Significance
Protein S-nitrosation (SNO-protein) is a posttranslational modification in which a cysteine (Cys) residue is modified by nitric oxide (SNO-Cys). SNO-proteins impact many biological systems, but their identification has been technically challenging. We developed a chemical proteomic strategy—SNOTRAP (SNO trapping by triaryl phosphine)—that allows improved identification of SNO-proteins by mass spectrometry. We found that S-nitrosation is elevated during early stages of neurodegeneration, preceding cognitive decline. We identified changes in the SNO-proteome during early neurodegeneration that are potentially relevant for synapse function, metabolism, and Alzheimer’s disease pathology. SNO-proteome analysis further reveals a potential linear motif for SNO-Cys sites that are altered during neurodegeneration. Our strategy can be applied to multiple cellular and disease contexts and can reveal signaling networks that aid drug development.
Keywords: S-nitrosation, Alzheimer’s disease, secretase pathway, presenilin pathway, neurodegeneration
Abstract
Protein S-nitrosation (SNO-protein), the nitric oxide-mediated posttranslational modification of cysteine thiols, is an important regulatory mechanism of protein function in both physiological and pathological pathways. A key first step toward elucidating the mechanism by which S-nitrosation modulates a protein’s function is identification of the targeted cysteine residues. Here, we present a strategy for the simultaneous identification of SNO-cysteine sites and their cognate proteins to profile the brain of the CK-p25–inducible mouse model of Alzheimer’s disease-like neurodegeneration. The approach—SNOTRAP (SNO trapping by triaryl phosphine)—is a direct tagging strategy that uses phosphine-based chemical probes, allowing enrichment of SNO-peptides and their identification by liquid chromatography tandem mass spectrometry. SNOTRAP identified 313 endogenous SNO-sites in 251 proteins in the mouse brain, of which 135 SNO-proteins were detected only during neurodegeneration. S-nitrosation in the brain shows regional differences and becomes elevated during early stages of neurodegeneration in the CK-p25 mouse. The SNO-proteome during early neurodegeneration identified increased S-nitrosation of proteins important for synapse function, metabolism, and Alzheimer’s disease pathology. In the latter case, proteins related to amyloid precursor protein processing and secretion are S-nitrosated, correlating with increased amyloid formation. Sequence analysis of SNO-cysteine sites identified potential linear motifs that are altered under pathological conditions. Collectively, SNOTRAP is a direct tagging tool for global elucidation of the SNO-proteome, providing functional insights of endogenous SNO proteins in the brain and its dysregulation during neurodegeneration.
Protein S-nitrosation (SNO-protein), in which a cysteine (Cys) thiol is converted to a nitrosothiol (RSNO), is an important posttranslational modification (PTM). Cys residues targeted for S-nitrosation often impact enzyme activity, protein localization, and protein–protein interactions (1). SNO begins with the production of nitric oxide radicals (NO•) via conversion of l-arginine to l-citrulline by nitric oxide synthase 1 (NOS1) (neuronal), NOS2 (inducible), and NOS3 (endothelial). NO-mediated SNO PTMs are thought to occur in vivo predominantly through radical recombination between NO• and a thiyl radical, transnitrosation by low-molecular weight NO carriers such as S-nitrosoglutathione (GSNO), or protein-assisted transnitrosation (2–8). In the healthy brain, low levels of NO and normal SNO PTMs play important roles in regulating synaptic plasticity, gene expression, and neuronal survival. In contrast, elevated NO levels associated with aging and environmental stress have been linked to neurological pathologies, including Alzheimer’s (AD), Parkinson’s, and Huntington’s disease (9). AD is the most prevalent form of human dementia, with a frequency that progressively increases in aging societies (10). A pivotal role in development and progression of late-onset AD, and other age-dependent dementias, has been attributed to inflammatory and oxidative stress cascades in the brain (11, 12), which are potentiated by elevated levels of nitrosating and oxidizing species (13, 14).
Despite the biological importance of this PTM, significant gaps exist regarding its in vivo specificity and origin. Characterization of endogenous proteins suggests that not all reduced Cys residues on a given protein and not all Cys-containing proteins are S-nitrosated, implying a biased selection. Although S-nitrosation has been frequently reported, the specific SNO residues for many of the proteins have not been determined and can be critical for determining their function. Currently, the identification of a specific SNO residue involves an iterative combination of mutagenic and mass spectrometry (MS)-based approaches. The prototypical method for detecting SNO-proteins is the biotin-switch technique (BST), which requires blocking of all free Cys-thiols, followed by selective ascorbate reduction of SNO-Cys residues that are biotinylated and isolated for analysis (9, 15–17). One limitation of the BST is that false positives can occur through incomplete blocking of free Cys-thiols, making them difficult to distinguish from true SNO-Cys residues. The variability of the BST and similar methods has driven a search for alternative approaches for accurate SNO-Cys detection and mapping.
In this context, we have developed a method that enables global, facile, and high-throughput identification of endogenous SNO-Cys residues. SNOTRAP (SNO trapping by triaryl phosphine) is a direct tagging technique that allows enrichment and identification of SNO-proteins and their cognate SNO-sites. Hyperactivation of cyclin-dependent kinase-5 (Cdk5) by its activator peptide, p25, leads to AD-like neurodegenerative pathology, and inhibition of p25 activity ameliorates AD phenotypes (18–20). The CK-p25 mouse model of AD-like neurodegeneration allows temporal characterization of neurodegeneration through inducible expression of the p25 activator peptide, leading to elevated amyloid-β levels and DNA damage, followed by synaptic loss, neuronal death, and cognitive impairments (21–24). SNOTRAP was used here to profile changes in the SNO-proteome of the CK-p25 mouse model of AD-like neurodegeneration and healthy controls (21, 22). Our data provide insights into signaling pathways that may be perturbed by SNO in the neurodegenerating brain that could provide novel avenues for AD-related therapies.
Results and Discussion
Site-Specific Identification of SNO-Peptides.
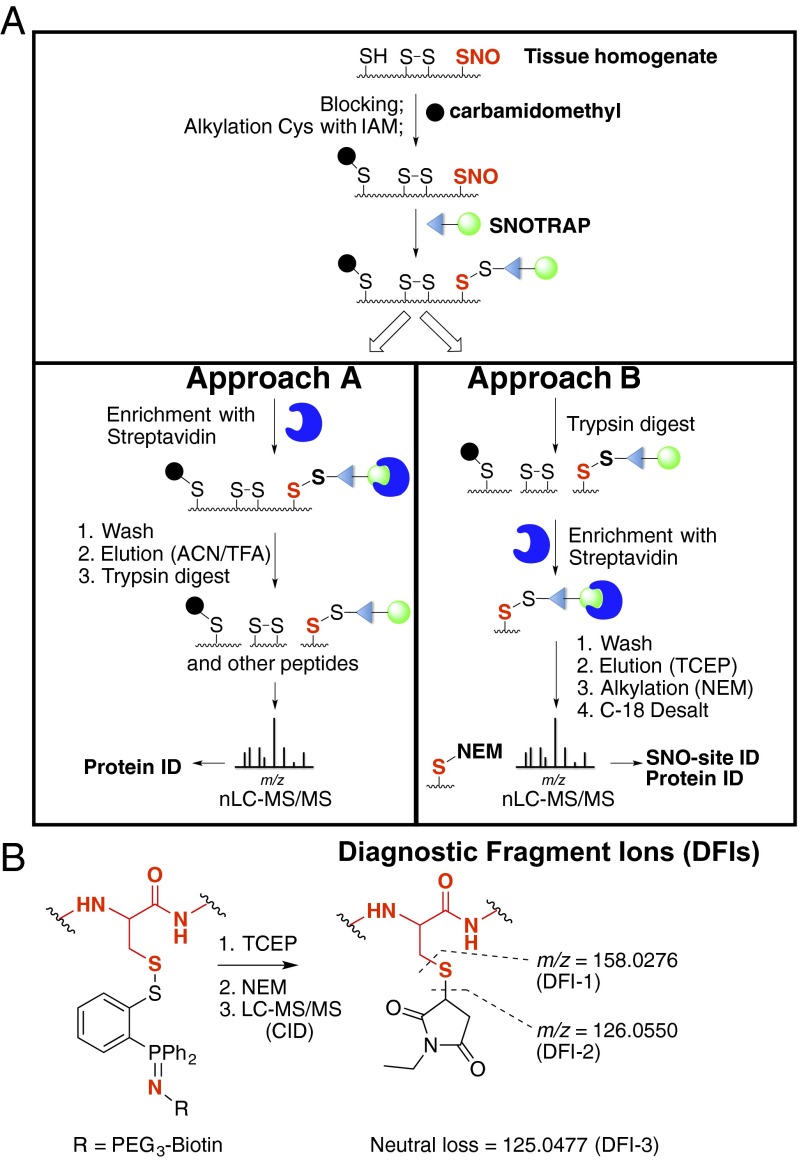
SNOTRAP is a proteomic extension of the method described earlier for detecting low-molecular--weight RSNOs using phosphine ester reagents (25). The SNOTRAP probe consists of a triphenylphosphine thioester linked to a biotin molecule through a polyethyleneglycol (PEG) spacer group. The method is based on reaction of the triphenylphosphine thioester with RSNO and subsequent biotin-mediated affinity capture of labeled peptides and/or proteins. The probe reacts with SNO groups, first yielding an azaylide intermediate, which through a properly positioned electrophile (thioester), rearranges to form a disulfide–iminophosphorane (Fig. S1A) (25, 26). The reaction proceeds through a Staudinger ligation-type mechanism that retains the nitrogen atom and thiol moiety, allowing unequivocal confirmation of specific SNO-sites in the peptide. Overall, the workflow consists of three main steps: (i) blocking of reduced Cys residues with iodoacetamide (IAM), (ii) capture and release of SNO-proteins or SNO-peptides, and (iii) nanoflow liquid chromatography (nLC)–MS/MS analysis (Fig. 1A). Blocking with IAM prevents transnitrosation during sample preparation, ensuring that the in vivo location of SNO-sites is retained.
