Synopsis
With earlier cancer diagnosis among older cancer patients, the possibility of curing cancer increases. However, cancer treatment may have long lasting impact on older cancer survivors. It is vital to screen, diagnose and properly manage the long term toxicities of cancer treatment, in order to maintain quality of life of older cancer survivors
Keywords: Older cancer survivors, Frailty, Cancer treatment, Toxicity, Quality of life
Introduction
The number of cancer survivors is increasing in the United States. In 2014, there were 14.5 million cancer survivors. By 2024, this number is expected to increase to 19 million with the significant portion of them being older than age 65 1. As more patients are diagnosed with earlier stages of cancer, the likelihood of cancer survivors living beyond 5 years after the initial cancer diagnosis has increased. 2 The role of primary care providers in the immediate and long-term follow up of cancer patients are still being defined, as there are significant differences between primary care providers and oncologists’ preferences toward follow up care of the cancer survivors. While 38% of primary care providers prefer shared care of the cancer survivors with the oncologists, only 16% of oncologists were in agreement with this model of care. More than half of primary care providers thought they have necessary skills to take care of the cancer survivors, while this was agreed to by only 23% of the oncologists 3. The primary care providers who were more confident in their skills to provide follow-up care for cancer patients, were more involved in the cancer patients’ care 4.
Interaction between aging, cancer, cancer treatment, and their impact on frailty
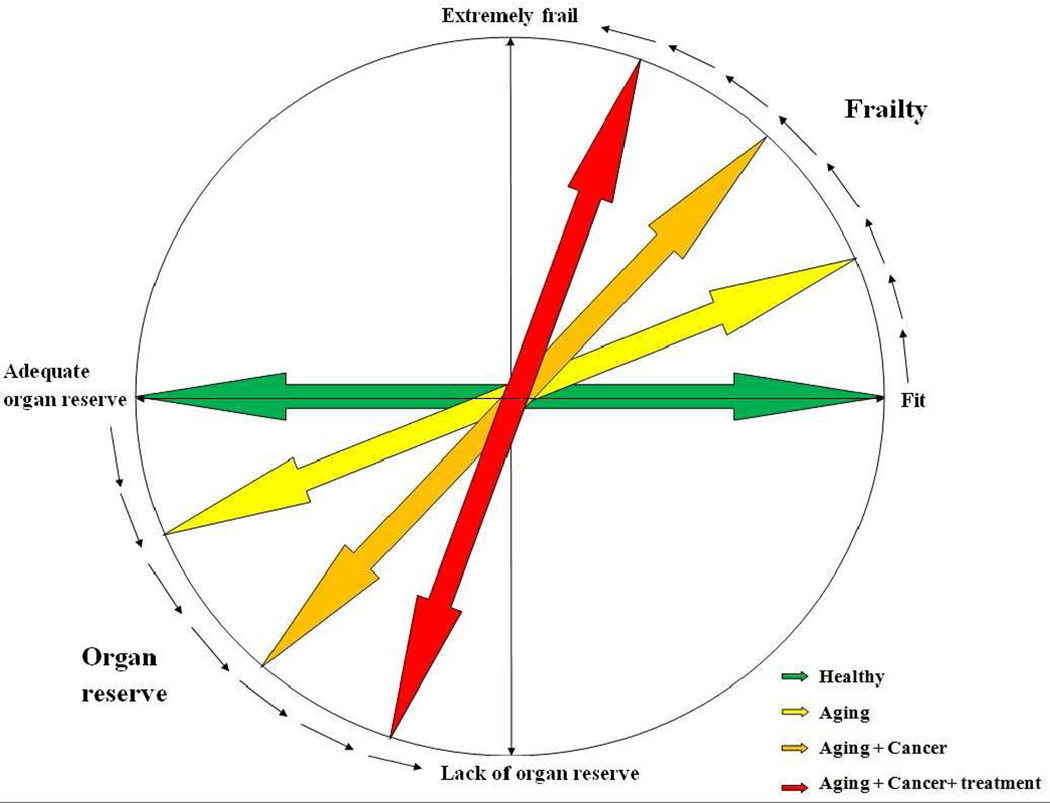
Frailty, broadly defined, is a state of decreased (or total lack of) reserve and resistance to physical and emotional stressors, due to continuous decline in various organ functions 5. As patients age, they tend to become more frail, although aging and frailty do not correlate with each other all the time 6. Cancer patients are more likely to be frail compared to non-cancer patients 7,8. Moreover, cancer treatment, itself, can lead to frailty 9. (Figure 1)
Figure 1.
Impact of aging, cancer, and cancer treatment on patients’ fitness and frailty.
Measuring frailty and geriatric deficits
Comprehensive Geriatric Assessment (CGA) (Table-1) performed by healthcare providers has been a useful tool to assess and manage frailty and geriatric deficits among older cancer patients and survivors 10,11. In the cancer setting, the data on usefulness of CGA in predicting short term toxicities of chemotherapy 12,13, complications and outcome after cancer surgery 14,15, and cancer treatment decision-making 16,17 is emerging.
Table 1.
Components of Comprehensive Geriatric Assessment
| Components of Comprehensive Geriatric Assessment |
|---|
| Activities of Daily Living (ADL) |
| Instrumental Activities of Daily Living (iADL) |
| Cognition |
| Social Support |
| Polypharmacy |
| Nutrition |
| Comorbid conditions |
| Emotional distress, Depression |
Therapy for elderly cancer patients
For many years, cancers were treated by surgery, chemotherapy, radiation, or hormonal treatments. Over the last 10–15 years, new class of cancer treatment has emerged which is known as targeted therapy 18. Considering the differences with the standard and well-known chemotherapy drugs regarding their route of administration, duration of treatment, and toxicity profile, this class of drug is discussed separately. Table 2 discusses basic facts on medical cancer treatment. In the next sections, we will discuss long-term toxicities of cancer treatment. Table 3 provides information for screening, diagnosis and management of each of long-term toxicities.
Table 2.
Basics of medical cancer treatment
| 1- Context | Timing | Goal | |
|---|---|---|---|
| A- Neoadjuvant | Administered before definitive surgery. |
To shrink the tumor so that surgery becomes feasible or easier |
|
| B- Adjuvant | After definitive surgery |
To treat microscopic disease, and to delay recurrence. |
|
| C- Palliative | Advanced cancer | Relieving symptoms (e.g. pain, shortness of breath, and to slow the progression of the disease. |
|
| 2- Route | |||
| A- Intravenous | Majority of cancer treatment | ||
| B- Oral agents | Mainly targeted therapies (e.g. Erlotinib, Lapatinib, Pazopanib) | ||
| C- Subcutaneous | Very few (e.g. Bortezomib for Multiple Myeloma) | ||
| D- Intramuscular | Very few (e.g. Fulvestrant for breast cancer, Leuprolide for prostate cancer) |
||
| 3- Number of agents | |||
| A- Multiple | Majority of chemotherapy regimens. At times, it is combined with targeted agents as well. In general, combined chemotherapy is more toxic than single agent chemotherapy. Used in neoadjuvant, adjuvant, and palliative setting |
||
| B- Single | Mainly used in the palliative setting. In frail patients, it can be used in the adjuvant setting. |
||
| 4- Dose | |||
| A- Standard | The concern over using standard dose in the elderly patients is due to limited number of older patients enrolled in the clinical trials. Many of those who are enrolled are not a true representation of community dwelling older patients with cancer. |
||
| B- Dose reduced | |||
Table 3.
