Abstract
Objectives
To assess clinicopathologic outcomes between two nodal assessment approaches in patients with endometrioid endometrial carcinoma and limited myoinvasion.
Methods
Patients with endometrial cancer at two institutions were reviewed. At one institution, a complete pelvic and para-aortic lymphadenectomy to the renal veins was performed in select cases deemed at risk for nodal metastasis due to grade 3 cancer and/or primary tumor diameter >2 cm (LND cohort). This is a historic approach at this institution. At the other institution, a sentinel lymph node mapping algorithm was used per institutional protocol (SLN cohort). Low risk was defined as endometrioid adenocarcinoma with myometrial invasion <50%. Macrometastasis, micrometastasis, and isolated tumor cells were all considered node-positive.
Results
Of 1135 cases identified, 642 (57%) were managed with an SLN approach and 493 (43%) with an LND approach. Pelvic nodes (PLNs) were removed in 93% and 58% of patients, respectively (P<0.001); para-aortic nodes (PANs) were removed in 14.5% and 50% of patients, respectively (P<0.001). Median number of PLNs removed was 6 and 34, respectively; median number of PANs removed was 5 and 16, respectively (both P<0.001). Metastasis to PLNs was detected in 5.1% and 2.6% of patients, respectively (P=0.03), and to PANs in 0.8% and 1.0%, respectively (P=0.75). The 3-year disease-free survival rates were 94.9% (95%CI, 92.4–97.5) and 96.8% (95%CI, 95.2–98.5), respectively.
Conclusions
Our findings support the use of either strategy for endometrial cancer staging, with no apparent detriment to the SLN algorithm. The clinical significance of disease detected on ultrastaging and the role of adjuvant therapy is yet to be determined.
Keywords: Endometrial carcinoma, sentinel lymph node, sentinel lymph node algorithm, SLN algorithm, comprehensive lymphadenectomy, ultrastaging
Introduction
The value of surgical staging in endometrial carcinoma is a subject of debate; as such, there is no uniform approach to lymph node assessment in clinically uterine-confined disease. Staging strategies range from no lymphadenectomy, with the use of preoperative imaging or intraoperative frozen section to guide management, to comprehensive lymphadenectomy. The results of two randomized trials showed no improvement in disease-free or overall survival for patients with early-stage endometrial carcinoma who had undergone systematic pelvic lymphadenectomy [1,2]. However, in a recent Classification and Regression Tree (CART) analysis, surgical staging, but not the total number of lymph nodes removed, was found to be the most important prognostic factor for overall survival in endometrial cancer [3]. Uterine factors have been shown to be less accurate predictors of recurrence than lymph node status [4,5]. Taken together, assessing nodal status at the time of initial staging in these patients is imperative.
In the landmark study by Creasman et al, pelvic nodal metastasis was found in 5% of patients with superficial myometrial invasion [6]. When taking into consideration the International Federation of Gynecology and Obstetrics (FIGO) 2009 staging system, Chi et al found that 5.5% of patients with endometrioid histology, all grades and myometrial invasion <50%, had nodal metastasis [7]. Despite the low incidence of nodal disease in these low-risk patients, omitting lymphadenectomy from their surgical management would result in inadequate staging, and consequently either preclude administration of adjuvant therapy when warranted or lead to the overtreatment of certain patients. Comprehensive lymphadenectomy is associated with intraoperative complications, such as increased operating time, nerve and vessel injury, higher blood loss, and postoperative morbidity [8]. The rate of long-term lymphedema directly attributed to lymphadenectomy was recently reported to be 23% [9]. The morbidity associated with comprehensive lymphadenectomy is of particular concern in the low-risk population. Sentinel lymph node (SLN) mapping is emerging as an acceptable approach for nodal assessment in endometrial carcinoma. This is reflected by the inclusion of an SLN algorithm in the 2014 National Comprehensive Cancer Network (NCCN) guidelines for the management of endometrial cancer [10]. However, when introducing a novel management strategy, we must take great care not to compromise oncologic outcome or inflict harm on our patients. With this in mind, we conducted the following study, in which we sought to assess and compare the clinicopathologic outcomes between two nodal assessment approaches in patients with “low-risk” endometrial carcinoma as defined by endometrioid histology and limited myoinvasion.
Materials and Methods
Patients with low-risk endometrial cancer at two institutions were identified using the uterine cancer institutional databases at the Mayo Clinic (Mayo) and Memorial Sloan Kettering Cancer Center (MSK). Low risk was defined as endometrioid adenocarcinoma of any grade with myometrial invasion <50%. At one of the institutions, tumor size is not routinely assessed intra-or postoperatively. Therefore, tumor size was not included in our risk classification. From the Mayo Clinic, the historic lymph node dissection (LND) cohort encompassed the years 2004 through 2008. The SLN cohort from MSK encompassed the years 2006 through 2013.