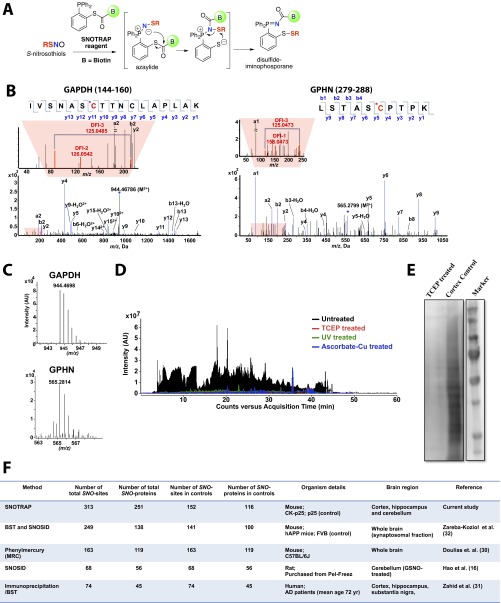
Fig. S1.
Site-specific identification of SNO-protein. (A) Schematic for selective tagging of protein S-nitrosothiols using the biotin-SNOTRAP (b-SNOTRAP) probe. The SNOTRAP reagent directly reacts with the –SNO group to yield an azaylide intermediate, which rearranges to form the disulfide–iminophosphorane compound. (B) Representative MS/MS spectra for SNO-Cys150 of GAPDH and SNO-Cys284 of GPHN (SNO-Cys is highlighted in red). (C) Representative MS1 spectrums of GAPDH (monoisotopic m/z 944.4688, MH+error 0.1 ppm) and GPHN (monoisotopic m/z 565.2814, MH+ error 0.2 ppm) identified in cortex with low ppm mass error. (D) Representative total ion chromatograms (TICs) after b-SNOTRAP capture showing ion intensity of untreated (black), TCEP-treated (red), UV-treated (green), and ascorbate-Cu–treated (blue) samples. Chromatogram corresponding to TCEP treatment was plotted with a y axis offset of 1E4. (E) Representative Western blot of total b-SNOTRAP–captured proteins of untreated (cortex control) and TCEP-treated samples. (F) Current methodologies used for detection of SNO-peptides and SNO-Cys sites (16, 30–32).
Fig. 1.
Site-specific identification of SNO-protein. (A) Schematic for selective labeling and analysis of SNO-proteins. Unmodified Cys-thiols were blocked, and SNO-Cys sites were labeled with the b-SNOTRAP probe. For approach A, b-SNOTRAP–tagged proteins were enriched using streptavidin beads, washed, eluted with denaturing conditions, trypsin-digested, and analyzed by LC–MS/MS. For approach B, the proteome was trypsin-digested, and b-SNOTRAP–tagged peptides were enriched using streptavidin beads, followed by release of the biotin linker by TCEP; alkylated with NEM; and analyzed by LC–MS/MS. (B) Generation of DFIs from NEM-modified peptides.
To identify SNO proteins, and their cognate SNO-sites, we used two complementary approaches (Fig. 1A). The first was to identify SNO proteins, whereby SNOTRAP-modified proteins were enriched by streptavidin affinity (Fig. 1A, approach A). Tryptic peptides were analyzed by LC–MS/MS, and proteins were identified by database searching. Identification of SNO-sites using approach A was hindered due to extra features in the mass spectra arising from the SNOTRAP tag. These features include (i) limited ionization due to the added bulkiness of the triphenylphosphine–PEG–biotin (SNOTRAP) tag, (ii) added features to the collision-induced dissociation (CID) spectra such as tag-related fragment ions and corresponding neutral losses, and (iii) ion suppression by the dominant ions created by the SNOTRAP tag.
Consequently, a second approach (B) was developed that substitutes N-ethyl maleimide (NEM) for the bulky SNOTRAP tag, which allows direct detection of SNO-sites by MS. Briefly, proteolytic digestion is performed before streptavidin capture to isolate modified peptide fragments that contain the SNOTRAP tag rather than intact SNO-proteins. (Fig. 1A, approach B). The SNOTRAP tag is subsequently cleaved, the peptides eluted with triscarboxyethylphosphine (TCEP), and the liberated Cys labeled with NEM before nLC–MS/MS. This strategy selectively enriches only SNO-containing peptides, which reduces the complexity of the nLC separation and improves the detection of SNO-sites. The diagnostic fragment ions (DFIs) at m/z 126.0550, 125.0477, and 158.0276 verify that this was a SNO-peptide (Fig. 1B and Fig. S1B) (27, 28). Our criteria for identification of SNO-proteins required detection of two or more tryptic peptides per SNO-protein using approach A and the SNO-Cys site-specific peptide identification using approach B.
Cortical tissues from CK-p25 mice were analyzed independently by capture of SNO-proteins (approach A) and SNO-peptides (approach B), using multiple biological replicates. Proteins were identified by Spectrum Mill (Agilent) for both approaches and pooled for analysis. By matching peptides for a given protein using a combination of both approaches, we identified 251 proteins (Dataset S1). DFIs in MS/MS spectra using approach B pinpointed 313 SNO-sites within these proteins (Dataset S1; MS/MS spectra can be viewed at web.mit.edu/toxms/www/SNOTRAP/). Detection of SNO-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on Cys-150, as previously reported by others using independent approaches, validated the robustness of the protocol (Fig. S1 B and C) (16, 29). SNOTRAP also detected previously unidentified SNO-sites, such as SNO-Cys284 of gephyrin (GPHN1), indicating the sensitivity of the method (Fig. S1 B and C). The SNOTRAP approach was able to detect and identify a large number of endogenous SNO-proteins and SNO-sites in the brain and is complementary to previous reports for mouse, rat, and human (Fig. S1F) (16, 30–32).
To control for false positives that may result from nonspecific interactions with the streptavidin beads, pretreatment with TCEP, UV, and ascorbate-Cu were used to displace SNO PTMs before reaction with the SNOTRAP probe and analyzed by MS (Fig. S1D). Samples pretreated with TCEP were also analyzed by Western blot (Fig. S1E). Approximately 3% of peptides and 5% of proteins (Materials and Methods) were identified as false positives (present in both the test samples and negative controls) and were removed from further analysis.
SNO-Proteins Identified During Early Neurodegeneration.