Long-term toxicity of cancer treatment, approach, and management.
| Toxicity | Diagnosis / screening | Management |
|---|---|---|
| Cognitive impairment | Mini-Cog 114, Mini Mental Status Exam 115, Montreal Cognitive Assessment 116 |
Rule out reversible causes of cognitive deficit (depression, hypothyroidism, vitamin B12 and folic acid deficiency) Referral to cognitive rehabilitation 117, when possible |
| Cardiotoxicity | Electrocardiogram, Echocardiogram, stress test |
Control other risk factors for cardiac condition (e.g. hypertension management, smoking cessation, lipid control, etc.) |
| Depression & Anxiety | Distress thermometer 118 Geriatric Depression Scale (GDS) 119, Patient Health Questionnaire (PHQ) 120 |
Cognitive behavioral and stress management 121, when possible Psychoeducational interventions to cope with stress 122 Selective serotonin reuptake inhibitor 123 Encouraging patients to be more physically active 124. |
| Ototoxicity | Hearing Handicap Inventory for the Elderly- Screening Version 125,126 |
Rule out other causes of hearing impairment (cerumen impaction, chronic otitis media) Referral to audiologists and otolaryngologists to assist with the diagnosis and proper hearing aides 127. |
| Imbalance and lack of coordination |
Mostly clinical. In rare circumstances, may consider NCV/EMG, skin and nerve biopsy to confirm the diagnosis 44. |
Control other causes of neuropathy (e.g. diabetes, vitamin B12 deficiency) Treatment with Duloxetine 42 or venlafaxine 128 Referral to physical and occupational therapy 129,130. |
| Osteoporosis | Bone densitometry 131 WHO Fracture Risk Assessment Tool (FRAX) |
Life style modifications: weight bearing exercises 132, Tai Chi 133. Home safety inspection134 Vitamin D and calcium supplement 135 Starting Bisphosphonates in patients with proven osteoporosis or fracture136 |
| Metabolic Syndrome | Assessment of weight, blood pressure, and waist circumference, measurement of glucose and lipid panel |
Recommendation for smoking cessation 137, excessive alcohol abstinence, healthy diet 138, and more physical activity 139 |
| Second malignancies | Assessment of symptoms not controlled with the conservative management Routine blood cell count Adherence to cancer screening guidelines |
Referral to medical oncologist. |
| Sexual and vaginal dysfunction | History taking (e.g. Erectile dysfunction, Dyspareunia) and pelvic examination (e.g. discomfort on examination, pelvic floor weakness) |
Vulvovaginal atrophy: vaginal lubricants and moisturizers, topical or systemic estrogen therapy (for non-hormone dependent cancers) Pelvic floor weakness: pelvic floor exercises, chronic pad use Vaginal pain or stenosis: dilators |
Chronic Toxicity from Cancer Therapy
1. Cardiotoxicity
Cardiotoxicity can present itself in various ways (Table 4). In general, patients with preexisting cardiac conditions are at higher risk for developing cardiotoxicity in the short -and long-term 19. Among breast cancer patients, the incidence of cardiotoxicity is 3 to 35% 20, and at times it competes with breast cancer for leading cause of death. 21 Anthracyclines (e.g. doxorubicin), frequently used chemotherapy agents in breast cancer patients, can cause cardiotoxicity even at low doses in patients with preexisting cardiac conditions 22. These patients are 5.4 and 6.25 times more likely to develop clinical and subclinical cardiotoxicity compared to those who did not receive anthracycline, respectively 23. More importantly, the risk of cardiac death was 4.94 times more compared to those who did not receive anthracycline. Older patients are at higher risk for developing cardiotoxicity, as for each 10-year increase in age, the risk of developing congestive heart disease doubled. 24
Table 4.
Cardiotoxicity of cancer treatment.
| Cardiotoxicity | Note | Drugs |
|---|---|---|
| 1- Heart Failure (left ventricular dysfunction) |
Most common | Anthracyclines Alkylating agents (e.g. cyclophosphamide) Inhibitors of microtubule polymerization (e.g. paclitaxel) Monoclonal antibodies (e.g. trastuzumab) |
| 2- Newly induced or worsening hypertension |
Class effect of VEGF inhibitors |
Bevacizumab |
| 3- Cardiac ischemia | Antimetabolites (e.g. 5-FU) Inhibitors of microtubule polymerization (e.g. paclitaxel) Targeted agents (e.g. bevacizumab) |
|
| 4- Arrhythmia | QT prolongation, Torsade de pointes |
Arsenic Trioxide Most of anti-emetics drugs |
VEGF: Vascular Endothelial Growth Factor
Prostate cancer patients on ADT may also have more cardiotoxicity, compared to those not on ADT 25,26. For every year increase in age, the risk of cardiac comorbidity increases by 3% 27. The 5-year cumulative risk of dying from cardiac causes in patients on ADT after prostatectomy reaches 5.5% compared to 2% of patients who only underwent prostatectomy 28. Patients receiving 5-fluouracil (5-FU) are also at higher risk for cardiotoxicity. 5-FU can cause cardiotoxicity in 1.2% to 18% of patients. The toxicity is usually short term and occurs while patient is receiving 5-FU 29. Capecitabine, an oral derivative of 5-FU, can also cause ischemia in up to 9% of patients 30. These toxicities are usually short term.
2. Emotional effects (depression, anxiety)
Cancer patients experience emotional disturbances even years after completion of the treatment. About 57% of patients with gynecologic cancer reported that they need help in dealing with cancer-related emotions, however only 35% had received such help, and 73% believed that physicians should ask whether patients with cancer want help in dealing with emotions 31. At least 11.6% and 17.9% of long term cancer survivors are suffering from depression, and significant level of anxiety 32. In extreme cases, patients may have suicidal ideation if distress and depression remain undiagnosed, and untreated 33.
3. Ototoxicity
Platinum agents (e.g. cisplatin) can cause ototoxicity 34. Ototoxicity can present itself as permanent bilateral hearing loss and/or tinnitus. Among platinum agents, cisplatin is the most common chemotherapeutic agent to cause ototoxicity, resulting in bilateral hearing loss and/or permanent tinnitus in 19 to 79% of the patients 35. Older patients with hearing difficulty are at higher risk for falls 36, accelerated cognitive decline 37, and poor quality of life 38.
