, whereas tumor vessels remained unaffected. Additionally, a low dose of VEGF blockade significantly inhibited the formation of thyroid vascular fenestrae, leaving tumor vascular structures unchanged. Along with vascular structural changes, the low dose of VEGF blockade inhibited vascular perfusion and permeability in thyroid, but not in tumors. Prolonged treatment with the low-dose VEGF blockade caused hypertension and significantly decreased circulating levels of thyroid hormone free-T3 and -T4, leading to functional impairment of thyroid. These findings show that the fenestrated microvasculatures in endocrine organs are more sensitive than tumor vasculatures in response to systemic anti-VEGF drugs. Thus, our data support the notion that clinically nonbeneficial treatments with anti-VEGF drugs could potentially cause adverse effects.
Antiangiogenic therapy is a commonly used therapeutic approach for treatment of various human cancers (1–8). Since the US Food and Drug Administration approval of the first anti-VEGF–based antiangiogenic drug, bevacizumab, for treatment of metastatic colorectal cancer (CRC) in human patients in 2004 (3), more than 10 years of clinical experiences with this agent and other antiangiogenic drugs have raised many clinically unfulfilled issues, including modest beneficial effects, lack of reliable biomarkers for patient selection, development of drug resistance, adverse effects, long-term therapy, and mechanistic rationale of combination therapy (1).
Systemic antiangiogenic treatment of cancer patients would indistinguishably cause drug exposure to tumor and healthy vasculatures. Although the anti-VEGF–based antiangiogenic drugs are designed to target the tumor vasculature, systemic delivery of these drugs produce broad impacts on healthy vasculatures. For example, treatment of mice with anti-VEGF drugs causes marked vascular regression in endocrine organs and other tissues (9, 10). Given the pivotal functions of VEGF in vascular homeostasis, it would probably not be surprising for disruption of VEGF-mediated physiological functions to cause broad disruptive effects in multiple tissues and organs (11). VEGF is a multifunctional growth factor that displays angiogenic activity, vascular remodeling, vascular permeability, vascular homeostasis, and other nonvascular functions (12). Under physiological conditions, persistent high expression levels of VEGF are essential for maintenance of endothelium fenestrations, which are crucial for maintenance of endocrine functions (13–19). Similarly, VEGF is also a requisite for maintenance of the sinusoidal vascular architecture in liver, bone marrow, and spleen (9, 10, 20–22).
VEGF binds to VEGFR1 and VEGFR2 to exert its biological functions (23). Although VEGFR1-mediated functions are still unclear, VEGFR2 transduces functional signals of angiogenesis, vascular permeability, and vascular homeostasis (24). In most experimental settings, inhibition of VEGFR2 would be sufficient to block the VEGF-triggered vascular and other nonvascular functions. Targeting the VEGF–VEGFR2 signaling axis has offered an excellent opportunity for development of antiangiogenic drugs for cancer therapy. In fact, commonly used antiangiogenic drugs for cancer therapy in human patients, including bevacizumab, ramucirumab, and numerous tyrosine kinase inhibitors are designed for targeting the VEGF–VEGFR2 signaling system (11). Clinical experience with these anti-VEGF–based antiangiogenic drugs shows that they often produce adverse effects related to the off-drug targets (25). Hypertension, protein urea, hypothyroidism, and gastrointestinal bleeding are common, and infrequent serious side effects, including gastrointestinal perforation and arterial thrombotic events, can also occur (25, 26). Systemic anti-VEGF therapy-induced adverse effects suggest that these drugs could potentially cause adverse effects in those cancer patients who do not benefit from treatment. In addition, the issue of drug sensitivity on tumor vasculatures versus healthy vasculatures remains poorly studied.
In the present work, we show in different tumor models that low doses of an anti-VEGF antibody significantly alter numbers and structures of microvasculatures in endocrine organs without affecting tumor vasculatures. Prolonged therapy with the low-dose anti-VEGF drug causes hypertension and endocrine dysfunction. Our findings demonstrate that vasculatures in endocrine organs are more perceptive than those in tumors in response to antiangiogenic treatment.
Results
Dose-Dependent Inhibition of Tumor Angiogenesis by VEGF Blockade.
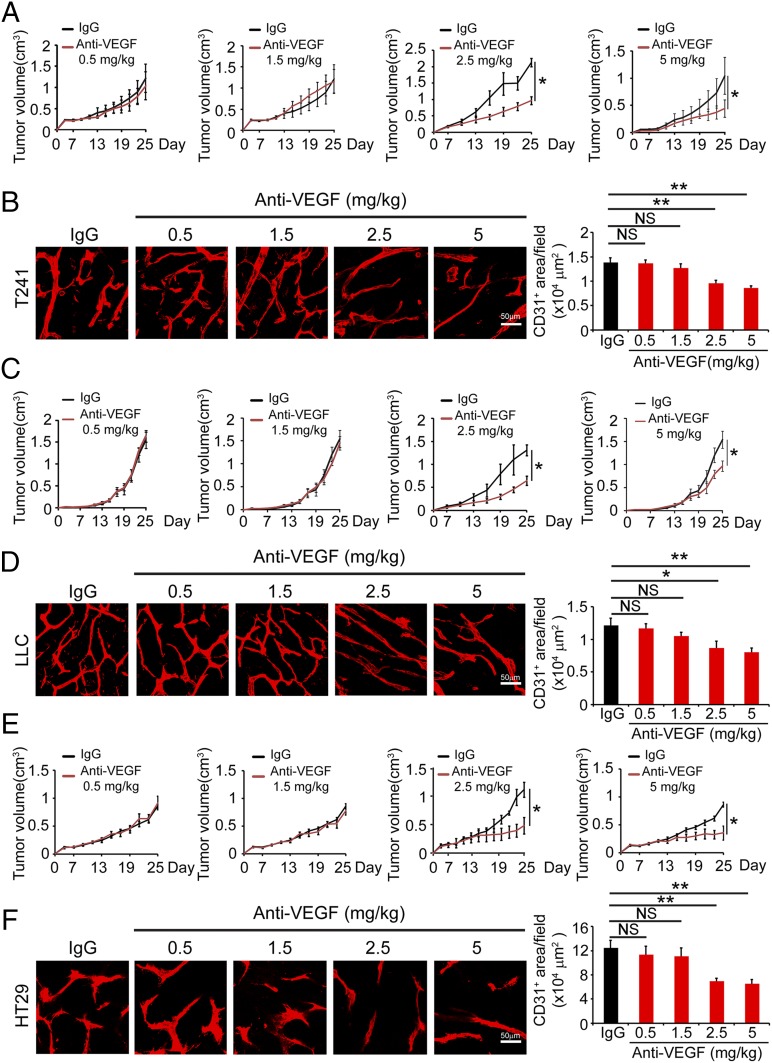
To study the impact of anti-VEGF drug therapy on tumor and healthy vasculatures, we used a rabbit anti-mouse VEGF neutralizing antibody (VEGF blockade) that has been shown to effectively inhibit tumor angiogenesis (10, 21, 27, 28). In two different mouse tumor models, including T241 fibrosarcoma and Lewis lung carcinoma (LLC), titration of different dosages of VEGF blockade showed dose-dependent inhibition of tumor growth. At 2.5 mg/kg and 5.0 mg/kg, VEGF blockade significantly inhibited tumor growth after 2-wk treatment (twice each week) (Fig. 1 A and C). However, low doses of 0.5 mg/kg and 1.5 mg/kg of VEGF blockade using the same treatment regimen did not significantly exhibit tumor growth relative to controls (Fig. 1 A and C). A similar pattern of the dose-dependent inhibition of tumor growth was also observed in a human HT29 CRC model (Fig. 1E).