The Mayo Clinic historical surgical algorithm for the study period was to perform a hysterectomy, bilateral salpingo-oophorectomy (BSO), peritoneal cytology, and bilateral pelvic and para-aortic lymphadenectomy. A gynecologic oncology pathologist performed frozen section of the uterine specimen to determine tumor size and depth of myometrial invasion. Lymphadenectomy was omitted in patients with disease of endometrioid histology with any grade or tumor size if there was no myometrial invasion, and in patients with disease of endometrioid histology, grade 1 or 2, with <50% myometrial invasion and tumor diameter ≤2 cm (Supplementary Figure 1) [11]. At MSK, a previously published SLN algorithm was used per institutional protocol (Supplementary Figure 2) [12]. Lymphatic mapping was performed by injecting blue dye or indocyanine green (ICG) into the cervical stroma at superficial and deep levels at the 3- and 9-o’clock positions for a total of 4 mL. Identified SLNs were excised and evaluated by the institutional SLN Pathologic Processing Protocol [13]. Any suspicious nodes were removed regardless of mapping. In cases with no mapping on a hemi-pelvis, a side-specific LND was performed. Para-aortic LND was performed at the surgeon’s discretion.
Macrometastasis, micrometastasis, and isolated tumor cells (ITCs) were all considered node-positive for this analysis. ITCs were defined as those cells measuring ≤0.2 mm, as seen on corresponding hematoxylin and eosin (H&E) sections and not just immunohistochemical (IHC) staining. Micrometastasis was defined as tumor within a lymph node larger than 0.2 mm but less than 2.0 mm in greatest diameter. Notably, when the tumor measurement was not delineated in the pathology report and the terms “isolated tumor cells” and “micrometastasis” were not used, a determination was made based on the pathology report, with clarification from a gynecologic pathologist when needed. For example, “rare scattered tumor cells” were classified as ITCs, whereas “diffuse clusters of cells” were defined as micrometastases. Lymph nodes with a tumor burden ≥2.0 mm were reported as metastatic lymph nodes without further delineation of number or cells or the size of the metastasis. Cytokeratin-positive cells not seen on H&E were considered node-negative. Adjuvant therapy was administered per recommended institutional guidelines.
We compared patient-, treatment-, and disease-specific parameters between cohorts using the chi square or Fisher exact test for categorical variables, the two-sample t test for age and body mass index (BMI), and the Wilcoxon rank-sum test for number of nodes removed and number of positive nodes. Disease-free survival, disease-specific survival, and overall survival were evaluated within the first 3 years after surgery. Survivorship was estimated using the Kaplan-Meier method and compared between the cohorts using the log-rank test. All calculated P values were two-sided, and P values <0.05 were considered statistically significant.
Results
A total of 1135 cases were identified: 642 in the SLN cohort and 493 in the selective LND cohort. Patient and tumor characteristics are shown in Table 1. Patients in the SLN cohort were significantly younger (mean age, 59.6 vs 63.1 years), had an overall lower mean BMI (31.7 vs 35.4 kg/m2), and more likely to not have myometrial invasion (57.0% vs 29.4%) compared with patients in the LND cohort (P<0.001 for each characteristic). The distribution of FIGO grade was similar in the two cohorts. In the SLN cohort, 15.2% of patients had lymphovascular space invasion (LVI) compared with 3.0% in the LND cohort (P<0.001). There are institutional differences in the diagnostic criteria for the presence of LVI.
Table 1.
Patient and tumor characteristics
| Characteristic | SLN cohort N=642 |
LND cohort N=493 |
P |
|---|---|---|---|
| Mean Age, years (SD) | 59.6 (9.9) | 63.1 (11.0) | <0.001 |
| Mean BMI, kg/m2 (SD) | 31.7 (8.4) | 35.4 (9.9) | <0.001 |
| Final FIGO grade, N (%) | 0.69 | ||
| 1 | 450 (70.1) | 336 (68.2) | |
| 2 | 146 (22.7) | 123 (24.9) | |
| 3 | 46 (7.2) | 34 (6.9) | |
| Myometrial invasion, N (%) | <0.001 | ||
| None | 366 (57.0) | 145 (29.4) | |
| >0 - <50% | 276 (43.0) | 348 (70.6) | |
| Lymphovascular space invasion, N (%) | <0.001 | ||
| No | 523/617 (84.8) | 478 (97.0) | |
| Yes | 94/617 (15.2) | 15 (3.0) | |
| Cervical invasion, N (%) | 0.38 | ||
| No invasion | 624 (97.2) | 485 (98.4) | |
| Mucosa | 13 (2.0) | 5 (1.0) | |
| Cervical stroma | 5 (0.8) | 3 (0.6) | |
| Peritoneal cytology, N (%) | <0.001 | ||
| Negative | 550 (85.7) | 370 (75.1) | |
| Positive | 68 (10.6) | 30 (6.1) | |
| Not sampled | 24 (3.7) | 93 (18.9) | |
| 1988 FIGO stage, N (%) | <0.001 | ||
| IA | 341 (53.1) | 139 (28.2) | |
| IB | 192 (29.9) | 308 (62.5) | |
| IC | - | - | |
| IIA | 2 (0.3) | 4 (0.8) | |
| IIB | 6 (0.9) | 1 (0.2) | |
| IIIA | 65 (10.1) | 25 (5.1) | |
| IIIC | 36 (5.6) | 14 (2.8) | |
| IVB | - | 2 (0.4) | |
| 2009 FIGO stage, N (%) | 0.02 | ||
| IA | 595 (92.7) | 471 (95.5) | |
| IB | - | - | |
| II | 8 (1.2) | 2 (0.4) | |
| IIIA/B | 3 (0.5) | 4 (0.8) | |
| IIIC1 | 31 (4.8) | 9 (1.8) | |
| IIIC2 | 5 (0.8) | 5 (1.0) | |
| IV | - | 2 (0.4) |
SLN, sentinel lymph node; LND, lymph node dissection; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; SD, standard deviation.
