Abstract
Many circuits in the mammalian brain are organized in a topographic or columnar manner. These circuits could be activated—in ways that reveal circuit function or restore function after disease—by an artificial stimulation system that is capable of independently driving local groups of neurons. Here we present a simple custom microscope called ProjectorScope 1 that incorporates off-the-shelf parts and a liquid crystal display (LCD) projector to stimulate surface brain regions that express channelrhodopsin-2 (ChR2). In principle, local optogenetic stimulation of the brain surface with optical projection systems might not produce local activation of a highly interconnected network like the cortex, because of potential stimulation of axons of passage or extended dendritic trees. However, here we demonstrate that the combination of virally mediated ChR2 expression levels and the light intensity of ProjectorScope 1 is capable of producing local spatial activation with a resolution of ∼200–300 μm. We use the system to examine the role of cortical activity in the experience-dependent emergence of motion selectivity in immature ferret visual cortex. We find that optogenetic cortical activation alone—without visual stimulation—is sufficient to produce increases in motion selectivity, suggesting the presence of a sharpening mechanism that does not require precise spatiotemporal activation of the visual system. These results demonstrate that optogenetic stimulation can sculpt the developing brain.
Keywords: optogenetics, activity-dependent plasticity, direction selectivity
a fundamental mystery of neuroscience is to understand how networks of neurons assemble and function in circuits to enable perception and behavior. Over the last decades, correlational studies have provided the bulk of our understanding of neural circuits in intact animals: a stimulus is delivered to an animal or a behavior is observed, a response is measured in the brain, and models are made of how the stimulus might be processed (Barlow and Levick 1965; Hubel and Wiesel 1963; O'Keefe 1976). A minority of studies have manipulated neural systems, either by silencing or cooling (e.g., Crook et al. 1991; Ferster et al. 1996; Kalil and Chase 1970; Long and Fee 2008; Nelson et al. 1994) or by stimulating a column with an electrode (Penfield and Jasper 1954; Salzman et al. 1990). Now optogenetic tools are enabling real-time manipulation of circuit elements (Boyden et al. 2005). These tools give us the ability to test specific hypotheses about neural circuit development and function.
Up to now, a majority of in vivo studies employing optogenetic methods have stimulated specific cell types with laser- or LED-driven optical fibers (Aravanis et al. 2007; Arenkiel et al. 2007; Cardin et al. 2009). These methods allow groups of neurons to be switched on or off with great temporal fidelity, but all of the stimulated neurons are modulated together. For many subcortical nuclei, simultaneous manipulations of all neurons of a particular subtype may be sufficient to test hypotheses about function or to therapeutically restore function via optogenetic prostheses.
To manipulate circuits at finer scales, a few groups have developed optical projection methods [using digital mirror device displays, liquid crystal on silicon (LCOS) spatial light modulators (SLMs), or custom-fabricated devices] for stimulating individual cells within small regions of circuits, often in vitro (Grossman et al. 2010; Munch et al. 2009; Nikolenko et al. 2008; Sakai et al. 2013; Watson et al. 2010) but also in vivo (Leifer et al. 2011; Packer et al. 2012, 2015; Rickgauer et al. 2014; Wilson et al. 2012). These devices offer promise for dissecting the role of individual cell types or individual neurons in circuit computation, although one has the burden of deciding which individual cells to stimulate.
Yet much of what we want to know about perception and behavior, or what we want to restore after disease or damage, will require the manipulation of brain circuits on a larger scale—a scale that spans a substantial portion of the representation of a sensory modality or even multiple sensory modalities. Conveniently, most primary and even secondary sensory areas are organized topographically: visual cortex is organized retinotopically, auditory cortex is organized tonotopically, somatosensory cortex is organized somatotopically, and the olfactory bulb is organized into glomeruli with distinct responses. In several mammalian species there is additional segregation of neurons into functional maps, such as the orientation- and direction-selective maps, ocular dominance patches, and cytochrome oxidase patches in visual cortex (Horton and Adams 2005). All of these structures provide a spatially organized substrate through which to address the nervous system with light. Some recent studies have taken advantage of this architecture by combined laser scanning stimulation of caged glutamate (Davison and Ehlers 2011) or optical projection onto the brain surface of animals expressing channelrhodopsin-2 (ChR2) (Dhawale et al. 2010; Huang et al. 2014; Stirman et al. 2012; Zhu et al. 2012).
Here we present a relatively simple custom microscope for in vivo spatial and temporal activation of cortical circuits at the level of columns. This “ProjectorScope 1” includes the ability to perform basic brain surface microscopy such as epifluorescence imaging and intrinsic signal imaging. When combined with viral expression of ChR2, we show that the projection system can drive robust neural activity with spatial specificity on the order of 200–300 μm. We show that the system can produce temporally modulated neural firing that matches our liquid crystal display (LCD) projector's temporal capabilities. With prolonged stimulation, the cortical neurons do not respond randomly but exhibit resonance at gamma frequencies.
As an example application of this device, we test hypotheses about how cortical circuits contribute to the emergence of motion selectivity in the developing ferret visual cortex. Motion processing requires that the cortex respond selectively to signals that vary in both space and time. Motion selectivity is very weak at the time of eye opening but develops rapidly with visual experience with a motion stimulus (Li et al. 2006, 2008; Van Hooser et al. 2012). Here we use the ProjectorScope 1 to explore whether direct stimulation of the cortex alone, without stimulation of the retina or direct stimulation of the lateral geniculate nucleus (LGN), can cause the emergence of direction selectivity. Second, we examine whether mimicking a moving image—by sweeping an optogenetic stimulus across the cortical surface, which sequentially activates horizontal connections—can cause the emergence of direction selectivity, as predicted in some models (Shon et al. 2004; Wenisch et al. 2005). We find that general activation of the cortical surface causes an increase in direction selectivity but a sweeping cortical stimulus does not influence the eventual direction preference that is acquired. These experiments suggest that direct activation of the cortex—without spatiotemporally specific activation of the retina or LGN—is sufficient to amplify weak initial direction biases into robust direction-selective responses.
METHODS
General design.
All experimental procedures were approved by the Brandeis University Animal Care and Use Committee and performed in compliance with National Institutes of Health guidelines. Ferrets [Mustela putorius furo; n = 21, age at time of terminal experiment postnatal day (P)30–36] were split into two primary study groups. Experimental animals (n = 15) were virally transfected with ChR2 at P19-23 and, after a recovery period of 10–14 days, used in a terminal experiment involving optogenetic activation and electrophysiological recordings. Control animals (n = 6, age P30-35) were used directly for electrophysiological recordings without ChR2 expression. The experimental group of animals was further split into two subgroups: the first group (n = 3, age P35-36) was used to test the spatiotemporal properties of the ProjectorScope 1, and the second group (n = 12, age P31-36) was used for cortical stimulation experiments designed to influence visual direction selectivity, with either optical constant stimuli (n = 4, age P31-36) or optical motion stimuli (drifting gratings; n = 8, age P32-36).
Viral expression of ChR2 in ferret V1.
ChR2 was expressed in ferret primary visual cortex by transfection with the virus AAV2/9-CaMKIIa-hChR2 (E123T/T159C)-EYFP-hGH (Berndt et al. 2011) obtained from the UPenn Vector Core. During injection surgeries, ferrets were intramuscularly (im) administered the analgesics ketoprofen (1 mg/kg) and tramadol (2–5 mg/kg), anesthetized with im injection of a ketamine-xylazine cocktail (20–30 mg/kg and 2–3 mg/kg, respectively), and given atropine (0.04 mg/kg) to reduce secretions. The animals' heads were shaved, sterilized with alternate Betadine and 70% isopropanol wipes, and held in a stereotactic device with ear bars and a bite bar. The temperature was continuously monitored and controlled by an FST TR-200 controller, heart rate was continuously monitored, and hydration was maintained throughout by subcutaneous injections of Ringer solution (3 ml·kg−1·h−1). Surgical margins were infused with 100–200 μl of bupivacaine. The cranium was exposed and a small craniotomy made over visual cortex. A very small durotomy was made with a 31-gauge needle on a cotton tip applicator. Glass injection micropipettes were pulled on a gravity-assisted puller, manually broken to size (20- to 30-μm tip diameter), and beveled (22°) with a Narishige beveler. With a volume injector (Nanoject, Drummond Scientific), 1–2 μl of virus solution (6.3 × 1012 genomes/ml) was delivered at two or three different depths (between 150 and 500 μm from the brain surface). The total volume was injected in small steps of 20–30 nl with 10- to 15-s rests between injections. Then the scalp incision was closed with nylon sutures, and Neosporin was applied. After the animals were ambulatory, they were transferred back to the cage with the rest of the litter. Analgesics were administered up to 48 h after surgery.