Fig. 1.
Dose-dependent effects of VEGF blockade on tumor growth and angiogenesis. (A, C, and E) Dose-dependent effects of VEGF blockade on T241 (A), LLC (C), and HT29 (E) tumor growth after a 2-wk systemic treatment schedule. Doses of 2.5 mg/kg and 5.0 mg/kg produced significantly inhibited tumor growth in all three tumor models. VEGF blockade at doses of 0.5 mg/kg and 1.5 mg/kg did not significantly inhibited tumor growth. (B, D, and F) CD31+ microvessel density in VEGF blockade- or nonimmune IgG-treated T241 (B), LLC (D), and HT29 (F) tumors and quantified from eight random fields per group. Six to eight mice per group were used. NS, not significant; *P < 0.05; **P < 0.01. (Scale bars, 50 μm.) Quantitative data are presented as mean determinants ± SEM.
Consistent with inhibition of tumor growth, VEGF blockade at dosages of 0.5 mg and 1.5 mg/kg did not suppress tumor angiogenesis, whereas 2.5 mg/kg and 5.0 mg/kg significantly inhibited tumor neovascularization in all three tumor models (Fig. 1 B, D, and F). Additionally, VEGF blockade also induced a normalized vascular phenotype by pruning excessive sprouts of vascular networks (Fig. 1 B, D, and F). These data demonstrate that VEGF blockade inhibited tumor angiogenesis in a dose-dependent manner.
A Nonantitumor Dosage of VEGF Blockade Regresses Thyroid Vasculatures.
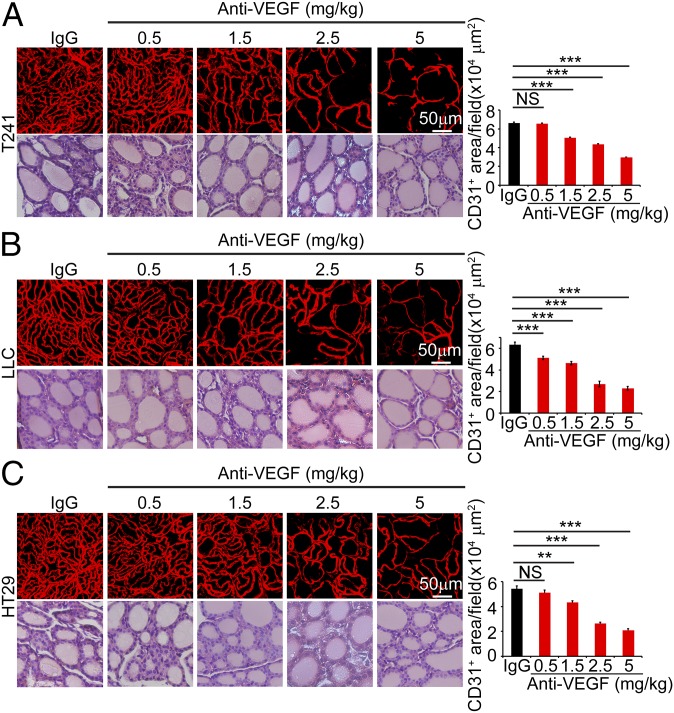
Knowing the effective dosages of VEGF blockade on tumor angiogenesis, we studied the impact of these doses on healthy vasculatures in adult animals. We previously showed that thyroid is one of the sensitive endocrine organs that manifest marked vascular regression in response to systemic anti-VEGF therapy (10). Interestingly, in T241 fibrosarcoma, LLC lung cancer, and human HT29 CRC models, systemic delivery of 1.5 mg/kg of VEGF blockade using the same regimen produced significantly repressive effects on thyroid vascular density (Fig. 2). Approximately, 20–30% of thyroid vasculatures became regressed after 2-wk treatment. However, this same dose of VEGF blockade did not inhibit tumor angiogenesis (Fig. 1). In the LLC lung cancer model, 0.5 mg/kg of VEGF blockade also caused significant regression of thyroid microvasculatures (Fig. 2B). This slight variation of sensitivity in different tumor models might reflect the differences of VEGF levels in tumors, which neutralize different amounts of antibodies. Expectedly, high doses of VEGF blockade at 2.5 mg/kg and 5.0 mg/kg produced profound effects of vascular regression in thyroid of tumor-bearing mice (Fig. 2). At the dose of 5.0 mg/kg, VEGF blockade produced more than 60% regression of thyroid vasculatures in all three tumor models. Apart from thyroid vascular regression, VEGF blockade did not affect the architecture of thyroid tissues at all dosages (Fig. 2). These data provide evidence that thyroid vasculatures are more sensitive than tumor microvasculatures in response to anti-VEGF therapy.
Fig. 2.
Impact of VEGF blockade on thyroid vasculatures in tumor-bearing mice. T241 (A), LLC (B), and HT29 (C) tumor-bearing mice were systemically treated with nonimmune IgG and various dosages of VEGF blockades and stained with CD31 and H&E. CD31+ microvessels were quantified from eight random fields per group. Six to eight mice per group were used. NS, not significant; **P < 0.01; ***P < 0.001. (Scale bars, 50 μm.) Quantitative data are presented as mean determinants ± SEM.
