Abstract
Objective
To determine the age- and ethnicity-specific prevalences of strabismus in African American and Hispanic/Latino children ages 6 to 72 months and of amblyopia in African American and Hispanic/Latino children 30 to 72 months.
Design
Cross-sectional study.
Participants
The Multi-ethnic Pediatric Eye Disease Study is a population-based evaluation of the prevalence of vision disorders in children ages 6 to 72 months in Los Angeles County, California. A comprehensive eye examination was completed by 77% of eligible children. This report focuses on results from 3007 African American and 3007 Hispanic/Latino children.
Methods
Eligible children in all enumerated households in 44 census tracts were identified. Participants underwent an in-home interview and were scheduled for a comprehensive eye examination and in-clinic interview. The examination included evaluation of ocular alignment, refractive error, and ocular structures, as well as determination of optotype visual acuity (VA) in children 30 months and older.
Main Outcome Measures
The proportion of 6- to 72-month-olds with strabismus on ocular examination and proportion of 30- to 72-month-olds with optotype VA deficits and amblyopia risk factors consistent with predetermined definitions of amblyopia.
Results
Strabismus was detected in 2.4% of Hispanic/Latino children and 2.5% of African American children (P = 0.81), and was more prevalent in older children than in younger children. Amblyopia was detected in 2.6% of Hispanic/Latino children and 1.5% of African American children, a statistically significant difference (P = 0.02), and 78% of cases of amblyopia were attributable to refractive error. Amblyopia prevalence did not vary with age.
Conclusions
Among Hispanic/Latino and African American children in Los Angeles County, strabismus prevalence increases with age, but amblyopia prevalence appears stable by 3 years of age. Amblyopia is usually caused by abnormal refractive error. These findings may help to optimize the timing and modality of preschool vision screening programs.
Amblyopia and strabismus are visual development disorders with onset in childhood, but their sequelae frequently persist into adulthood. Early detection and treatment result in improved outcomes,1–3 but health care policy is currently hampered by a lack of basic information regarding the true prevalence of these diseases and the best screening approaches.
Amblyopia is the most important cause of unilateral visual impairment in children and adults under 60 years and accounts for 50% to 73% of all such vision loss.4–7 Unilateral amblyopia induces a lifetime risk of 1.2% to 3.3% for bilateral blindness or visual impairment due to acquired disease in the fellow eye.8,9 Strabismus contributes to amblyopia10 and has significant psychosocial consequences.11,12 Both conditions preclude normal binocular depth perception.
Population-based prevalence estimates in infants and children younger than 8 years range from 0.3% to 4.4% for strabismus13–35 and from 0.5% to 3.5% for amblyopia.* Several of these estimates rely on the review of medical records rather than examinations.15,24,29,33,35,39 The age range studied varies, and no study has addressed age-related prevalence. Amblyopia definitions vary and do not always explicitly require amblyopia risk factors22,28–31,37,38,41,42 or 2-line interocular optotype acuity differences for unilateral amblyopia.† In populations with high rates of prior amblyopia treatment, using current visual acuity (VA) measures may underestimate the overall burden,10,30,41 but diagnosing treated amblyopia by history is also problematic.
There are few data regarding non-Caucasian children. There are no population-based studies of amblyopia or strabismus prevalence in children of African descent in the United States or anywhere in the world, and even school-based studies are rare.20,43 Hispanic/Latino children ages 5 to 14 years were assessed in one large (but not clearly population based) study in Colombia, which showed a strabismus prevalence of 3.1% and amblyopia (definition not specified) prevalence of 1.3%.44 Data from Caucasian populations should not be extrapolated to minority children in the U.S. because of potential racial variations in disease susceptibility, and health care disparities,45 which can result in a higher burden of disease in minority children.46,47
The population-based Multi-ethnic Pediatric Eye Disease Study (MEPEDS) is evaluating the prevalence of strabismus, amblyopia, and refractive error in 12 000 6- to 72-months from 4 ethnic groups—African American, Asian American, Hispanic/Latino, and non-Hispanic Caucasian. This report focuses on the age- and ethnicity-specific prevalences of strabismus and amblyopia in African American and Hispanic/Latino children.
Materials and Methods
Study Cohort
The study design is reported elsewhere.48 Forty-four census tracts from the city of Inglewood and adjacent communities in Los Angeles County, California were selected because they encompass residential areas with high proportions of African American and Hispanic/Latino residents and a high enough census for precise prevalence estimates.
Eligible children were between 5 and 70 months old on the day of the household screening, with a parent or legal guardian confirming that the child resided in an MEPEDS census tract. An in-home interview was conducted after informed consent in the participant’s native language (Spanish or English). This included inquiry about prior strabismus surgery and any history of form deprivation from pathologic visual axis obstruction, to identify amblyopia risk factors that might not be apparent on current examination. The interviewer also administered the 800– and 400–arc-second booklet of the Randot Preschool Stereoacuity test to eligible children. An appointment was then scheduled for examination at the local MEPEDS examination center. Institutional review board/ethics committee approval was obtained from the Los Angeles County/University of Southern California Medical Center Institutional Review Board. Written informed consent was obtained from a parent or legal guardian of each participant.
Clinic Ocular Examination and Interview
The clinic visit included a structured interview and comprehensive eye examination performed by MEPEDS optometrists or ophthalmologists who specialize in pediatric eye care and are trained and certified using standardized protocols.48 Details of the interview can be found elsewhere.48 The interviewer recorded whether the child had ever been diagnosed with and treated for strabismus or amblyopia (after defining these conditions), but these responses were not used to diagnose either condition. Ocular alignment was tested by unilateral cover (cover–uncover) testing (UCT) and alternate cover testing (ACT) of standardized duration, at 6-m fixation (cartoon video) and 40-cm fixation (Lang cube [Fresnel Prism & Lens Co., Eden Prairie, MN] or colored sticker), with and without correction (if worn). Transient misalignment after ACT was classified as strabismus only if confirmed by repeat UCT. Strabismus was measured by simultaneous prism and cover test and ACT. Hirschberg and Krimsky testing at near were used, respectively, when UCT or the simultaneous prism and cover test could not be performed (Krimsky not used if <10 prism diopters [PD]).