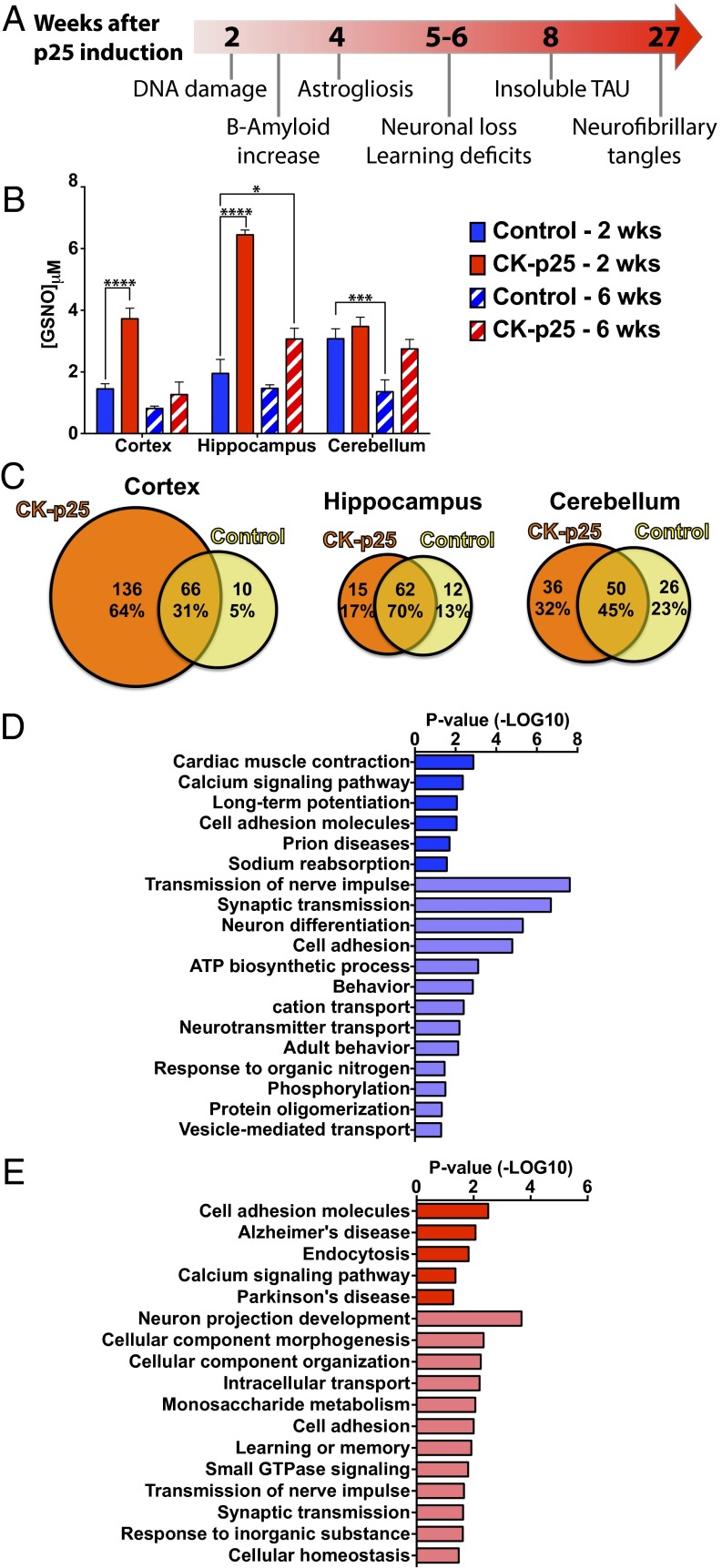
To determine temporal changes during neurodegeneration of SNO-proteins and their cognate SNO-Cys sites, we used the CK-p25 mouse model of AD-like neurodegeneration. CK-p25 mice show early signs of neurodegeneration, including DNA damage, increased amyloid-β, and the onset of neuroinflammation, before behavior abnormalities (Fig. 2A) (21, 22, 33). After 6 wk of p25 induction, mice exhibit learning and memory impairments and signs of advanced neurodegeneration, such as neuronal death and reduced synapse number (23, 24). To assess changes in SNO in the brain during the progression of neurodegeneration, we first measured GSNO levels in the hippocampus, cortex, and cerebellum of CK-p25 mice and p25 control littermates during early (2 wk) and later (6 wk) stages of neurodegeneration. In control mice, the levels of GSNO were highest in the cerebellum, reflecting previous observations that NOS1 expression is high in the cerebellum of adult mice (13). During early stages of neurodegeneration, GSNO increased in the cortex and the hippocampus to levels (twofold and threefold, respectively) that either resembled or surpassed that of the cerebellum (Fig. 2B). Increased SNO levels in 2-wk–induced CK-p25 mice correlate with elevated DNA damage previously observed in these regions (24). In the cerebellum, no increase in GSNO was observed at 2 wk, which likely reflects the low level of p25 induction in this brain region (21). GSNO levels in the hippocampus and cortex during a later stage of neurodegeneration return to levels similar to controls. Low levels of NO lead to S-nitrosation, whereas high levels cause cell death (14). The reduction of GSNO levels at a later stage of neurodegeneration could reflect a change in the proportion of cell types in these regions, including a loss of neurons and gliosis (21), although the possibility of lowered SNO production cannot be excluded. Collectively, our results suggest that elevated GSNO in the hippocampus and cortex is an early indicator of neurodegeneration and that elevated SNO may be a driving mechanism for disease progression.
Fig. 2.
Altered levels of SNO are detected in CK-p25 mice during early stages of neurodegeneration. (A) Timeline of the pathological progression of the CK-p25 mouse model of neurodegeneration. (B) GSNO concentration (μM) in the cortex, hippocampus, and cerebellum of control or CK-p25 mice following induction of p25 expression for 2 wk or 6 wk. Two-way ANOVA; Dunnett’s multiple comparisons; ****P < 0.0001, ***P < 0.001, *P < 0.05; n = 4 for 2-wk samples and n = 3 for 6-wk samples; mean ± SEM. (C) Number of SNO-proteins identified in the cortex, hippocampus, and cerebellum of control and CK-p25 mice following 2-wk induction. (D) Gene ontology analysis of total SNO-proteins identified in the cortex of control mice (dark blue, KEGG pathways; light blue, BPs). (E) Gene ontology analysis of SNO-proteins exclusively identified in the cortex of CK-p25 mice following 2-wk induction (dark red, KEGG pathways; light red, BPs).
To assess the endogenous SNO-proteome profile in the brains of CK-p25 and control mice during early stages of neurodegeneration (2 wk), SNO-proteins were isolated and identified in the cortex, hippocampus, and cerebellum. We detected a larger number of SNO-proteins and SNO-sites in CK-p25 mice compared with controls. In control mice, 152 SNO-sites and 116 SNO-proteins were identified, compared with 292 SNO-sites and 237 SNO-proteins in the CK-p25 mice (Dataset S1). The largest increase in CK-p25–specific SNO-proteins was detected in the cortex (Fig. 2C), which is consistent with elevated levels of GSNO during early neurodegeneration (Fig. 2B). Of the 212 SNO-proteins identified in the cortex, almost two-thirds (64%) were detected only in the CK-p25 mice (Fig. 2C and Dataset S1). A total of 264 SNO-Cys sites were identified in the cortex, of which 160 (61%) were found exclusively in CK-p25 mice (Dataset S1).
We detected 89 SNO-proteins in the hippocampus. Although AD pathology is readily observed in the hippocampus and our data show elevated GSNO, the majority of detected SNO-proteins (70%) were common between CK-p25 and controls (Fig. 2C). The reduced number of proteins may reflect a limitation in the total protein we obtained from the hippocampus (Fig. 2C and Dataset S1). The cerebellum has an approximately equal distribution of SNO-proteins (32%, 45%, and 23%) between control and CK-p25 mice (Fig. 2C and Dataset S1) and mirrors observations of little change in GSNO for this brain region (Fig. 2B).
Bioinformatic Analysis of the S-Nitrosoproteome.