Electrophysiology.
Ferrets were sedated with ketamine (20 mg/kg im). Atropine (0.16–0.8 mg/kg im) and dexamethasone (0.5 mg/kg im) were administered to reduce bronchial and salivary secretion and to reduce inflammation, respectively. The animal was anesthetized with a mixture of isoflurane, oxygen, and nitrous oxide through a mask while a tracheostomy was performed. Animals were ventilated with 1.5–3% isoflurane in a 2:1 mixture of nitrous oxide and oxygen. A cannula was inserted into the intraperitoneal (ip) cavity for delivery of neuromuscular blockers and Ringer solution (3 ml·kg−1·h−1), and the animal was inserted in a custom stereotaxic frame that did not obstruct vision. All wound margins were infused with bupivacaine. Silicone oil was placed on the eyes to prevent corneal damage. A craniotomy (4 × 4 mm) was made in the right hemisphere, and the dura was removed with a 31-gauge needle. Black aluminum foil was glued to the animal's head, above the eyes, to block the projector light from reaching the eyes. The light block was carefully tested with bright light while recording in visual cortex (Fig. 1E).
Fig. 1.
ProjectorScope 1. A: schematic. Projector path (dashed cyan lines): the image of a liquid crystal display (LCD) projector is brought into focus on the surface of the brain by a pair of lenses (L1 and L2) and a ×4 or ×10 objective (OBJ). A dichroic mirror (DM) reflects light of certain wavelengths while permitting light of other wavelengths to be imaged at the CCD camera (CCD) via a tube lens (TL). When the projector was driven at its maximum luminance on the blue channel, the irradiance measured at the objective ranged from 8 to 11 mW/mm2. Brightfield path (solid yellow lines): external light (LIGHT) is provided to permit brightfield imaging of the brain surface. Epifluorescence path (short-dashed green and yellow lines): for epifluorescence imaging, the projector dichroic mirror is removed, and a new epifluorescence dichroic mirror is installed (DM, gray). A halide light source (via a liquid light guide, LLG) is used along with excitation (EX) and emission (EM) filters to perform epifluorescence microscopy. B: test image (Boston Red Sox logo) (using a half-mirror for DM) on a US dime, illustrating minification of the projector image. C: epifluorescence yellow fluorescent protein (YFP) image of the brain surface, showing area with strong channelrhodopsin-2 (ChR2)-YFP expression, for guiding electrode placement. D: images of surface stimulation. Left: brightfield image of brain surface under thin layer of agarose, with superimposed outline of target stimulation site. Right: activation of stimulation site, as viewed by CCD camera. E, left: average optogenetically evoked response rates with a full-field flash stimulus (solid lines) or a grating stimulus (spatial frequency = 2.5 cycles/mm, dashed lines), using full spectral illumination (black lines) or blue channel only illumination (cyan lines), for 2 animals. Right: lack of optogenetically driven responses in 7 animals without ChR2. This shows that the bright optogenetic stimulus on the brain did not drive visual cortex through the eyes via reflection or via some other process. F: histogram of the maximum response rates evoked by optogenetic stimulation for all single-unit recordings and multiunit sites (N = 117) in ChR2 animals. On average, 28 spikes/s were evoked. Visually evoked responses at this age range from 3 to 10 Hz (Clemens et al. 2012; Van Hooser et al. 2014), so, on average, we are able to evoke stronger responses optogenetically than with visual stimuli. Optogenetic stimulus intensity can be reduced to match visually evoked rates.
Next, ferrets were paralyzed with the neuromuscular blocker gallamine triethiodide (10–30 mg·kg−1·h−1) through the ip cannula to suppress spontaneous eye movements, and the nitrous oxide-oxygen mixture was adjusted to 1:1. The animal's ECG was continuously monitored to ensure adequate anesthesia, and the percentage of isoflurane was increased if the ECG indicated any distress. Body temperature was maintained at 37°C.
In most experiments, multichannel silicon probes (NeuroNexus, A1x32-poly2-10mm-50-177) were used to record from the ferret visual cortex. The probes were coated with DiI for later histological examination and lowered into the ChR2-expressing areas of V1 guided by the fluorescence signal visualized through the ProjectorScope 1. The probe was lowered 800-1,200 μm into the brain until all pads were inserted into the brain, and 2–3% agarose was applied to prevent brain pulsations. Signals were amplified with a preamplifier/amplifier system by Multichannel Systems. One channel with clear spikes was acquired and clustered with a Micro1401 acquisition board and Spike2 software (Cambridge Electronic Design) and monitored throughout the experiment. Data from all 32 channels were acquired with custom software in LabVIEW and a National Instruments 6071e data acquisition board. Individual spike waveforms were extracted from the signal from all 32 channels using 4–8 standard deviation as threshold. Principal component analysis (PCA) was performed on the isolated waveforms from each channel, clusters were drawn using the first 3 PCA components, and finally the clusters were sorted with a T-distribution expectation maximization algorithm. All sorting procedures were performed with Plexon Offline Spike Sorter. Tuning curves were analyzed with custom software in MATLAB (Heimel et al. 2005; Van Hooser et al. 2006).
Visual stimulation and data analysis.
Visual stimuli were created in MATLAB with the Psychophysics Toolbox (Brainard 1997; Pelli 1997) on a Macintosh Pro running OSX and displayed on a Sony monitor (GDM-520).
For each cell, we examined both the mean response to drifting grating stimulation (F0) as well as the modulation at the stimulus frequency (F1). If a cell's F1 response was greater than the mean response (F0), then the F1 component was used to calculate index values. Otherwise, the F0 component was used (Heimel et al. 2005; Movshon et al. 1978a, 1978b). Direction selectivity was examined in recorded cells that exhibited significant variation across all stimuli by an ANOVA test, and tuning curves were fit with a double Gaussian (Van Hooser et al. 2012). The direction index (DI) was defined to be (Rp − Rn)/Rp, where Rp is the fitted response in the preferred direction and Rn is the response in the direction that is 180° opposite to the preferred direction. Circular variance was calculated as described previously (Mazurek et al. 2014; Ringach et al. 2002).
Optogenetic receptive zones.
Circular light spots were flashed onto the brain surface for 100 ms each. Multiple spot sizes were used, ranging from 90 μm to 360 μm in diameter. For each spot size, the whole imaging area was scanned in steps that were half the diameter of the spot size. Presentation order was randomized. For each stimulus diameter, we determined whether a cell's response at any of the stimulus positions was significantly different from the response to a “blank” stimulus by performing an ANOVA test (P < 0.05). Responses from diameters that showed significant modulation were included in model fits.
Responses were fitted by a bivariate Gaussian function to estimate the region over which each cell was strongly activated:
where Is(x,y) is the intensity at point x,y for stimulus s, G(x,y,μ,∑) is the bivariate Gaussian with mean μ and covariance matrix ∑, and NR(c,a,c50,n) is the Naka-Rushton function:
where a is the maximum cell response, c is the stimulus intensity, and c50 is the intensity of a stimulus that produces half of the maximum response. Variables a, c50, n, μ, and ∑ were used as free parameters for the fit. The optogenetic receptive zone (ORZ) size was taken to be the full width at half-height (FWHH) along the major and minor axes of G(x,y,μ,∑).
Temporal resolution measures.
Several indexes were calculated for responses to trains of optical pulses (see Fig. 3). For a given cell, the response latency was taken to be the median time of the first spike across all sixty 500-ms pulses. The first spike jitter was the standard deviation of spike arrival times around this response latency measure.
Fig. 3.
Temporal modulation of cortical neurons with trains of optogenetic full-field pulses. A and B: responses of 2 example neurons to a train of 10 pulses of 500-ms duration (A; 500 ms ON/500 ms OFF) or 33.4-ms duration (B; 33.4 ms ON/33.4 ms OFF). C: fraction of pulses that resulted in at least 1 spike (“success rate”) for different pulse durations. D: average 1st pulse firing rate of sites (N = 95) for different pulse durations; responses are the number of spikes divided by the duration of the stimulation (spikes/s). E: adaptation of firing rate response as a function of pulse number within a train. F and G: distribution across recording sites of response latencies (F) and 1st spike jitter (G) for all 60 pulses within the 500 ms ON/500 ms OFF train. The projector light response, which depends on the liquid crystal reaction time, is superimposed in blue in F. H–K: response resonances. H: raster and peristimulus time histogram (PSTH) of responses to all 60 pulses in the 500 ms ON/500 ms OFF case. I: autocorrelograms (ACH) of same sites. Yellow lines indicate smoothed ACH for oscillation score (Muresan et al. 2008). J: peak frequency of smoothed ACH for each site. K: oscillation scores indicate substantial resonance.