Monocular distance VA was tested in children ≥30 months old using a single-surround HOTV optotype on the electronic VA tester49 according to the Amblyopia Treatment Study protocol,50 using naming or matching of letters. Initial VA was tested with correction, if worn. If VA was retested for quality control, the initial VA for each eye was the better of the two results. If initial VA was decreased in either eye (worse than 20/50 for 30- to 47-month-olds, or worse than 20/40 for ≥48-month-olds) or if there was a 2-line interocular difference with VA 20/32 or worse in the worse eye, VA was retested after cycloplegic refraction, with full correction. Taking the best of all test results for each eye, children who still had subnormal VA in either eye or a 2-line interocular difference with VA of 20/32 or worse in the worse eye and an amblyopia risk factor (see below) were scheduled for return-visit retesting with correction to confirm that poor corrected VA was not an artifact of testing after dilation. For uncyclopleged VA testing, spherical equivalent (SE) hyperopia was undercorrected by up to 1.50 diopters (D) to allow for incomplete relaxation of accommodation. Children unable to perform VA testing at the original visit were also scheduled for a return visit and sent home with HOTV letters for practice. The final best-corrected VA (BCVA) for each eye was the best of all test results recorded for that eye.
Cycloplegic refraction was performed with the Retinomax Autorefractor (Right Manufacturing, Virginia Beach, VA) at least 30 minutes after cycloplegia with 2 drops of 1% cyclopentolate (0.5% for <12-month-olds) given 5 minutes apart. Cycloplegic retinoscopy was performed if Retinomax readings with confidence ratings of ≥8 were not obtained in both eyes after 3 attempts. If parents did not allow cycloplegic eyedrops, noncycloplegic retinoscopy was performed. Anterior segment and fundus examinations followed refraction.
Definitions of Strabismus and Amblyopia
Strabismus was defined as constant or intermittent tropia of any magnitude at distance and/or near fixation. To avoid artifacts of repeated dissociation, quality control repeat test results were not factored in. Children tested at only one fixation distance and without strabismus on that test were considered nonstrabismic. There were no instances of the direction of strabismus changing between UCT and ACT or near and distance. Tropias that could not be measured by either simultaneous prism and cover test or Krimsky testing were considered to be of unknown magnitude.
Unilateral amblyopia was defined as a 2-line interocular difference in BCVA with 20/32 or worse in the worse eye and at least one of the following unilateral amblyopia risk factors: strabismus on examination, history of strabismus surgery (from in-home interview), anisometropia consistent with the eye with worse VA (≥1.00-D SE anisohyperopia, ≥3.00-D SE anisomyopia, or ≥1.50-D anisoastigmatism), evidence of past or present visual axis obstruction (e.g., cataract, pseudophakia, aphakia, corneal opacity, ptosis, eyelid hemangioma), or history of such obstruction for at least 1 week (from in-home interview).
Bilateral amblyopia was defined as bilateral subnormal BCVA (worse than 20/50 in 30- to 47-month-olds or worse than 20/40 in ≥48-month-olds) with either bilateral evidence of visual axis obstruction (see above) or bilateral ametropia (≥4.00-D SE hyperopia, ≥6.00-D SE myopia, or ≥2.50-D astigmatism). Children meeting both unilateral and bilateral amblyopia criteria were classified as unilateral. Children with coexisting fundus or anterior segment abnormalities precluding normal vision were not considered amblyopic.
Statistical Analysis
Prevalence was calculated as the ratio of the number of individuals with any type of strabismus or amblyopia to the total number evaluated. Strabismus prevalence was evaluated in participants 6 to 72 months old; amblyopia prevalence was evaluated in participants 30 to 72 months old, because the diagnosis required optotype VA testing, which was not performed in children younger than 30 months. A chi-square test was used to compare the proportions of children with a given diagnosis between ethnic groups and genders. The association of strabismus and amblyopia prevalence with age was examined by the Cochran trend test, stratifying by age into 6 categories. Strabismus prevalence as related to age was also evaluated by chi-square testing after stratifying by age into 2 categories. A secondary analysis of strabismus and amblyopia prevalence was performed by randomly selecting one child from each household to test for clustering effects. All analyses employed SAS 9.1 software (SAS Institute, Inc., Cary, NC) and a 0.05 significance level. P values refer to chi-square testing unless otherwise specified.
Results
Study Cohort
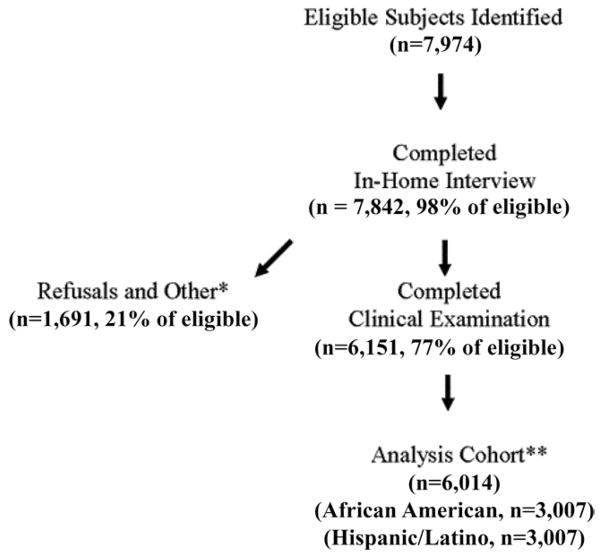
Of the 7974 eligible individuals in the census tracts surveyed, 132 (2%) did not complete the in-home interview, 1691 (21%) completed only the in-home interview, and 6151 (77%) completed the in-home interview and clinical examination (Fig 1 [available at http://aaojournal.org]). We compared eligible individuals who completed the in-home interview but did not complete a clinical examination with those who were examined. Those not examined had significantly higher proportions of males (54%, compared with 51% for participants completing examination), families with income > $30 000 (25%, vs. 22% for participants completing examination), and very good to excellent self-reported health (94% vs. 91%). There were no significant differences in age, insurance coverage, self-reported eye health, race (distribution of African American and Hispanic children), or the proportion of children tested scoring 400 arc-seconds of stereopsis on the in-home Randot Preschool Stereoacuity test.