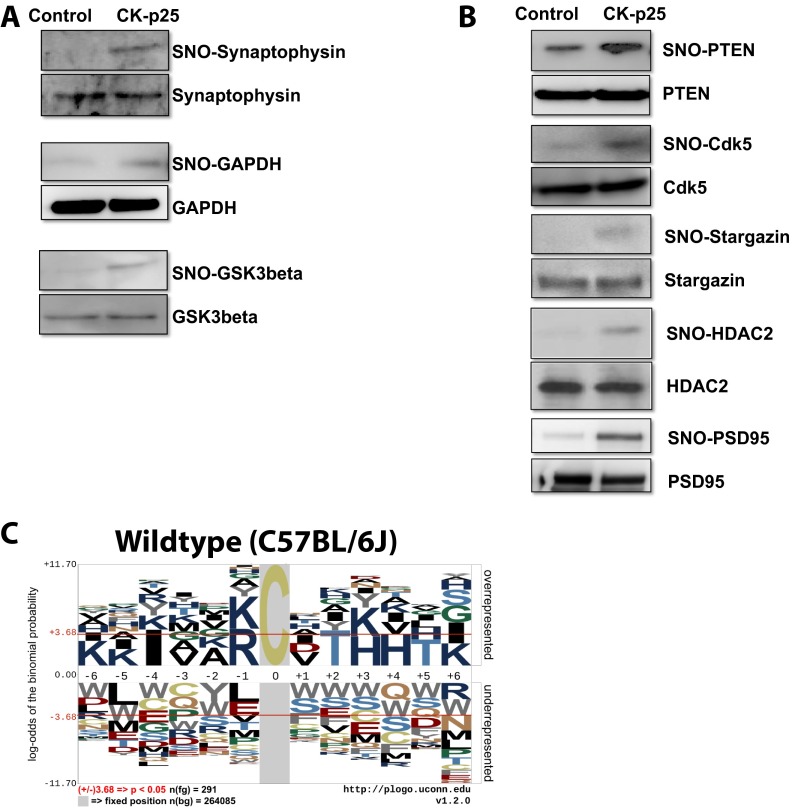
To decipher the possible impact of SNO-proteins on molecular and cellular systems during early neurodegeneration, we performed gene ontology analysis of biological processes (BPs) and KEGG pathways using the total control SNO-proteome (Fig. 2D) and the CK-p25–specific SNO-proteome (Fig. 2E) of the cortex (34, 35). Gene ontology clusters associated with synaptic functions were observed for SNO-proteins in control and CK-p25 mice (BP_Synaptic transmission and BP_Transmission of nerves impulse). The synaptic protein synaptophysin was validated by SNOTRAP-Western blot (Fig. S2A). Pathways associated with cognition were identified in both control (KEGG_Long-term potentiation and BP_Behavior) and CK-p25 mice (BP_Learning or memory). Collectively, these data suggest that regulation of synaptic SNO-proteins is important for normal neuronal functions associated with learning and memory and also that the synapse is vulnerable to aberrant SNO-signaling. NOS1, the major source of neuronal NO, is tethered to the synapse by postsynaptic density protein 95 (PSD95; also known as DLG4) (36). Although we did not detect SNO-PSD95 by MS, SNOTRAP-Western blot analysis shows a strong elevation of SNO-PSD95 in CK-p25 mice (Fig. S2B), indicating a susceptibility of the synapse to increased SNO during early neurodegeneration.
Fig. S2.
Altered SNO-proteins during early neurodegeneration. (A) SNO-proteins identified by MS were validated by SNOTRAP Western blot analysis. Representative Western blot of SNO-Synaptophysin, SNO-GAPDH, and SNO-GSK3β in control and CK-p25 mice following 2-wk induction (n = 2). (B) Neuronal proteins previously identified as S-nitrosated that were not in our MS datasets were examined by SNOTRAP Western blot. Representative Western blot of SNO-PTEN, SNO-Cdk5, SNO-Stargazin, SNO-HDAC2, and SNO-PSD95 in control and CK-p25 mice following 2-wk induction (n = 2). (C) Linear motif generated by pLOGO using published sequences that flank SNO-Cys sites detected in C57BL/6 wild-type mice (47).
SNO-proteins linked to AD were detected in the CK-p25 cortex and not in controls, as identified by the AD KEGG pathway (Fig. 2E; GRIN2B, TAU, GSK3β, LRP1, NDUFS1, COX6B1, and GAPDH). NOS1 is activated by N-methyl-d-aspartate receptor (NMDAR)-mediated influx of Ca2+; subsequent S-nitrosation of NMDAR is thought to modulate its activity. However, hyperexcitation of NMDAR leads to excessive Ca2+ signaling and elevated NO production, conditions thought to occur during neurodegeneration. This could explain detection of SNO-GRIN2B, a subunit of the NMDAR, in the CK-p25 mice. Amyloid activation of the NMDAR can increase p25 production, possibly providing a feed-forward mechanism for SNO production (18). NMDAR-mediated p25 production and Cdk5 activation is thought to increase glycogen synthase kinase-3β (GSK3β) and TAU (Mapt) phosphorylation, two key mediators of neuronal death in AD, both of which we identified in the CK-p25 mice. Elevated SNO-GSK3β was validated by SNOTRAP-Western blot analysis (Fig. S2A). Cdk5 itself has been reported to be S-nitrosated, which enhances its serine/threonine kinase activity (37). Although SNO-Cdk5 was not detected by MS, it was elevated in CK-p25 mice (shown by SNOTRAP-Western blot; Fig. S2B), possibly contributing to elevated Cdk5 activity and acting as a feed-forward mechanism during neurodegeneration. In addition, protein kinase C (PKC) epsilon and gamma isoforms (Prkce and Prkcg) were S-nitrosated in CK-p25 mice. PKC inhibits GSK3β and promotes nonamyloidogenic processing of amyloid precursor protein (APP) through activation of α-secretase (38). S-nitrosation inhibits PKC activity (39, 40) and therefore may reduce APP processing through the nonamyloidogenic α-secretase pathway.
Additional AD-related pathways impacted by SNO during neurodegeneration included apolipoprotein E (ApoE)-mediated amyloid clearance (SNO-LRP1) and mitochondrial dysfunction (SNO-NDUFS1 and SNO-COX6B1, which are components of CxI and CxIV of the respiratory chain). Furthermore, Gene Ontology (GO) analysis indicated that proteins regulating glycolysis were enriched in the CK-p25–specific SNO-proteome (BP_Monosaccharide metabolism), suggesting that elevated SNO may affect metabolic processes during early neurodegeneration. In particular, GAPDH was S-nitrosated in CK-p25 mice but not in control mice and was validated by SNOTRAP-Western blot (Fig. S2A). Nuclear SNO-GAPDH transnitrosates SIRT1, HDAC2, and DNA-PK (DNA-dependent protein kinase, catalytic subunit), impacting metabolic pathways, aging, and chromatin remodeling (41–43). HDAC2 was not detected in our SNO-proteome, although we observed elevated SNO-HDAC2 in CK-p25 mice by SNOTRAP-Western blot (Fig. S2B).
Linear Motifs for S-Nitrosation.
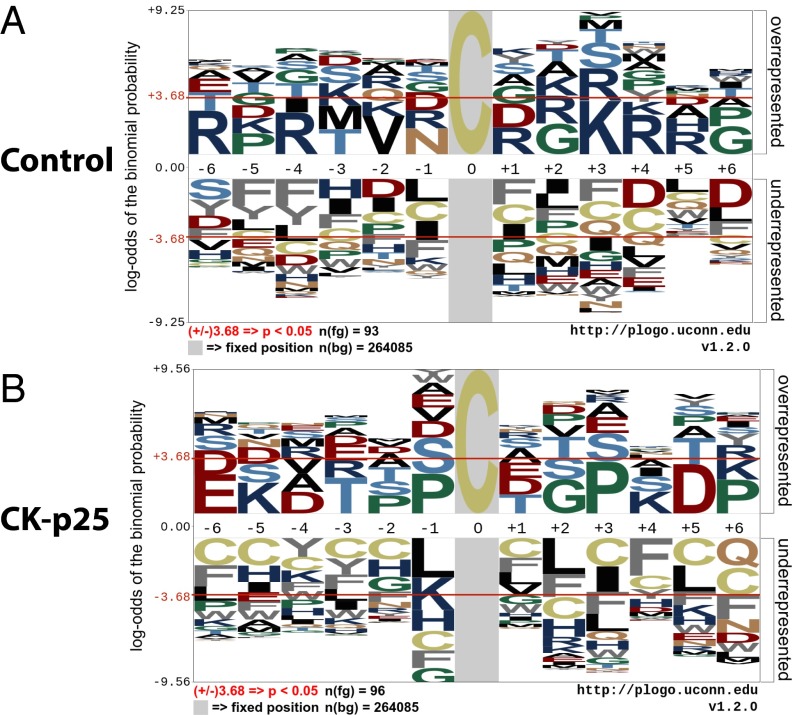
Recent studies indicate that the specificity of SNO-Cys sites may be dependent on the spatial proximity of charged amino acids, which are hypothesized to allow protein–protein interactions that facilitate transnitrsation (29, 44). To elucidate common features of SNO-Cys sites, we used the probability LOGO (pLOGO) tool to analyze flanking sequences of SNO-Cys residues for linear motifs (45). Motif analysis of SNO-Cys sites in control and CK-p25 mice revealed an overrepresentation of charged flanking amino acids; however, this was not consistent for all SNO-Cys sites (Fig. 3 A and B). Specifically, in control mice, basic residues Arg (R) and Lys (K) were overrepresented at the –6, –4, +1, +3, +4, and +5 positions (Fig. 3A); these residues may be required for base-catalyzed transnitrosation (1, 46). To test the robustness of the motif, we performed pLOGO analysis using an independently published SNO-proteome from brains of wild-type (C57BL/6J) mice (47) and found an enrichment of the basic residues—Lys (K), Arg (R), and His (H)—surrounding the SNO-Cys position (Fig. S2C, –6, –5, –1, +3, and +4). In the CK-p25 mice, we observed Lys (K) basic residues at the –5 and +4 positions and Glu (E) and Asp (D) acidic residues at the –6, –4, and +5 positions (Fig. 3B). This motif may represent charge clustering and has been previously proposed to allow acid–base catalysis for nitrosation (base) and denitrosation (acid) of Cys residues. Despite these observations, many SNO-Cys sites identified in both control and CK-p25 mice did not have charged amino acids within a proximal location of the primary sequence. Previous studies indicate that tertiary structural elements may be required for localizing charged residues to SNO-Cys sites for many proteins (29, 44). Collectively, these data suggest that the specificity for S-nitrosation of a subset of Cys may be dependent on proximal charged amino acids, which possibly provide docking sites for nitrosating and denitrosating agents (29).