The oscillation index was computed as described previously (Muresan et al. 2008). In brief, for each spiking unit autocorrelograms (ACHs) were computed from spike times across all trials of stimulus-matched cycles using a 1-ms bin size. The ACH was smoothed with a fast Gaussian kernel, with σ given by σfast = min(2, 134/1.5fmax) × fc/1,000, where fc = 1/bin size and fmin is the lowest frequency of interest (15 Hz here). A second independent smoothing of the original ACH is calculated using a slow Gaussian kernel to facilitate identification of the boundaries of the central peak, detected at the bin where the slope of the smoothed ACH exceeds 10°. The slope was calculated as a numerical derivative multiplied by a rescaling factor, w/ACHslow(t = 0), setting the y-axis of the slow-smoothed ACH in units of x to remove dependence on firing rate, where w = 2 ^ (floor(max[log2(fc/fmin),log2(fc/4)]) + 1). In the fast-smoothed ACH, all bin values formerly comprising the peak are then replaced with the mean value of the bins at the ends of the peak boundaries. An amplitude spectrum is computed for the resulting ACH with a fast Fourier transform with Blackman windowing. Finally, the oscillation score is computed as the ratio between the peak amplitude detected in the frequency band of interest and the mean amplitude of the full frequency spectrum.
Cortical stimulation experiments.
For optical motion training, drifting gratings moving in one specific direction were shone for 3 s, followed by a 15-s interstimulus interval (ISI). This stimulation was continued for 20 min, followed by 10 min of rest (Li et al. 2008), and two such blocks of 30 min comprised 1 h of training. For constant optical stimulation full-screen white or blue light was flashed on the brain for 1 s, followed by 10-s ISI and repeated in the same 20 min on-10 min off pattern. After every hour of either training protocol a posttraining visual direction tuning curve was obtained, and a brightness-response curve was taken every 2 h. This protocol was continued for up to 8–9 h.
Histology.
After transcardial perfusion with 0.9% saline and 4% paraformaldehyde, brains were removed and placed in 4% paraformaldehyde in 0.1 M PBS for an additional 12–24 h. Brains were then incubated in 10% sucrose for 24–48 h, followed by 30% sucrose for 24–48 h. Frozen sections (50 μm) were cut on a sliding microtome in the parasagittal-horizontal anatomical plane.
Sections were collected in 0.1 M PBS, washed three times, 5 min each, in PBS, and agitated for 2 h in 0.3% Triton X-100 (MP Biomedicals, ICN807423) and 1% normal donkey serum in 0.1 M PBS at room temperature. After three 5-min washes in PBS, sections were incubated in rabbit anti-GFP (Invitrogen, A-11122; 1:500) diluted in 0.1 M PBS overnight (>12 h) at 4°C on a shaker. Sections were then rinsed three times, 5 min each, in PBS, incubated with donkey anti-rabbit (Invitrogen, A-31572; 1:200) and fluorescent anti-NeuN (Millipore, MAB377x; 1:200) at room temperature for 2 h, and washed three times, for 5 min each, in 0.1 M PBS. Sections were then mounted on slides and coverslipped with Fluoromount-G (Cell Lab, 731604). Slices were photographed with a Leica MZ16 F (×1) or scanned with a Leica SP2 confocal microscope (×20) and examined with ImageJ.
RESULTS
A schematic diagram of the ProjectorScope 1 is shown in Fig. 1A. A pair of lenses is used to bring the image displayed by a standard LCD projector into focus at the working end of a long-working-distance objective (×4 or ×10). A dichroic mirror allows blue wavelengths of light (that activate ChR2) to be deflected down to the cortical surface, while at the same time permitting green, yellow, and red light to be passed up from the objective to the CCD camera for brightfield microscopy or intrinsic signal imaging. A separate input port allows access for a high-quality halide light source that can be used with an exchangeable dichroic mirror (DM) for epifluorescent imaging of the cortical surface. No special software or hardware is needed to drive the LCD projector; it can be driven with standard video signals at 60 Hz.
The ProjectorScope 1 is mounted on a rail and can be moved with respect to the stereotaxic frame. In addition, it is mounted on a rotating bracket such that the objective can be rotated by tens of degrees in order to image cortical areas that are either perfectly dorsal or are on a dorso-lateral slope. The eyes of the animal are protected from responding to the bright light of the minified image (blue projector channel reflected by the dichroic mirror: 8–11 mW/mm2, all channels reflected by the dichroic mirror: 27–35 mW/mm2) by black aluminum foil. Full technical plans for ProjectorScope 1 are provided in the appendix (see Fig. A1 and Fig. A2) and the ProjectorScope 1 Parts and Construction Guide (http://go.brandeis.edu/projectorscope1-0); the spatial resolution of the system is described in the appendix (see Fig. A3).
In these experiments, ferrets were injected with an adeno-associated virus (AAV) to cause expression of ChR2. After 10–14 days incubation time, animals were prepared for visual and optogenetic stimulation and electrode recording. Fluorescent imaging of the brain surface and staining of postmortem tissue showed that ChR2 was expressed over a broad area, >4 mm in extent (see appendix, Fig. A5). Using this system, we were able to drive robust spiking of neurons in visual cortex (Fig. 1, E and F).
Spatial control of surface circuits.
While it is straightforward—optically—to obtain precise control of the size of the projected light stimulus (resolutions of our configurations ranged from 1.3 to 7.7 μm/pixel), it was initially unclear whether we would have reasonably local control of the stimulated cortex. The dendrites of cortical neurons extend for several hundred micrometers out from the cell body (Braitenberg and Schüz 1991), and cortical axons extend for several millimeters across the cortical surface (Bosking et al. 1997; Gilbert and Wiesel 1989; Rockland et al. 1982). Present viral methods that are capable of driving action potentials in vivo produce ChR2 throughout each cell's membrane, including dendrites and axons (Petreanu et al. 2007; Sato et al. 2014). Therefore, if ChR2 expression were sufficiently high, or if the optical stimulus light were sufficiently bright, we would expect that a small spot of light would activate cells over a distance of several millimeters and the fine spatial control of light that our projector provides would not translate into fine spatial control of neural circuits.
To clarify the degree to which we could provide local control of cortical circuits, we introduced multichannel electrodes into the cortex and sought to determine the ORZ for each recorded neuron or multiunit site. We projected circular stimuli of varying sizes at varying positions to map responses of a given cell to optogenetic stimulation across the cortical surface (Fig. 2), much as one would map the receptive field of a visual neuron with retinal stimulation. We modeled the ORZ of each cell as a two-dimensional Gaussian; the summed activity over the two-dimensional Gaussian was passed through a nonlinearity—a Naka-Rushton function (Naka and Rushton 1966)—to generate the model response.
Fig. 2.
Optogenetically driven surface receptive zones indicate local control of cortical circuits with ProjectorScope 1. A: optogenetic receptive zones (ORZs) for 4 recording sites. False color: average response to flashed circles of 90-μm diameter, with maximum response noted at bottom. Black line, fit of full width at half-height (FWHH) of a Gaussian surface receptive field fit with a Naka-Rushton summation response to light pulses of several sizes (see methods). B: fits of ORZ for several cells recorded on the same multichannel electrode from the same animal, showing locations of ORZ locations across the cortical surface. Color indicates electrode pad (key at right). C: average FWHH for 2 animals and with 2 light levels for 1 animal (∼30 mW/mm2 and 15 mW/mm2). FWHH is shown for each axis (major and minor) of the elliptical ORZ fit. ORZs are typically smaller than 200–300 μm in diameter and are smaller when lower light intensity is used. D: fraction of sites that exhibited a significant response as a function of spot size. E: maximum firing rate of response to increasing spot sizes. F: coefficient of variation (CV) of responses to increasing spot sizes. As spot size increases, the variability of the response is reduced.
The ORZ size varied from cell to cell, from animal to animal, and with stimulation power. Figure 2A shows example responses for four sites that were stimulated with 90-μm spots. Cells recorded with multichannel electrodes that were inserted at an angle to the cortical surface had different ORZ positions across the cortical surface (Fig. 2B), ruling out any concerns that the ORZ might be purely determined by the location of ChR2 expression, which would have yielded a common ORZ for all cells. On average, ORZs were relatively compact, with a FWHH smaller than 300 μm (Fig. 2C). The variation in these zones across animals could arise from many factors, including differences in expression from injection to injection. This size is less than half the average width of the average center-to-center spacing of orientation columns in ferret (∼700 μm) (Rao et al. 1997). Cells exhibited reliable responses to these spot stimuli regardless of spot size (Fig. 2D) and gave stronger responses (Fig. 2E) and less variable responses (Fig. 2F) as spot size increased.
Temporal control of surface circuits.