4. Balance and coordination
Lack of balance and falls may occur in cancer patients and can lead to injuries such as bone fracture 39. Maintaining proper balance is a result of complex interaction between cognition 40, orientation to space, biomechanical changes, and sensors 39,41. Chemotherapy induced peripheral neurotoxicity (CIPN) may happen in 20 to 40% of cancer patients receiving neurotoxic chemotherapy agents 42, and can increase risk of falls and associated fractures in cancer survivors 43. Taxanes and platinum agents are the most common drugs that can cause CIPN 44–46. Patients with preexisting neurological deficits such as diabetic neuropathy are at higher risk for developing CIPN 47. Most common presenting symptoms are numbness and tingling especially in the lower limbswhich at times could be painful 48,49. Vinca alkaloids (vincristine, vinblastine, vinorelbine) and bortezomib can also cause significant chronic neurotoxicity 50.
5. Effect on muscle and bone health
Cancer survivors are at higher risk for osteoporosis compared to the general population 51, and as a result, they are at higher risk for fractures 52. Certain breast cancer treatments increase the risk of osteoporosis. Up to 70% of patients may experience menopause during adjuvant chemotherapy for breast cancer. The earlier the induced menopause occurs, the higher the risk of osteoporosis 53. Many older breast cancer patients receive adjuvant hormonal therapy. While tamoxifen is associated with a decreased risk of osteoporosis if used in postmenopausal women, it may lead to an increase in the incidence of osteoporosis in premenopausal women 54. Compared to tamoxifen, aromatase inhibitors (AIs) are associated with higher risk of low bone density and fractures 55. Surgical or medical ovarian ablation also leads to a decrease in estrogen production resulting in bone loss 56. Prostate cancer survivors are also at high risk for developing osteoporosis. In one study, five years after diagnosis of prostate cancer and receiving ADT, 19.4% of the patients suffered a fracture 57. In another study, prostate cancer survivors were at least 2.49 times more likely to have osteoporosis compared to those without prostate cancer 58. Despite higher risk for osteoporosis and fracture, one study showed that 77% of survivors with osteoporosis were undiagnosed by their primary care providers 59. This finding has been confirmed by other studies 58,60,61. The American Society of Clinical Oncology (ASCO) 62 and National Comprehensive Cancer Network (NCCN) 63 have proposed guidelines for diagnosis and management of the osteoporosis in cancer patients.
6. Metabolic Syndrome
Metabolic syndrome (MS) is a constellation of states that increases the risk of cardiovascular events, diabetes, fatty liver, and sleep disturbances 64. The majority of the studies on incidence of MS in long term cancer survivors have focused on testicular and early adulthood diagnosis of leukemia / lymphoma 65,66. Two known causes of the MS are testosterone 67 and estrogen deficiency 68. In one study, 50% of men with prostate cancer receiving long term ADT had MS 69. Another study on men with recurrent or locally advanced prostate cancer receiving leuprolide for 12 months showed that the mean weight, body mass index, waist circumference, and fat mass increased , while the percentage of lean body mass decreased compared to the baseline 70. Breast cancer survivors are also at higher risk for development of MS. A study of 53 breast cancer survivors showed that compared to surgery alone, patients undergoing chemotherapy are at higher risk for weight gain, increase in body fat percentage and fat mass, and decrease in lean body mass 71. Breast cancer survivors with MS are at higher risk for cancer recurrence than those without MS 72.
7. Secondary malignancies:73
Cancer survivors are at high risk to develop second cancers 74. This increased risk could be due to genetic predisposition, consequence of previous cancer treatment, undergoing surveillance following first cancer treatment completion, or environmental factors 75,76. In particular patients who receive chemotherapy are at 4.7 fold higher risk for developing treatment-related acute myeloid leukemia (AML) compared to the general population. Nearly half of 801 treatment-related AML from 1975 to 2008 occurred in breast or non-Hodgkin Lymphoma (NHL) survivors 77. Patients who have received topoisomerase II inhibitors (e.g. doxorubicin, etoposide, irinotecan) usually develop leukemia within 5 years, and those who receive alkylating agents (e.g. cyclophosphamide) develop leukemia after 5 years 78. The incidence of leukemia also correlates with the dose of chemotherapy patients receive 79,80. Hodgkin lymphoma survivors are particularly at high risk for developing leukemia 81 which is particularly related to dose of alkylating agents. Although with recent treatments 82, the incidence of leukemia has been shown to have decreased, it still is worth considering when taking care of the cancer survivors. In similar fashion, patients with non-Hodgkin lymphoma are at higher risk for developing leukemia within 10 years of treatment completion 83.
8. Sexual and Vaginal Dysfunction
Cancer and its associated treatment can have a devastating effect on vaginal health and sexuality. Disease type, stage of disease, and type of treatment can contribute or compound atrophy of the vagina and vulvar tissues, resulting in painful gynecological exams, sexual difficulties, and other long-term issues 84–87 Estrogen deprivation effects include vulvovaginal atrophy (VVA), with the loss of genital tissue elasticity and lubrication, and symptoms of dryness, irritation, itching, discharge, and dyspareunia. Estrogen-deprivation-associated VVA can lead to loss of sexual desire and arousal, and orgasm difficulties stemming from vaginal dryness, pain, and stenosis 88 Older cancer patients may mistakenly believe that sexual/vaginal changes are an inevitable result of aging rather than recognizing that cancer treatment may be a contributing factor 89 For example, many women treated with extended endocrine therapy, specifically aromatase inhibitors (AIs), develop vaginal dryness, gross architectural vulvar changes, etc.. Radiation therapy to the pelvis can cause agglutination, ulceration, stenosis, scarring , and a reduction in vaginal depth, elasticity and sexual function Long-term bowel issues and fear of urinary and fecal incontinence post-treatment are significant concerns that can also interfere with sexual activity. Furthermore, radical vulvar excisions are significantly associated with lower sexual function and quality of life, particularly in older women. Patients have indicated a need for basic advice on the prevention and treatment of vaginal and sexual toxicities and welcome discussions on these topics with their doctors These issues do not spontaneously resolve over time without appropriate intervention Early identification and treatment strategies are essential in addressing these long-term challenges; physician-patient communication is imperative, and may be enhanced with the use of brief surveys and checklists90
9. Fatigue
One of the most common long term side effects of cancer therapy is fatigue. The symptom of fatigue that the cancer patient experiences is different from the symptoms that health people experience. The feelings of fatigue that healthy people feel is often alleviated by sleep and rest. Patients who have undergone cancer treatment get fatigued after less activity than those who had had cancer. Fatigue can definitely affect quality of life. The cause of this symptoms is multifactorial and can include the long term affects of therapy (chemotherapy, radiation, biologic therapy, surgery, etc.), anemia, nutrition, anxiety and depression, sleep disorders and drugs. Polypharmacy which is common in the elderly can contribute. Specific drugs such as anxiolytics, sleeping medicine, narcotics, drugs which treat neuropathy (gabapentin, pregabalin) contribute to this syndrome. Pharmacologic interventions have been unsuccessful unless a specific diagnosis (ie. depression) can be made 91.