Figure 1.
Recruitment of the study cohort. Eligible subjects were identified through direct in-person contact with the occupants of an enumerated dwelling to determine the presence or absence of eligible children in the home. *Includes respondents who had moved out of the area or were medically incapable, deceased, or never available. **One hundred thirty-seven children examined were of other races (e.g., non-Hispanic Caucasian, Asian American). An in-clinic examination was completed by 5256 (87.4%) of the 6014 children in the analysis cohort. An examination in a mobile clinic was completed by 758 children (12.6%).
Of the 6151 children who completed a clinical examination, 3007 (49%) were Hispanic/Latino, 3007 (49%) were African American, and 137 (2%) were of another race/ethnicity. The 6014 Hispanic/Latino and African American children (3059 male, 2955 female) constitute the analysis cohort (Table 1 [available at http://aaojournal.org]). Participants were representative of children nationwide in terms of U.S. Census age and gender distributions. Only 2.8% of participants had noncycloplegic refraction when parents did not consent to cycloplegic eyedrops.
Table 1.
Demographic Characteristics of Participants
| Hispanic/Latino (n = 3007) [n (%)] | African American (n = 3007) [n (%)] | Total (n = 6014) [n (%)] | |
|---|---|---|---|
| Age | |||
| 6–11 mos | 295 (10) | 278 (9) | 573 (9) |
| 12–23 mos | 536 (18) | 556 (19) | 1092 (18) |
| 24–35 mos | 568 (19) | 546 (18) | 1114 (19) |
| 36–47 mos | 532 (17) | 535 (18) | 1067 (18) |
| 48–59 mos | 539 (18) | 549 (18) | 1088 (18) |
| 60–72 mos | 537 (18) | 543 (18) | 1080 (18) |
| Gender | |||
| Male | 1541 (51) | 1518 (50) | 3059 (51) |
| Female | 1466 (49) | 1489 (50) | 2955 (49) |
Prevalence of Strabismus
Of the 6014 Hispanic/Latino and African American participants ages 6 to 72 months, 6 were excluded because ocular alignment could not be assessed by at least one of the following: cover testing at distance or at near or Hirschberg testing at near. Among the 6008 remaining children were 346, mostly infants, in whom ocular alignment could only be evaluated at near. Of the 6008 children, 149 (2.5%) were diagnosed with strabismus (Table 2). There was no difference between ethnicities (P = 0.81), with 2.4% of Hispanic/Latino children and 2.5% of African American children having strabismus. Esotropia prevalence did not differ significantly between African American children (1.1%) and Hispanic/Latino children (0.9%) (P = 0.36). Exotropia was more common than esotropia and did not differ significantly between Hispanic/Latino children (1.5%) and African American children (1.4%) (P = 0.74). Three Hispanic/Latino children and 1 African American child had pure or primary vertical strabismus (hypertropia in 2 cases, and isolated dissociated vertical deviation in 2 cases, 1 postoperative). Seven additional children had a hypertropia other than dissociated vertical deviation associated with large-angle horizontal strabismus, and were classified according to the latter. There was no significant difference in strabismus prevalence between males (2.2%) and females (2.7%) (P = 0.20).
Table 2.
Strabismus Prevalence and Subtypes by Ethnicity
| Hispanic/Latino (n = 3003) | African American (n = 3005) | |
|---|---|---|
| Prevalence (%) (95% confidence interval) (n) | ||
| Any*† | 2.4 (1.9–3.0) (73) | 2.5 (2.0–3.1) (76) |
| Exotropia | 1.5 (1.0–1.9) (44) | 1.4 (0.9–1.8) (41) |
| Esotropia | 0.9 (0.5–1.2) (26) | 1.1 (0.7–1.5) (33) |
| Strabismus type at distance [n (%)]* | ||
| Intermittent exotropia | 16 (21.9) | 24 (31.6) |
| Constant exotropia | 12 (16.4) | 8 (10.5) |
| Intermittent esotropia | 5 (6.8) | 11 (14.5) |
| Constant esotropia | 17 (23.3) | 17 (22.4) |
| Strabismus identified only at near‡ | 20 (27.4) | 15 (19.7) |
| Strabismus type at near [n (%)]*†§ | ||
| Intermittent exotropia | 18 (24.7) | 22 (28.9) |
| Constant exotropia | 10 (13.7) | 4 (5.3) |
| Intermittent esotropia | 5 (6.8) | 9 (11.8) |
| Constant esotropia | 20 (27.4) | 19 (25.0) |
| Strabismus identified only at distance‡ | 16 (21.9) | 20 (26.3) |
| Strabismus magnitude at distance* (horizontal SPCT) [n (%)] | ||
| 1–9 PD | 3 (4.1) | 10 (13.2) |
| 10–30 PD | 34 (46.6) | 34 (44.7) |
| >30 PD | 6 (8.2) | 9 (11.8) |
| Unable to measure | 7 (9.6) | 7 (9.2) |
| Strabismus identified only at near‡ | 20 (27.4) | 15 (19.7) |
| Strabismus magnitude at near* (Horizontal SPCT) [n (%)] | ||
| 1–9 PD | 5 (6.8) | 5 (6.6) |
| 10–30 PD | 33 (45.2) | 33 (43.2) |
| >30 PD | 7 (9.6) | 11 (14.5) |
| Unable to measure | 9 (12.3) | 6 (7.9) |
| Strabismus identified only at distance‡ | 16 (21.9) | 20 (26.3) |
PD = prism diopters; SPCT = simultaneous prism and cover test.
Three Hispanic children and 1 African American child had a purely or primarily vertical strabismus.
One African American child with bilateral aphakia had a deviation that varied from exotropia to esotropia.
These strabismic children were nonstrabismic at one of the fixation distances tested or could be evaluated only at one fixation distance (usually near) due to young age and inattention.
One Hispanic child had an exotropia at near that could not be classified as either intermittent or constant.