To understand the temporal modulation possible with ProjectorScope 1, we provided through ProjectorScope 1 trains of full-field optogenetic stimuli with different pulse durations. It is likely that the temporal response characteristics would depend on stimulus size; here, only full-field stimuli were investigated. Figure 3, A and B, show the responses of two recording sites to stimuli of two different on-off combinations. On average, recording sites exhibited spiking activity in response to a majority of individual pulses (Fig. 3C) with reasonably high firing rates (Fig. 3D). Across a train of 10 pulses, neural responses exhibited modest adaptation that varied with the interpulse interval (Fig. 3E; see appendix, Fig. A6).
Unlike LEDs or laser light that is switched through a fast shutter, LCDs do not permit submillisecond changes in light intensity. Instead, upon receipt of a command to switch from OFF to ON, rotation and alignment of the crystal must take place that alters the transparency of the liquid crystal over a period of several milliseconds (Fig. 3F). Despite this slow ramp up of the projector light, a majority of sites exhibited reliable spike timing, with first spike jitter values < 10 ms (Fig. 3G).
In principle, one could imagine two possibilities for the neural firing statistics generated by constant optogenetic stimulation. First, one might imagine that constant optogenetic stimulation causes neurons to fire at an elevated firing rate but with random spike timing. Second, one might imagine that the stimulation engages the cortical network in such a way that the neural firing exhibits network-dependent resonances (Cardin et al. 2009; Sohal et al. 2009). We found strong evidence for this second possibility. Pulse-triggered histograms of the responses at the same recording sites shown in Fig. 3, A and B, show clear resonances (Fig. 3, H and I) that correspond to modulation in the gamma band. On average, recording sites exhibited strong modulation at these frequencies (Fig. 3, J and K). This is consistent with the idea that the network is being strongly engaged at this stimulus intensity, with contributions from neurons that are both directly and indirectly activated by light.
We also provided stimulation to the cortex with sinusoidal gratings. Firing rates were strongly modulated with a temporal frequency that matched the stimulus temporal frequency (Fig. 4A). On average, the strength of modulation at the stimulus temporal frequency, known as the “F1 response,” increased with increasing temporal frequency, indicating that neurons tended to fire in an increasingly phase-locked manner as the temporal frequency increased (Fig. 4B). Fourier analysis revealed that, on average, the strongest temporal frequency component matched the temporal frequency of the stimulus (Fig. 4, C and D) and also revealed a weaker stimulus-evoked gamma component at similar frequencies (35 Hz) as the full-field stimuli in Fig. 3, H and I.
Fig. 4.
Temporal modulation of cortical neurons with optogenetic sinusoidal gratings. A: responses of a single neuron to sinusoidal gratings at different temporal frequencies (TFs). In each case, the neuron responds at a consistent phase at the stimulus temporal frequency. Firing rates are shown by PSTHs with bin size equal to 1/TF/10. B: average modulation at the stimulus temporal frequency (F1 component) for a range of stimulus temporal frequencies. Higher modulation rates at higher temporal frequencies reflect precise spike timing at those higher temporal frequencies. C: log-log plot of the average Fourier amplitude coefficients of spike responses to 2-Hz stimulation, which was commonly employed in optogenetic training experiments. There is a clear peak at the stimulus temporal frequency (2 Hz) and weak modulation at ∼35 Hz. D: average Fourier amplitude coefficients of responses to several stimulus temporal frequencies. Each column is the spectrum for a different stimulus temporal frequency. Shading indicates coefficient value (white is 0, black is maximum for each column). In each case, the largest modulation component (darkest bar) is at the stimulus temporal frequency.
Testing hypotheses of the development of motion selectivity.
Having established that we can achieve spatially and temporally localized control of the visual cortical circuit, we then used the ProjectorScope 1 to test specific models of experience-dependent (activity-dependent) development of cortical circuits.
At eye opening, neurons in the ferret visual cortex are already orientation selective (Chapman and Stryker 1993), but these neurons have not yet acquired the robust selectivity for stimulus direction of motion that is observed in adult animals (Clemens et al. 2012; Li et al. 2006, 2008; Weliky et al. 1996). Under normal rearing conditions, strong direction selectivity is acquired in the first 2 wk after eye opening. However, if animals are deprived of visual experience during this critical period by dark-rearing, then the formation of strong direction selectivity is abolished, even if normal visual experience is reintroduced later in life (Li et al. 2006). That is, visual experience is required for the development of direction selectivity.
This experience-dependent development of direction selectivity can be studied in the laboratory. When immature, anesthetized animals are shown moving sinusoidal gratings (but not flashing gratings), direction selectivity of visual cortical neurons increases rapidly, in 3–6 h, in a single experimental session (Li et al. 2008; Van Hooser et al. 2012). Here we sought to investigate the circuit mechanisms that underlie this process by providing direct stimulation to cortex with the ProjectorScope 1, while avoiding visual stimulation of the retina.
There is a large set of possible model circuits that could, in principle, underlie the responses in the immature state and—with experience-dependent plasticity—acquire the direction-selective responses that are eventually observed in the animal. Here we divide some of these model circuits into three conceptual groups.
First, one could imagine a set of circuit models where the activity of a cortical neuron is completely determined by the converging inputs of LGN neurons with different position preferences and stimulus latencies (Feidler et al. 1997; Gjorgjieva et al. 2011; Reichardt 1961; Saul and Humphrey 1990 1992). One example of such a model is shown in Fig. 5A (Blais et al. 2000; Feidler et al. 1997). In this example, the cortical neuron initially receives net LGN input that produces a slight direction bias, and this bias is strengthened (according to the BCM learning rule or modified spike-timing-dependent plasticity) through experience with motion stimulation (Blais et al. 2000; Gjorgjieva et al. 2011). If a member of this set of circuit models were operating in the developing brain, then spatiotemporally precise coactivation of retinal ganglion cells, LGN cells, and cortex would be required to produce increases in direction selectivity, and we would predict that cortical stimulation alone would be insufficient.
Fig. 5.
Different categories of model circuits and plasticity mechanisms that could, in principle, underlie the development of direction selectivity. A: example of a circuit that becomes direction selective on the basis of changes in synaptic weights from feedforward lateral geniculate nucleus (LGN) connections (Blais et al. 2000; Feidler et al. 1997). After sufficient exposure, weights from LGN cells at specific latencies and spatial positions become strengthened, and the cortical cell exhibits direction selectivity. Under this circuit, activation of the cortex itself would not produce direction selectivity. B: example of a circuit that becomes direction selective on the basis of sharpening of initial biases within cortical circuits. The model depicted here undergoes activity-dependent increases in intracortical inhibition (see Garkun and Maffei 2014; Van Hooser et al. 2014), which would increase any difference between suprathreshold responses to preferred and null directions that existed initially. Under this circuit, activating cortex by itself should cause increases in direction selectivity; however, the spatiotemporal characteristics of the cortical activation should not influence the direction preference that emerges. C: example of a circuit that develops direction selectivity on the basis of space-specific strengthening and weakening of horizontal connections (Shon et al. 2004; Wenisch et al. 2005). In the initial circuit, horizontal connections have moderate strengths. With stimulation in 1 direction, say rightward, spike-timing-dependent plasticity at the horizontal connections causes an asymmetric strengthening of connections from cells whose receptive fields are opposite the preferred direction (to the left here) and a commensurate weakening of connections from cells whose receptive fields are in the preferred direction (to the right here). These altered connections cause an amplification of responses in the preferred direction and a reduction of responses in the null direction, producing increased direction selectivity. Under this circuit, activating cortex with drifting sinusoidal waves should cause an increase in direction selectivity, and the direction preference that is acquired should match the sweep direction. Spatially broad activation of the cortex should not cause an increase in direction selectivity in this class of circuit models.
One could imagine a second set of circuit models very similar to the first, where the activity of cortical neurons is largely determined by feedforward biases but, in addition, these subcortical inputs are sharpened by some process that is local to the cortex. A specific example of such a model is shown in Fig. 5B (Van Hooser et al. 2014). In this model, cortical inhibitory neurons exhibit responses that are unselective for direction, and the weight of the synapses from cortical inhibitory neurons onto cortical excitatory neurons increases with activity in a spike-timing-independent manner (Garkun and Maffei 2014). As this inhibitory weight increases, the direction tuning of the cortical excitatory neurons is sharpened (Van Hooser et al. 2014). If a member of this set of cortical models were operating in the developing brain, then directly activating the cortex with spatially diffuse optogenetic stimulation would be expected to cause an increase in direction selectivity.