Pelvic lymph nodes were excised in 92.8% of the patients in the SLN cohort compared with 57.8% in the LND cohort (P<0.001; Table 2). Among those who underwent pelvic nodal assessment, the median number of pelvic lymph nodes excised per patient was 6 (interquartile range [IQR]: 3,11) in the SLN cohort versus 34 (IQR: 26,45) in the LND cohort (P<0.001). Positive pelvic lymph nodes were detected in 5.1% (33/642; 95% CI, 3.4–6.9%) and 2.6% (13/493; 95% CI, 1.2–4.1%) of patients, respectively (P=0.03). These last percentages are not identical to the detection rate of stage IIIC1 since some patients with positive pelvic lymph nodes also had positive para-aortic lymph nodes and belong in stage IIIC2. The median number of positive pelvic nodes per patient among those patients with positive pelvic nodes was 1 (IQR: 1,2) for both groups.
Table 2.
Lymphadenectomy characteristics and adjuvant therapy
| SLN cohort N=642 |
LND cohort N=493 |
P | |
|---|---|---|---|
| Pelvic LND, N (%) | <0.001 | ||
| No | 46 (7.2) | 208 (42.2) | |
| Yes | 596 (92.8) | 285 (57.8) | |
|
Median number of pelvic nodes removed among patients undergoing pelvic LND (IQR) |
6 (3, 11) | 34 (26, 45) | <0.001 |
| Positive pelvic nodes, N (%) | 0.03 | ||
| No or pelvic LND not performed | 609 (94.9) | 480 (97.4) | |
| Yes | 33 (5.1) | 13 (2.6) | |
|
Positive pelvic nodes among patients undergoing pelvic LND, N (%) |
0.54 | ||
| No | 563/596 (94.5) | 272/285 (95.4) | |
| Yes | 33/596 (5.5) | 13/285 (4.6) | |
| Para-aortic LND, N (%) | <0.001 | ||
| No | 549 (85.5) | 248 (50.3) | |
| Yes | 93 (14.5) | 245 (49.7) | |
|
Median number of para-aortic nodes removed among patients undergoing para- aortic LND (IQR) |
5 (3, 8) | 16 (11, 23) | <0.001 |
| Positive para-aortic nodes, N (%) | 0.75 | ||
| No or para-aortic LND not performed | 637 (99.2) | 488 (99.0) | |
| Yes | 5 (0.8) | 5 (1.0) | |
|
Positive para-aortic nodes among patients undergoing para-aortic LND, N (%) |
0.15 | ||
| No | 88/93 (94.6) | 240/245 (98.0) | |
| Yes | 5/93 (5.4) | 5/245 (2.0) |
SLN, sentinel lymph node; LND, lymph node dissection; IQR, interquartile range
Para-aortic nodes were excised in 14.5% of the patients in the SLN cohort compared with 49.7% in the LND cohort (P<0.001). Among those who underwent para-aortic nodal assessment, the median number of para-aortic lymph nodes excised was 5 (IQR: 3,8) in the SLN cohort versus 16 (IQR: 11,23) in the LND cohort (P<0.001). Positive para-aortic lymph nodes were detected in 0.8% (5/642; 95% CI, 0.1–1.5%) and 1.0% (5/493; 95% CI, 0.1–1.9%) of patients, respectively (P=0.75). Among the patients with positive para-aortic nodes (5 in each cohort), 4 in the SLN cohort had a single positive node and 1 had 2 positive nodes; in the LND cohort, 2 patients had 1 positive node, 1 had 3 positive nodes, and 2 had 5 positive nodes. Of the 36 cases with positive pelvic or para-aortic nodes in the SLN cohort, 11 were positive by macrometastasis, 2 by micrometastasis, and 23 by ITCs (Supplementary Table 1).
Adjuvant therapy was administered to 27.1% of patients in the SLN cohort and 10.8% of patients in the LND cohort (P<0.001). Intravaginal brachytherapy (IVRT) alone was administered to 17.6% of patients in the SLN cohort vs 6.1% in the LND cohort (P<0.001). The various combinations of adjuvant therapy are shown in Table 3.