When the results were stratified into 6 age groups, the proportion having strabismus significantly increased with age (P<0.0001, trend test; Table 3). This trend was significant in both males (P = 0.01) and females (P = 0.002). It was also significant in African American children (P = 0.0002, trend test), though not in Hispanic/Latino children (P = 0.054, trend test). Due to small samples for strabismus subtypes using 6 strata, age was also stratified into 2 levels: 6 to 35 months (younger) and 36 to 72 months (older). There was significantly more strabismus in older children than in younger children among both African American (P = 0.003) and Hispanic/Latino children (P = 0.009). The overall prevalence of strabismus ≥10 PD in amplitude was also significantly higher in older children (P = 0.003). There was a higher prevalence of esotropia in older children in both Hispanic/Latino (P = 0.02) and African American children (P = 0.01). Exotropia prevalence did not differ significantly between older and younger groups in either Hispanic/Latino (P = 0.29) or African American children (P = 0.07).
Table 3.
Strabismus Prevalence in Hispanic/Latino and African American Children by Age (Months)
| Age | Hispanic/Latino
|
Age | African American
|
||||
|---|---|---|---|---|---|---|---|
| Any Strabismus (Prevalence [(n) (95% CI)])* | Any Exotropia [Prevalence (n)] | Any Esotropia [Prevalence (n)] | Any Strabismus (Prevalence [(n) (95% CI)])* | Any Exotropia [Prevalence (n)] | Any Esotropia [Prevalence (n)] | ||
| 6–11 (n = 295) | 2.0% (6) (0.4–3.6) | 1.7% (5) | 0.3% (1) | 6–11 (n = 278) | 1.1% (3) (0.0–2.3) | 0.7% (2) | 0.4% (1) |
| 12–23 (n = 536) | 2.2% (12) (1.0–3.5) | 1.7% (9) | 0.6% (3) | 12–23 (n = 555) | 1.3% (7) (0.3–2.2) | 0.7% (4) | 0.5% (3) |
| 24–35 (n = 566) | 0.9% (5) (0.1–1.6) | 0.5% (3) | 0.4% (2) | 24–35 (n = 545) | 2.2% (12) (1.0–3.4)† | 1.3% (7) | 0.7% (4) |
| 36–47 (n = 530) | 2.6% (14) (1.3–4.0) | 1.5% (8) | 1.1% (6) | 36–47 (n = 535) | 1.9% (10) (0.7–3.0) | 1.1% (6) | 0.7% (4) |
| 48–59 (n = 539) | 3.9% (21) (2.3–5.5)† | 2.0% (11) | 1.5% (8) | 48–59 (n = 549) | 4.2% (23) (2.5–5.9)‡ | 2.0% (11) | 2.0% (11) |
| 60–72 (n = 537) | 2.8% (15) (1.4–4.2)† | 1.5% (8) | 1.1% (6) | 60–72 (n = 543) | 3.9% (21) (2.2–5.5) | 2.0% (11) | 1.8% (10) |
CI = confidence interval.
Reported by age and race/ethnicity for overall strabismus prevalence only.
Three Hispanic children and 1 African American child had a purely or primarily vertical strabismus.
One African American child with bilateral aphakia had strabismus that was at times exotropia and at times esotropia.
At distance fixation, 68% of esotropias (34/50) were constant rather than intermittent, whereas only 33% of exotropias (20/60) were constant. Similarly, at near fixation 74% of esotropias (39/53) were constant, whereas only 26% of exotropias (14/54) were constant (Table 2). Of the 59 children with esotropia, 29 (49%) had constant strabismus at both distance and near. Of the 85 children with exotropia, only 9 (11%) had constant strabismus at both distance and near. Thus, 76% of strabismus cases that were constant at both distance and near (29/38) were esotropias.
The magnitudes of horizontal strabismus at distance, when measurable, were predominantly in the range of 10 to 30 PD, with only 13 of 96 (14%) smaller than 10 PD and 15 of 96 (16%) larger than 30 PD (Table 2). The magnitudes at near showed a similar distribution, with only 10 of 94 (11%) smaller than 10 PD and 18 of 94 (19%) larger than 30 PD.
Prevalence of Amblyopia
Of the 3817 participants ages 30 to 72 months who were evaluated for amblyopia, 467 were excluded from analysis because they were unable to perform VA testing in one or both eyes. Of the 467 excluded children, 311 were 30 to 35 months old (53% of the 582 children in this age group), 133 were 36 to 48 months, 17 were 48 to 60 months, and 6 were 60 to 72 months. In the 30- to 35-month age group, the prevalence of amblyopia risk factors (strabismus, amblyogenic anisometropia, or amblyogenic bilateral ametropia) was higher in excluded children (9.3%) than in included children (4.8%) (P = 0.04). Of the 3350 children included in the analysis, 69 (2.1%) were diagnosed with amblyopia. Two additional children met amblyopia VA and risk factor criteria but were not considered amblyopic due to coexisting pathology (aniridia with foveal hypoplasia and corneal scar). Of the 69 amblyopic children, 46 (67%) attended a return-visit retest of corrected VA without cycloplegia. The remaining 23 participants did not attend a return visit within 3 months of initial testing and were diagnosed with amblyopia based on the results from the first visit (the better of presenting acuity and corrected acuity after cycloplegic refraction). Retest data suggest that up to 4 of these might not have met amblyopia criteria had they attended a return visit. No diagnoses of amblyopia depended on self-reported amblyopia risk factors alone.
The prevalence of amblyopia in Hispanic/Latino children was 2.6% higher than the prevalence of 1.5% seen in African American children (P = 0.02) (Table 4). The largest differences were seen in the prevalence of amblyopia associated with anisometropia (Table 4). The prevalence of anisometropia itself appeared to be slightly higher in Hispanic/Latino ≥30-month-olds (5%) than in African American ≥30-month-olds (3.9%), but this difference was not statistically significant (P = 0.13). No difference in amblyopia prevalence (P = 0.91) was found between genders (male, 2.1%; female, 2.0%). In addition, there was no significant relationship between amblyopia frequency and age for Hispanic/Latino children (P = 0.11, trend test) or African American children (P = 0.73, trend test) (Table 5) or for males (P = 0.52, trend test) or females (P = 0.16, trend test).
Table 4.