Low-Dose VEGF Blockade Regresses Adrenal and Pancreatic Islet Vasculatures.
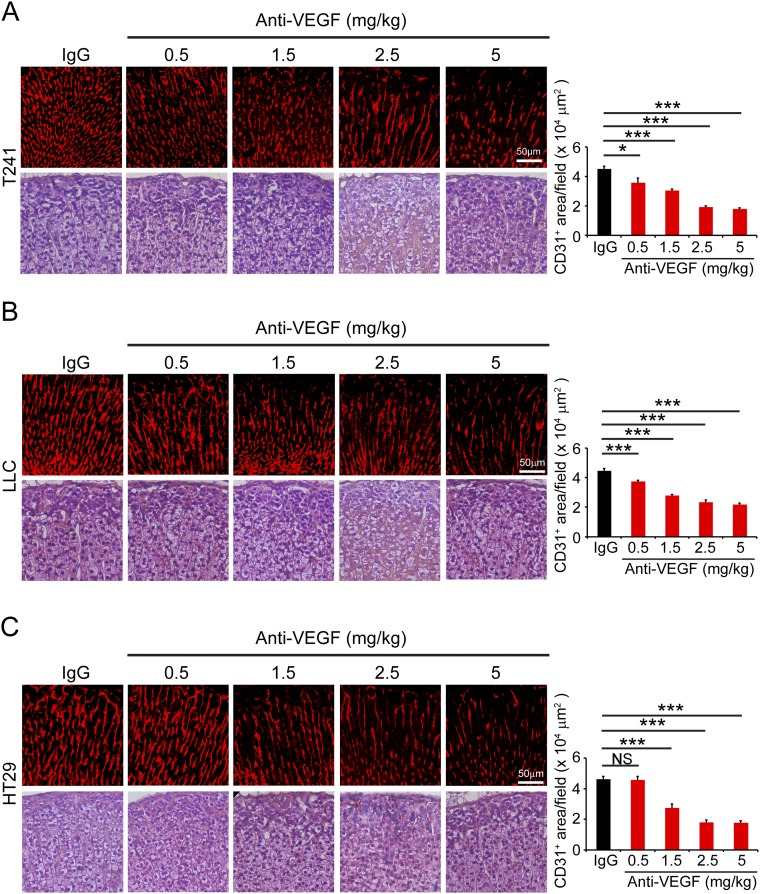
We extended our findings in adult thyroid to other endocrine organs, including adrenal glands and pancreatic islets. Similar to thyroid, VEGF blockade at a low dose of 1.5 mg/kg produced significantly regressive effects on adrenal microvasculatures in tumor-bearing mice (Fig. S1). It appeared that adrenal micorvasculatures were more prone to anti-VEGF treatment. At a very low-dose of 0.5 mg/kg, a significant decrease of adult adrenal microvessels was seen in T241 and LLC tumor-bearing mice (Fig. S1 A and B). However, this low-dose of VEGF blockade did not significantly regress adrenal microvasculatures in HT29 CRC tumor-bearing mice (Fig. S1C).
Fig. S1.
Impact of VEGF blockade on adrenal gland vasculatures in tumor-bearing mice. T241 (A), LLC (B), and HT29 (C) tumor-bearing mice were systemically treated with nonimmune IgG and various dosages of VEGF blockades and stained with CD31 and H&E. CD31+ microvessels were quantified from eight random fields per group. Six to eight mice per group were used. NS, not significant; *P < 0.05; ***P < 0.001. (Scale bars, 50 μm.) Quantitative data are presented as mean determinants ± SEM.
Dose-dependent vascular regression in adrenal glands was present in all three tumor models and 2.5 mg/kg reached the maximally regressive effect. Despite significant vascular regression, the adrenal tissue architecture was not affected by anti-VEGF treatment (Fig. S1).
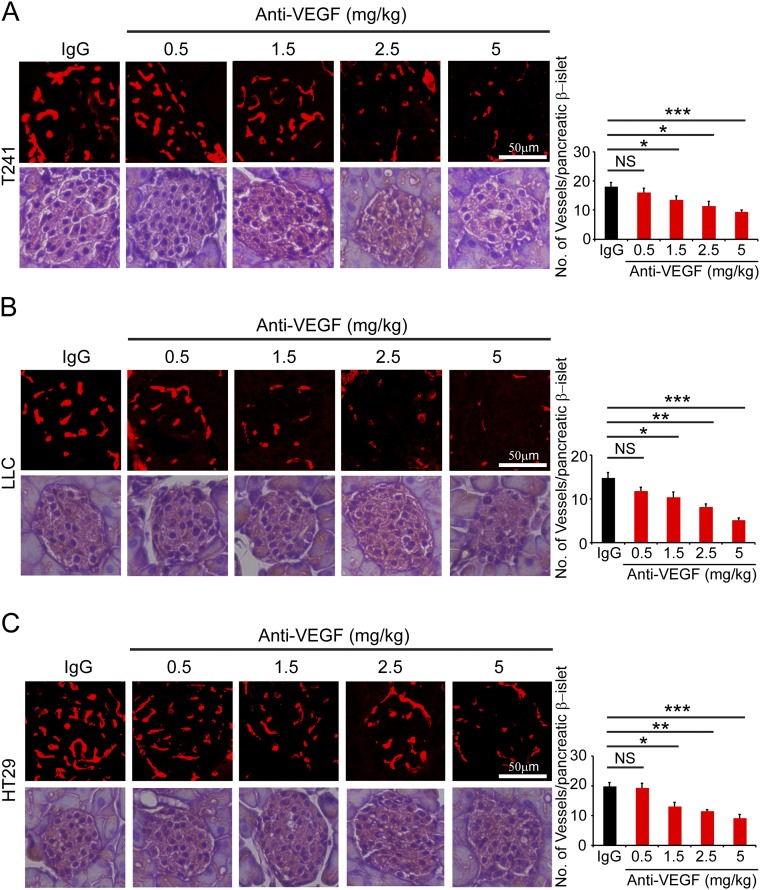
Similar to adrenal vasculatures, vessel density in pancreatic β-islets was also significantly decreased in response to anti-VEGF systemic treatment (Fig. S2). Again, VEGF blockade at 1.5 mg/kg significantly reduced microvessel numbers in tumor-bearing mice (Fig. S2). VEGF blockade induced dose-dependent responses of vascular regression in pancreatic β-islets. No obvious tissue architecture changes were present in all treated groups. Taken together, these results provide further evidence that vasculatures in endocrine organs are more sensitive to anti-VEGF treatment.
