Abstract
The aim of this study was to investigate aerobic exercise training as a means to prevent erectile dysfunction (ED) and coronary artery disease (CAD) development associated with inactivity and diet-induced obesity. Male Sprague-Dawley rats were fed a Western diet (WD) or a control diet (CD) for 12 wk. Subgroups within each diet remained sedentary (Sed) or participated in aerobic interval treadmill running throughout the dietary intervention. Erectile function was evaluated under anesthesia by measuring the mean arterial pressure and intracavernosal pressure in response to electrical field stimulation of the cavernosal nerve, in the absence or presence of either apocynin, an NADPH oxidase inhibitor, or sepiapterin, a tetrahydrobiopterin precursor. Coronary artery endothelial function (CAEF) was evaluated ex vivo with cumulative doses of ACh applied to preconstricted segments of the left anterior descending coronary artery. CAEF was assessed in the absence or presence of apocynin or sepiapterin. Erectile function (P < 0.0001) and CAEF (P < 0.001) were attenuated in WD-Sed. Exercise preserved erectile function (P < 0.0001) and CAEF (P < 0.05) within the WD. Erectile function (P < 0.01) and CAEF (P < 0.05) were augmented by apocynin only in WD-Sed, while sepiapterin (P < 0.05) only augmented erectile function in WD-Sed. These data demonstrate that a chronic WD induces impairment in erectile function and CAEF that are commonly partially reversible by apocynin, whereas sepiapterin treatment exerted differential functional effects between the two vascular beds. Furthermore, exercise training may be a practical means of preventing diet-induced ED and CAD development.
Keywords: diet-induced obesity, NADPH oxidase, high-fat high-sucrose, endothelial nitric oxide synthase uncoupling, high-intensity interval training
obesity is no longer strictly an American problem. Obesity rates have drastically risen in much of the developed world since the 1980s, and continue to rise in much of the developing world (16). Corresponding to these trends is the increasing worldwide consumption of the Western diet (WD), consisting of high levels of saturated fat, omega-6 (n-6) polyunsaturated fatty acids (PUFA), particularly n-6 linoleic acid, as well as added sugar (46). Obesity has proven to be a significant, independent risk factor for erectile dysfunction (ED); thus, the prevalence of ED is projected to rise alongside the rising obesity rates (5, 30). Furthermore, clinical data suggest that the presence of ED in otherwise healthy men may be associated with early, subclinical signs of coronary artery disease (CAD) that may not be detectable during stress testing (8, 31, 40). A recent metaanalysis of 92,757 subjects followed for a mean of 6.1 yr confirms that patients with ED have a 62% increased risk for myocardial infarction compared with patients without ED, which is independent of traditional cardiovascular risk factors (61). We have recently demonstrated that rats fed a WD develop ED prior to coronary artery endothelial dysfunction (36). Thus, ED may be a powerful predictor of CAD, and therapeutic strategies aimed at treatment of both of these diseases warrants investigation.
Oxidative stress, marked by excessive production of reactive oxygen species (ROS), is thought to be elevated in obese individuals and is a prominent feature of vascular diseases (9, 19). NADPH oxidase (NOX), in particular, is considered a prominent source of vascularly derived ROS, the expression of which has been shown to be elevated in arteries of human diabetes and CAD patients (22, 23). A potential consequence of increased NOX activity is oxidation of tetrahydrobiopterin (BH4), an essential cofactor of endothelial nitric oxide synthase (eNOS) (11). When BH4 is deficient, eNOS dimers destabilize, resulting in formation of superoxide rather than nitric oxide (NO) (11). Production of superoxide from eNOS is termed uncoupling, which represents a potential vicious cycle of vascular dysfunction by promoting depressed NO bioavailability and increased oxidative stress through excessive superoxide production. Neuronal nitric oxide synthase (nNOS) may also uncouple by a similar mechanism (49), which has recently been shown to potentiate endothelial dysfunction in penile arteries (48).
Exercise training is known to protect against cardiovascular disease (35). Aerobic interval training, in particular, has shown to produce several positive health benefits in patients with heart failure or the metabolic syndrome, including increases in aerobic capacity, flow-mediated dilation, insulin sensitivity, and decreases in fatty acid synthase (56, 66). The purpose of this study was to determine whether aerobic interval training could protect against WD-induced ED and coronary artery endothelial dysfunction. Further, we sought to investigate the efficacy of acute apocynin (NOX1/2 inhibitor) and sepiapterin (BH4 precursor) treatment for erectile function and coronary artery endothelial function (CAEF) in rats fed a WD.
METHODS
Experimental animals and diets.
Male Sprague-Dawley rats were purchased at 5 wk of age (Charles River Laboratories, Wilmington, MA) and housed in the Department of Comparative Medicine at East Carolina University, in pairs when possible, in a temperature-controlled room (22 ± 1°C) with a 12:12-h light-dark cycle. Rats were fed a high-fat, high-sucrose diet with a Western pattern fatty acid distribution (Teklad Diets 110365; Harlan Laboratories, Madison, WI) (WD) or a control diet (Teklad Diets 110367) (CD) for the final 12 wk of life. The Western diet (4.68 kcal/g, 44.6% kcal fat, 40.7% kcal carbohydrate, 340 g/kg sucrose, 27 n-6:n-3 ratio), and a control diet (3.67 kcal/g, 12.7% kcal fat, 72.4% kcal carbohydrate, 150 g/kg sucrose, 9.6 n-6:n-3 ratio) contained equivalent levels of vitamins, minerals, and protein when considered on a basis of kilocalorie density, as further described (36). All rats were killed at 19–20 wk of age. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee at East Carolina University.
Exercise intervention.
Rats remained sedentary (n = 12 CD and n = 11 WD) or underwent an aerobic interval training intervention (n = 8 CD and n = 11 WD) throughout the duration of the dietary intervention. Rats were acclimated to exercise by walking on a treadmill at 15 m/min at 0° inclination for 10 min/day for seven consecutive days prior to induction of the training/dietary intervention. The training protocol consisted of a maximal work capacity test at the beginning of each week, followed by aerobic interval training 4 days/wk, for a total of five exercise days/week. The maximal work capacity test consisted of a 20-min warm-up at ∼50% V̇o2max, followed by incremental increases in speed of 2.0 m/min every 2 min until volitional fatigue, followed by a 10-min cool-down at ∼50% V̇o2max. The aerobic interval protocol consisted of a 10-min warm-up followed by eight intervals, each consisting of a 4-min high-intensity phase and a 3-min low-intensity phase, followed by a 4-min cool-down as detailed in Table 1. Running intensity increased each week until a plateau was reached in maximal work capacity at week 9. Rats were encouraged to run primarily by compressed air, with every effort made to minimize the use of the electric shock grid at the back of the treadmill belt. This protocol was designed to approximate the intensities utilized in previous studies, which demonstrate the beneficial impact of aerobic interval training on endothelial function and aerobic capacity relative to continuous moderate-intensity exercise protocols (25, 26, 28, 65).
Table 1.
Treadmill aerobic interval training protocol
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7° | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incline | 17° | 17° | 17° | 17° | 17° | 17° | 25 | 25° | 25° | 25° | 25° | 25° |
| Minute | Speed, m/min | |||||||||||
| 0 | 10 | 12 | 14 | 14 | 15 | 16 | 14 | 14 | 14 | 14 | 14 | 14 |
| 10 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 14 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 17 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 21 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 24 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 28 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 31 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 35 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 38 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 42 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 45 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 49 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 52 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 56 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 59 | 19 | 20 | 21 | 22 | 23 | 24 | 21 | 22 | 22 | 22 | 22 | 22 |
| 63 | 14 | 15 | 16 | 17 | 18 | 19 | 16 | 17 | 17 | 17 | 17 | 17 |
| 66 | 10 | 12 | 13 | 14 | 15 | 16 | 13 | 14 | 14 | 14 | 14 | 14 |
| 70 | stop | |||||||||||
Glucose, insulin, lipids, and anthropometrics.