Finally, one could imagine a third set of circuit models where the activity of cortical neurons is determined by converging inputs that arise both from LGN cells and from cortical neurons at different locations across the cortical surface (Shon et al. 2004; Wenisch et al. 2005). An example of such a model is shown in Fig. 5C (Wenisch et al. 2005). In this model, the horizontal cortico-cortical connections undergo spike-timing-dependent plasticity. Consider the center cortical cell in Fig. 5C as a reference. As the cortex gains experience with objects moving in a given direction, say rightward, then the horizontal connections from cells with receptive field locations to the left of the reference cell will be activated earlier than the reference cell, and these synapses will be strengthened by spike-timing-dependent plasticity. Cells with receptive field locations to the right of the reference cell will be activated later than the reference cell, and the connections from these cells will be weakened. Eventually, the input from the cortical cells will amplify responses in the experienced (preferred) direction, while decreasing responses to the nonexperienced (nonpreferred) direction. If convergence of responses across the cortical surface contributes to direction selectivity, then we expect that directly activating the cortex with an optogenetic stimulus that sweeps across the cortical surface would cause two changes: 1) cells should exhibit an overall increase in direction selectivity, and 2) the preferred direction that these cells acquire should match the stimulated direction.
Because the surface of the visual cortex is organized retinotopically (Law et al. 1988), it is possible to make an approximate correspondence between the direction of an object moving on the retina and the progression of activity across the cortical surface, as illustrated in Fig. 6B and in the appendix (see Fig. A4). Therefore, if horizontal connections participated in the formation of direction selectivity, we would predict that sweeping activity across the cortical surface in a medial-to-lateral direction would mimic upward motion, and that if neurons that were stimulated in this manner were tested with a visual stimulus, they might show increased direction selectivity for upward visual motion.
Fig. 6.
Prolonged optogenetic stimulation experiment. A: schematic of the experiment. B: simplified diagram of the mapping of retinal space onto the surface of primary visual cortex in the ferret (Law et al. 1988). C and D: schematic drawings of the optogenetic constant stimulus (C), which was a full-field stimulus 1 s in duration, and the optogenetic motion stimulus (D), which was a drifting sinusoidal grating moving across the cortical surface.
We sought to look for evidence of these three broad classes of models by performing three types of experiments. In all experiments, animals were prepared for optogenetic stimulation and electrodes were introduced into the primary visual cortex (Fig. 6A). Two groups of animals had been previously injected with AAV viruses encoding ChR2, while another group served as a no-ChR2 control. We made an initial assessment of orientation and direction selectivity by showing conventional visual stimuli to the eyes (Clemens et al. 2012). Then we turned off our visual stimulus monitor and provided direct optogenetic “training” stimuli to the brain through the ProjectorScope 1.
In some ChR2 experiments, we trained with an “optogenetic constant stimulus” that consisted of a broad activation of the entire field of view for 1 s followed by 10 s of rest (Fig. 6C). In other ChR2 experiments and all no-ChR2 experiments, we trained with an “optogenetic motion stimulus,” which consisted of a drifting grating whose orientation matched the prevailing orientation preferences we observed on the electrode (Fig. 6D). The optogenetic motion stimulus moved in a single direction and had a spatial frequency of 1.5 cycles/mm and a temporal frequency that ranged from 2 to 4 Hz (Supplemental Video S1).1 After each hour of optogenetic training, we reassessed visually driven orientation and direction selectivity with conventional visual stimuli delivered to the eyes (with optogenetic stimulation turned off). We continued this protocol for several hours. Example raw recordings from one multiunit site before and after 6 h of cortical stimulation are shown in the appendix (see Fig. A7). The direction tuning curves of three sites before and after stimulation are shown in Fig. 7, A–C.
Fig. 7.
Direct surface stimulation of the immature visual cortex causes a rapid increase in direction selectivity. A–C: visually driven direction tuning curves measured before and after several hours of training with an optogenetic motion stimulus. The estimated direction, in retinal coordinates, of the optical motion stimulus is shown in gray. Time indicates time since initial recording; ∼1.5 h of surface training was provided during each 3-h recording interval. DI, direction index; ΔDIs, change in signed DI. The signed DI is the absolute DI multiplied by +1 if the empirically measured direction preference of the cell is within 90° of the training direction (in retinal coordinates) and −1 if the measured direction preference differs by >90°. Some cells increased DI without switching their empirical direction preference (A); other cells reversed to match the training direction (B), while others reversed to be opposite the training direction (C). D: DI measurements at each recording site vs. time for optogenetic constant training stimuli (i), optogenetic motion training stimuli (ii), and optogenetic motion training stimuli delivered to control animals that lacked ChR2 (iii). Left: scatterplots. Right: histograms of mean values that were obtained over indicated time intervals (error bars are SE; individual points are shown on right of each bar). Animals with ChR2 that were provided optogenetic motion or optogenetic constant training exhibited robust increases in direction selectivity (regression slope > 0 with P < 0.001 for both cases), but no significant increase was observed in animals that lacked ChR2 (slope not different from 0, P = 0.13). While the regression slopes in Di and Dii were highly significant, other factors would be needed to more completely describe the variation in the data around the mean (r2 = 0.14, 0.10, 0.09, for Di–Diii, respectively). *Significant relationships in means over intervals (ANOVA, P < 0.05). E: changes in DI at individual sites for optogenetic motion stimulation (red) and optogenetic constant stimulation (green). Individual sites are linked by lines. Data are separated into sites with initial DI < 0.5 (left) and those with initial DI ≥ 0.5 (right). Sites with weak initial DI values tended to exhibit increases in DI, while sites with strong initial DI values tended to maintain those values, indicating that the increases in Di and Dii are due to increases in DI at individual sites, and not due to a loss of visual responsiveness of weakly selective sites.
General cortical activation causes an increase in direction selectivity in developing ferret visual cortex.
Over the course of the experiment, we observed a robust increase in direction selectivity for animals that had received ChR2 and either optogenetic constant stimulation or optogenetic motion stimulation but no corresponding increase in direction selectivity for the no-ChR2 optogenetic motion stimulation group (Fig. 7D; appendix, Fig. A8). These data show that activating the brain optogenetically caused an increase in direction selectivity and that this increased direction selectivity was not a result of the brief visual experience with the testing stimuli that were periodically delivered through the eyes or due to some other activation of the cortex (e.g., via heat from the stimulator).
Recording efficiency over the several hours of monitoring was similar in the three conditions. In most experiments, we employed silicon multichannel electrodes that were stabilized by agarose in place. The fraction of recording sites that continued to exhibit significant responses decreased over time in all conditions, and the overall maximum responsiveness of the cells, on average, decreased for all conditions (appendix, Fig. A9). In examining the increases in direction selectivity in Fig. 7, Di and Dii, one might wonder if individual sites exhibited increases or if sites with poor direction selectivity merely became unresponsive more frequently than sites with strong selectivity. The data in Fig. 7E are responses at single recording sites over time; the data have been divided into cells that initially exhibited a DI value <0.5 or ≥0.5. Sites that initially exhibited low DI values tended to increase, while sites that initially exhibited high DI values rarely decreased. This evidence suggests that neurons at individual sites tended to exhibit increased direction selectivity over time with optogenetic stimulation.
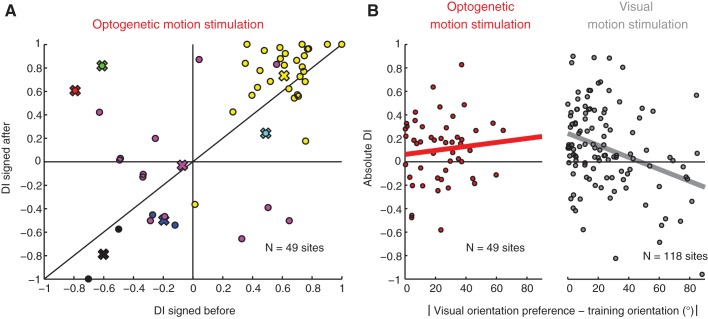
While the previous experiments provided evidence that constant cortical activation is sufficient to induce direction-selective responses in visual cortex, in principle it remained possible that sequential activation of horizontal connections in a particular direction could influence the final direction preference that was acquired. That is, it remained possible that the cortical circuit contained both an activation-enhanced sharpening mechanism (as in Fig. 5B) and a sequential cortical activation mechanism (as in Fig. 5C). To examine this possibility, we plotted the signed DI for cells before and after training (Fig. 8A). This index is the product of the absolute DI and a sign parameter. This sign parameter is +1 if the estimated retinal direction of the surface training stimulus matched (within 90°) the cell's empirical direction preference and is −1 if the empirical direction preference is >90° away. If the direction of cortical stimulation determined the direction angle preference that cells acquired, we would predict that the majority of the data should lie above the unity line; that is, the signed DIs should uniformly increase. If there were no relation between the direction preference that was acquired and the stimulation direction, then we would expect to see many cells both above and below the unity line. In fact, we observed this second possibility; in different experiments, cells sometimes increased their direction selectivity to follow the retinal direction of the optogenetic training stimulus, but other times the opposite direction emerged (Fig. 7C, Fig. 8A). Thus we did not observe evidence consistent with the idea that cortical sweep direction determined the acquired direction preference.