Fig. S2.
Impact of VEGF blockade on pancreatic β-islet vasculatures in tumor-bearing mice. T241 (A), LLC (B), and HT29 (C) tumor-bearing mice were systemically treated with nonimmune IgG and various dosages of VEGF blockades and stained with CD31 and H&E. CD31+ microvessels were quantified from eight random fields per group. Six to eight mice per group were used. NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. (Scale bar, 50 μm.) Quantitative data are presented as mean determinants ± SEM.
A Nonantitumor Dosage of VEGF Blockade Alters Vascular Functions in Thyroid.
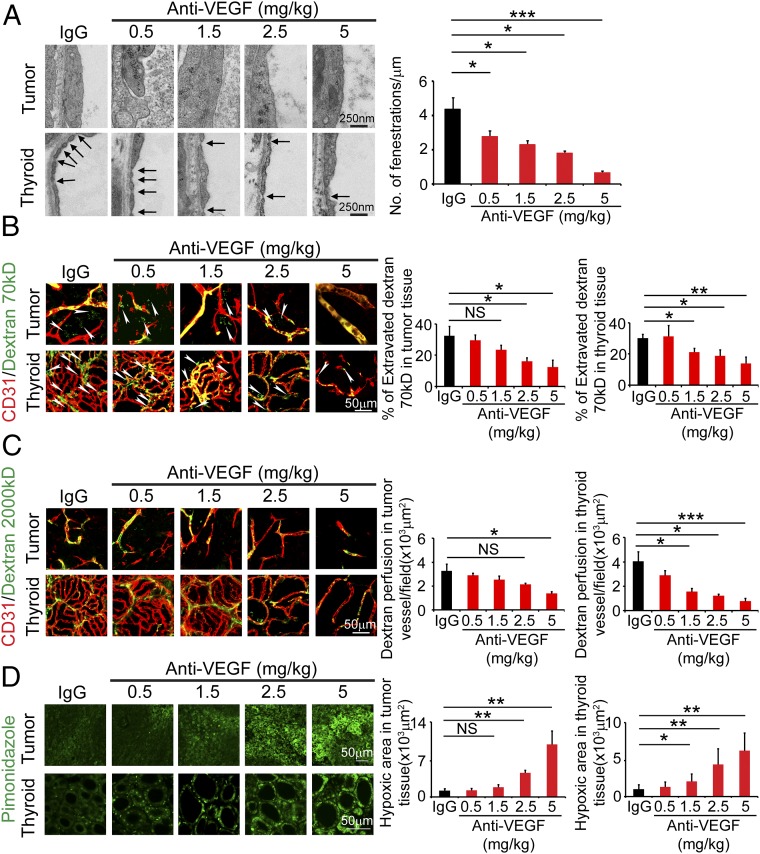
Giving the significant impact of a nonantitumor low-dose of VEGF blockade on endocrine vasculatures, we further investigated the functional consequences of VEGF blockade-treated and nontreated tumor-bearing animals. Because T241 fibrosarcoma-, LLC lung cancer-, and HT29 tumor-bearing mice showed similar vascular effects in endocrine organs, we chose the T241 tumor model as an example. It is known that thyroid vasculatures are constituted with fenestrated endothelium (9, 10). Indeed, transmission electron microscopic analysis revealed highly fenestrated endothelium in thyroid (Fig. 3A). Systemic anti-VEGF treatment of tumor-bearing mice significantly inhibited vascular fenestrations in a dose-dependent manner (Fig. 3A). At the dose of 1.5 mg/kg, significant inhibition of thyroid vascular fenestrations was observed (Fig. 3A). Unlike thyroid vasculatures, tumor microvessels lacked obvious fenestrations in the T241 fibrosarcoma model (Fig. 3A).
Fig. 3.
VEGF blockade-induced alterations of vascular fenestrations, permeability, perfusion, and hypoxia in thyroid glands of T241 tumor-bearing mice. (A) Inhibition of thyroid, but not tumor, endothelium fenestrations by all doses of VEGF blockades. Nonimmune IgG was used as a control. Fenestrae numbers were quantified as per micrometer from 8 to 10 vessels per group. Arrows point to endothelium fenestrae. (Scale bar, 250 nm.) (B) Leakage of fluorescein-labeled 70-kDa dextran (green) in tumors and thyroid gland. Thyroid vasculatures were stained with CD31 (red). Arrowheads point to extravasated fluorescein-dextran molecules. (Scale bar, 50 μm.) Data were quantified from eight random fields per group (n = 6–8 mice per group). (C) Perfusion of fluorescein-labeled 2,000-kDa dextran (green) in tumors and thyroid gland. Thyroid vasculatures were stained with CD31 (red). (Scale bar, 50 μm.) Data were quantified from eight random fields per group (n = 6–8 mice per group). (D) Tumor and thyroid tissue hypoxia was measured by pimonidazole positive signals. (Scale bar, 50 μm.) Data were quantified from eight random fields per group (n = 6–8 mice per group). NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Quantitative data are presented as mean determinants ± SEM.
The key function of fenestrated endothelium in endocrine organs is to mediate exchanges of large protein molecules in the endocrine local microenvironment (11). In control nonimmune IgG-treated tumor-bearing mice, thyroid vasculatures exhibited hyperpermeability by extravasating 70-kDa dextran molecules (Fig. 3B). In 1.5 mg/kg of VEGF blockade-treated thyroid, vascular permeability was significantly inhibited (Fig. 3B). However, this dose did not show significant inhibition of vascular leakage in tumors (Fig. 3B). High doses of VEGF blockade inhibited vascular permeability of both tumor and thyroid vessels (Fig. 3B). In addition to vascular permeability, 1.5 mg/kg of blockade also significantly inhibited blood vessel perfusion in thyroid gland, but not in tumors (Fig. 3C). Consistent with inhibition of vascular perfusion, this low dose of VEGF blockade also significantly increased thyroid, but not tumor, tissue hypoxia (Fig. 3D). These findings show that treatment with a low-dose VEGF blockade results in functional alterations of thyroid—but not tumor—vasculatures.