Approximately 0.3 ml of blood was drawn from the tail vein prior to anesthesia, from which fasting glucose concentration was measured from whole blood (Accu-Check, Roche, Basel, Switzerland). Plasma was separated and stored at −80°C until analysis of insulin with a rat/mouse insulin assay kit (Millipore, Billerica, MA). Serum was collected during the erection surgery, as described previously (36), separated and stored at −80°C until analysis. Total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides were measured with a clinical mass analyzer (UniCel DxC 600; Beckman Coulter, Indianapolis, IN). Serum 4-hydroxynonenal (HNE) adducts were measured with a modified ELISA approach, as described previously (17). Body weight was measured prior to surgery with a triple beam balance. Lean mass and fat mass were measured immediately prior to surgery by nuclear magnetic resonance-magnetic resonance imaging (NMR-MRI) (EchoMRI 700; Echo Medical Systems, Houston, TX).
Erectile response measurements.
All rats were fasted overnight prior to erectile function studies. Rats were anesthetized with an intraperitoneal injection of 90 mg/kg ketamine and 10 mg/kg xylazine, and supplemented with an intramuscular injection as needed. The left carotid artery was cannulated with a 20-gauge needle connected via polyethylene (PE) tubing to a pressure transducer, anti-coagulated with 2.0 U/ml heparinized saline, allowing for continuous measurement of systemic blood pressure and intra-arterial administration of saline. The right corpus cavernosum was cannulated with a 27-gauge needle connected via PE tubing to a pressure transducer, and the left corpus cavernosum was cannulated with a 27-gauge needle connected via PE tubing to a syringe. Platinum bipolar electrodes attached to a Grass Stimulator (Grass S09, Grass Technologies, West Warwick, RI) were positioned near the cavernosal nerve to deliver electrical field stimulation, consisting of a successive, increasing voltage series of 1–5 V for 30 s at each voltage, delivered in 5-ms pulses at 13 Hz (36). Mean arterial pressure (MAP) and intracavernosal pressure (ICP) were recorded throughout each voltage series with LabChart 7 software (ADInstruments, Sydney, Australia). Three voltage series were conducted and averaged as the baseline erectile response. ICP returned to baseline between each series. We have found the erectile response to these stimulation parameters to be stable for at least 2 h. Following the three voltage series, either 12 μl of 1 mM apocynin (Sigma Aldrich, St. Louis, MO) or 12 μl of 10 μM sepiapterin (Cayman Chemical, Ann Arbor, MI), prepared in heparinized saline, was injected into the left corpus cavernosum. A voltage series was conducted 20-min postapocynin injection or 30-min postsepiapterin injection, when treatment effects appeared to be maximal.
Coronary artery endothelium-dependent and -independent vasorelaxation.
Immediately following the erection surgery, rats were euthanized by exsanguination and double pneumothorax. Hearts were excised, and up to four segments of the left anterior descending (LAD) coronary artery were carefully dissected and freed from adhering myocardium. Two, 40-μm diameter, stainless-steel wires were passed through each arterial segment and mounted in a multiwire myograph (DMT 620 M, Aarhus, Denmark). Vessel segments were bathed in physiological saline solution (PSS) at pH 7.4, 37°C, gassed with medical-grade air, as described previously (64). Vasorelaxation assessments were performed as described previously (36). Briefly, vessels were gradually stretched to an optimum resting tension, established by determining diameter-tension relationship of each segment and setting it to 90% of the lumen circumference achieved at 13.3 kPa, and viability was tested with a 109 mM K+ physiological saline solution (KPSS) challenge. Vessels that failed to constrict to at least 1.0 mN/mm2 were excluded from experimentation. Following 30 min of repeated washes of PSS at 10-min intervals, vessels were preconstricted with 3.0 μM 5-HT, and endothelial function was tested with cumulative concentrations of ACh (0.001–10.0 μM). Following 30 min of repeated washes of PSS at 10-min intervals, vessel segments were incubated in either 300 μM apocynin or 10 μM sepiapterin for 30 min, and the ACh-cumulative concentration response following 5-HT preconstriction was repeated. Vessels were washed for 30 min with repeated washes of PSS at 10-min intervals, and endothelium-independent relaxation was tested with cumulative concentrations of sodium nitroprusside (SNP) (0.0001–1.0 μM) following 5-HT preconstriction.
Aortic NADPH oxidase activity and TBARS.
Following excision of the heart, the thoracic aorta was excised and placed in ice-cold PSS. Adhering connective tissue and adipose tissue were removed. Aortas were snap frozen in liquid nitrogen and stored at −80°C until analysis. Aortas were homogenized in 10× (wt/vol) TEE buffer (10 mM Tris base, 1 mM EDTA, and 1 mM EGTA) using a tissue grinder (Kimble Chase, Vineland, NJ). 0.1% Triton-X was added following homogenization. Each homogenate was divided into two aliquots. One aliquot was centrifuged at 1,600 g for 10 min at 4°C, and the supernatant was separated and stored on ice until measurement of NOX activity. For this assay, 30 μl of sample was added to 170 μl of a cocktail containing 10 μM Amplex Red (Invitrogen, Eugene, OR), 1.0 U/ml horseradish peroxidase (Sigma Aldrich, St. Louis, MO), and 10 U/ml superoxide dismutase (Sigma Aldrich) in PBS. Fluorescence of resorufin (563 ex/587 em) was measured continuously with a spectrofluorometer (Horiba Jobin Yvon, Ann Arbor, MI) with temperature control and magnetic stirring at 37°C (3). After establishing baseline rate of ΔF/dt in the absence of substrate, NOX activity was stimulated with the addition of 500 μM NADPH, following which ΔF/dt was continuously measured for ∼5 min. Total tissue NOX activity was calculated as ΔF/dt of resorufin, where total NADPH-dependent H2O2 generated in the sample was used as an index of NOX activity. Activity was normalized to total protein content, as determined by a bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL).
The other aliquot was sonicated for 15 s at 40 V (Branson sonifer 450) over ice and then centrifuged. The supernatant was then stored at −80°C until analysis of thiobarbituric acid reactive substances (TBARS), an index of lipid peroxidation and associated oxidative stress. TBARS were measured in duplicate with a commercial assay kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer's instructions. Protein concentrations from these samples were determined using a BCA assay kit. Sample malondialydehyde (MDA) concentration was calculated from a MDA standard curve, and the concentration was normalized to total protein content.
Data and statistical analysis.