10. Cognitive Impairment
Many patients undergoing chemotherapy complain of cognitive changes (chemotherapy-related cognitive impairment [CRCI]) 92. These complaints are usually broad and range from distraction, lack of focus, to inability to perform daily cognitive routines (e.g. paying bills) 93. Although at times subjective complaints do not correlate with the objective assessments 94, it is vital to appreciate such complaints. The majority of studies on CRCI have been conducted in breast cancer survivors. In this setting, cognitive deficit usually involves certain domains of cognition (e.g. verbal ability or visuospatial) 95 which could be long lasting 96. It can develop after completion of the treatment 97, however, in some cases, cognition may improve following completion of the treatment 98. Hormone receptor positive breast cancer patients usually take anti-estrogen treatments (e.g. tamoxifen, exemestane) which may impact their cognition. Patients on the 5-year tamoxifen regimen reported memory complaints more than those who were not taking tamoxifen 99. As with chemotherapy, cognitive deficit occurred in specific domains of cognition (verbal memory, verbal functioning, verbal fluency and information processing speed) 100,101. The other common cancer is prostate cancer. Patients may require androgen deprivation therapy (ADT) aiming at reducing the testosterone level. About half of prostate cancer patients on ADT could have cognitive decline in at least one domain of cognition 102,103. Like breast cancer treatment, prolonged use of ADT leads to decline in specific domains of cognition as noted previously 104. Patients with other types of cancer (e.g. colorectal) experience the same phenomena 105.
Effect of other modalities
1. Targeted Therapies
In the past 10–15 years, the emergence of a new class of cancer treatment known as targeted agents has changed the spectrum of cancer treatment. In some instances, patients with metastatic disease can receive targeted agents for months or even years. In brief, targeted therapies are either monoclonal antibodies to certain proliferation or anti-apoptotic proteins, or are inhibitors of pathways that signal cell proliferation 18. These therapies are not often associated with long-term toxicities. Many of the adverse events are short lived or reversible (Table 5). The most common targets for these agents are Epidermal Growth Factor Receptor (EGFR), Vascular Endothelial Growth Factor (VEGF), Human Epidermal Receptor-2 (HER-2), mammalian target of rapamycin (m-TOR) and BRAF kinase. Agents that target EGFR can cause skin rash, diarrhea, and electrolyte abnormalities (e.g. hypomagnesemia). The toxicity of VEGF targeted agents include hypertension, fatigue, wound healing, and thrombosis. Due to vascular toxicity they are associated with increased risk in older patients 106. m-TOR inhibitors, especially temsirolimus can cause hyperlipidemia and hyperglycemia. Those who take BRAF inhibitors can develop skin cancer. Trastuzumab is associated with cardiomyopathy.
Table 5.
Toxicities of targeted agents.
| Cancer | Name of agent | Target | Common side effects |
|---|---|---|---|
| Non-small cell Lung Cancer |
Erlotinib Gefitinib Crizotinib |
EGFR EGFR ALK-4 |
Rash, fatigue, appetite loss Rash, diarrhea Edema, fatigue, diarrhea, visual disturbances |
| Renal cell carcinoma |
Sunitinib pazopanib Temsirolimus Axitinib |
Mutikinase Multikinase m-TOR VEGF |
Hand and foot syndrome, hypertension, fatigueRash, edema, hyperlipidemia, hyperglycemia Hypertension, fatigue, diarrhea Hypertension, rash, diarrhea, fatigue |
| Colorectal cancer | Cetuximab Panitumumab Regorafenib Aflibercept |
EGFR EGFR Kinase, VEGF VEGF |
Rash, diarrhea Rash, diarrhea Hypertension, Fatigue, hand and foot syndrome, proteinuria Hypertension, fatigue, diarrhea. |
| Breast Cancer | Trastuzumab Pertuzumab Trastuzumab emtansine (T-DM1) Lapatinib |
HER2 HER 2 HER 2 Kinase |
Heart failure Diarrhea, skin rash, heart failure Fatigue, skin rash, arthralgia, heart failure Skin rash, hand and foot syndrome, diarrhea |
| Renal cell carcinoma, hepatocellular carcinoma |
Sorafenib | Multikinase | Hypertension, diarrhea, fatigue, hand and foot syndrome |
| Colorectal cancer, Ovarian cancer |
Bevacizumab | VEGF | Hypertension, thrombosis, proteinuria , delayed wound healing |
| Renal cell carcinoma, Breast cancer |
Everolimus | m-TOR | Stomatitis, diarrhea, |
| Melanoma | Vemurafenib Dabrafenib Ipilimumab |
BRAF kinase BRAF kinase CTLA-4 |
Fatigue, arthralgia, skin cancer Fatigue, fever, arthralgia Immune-mediated reactions (diarrhea, fever, fatigue, etc…) |
| Chronic Myeloid Leukemia, Gastrointestinal Stromal Tumors (GIST) |
Imatinib | Kinase | Edema, diarrhea, rash. |
EGFR: Epidermal Growth Factor Receptor, m-TOR: Mammalian target of rapamycin inhibitor, HER: Human Epidermal Receptor, VEGF: Vascular Endothelial Growth Factor , CTLA4: Cytotoxic T-Lymphocyte-associated protein 4.
2. Long term toxicities of radiation
Radiation therapy has a substantial role in treating many prevalent and frequently curable malignancies, notably breast and prostate cancer. Radiotherapy can induce chronic, nonlethal changes in non-proliferating normal tissues, with fibrosis being the prototypical example. The potential late toxicities in a given patient depend upon the anatomic region, volume of tissue that was irradiated, radiation dose and use of concurrent chemotherapy. Modern tools such as intensity-modulated radiation therapy (IMRT), image-guided radiation therapy, and proton therapy reduce the incidence and severity of late toxicity.
Central Nervous System
Brain radiation is most commonly employed for patients with brain metastases or for gliomas. However, brain radiation is also utilized for less lethal tumors such as meningioma. Studies indicate that short-term memory is the faculty most likely to be chronically impaired when radiating the brain 107. Stereotactic radiosurgery, which can treat meningiomas, isolated brain metastases, or benign conditions such as arteriovenous malformations, also carries a risk of necrosis in the brain tissue adjacent to the target. This can cause seizures or focal neurologic deficits, months or years after treatment 108.