Prolonged Treatment with a Low Dose of VEGF Blockade Causes Endocrine Functional Changes.
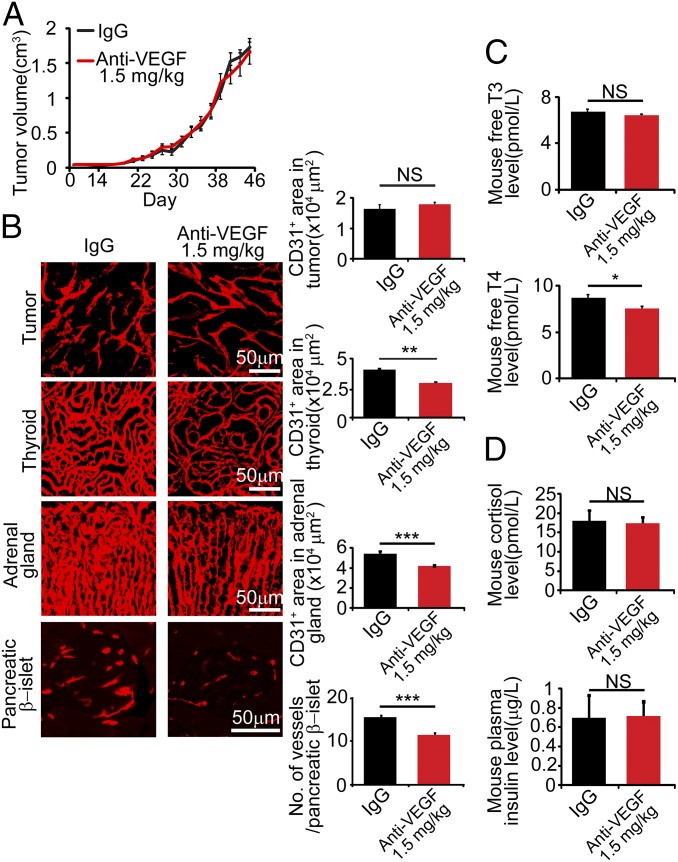
To link vascular changes to endocrine functions, we extended the treatment schedule of 1.5 mg/kg VEGF blockade for 4 wk in tumor-bearing mice and measured the plasma levels of endocrine hormones. Because of the ethical limit of tumor size, further extension of the treatment timeline was impossible. During the 4-wk treatment period, mouse EO771 breast cancer growth was not significantly affected relative to controls (Fig. 4A). In concordance with other tumor models, microvessel density in thyroid gland, adrenal gland, and pancreatic islets were all significantly decreased in response to this low-dose anti-VEGF therapy (Fig. 4B). Interestingly, the circulating level of free-T4, but not free-T3, thyroid hormone was significantly reduced (Fig. 4C). Other hormones including cortisol and insulin remained unchanged in response to the low dose of anti-VEGF therapy (Fig. 4D).
Fig. 4.
Prolonged anti-VEGF treatment at 1.5 mg/kg reduces thyroid free T4 hormone. (A) Anti-VEGF 4-wk treatment at 1.5 mg/kg did not inhibit EO771 tumor growth compared with nonimmune IgG (n = 6–8 mice per group). (B) Microvascular changes in tumors, thyroid glands, adrenal glands, and pancreatic β-islets. (Scale bar, 50 μm.) Data were quantified from eight random fields per group (n = 6–8 mice/group). (C and D) Measurement of thyroid free-T3 and T4 hormones, cortisol, and insulin in plasma of nonimmune IgG- and anti-VEGF–treated mice (n = 6–8 samples per group). NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Quantitative data are presented as mean determinants ± SEM.
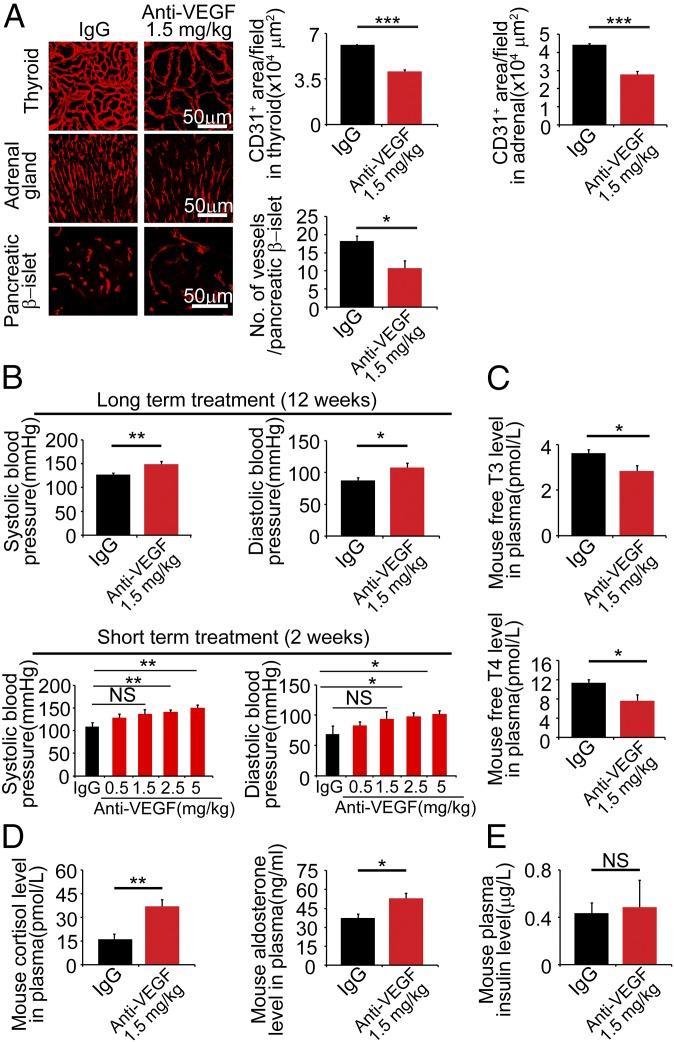
To further prolong the treatment timeline, we treated tumor-free mice with 1.5 mg/kg VEGF blockade for 12 wk. This treatment regimen produced a marked effect on vascular regression in the thyroid gland (Fig. 5A). Thus, prolonged treatment with a low-dose VEGF blockade produced a greater impact on regression of thyroid microvasculatures. Similar enhanced vascular regression effects were also seen in adrenal glands and pancreatic β-islets (Fig. 5A). Prolonged, but not short-term anti-VEGF therapy also caused hypertension in mice (Fig. 5B). Importantly, prolonged treatment with the low-dose of VEGF blockade marked reduced circulating levels of free-T3 and free-T4 thyroid hormone, leading to development hypothyroidism (Fig. 5C). In contrast, measurements of glucocorticoids, including cortisol and aldosterone, demonstrated marked increased levels of cortisol and aldosterone in the anti-VEGF–treated group compared with the control nonimmune IgG-treated group (Fig. 5D). Conversely, plasma insulin levels remained unchanged (Fig. 5E), consistent with our previous findings (29). These data provide compelling evidence that prolonged treatments with a low nonantitumor dose of an anti-VEGF drug produced profound functional impairments in endocrine organs.