The erectile response was calculated from the ratio of the average ICP divided by the average MAP during the final 25 s of each 30-s voltage stimulation level. Effects of acute apocynin or sepiapterin treatment on the erectile response were compared with the pretreatment response within the same rat. Coronary artery endothelium-dependent and -independent vasodilation was assessed by the average stress produced by each vessel in the final 30 s of each 5-min concentration exposure. Vessel stress was calculated by subtracting basal vessel force produced from the force produced at each respective concentration and normalized to vessel surface area. The relaxation responses from the vessel segments were averaged for each rat and analyzed as a single observation. The effects of apocynin or sepiapterin treatment were compared with the untreated response within the same segment. Concentration response curves were generated against the logarithm of each concentration and fit by nonlinear regression to generate EC50 values to describe each curve (GraphPad Prism v. 5.0). Emax values were determined as the maximal relaxation of each segment, expressed as a percentage of 5-HT induced constriction. Statistical differences for the voltage-dependent erectile response and coronary artery agonist-stimulated vasorelaxation were determined by two-way repeated-measures ANOVA with Bonferroni's post hoc analysis. Between-group differences for EC50 and Emax values were determined by one-way ANOVA with Tukey's multiple-comparison post hoc analysis. Treatment effects of apocynin or sepiapterin on EC50 and Emax values were determined by a paired t-test. Statistical differences for metabolic parameters, NADPH oxidase activity, and TBARS were determined by one-way ANOVA with Tukey's multiple-comparison post hoc analysis. Data were presented as means ± SE. Statistical analyses were performed with GraphPad Prism v. 5.0 or SPSS v. 19.0, with an α level of 0.05.
RESULTS
Metabolic parameters.
The body weight from the CD-Ex rats was lower than WD-Sed; however, WD-Sed rats had greater fat mass than all other groups, while lean mass was not different among any group (Table 2). Despite increased blood glucose in the WD-Sed rats, no differences in insulin or homeostasis model assessment-estimated insulin resistance (Table 2) were observed between groups. WD-Ex rats had lower total cholesterol levels than CD-Sed rats, while no differences existed for HDL-cholesterol, LDL-cholesterol, or triglyceride levels between groups (Table 2). Systemic oxidative stress was lower in both exercise groups compared with the WD-Sed group, as indicated by serum HNE (HNE-adducts; Table 2).
Table 2.
Metabolic parameters
| CD-Sed | CD-Ex | WD-Sed | WD-Ex | P value | |
|---|---|---|---|---|---|
| Body weight, g | 458 ± 8.0 | 424 ± 12.5 | 499 ± 11.1† | 456 ± 17.2 | 0.003 |
| Fat mass, g | 52.0 ± 5.97 | 34.8 ± 7.48 | 76.9 ± 4.93*†‡ | 44.3 ± 8.48 | 0.001 |
| Lean mass, g | 341 ± 6.1 | 328 ± 7.8 | 356 ± 8.3 | 349 ± 10.3 | 0.154 |
| Body fat, % | 13.1 ± 1.30 | 9.3 ± 1.82 | 17.7 ± 0.98†‡ | 10.9 ± 1.52 | 0.001 |
| Glucose, mg/dl | 107 ± 3.5 | 99 ± 1.6 | 121 ± 3.5*† | 109 ± 3.7 | <0.001 |
| Insulin, pM | 125 ± 43.5 | 142 ± 28.1 | 174 ± 24.3 | 116 ± 23.4 | 0.482 |
| HOMA-IR | 4.94 ± 1.84 | 4.87 ± 0.95 | 7.34 ± 1.04 | 4.51 ± 1.09 | 0.25 |
| Cholesterol, mg/dl | 39.0 ± 4.53‡ | 34.0 ± 2.59 | 30.2 ± 1.91 | 27.1 ± 2.14 | 0.029 |
| HDL-C, mg/dl | 25.9 ± 2.36 | 22.9 ± 2.24 | 24.6 ± 1.67 | 21.8 ± 1.80 | 0.542 |
| LDL-C, mg/dl | 8.6 ± 1.44 | 9.8 ± 0.66 | 7.7 ± 0.42 | 8.0 ± 0.50 | 0.129 |
| Triglycerides, mg/dl | 58.0 ± 9.8 | 54.8 ± 12.2 | 45.3 ± 10.0 | 27.3 ± 6.1 | 0.128 |
| HNE-adducts, mM | 0.706 ± 0.07 | 0.500 ± 0.06 | 1.02 ± 0.10† | 0.407 ± 0.06 | 0.003 |
Values are expressed as means ± SE; n = 5–11 animals per group.
P < 0.05 vs. CD-Sed;
P < 0.05 vs. CD-Ex;
P < 0.05 vs. WD-Ex. CD, control diet; WD, Western diet; Sed, sedentary; Ex, exercise; HOMA-IR, homeostasis model assessment-estimated insulin resistance.
Voltage-dependent erectile response.
The ICP/MAP in response to the intermediate voltages (2, 3, and 4 V) was significantly attenuated by the Western diet (Fig. 1A) and was preserved by exercise training within the Western diet (Fig. 1). However, the ICP/MAP at 2 and 3 V was significantly attenuated by exercise training within the control diet (Fig. 1D). Intracavernosal treatment with apocynin (Fig. 2) or sepiapterin (Fig. 3) had no effect on the voltage-dependent erectile response of the CD-Sed, CD-Ex, or WD-Ex rats, but significantly augmented the erectile response of WD-Sed rats.
Fig. 1.
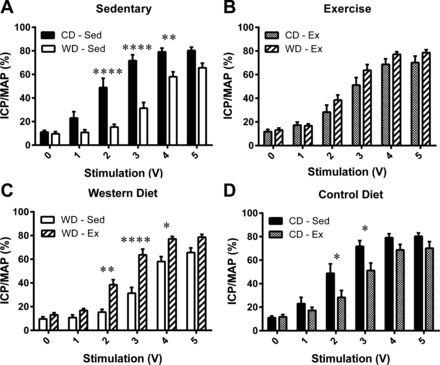
Voltage-dependent erectile responses. A: intracavernosal pressure/mean arterial pressure (ICP/MAP) was attenuated by the Western diet (WD). B: ICP/MAP was equivalent with exercise between diets. C: WD-induced impairment of ICP/MAP was prevented by exercise training. D: ICP/MAP was attenuated by exercise training within the control diet. Values are expressed as means ± SE for 8–11 animals in each group. *P < 0.05, **P < 0.01, ****P < 0.0001. CD, control diet; Ex, exercise; Sed, sedentary.
Fig. 2.
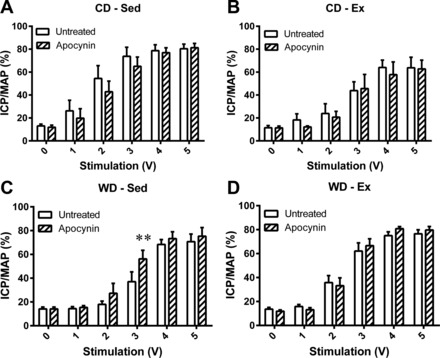
Effect of acute apocynin administration on voltage-dependent erectile response. Following the untreated voltage series' (open bars), 1 mM apocynin was injected intracavernosally, and a voltage series was applied 20 min postinjection (hatched bars). No treatment effects were observed in control diet-sedentary (CD-Sed) (A), control diet-exercise (CD-Ex) (B), or Western diet-exercise (WD-Ex) (D), while apocynin enhanced the erectile response in Western diet-sedentary (WD-Sed) rats (C). Values are expressed as means ± SE for 4 or 5 animals in each group **P < 0.01 vs. untreated.
Fig. 3.