Fig. 8.
No evidence of a relationship between the optogenetic surface motion training direction and the final visual direction preference. A: signed DI of the initial and final measurement of each site. Positive DI indicates that the visual direction preference was within 180° of the estimated corresponding retinal direction of the optogenetic surface motion training; negative DI indicates that the visual direction preference differed from the estimated corresponding retinal direction by >180°. Cells from individual animals are indicated in different colors; the mean for each animal is indicated by an X. Some cells switched sign to match the corresponding retinal training direction (top left quadrant) while others switched sign to differ from the corresponding retinal training direction (bottom right quadrant), and many cells kept the same sign, regardless of whether they initially matched (positive signs, top right quadrant) or differed from (negative signs, bottom left quadrant) the corresponding retinal training direction. So while direction selectivity increased overall, visual direction preference was not related to the corresponding retinal training direction. B: relationship between the initial visual orientation preference of neurons (rotated so the training orientation is 0) and the increase in direction selectivity. Left: for brain surface training with ProjectorScope 1, there was no relationship between the visual orientation preference and the increase in direction selectivity (P = 0.8220). Right: for visual motion training stimulation through the eyes, there was a strong relationship (P < 0.001); cells whose orientation preferences match the visual training orientation tend to show much stronger increases in direction selectivity after training. This indicates that the increases in direction selectivity with brain surface training were nonspecific and were not influenced by the direction of the stimulus for brain surface motion training.
One final piece of data is also inconsistent with the idea that the sweep direction is driving the direction selectivity we observed. In visual motion training experiments (through the eyes) as in Fig. 8B, right, experience-dependent increases in direction selectivity are restricted to cells whose orientation preferences match the visual training direction (see also Li et al. 2008). If expression of this selectivity required sequential activation of the patchy horizontal connections among areas with similar orientation preferences that exist in the cortex (Bosking et al. 1997; Gilbert and Wiesel 1989; Malach et al. 1993), then we might expect that increases in direction selectivity with the optogenetic motion training stimulus would be similarly limited to orientations that match the direction of the optogenetic stimulus. Instead, we found that subsequent increases in direction selectivity were not related to the initial orientation preference of cells (Fig. 8B). These data are consistent with the idea that stimulation causes a general increase in direction selectivity that is unrelated to cortical sweep direction.
DISCUSSION
We have described a relatively simple system for combining patterned light stimulation of the brain surface with a microscope that is appropriate for in vivo epifluorescent microscopy and intrinsic signal imaging. Given the efficacy of optogenetic tools using common viral methods, and the irradiance of common projector sources, it is now feasible to manipulate the activity of structures on the brain surface in both space—at the resolution of hundreds of micrometers—and time—at a resolution limited in large part by the projection system. Using this system we showed, for the first time to our knowledge, that prolonged stimulation of the immature cortex could cause the rapid emergence of an experience-dependent functional property of the cortical circuit. These results open possibilities for future therapeutic approaches involving cortical function restoration in developmental brain diseases.
Design of patterned optogenetic stimulation systems for brain circuit activation.
There are now a handful of systems that have been designed for patterned optogenetic stimulation through a microscope objective, each with its own advantages.
Some LCD systems are based on LCOS SLMs, such as those created by Ian Davison (I. Davison, personal communication) and adapted for in vivo use by Yishai Elyada (Huang et al. 2011). The compact system in Huang et al. is a single structure and is driven by a halide source via a liquid light guide. It has the advantage of being able to rotate without the need to adjust any of the optical elements. The ProjectorScope 1 can only be rotated about a single axis (in the coronal plane), and one must carefully make adjustments to the arms to retain maximum power, so it is less convenient. Budget constraints led us to build our system around a standard LCD projector with a halogen light. We were surprised to find that our system, despite its larger and less convenient footprint, has an irradiance that is ∼5–10 times that of the LCOS SLM and halide-based system. This extra power could be a crucial advantage for some experiments (such as the developmental experiments described here) where one cannot wait indefinitely long for the expression of optogenetic proteins.
Another strategy that allows significant additional power is the use of a laser paired with galvanometric mirrors. Davison and Ehlers (2011) used a UV laser, galvanometric mirrors, and caged glutamate to activate sets of glomeruli in the olfactory bulb, to understand which combinations could drive pyramidal neurons in olfactory cortex. These mirrors have excellent temporal fidelity for activating a few small spots in rapid succession but can only activate a single spot at a time and are slower than projectors for drawing arbitrary images. The drawing time would depend on how long the light had to remain at each spot in order to activate the tissue, but rates of 1–2 Hz, or up to 30 Hz for resonant scanners, would be possible.
An ideal system for surface stimulation experiments would be to use a compact design (like the Huang et al. system) that incorporates a DMD device driven by an arbitrary external light source, such as that described in von Reyn et al. (2014) or Pandarinath et al. (2013). This system would have tremendous irradiance and would—in principle—be able to modulate neurons at the speed of the nervous system (submillisecond resolution). Such a system could be built for substantially less money than a two-photon microscope.
Finally, some circuits of interest are either deep within the brain or buried in sulci and are not amenable to surface stimulation. Ed Boyden's lab has developed 3D “combs,” where each tooth has an array of LEDs spaced some 500 μm apart (Zorzos et al. 2012). This technology allows circuits to be addressed in three spatial dimensions, albeit with lower resolution than is permitted by projectors.
Overcoming current limitations.
While many in vivo studies in adult animals have employed ChR2 and allowed 6–8 wk of expression (Huang et al. 2014), our developmental studies can only tolerate short expression durations. We observed strong responses after only 1–2 wk of expression with a third-generation version of ChR2 (T159C/E123T), which exhibits a larger current and sensitivity to light (Berndt et al. 2011). More sensitive optogenetic channels could further reduce the expression time required.
We were able to achieve local control of cortical circuitry because the intensity of our stimulation light and the expression level of ChR2 were compatible. Stronger expression or a stronger light source might have caused activation of small axonal or dendritic processes far from the cell body, thus destroying local control. ORZ sizes could be actively managed if one could restrict a very sensitive version of ChR2 to somas or axon initial segments. At present, these tools are suitable for in vitro but not in vivo use (Grubb and Burrone 2010).
A difficulty that reduced our success rate was the formation of scar tissue on the surface of the brain after the virus injections. Often, thick scar tissue either prevented us from inserting electrodes near the injection site or limited our optical access to the surface tissue. Methods to reduce scar tissue would greatly increase efficacy.
An increase in direction selectivity after repeated cortical activation.
We found strong evidence that general activation of the visual cortical surface over a period of 3–9 h was sufficient to increase direction selectivity in the developing ferret visual cortex. The duration of optogenetic stimulation that is required for this increase is comparable to the duration of visual stimulation that is necessary to produce a similar increase in direction selectivity (Li et al. 2008; Van Hooser et al. 2012).
Because motion-sensitive responses require joint selectivity to visual stimuli in both space and time, many models have posited that natural stimulation coupled with spike-timing-dependent plasticity mechanisms (either feedforward or recurrent) underlie the development of direction selectivity (Blais et al. 2000; Feidler et al. 1997; Honda et al. 2011; Shon et al. 2004; Van Hooser et al. 2014; Wenisch et al. 2005). The experimental observations here indicate that there is at least one neural mechanism that can sharpen direction selectivity that does not depend on the precise spatiotemporal patterns of natural stimuli in the external world. Because this mechanism can be activated by prolonged bouts of constant optogenetic stimulation, this mechanism is either spike timing independent or depends only on the timings produced by the internal rhythms in the generically activated circuits of the visual system, rather than the timings produced by external patterns of stimulation.
As a concrete example, it is worth noting that even if LGN were activated via feedback connections by the surface stimulation, the response latencies of those LGN cells would not be related to the latencies that these cells exhibit when they are activated by a visual stimulus. So any spike-timing-dependent plasticity mechanisms that might be operating during visual stimulation would be activated in a very different way through full-field direct cortical activation compared with how they would be activated by a moving stimulus.
The location of this mechanism is less certain. The resonant cortical activation that we observed with constant optogenetic activation of the cortex (Fig. 4) implies that the optogenetic stimulation caused the activation of cells that directly expressed ChR2 as well as coupled cells in the network. This activity is likely to have propagated back to LGN, possibly even driving cortex via LGN. So this stimulus-timing-independent mechanism could be present in LGN projections to visual cortex, within visual cortex itself, or in higher cortical areas.