Fig. 5.
Prolonged anti-VEGF therapy causes functional changes in endocrine organs in a tumor-free mouse model. (A) Microvascular changes in thyroid glands, adrenal glands, and pancreatic β-islets in tumor-free mice after treatment with VEGF blockade for 12-wk at 1.5 mg/kg compared with nonimmune IgG (n = 6–8 mice per group). (Scale bar, 50 μm.) Data were quantified from eight random fields per group (n = 6–8 mice per group). (B) Systolic and diastolic blood pressure changes after 12-wk 1.5 mg/kg VEGF blockade treatment (n = 6–8 mice per group). However, 1.5 mg/kg VEGF blockade has no impact on blood pressures after 2-wk short-term treatment (n = 6–8 mice per group). (C) Measurements of plasma free-T3 and -T4 thyroid hormones after 12-wk anti-VEGF treatment (n = 6–8 mice per group). (D) Measurements of plasma glucocorticoid hormones after 12-wk anti-VEGF treatment (n = 6–8 mice per group). (E) Measurements of plasma insulin levels after 12-wk anti-VEGF treatment (n = 6–8 mice per group). NS, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Quantitative data are presented as mean determinants ± SEM.
Discussion
Clinical practice with antiangiogenic drugs for cancer treatment often raises the concern of specific targets by antiangiogenic agents. Although there is a lack of supportive evidence, it has been speculated that angiogenic vessels in growing tumors are more sensitive to antiangiogenic drugs than those quiescent vasculatures in healthy tissues and organs. This is probably true for certain angiogenesis inhibitors, such as angiostatin and endostatin that specifically target proliferating endothelial cells (30, 31). However, these generic angiogenesis inhibitors are not commonly used for treatment of human patients and molecular mechanisms underlying their antiangiogenic function are poorly understood, despite their early discoveries. Given the broad physiological functions of VEGF, anti-VEGF–based antiangiogenic drugs would, in theory, indiscriminately target multiple healthy vasculatures in different tissues and organs. In particular, VEGF has been described as a potent survival factor for maintenance of vascular networks in different tissues (32). Interference of these physiological functions with systemic delivery of anti-VEGF drugs would inevitably affect vascular and organ functions.
The key question is which vasculatures in tumor and healthy tissues are more sensitive to systemic treatment with anti-VEGF drugs. Despite anti-VEGF drugs being used in human cancer patients for more than 10 y (1), this crucial question remains unknown. This is particularly important for those patients who do not benefit from anti-VEGF therapy. Would the nonbeneficial patient population develop adverse effects? In various mouse tumor models, we provide comprehensive evidence that endocrine vasculatures—including thyroid, adrenal gland, and pancreatic islets—are more sensitive to anti-VEGF therapy. At a low nonantitumor dose, systemic treatment with VEGF blockade significantly causes regression of healthy vasculatures in these endocrine organs. Why are the structural molecular bases for endocrine microvasculatures being more sensitive to anti-VEGF treatment? The endocrine microvasculature contains highly fenestrated endothelium for mediating hormone transport to the targeted organs. VEGF is a crucial factor for maintaining vascular fenestrations in endocrine organs and inhibition of VEGF function completely blocked vascular fenestrations in endocrine organs (10). VEGF appears to have two critical functions in modulation of endocrine vasculatures: (i) vascular homeostasis and survival, and (ii) maintenance of vascular fenestrations and architectures. Compelling evidence shows that vascular survival and fenestration maintenance are completely dependent on VEGF (12, 13, 17). Unlike endocrine organs, tumors use multiple angiogenic factors to stimulate neovascularization and inhibition of the VEGF–VEGFR signaling may not always produce profound antiangiogenic effects. In this regard, tumors originating from endocrine organs and neuroendocrine tumors are probably more susceptible to antiangiogenic therapy (33, 34).
A substantial number of cancers are intrinsically resistant to anti-VEGF therapy and intrinsic resistance is frequently seen in cancer patients (35). Antiangiogenic treatment of these patients would not only be nonbeneficial, but could also potentially produce adverse effects, because off-tumor vasculatures in endocrine organs are preferable targets. Particularly, we show that prolonged low-dose treatment causes functional impairments of endocrine organs and hypertension, which are also often manifested in human cancer patients (25). Hypothyroidism is another commonly seen adverse effect in human cancer patients in response to antiangiogenic therapy (36). Thus, our preclinical findings provide further mechanistic insights into development of clinical adverse effects in patients receiving antiangiogenic therapy. In light of this view, defining reliable biomarkers would not only improve beneficial outcomes but also avoid development of unnecessary adverse effects in nonresponders. From the patient point of view, one would not like to buy an expensive, but ineffective drug for development of side effects. These important issues warrant further clinical validation in cancer patients.
Materials and Methods
Cell Line.
Monolayers of T241, LLC, HT29, and EO771 tumor cells were cultured in DMEM supplemented with 10% (vol/vol) heat-inactivated FBS (HyClone; Cat. No. SH30160.03), 100 U/mL penicillin, and 100 μg/mL streptomycin (HyClone; Cat. No. SV30010).
Animals.
All animal studies were approved by the North Stockholm Animal Ethical Committee. C57BL/6 mice were provided by the breeding unit of the animal facility of the Department of Microbiology, Tumor and Cell Biology, Karolinska Institute, Sweden. Immunodeficient CB17/Icr-Prkdcscid/IcrCrl mice were purchased from Charles River Laboratories. Mice at age 6–8 wk were used for all tumor studies.
Mouse Tumor Model and Anti-VEGF Treatment.