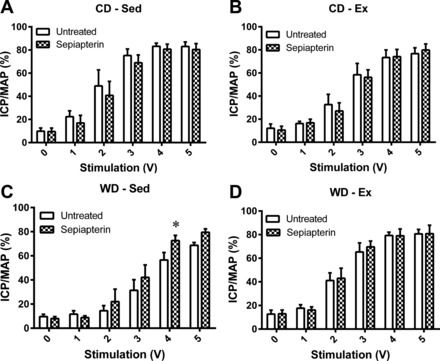
Effect of acute sepiapterin administration on voltage-dependent erectile response. Following the untreated voltage series' (open bars), 10 μM sepiapterin was injected intracavernosally and a voltage series was applied 30-min postinjection (checkered bars). No treatment effects were observed in control diet-sedentary (CD-Sed) (A), control diet-exercise (CD-Ex) (B), or Western diet-exercise (WD-Ex) (D), while sepiapterin enhanced the erectile response in Western diet-sedentary (WD-Sed) rats (C). Values are expressed as means ± SE for 4–6 animals in each group *P < 0.05 vs. untreated.
Coronary artery endothelium-dependent and -independent relaxation.
Vasoconstriction tested by KPSS challenge (P = 0.828) or 3.0 μM 5-HT (P = 0.945) was not different between any groups (Table 3). The ACh-stimulated relaxation curves are presented in Fig. 4. The relaxation profile was significantly attenuated by the WD at two ACh concentrations (Fig. 4A). Similarly, exercise augmented the relaxation profile within the WD at two ACh concentrations (Fig. 4C). Corresponding relaxation profile analysis reveals a greater EC50 value and depressed Emax response to ACh in the WD-Sed group (Table 3). Treatment of the vessel segments with apocynin augmented ACh-stimulated vasorelaxation profile in WD-Sed but had no significant effect on CD-Sed, Con-Ex, or WD-Ex coronary artery segments (Fig. 5). The effect of apocynin treatment on EC50 (P = 0.065) and Emax (P = 0.079) failed to reach statistical significance (Table 3). Treatment with sepiapterin did not improve ACh-stimulated vasorelaxation in any group, while sepiapterin attenuated the vasorelaxation profile, EC50, and Emax in CD-Sed (Fig. 6, Table 3), and Emax in WD-Ex (Table 3). The coronary artery endothelium-independent relaxation was assessed from the concentration-response profile to the NO donor sodium nitroprusside. There were no significant differences between groups for the relaxation response to SNP (Fig. 7); however, the Emax was greater in the WD-Sed group compared with the CD-Ex group (Table 3).
Table 3.
Coronary artery vasoconstriction and vasorelaxation characteristics
| CD-Sed | CD-Ex | WD-Sed | WD-Ex | |
|---|---|---|---|---|
| StressKPSS, mN/mm2 | 2.24 ± 0.21 | 2.32 ± 0.44 | 2.36 ± 0.32 | 2.65 ± 0.40 |
| Stress5-HT, mN/mm2 | 2.96 ± 0.32 | 2.82 ± 0.48 | 2.83 ± 0.27 | 3.09 ± 0.39 |
| ACh EC50, mM | 0.120 ± 0.015 | 0.204 ± 0.075 | 0.509 ± 0.145* | 0.218 ± 0.073 |
| ACh Emax | 96.3 ± 1.05 | 93.5 ± 2.10 | 85.0 ± 3.55* | 92.6 ± 3.00 |
| SNP EC50 (nM) | 19.4 ± 3.69 | 17.1 ± 3.86 | 17.3 ± 3.70 | 33.1 ± 7.82 |
| SNP Emax | 99.2 ± 0.43 | 94.5 ± 1.46 | 102 ± 1.70§ | 97.4 ± 1.67 |
| Apo-ACh EC50 | 0.232 ± 0.081 | 0.111 ± 0.046 | 0.252 ± 0.154 | 0.177 ± 0.054 |
| Apo-ACh Emax | 90.0 ± 2.99 | 94.3 ± 2.04 | 90.6 ± 3.03 | 96.2 ± 0.95 |
| Sep-ACh EC50 | 0.205 ± 0.030† | 0.335 ± 0.093 | 0.526 ± 0.180 | 0.616 ± 0.223 |
| Sep-ACh Emax | 92.7 ± 2.07† | 86.3 ± 4.18 | 86.8 ± 5.23 | 85.5 ± 3.28† |
Values are expressed as means ± SE, n = 6–11 animals per group.
P < 0.05 vs. CD-Sed. §P < 0.05 vs. CD-Ex.
P < 0.05, significant treatment effect. CD, control diet; WD, Western diet, Sed, sedentary; Ex, exercise; ACh, acetylcholine; SNP, sodium nitroprusside; Apo, apocynin; Sep, sepiapterin.
Fig. 4.
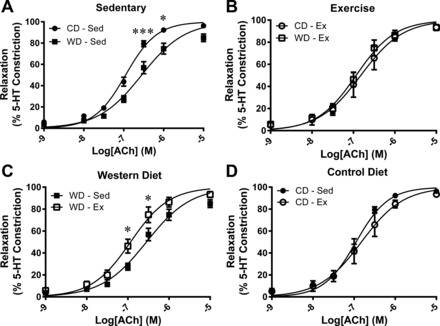
Mean concentration-response of coronary artery segments following 3.0 μM 5-HT preconstriction to ACh stimulation (0.001–10.0 μM). ACh-stimulated vasorelaxation was impaired by the Western diet (A), and equivalent with exercise between diets (B). WD-induced impairment of ACh-stimulated vasorelaxation was prevented by exercise training (C), while exercise training had no effect within the control diet (D). Values are expressed as means ± SE for 6–11 animals in each group. *P < 0.05, ***P < 0.001.
Fig. 5.
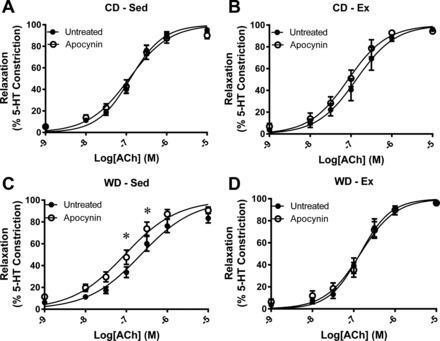
Effect of apocynin on ACh-stimulated vasorelaxation of coronary artery segments. Vasorelaxation was examined in the absence (●) and presence (○) of 300 μM apocynin. No treatment effects were observed in control diet-sedentary (CD-Sed) (A), control diet-exercise (CD-Ex) (B), or Western diet-exercise (WD-Ex) (D), while apocynin enhanced ACh-stimulated vasorelaxation in Western diet-sedentary (WD-Sed) rats (C). Values are expressed as means ± SE for 6–10 animals in each group. *P < 0.05 vs. untreated.
Fig. 6.
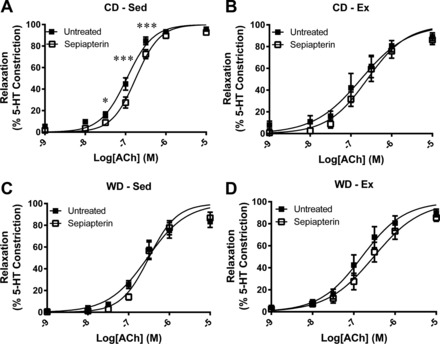
Effect of sepiapterin on ACh-stimulated vasorelaxation of coronary artery segments. Vasorelaxation was examined in the absence (■) and the presence (▫) of 10 μM sepiapterin. Sepiapterin treatment attenuated ACh-stimulated vasorelaxation in control diet-sedentary rats (A), while no treatment effects were observed in control diet-exercise (B), Western diet-sedentary (WD-Sed) (C), or Western diet-exercise (WD-Ex) (D) rats. Values are expressed as means ± SE for 6–10 animals in each group. *P < 0.05 vs. untreated; ***P < 0.001 vs. untreated.
Fig. 7.