Table 3.
Adjuvant therapy
| SLN cohort N=642 |
LND cohort N=493 |
P | |
|---|---|---|---|
| Adjuvant therapy, N (%) | <0.001 | ||
| No adjuvant therapy | 466 (72.6) | 432 (87.6) | |
| IVRT only | 113 (17.6) | 30 (6.1) | |
| EBRT ± IVRT | 6 (0.9) | 3 (0.6) | |
| Chemotherapy ± IVRT | 30 (4.7) | 13 (2.6) | |
| Chemotherapy and EBRT ± IVRT | 17 (2.6) | 7 (1.4) | |
| Hormones | 8 (1.2) | - | |
| Unknown | 2 (0.3) | 8 (1.6) |
SLN, sentinel lymph node; LND, lymph node dissection; IVRT, intravaginal brachytherapy; EBRT, external beam radiation therapy
The median follow-up time was 2.1 years (IQR: 1.2,3.3) in the SLN cohort compared to 3.5 years (IQR: 2.5,4.9) in the LND cohort (P<0.001). Disease-free survival, disease-specific survival, and overall survival were evaluated within the first 3 years following surgery. There were 19 recurrences within 3 years in the SLN cohort and 14 recurrences within 3 years in the selective LND cohort. Of these, there were 2 isolated lymph node recurrences within each cohort, yielding a 3-year isolated nodal-free recurrence of 99.6% in both cohorts. The nodal recurrences in the SLN cohort occurred in an 83-year-old patient with FIGO 2009 stage IA disease, a grade 1 tumor with no LVI, negative peritoneal washings, and 6 normal pelvic nodes. She recurred 5 months postoperatively in her aortocaval and right common iliac lymph nodes. The second patient was a 61-year-old with stage IIIC1 disease, a grade 1 tumor, and positive LVI who received adjuvant IVRT and chemotherapy. She recurred 16 months postoperatively with presacral adenopathy. The isolated nodal recurrences in the LND cohort occurred in a 63-year-old patient with FIGO 2009 stage IIIC2 disease, a grade 1 tumor, no LVI, no cervical invasion, and negative peritoneal cytology who was administered chemotherapy ± IVRT; nodal recurrence was documented 8 months later. And in a 50-year-old patient with FIGO 2009 stage IIIC1 disease, a grade 2 tumor, no LVI, mucosa cervical invasion, and negative peritoneal cytology who received EBRT ± IVRT; this patient recurred 7 months later.