Amblyopia Prevalence by Ethnicity
| Amblyopia Type | Hispanic/Latino (n = 1687) (Prevalence [(n) (95% CI)])* | African American (n = 1663) (Prevalence [(n) (95% CI)])* |
|---|---|---|
| Any | 2.6% (44) (1.8–3.4) | 1.5% (25) (0.9–2.1) |
| Unilateral anisometropic | 1.5% (25) | 0.8% (14) |
| Unilateral strabismic | 0.4% (6) | 0.2% (4) |
| Unilateral combined strabismic/anisometropic | 0.2% (3) | 0.0% (0) |
| Unilateral deprivational | 0.1% (1) | 0.0% (0) |
| Bilateral† | 0.5% (9) | 0.4% (7) |
CI = confidence interval.
Reported by race/ethnicity for overall amblyopia prevalence only.
One African American child had bilateral aphakia with a history of congenital cataracts; all other cases of bilateral amblyopia were due to bilateral ametropia alone. One additional African American child with high myopia was classified as having unilateral amblyopia but also met the criteria for bilateral amblyopia.
Table 5.
Amblyopia Prevalence in Hispanic/Latino and African-American Children by Age (Months)
| Age | Hispanic/Latino
|
Age | African American
|
||
|---|---|---|---|---|---|
| Any Amblyopia (Prevalence [(n) (95% CI)]) | Anisometropic Amblyopia [Prevalence (n)] | Any Amblyopia (Prevalence [(n) (95% CI)]) | Anisometropic Amblyopia [Prevalence (n)] | ||
| 30–35 (n = 143) | 0.7% (1) (0.0–2.1) | 0.0% (0) | 30–35 (n = 128) | 0% (0) (NA) | 0% (0) |
| 36–47 (n = 478) | 2.1% (10) (0.8–3.4) | 1.0% (5) | 36–47 (n = 456) | 1.8% (8) (0.5–3.0) | 1.1% (5) |
| 48–59 (n = 531) | 3.2% (17) (1.7–4.7) | 1.7% (9) | 48–59 (n = 540) | 1.9% (10) (0.7–3.0) | 0.9% (5) |
| 60–72 (n = 535) | 3.0% (16) (1.5–4.4) | 2.1% (11) | 60–72 (n = 539) | 1.3% (7) (0.3–2.3) | 0.7% (4) |
CI = confidence interval; NA = not applicable.
The majority of amblyopic children had purely anisometropic unilateral amblyopia without strabismus (39 participants [57%]), and another 15 children (22%) had bilateral ametropic amblyopia. Therefore, 54 cases, or 78% of all amblyopia cases, were attributable to abnormal refractive error. Only 13 cases (19%) were attributable even in part to strabismus.
To evaluate possible bias from familial clustering, the variance estimates of the age-adjusted prevalence rate with and without adjustment for familial clustering were compared using a mixed effects model (with household as the random factor). No significant difference was observed in the strabismus prevalence variance estimates (0.023 with adjustment, 0.024 without adjustment). There was a 0.1% improvement in the goodness of fit after the adjustment. Similarly, the variance estimate for amblyopia prevalence was 0.020 both with and without adjustment, with no improvement in goodness of fit.
To evaluate the impact of familial clustering further, one child was randomly selected to represent each household. Amblyopia prevalence by household was 2.1%, compared with 2.1% in all children, and strabismus prevalence by household was 2.4%, compared with 2.5% in all children, suggesting no clustering bias.
History of Treatment
Less than 0.9% of children wore glasses. No parents reported their children having been previously diagnosed with amblyopia. A previous diagnosis of strabismus was reported in 1.7% of participants (62% of whom had strabismus on examination). Only 7 children had ever received patching treatment, and only 2 had received eyedrop treatment. Thus, few children are likely to have been cured of previous amblyopia and, therefore, are not identified in this analysis.
Discussion
In this large, population-based cross section of children in Los Angeles County, strabismus prevalence in Hispanic/Latino and African American children ages 6 to 72 months is 2.5%, with no significant difference between genders or ethnicities. Strabismus prevalence is higher in older children, and exotropia is more common than esotropia. Amblyopia prevalence in children 30 to 72 months is higher in Hispanic/Latino children (2.6%) than in African American children (1.5%); it does not vary by gender. In this population, previous diagnosis and treatment of amblyopia are uncommon. Therefore, these prevalence estimates reflect the natural frequency of pathology in the absence of effective surveillance and intervention, indicating the full magnitude of the public health challenge.
The higher prevalence of strabismus and, specifically, of esotropia in older children than in younger children in both Hispanic/Latino and African American populations is unlikely to be an artifact of the difficulty of detecting strabismus in younger children, because strabismus prevalence increases with age even when excluding small-angle deviations. We know of no previous study that has demonstrated a relationship of strabismus with age, although it is consistent with studies showing little strabismus in the first year of life.21,25,32 Increasing esotropia prevalence with age may reflect the development of accommodative esotropia in hyperopic children once they begin consistently accommodating to compensate for their hyperopia, revealing the accommodative convergence associated with these efforts.
Esotropia is a less common form of strabismus than exotropia in both African American and Hispanic children. However, constant ocular misalignment at both distance and near—which precludes any normal binocular function—is more often due to esotropia than exotropia. Small-angle deviations <10 PD, which may be compatible with subnormal but not absent binocularity, represent only a small proportion of strabismus cases.
Amblyopia prevalence does not vary significantly with age between 30 months and 72 months. There is a suggestion of lower prevalence in the 30- to 35-month age group; however, the sample size is smaller than for other age groups, due to the shorter age span and lower optotype testability rates, with confidence intervals overlapping broadly with those of older age groups. Furthermore, in this age group children who completed VA testing were less likely to have amblyopia risk factors than those who could not be tested, biasing toward lower prevalence estimates. Finally, variability of test performance in normal eyes at younger ages could mask true interocular acuity differences, again biasing toward lower amblyopia prevalence. The retest protocol minimizes amblyopia overdiagnosis but leaves room for underdiagnosis, because children with normal-for-age VA and no interocular difference are not retested even if they have an amblyopia risk factor and room for an interocular difference to develop upon retesting. Above 3 years of age, amblyopia prevalence appears stable. Assuming there are no significant cohort differences between younger and older participants, such a cross-sectional pattern implies either a low incidence of new cases of amblyopia after age 3 or an incidence rate that is matched by resolution of existing cases. Because amblyopia treatment is uncommon in this population and amblyopia is not known to resolve spontaneously,51 our data suggest that most cases of amblyopia have developed and can be detected by 3 to 4 years of age. This, in turn, implies that preschool vision screening should detect most cases of amblyopia.