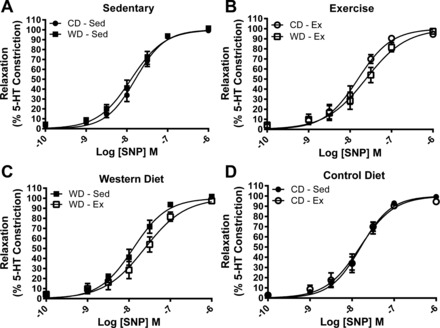
Mean concentration-response of coronary artery segments following 3.0 μM 5-HT preconstriction to SNP stimulation (0.0001–1.0 μM). There were no significant differences between groups. Values are expressed as means ± SE for 6–11 animals in each group.
Vascular tissue oxidative stress.
All attainable LAD tissue was utilized for the myography experiments. Use of cavernosal tissue to perform NOX activity and oxidative stress measurements was untenable following apocynin/sepiapterin treatment; therefore, the thoracic aorta was utilized as a surrogate vascular tissue to investigate these parameters. NADPH-stimulated ROS production was elevated in the WD-Sed group compared with both the CD-Sed and WD-Ex groups (Fig. 8A). Aortic TBARS was measured as an index of vascular tissue lipid peroxidation (Fig. 8B), and there were no statistically significant differences found between the groups (P = 0.227).
Fig. 8.
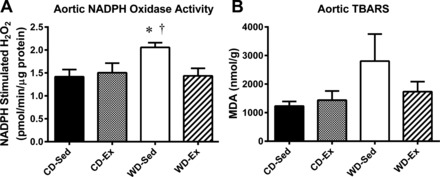
Vascular tissue indices of oxidative stress measured in the thoracic aorta. NADPH-stimulated H2O2 production was measured as an index of NADPH oxidase activity (A), which was elevated in the WD-Sed group. Thiobarbituric acid reactive substances (TBARS) were measured as an index of lipid peroxidation (B), which was not significantly elevated in any group. *P < 0.05 vs. CD-Sed; †P < 0.05 vs. WD-Ex. Values are expressed as means ± SE for 8–11 animals in each group. MDA, malondialdehyde; CD, control diet; WD, Western diet; Ex, exercise; Sed, sedentary.
DISCUSSION
In the present study, we investigated the efficacy of an aerobic interval training intervention on prevention of WD-induced ED and coronary artery endothelial dysfunction. Furthermore, we investigated the differential effects of acute apocynin treatment and acute sepiapterin treatment on erectile function and CAEF following a 12-wk WD that is high in sucrose and fat derived from saturated and n-6 PUFA. Rats demonstrated an impaired erectile response to electrical field stimulation of the cavernosal nerve, and an impaired coronary artery relaxation response to ACh following the chronic WD, both of which were prevented by the exercise intervention. Acute apocynin treatment significantly improved erectile function and CAEF in the WD-Sed group, while having no impact in the CD or Ex groups. In contrast, stimulation of intracellular BH4 production with acute sepiapterin treatment significantly improved erectile function in the WD-Sed group, but had no effect on CAEF. Acute sepiapterin treatment attenuated CAEF in the CD-Sed and WD-Ex group, while having no effect on the WD-Sed group. These findings suggest that erectile function and CAEF are impaired commonly by a mechanism that may be targeted by acute apocynin treatment in a 12-wk WD-induced obesity model. However, despite the common functional improvement mediated by apocynin treatment, the two vascular beds demonstrated a differential functional response to BH4 production stimulation with sepiapterin.
Oxidative stress has previously been demonstrated in cavernosal tissue of LDLR−/− mice fed a hypercholesterolemic diet (42), cholesterol-fed rabbits (50), and high-fat diet-fed pigs (43). Oxidative stress is a likely contributor to ED, as treatment with various antioxidants (4, 53) or transfection of SOD (7) normalize markers of cavernosal oxidative stress and augments cavernosal vasorelaxation in streptozotocin (STZ)-induced diabetic rats. Mitochondria-derived oxidative stress may be a pathological consequence of the metabolic syndrome that contributes to vascular dysfunction (63); however, the impact of mitochondria-derived ROS on erectile function remains unknown. Cytosol-derived ROS have more traditionally been associated with erectile dysfunction, as elevated expression of NOX subunits have been found in cavernosal tissue of STZ-induced diabetic rats (38) and LDLR−/− mice fed a hypercholesterolemic diet (42). Additionally, chronic supplementation of apocynin to drinking water prevents hypercholesterolemic diet- (42) and hypertension (33)-associated vascular insult and preserves the voltage-dependent erectile response. The present study suggests that an apocynin-targeted mechanism partially reverses WD-associated ED. This results in an important distinction from a chronic supplementation study, as we postulate that acute apocynin treatment only restores functionality, presumably by inhibiting NOX1/2-derived ROS production, whereas chronic apocynin supplementation may also inhibit progressive atherosclerotic development and vascular remodeling resulting from chronic oxidative stress.
One potentially vasodestructive consequence of elevated NOX activity is the quenching of NO by superoxide, resulting in the production of peroxynitrite. Further, peroxynitrite may then deplete intracellular BH4 levels, by direct oxidation of BH4 to BH2 (37). Adequate presence of BH4 is a critical requirement for normal eNOS functioning, which acts to stabilize eNOS dimers and allows for the oxidation of l-arginine and the subsequent generation of NO (11). Destabilization of eNOS dimers through BH4 depletion results in “eNOS uncoupling”, where electron flow from NADPH through eNOS to O2 is not inhibited but results in the formation of superoxide rather than NO (11). The resulting situation is a potential vicious cycle where NOX activity is elevated in metabolic syndrome conditions, which promotes endothelial dysfunction through NO quenching and eNOS uncoupling, and further endothelial dysfunction via progressive loss of NO bioavailability and superoxide production from eNOS. LDLR−/− mice fed a hypercholesterolemic diet demonstrate a decreased cavernosal eNOS dimer-monomer ratio, which is prevented by chronic drinking water supplementation of apocynin (42). Although nNOS uncoupling is studied much less than eNOS uncoupling, nNOS may become uncoupled via BH4 oxidation (49). This may be a particularly important mechanism regulating erectile function, as nNOS-derived NO is critical for the initiation of erection (47). The concept of nNOS uncoupling mediating ED has recently been brought to light by Sánchez et al. (48), who demonstrated improved nitrergic vasorelaxation of penile arteries in response to BH4 or apocynin in a genetically obese rat model. In the present study, we observed a partial reversal of obesity-associated ED in response to acute BH4 production stimulation with sepiapterin. While we cannot be certain that NOX was the predominant mechanism by which cavernosal NOS was apparently dysfunctional in response to the WD, apocynin treatment and BH4 production stimulation with sepiapterin both augmented the voltage-dependent erectile response to a similar degree.