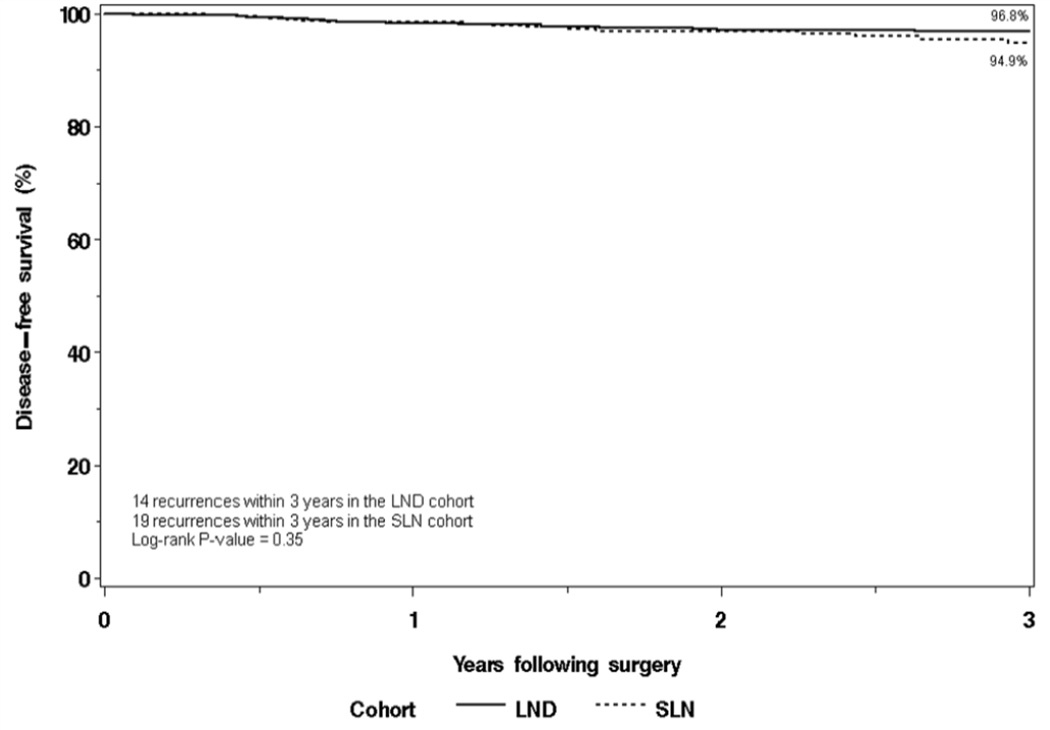
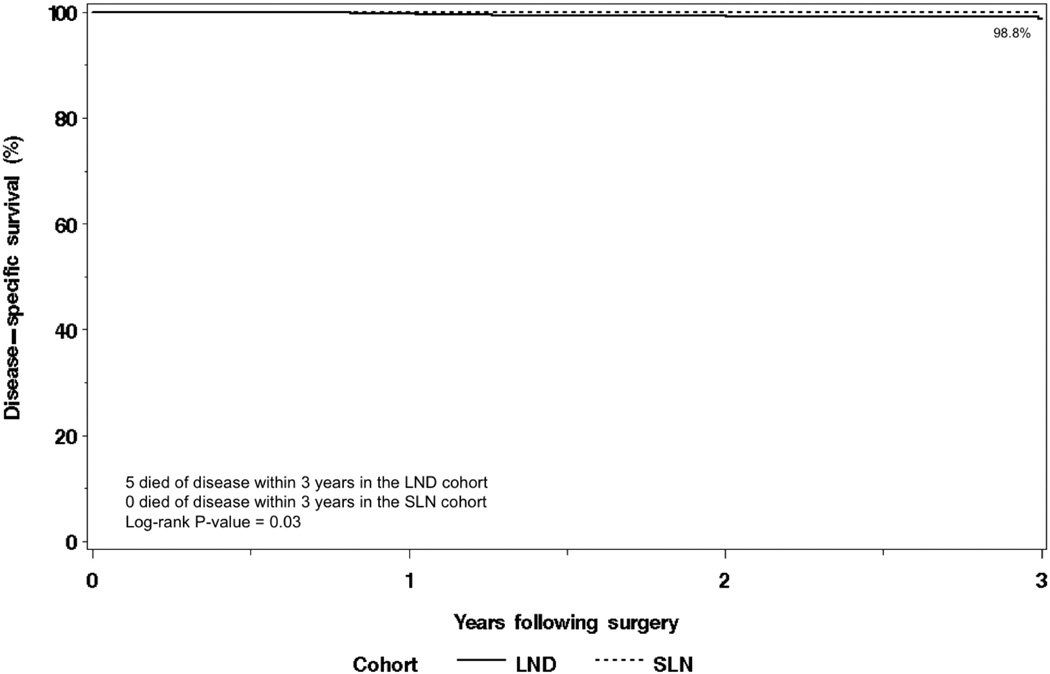
The distribution of recurrences for both cohorts is shown in Table 4. The 3-year disease-free survival rate was 94.9% (95% confidence interval [CI], 92.4–97.5) in the SLN cohort versus 96.8% (95% CI 95.2–98.5) in the selective LND cohort (Figure 1). There were 9 deaths within the first 3 years following surgery in the SLN cohort, none of which were due to disease. There were 20 deaths within the first 3 years following surgery in the LND cohort, 5 of which were due to disease. Overall survival was not significantly different between the two cohorts (P=0.07); the 3-year overall survival rates were 97.4% (95% CI, 95.7–99.2%) and 95.4% (95% CI, 93.4–97.4%) in the SLN and LND cohorts, respectively. Disease-specific survival was significantly different between the two cohorts (P=0.03; Figure 2); the 3-year disease-specific survival rates were 100% and 98.8% (95% CI, 97.7–99.9), respectively. Multivariate analyses adjusting for other features was not possible due to the small number of disease-related deaths in both cohorts (0 vs 5).
Table 4.
Recurrences
| SLN cohort N=642 |
LND cohort N=493 |
P | |
|---|---|---|---|
|
Disease progression/recurrence within 3 years, N (%) |
19 (3.0) | 14 (2.8) | 0.35 |
| 3-year disease-free survival, % (95% CI) | 94.9 (92.4–97.5) | 96.8 (95.2–98.5) | |
| Route of first recurrence, N | |||
| Hematogenous only | 4 | - | |
| Hematogenous and lymphatic | - | 1 | |
| Hematogenous and peritoneal | 1 | 1 | |
| Hematogenous, lymphatic and peritoneal | 1 | 1 | |
| Lymphatic only | 2 | 2 | |
| Lymphatic and peritoneal | 1 | - | |
| Vaginal only | 9 | 4 | |
| Vaginal and peritoneal | - | 1 | |
| Peritoneal only | 1 | 4 |
SLN, sentinel lymph node; LND, lymph node dissection
Figure 1.
Disease-free survival.
Figure 2.
Disease-specific survival.