Neck and upper aerodigestive tract
Radiation therapy is often employed as curative or post-surgical treatment of primary head and neck tumors. Though highly effective and often allowing for organ preservation, radiation therapy to this region is associated with perhaps the most frequently apparent late radiation toxicity. Permanent xerostomia due to incidental irradiation of the parotid glands is very common and significantly impacts patient quality of life. Fibrosis of the skin and connective tissue can lead to trismus and restricted range of motion in the neck. Hypothyroidism is commonly induced by head-and-neck radiotherapy, and incidence of carotid artery stenosis after radiation has been reported as high as 50% 109. Brachial plexus injury is also possible.
Thorax
Breast or chest-wall radiation for breast cancer is among the most common indications for radiation therapy and long-term survival is likely. The most common late effects include poor cosmesis (e.g. skin hyperpigmentation), fibrosis limiting range of motion in the arm, and lymphedema. Radiation pneumonitis, which is a delayed inflammatory response to lung irradiation, is a subacute toxicity typically occurring within a few months to one year after radiotherapy. It can recur and increase the risk of radiation fibrosis, which is a chronic scarring and inactivation of lung tissue. Radiation pneumonitis is typically treated successfully with corticosteroids, but there is no established therapy for radiation-induced lung fibrosis 110.Cardiac irradiation increases the risk of heart disease, as has been apparent from the experience with long-term survivors of Hodgkin lymphoma, and also from patients with left-sided breast cancer 111,112.
Gastrointestinal
The largest population of gastrointestinal patients with potential late radiation toxicity is rectal cancer patients, owing to the routine use of preoperative radiation in this prevalent and frequently cured disease. Pelvic radiation therapy can diminish bowel function, leading to chronic diarrhea, rectal bleeding, or incontinence 113. Radiotherapy to the abdomen or pelvis also increases the risk of small bowel obstruction. Radiation for pancreatic and esophagogastric cancers increases the risk of serious mucosal injury to the stomach, duodenum, or bowel.
Conclusion
The aging of the population and the success of cancer therapy has resulted in a large number of older cancer survivors. The chronic toxicity of therapy combined with the comorbidities seen in this population make long term management challenging. To provide optimum care, survivorship guidelines are being formulated. This will provide the oncologist, primary care physician and geriatrician an organized framework to take care of these patients. In 2005, the Institute of medicine published a report entitled “From cancer patient to cancer survivor: Lost in transition". This report describes recommendations for ongoing guidelines for cancer survivors, the cancer team, primary care physicians, and other healthcare providers. The report recommends that at the completion of cancer treatment, clinicians provide a Survivorship Care Plan that includes the summary of treatment delivered and a detailed plan of ongoing care, as well as surveillance guidelines, potential late effects, and potential behavioral modifications that patients can make such as weight management, alcohol and regular exercise (https://www.iom.edu/Reports/2005/From-Cancer-Patient-to-Cancer-Survivor-Lost-in-Transition.aspx; accessed June 28, 2015).
Key Points.
The number of older cancer survivors is expected to rise in the next few decades due to aging population, earlier cancer stage diagnosis, and proper cancer treatment.
Although effective on cancer treatment, both chemotherapy and radiation therapy may have long lasting negative impact on older cancer survivors’ quality of life.
Long term toxicities of breast and prostate cancer treatment on cognition, cardiac function, emotional wellbeing, muscle and bone health, balance and coordination, and sexual health are well known.
In order to maintain older cancer survivors’ quality of life, it is critical that primary care providers screen, diagnose, and properly manage long term toxicities of cancer treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Potosky AL, Han PK, Rowland J, et al. Differences between primary care physicians' and oncologists' knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med. 2011;26:1403–1410. doi: 10.1007/s11606-011-1808-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klabunde CN, Han PKJ, Earle CC, et al. Physician Roles in the Cancer-Related Follow-Up Care of Cancer Survivors. Family medicine. 2013;45:463–474. [PMC free article] [PubMed] [Google Scholar]
- 5.Rockwood K. What would make a definition of frailty successful? Age and Ageing. 2005;34:432–434. doi: 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- 6.Schuurmans H, Steverink N, Lindenberg S, et al. Old or Frail: What Tells Us More? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2004;59:M962–M965. doi: 10.1093/gerona/59.9.m962. [DOI] [PubMed] [Google Scholar]
- 7.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009;101:1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flood KL, Carroll MB, Le CV, et al. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24:2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 9.Bylow K, Mohile SG, Stadler WM, et al. Does androgen-deprivation therapy accelerate the development of frailty in older men with prostate cancer? Cancer. 2007;110:2604–2613. doi: 10.1002/cncr.23084. [DOI] [PubMed] [Google Scholar]
- 10.Caplan GA, Williams AJ, Daly B, et al. A Randomized, Controlled Trial of Comprehensive Geriatric Assessment and Multidisciplinary Intervention After Discharge of Elderly from the Emergency Department—The DEED II Study. Journal of the American Geriatrics Society. 2004;52:1417–1423. doi: 10.1111/j.1532-5415.2004.52401.x. [DOI] [PubMed] [Google Scholar]
- 11.Vidán M, Serra JA, Moreno C, et al. Efficacy of a Comprehensive Geriatric Intervention in Older Patients Hospitalized for Hip Fracture: A Randomized, Controlled Trial. Journal of the American Geriatrics Society. 2005;53:1476–1482. doi: 10.1111/j.1532-5415.2005.53466.x. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 14.Kristjansson SR, Nesbakken A, Jordhøy MS, et al. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Critical reviews in oncology/hematology. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Kristjansson SR, Jordhøy MS, Nesbakken A, et al. Which elements of a comprehensive geriatric assessment (CGA) predict post-operative complications and early mortality after colorectal cancer surgery? Journal of Geriatric Oncology. 1:57–65. [Google Scholar]
- 16.Caillet P, Canoui-Poitrine F, Vouriot J, et al. Comprehensive Geriatric Assessment in the Decision-Making Process in Elderly Patients With Cancer: ELCAPA Study. Journal of Clinical Oncology. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 17.Chaïbi P, Magné N, Breton S, et al. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Critical reviews in oncology/hematology. 2011;79:302–307. doi: 10.1016/j.critrevonc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Kelly CM, Power DG, Lichtman SM. Targeted therapy in older patients with solid tumors. J Clin Oncol. 2014;32:2635–2646. doi: 10.1200/JCO.2014.55.4246. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz KH, Prosnitz RG, Schwartz AL, et al. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer. 2012;118:2270–2276. doi: 10.1002/cncr.27462. [DOI] [PubMed] [Google Scholar]