Contrary to the voltage-dependent erectile response, acute sepiapterin treatment did not improve coronary artery endothelial dysfunction in the WD-Sed rats. 10 μM sepiapterin treatment has previously restored endothelial function in aortic segments of ApoE−/− mice (37). Furthermore, oral administration of sepiapterin has been found to augment left ventricular function in STZ-induced diabetic mice (34), while intravenous administration of sepiapterin restores the cardioprotective effect of ischemic preconditioning that is completely blocked by severely hyperglycemic conditions (62). However, investigations on the effect of sepiapterin treatment specifically on CAEF have been limited: 100 μM sepiapterin has previously been found to attenuate endothelium-dependent vasorelaxation responses in canine middle cerebral arteries (57) and rabbit aortas (58), which agrees with the findings of the present study in which 10 μM sepiapterin attenuated ACh-stimulated vasorelaxation in coronary artery segments of rats with preserved endothelial function. It has previously been suggested that sepiapterin supplementation to normally functional eNOS could stimulate eNOS-dependent superoxide generation, resulting in the potential for attenuation of endothelial function (59). Additionally, the hypothesis of NOX-induced eNOS uncoupling suggests that NOX activity is increased prior to any depletion of BH4 and resultant eNOS uncoupling (2). The WD used in the present study has previously been demonstrated to induce ED more rapidly than coronary artery endothelial dysfunction (36). Thus, it is probable that NOX activity is also increased in the cavernosum prior to the LAD coronary artery. It is likely that 12 wk of exposure to the WD represents a very early time point in development of CAD in this rat model (36). These WD-Sed rats demonstrated mild elevations in adiposity and blood glucose levels, with no substantial alterations in blood lipids or insulin sensitivity. Thus, it is possible that prolonging the dietary intervention may deplete intracellular BH4 levels and induce NOS uncoupling in the coronary vasculature, as prior studies demonstrating increased vasoprotection or cardioprotection with sepiapterin treatment have utilized overtly hypercholesterolemic, hyperglycemic, or diabetic models (34, 37, 62). This study is in agreement with prior investigations, where apocynin has been used by the Zhang group (20, 44, 45) to reverse endothelial dysfunction in coronary microvessels of insulin-resistant obese Zucker rats (45), type II diabetic mice (20), and high-fat, diet-induced obese mice (44). This study suggests that elevated NOX activity is an important contributor to early WD-associated coronary artery endothelial dysfunction, in which functionality is not restored with BH4 production stimulation.
The potential of lifestyle interventions, including dietary and/or physical activity alterations on treating ED, has been discussed in several review articles (14, 21, 24, 39). Across general populations of men, physical activity is generally associated with better erectile function (13, 29, 32). However, investigations on the impact of exercise interventions on obesity-associated erectile dysfunction are rather limited. In a groundbreaking study by Esposito et al. (15), obese men with ED who ate a calorie-restricted diet for two years and received advice to increase physical activity reported reduced severity of erectile dysfunction coinciding with substantial weight loss. Although the present study fails to address the question of whether exercise is exclusively capable of reversing ED, exercise completely preserved erectile function despite ad libitum access to the obesogenic WD, which rapidly induces ED (36). There are previous implications for a protective effect of exercise through eNOS coupling, as treadmill exercise of pigs has been shown to preserve the cavernosal eNOS dimer:monomer ratio that is attenuated in response to a hypercholesterolemic diet (43). The present study adds to these findings, as exercise preserves erectile function despite chronic WD consumption, whereby chronic WD consumption induced impaired erectile functionality that was partially reversible with stimulation of BH4 production. An unexpected finding from this study was that exercise attenuated erectile function within the control diet. The exercise protocol was challenging, which could create undesirable stress for the rats. High-volume, high-intensity exercise training has previously been found to attenuate peripheral vascular function in lean humans (6) and aortic endothelial function in lean rats (52). However, WD-Ex rats were subjected to the same stress that CD-Ex were, which highlights the protective effects of exercise in the WD condition. Failure of apocynin to alter erectile function in either exercised condition may suggest that NOX activity was not upregulated in the cavernosum, as was demonstrated in the aorta and supported by low levels of systemic oxidative stress measured by serum HNE adducts.
Treadmill exercise has previously proven to preserve CAEF through a NO-mediated mechanism in a hypercholesterolemic pig model (55). Importantly, exercise has shown to improve endothelial function in internal mammary artery segments and attenuate NOX subunit expression in CAD patients (1). Similar to the present study, the apocynin-induced augmentation of CAEF in high-fat, diet-fed mice is lost with the addition of voluntary wheel running (44). Protection from ROS provided by the endogenous antioxidant system is critical to the maintenance of redox balance and protection from vascular insult; however, the response of antioxidants to diet, exercise, and pathologies have been variable. Penile SOD expression is decreased (53), while SOD activity is not changed with STZ-induced diabetes (7). SOD-1 expression does not change with high-fat diet in mesenteric arteries but increases with exercise irrespective of diet (12). SOD-1 expression is depressed with high-fat diet in coronary arteries (44, 55), while SOD-1 increases (44) or does not change (55) with exercise training. Gene expression of an array of antioxidant genes was upregulated with WD and/or Ex in myocardial tissue from the rats in the present study (17), which may promote a cardioprotective phenotype at the time point in which these rats were studied. Transient, exercise-induced ROS production induces a hormesis effect, whereby increased ROS production provides a signal to stimulate the antioxidant defense system (18, 67). Exercise-induced cardioprotection has been shown to be strongly associated with myocardial SOD activity (67). Interestingly, it was recently reported that NOX-derived ROS are responsible for exercise-induced cardioprotection as an intraperitoneal injection of apocynin prior to exercise prevented cardioprotection, whereas an intraperitoneal injection of mitochondrial targeted ROS scavengers were ineffective (18). These findings are exciting; however, the implications for transient exercise-induced NOX activation in pathological states where NOX activity is constitutively elevated remain a fascinating area of future investigation.
Aerobic interval training may be a particularly advantageous mode of intervention in the metabolic syndrome. Interval training protocols similar to that utilized in the present study have been shown to substantially augment endothelial function and aerobic capacity in human metabolic syndrome patients (56), heart failure patients (66), and CAD patients (41), as well as rodents bred to have a low aerobic capacity (26). The present study demonstrates that aerobic interval training is an effective method for preserving sexual function and CAEF despite consumption of an obesogenic diet, which promotes vascular dysfunction.
Several limitations exist in the present study. Apocynin is classically reported to inhibit the assembly of p47phox and p67phox within the membrane complex, and thus inhibit activation of NOX isoforms that require subunit translocation (51). However, apocynin has been reported to be both an antioxidant (27) and a prooxidant (10). Thus, we cannot be certain that apocynin acted specifically to inhibit NOX activity in our model. Furthermore, we demonstrated elevated NOX activity in aortas of the WD-Sed group, which support our functional data with apocynin treatment; however, we cannot be certain that NOX activity of the aorta translates to the cavernosum or the coronary artery. Also, BH4 may act as an antioxidant (11). Thus, in the absence of NOS dimer:monomer measurements, we cannot be certain that sepiapterin induces NOS coupling rather than oxidant scavenging. However, apocynin and sepiapterin elicited similar treatment effects on erectile function, but differing effects on CAEF. Thus, it is unlikely that both agents are acting merely as antioxidants. It should also be noted that treatment effects were compared with untreated responses within the same rat or same vessel segment. There was no vehicle-treated group in these experiments to assess the effect of time on responsiveness because the vehicle was our PSS solution, and all drugs were PSS soluble. We have previously performed parallel tissue experiments to ensure reproducibility of the vessel responses and erectile responses over time and found no significant diminution in repeated responses over the time course in which these studies were carried out. We acknowledge that without a specific vehicle-treated group in these experiments, it remains possible that a time effect may have masked potential effects of apocynin in the exercised and CD-Sed group. Additionally, the study design using an exercise intervention initiated at the onset of the dietary intervention does not allow one to infer whether the exercise training effects observed with the WD are merely due to the prevention of obesity or a more complex interaction between diet and exercise.