It is striking that more than three quarters of all amblyopia cases in Hispanic/Latino and African American children are attributable to refractive error alone. Conversely, only 0.4% of the population has amblyopia that is even partially attributable to strabismus, so clearly, many cases of strabismus, even of esotropia, are not associated with amblyopia. This implies that in these minority populations screening for high-risk refractive error should detect most cases of amblyopia. However, because the likelihood of amblyopia at any given level of refractive error has yet to be defined, selecting optimal referral criteria remains challenging.
Bilateral ametropic amblyopia, which may have a greater impact on a child’s habitual visual function than unilateral disease, was diagnosed in 0.45% of children. This study is the first to provide a population-based estimate of bilateral amblyopia prevalence in a population with little prior treatment. Robaei et al diagnosed it in 0.12% of children in Australia,10 who had higher rates of prior amblyopia treatment and spectacle wear (1.3% and 4.4% of all children, respectively)10,52 than MEPEDS participants.
Amblyopia prevalence is significantly higher in Hispanic/ Latino children than in African American children. The largest differences are seen for anisometropic amblyopia. Anisometropia prevalence in the age group evaluated for amblyopia was somewhat higher in Hispanic/Latino children, although this difference was not statistically significant. It may be that anisometropia in Hispanic/Latino children is more likely to result in amblyopia than in African American children, because of an underlying susceptibility or differences in type or magnitude of anisometropia. Future analysis of ethnicity-specific refractive error distributions and refractive error associations with amblyopia will help to distinguish between these possibilities.
Population-based data, rigorous protocols and quality control,48 high participation rates, and a large sample support the validity of the MEPEDS findings. We believe our findings are generalizable to African American and Hispanic/Latino children throughout the U.S., and completion of the MEPEDS evaluation of non-Hispanic Caucasian and Asian American children will reveal whether our findings are also typical of these children. Despite its strengths, the study has several limitations. As discussed above, amblyopia prevalence may be underestimated in the 30- to 35-month age group. Because optotype VA was not assessed in children younger than 30 months, we cannot estimate the prevalence of amblyopia as defined by VA in younger age groups. Thus, we cannot determine the likely age of amblyopia onset, though it is probably present by 3 years of age in most cases. The inclusion of data from children who did not return for VA retesting might have resulted in a few cases of amblyopia overdiagnosis. Conversely, we could have underdiagnosed amblyopia in children who met VA criteria but no longer had an amblyogenic refractive error; however, because amblyopia is associated with impaired emmetropization,53–57 this is unlikely.
In summary, the MEPEDS is the first rigorous population-based investigation of the prevalence of strabismus and amblyopia in African American and Hispanic/Latino infants and young children and the first study in any population to investigate the prevalence of these disorders as a function of cross-sectional age. Our findings that most amblyopia in these populations is caused by refractive error and that amblyopia prevalence is stable by 3 to 4 years of age have direct implications for public health policy with regard to the optimal timing and modality for vision screening.
Acknowledgments
Supported by the National Eye Institute, Bethesda, Maryland (grant nos. EY14472, EY03040), and an unrestricted grant from Research to Prevent Blindness, New York, New York. Dr Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Appendix
Writing Committee
Kristina Tarczy-Hornoch, MD, DPhil, Rohit Varma, MD, MPH, Susan Cotter, OD, MS, Anne DiLauro, MPH, Ying Wang, MS, Mark Borchert, MD, Stanley P. Azen, PhD, Allyson Collins, BA.
Multi-ethnic Pediatric Eye Disease Study Group
University of Southern California
Rohit Varma, MD, MPH (Principal Investigator), LaVina Abbott (2002–2005), George Ayala (2005–2006), Stanley P. Azen, PhD, Tal Barak, OD, Mark Borchert, MD, Jessica Chang, OD, Felicia K. Chen, OD, Susan Cotter, OD, MS (Co–Principal Investigator), Jennifer Deneen, MPH, Jackie Diaz, Anne Di-Lauro, MPH, Jill Donofrio, MPH (2003–2005), Claudia Dozal (2003–2004), Athena Foong, James Gardner, Jackson Lau, OD, Jesse Lin, MS, George Martinez, Roberta McKean, PhD, Kisha Milo, Carlos Moya, Sylvia Paz, MS (2002–2005), Ana Penate, Amanda Reiner, MPH, Claudia Salazar, Erin Song, OD, Kristina Tarczy-Hornoch, MD, DPhil, Mina Torres, MS, Natalia Uribe, OD, Ivania Verrico, Ying Wang, MS, Peng Zhao, MS, Amy Zhu, MPH.
Battelle Survey Research Center
Charles Aders (2003–2006), Candace Kwong, MPH, Nancy Noedel, Michael Preciado, Karen Tucker, MA.
Data Monitoring Oversight Committee
Jonathan M. Holmes, MD (Chair), Eileen E. Birch, PhD, Karen Cruiskshanks, PhD, Natalie Kurinij, PhD, Graham E. Quinn, MD, Maureen G. Maguire, PhD, Joseph M. Miller, MD, Karla Zadnik, OD, PhD.