- 20.Yeh ETH, Bickford CL. Cardiovascular Complications of Cancer Therapy: Incidence, Pathogenesis, Diagnosis, and Management. Journal of the American College of Cardiology. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 21.Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryberg M, Nielsen D, Cortese G, et al. New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst. 2008;100:1058–1067. doi: 10.1093/jnci/djn206. [DOI] [PubMed] [Google Scholar]
- 23.Smith L, Cornelius V, Plummer C, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337. doi: 10.1186/1471-2407-10-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinder M, Duan Z, Goodwin J, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 25.O'Farrell S, Garmo H, Holmberg L, et al. Risk and Timing of Cardiovascular Disease After Androgen-Deprivation Therapy in Men With Prostate Cancer. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 26.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer. 2009;115:2388–2399. doi: 10.1002/cncr.24283. [DOI] [PubMed] [Google Scholar]
- 27.Saigal CS, Gore JL, Krupski TL, et al. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 28.Tsai HK, D’Amico AV, Sadetsky N, et al. Androgen Deprivation Therapy for Localized Prostate Cancer and the Risk of Cardiovascular Mortality. Journal of the National Cancer Institute. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 29.Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8:191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]
- 30.Curigliano G, Mayer EL, Burstein HJ, et al. Cardiac toxicity from systemic cancer therapy: a comprehensive review. Prog Cardiovasc Dis. 2010;53:94–104. doi: 10.1016/j.pcad.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Miller BE, Pittman B, Strong C. Gynecologic cancer patients' psychosocial needs and their views on the physician's role in meeting those needs. International Journal of Gynecological Cancer. 2003;13:111–119. doi: 10.1046/j.1525-1438.2003.13001.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell AJ, Ferguson DW, Gill J, et al. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. The Lancet Oncology. 2013;14:721–732. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 33.Recklitis CJ, Zhou ES, Zwemer EK, et al. Suicidal ideation in prostate cancer survivors: Understanding the role of physical and psychological health outcomes. Cancer. 2014;120:3393–3400. doi: 10.1002/cncr.28880. [DOI] [PubMed] [Google Scholar]
- 34.Ding D, Allman BL, Salvi R. Review: Ototoxic Characteristics of Platinum Antitumor Drugs. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 2012;295:1851–1867. doi: 10.1002/ar.22577. [DOI] [PubMed] [Google Scholar]
- 35.Brydøy M, Oldenburg J, Klepp O, et al. Observational Study of Prevalence of Long-term Raynaud-Like Phenomena and Neurological Side Effects in Testicular Cancer Survivors. Journal of the National Cancer Institute. 2009;101:1682–1695. doi: 10.1093/jnci/djp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin FR, Ferrucci L. HEaring loss and falls among older adults in the united states. Archives of Internal Medicine. 2012;172:369–371. doi: 10.1001/archinternmed.2011.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin FR, Yaffe K, Xia J, et al. HEaring loss and cognitive decline in older adults. JAMA Internal Medicine. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciorba A, Bianchini C, Pelucchi S, et al. The impact of hearing loss on the quality of life of elderly adults. Clinical Interventions in Aging. 2012;7:159–163. doi: 10.2147/CIA.S26059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013;75:51–61. doi: 10.1016/j.maturitas.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age and Ageing. 2012;41:299–308. doi: 10.1093/ageing/afs012. [DOI] [PubMed] [Google Scholar]
- 41.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age and Ageing. 2006;35:i7–ii11. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 42.Smith EML, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA : the journal of the American Medical Association. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward PR, Wong MD, Moore R, et al. Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: A retrospective cohort study. Journal of Geriatric Oncology. 2014;5:57–64. doi: 10.1016/j.jgo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-Oncology. 2012;14:v45–iv54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lichtman SM, Hurria A, Cirrincione CT, et al. Paclitaxel efficacy and toxicity in older women with metastatic breast cancer: combined analysis of CALGB 9342 and 9840. Ann Oncol. 2012;23:632–638. doi: 10.1093/annonc/mdr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtman SM, Wildiers H, Chatelut E, et al. International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients--an analysis of the medical literature. J Clin Oncol. 2007;25:1832–1843. doi: 10.1200/JCO.2007.10.6583. [DOI] [PubMed] [Google Scholar]
- 47.Chaudhry V, Chaudhry M, Crawford TO, et al. Toxic neuropathy in patients with pre-existing neuropathy. Neurology. 2003;60:337–340. doi: 10.1212/01.wnl.0000043691.53710.53. [DOI] [PubMed] [Google Scholar]
- 48.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 49.Cavaletti G, Nobile-Orazio E. Bortezomib-induced peripheral neurotoxicity: still far from a painless gain. Haematologica. 2007;92:1308–1310. doi: 10.3324/haematol.11752. [DOI] [PubMed] [Google Scholar]
- 50.Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Curr Opin Neurol. 2015 doi: 10.1097/WCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 51.VanderWalde A, Hurria A. Aging and osteoporosis in breast and prostate cancer. CA: A Cancer Journal for Clinicians. 2011;61:139–156. doi: 10.3322/caac.20103. [DOI] [PubMed] [Google Scholar]
- 52.Chen Z, Maricic M, Bassford TL, et al. Fracture risk among breast cancer survivors: Results from the women’s health initiative observational study. Archives of Internal Medicine. 2005;165:552–558. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 53.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–571. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 54.Taxel P, Choksi P, Van Poznak C. The management of osteoporosis in breast cancer survivors. Maturitas. 2012;73:275–279. doi: 10.1016/j.maturitas.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Becker T, Lipscombe L, Narod S, et al. Systematic Review of Bone Health in Older Women Treated with Aromatase Inhibitors for Early-Stage Breast Cancer. Journal of the American Geriatrics Society. 2012;60:1761–1767. doi: 10.1111/j.1532-5415.2012.04107.x. [DOI] [PubMed] [Google Scholar]
- 56.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 57.Shahinian VB, Kuo Y-F, Freeman JL, et al. Risk of Fracture after Androgen Deprivation for Prostate Cancer. New England Journal of Medicine. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 58.Khan NF, Mant D, Carpenter L, et al. Long-term health outcomes in a British cohort of breast, colorectal and prostate cancer survivors: a database study. Br J Cancer. 2011;105:S29–S37. doi: 10.1038/bjc.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Z, Maricic M, Pettinger M, et al. Osteoporosis and rate of bone loss among postmenopausal survivors of breast cancer. Cancer. 2005;104:1520–1530. doi: 10.1002/cncr.21335. [DOI] [PubMed] [Google Scholar]
- 60.Hoff AO, Gagel RF. Osteoporosis in breast and prostate cancer survivors. Oncology (Williston Park, N.Y.) 2005;19:651–658. [PubMed] [Google Scholar]