Perspectives and Significance
The findings of this study show that rats fed 12 wk of a high-fat, high-sucrose diet with a Western pattern fatty acid distribution demonstrate ED and coronary artery endothelial dysfunction. Dysfunctionality of both vascular beds was partially reversed by acute apocynin treatment. Despite the common effect of apocynin treatment, the two vascular beds demonstrated a differential response to acute sepiapterin treatment. Furthermore, these dysfunctions are preventable with concomitant aerobic interval exercise training. In the face of a worldwide obesity pandemic resulting from global spread of the Western diet (46), novel approaches to treatment and prevention of CAD are increasingly necessary. The association between ED and CAD has been shown to be nearly identical to the risk associated with current smoking or family history of myocardial infarction (54). This study demonstrates common efficacy of acute, localized pharmacologic treatment of ED and CAD at a time point that likely translates to a progressed stage of ED and a very early stage of development of CAD (36). These findings come at the heels of a recent review discussing clinical ED management strategies for patients with varying degrees of cardiovascular disease (CVD) risk, which also calls for sexual health counseling of CVD patients (60). The finding that exercise prevents WD-associated ED and CAD progression translates to an intensively active lifestyle throughout the duration of the “junk food” diet. It remains to be seen whether a moderately active lifestyle, or an active lifestyle initiated after a prolonged duration of a sedentary lifestyle combined with a “junk food” diet is effective at reversing functional impairment. While presentation of ED represents an attractive time point for lifestyle and/or therapeutic intervention aimed at cardioprotection, future studies are necessary to establish these relationships.
GRANTS
This work was supported by a Trainee grant from the Sexual Medicine Society of North America (to J. D. La Favor).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.D.L.F., E.J.A., R.C.H., and C.J.W. conception and design of research; J.D.L.F. and J.T.D. performed experiments; J.D.L.F. and C.J.W. analyzed data; J.D.L.F. and C.J.W. interpreted results of experiments; J.D.L.F. prepared figures; J.D.L.F. drafted manuscript; J.D.L.F., E.J.A., R.C.H., and C.J.W. edited and revised manuscript; J.D.L.F., E.J.A., R.C.H., and C.J.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We would like to thank Kathleen Thayne for assistance with phlebotomy, and Mike McCammon for the use of mass analyzer reagents.
REFERENCES
- 1. Adams V, Linke A, Krankel N, Erbs S, Gielen S, Möbius-Winkler S, Gummert JF, Mohr FW, Schuler G, Hambrecht R. Impact of regular physical activity on the NAD(P)H oxidase and angiotensin receptor system in patients with coronary artery disease. Circulation 111: 555– 562, 2005. [DOI] [PubMed] [Google Scholar]
- 2. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413– 420, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem 282: 31257– 31266, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Angulo J, Peiró C, Cuevas P, Gabancho S, Fernández A, González-Corrochano R, La Fuente JM, Baron AD, Chen KS, Saenz de Tejada I. The novel antioxidant, AC3056 (2,6-di-t-butyl-4-([dimethyl-4-methoxyphenylsilyl] methyloxy) phenol), reverses erectile dysfunction in diabetic rats and improves NO-mediated responses in penile tissue from diabetic men. J Sex Med 6: 373– 387, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 84: 50– 56, 1999. [DOI] [PubMed] [Google Scholar]
- 6. Bergholm R, Mäkimattila S, Valkonen M, Liu ML, Lahdenperä S, Taskinen MR, Sovijärvi A, Malmberg P, Yki-Järvinen H. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilation in vivo. Atherosclerosis 145: 341– 349, 1999. [DOI] [PubMed] [Google Scholar]
- 7. Bivalacqua TJ, Usta MF, Kendirci M, Pradhan L, Alvarez X, Champion HC, Kadowitz PJ, Hellstrom WJG. Superoxide anion production in the rat penis impairs erectile function in diabetes: influence of in vivo extracellular superoxide dismutase gene therapy. J Sex Med 2: 187– 198, 2005. [DOI] [PubMed] [Google Scholar]
- 8. Borgquist R, Gudmundsson P, Winter R, Nilsson P, Willenheimer R. Erectile dysfunction in healthy subjects predicts reduced coronary flow velocity reserve. Int J Cardiol 112: 166– 170, 2006. [DOI] [PubMed] [Google Scholar]
- 9. Cai H, Harrison DG. Endothelial dysfunction in cardiovascular disease: the role of oxidant stress. Circ Res 87: 840– 844, 2000. [DOI] [PubMed] [Google Scholar]
- 10. Castor LR, Locatelli KA, Ximenes VF. Pro-oxidant activity of apocynin radical. Free Radic Biol Med 48: 1636– 1643, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Channon KM. Tetrahydrobiopterin: regulator of endothelial nitric oxide synthase in vascular disease. Trends Cardiovasc Med 14: 323– 327, 2004. [DOI] [PubMed] [Google Scholar]
- 12. de Moraes C, Davel AP, Rossoni LV, Antunes E, Zanesco A. Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol 8: 12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Derby CA, Mohr BA, Goldstein I, Feldman HA, Johannes CB, McKinlay JB. Modifiable risk factors and erectile dysfunction: can lifestyle changes modify risk? Urology 56: 302– 306, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Esposito K, Giugliano D. Obesity, the metabolic syndrome, and sexual dysfunction. Int J Impot Res 17: 391– 398, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men. JAMA 291: 2978– 2984, 2004. [DOI] [PubMed] [Google Scholar]
- 16. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377: 557– 567, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher-Wellman KH, Mattox TA, Thayne K, Katunga LA, La Favor JD, Neufer PD, Hickner RC, Wingard CJ, Anderson EJ. Novel role for thioredoxin reductase-2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J Physiol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frasier CR, Moukdar F, Patel HD, Sloan RC, Stewart LM, Alleman RJ, La Favor JD, Brown DA. Redox-dependent increases in glutathione reductase and exercise preconditioning: role of NADPH oxidase and mitochondria. Cardiovasc Res 98: 47– 55, 2013. [DOI] [PubMed] [Google Scholar]
- 19. Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol 292: H904– H911, 2007. [DOI] [PubMed] [Google Scholar]
- 20. Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation 115: 245– 254, 2007. [DOI] [PubMed] [Google Scholar]
- 21. Glina S, Sharlip ID, Hellstrom WJ. Modifying risk factors to prevent and treat erectile dysfunction. J Sex Med 10: 115– 119, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105: 1656– 1662, 2002. [DOI] [PubMed] [Google Scholar]
- 23. Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, Wierzbicki K, Korbut R, Harrison DG, Channon KM. Coronary artery superoxide production and Nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol 26: 333– 339, 2006. [DOI] [PubMed] [Google Scholar]
- 24. Hannan JL, Maio MT, Komolova M, Adams MA. Beneficial impact of exercise and obesity interventions on erectile function and its risk factors. J Sex Med 6 Suppl 3: 254–261, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Haram PM, Adams V, Kemi OJ, Brubakk AO, Hambrecht R, Ellingsen Ø, Wisløff U. Time-course of endothelial adaptation following acute and regular exercise. Eur J Cardiovasc Prev Rehabil 13: 585– 591, 2006. [DOI] [PubMed] [Google Scholar]
- 26. Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res 81: 723– 732, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211– 217, 2008. [DOI] [PubMed] [Google Scholar]