Fig. 3.
SNO-site linear motif analysis indicates differential preference for SNO-Cys sites during early neurodegeneration. (A) Linear motif generated by pLOGO using sequences flanking SNO-Cys sites detected in control mice. (B) Linear motif generated by pLOGO using sequences flanking SNO-Cys sites exclusively detected in 2-wk–induced CK-p25 mice.
Materials and Methods
Reagents.
Chemical reagents were obtained from commercial sources (Sigma-Aldrich for chemicals, Cambridge Isotope Laboratories for deuterated solvents, Isotec for 13C2,15N-labeled G*SH, Creative PEGWorks for maleimide–PEG–maleimide, and ChemPep, Inc., for biotin–PEG3–propionic acid) and were used without additional purification. Sequencing-grade modified trypsin was obtained from Promega. Complete protease inhibitor mixture was from Sigma. Extraction and silica chromatography solvents were reagent grade. LC–MS and HPLC solvents were HPLC grade. Acetonitrile was distilled for HPLC and LC–MS. Distilled water was obtained in house and redistilled for HPLC and LC–MS experiments. Vivaspin 3,000, 10,000, and 30,000 molecular-weight cutoff (MWCO) membrane filters were from Sartorius Stedim NA. The primary antibodies used were as follows: Synaptophysin (Sigma, S5768), GAPDH (Cell Signaling Tech., 2118), Gsk3b (R&D), phosphatase and tensin homolog (PTEN) (Cell Signaling Tech., 9552), Cdk5 (Cell Signaling Tech., 2506), Stargazin (Santa Cruz, sc-374123), HDAC2 (Cell Signaling Tech., 2540), and PSD95 (NeuroMab, P78352). Secondary HRP-conjugated antibodies were purchased from Santa Cruz Biotechnology (sc-2030 and sc-2005). ECL reagent was from GE Healthcare (RPN2232). Unless otherwise stated, all sample preparations, nitrosothiol preparations, and SNO-probe reactions were carried out in the dark at room temperature. For NMR analysis, 1H NMR spectra were recorded on Bruker Avance-600 and Varian Inova-500 instruments at 600.13 and 500.13 MHz, respectively. The 13C NMR spectra were recorded on a Varian Inova-500 instrument operating at 125.76 MHz. 31P NMR spectra were recorded on a Varian Inova-500 instrument operating at 202.46 MHz, and 31P chemical shifts are relative to 3% (vol/vol) H3PO4 (δ = 0 ppm) contained in a concentric internal capillary (Wilmad). NMR spectra were obtained using Bruker 5-mm TXI cryo-probes and Varian 5-mm pulsed-field gradient (PFG)-probes held at 22 °C unless otherwise stated.
Animals.
All mouse experiments were approved by the Committee on Animal Care of the Division of Comparative Medicine at MIT. Adult (2.5–3 mo old) male double-transgenic CK-p25 mice and respective p25 control littermates were used (21). Tissue was collected at 2 or 6 wk after induction of p25 expression. Three brain regions were dissected from each mouse (cortex, hippocampus, and cerebellum). Dissections were performed on ice, immediately flash frozen in liquid nitrogen, and stored at –80 °C. Each biological replicate represents data obtained using tissue from an individual mouse. For the GNSO experiments, four biological replicates were used per group for 2-wk induction, and three biological replicates were used per group for 6-wk induction. For MS analysis, three biological replicates and two technical replicates were used per group for both method A and method B, and each sample was preseparated into five fractions.
Synthesis of SNOTRAP-Biotin.
To a stirred 2-(diphenylphosphino)-benzenethiol (48, 49) (100 mg, 0.34 mmol) in dry DMF (5 mL) we added biotin–PEG3–propionic acid (100 mg, 0.22 mmol; ChemPep, Inc.), N,N’-dicyclohexylcarbodiimide (70 mg, 0.34 mmol), and dimethylaminopyridine (4 mg, 0.03 mmol) successively. The resulting mixture was stirred for 7 h at room temperature, and the resulting clear solution was then concentrated under reduced pressure and purified by flash chromatography (hexane/EtOAc/MeOH gradient) to give the desired product (yield 30%). The SNOTRAP probe was repurified on an 1100 HPLC system with a photodiode array UV detector at 254 nm (Agilent Technologies). HPLC columns and solvent systems were as follows: A semipreparative Phenomenex Luna C18 (25 cm × 9.4 mm, 10 μm) column was eluted with a linear gradient of 0.1% formic acid in water (A) and acetonitrile (B) at a flow rate of 2.5 mL/min. Solvent composition was initially at 40% for 5 min, 70% at 10 min, 90% at 20 min, and then further to 95% B over 8 min: 1H NMR (500 MHz, CD3CN, δ), 7.42–7.38 (m, 9H), 7.23–7.18 (m, 4H), 7.84 (m, 1H), 4.60–4.51 (m, 2H), 3.67–3.51 (m, 12H), 3.2 (m, 3H), 2.8 (m, 2H), 2.55 (t, 2H), 2.15 (t, 2H), 1.57–3.51 (m, 6H); 13C NMR (125 MHz, CD3CN, δ), 199.19, 172.5, 164.5, 144.8, 138.1, 137.0, 134.8, 129.9, 129.6, 129.6, 118.3, 69.2, 63.1, 62.3, 45.9, 42.5, 38.2, 27.1, 23.1, 22.5; 31P NMR (202 MHz, CD3CN, δ), −10.3; and HRMS-ESI+ (m/z) [M + H+]+ calculated for C37H47N3O6PS2, 724.2638, and found, 724.2632.
Sample Preparation for Tissue GSNO Analysis.
Samples were first spiked with 500 fmol of isotopically labeled internal standard (13C2,15N-labeled G*SNO) and mixed with maleimide–PEG–maleimide (20 mM, Creative PEGWorks) in PBS buffer containing 1 mM EDTA. For complete cell lysis, samples were subjected to three freeze/thaw cycles (dry ice to RT) with frequent vortexing. Lysed samples were kept 15 min at room temperature to allow complete blocking by maleimide-PEG-maleimide. Samples were centrifuged (16,000 × g, 10 min at 4 °C) to remove cell debris, followed by filtration using 3,000 MWCO membrane filters (15,000 × g, 15 min at 4 °C). The filtrate (low-molecular weight fraction) was then treated with the phosphine probe 3, as described in an earlier publication (3 mM final concentration, prepared by dissolving 4 mg of phosphine in 400 μL of 2:1 acetonitrile/methanol mixture) (25). The resulting clear solution was then freeze-dried to a final volume of 30 μL, and 8 μL was injected into the LC–MS/MS. To generate negative controls, the low molecular weight (LMW) filtrate was treated with 3 mM DTT (15 min, 24 °C).
Endogenous GSNO Quantification.
LC–MS quantitation was performed by multiple-reaction monitoring (MRM), using the internal standard (G*SNO). A method calibration curve for LC–MS/MS was obtained by spiking 500 fmol of 13C2,15N-labeled internal standard (G*SNO) into the reaction between phosphine 3 (5 mM) and GSNO standards (0–10 pmol, in PBS buffer containing 1 mM EDTA). Regression analysis of the relative response ratio, calculated from LC–MS/MS peak area ratios corresponding, respectively, to analytes and internal standards, was then used to calculate the amount of GSNO (in pmols). This was then multiplied by 3.8 (total volume 30 μL/8 μL injection to a MS of 3.8) to obtain the total amount of analyte in the cell lysate. The GSNO concentration was determined by dividing the total amount of analyte (pmol) per million cells by the wet weight per million cells (mg), assuming that the wet weight of cells (10 million) was equal to the weight of water (i.e., 1 mg = 1 μL).