Footnotes
References
- 1.Fawcett SL, Birch EE. Risk factors for abnormal binocular vision after successful alignment of accommodative esotropia. J AAPOS. 2003;7:256–62. doi: 10.1016/s1091-8531(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 2.Birch EE, Stager DR., Sr Long-term motor and sensory outcomes after early surgery for infantile esotropia. J AAPOS. 2006;10:409–13. doi: 10.1016/j.jaapos.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Stewart CE, Fielder AR, Stephens DA, Moseley MJ. Treatment of unilateral amblyopia: factors influencing visual outcome. Invest Ophthalmol Vis Sci. 2005;46:3152–60. doi: 10.1167/iovs.05-0357. [DOI] [PubMed] [Google Scholar]
- 4.Robaei D, Huynh SC, Kifley A, Mitchell P. Correctable and non-correctable visual impairment in a population-based sample of 12-year-old Australian children. Am J Ophthalmol. 2006;142:112–8. doi: 10.1016/j.ajo.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 5.Robaei D, Rose K, Ojaimi E, et al. Visual acuity and the causes of visual loss in a population-based sample of 6-year-old Australian children. Ophthalmology. 2005;112:1275–82. doi: 10.1016/j.ophtha.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Kessel L, Hougaard JL, Mortensen C, et al. Visual acuity and refractive errors in a suburban Danish population: Inter99 Eye Study. Acta Ophthalmol Scand. 2004;82:19–24. doi: 10.1111/j.1395-3907.2004.0179.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clin Experiment Ophthalmol. 2000;28:268–73. doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 8.Rahi J, Logan S, Timms C, et al. Risk, causes, and outcomes of visual impairment after loss of vision in the non-amblyopic eye: a population-based study. Lancet. 2002;360:597–602. doi: 10.1016/s0140-6736(02)09782-9. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson P, Kvarnstrom G, Abrahamsson M, et al. The frequency of amblyopia among visually impaired persons. Acta Ophthalmol Scand. 2002;80:44–6. doi: 10.1034/j.1600-0420.2002.800109.x. [DOI] [PubMed] [Google Scholar]
- 10.Robaei D, Rose KA, Ojaimi E, et al. Causes and associations of amblyopia in a population-based sample of 6-year-old Australian children. Arch Ophthalmol. 2006;124:878–84. doi: 10.1001/archopht.124.6.878. [DOI] [PubMed] [Google Scholar]
- 11.Jackson S, Harrad RA, Morris M, Rumsey N. The psychosocial benefits of corrective surgery for adults with strabismus. Br J Ophthalmol. 2006;90:883–8. doi: 10.1136/bjo.2005.089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goff MJ, Suhr AW, Ward JA, et al. Effect of adult strabismus on ratings of official U.S. Army photographs. J AAPOS. 2006;10:400–3. doi: 10.1016/j.jaapos.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Nawratzki I, Oliver M, Newmann E. Screening for amblyopia: screening for amblyopia in children under three years of age in Israel. Isr J Med Sci. 1972;8:1469–72. [PubMed] [Google Scholar]
- 14.Kohler L, Stigmar G. Vision screening of four-year-old children. Acta Paediatr Scand. 1973;62:17–27. doi: 10.1111/j.1651-2227.1973.tb08060.x. [DOI] [PubMed] [Google Scholar]
- 15.Krause U, Rantakallio P. Paediatric ophthalmology in Northern Finland—a population investigation. Acta Ophthalmol Suppl. 1974;123:154–6. [PubMed] [Google Scholar]
- 16.Graham PA. Epidemiology of strabismus. Br J Ophthalmol. 1974;58:224–31. doi: 10.1136/bjo.58.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornder LD, Nursey JN, Pratt-Johnson JA, Beattie A. Detection of manifest strabismus in young children. I. A prospective study. Am J Ophthalmol. 1974;77:207–10. doi: 10.1016/0002-9394(74)90674-6. [DOI] [PubMed] [Google Scholar]
- 18.Friedmann L, Biedner B, David R, Sachs U. Screening for refractive errors, strabismus and other ocular anomalies from ages 6 months to 3 years. J Pediatr Ophthalmol Strabismus. 1980;17:315–7. doi: 10.3928/0191-3913-19800901-11. [DOI] [PubMed] [Google Scholar]
- 19.Friedman Z, Neumann E, Hyams SW, Peleg B. Ophthalmic screening of 38,000 children, age 1 to 2½ years, in child welfare clinics. J Pediatr Ophthalmol Strabismus. 1980;17:261–7. doi: 10.3928/0191-3913-19800701-16. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Screening results on the ocular status of 651 prekin-dergarteners. Am J Optom Physiol Opt. 1981;58:648–62. [PubMed] [Google Scholar]
- 21.Atkinson J, Braddick OJ, Durden K, et al. Screening for refractive errors in 6–9 month old infants by photorefraction. Br J Ophthalmol. 1984;68:105–12. doi: 10.1136/bjo.68.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson A, Kirkland C, Silva PA. Vision and eye problems in seven year olds: a report from the Dunedin Multidisciplinary Health and Development Research Unit. N Z Med J. 1984;97:445–9. [PubMed] [Google Scholar]
- 23.Rasmussen F, Thoren K, Caines E, et al. Suitability of the Lang II Random Dot Stereotest for detecting manifest strabismus in 3-year-old children at child health centres in Sweden. Acta Paediatr. 2000;89:824–9. [PubMed] [Google Scholar]
- 24.Stayte M, Johnson A, Wortham C. Ocular and visual defects in a geographically defined population of 2-year-old children. Br J Ophthalmol. 1990;74:465–8. doi: 10.1136/bjo.74.8.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson J, Braddick O, Robier B, et al. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10:189–98. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- 26.Newman DK, Hitchcock A, McCarthy H, et al. Preschool vision screening: outcome of children referred to the hospital eye service. Br J Ophthalmol. 1996;80:1077–82. doi: 10.1136/bjo.80.12.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasmann-Kellner B, Heine M, Pfau B, et al. Screening for amblyopia, strabismus and refractive abnormalities in 1,030 kindergarten children [in German] Klin Monatsbl Augenheilkd. 1998;213:166–73. doi: 10.1055/s-2008-1034968. [DOI] [PubMed] [Google Scholar]
- 28.Robinson B, Bobier WR, Martin E, Bryant L. Measurement of the validity of a preschool vision screening program. Am J Public Health. 1999;89:193–8. doi: 10.2105/ajph.89.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams C, Harrad RA, Harvey I, Sparrow JM. Screening for amblyopia in preschool children: results of a population-based, randomised controlled trial. Ophthalmic Epidemiol. 2001;8:279–95. doi: 10.1080/09286586.2001.11644257. [DOI] [PubMed] [Google Scholar]