- 28. Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14: 753– 760, 2007. [DOI] [PubMed] [Google Scholar]
- 29. Hsiao W, Shrewsberry AB, Moses KA, Johnson TV, Cai AW, Stuhldreher P, Dusseault B, Ritenour CWM. Exercise is associated with better erectile function in men under 40 as evaluated by the international index of erectile function. J Sex Med 9: 524– 530, 2012. [DOI] [PubMed] [Google Scholar]
- 30. Jackson G, Montorsi P, Adams MA, Anis T, El-Sakka A, Miner M, Vlachopoulos C, Kim E. Cardiovascular aspects of sexual medicine. J Sex Med 7: 1608– 1626, 2010. [DOI] [PubMed] [Google Scholar]
- 31. Jackson G, Padley S. Erectile dysfunction and silent coronary artery disease: abnormal computed tomography coronary angiogram in the presence of normal exercise ECGs. Int J Clin Pract 62: 973– 976, 2008. [DOI] [PubMed] [Google Scholar]
- 32. Janiszewski PM, Janssen I, Ross R. Abdominal obesity and physical inactivity are associated with erectile dysfunction independent of body mass index. J Sex Med 6: 1990– 1998, 2009. [DOI] [PubMed] [Google Scholar]
- 33. Jin L, Lagoda G, Leite R, Webb RC, Burnett AL. NADPH oxidase activation: a mechanism of hypertension-associated erectile dysfunction. J Sex Med 5: 544– 551, 2008. [DOI] [PubMed] [Google Scholar]
- 34. Jo H, Otani H, Jo F, Shimazu T, Okazaki T, Yoshioka K, Fujita M, Kosaki A, Iwasaka T. Inhibition of nitric oxide synthase uncoupling by sepiapterin improves left ventricular function in streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol 38: 485– 493, 2011. [DOI] [PubMed] [Google Scholar]
- 35. Joyner MJ, Green DJ. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J Physiol 587: 5551– 5558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. La Favor JD, Anderson EJ, Hickner RC, Wingard CJ. Erectile dysfunction precedes coronary artery endothelial dysfunction in rats fed a high-fat, high-sucrose, Western pattern diet. J Sex Med 10: 694– 703, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in ApoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282– 1288, 2001. [DOI] [PubMed] [Google Scholar]
- 38. Long T, Liu G, Wang Y, Chen Y, Zhang Y, Qin D. TNF-α, erectile dysfunction, and NADPH oxidase-mediated ROS generation in corpus cavernosum in high-fat diet/streptozotocin-induced diabetic rats. J Sex Med 9: 1801– 1814, 2012. [DOI] [PubMed] [Google Scholar]
- 39. Meldrum DR, Gambone JC, Morris MA, Esposito K, Giugliano D, Ignarro LJ. Lifestyle and metabolic approaches to maximizing erectile and vascular health. Int J Impot Res 24: 61– 68, 2012. [DOI] [PubMed] [Google Scholar]
- 40. Montorsi F, Briganti A, Salonia A, Rigatti P, Margonato A, Macchi A, Galli S, Ravagnani PM, Montorsi P. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol 44: 360– 364, 2003. [DOI] [PubMed] [Google Scholar]
- 41. Munk PS, Staal EM, Butt N, Isaksen K, Larsen AI. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation: a randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J 158: 734– 741, 2009. [DOI] [PubMed] [Google Scholar]
- 42. Musicki B, Liu T, Lagoda GA, Strong TD, Sezen SF, Johnson JM, Burnett AL. Hypercholesterolemia-induced erectile dysfunction: endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J Sex Med 7: 3023– 3032, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Musicki B, Liu T, Strong T, Jin L, Laughlin MH, Turk JR, Burnett AL. Low-fat diet and exercise preserve eNOS regulation and endothelial function in the penis of early atherosclerotic pigs: a molecular analysis. J Sex Med 5: 552– 561, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Park Y, Booth FW, Lee S, Laye MJ, Zhang C. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol 590: 4255– 4268, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res 99: 69– 77, 2006. [DOI] [PubMed] [Google Scholar]
- 46. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70: 3– 21, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res 20: 17– 29, 2008. [DOI] [PubMed] [Google Scholar]
- 48. Sánchez A, Contreras C, Martínez MP, Climent B, Benedito S, Garcia-Sacristán A, Hernández M, Prieto D. Role of neural NO synthase (nNOS) uncoupling in the dysfunctional nitrergic vasorelaxation of penile arteries from insulin-resistant obese Zucker rats. PLos One 7: e36027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shang T, Kotamraju S, Kalivendi SV, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J Biol Chem 279: 19099– 19112, 2004. [DOI] [PubMed] [Google Scholar]
- 50. Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol 517: 224– 231, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol 11: 95– 102, 1994. [DOI] [PubMed] [Google Scholar]
- 52. Sun MW, Zhong MF, Gu J, Qian FL, Gu JZ, Chen H. Effects of different levels of exercise volume on endothelium-dependent vasodilation: roles of nitric oxide synthase and heme oxygenase. Hypertens Res 31: 805– 816, 2008. [DOI] [PubMed] [Google Scholar]
- 53. Suresh S, Prakash S. Effect of Mucuana pruriens (Linn.) on oxidative stress-induced structural alteration of corpus cavernosum in streptozotocin-induced diabetic rat. J Sex Med 8: 1943– 1956, 2011. [DOI] [PubMed] [Google Scholar]
- 54. Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpoiur CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA 294: 2996– 3002, 2005. [DOI] [PubMed] [Google Scholar]
- 55. Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114– 1126, 2004. [DOI] [PubMed] [Google Scholar]
- 56. Tjønna AE, Lee SJ, Rognmo Ø, Stolen TO, Bye A, Haram PM, Loennechen JP, Al-Share QY, Skogvoll E, Slørdahl AT, Kemi OJ, Najjar SM, Wisløff U. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation 118: 346– 354, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tsutsui M, Milstien S, Katusic ZS. Effect of tetrahydrobiopterin on endothelial function in canine middle cerebral arteries. Circ Res 79: 336– 342, 1996. [DOI] [PubMed] [Google Scholar]
- 58. Vásquez-Vivar J, Duquaine D, Whitsett J, Kalyanaraman B, Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler Thromb Vasc Biol 22: 1655– 1661, 2002. [DOI] [PubMed] [Google Scholar]
- 59. Vásquez-Vivar J, Martasek P, Whitsett J, Joseph J, Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem J 362: 733– 739, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Vlachopoulos C, Jackson G, Stefanadis C, Montorsi P. Erectile dysfunction in the cardiovascular patient. Eur Heart J In press. [DOI] [PubMed] [Google Scholar]
- 61. Vlachopoulos CV, Terentes-Printzios DG, Ioakeimidis NK, Aznaouridis KA, Stefanadis CI. Prediction of cardiovascular events and all-cause mortality with erectile dysfunction: a systemic review and meta-analysis of cohort studies. Circ Cardiovasc Qual Outcomes 6: 99– 109, 2013. [DOI] [PubMed] [Google Scholar]
- 62. Vladic N, Ge ZD, Leucker T, Brzezinska AK, Du JH, Shi Y, Warltier DC, Pratt PF, Jr, Kersten JR. Decreased tetrahydrobiopterin and disrupted association of Hsp90 with eNOS by hyperglycemia impair myocardial ischemic preconditioning. Am J Physiol Heart Circ Physiol 301: H2130– H2139, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15: 1517– 1530, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wingard C, Fulton D, Husain S. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J Sex Med 4: 348– 363, 2007. [DOI] [PubMed] [Google Scholar]
- 65. Wisløff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: V̇o2max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H1301– H1310, 2001. [DOI] [PubMed] [Google Scholar]
- 66. Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation 115: 3086– 3094, 2007. [DOI] [PubMed] [Google Scholar]
- 67. Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189: 1699– 1706, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