Approximately 1 × 106 mouse T241, LLC, EO771, and 3 × 106 human HT29 tumor cells were suspended in 100 μL PBS, and subcutaneously injected into the middorsal region on the back of each mouse. Tumors were measured every other day and the tumor size was calculated by width2 × length × 0.52. A rabbit anti-mouse VEGF-specific neutralizing antibody (BD0801) was kindly provided by the Simcere Pharmaceutical Company (Nanjing, Jiangsu, China). A rabbit IgG isotype (Cat. No. 10500C, Invitrogen) was used as a control vehicle. Both IgG and the anti-VEGF antibody at different doses were injected intraperitoneally twice a week into each mouse when tumor size reached 0.2 cm2 for a total of a 2-wk duration for T241, LLC, and HT29 tumor models. For EO771 tumor and tumor-free mouse models, anti-VEGF treatment at the dose of 1.5 mg/kg was administered for 4 and 12 wk, respectively.
Blood Pressure Measurement.
Mouse systolic and diastolic blood pressures were measured by a noninvasive CODA tail-cuff blood pressure system using the volume pressure R\recording (VPR) technique (Cat.No. CODA HT2, Kent Scientific). Briefly, mice were accustomed to the testing tube before measurement. Mice were warmed up on a 37 °C warming plate, and connected to the CODA blood pressure system through the tail vein. Each mouse was measured for 25 cycles and an average value was presented as the final result for each mouse (n = 6–8 mice per group).
Statistics.
Randomized micrographs from six to eight different fields per group were quantified. For each experiment, six to eight animals were recruited to each group and were repeated twice. An Adobe Photoshop CS5 software program was used with a color range tool and a count tool to detect positive areas or numbers. Statistical analyses were performed using the standard two-tailed Student t test, and values of P < 0.05, P < 0.01, and P < 0.001 were deemed statistically significant. Data were presented as mean ± SEM.
SI Materials and Methods
Histology, Immunohistochemistry, and Whole-Mount Staining.
Paraffin-embedded thyroid, adrenal gland, and pancreas tissues were cut into 5-μm thickness sections and baked on slides at 60 °C overnight. Sections were de-paraffinized in Tissue-Clear (Cat. No. 1466, Sakura), and rehydrated with 99–95% (vol/vol) to 70% (vol/vol) ethanol. Sections were stained with Hematoxylin for 5 min, followed by washing with H2O for 10 min. Subsequent Eosin staining was executed for 30 s, followed by dehydration with 95–99% (vol/vol) ethanol. Stained slides were mounted with PERTEX (Cat. No. 00801, HistoLab). Images were randomly taken using a light microscope (Nikon Eclipse TS100). Some tissue sections were subjected to immunofluorescent staining for CD31 and pimonidazole after rehydration. Antigen retrieval was done with a retrieval buffer (Cat. No. H3300, Vector laboratories) by boiling for 10 min. Sections were blocked with 4% (vol/vol) goat serum for 30 min at room temperature, followed by incubation at 4 °C overnight with an anti-CD31 primary antibody (Cat. No. AF3628, R&D; 1:200 dilution) or a FITC conjugated anti-pimonidazole (Cat. No. HP2-1000Kit, Hypoxyprobe; 1:200 dilution) antibody. Sections were further stained with a secondary donkey anti-goat Alexa 555 antibody (Cat. No. A21432, Invitrogen). Images were captured under fluorescent microscopy (ECLIPSE 90i, NIS-Element D, Nikon).
Whole-mount staining was performed as previously described (10, 29, 37). Briefly, PFA-fixed tumor and thyroid tissues were cut into small pieces, and digested with proteinase K (20 μg/mL). A primary goat anti-mouse CD31 antibody (Cat. No. AF3628, R&D) was incubated overnight at 4 °C. A secondary donkey anti-goat Alexa 555 antibody in 3% (wt/vol) skim milk (Cat. No. A21432, Invitrogen; diluted 1:400) was added to the tissue sections and incubated 2 h at room temperature. Stained samples were analyzed under a confocal microscope (ECLIPSE 90i, EZ-C1, Nikon). Three-dimensional images of each tissue sample were captured and at least six to eight different tissue sections of each group were quantitatively analyzed using an Adobe Photoshop CS5 software program.
Vascular Perfusion, Permeability, and Hypoxia.
Mice treated with different doses of an anti-VEGF antibody were anesthetized, followed by injection into the tail vein with a lysinated fluorescein-labeled dextran (2,000 kDa; 1 mg per mouse; Cat. No. D7139, Invitrogen). After 5 min, animals were killed, and tissues and organs were dissected, followed by immediate fixation with 4% (wt/vol) PFA at 4 °C. For permeability assay, a lysinated fluorescein-labeled dextran (70 kDa; 1.25 mg per mouse; Cat. No. D1818, Invitrogen) was injected into the tail vein of each mouse. After 15 min, animals were killed. Tissues were carefully dissected, whole-mount stained, and examined by confocal microscopy. For detection of hypoxia in tumor and thyroid tissues, a hypoxia probe, pimonidazole hydrochloride, was intravenously injected into the tail vein of each mouse at the dose of 30 mg/kg. Mice were killed 30-min later after injection, and tissues were dissected and fixed in 4% (wt/vol) PFA immediately.
Transmission Electron Microscopy.
Transmission electron microscopy was performed as previously described (10). Briefly, tumor-bearing mice were perfused with a fixation buffer containing 2.5% (vol/vol) glutaraldehyde. Tumor and thyroid tissues were dissected, cut into small pieces, and kept in the fixative until use. Ultrathin tissue sections in 40–50 nm in thickness were prepared and examined under a Tecnai 12 Spirit Bio TWIN transmission electron microscope (Fei Company) at 100 kV. Digital images were captured using a Veleta camera (Olympus Soft Imaging Solutions).
ELISA.
Plasma-free thyroxine (T3), free triiodothyronine (T4), mouse cortisol, and mouse aldosterone were measured by Elisa kits (mFT3, Cat. No. MBS705057; mFT4, Cat. No. MBS700444; mouse Cortisol Cat. No. MBS1604, mouse Aldosterone, Cat. No. MBS035007, MyBiosource). Blood samples were collected with EDTA-coated microtubes (Cat. No. 365975, BD), followed by centrifugation at 2,000 × g for 20 min. Plasma samples were collected and kept in −80 °C. ELISA was performed according to the manufacturer’s instructions.