- 61.Saad F, Adachi JD, Brown JP, et al. Cancer Treatment-Induced Bone Loss in Breast and Prostate Cancer. Journal of Clinical Oncology. 2008;26:5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 62.Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 Update on the Role of Bisphosphonates and Bone Health Issues in Women With Breast Cancer. Journal of Clinical Oncology. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 63.Gralow JR, Biermann JS, Farooki A, et al. NCCN Task Force Report: Bone Health in Cancer Care. Journal of the National Comprehensive Cancer Network. 2009;7 doi: 10.6004/jnccn.2009.0076. S-1-S-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grundy SM, Brewer HB, Cleeman JI, et al. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 65.Nuver J, Smit AJ, Postma A, et al. The metabolic syndrome in long-term cancer survivors, and important target for secondary preventive measures. Cancer Treatment Reviews. 2002;28:195–214. doi: 10.1016/s0305-7372(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 66.de Haas EC, Oosting SF, Lefrandt JD, et al. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010;11:193–203. doi: 10.1016/S1470-2045(09)70287-6. [DOI] [PubMed] [Google Scholar]
- 67.Traish AM, Guay A, Feeley R, et al. The Dark Side of Testosterone Deficiency: I. Metabolic Syndrome and Erectile Dysfunction. Journal of Andrology. 2009;30:10–22. doi: 10.2164/jandrol.108.005215. [DOI] [PubMed] [Google Scholar]
- 68.Carr MC. The Emergence of the Metabolic Syndrome with Menopause. The Journal of Clinical Endocrinology & Metabolism. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 69.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. Journal of Clinical Oncology. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 70.Smith MR, Lee H, McGovern F, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: differences from the classic metabolic syndrome. Cancer. 2008;112:2188–2194. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Demark-Wahnefried W, Peterson BL, Winer EP, et al. Changes in Weight, Body Composition, and Factors Influencing Energy Balance Among Premenopausal Breast Cancer Patients Receiving Adjuvant Chemotherapy. Journal of Clinical Oncology. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 72.Calip G, Malone K, Gralow J, et al. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Research and Treatment. 2014;148:363–377. doi: 10.1007/s10549-014-3157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Demark-Wahnefried W, Aziz NM, Rowland JH, et al. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aziz NM, Rowland JH. Trends and advances in cancer survivorship research: challenge and opportunity. Semin Radiat Oncol. 2003;13:248–266. doi: 10.1016/S1053-4296(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 75.Aziz NM. Cancer survivorship research: challenge and opportunity. J Nutr. 2002;132:3494s–3503s. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- 76.Travis LB. Therapy-associated Solid Tumors. Acta Oncologica. 2002;41:323–333. doi: 10.1080/028418602760169361. [DOI] [PubMed] [Google Scholar]
- 77.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975–2008. Blood. 2013;121:2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedersen-Bjergaard J, Pedersen M, Roulston D, et al. Different genetic pathways in leukemogenesis for patients presenting with therapy-related myelodysplasia and therapy-related acute myeloid leukemia. 1995 [PubMed] [Google Scholar]
- 79.Praga C, Bergh J, Bliss J, et al. Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol. 2005;23:4179–4191. doi: 10.1200/JCO.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 80.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 81.Hodgson D, van Leeuwen F. Second Malignancy Risk After Treatment of Hodgkin Lymphoma. In: Engert A, Younes A, editors. Hodgkin Lymphoma. Hematologic Malignancies: Springer International Publishing; 2015. pp. 375–409. [Google Scholar]
- 82.Koontz MZ, Horning SJ, Balise R, et al. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol. 2013;31:592–598. doi: 10.1200/JCO.2012.44.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Armitage JO, Carbone PP, Connors JM, et al. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin's lymphoma patients. J Clin Oncol. 2003;21:897–906. doi: 10.1200/JCO.2003.07.113. [DOI] [PubMed] [Google Scholar]
- 84.Palacios S, Tobar AC, Menendez C. Sexuality in the climacteric years. Maturitas. 2002;43(Suppl 1):S69–S77. doi: 10.1016/s0378-5122(02)00150-0. [DOI] [PubMed] [Google Scholar]
- 85.Matulonis UA, Kornblith A, Lee H, et al. Long-term adjustment of early-stage ovarian cancer survivors. Int J Gynecol Cancer. 2008;18:1183–1193. doi: 10.1111/j.1525-1438.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 86.Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 88.Carter J, Goldfrank D, Schover LR. Simple strategies for vaginal health promotion in cancer survivors. J Sex Med. 2011;8:549–559. doi: 10.1111/j.1743-6109.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- 89.Katz A. Breaking the silence on cancer and sexuality: a handbook for healthcare providers. Pittsburg. 2007 [Google Scholar]
- 90.Stabile C, Steed R, Carter J. Sexual medicine in the management of older gynecologic cancer patients. In: Lichtman SM, Audisio RA, editors. Management of gyneoclogical cancers in older women. London: Springer; 2013. pp. 349–366. [Google Scholar]
- 91.Mucke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015;5:CD006788. doi: 10.1002/14651858.CD006788.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tannock IF, Ahles TA, Ganz PA, et al. Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol. 2004;22:2233–2239. doi: 10.1200/JCO.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 93.Hess LM, Insel KC. Chemotherapy-related change in cognitive function: a conceptual model. Oncol Nurs Forum. 2007;34:981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- 94.Hutchinson AD, Hosking JR, Kichenadasse G, et al. Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treatment Reviews. 38:926–934. doi: 10.1016/j.ctrv.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 95.Jim HSL, Phillips KM, Chait S, et al. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. Journal of Clinical Oncology. 2012;30:3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ganz PA, Kwan L, Castellon SA, et al. Cognitive Complaints After Breast Cancer Treatments: Examining the Relationship With Neuropsychological Test Performance. Journal of the National Cancer Institute. 2013 doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wefel JS, Saleeba AK, Buzdar AU, et al. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348–3356. doi: 10.1002/cncr.25098. [DOI] [PubMed] [Google Scholar]
- 98.Ono M, Ogilvie JM, Wilson JS, et al. A Meta-Analysis of Cognitive Impairment and Decline Associated with Adjuvant Chemotherapy in Women with Breast Cancer. Frontiers in Oncology. 2015;5:59. doi: 10.3389/fonc.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paganini-Hill A, Clark L. Preliminary Assessment of Cognitive Function in Breast Cancer Patients Treated with Tamoxifen. Breast Cancer Research and Treatment. 2000;64:165–176. doi: 10.1023/a:1006426132338. [DOI] [PubMed] [Google Scholar]