While the space of models consistent with the data is still large, one possible circuit change that could underlie these results is an increase in local inhibition (either feedforward or feedback). Activity-dependent increase in inhibition onto layer 4 cortical excitatory neurons begins at the time of eye opening and ends at the onset of the critical period for ocular dominance (Garkun and Maffei 2014), which is precisely the time of our experiments. A recent modeling study from our lab showed that this increase in inhibition could enhance competition among the synapses that support firing in the two opposite directions to promote the robust development of direction selectivity (Van Hooser et al. 2014).
Another possible model in the “cortical sharpening” family is that local sharpening could occur through increases in local cortico-cortical excitatory connections, which could cause increased cortical amplification of feedforward biases, as in Suarez et al. (1995). An increase in cortical amplification would cause the difference in responses to preferred and null directions to increase.
Further experiments will be needed to identify the specific circuit mechanisms that are participating in the increases in direction selectivity, but these experiments provide strong evidence that activation of the cortex in a spatiotemporally nonspecific manner is sufficient to invoke these mechanisms.
In all, we found no compelling relationship between the optogenetic motion training direction and the eventual visual direction preference that was acquired (Fig. 8). This is a negative result, a failure to find positive evidence, so we cannot rule out that we might have seen an influence of the direction of the optogenetic training stimulus on the eventual visual direction preference if we had altered our protocol in some way. It is possible that a more natural activation of the cortical circuit would allow a spike-timing-dependent plasticity process that is somehow blocked under the conditions of our experiment. However, we did activate the cortex with sweeps of activity that drive the cells at temporal rates similar to those observed in vivo. Preliminary evidence from visual training experiments in which experience was provided at one of a range of different speeds indicates that direction selectivity develops after experience with any of the speeds (Ritter et al. 2013), so we feel it is unlikely that a small change in the cortical sweep speeds that we tried would have suddenly shown a strong sweep dependence. These results are inconsistent with models that rely on spike-timing-dependent plasticity in horizontal connections and sweeps of activity across the visual cortex (Shon et al. 2004; Wenisch et al. 2005).
Other very recent evidence argues against the horizontal connection activation hypothesis as a primary mechanism for the development of direction selectivity. A necessary condition for the horizontal connection hypothesis is that natural stimulation should produce waves of activity across the horizontal surface. On the scale of millimeters, this must be true because receptive field locations are already established at this age. However, imaging experiments have found that, at the local scale of <1 mm, waves of endogenous activity do occur in the developing ferret visual cortex at this age but the wave sweep direction does not align with the direction of visual stimulation (Smith et al. 2015). Instead, these waves have an intrinsic sweep pattern that can be induced with any visual stimulus regardless of the direction of visual stimulation. These data argue that if the horizontal connection hypothesis is true, it must involve connections that are farther away than ∼1 mm.
Visual activation vs. cortical activation.
In previous studies, experience with visual grating stimuli for 3–6 h produced a rapid increase in direction selectivity in visual cortex of visually naive animals (Li et al. 2008; Van Hooser et al. 2012). However, stimulation with a flashing stimulus did not produce an increase in direction selectivity (Li et al. 2008). Here, stimulation with a constant stimulus directly on the cortical surface did cause an increase in direction selectivity. At a glance, these results seem incongruent. With more thought, two explanations are plausible. First, it is possible that the direct cortical activation is somehow stronger, or produces more effective plasticity, than the flashing visual stimulus. Under this view, if previous studies had been able to provide longer flashing visual stimulation to the animal, then the authors might have eventually observed an increase in direction selectivity. However, strobe-reared animals lack direction-selective neurons in mature cortex (Cremieux et al. 1987; Humphrey et al. 1998; Humphrey and Saul 1998; Kennedy and Orban 1983), suggesting that additional stimulation is not enough to develop direction selectivity. A second possibility seems more likely: there may be an interaction between feedforward inputs and the intracortical circuitry. If visual stimulation strengthens connections from LGN to cortex (Blais et al. 2000; Feidler et al. 1997; Gjorgjieva et al. 2011; Van Hooser et al. 2014), then it is likely that a different subset of feedforward inputs are strengthened with flashing or strobe stimulation compared with stimulation with a drifting grating.
Potential therapeutic use.
Neural prosthetic devices that would stand in for injured senses such as vision or touch must provide sufficiently many independent inputs to the nervous system so the user has enough sensory resolution to make use of the injured sense. Systems that address neural tissue with imaging technology or arrays have the potential to provide millions of independent inputs. For a visual prosthesis, these numbers are comparable to the number of inputs that one would want to replace, as the number of optic nerve fibers in humans is ∼1 million (Jonas et al. 1992). The columnar organization of the primate sensory cortex offers additional advantages for these optical approaches. Nearby neurons tend to have similar receptive field properties—visual space, orientation columns in V1 and direction columns in MT (Albright et al. 1984; Hubel and Wiesel 1963), color globules in area V4 (Conway et al. 2007), somatosensory modalities (Mountcastle 1997)—that could potentially allow columnar stimulation to effectively stand in for sensory input (Salzman et al. 1990).
The temporal precision with which we could produce spikes depended on resonant frequencies within the cortical circuit. This dependence could burden or enhance efforts to activate cortical neurons with precise temporal codes, as has been shown in the retina (Nirenberg and Pandarinath 2012). These resonances could either be an obstacle, limiting the ability to encode stimuli, or a feature that makes such encoding easier. Activating thalamocortical axons instead of cortical neurons could overcome any limitations due to cortical resonances.
Finally, a majority of previous prosthetic approaches have focused on devices that would stand in for a part of the nervous system in real time. Here, however, we provided optogenetic stimulation during development that resulted in circuit changes that altered responses to subsequent visual stimuli. These results suggest an alternative possible use of neural prosthetics, one that involves transient activation during a “training period” that modifies circuits so that they are capable of responding differently to future endogenous stimuli.
GRANTS
This work was funded by National Science Foundation IOS 1120938 (J. Fiser, S. D. Van Hooser) and National Eye Institute Grant EY-022122 (S. D. Van Hooser).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.R., J.J.O., N.J.R., J.F., and S.D.V.H. conception and design of research; A.R., J.J.O., N.J.R., S.W., and J.T.S. performed experiments; A.R., J.J.O., N.J.R., S.W., and S.D.V.H. analyzed data; A.R., J.J.O., N.J.R., S.W., J.T.S., and S.D.V.H. interpreted results of experiments; A.R., J.J.O., N.J.R., and S.D.V.H. prepared figures; A.R., J.J.O., and S.D.V.H. drafted manuscript; A.R., J.J.O., N.J.R., S.W., J.T.S., J.F., and S.D.V.H. edited and revised manuscript; A.R., J.J.O., N.J.R., S.W., J.T.S., J.F., and S.D.V.H. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Krista Disano and Meredith Blankenship for help in building and improving the projection system during laboratory rotations. We thank Ian Davison at Boston University, Yishai Elyada, Sharon Wang, William H. Bosking, and Gordon B. Smith from David Fitzpatrick's lab at Max Planck Florida Institute, and Todd Roberts at University of Texas Southwestern for valuable discussions of optics and viral methods, Jennifer Wolff, Heather Bernstein, Julie Miller, Nora Anderson, and Benyamin Meschede-Krasa for technical and surgical assistance, members of the Van Hooser lab for comments on the manuscript, and Rebekah Corlew and Elizabeth Johnson for helpful discussions.
APPENDIX
This appendix contains supporting technical information and observations from the study. Figure A1 shows a technical diagram of the ProjectorScope 1.0. Photographs of the complete system are shown in Fig. A2. The modulation transfer function is plotted in Fig. A3. Figure A4 illustrates the coarse transformation between retinal space and cortical space for the ferret. Figure A5 shows histological sections of ChR2 expression in ferret visual cortex.
Fig. A1.
ProjectorScope 1 technical diagram (see text).
Fig. A2.
Photographs of ProjectorScope 1. Left: the base of the ProjectorScope 1, removed from the rig and projector. Right: the ProjectorScope 1 in place with the projector.
Fig. A3.

Modulation transfer function: the empirical brightness of alternating black and white bars passed through the ProjectorScope 1 alone (blue ○) and through ∼1.5 mm of agarose (red ×) toward a target (a glass slide). Brightness was examined on the opposite side of the slide with a second camera (IDS u-Eye) through a ×4 lens, such that the light only passed through the agarose once. Fits are 3rd-order polynomial fits of the data points.
Fig. A4.