Discussion
There is no uniform practice for surgical staging of uterine-confined endometrial carcinoma. Patients with <50% myometrial invasion have a low risk of nodal involvement [7]; therefore, a comprehensive lymphadenectomy may not be necessary in this patient population considering the associated morbidity [8]. Neither the ASTEC trial nor a study by Panici and colleagues showed a benefit of lymphadenectomy on overall survival [1,2]; however, both studies have been extensively criticized. Nearly 50% of the patients in the LND arm of the ASTEC trial had no or ≤9 lymph nodes removed, and 16% of the patients in the non-LND arm in the study by Panici et al had ≥6 lymph nodes removed. Furthermore, both studies allowed bulky nodes to be removed in the non-LND arms, para-aortic LND was left up to the surgeons’ discretion, and there was an unequal distribution of high-risk patients and of adjuvant therapy between the study arms, which was not taken into account in their final analysis.
In most institutions, low-risk endometrial cancer is a postoperative diagnosis based on final pathology. The goal of staging women with uterine-confined endometrial cancer is to do as little harm as possible intraoperatively while evaluating nodal status adequately. Such evaluation is important to allow for the tailoring of adjuvant therapy, as uterine factors have been shown to be less accurate predictors of recurrence compared with lymph node status [4,5]. The Mayo Clinic has published extensively on their approach to intraoperative frozen section to triage patients to no lymphadenectomy or comprehensive lymphadenectomy [14,15]. In particular, the intraoperative utilization of tumor diameter, in combination with frozen section, can help select patients (27% of the total endometrial cancer population) who can potentially avoid surgical staging [14]. Moreover, even in the absence of frozen section, the intraoperative use of tumor diameter may select those patients with preoperative endometrioid grade 1 or 2 cancer who may not benefit from surgical staging [16]. A French prospective multicenter study has shown that SLN biopsy can accurately diagnose lymph node involvement in patients with low-risk or intermediate-risk endometrial cancer [17], as have other prospective and retrospective studies [18–20]. It is of great importance to adhere to a surgical SLN algorithm to minimize the false-negative rate when applying the SLN technique [20]. The SENTI-ENDO study’s long-term results support the relevance of SLN biopsy on surgical management, as well as on indications for adjuvant therapies [5].
In our study, we were able to retrospectively compare the historical Mayo LND algorithm to the MSK institutional SLN algorithm [12]. We found that for this low-risk cohort, the SLN algorithm results in significantly more patients having pelvic nodes excised, a lower number of lymph nodes removed per patient, and a higher detection rate in FIGO 2009 stage IIIC1 disease. Theoretically, SLN mapping should reduce the risk of lower extremity lymphedema, but this has not yet been objectively assessed. When looking at para-aortic nodes, the SLN algorithm results in fewer patients having nodes removed from this anatomic basin, a lower number of nodes excised per patient, and a similar detection rate in stage IIIC2 disease compared with the Mayo LND algorithm. Interestingly, the median number of positive pelvic nodes was the same with both approaches (1; IQR: 1,2), suggesting that the SLN approach is able to identify the positive pelvic lymph node in cases with FIGO 2009 stage IIIC1 disease, and that it is an adequate method for surgical staging. In fact, isolated paraaortic metastases, with negative pelvic lymph nodes, are extremely rare in patients with endometrioid cancer and limited myometrial invasion [21]. For the para-aortic nodes, the median number of positive nodes was 1 versus 3, respectively, suggesting that in those patients with a positive para-aortic lymph node, there may be residual nodal metastasis with the SLN approach. The importance of this latter finding on disease-free survival and overall survival is unclear, as these patients will receive tailored adjuvant therapy.
The differences between cohorts in age and BMI may have influenced the disease-specific survival in disfavor of the LND cohort. The disproportion of patients without myometrial invasion between the groups may also have played a favorable role for the SLN cohort. However, the SLN cohort had a significantly higher number of patients with LVI, which is a negative predictive factor. There is institutional variation in the pathologic criteria for the presence of LVI, which limits the interpretation of this finding. Regardless, disease-specific survival was excellent in both cohorts, with a small number of patients dying from their disease. Adjusting for other factors was not possible because of this low number of deaths. At a minimum, this favorable disease-specific survival outcome in the SLN cohort provides additional reassurance that an SLN mapping algorithm will not lead to unnecessary deaths due to false-negative staging. This finding would have to be proven in a randomized trial.
The SLN cohort received significantly more adjuvant therapy, mainly IVRT, but also more chemotherapy with or without radiation therapy, compared with the LND cohort. When we excluded the use of IVRT, however, differences in adjuvant treatment were minimal. There may be institutional differences in guiding the administration of adjuvant therapy. Irrespective of this, the higher number of patients with documented LVI in the MSK cohort was responsible for the increased administration of IVRT, and the increased use of chemotherapy with or without radiation therapy corresponds to the higher number of patients with detected stage IIIC disease. It is extremely important to consider the potential added morbidity of any adjuvant therapies, especially in the presence of low-volume metastases in a low-risk cohort of endometrial cancer patients.