- 100.Schilder CM, Seynaeve C, Beex LV, et al. Effects of Tamoxifen and Exemestane on Cognitive Functioning of Postmenopausal Patients With Breast Cancer: Results From the Neuropsychological Side Study of the Tamoxifen and Exemestane Adjuvant Multinational Trial. Journal of Clinical Oncology. 2010;28:1294–1300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 101.Schilder CM, Eggens PC, Seynaeve C, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: Cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncologica. 2009;48:76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- 102.Nelson CJ, Lee JS, Gamboa MC, et al. Cognitive effects of hormone therapy in men with prostate cancer: a review. Cancer. 2008;113:1097–1106. doi: 10.1002/cncr.23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jamadar RJ, Winters MJ, Maki PM. Cognitive changes associated with ADT: a review of the literature. Asian J Androl. 2012;14:232–238. doi: 10.1038/aja.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jim HSL, Small BJ, Patterson S, et al. Cognitive Impairment in Men Treated with Luteinizing Hormone-Releasing Hormone Agonists for Prostate Cancer: A Controlled Comparison. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2010;18:21–27. doi: 10.1007/s00520-009-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vardy J, Dhillon HM, Pond GR, et al. Cognitive function and fatigue after diagnosis of colorectal cancer. Annals of Oncology. 2014;25:2404–2412. doi: 10.1093/annonc/mdu448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sclafani F, Cunningham D. Bevacizumab in elderly patients with metastatic colorectal cancer. J Geriatr Oncol. 2014;5:78–88. doi: 10.1016/j.jgo.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 107.Sun A, Bae K, Gore EM, et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: neurocognitive and quality-of-life analysis. J Clin Oncol. 2011;29:279–286. doi: 10.1200/JCO.2010.29.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Soussain C, Ricard D, Fike JR, et al. CNS complications of radiotherapy and chemotherapy. The Lancet. 2009;374:1639–1651. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- 109.Abayomi OK. Neck irradiation, carotid injury and its consequences. Oral Oncol. 2004;40:872–878. doi: 10.1016/j.oraloncology.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Graves PR, Siddiqui F, Anscher MS, et al. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20:201–207. doi: 10.1016/j.semradonc.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 111.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin's disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 112.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 113.Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients--a Dutch colorectal cancer group study. J Clin Oncol. 2005;23:6199–6206. doi: 10.1200/JCO.2005.14.779. [DOI] [PubMed] [Google Scholar]
- 114.Borson S, Scanlan JM, Chen P, et al. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451–1454. doi: 10.1046/j.1532-5415.2003.51465.x. [DOI] [PubMed] [Google Scholar]
- 115.Crum RM, Anthony JC, Bassett SS, et al. Population-based norms for the Mini-Mental State Examination by age and educational level. Jama. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 116.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 117.King S, Green HJ. Psychological Intervention for Improving Cognitive Function in Cancer Survivors: A Literature Review and Randomized Controlled Trial. Frontiers in Oncology. 2015;5:72. doi: 10.3389/fonc.2015.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holland JC, Andersen B, Breitbart WS, et al. Distress management. J Natl Compr Canc Netw. 2013;11:190–209. doi: 10.6004/jnccn.2013.0027. [DOI] [PubMed] [Google Scholar]
- 119.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 120.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a Brief Depression Severity Measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Penedo FJ, Traeger L, Dahn J, et al. Cognitive behavioral stress management intervention improves quality of life in Spanish monolingual hispanic men treated for localized prostate cancer: results of a randomized controlled trial. Int J Behav Med. 2007;14:164–172. doi: 10.1007/BF03000188. [DOI] [PubMed] [Google Scholar]
- 122.Gil K, Mishel M, Belyea M, et al. Benefits of the uncertainty management intervention for African American and white older breast cancer survivors: 20-Month Outcomes. International Journal of Behavioral Medicine. 2006;13:286–294. doi: 10.1207/s15327558ijbm1304_3. [DOI] [PubMed] [Google Scholar]
- 123.Hart SL, Hoyt MA, Diefenbach M, et al. Meta-Analysis of Efficacy of Interventions for Elevated Depressive Symptoms in Adults Diagnosed With Cancer. Journal of the National Cancer Institute. 2012 doi: 10.1093/jnci/djs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fong DYT, Ho JWC, Hui BPH, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. 2012 doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lichtenstein MJ, Bess FH, Logan SA. Diagnostic performance of the hearing handicap inventory for the elderly (screening version) against differing definitions of hearing loss. Ear Hear. 1988;9:208–211. doi: 10.1097/00003446-198808000-00006. [DOI] [PubMed] [Google Scholar]
- 126.Chou R, Dana T, Bougatsos C, et al. Screening Adults Aged 50 Years or Older for Hearing Loss: A Review of the Evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2011;154:347–355. doi: 10.7326/0003-4819-154-5-201103010-00009. [DOI] [PubMed] [Google Scholar]
- 127.Yueh B, Shapiro N, MacLean CH, et al. Screening and management of adult hearing loss in primary care: Scientific review. JAMA. 2003;289:1976–1985. doi: 10.1001/jama.289.15.1976. [DOI] [PubMed] [Google Scholar]
- 128.Aziz MT, Good BL, Lowe DK. Serotonin-Norepinephrine Reuptake Inhibitors for the Management of Chemotherapy-Induced Peripheral Neuropathy. Annals of Pharmacotherapy. 2014;48:626–632. doi: 10.1177/1060028014525033. [DOI] [PubMed] [Google Scholar]
- 129.Grisold W, Vass A, Schmidhammer R, et al. Rehabilitation of Neuropathies. 2007;19:19–53. [Google Scholar]
- 130.Stubblefield MD. Cancer Rehabilitation. Seminars in Oncology. 2011;38:386–393. doi: 10.1053/j.seminoncol.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 131.Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526–528. doi: 10.7326/0003-4819-137-6-200209170-00014. [DOI] [PubMed] [Google Scholar]
- 132.Feskanich D, Willett W, Colditz G. WAlking and leisure-time activity and risk of hip fracture in postmenopausal women. JAMA. 2002;288:2300–2306. doi: 10.1001/jama.288.18.2300. [DOI] [PubMed] [Google Scholar]
- 133.Li F, Harmer P, Fisher KJ, et al. Tai Chi and fall reductions in older adults: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2005;60:187–194. doi: 10.1093/gerona/60.2.187. [DOI] [PubMed] [Google Scholar]
- 134.Nikolaus T, Bach M. Preventing Falls in Community-Dwelling Frail Older People Using a Home Intervention Team (HIT): Results From the Randomized Falls-HIT Trial. Journal of the American Geriatrics Society. 2003;51:300–305. doi: 10.1046/j.1532-5415.2003.51102.x. [DOI] [PubMed] [Google Scholar]
- 135.Holick MF. Vitamin D Deficiency. New England Journal of Medicine. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 136.Hadji P, Aapro MS, Body JJ, et al. Management of aromatase inhibitor-associated bone loss in postmenopausal women with breast cancer: practical guidance for prevention and treatment. Ann Oncol. 2011;22:2546–2555. doi: 10.1093/annonc/mdr017. [DOI] [PubMed] [Google Scholar]
- 137.Sun K, Liu J, Ning G. Active smoking and risk of metabolic syndrome: a meta-analysis of prospective studies. PLoS One. 2012;7:e47791. doi: 10.1371/journal.pone.0047791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kastorini CM, Milionis HJ, Esposito K, et al. The effect of Mediterranean diet on metabolic syndrome and its components: a meta-analysis of 50 studies and 534,906 individuals. J Am Coll Cardiol. 2011;57:1299–1313. doi: 10.1016/j.jacc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 139.Strasser B. Physical activity in obesity and metabolic syndrome. Annals of the New York Academy of Sciences. 2013;1281:141–159. doi: 10.1111/j.1749-6632.2012.06785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]