ESI+–QqQ–MS Analyses.
MRM and precursor-ion analyses were carried out in positive-ion mode, as described earlier (25), using an Agilent 1200 capillary HPLC system with an Agilent Triple Quad LC/MS (model 6430). The chromatography was done on an Agilent Eclipse XDB-C18 reverse-phase column (1.0 mm × 50 mm, 3.5 μm) with acetonitrile/water/formic acid gradients at 20 μL/min. The instrument parameters were optimized for maximum response during infusions of standard solutions.
Isolation and Preparation of Mouse Brain Protein Homogenates.
Intact brains were collected, immediately frozen in liquid nitrogen, and stored at −80 °C. Isolated brain regions (cerebral cortex, hippocampus, and cerebellum) were homogenized into 3 mL lysis buffer (250 mM Hepes–NaOH, pH 7.7, containing 1 mM diethylene triamine pentaacetic acid, 0.1 mM neocuproine, 1% Triton X-100, 20 mM IAM, and protease inhibitors) on ice using a Teflon pestle and a Jumbo Stirrer (Fisher Scientific). The homogenates were centrifuged at 10,000 × g for 10 min at 4 °C. The soluble protein fraction was collected, and the protein concentration was determined by the Bradford assay. Each sample consisted of 2 mg of protein in 4 mL of lysis buffer (0.5 mg/mL). Lysates were then split into equal aliquots. Negative controls were generated by treatment with 10 mM TCEP for 30 min at room temperature or with Cu-ascorbate. Following treatment with 2.5% SDS (final) and 300 mM IAM in 250 mM Hepes, pH 7.7, buffer, samples were alkylated by incubation in a Thermomixer in the dark at 37 °C and shaking for 30 min. To minimize sample loss during sample processing, filter-aided sample preparation (FASP) was used to remove excess reagents and the MWCO filter was used as a reaction vessel (50). After alkylation, excess IAM reagents were removed by buffer exchange with 8 M urea three times and Milli-Q water once by centrifugation at 4,000 × g for 30 min at 4 °C with 10,000 MWCO spin filters (Sartorius Corporation).
Probe Labeling.
SNOTRAP stock solutions [in 40% acetonitrile (ACN)] were added to all samples to reach a final concentration of 1.2 mM (in 25 mM Hepes buffer at pH 7.7) to selectively convert SNO to stable disulfide–iminophosphorane. Samples were incubated with SNOTRAP solution at room temperature for 2 h for complete labeling. Excess reagent was removed by three washes with 25 mM Hepes, pH 7.7, buffer and centrifuged at 4,000 × g for 30 min at 4 °C with 10,000 MWCO spin filters.
Streptavidin Affinity Chromatography of SNOTRAP-Derivatized SNO-proteins and Protein Digestion.
Streptavidin was used to enrich for SNOTRAP-derivatized proteins according to the protocol detailed in Slade et al. (51). Briefly, following addition of the samples to the streptavidin columns, the columns were washed with PBS/CHAPS and distilled water, eluted with TFA/acetonitrile, and then frozen, lyophilized, and stored at –20 °C. In-solution protein digestion (in 25 mM Hepes, pH 7.7, buffer) for each sample was carried out in the dark at 37 °C for 3 h by adding 8.8 μg trypsin (sequencing grade-modified trypsin was obtained from Promega) and 1 mM CaCl2 to each sample. The digests were transferred to 30-kDa cutoff filters (previously rinsed with methanol and washed with water) to remove trypsin and other higher molecular-weight components. Peptide-containing fractions were desalted using Agilent 100-μL C18 Bond Elut Zip tips and eluted with step gradients of acetonitrile fractions (20%, 40%, 60%, 80%, and 100%). Samples were reduced by Speedvac to less than 5 μL and adjusted to 10 μL with Millipore water. Eluted peptides (5 μL) were analyzed by LC–MS/MS, as described in MS Analysis for Protein Identification.
Protein Digestion and SNOTRAP-Biotin–Streptavidin Affinity Peptide Capture.
For peptide-level enrichment, in-solution protein digestion (in 25 mM Hepes, pH 7.7, buffer) for each sample was carried out in the dark at 37 °C for 3 h by adding 8.8 μg trypsin and 1 mM CaCl2 to each sample. The digests were transferred to 30-kDa cutoff filters (previously rinsed with methanol and washed with water) to remove trypsin and other higher molecular-weight components. The filtrate containing the biotinylated peptides was recovered and incubated with Neutravidin beads (200 μL slurry per milligram initial protein; washed with 10 volumes of 0.1 M ammonium bicarbonate) for 2 h at room temperature under gentle rocking. Samples were centrifuged at 1,000 × g for 4 min, and the unbound fraction was removed. The beads were washed four times with 2.5 volumes of 1 M ammonium bicarbonate containing 50 mM NaCl, four times with 2.5 volumes of 0.1 M ammonium bicarbonate, two times with 2.5 volumes of 0.025 M ammonium bicarbonate, and finally four times with deionized water. Between each wash, beads were rocked for 2 min and centrifuged at 1,000 × g for 1 min. Bound peptides were eluted with 5 mM of TCEP by shaking for 30 min at room temperature. Eluted, Cys-containing peptides were alkylated using 100 mM NEM. Peptides containing fractions were desalted using Agilent 100-μL C18 Bond Elut Zip tips and eluted to a gradient of acetonitrile fractions (20%, 40%, 60%, 80%, and 100%). Samples were reduced in volume by Speedvac to less than 5 μL and adjusted to 10 μL with Millipore water. Eluted peptides (5 μL) were analyzed by LC–MS/MS, as described in MS Analysis for Protein Identification.
Western Blot Detection of Endogenous S-Nitrosoproteome in Mouse Brains.
To detect endogenous SNO-proteins, control and CK/p25 samples were labeled using SNOTRAP as described above. UltraLink Immobilized Streptavidin beads (Thermo Scientific, 20349) (100 μL of slurry) were first washed with 25 mM Hepes buffer (pH 7.7) (3 × 200 μL), and probe-labeled proteins were added to the beads and pull-down was carried out for 1 h in pull-down buffer (25 mM Hepes, 150 mM NaCl, 0.05% SDS) at room temperature. Then beads were washed with (3 × 200 μL) wash buffer (25 mM Hepes buffer, pH 7.7, 0.1% SDS), (3 × 200 μL) 25 mM Hepes buffer (pH 7.7), and finally (2 × 200 μL) ddH2O. Biotinylated proteins were eluted from beads by boiling in the presence of electrophoresis sample loading reducing buffer (contains DTT), followed by SDS/PAGE and transfer to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked and incubated with monoclonal/polyclonal antibody, followed by respective secondary HRP-conjugated antibodies. Protein bands were visualized with ECL reagent, and bands were imaged using Fluorchem 8900 (Alpha Innotech). To detect endogenous SNO-biotinylated proteins, equal amounts of SNOTRAP-labeled proteins from control and CK/p25 samples (10 μg) were loaded into the SDS/PAGE gel for electrophoresis. The proteins were transferred into PVDF membranes, and the blot was probed against streptavidin–HRP to visualize total biotinylated proteins. The bands were captured using Fluorchem 8900 from Alpha Innotech. For negative controls, SNO modifications were reduced using TCEP and processed as with SNOTRAP labeling.
MS Analysis for Protein Identification.