Twenty-five of 36 patients with stage IIIC disease in the SLN cohort were staged based on findings of low-volume metastasis. The clinical significance of low-volume metastasis and how it should guide adjuvant therapy remains uncertain. However, in a retrospective case-control surgicopathological study of women with early-stage cervical cancer, low-volume metastases were found to be an important risk factor of tumor recurrence in patients with no apparent lymph node metastasis [22]. In other studies, immunohistochemical expression of cytokeratin in lymph nodes with undetected metastasis predicted occult metastasis and was a risk factor for recurrence in early-stage endometrial cancer [23,24]. These findings, though limited in numbers, support the removal of low-volume metastasis and adjuvant treatment for these patients. However, in all these reported cases with micrometastases, recurrences occurred when the primary cancers presented with high-risk characteristics and may not specifically apply to our series of low-risk patients [23,25]. The optimal management of patients with ITCs and micrometastasis in endometrial cancer remains to be determined.
There are limitations to this retrospective study. It is a comparison between two institutions with two sets of pathologists evaluating the specimens; this may have influenced the discrepancy in reported uterine characteristics, such as the large difference in the number of tumors with LVI. Tumor size was not available in the SLN cohort, and subsequently the comparison of this subgroup could not be performed. Another limitation is the lack of standardized postoperative treatment approaches both between the two institutions and within each one. This heterogeneity could likely have affected our survival outcomes. The median follow-up of the SLN cohort was significantly shorter than that of the LND cohort. However, both follow-up times were >24 months, and the majority of recurrences will occur during this period, and the evaluation of outcomes in this analysis was limited to the first 36 months [25,26].
It is important to recognize that the Mayo Clinic algorithm described here is historical, and it has evolved since the study time period in this report. In 2009, the Mayo Clinic modified their protocol, leading to fewer patients requiring a para-aortic lymphadenectomy. In brief, a pelvic and para-aortic lymphadenectomy is performed in patients with either deep (>50%) myoinvasion or Type 2 carcinoma. In cases without either of these features, a pelvic lymphadenectomy is performed if any one of 3 other features are found (cervical invasion, FIGO grade 3 (any myoinvasion), or tumor diameter >2 cm). A para-aortic lymphadenectomy is reserved only in these cases if they are found to have pelvic nodal metastases. SLN mapping has been increasingly incorporated since 2013 [27].
In conclusion, when comparing these two approaches to surgical staging of low-risk endometrial carcinoma, we found that pelvic lymph nodes were excised in a larger proportion of patients when applying an SLN algorithm versus a selective LND algorithm; however, fewer lymph nodes were removed per patient with the SLN algorithm, the algorithm yielded a higher detection rate in stage IIIC1 disease, and the median number of positive pelvic nodes per patient was the same. For stage IIIC2 disease, both algorithms achieved the same detection rate. The application of an SLN algorithm does not appear to compromise oncologic outcomes within this short follow-up. Our findings strongly support the use of an SLN mapping algorithm, instead of a comprehensive lymphadenectomy, in patients with endometrioid endometrial cancer and myometrial invasion <50%. Of note, patients with grade 1 or 2 cancer and tumor diameter of 2 cm or less may be able to avoid any type of nodal assessment if they can be reliably identified pre- and intraoperatively. The clinical significance of disease detected on ultrastaging and the role of adjuvant therapy in these cases is yet to be determined. Prospective assessment of the SLN algorithm is needed and currently underway [28].
Supplementary Material
Research Highlights.
SLN algorithm in low-risk endometrial carcinoma allows for lymph node assessment in more patients
Fewer lymph nodes are removed in patients undergoing an SLN approach vs selective lymphadenectomy
Oncologic outcomes are similar for SLN algorithm compared to selective lymphadenectomy
Acknowledgments
Financial Support
Supported in part by the MSK Cancer Center Support Grant P30 CA008748
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
References
- 1.Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial) Lancet. 2009;373:125–136. doi: 10.1016/S0140-6736(08)61766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedetti Panici P, Basile S, Maneschi F, Alberto Lissoni A, Signorelli M, Scambia G, et al. Systematic pelvic lymphadenectomy vs no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100:1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 3.Barlin J, Zhou Q, St Clair CM, Iasonos A, Soslow RA, Alektiar KM, et al. Classification and regression tree (CART) analysis of endometrial carcinoma: Seeing the forest for the trees. Gynecol Oncol. 2013;130:452–456. doi: 10.1016/j.ygyno.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nugent EK, Bishop EA, Mathews CA, Moxley KM, Tenney M, Mannel RS, et al. Do uterine risk factors or lymph node metastasis more significantly affect recurrence in patients with endometrioid adenocarcinoma? Gynecol Oncol. 2012;125:94–98. doi: 10.1016/j.ygyno.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 5.Darai E, Dubernard G, Bats AS, Heitz D, Mathevet P, Marret H, et al. Sentinel node biopsy for the management of early stage endometrial cancer: Long-term results of the SENTI-ENDO study. Gynecol Oncol. 2015;136:54–59. doi: 10.1016/j.ygyno.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical Pathologic Spread Patterns of Endometrial Cancer A Gynecologic Oncology Group Study. Cancer. 1987;60:2035–2041. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Chi DS, Barakat RR, Palayekar MJ, Levine DA, Sonoda Y, Alektiar K, et al. The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int J Gynecol Cancer. 2008;18:269–273. doi: 10.1111/j.1525-1438.2007.00996.x. [DOI] [PubMed] [Google Scholar]