Acknowledgments
Y.C.’s laboratory is supported through research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Swedish Diabetes Research Foundation, the Swedish Childhood Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute Distinguished Professor award, the Torsten Soderbergs Foundation, European Research Council Advanced Grant ANGIOFAT (Project 250021), the Knut Alice Wallenberg Foundation, the NOVO Nordisk Foundation for the advanced grant, Program of Introducing Talents of Discipline to Universities (Grant B07035), and the State Program of National Natural Science Foundation of China for Innovative Research Group (Grant 81321061).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1601649113/-/DCSupplemental.
References
- 1.Cao Y, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3(114):114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Agostino RB., Sr Changing end points in breast-cancer drug approval—The Avastin story. N Engl J Med. 2011;365(2):e2. doi: 10.1056/NEJMp1106984. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 7.Tannock IF, et al. VENICE investigators Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): A phase 3, double-blind randomised trial. Lancet Oncol. 2013;14(8):760–768. doi: 10.1016/S1470-2045(13)70184-0. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Angiogenesis: An organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 9.Kamba T, et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290(2):H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, et al. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci USA. 2013;110(29):12018–12023. doi: 10.1073/pnas.1301331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao Y. VEGF-targeted cancer therapeutics-paradoxical effects in endocrine organs. Nat Rev Endocrinol. 2014;10(9):530–539. doi: 10.1038/nrendo.2014.114. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29(6):789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 13.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 14.Alfer J, Neulen J, Gaumann A. Lactotrophs: The new and major source for VEGF secretion and the influence of ECM on rat pituitary function in vitro. Oncol Rep. 2015;33(5):2129–2134. doi: 10.3892/or.2015.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita S, et al. Vascular endothelial growth factor-dependent angiogenesis and dynamic vascular plasticity in the sensory circumventricular organs of adult mouse brain. Cell Tissue Res. 2015;359(3):865–884. doi: 10.1007/s00441-014-2080-9. [DOI] [PubMed] [Google Scholar]
- 16.Iwashita N, et al. Impaired insulin secretion in vivo but enhanced insulin secretion from isolated islets in pancreatic beta cell-specific vascular endothelial growth factor-A knock-out mice. Diabetologia. 2007;50(2):380–389. doi: 10.1007/s00125-006-0512-0. [DOI] [PubMed] [Google Scholar]
- 17.LeCouter J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412(6850):877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 18.Vittet D, Ciais D, Keramidas M, De Fraipont F, Feige JJ. Paracrine control of the adult adrenal cortex vasculature by vascular endothelial growth factor. Endocr Res. 2000;26(4):843–852. doi: 10.3109/07435800009048607. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard I, et al. ACTH-regulated expression of vascular endothelial growth factor in the adult bovine adrenal cortex: A possible role in the maintenance of the microvasculature. J Cell Physiol. 2000;185(2):226–234. doi: 10.1002/1097-4652(200011)185:2<226::AID-JCP7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell RK, et al. VEGF-A/VEGFR inhibition restores hematopoietic homeostasis in the bone marrow and attenuates tumor growth. Cancer Res. 2016;76(3):517–524. doi: 10.1158/0008-5472.CAN-14-3023. [DOI] [PubMed] [Google Scholar]
- 21.Lim S, et al. VEGFR2-mediated vascular dilation as a mechanism of VEGF-induced anemia and bone marrow cell mobilization. Cell Reports. 2014;9(2):569–580. doi: 10.1016/j.celrep.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 22.DeLeve LD. Liver sinusoidal endothelial cells and liver regeneration. J Clin Invest. 2013;123(5):1861–1866. doi: 10.1172/JCI66025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 24.Shibuya M. VEGF-VEGFR signals in health and disease. Biomol Ther (Seoul) 2014;22(1):1–9. doi: 10.4062/biomolther.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott LJ, Chakravarthy U, Reeves BC, Rogers CA. Systemic safety of anti-VEGF drugs: A commentary. Expert Opin Drug Saf. 2015;14(3):379–388. doi: 10.1517/14740338.2015.991712. [DOI] [PubMed] [Google Scholar]
- 26.Afranie-Sakyi JA, Klement GL. The toxicity of anti-VEGF agents when coupled with standard chemotherapeutics. Cancer Lett. 2015;357(1):1–7. doi: 10.1016/j.canlet.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, et al. Vascular endothelial growth factor-dependent spatiotemporal dual roles of placental growth factor in modulation of angiogenesis and tumor growth. Proc Natl Acad Sci USA. 2013;110(34):13932–13937. doi: 10.1073/pnas.1309629110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Y, et al. A humanized anti-VEGF rabbit monoclonal antibody inhibits angiogenesis and blocks tumor growth in xenograft models. PLoS One. 2010;5(2):e9072. doi: 10.1371/journal.pone.0009072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Honek J, et al. Modulation of age-related insulin sensitivity by VEGF-dependent vascular plasticity in adipose tissues. Proc Natl Acad Sci USA. 2014;111(41):14906–14911. doi: 10.1073/pnas.1415825111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Reilly MS, et al. Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 31.O’Reilly MS, et al. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 32.Ellis LM, Reardon DA. Cancer: The nuances of therapy. Nature. 2009;458(7236):290–292. doi: 10.1038/458290a. [DOI] [PubMed] [Google Scholar]
- 33.Arbiser ZK, Arbiser JL, Cohen C, Gal AA. Neuroendocrine lung tumors: Grade correlates with proliferation but not angiogenesis. Mod Pathol. 2001;14(12):1195–1199. doi: 10.1038/modpathol.3880459. [DOI] [PubMed] [Google Scholar]
- 34.Chan JA, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30(24):2963–2968. doi: 10.1200/JCO.2011.40.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boehm S, Rothermundt C, Hess D, Joerger M. Antiangiogenic drugs in oncology: A focus on drug safety and the elderly—A mini-review. Gerontology. 2010;56(3):303–309. doi: 10.1159/000262450. [DOI] [PubMed] [Google Scholar]
- 37.Jensen LD, et al. VEGF-B-Neuropilin-1 signaling is spatiotemporally indispensable for vascular and neuronal development in zebrafish. Proc Natl Acad Sci USA. 2015;112(44):E5944–E5953. doi: 10.1073/pnas.1510245112. [DOI] [PMC free article] [PubMed] [Google Scholar]