These were carried out on the Agilent HPLC-Chip/MS System, consisting of a micro-autosampler with a thermostat (set to 4 °C), a capillary and nanoflow pump with microdegasser, and the Chip-Cube that interfaces LC modules and the MS instrument. HPLC-grade water [0.1% formic acid (FA)] and ACN (0.1% FA) were used as mobile phases A and B, respectively. Separations were conducted on a Polaris-HR-Chip 3C18 (150 mm × 50 μm) 80A 3-µm C18 chip with 360 nL trap column (Agilent Technologies). Sample analysis used a 60-min gradient operating on the nanopump. The capillary pump provides a constant flow of 2 μL/min for delivery of samples from the autosampler to the HPLC–Chip interface. Two LC gradients were used. Mobile phases were 0.1% formic acid in HPLC-grade water (phase A) and 0.1% formic acid in acetonitrile (phase B). In MS approach A, the gradient started at 3% B at 300 nL/min for 5 min and increased to 30% B from 2 to 102 min, to 60% B at 112 min, to 90% B at 125 min, and to 97% at 126 min, was held for 4 min, and was followed by a 10-min postrun at 3% B. For MS approach B, the gradient started at 3% B at 300 nL/min for 5 min and increased to 30% B from 2 to 35 min, to 60% B at 40 min, to 90% B at 45 min, to 97% at 46 min, was held for 4 min, and was followed by a 10-min postrun at 3% B. Mass detection was performed with an Agilent 6550 Accurate-Mass Ion Funnel QTOF Chip-MS System operated in positive-ion mode. Mass spectra were acquired in the 1,700 Da extended dynamic range mode (2 GHz) using the following settings: ESI capillary voltage, 1,960 V; fragmentor, 360 V; Octopole RF peak, 750 V; drying gas, 13 L/min; drying temperature, 225 °C. Data were acquired at a rate of 6 MS spectra per second and 3 MS/MS spectra per second in the mass ranges of m/z 295–1,700 for MS and 50–1,700 for MS/MS and stored in profile mode with a maximum of 20 precursors per cycle with a threshold of 5,000 ions in a precursor abundance-based scan speed in a peptide isotope model, with +2, +3, and above charge state preference, with active exclusion after 1 spectra, and released after 0.15 min. Fragmentation energy was applied at a slope of 3.0 V/100 Da with a 3.0 offset. Mass accuracy was maintained by using internal reference ion m/z 1221.9906, in positive mode.
Data Processing.
Agilent MassHunter Workstation software was used for data acquisition and processing, and Agilent Spectrum Mill MS Proteomics Workbench was used for database searching, with the false discovery rate set at 1% (see Ravindra et al., 2015, for details) (52). The data were additionally processed with X!Tandem. Reverse/random database searches and manual inspection of spectra were used to validate the peptide/protein identifications. BSA standards were run daily for quality control. Three biological and two technical replicate experiments were performed, and detection of SNO-peptides in at least two was required for identification. In addition, our criteria for identification of SNO-proteins required detection of two or more tryptic peptides per SNO-protein using approach A and the SNO-Cys site-specific peptide identification using approach B. Using the Spectrum Mill search engine (B.04.00.127, Agilent Technologies), raw MS/MS spectra were processed to extract MS/MS spectra and searched against the SwissProt database. During searching, the following parameters were used: carbamidomethylation as a fixed modification; trypsin; maximum of two missed cleavages; precursor mass tolerance, ±20 ppm; product mass tolerance, ±50 ppm; and maximum ambiguous precursor charge, 4. Data were evaluated, and protein identifications were considered significant if the following confidence thresholds were met: protein score, >13; individual peptide scores of at least 10; and Scored Peak Intensity, 60%. For approach B (SNO-site identification), methionine oxidation, N-ethylmaleimide on Cys, carbamidomethyl on Cys, N-ethylmaleimide + H2O on Cys, and protein N terminus acetylation were set as variable PTMs. Minimal peptide length was set to six amino acids, and a maximum of two missed cleavages was allowed. Peptide sequence and fragmentation spectrum information were visualized using Scaffold software (v.3.6, Proteome Software, Inc.) with the imported peptide hits from Spectrum Mill.
Label-Free Quantitation Using Precursor Intensity with Scaffold Q+.
Precursor intensity was used for the fold-change quantitation of common proteins detected in cortical and hippocampal samples. The method is to work backward from the peptides that have been identified through their MS/MS spectra and compare the intensities of the MS peaks from which they were derived. Precursor intensity quantitation is based on the principle that the area of the peak in the MS1 chromatogram provides a measure of the relative abundance of the corresponding peptide in the sample. Peptides are identified based on their MS/MS spectra, and then the corresponding MS1 peaks are identified in each LC–MS/MS run. The areas under these peaks are calculated and normalized, and their ratios are used as a measure of the relative abundance of the peptides in different samples by Scaffold Q+ (Proteome Software, Inc.). Relative quantities of proteins are estimated by combining the precursor intensities of the constituent peptides. Using the quantitative values based on precursor intensity, fold changes were calculated by selecting control samples as the reference using Scaffold.
Bioinformatics.
To assess functional enrichment, the lists of SNO-proteins were submitted to DAVID (https://david.ncifcrf.gov) (34, 53). “Fold enrichment” was defined as the number of proteins detected in the sample compared with the total number of proteins expected in the mouse or human proteome in each GO BP and pathway. P values for term enrichment were calculated using the right-sided hypergeometric test. GO and KEGG pathway enrichment (P < 0.05) analyses were performed by using the functional annotation tool in DAVID. The consensus sequence motifs of SNO-Cys were visualized by pLogo, a linear sequence prediction algorithm based on their statistical significance (P < 0.05). Motif_x was used to align the Cys-containing peptide sequence for pLogo analysis (45).
Conclusion
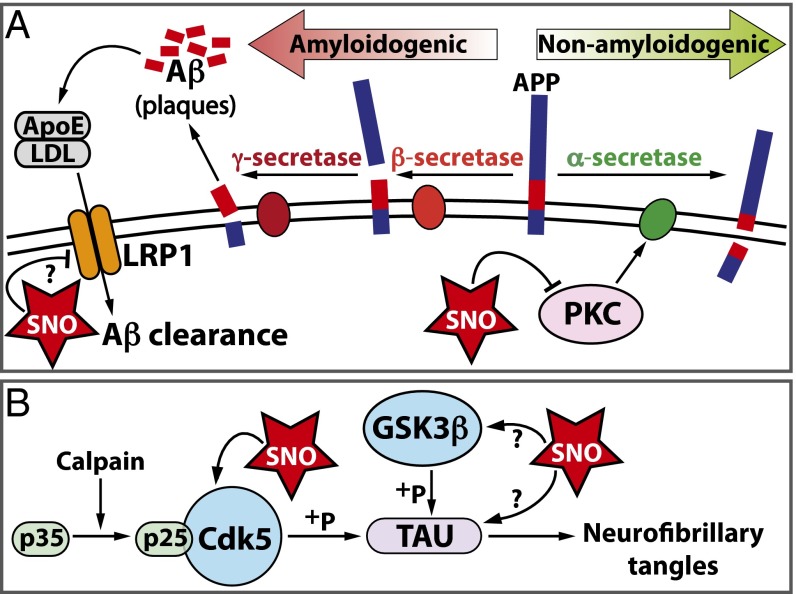
Through development of SNOTRAP, a direct SNO-tagging MS method for detecting both small and large molecular RSNOs, we identified an expanded endogenous SNO-proteome in the mouse brain. We identified the hippocampus and cortex as regions subjected to elevated levels of SNO during early stages of neurodegeneration that precede the onset of detrimental behavioral changes. The identification of SNO-PKC and SNO-LRP1 during neurodegeneration suggests that elevated SNO may impact amyloid processing and clearance, thus contributing to amyloid plaque deposition (Fig. 4A). Furthermore, detection of SNO-GSK3β and SNO-TAU in the CK-p25 mice indicates that aberrant SNO signaling may affect neurofibrillary tangle formation, another hallmark of AD pathology (Fig. 4B). The susceptibility of multiple proteins to S-nitrosation during early neurodegeneration relevant to synaptic function, metabolism, and AD pathology may drive cellular pathologies observed at later stages, such as neuronal loss, reduced synapse integrity, and ultimately memory impairment (Fig. 4 A and B). Increased protein aggregation and cell death linked to SNO-proteins in CK-p25 mice likely lead to activation of inflammatory responses, an important component of AD pathology and progression (33). The SNOTRAP strategy provided a comprehensive assessment of S-nitrosation in the neurodegenerating brain that can be applied to multiple cellular and disease contexts and has therapeutic potential for biomarker discovery and drug development.
Fig. 4.
Pathways affected by S-nitrosation during neurodegeneration. (A) SNO-LRP1 and SNO-PKC isoforms were identified in CK-p25 mice after 2-wk induction and have been implicated in secretase processing of APP and amyloid clearance. (B) SNO-GSK3β and SNO-TAU were identified in CK-p25 mice after 2-wk induction and have been implicated in neurofibrillary tangle formation and AD pathology. The functional implications of previously unidentified SNO-proteins are represented with a question mark.
Animals.
All mouse experiments were approved by the Committee on Animal Care of the Division of Comparative Medicine at Massachusetts Institute of Technology.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant CA26731, MIT Center for Environmental Health Sciences Grant ES002109, a grant from the Simons Foundation to the Simons Center for the Social Brain at MIT (S.R.T.), and National Institutes of Health Grant R01 NS051874 (to L.-H.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The mass spectrometry proteomics data reported in this paper have been deposited to the ProteomeXchange Consortium, www.ebi.ac.uk/pride/archive via the PRIDE partner repository (accession no. PXD003802).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1521318113/-/DCSupplemental.