- 8.Dowdy SC, Borah BJ, Bakkum-Gamez JN, Weaver AL, McGree ME, Haas LR, et al. Prospective assessment of survival, morbidity, and cost associated with lymphadenectomy in low-risk endometrial cancer. Gynecol Oncol. 2012;127:5–10. doi: 10.1016/j.ygyno.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124:307–315. doi: 10.1097/AOG.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology. Uterine Neoplasms. Version 2. 2015 Available at: http://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 11.Mariani A, Dowdy SC, Keeney GL, Long HJ, Lesnick TG, Podratz KC. High-risk endometrial cancer subgroups: candidates for target-based adjuvant therapy. Gynecol Oncol. 2004;95:120–126. doi: 10.1016/j.ygyno.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 12.Barlin JN, Khoury-Collado F, Kim CH, Leitao MM, Jr, Chi DS, Sonoda Y, et al. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol Oncol. 2012;125:531–535. doi: 10.1016/j.ygyno.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic ultrastaging improves micrometastases detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–970. doi: 10.1097/IGC.0b013e3182954da8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: A paradigm shift in surgical staging. Gynecol Oncol. 2008;109:11–18. doi: 10.1016/j.ygyno.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-risk corpus cancer: Is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol. 2000;182:1506–1519. doi: 10.1067/mob.2000.107335. [DOI] [PubMed] [Google Scholar]
- 16.AlHilli MM, Podratz KC, Dowdy SC, Bakkum-Gamez JN, Weaver AL, McGree ME, Kumar S, Keeney GL, Cliby WA, Mariani A. Preoperative biopsy and intraoperative tumor diameter predict lymph node dissemination in endometrial cancer. Gynecol Oncol. 2013;128:294–299. doi: 10.1016/j.ygyno.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: a prospective multicenter study (SENTI-ENDO) Lancet Oncol. 2011;12:469–476. doi: 10.1016/S1470-2045(11)70070-5. [DOI] [PubMed] [Google Scholar]
- 18.How J, Lau S, Press J, Ferenczy A, Pelmus M, Stern J, et al. Accuracy of sentinel lymph node detection following intra-operative cervical injection for endometrial cancer: A prospective study. Gynecol Oncol. 2012;127:332–337. doi: 10.1016/j.ygyno.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Jewell EL, Huang JJ, Abu-Rustum NR, Gardner GJ, Brown CL, Sonoda Y, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol. 2014;133:274–277. doi: 10.1016/j.ygyno.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinno AK, Fader AN, Roche KL, Giuntoli RL2nd, Tanner EJ. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–286. doi: 10.1016/j.ygyno.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S, Podratz KC, Bakkum-Gamez JN, Dowdy SC, Weaver AL, McGree ME, et al. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol Oncol. 2014;132:38–43. doi: 10.1016/j.ygyno.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchiolé P, Buénerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lympho vascular space involvement and lymph node micrometastases I early-stage cervical cancer: A retrospective case-control surgico-pathological study. Gynecol Oncol. 2005;97:727–732. doi: 10.1016/j.ygyno.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez Bosquet J, Keeney GL, Mariani A, Webb MJ, Cliby WA. Cytokeratin staining of resected lymph nodes may improve the sensitivity of surgical staging for endometrial cancer. Gynecol Oncol. 2003;91:518–525. doi: 10.1016/j.ygyno.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Yabushita H, Shimazu M, Yamada H, Sawaguchi K, Noguchi M, Nakanishi M, et al. Occult Lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in node-negative endometrial cancer. Gynecol Oncol. 2001;80:139–144. doi: 10.1006/gyno.2000.6067. [DOI] [PubMed] [Google Scholar]
- 25.Todo Y, Kato H, Okamoto K, Minobe S, Yamashiro K, Sakuragi N. Isolated tumor cells and micrometastases in regional lymph nodes in FIGO stage I to II endometrial cancer. J Gynecol Oncol. 2015 doi: 10.3802/jgo.2016.27.e1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greer BE, Goff BA, Koh WJ. Endometrial carcinom a. In: Johnson FE, Virgo KS, editors. Cancer Patient Follow-up. St. Louis: Mosby; 1997. pp. 357–377. [Google Scholar]
- 27.Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Rossetti D, Mariani A. Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: current evidence. J Obstet Gynaecol Res. 2014;40:301–311. doi: 10.1111/jog.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi EC, Boggess JF. NLM Identifier: NCT01673022. ClinicalTrials.gov. Bethesda: National Library of Medicine; 2015. Fluorescence imaging for robotic endometrial cancer sentinel node mapping (FIRES) trial. Available at: https://clinicaltrials.gov/show/NCT01673022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.