- 30.Kvarnstrom G, Jakobsson P, Lennerstrand G. Visual screening of Swedish children: an ophthalmological evaluation. Acta Ophthalmol Scand. 2001;79:240–4. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- 31.Barry JC, Konig HH. Test characteristics of orthoptic screening examination in 3 year old kindergarten children. Br J Ophthalmol. 2003;87:909–16. doi: 10.1136/bjo.87.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anker S, Atkinson J, Braddick O, et al. Identification of infants with significant refractive error and strabismus in a population screening program using noncycloplegic videore-fraction and orthoptic examination. Invest Ophthalmol Vis Sci. 2003;44:497–504. doi: 10.1167/iovs.02-0070. [DOI] [PubMed] [Google Scholar]
- 33.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia. A population-based study. Ophthalmology. 2005;112:104–8. doi: 10.1016/j.ophtha.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 34.Robaei D, Rose KA, Kifley A, et al. Factors associated with childhood strabismus. Findings from a population-based study. Ophthalmology. 2006;113:1146–53. doi: 10.1016/j.ophtha.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Greenberg AE, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood esotropia. A population-based study. Ophthalmology. 2007;114:170–4. doi: 10.1016/j.ophtha.2006.05.072. [DOI] [PubMed] [Google Scholar]
- 36.Flom MC, Neumaier RW. Prevalence of amblyopia. Public Health Rep. 1966;81:329–41. [PMC free article] [PubMed] [Google Scholar]
- 37.Oliver M, Nawratzki I. Screening of pre-school children for ocular anomalies. II. Amblyopia: prevalence and therapeutic results at different ages. Br J Ophthalmol. 1971;55:467–71. doi: 10.1136/bjo.55.7.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross E, Murray AL, Stead S. Prevalence of amblyopia in grade 1 schoolchildren in Saskatoon. Can J Public Health. 1977;68:491–3. [PubMed] [Google Scholar]
- 39.Thompson JR, Woodruff G, Hiscox FA, et al. The incidence and prevalence of amblyopia detected in childhood. Public Health. 1991;105:455–62. doi: 10.1016/s0033-3506(05)80616-x. [DOI] [PubMed] [Google Scholar]
- 40.Lithander J. Prevalence of amblyopia with anisometropia or strabismus among schoolchildren in the Sultanate of Oman. Acta Ophthalmol Scand. 1998;76:658–62. doi: 10.1034/j.1600-0420.1998.760604.x. [DOI] [PubMed] [Google Scholar]
- 41.Kvarnstrom G, Jakobsson P, Lennerstrand G. Screening for visual and ocular disorders in children, evaluation of the system in Sweden. Acta Paediatr. 1998;87:1173–9. doi: 10.1080/080352598750031176. [DOI] [PubMed] [Google Scholar]
- 42.Newman DK, East MM. Prevalence of amblyopia among defaulters of preschool vision screening. Ophthalmic Epidemiol. 2000;7:67–71. [PubMed] [Google Scholar]
- 43.Preslan MW, Novak A. Baltimore Vision Screening Project. Ophthalmology. 1996;103:105–9. doi: 10.1016/s0161-6420(96)30753-7. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez MA, Castro Gonzalez M. Visual health of schoolchildren in Medellin, Antioquia, Colombia [in Spanish] Bol Oficina Sanit Panam. 1995;119:11–4. [PubMed] [Google Scholar]
- 45.Shi L, Stevens GD. Vulnerability and the receipt of recommended preventive services: the influence of multiple risk factors. Med Care. 2005;43:193–8. doi: 10.1097/00005650-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Stevens GD, Seid M, Mistry R, Halfon N. Disparities in primary care for vulnerable children: the influence of multiple risk factors. Health Serv Res. 2006;41:507–31. doi: 10.1111/j.1475-6773.2005.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flores G, Olson L, Tomany-Korman SC. Racial and ethnic disparities in early childhood health and health care. [Accessed July 28, 2007];Pediatrics [online article] 2005 115:e183–93. doi: 10.1542/peds.2004-1474. Available at: http://pediatrics.aappublications.org/cgi/content/full/115/2/e183. [DOI] [PubMed] [Google Scholar]
- 48.Varma R, Deneen J, Cotter S, et al. The Multi-ethnic Pediatric Eye Disease Study: design and methods. Ophthalmic Epidemiol. 2006;13:253–62. doi: 10.1080/09286580600719055. [DOI] [PubMed] [Google Scholar]
- 49.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the Amblyopia Treatment Study visual acuity testing protocol. Am J Ophthalmol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 50.Holmes JM, Beck RW, Repka MX, et al. The Amblyopia Treatment Study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 51.Simons K, Preslan M. Natural history of amblyopia untreated owing to lack of compliance. Br J Ophthalmol. 1999;83:582–7. doi: 10.1136/bjo.83.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robaei D, Rose K, Kifley A, Mitchell P. Patterns of spectacle use in young Australian school children: findings from a population-based study. J AAPOS. 2005;9:579–83. doi: 10.1016/j.jaapos.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Abrahamsson M, Fabian G, Andersson AK, Sjostrand J. A longitudinal study of a population based sample of astigmatic children. I. Refraction and amblyopia. Acta Ophthalmol (Copenh) 1990;68:428–34. doi: 10.1111/j.1755-3768.1990.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 54.Abrahamsson M, Fabian G, Sjostrand J. A longitudinal study of a population based sample of astigmatic children. II. The changeability of anisometropia. Acta Ophthalmol (Copenh) 1990;68:435–40. doi: 10.1111/j.1755-3768.1990.tb01672.x. [DOI] [PubMed] [Google Scholar]
- 55.Burtolo C, Ciurlo C, Polizzi A, et al. Echobiometric study of ocular growth in patients with amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:209–14. doi: 10.3928/0191-3913-20020701-08. [DOI] [PubMed] [Google Scholar]
- 56.Leffertstra LJ. A comparative study of the difference in the evolution of refraction in the two eyes in patients with convergent strabismus [in German] Klin Monatsbl Augenheilkd. 1977;170:74–9. [PubMed] [Google Scholar]
- 57.Smith EL, III, Hung LF, Harwerth RS. Developmental visual system anomalies and the limits of emmetropization. Ophthalmic Physiol Opt. 1999;19:90–102. [PubMed] [Google Scholar]