References
- 1.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6(2):150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 2.Smith BC, Marletta MA. Mechanisms of S-nitrosothiol formation and selectivity in nitric oxide signaling. Curr Opin Chem Biol. 2012;16(5-6):498–506. doi: 10.1016/j.cbpa.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Ruiz A, Cadenas S, Lamas S. Nitric oxide signaling: Classical, less classical, and nonclassical mechanisms. Free Radic Biol Med. 2011;51(1):17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Keshive M, Singh S, Wishnok JS, Tannenbaum SR, Deen WM. Kinetics of S-nitrosation of thiols in nitric oxide solutions. Chem Res Toxicol. 1996;9(6):988–993. doi: 10.1021/tx960036y. [DOI] [PubMed] [Google Scholar]
- 5.Keszler A, Zhang Y, Hogg N. Reaction between nitric oxide, glutathione, and oxygen in the presence and absence of protein: How are S-nitrosothiols formed? Free Radic Biol Med. 2010;48(1):55–64. doi: 10.1016/j.freeradbiomed.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madej E, Folkes LK, Wardman P, Czapski G, Goldstein S. Thiyl radicals react with nitric oxide to form S-nitrosothiols with rate constants near the diffusion-controlled limit. Free Radic Biol Med. 2008;44(12):2013–2018. doi: 10.1016/j.freeradbiomed.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell DA, Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1(3):154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 8.Seth D, Stamler JS. The SNO-proteome: Causation and classifications. Curr Opin Chem Biol. 2011;15(1):129–136. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, et al. Aberrant protein S-nitrosylation contributes to the pathophysiology of neurodegenerative diseases. Neurobiol Dis. 2015;84:99–108. doi: 10.1016/j.nbd.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbott A. Cognition: The brain’s decline. Nature. 2012;492(7427):S4–S5. doi: 10.1038/492S4a. [DOI] [PubMed] [Google Scholar]
- 11.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des. 2010;16(25):2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 12.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer’s disease. J Alzheimers Dis. 2010;19(1):341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 13.Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994;13(2):301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 14.Dedon PC, Tannenbaum SR. Reactive nitrogen species in the chemical biology of inflammation. Arch Biochem Biophys. 2004;423(1):12–22. doi: 10.1016/j.abb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Forrester MT, et al. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotechnol. 2009;27(6):557–559. doi: 10.1038/nbt.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao G, Derakhshan B, Shi L, Campagne F, Gross SS. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci USA. 2006;103(4):1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 18.Seo J, et al. Activity-dependent p25 generation regulates synaptic plasticity and Aβ-induced cognitive impairment. Cell. 2014;157(2):486–498. doi: 10.1016/j.cell.2014.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shukla V, et al. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013;27(1):174–186. doi: 10.1096/fj.12-217497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shukla V, Skuntz S, Pant HC. Deregulated Cdk5 activity is involved in inducing Alzheimer’s disease. Arch Med Res. 2012;43(8):655–662. doi: 10.1016/j.arcmed.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruz JC, Tseng H-C, Goldman JA, Shih H, Tsai L-H. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40(3):471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 22.Cruz JC, et al. p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci. 2006;26(41):10536–10541. doi: 10.1523/JNEUROSCI.3133-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai L-H. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48(5):825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, et al. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60(5):803–817. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seneviratne U, Godoy LC, Wishnok JS, Wogan GN, Tannenbaum SR. Mechanism-based triarylphosphine-ester probes for capture of endogenous RSNOs. J Am Chem Soc. 2013;135(20):7693–7704. doi: 10.1021/ja401565w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Wang H, Xian M. An unexpected Bis-ligation of S-nitrosothiols. J Am Chem Soc. 2009;131(11):3854–3855. doi: 10.1021/ja900370y. [DOI] [PubMed] [Google Scholar]
- 27.Levsen K, et al. Structure elucidation of phase II metabolites by tandem mass spectrometry: An overview. J Chromatogr A. 2005;1067(1-2):55–72. doi: 10.1016/j.chroma.2004.08.165. [DOI] [PubMed] [Google Scholar]
- 28.Yang J, Gupta V, Carroll KS, Liebler DC. Site-specific mapping and quantification of protein S-sulphenylation in cells. Nat Commun. 2014;5:4776. doi: 10.1038/ncomms5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doulias P-T, et al. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci USA. 2010;107(39):16958–16963. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doulias P-T, Tenopoulou M, Greene JL, Raju K, Ischiropoulos H. Nitric oxide regulates mitochondrial fatty acid metabolism through reversible protein S-nitrosylation. Sci Signal. 2013;6(256):rs1. doi: 10.1126/scisignal.2003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zahid S, Khan R, Oellerich M, Ahmed N, Asif AR. Differential S-nitrosylation of proteins in Alzheimer’s disease. Neuroscience. 2014;256:126–136. doi: 10.1016/j.neuroscience.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Zaręba-Kozioł M, Szwajda A, Dadlez M, Wysłouch-Cieszyńska A, Lalowski M. Global analysis of S-nitrosylation sites in the wild type (APP) transgenic mouse brain-clues for synaptic pathology. Mol Cell Proteomics. 2014;13(9):2288–2305. doi: 10.1074/mcp.M113.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gjoneska E, et al. Conserved epigenomic signals in mice and humans reveal immune basis of Alzheimer’s disease. Nature. 2015;518(7539):365–369. doi: 10.1038/nature14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 35.Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8(8):1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274(39):27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 37.Qu J, et al. S-Nitrosylation activates Cdk5 and contributes to synaptic spine loss induced by beta-amyloid peptide. Proc Natl Acad Sci USA. 2011;108(34):14330–14335. doi: 10.1073/pnas.1105172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Barry J, Liégeois CM, Janoshazi A. Protein kinase C as a peripheral biomarker for Alzheimer’s disease. Exp Gerontol. 2010;45(1):64–69. doi: 10.1016/j.exger.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Choi H, Tostes RC, Webb RC. Thioredoxin reductase inhibition reduces relaxation by increasing oxidative stress and s-nitrosylation in mouse aorta. J Cardiovasc Pharmacol. 2011;58(5):522–527. doi: 10.1097/FJC.0b013e31822d80a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gopalakrishna R, Chen ZH, Gundimeda U. Nitric oxide and nitric oxide-generating agents induce a reversible inactivation of protein kinase C activity and phorbol ester binding. J Biol Chem. 1993;268(36):27180–27185. [PubMed] [Google Scholar]
- 41.Hara MR, et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat Cell Biol. 2005;7(7):665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 42.Kornberg MD, et al. GAPDH mediates nitrosylation of nuclear proteins. Nat Cell Biol. 2010;12(11):1094–1100. doi: 10.1038/ncb2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455(7211):411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 44.Marino SM, Gladyshev VN. Structural analysis of cysteine S-nitrosylation: A modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395(4):844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Shea JP, et al. pLogo: A probabilistic approach to visualizing sequence motifs. Nat Methods. 2013;10(12):1211–1212. doi: 10.1038/nmeth.2646. [DOI] [PubMed] [Google Scholar]
- 46.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18(5):691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 47.Raju K, et al. Regulation of brain glutamate metabolism by nitric oxide and S-nitrosylation. Sci Signal. 2015;8(384):ra68. doi: 10.1126/scisignal.aaa4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Block E, Ofori-Okai G, Zubieta J. 2-phosphino- and 2-phosphinylbenzenethiols: New ligand types. J Am Chem Soc. 1989;111(6):2327–2329. [Google Scholar]
- 49.Figuly GD, Loop CK, Martin JC. Directed ortho-lithiation of lithium thiophenolate. New methodology for the preparation of ortho-substituted thiophenols and related compounds. J Am Chem Soc. 1989;111(2):654–658. [Google Scholar]
- 50.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6(5):359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 51.Slade PG, et al. Proteins modified by the lipid peroxidation aldehyde 9,12-dioxo-10(E)-dodecenoic acid in MCF7 breast cancer cells. Chem Res Toxicol. 2010;23(3):557–567. doi: 10.1021/tx9002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravindra KC, et al. Untargeted proteomics and systems-based mechanistic investigation of artesunate in human bronchial epithelial cells. Chem Res Toxicol. 2015;28(10):1903–1913. doi: 10.1021/acs.chemrestox.5b00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang DW, et al. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8(9):R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.