Conversion from cortical space to visual space. We used the visuotopic map of Law et al. (1988) to produce a rough projection between retinotopic space and cortical space. Colors indicate directions in visual and cortical space. That is, the green arrow indicates that medial-to-lateral travel in cortical space corresponds to movement in an upward direction in visual space. Degree numbers on visual space panel indicate the transformation between visual space and the coordinate frame of our visual stimulus monitor (that is, 0° stimulus on visual stimulus monitor corresponds to upward motion). Degree numbers in cortex panel indicate the transformation between the cortical directions and the projector (empirically determined from the rotation of the image of the projector on the cortical surface). That is, a 0° stimulus sweeps in posterior-medial direction on the cortical surface, whereas a 120° stimulus sweeps in medial-to-lateral motion that corresponds to upward motion in visual space.
Fig. A5.
A: adeno-associated viruses (AAVs) caused widespread expression of ChR2 over several millimeters and across all cortical layers. Left: anti-green fluorescent protein (GFP) (ChR2) staining (in magenta) shown in a horizontal section through the expression zone in V1 (×4 magnification). A-P axis is anterior-posterior axis; M-L axis is medial-lateral axis. Right: anti-NeuN (all cells, in green) and anti-GFP (only ChR2-expressing cells, in blue) staining are shown at higher magnification (×10) from the same section at location indicated by yellow arrow. Inset, right: higher-magnification view (×20). High-resolution images of neurons stained for both NeuN and YFP indicated that virtually all cells that were positive for NeuN also expressed ChR2, suggesting wide staining of both excitatory and inhibitory neurons, as documented previously for high-titer, high-volume injections (Nathanson et al. 2009). B: normalized fluorescence intensity measured along a path across each slice (see yellow line in A), averaged from several slices from each of 4 animals.
Figures A6–A9 show additional experimental measurements. Figure A6 shows within-train adaptation for full-screen optical stimulus trains. Figure A7 shows an example extracellular recording. Figure A8 shows an alternative analysis of the increase of direction selectivity following optical stimulation that uses circular variance methods. Figure A9 illustrates the responsiveness of the neurons across time.
Fig. A6.
Within-train adaptation for all stimulus ON and OFF times. For moderate and long stimulus ON times, neurons exhibited modest adaptation. For very short stimulus ON times, neurons exhibited some weak facilitation. These adaptation data indicate that there are limits to the action potential trains that can be produced in the cortex through the ProjectorScope 1. FR, firing rate.
Fig. A7.
Example extracellular recording data from 1 multiunit site. Top: before cortical stimulation. Bottom: after 6 h of stimulation. In each panel, single-trial raw voltage trace and spike rasters for 5 trials for visual responses to 2 opposite direction angles 90 and 270 are shown on left. On right, the individual spike waveforms (black) and average spike waveforms are shown. While responses to the 2 opposite directions were similar before cortical stimulation, after prolonged stimulation the responses to angle 90 were stronger in this case. These data show an example of spiking activity that remained robust over time.
Fig. A8.
Direction selectivity and orientation selectivity as assessed by circular variance methods. Several studies have indicated that selectivity indexes that are based on circular variance are more robust than those based purely on a preferred-to-null ratio (Grabska-Barwinska et al. 2012; Mazurek et al. 2014; Ringach et al. 2002). A: direction selectivity of neurons in the 3 experimental groups as assessed by 1-direction circular variance (1-DCV). The statistically significant differences are identical to those reported for the direction index DI that was used in Fig. 7D. B: orientation selectivity in the 3 experimental groups measured over time. There were no differences in orientation selectivity across time in the optical motion stimulation case (ANOVA, P = 0.87) or the optical constant stimulation case (ANOVA, P = 0.73), but we did observe an increase in orientation selectivity in the control case, where we performed optical motion stimulation without ChR2 (ANOVA, P < 0.001).
Fig. A9.
Changes in responsiveness over time. A: raw responses tended to decline throughout the duration of the experiment in all conditions. B: normalized response magnitude across conditions and time. Declines were similar across conditions, except that neurons exposed to optical constant stimulation exhibited slightly higher responses in the time interval 1–4 h than the other conditions (ANOVA, Tukey-Kramer post hoc test, P < 0.05). C: fraction of electrodes with neurons that exhibited a significant degree of orientation tuning via an ANOVA test across responses and blank (with P < 0.05). No statistically significant differences were found across the 3 experimental conditions (P = 0.06). D: a small amount of the increase in direction selectivity over time in the optical motion stimulation and optical constant stimulation cases can be explained by an overall reduction in firing rate in the neurons across time. The slope of the correlation is significant (P < 0.001), but the amount of variance explained by response magnitude is relatively small (r2 = 0.12).
The optical system collects spatiotemporal patterns as emitted through a conventional microlens array-liquid crystal display (MLA-LCD) projector (Projector; NEC NP510, 3,000 lumens max output), of the type commonly in use in modern lecture halls, and condenses the images to millimeter and micrometer scale, effectively maintaining resolution while significantly increasing (∼10×) power per unit area. While a pattern-generating light source of this type represents the most cost-effective “out-of-the-box” solution for quick setup and ChR2 stimulation, the optical system is readily adaptable to any number of light-modulating technologies of sufficient power (e.g., laser in conjunction with SLM, etc.). The projector was mounted on a series of stacked translation stages (Newport M-443-4; ThorLabs L490) and projector mounts (Chief RMAO universal projector mount in SMLO configuration), giving complete control of the projector's x-, y-, and z-positions as well as the pitch and roll. The pitch of the projector mount was adjusted −15° to compensate for the 30° throw angle inherent to the model's projected beam. The projector zoom was set to the minimum setting, while the output mode was set to maximum luminance.
Projector path.
Light was collected from the projector (with its original lens in place, focus set in middle of range) first through a 50-mm SLR zoom lens (L1; Nikon Nikkor, focal length 50 mm, f/1.2 AI-s, flange toward projector) set to maximum aperture using f/1.2 and infinity focus and second through a visible achromat lens (L2; ThorLabs AC254-030-A, focal length 30 mm). After inverting twice, the light beam is reflected at the band-pass dichroic filter (DM; Semrock FF483/639) and focused onto the brain surface through a Nikon ×4 or ×10 objective (OBJ; Nikon ×4, NA 0.13, WD 17.2, Plan Fluor or Nikon ×10, NA 0.3, WD16, CFI Plan Fluor). The ∼2 × 50 mm distance between L1 and L2 facilitates collection of the light beam at the size of L2, which with a diameter of 25.4 mm is ∼50% of the aperture size of L1 but matches the size of the projector field as it exits the projector aperture. Because the distance between the projector aperture and L1 is kept to a minimum (i.e., is effectively zero), at infinity-focus the L1 zoom lens can be treated according to the thin-lens equation. Together L1 and L2 form an infinite-finite zoom system with an effective focal length of ∼62.5 mm (see Eq. A1).
| (A1) |
where d is distance between L1 and L2. A distance of 219 mm was then needed between L2 and DM in the cube-body to resize the image to the 2-in. cube aperture. Focal length distances were adjusted in both the imaging and stimulation paths to achieve near-parfocality. A tube lens (TL; ThorLabs LA1229-A, f = 175 mm) focused the infinity-corrected image onto a CCD camera (CCD; Dalsa, 1M60).
Epifluorescence path.
To image yellow fluorescent protein (YFP)-tagged ChR2 expression, a paraxial filter housing was constructed and affixed to the end of the ProjectorScope main cube-body, opposite the stimulator arm. The housing contains a YFP excitation filter (EX; Semrock FF01-500/24-25) and a series of centered washer rings into which is fed the front end of a liquid light guide, powered by a halide white-light source (Halide Light; Xcite 200DC, Lumen Dynamics). The DM used in the projector path is removed, and a rotating bracket fitted with a 2-in. round dichroic filter specialized for YFP imaging (DM′; Semrock FF520-Di02-50) was positioned in the main cube-body of the ProjectorScope 1 at a 45° angle with respect to the halide light input. Excitation light was focused onto the brain through the objective (OBJ). The image of excited expression zones on the brain (emitting Stokes-shifted light) was further refined by passage back through the dichroic DM′ as well as through a removable YFP emission filter (EM; Semrock FF01/542/27-25) slotted into the lens tube and imaged with the TL on the CCD.
Optomechanics.
In brief, the ProjectorScope 1 was constructed with a cage system (ThorLabs). The 52-mm thread of the nonflange end of L1 was directly screwed into the cage system via an adapter (LCP01 and SM2T2, ThorLabs); the projector arm and CCD arm came together in a 50.4-cm cube (LC6W, ThorLabs). L2 was held with a 50.48- to 25.4-mm cage conversion piece (LCP02, ThorLabs). The dichroic mirrors DM and DM′ were mounted and rotated with mirror rotation mounts (LB3C, ThorLabs).
For a full parts list and construction tips, see the ProjectorScope 1 Parts and Construction Guide (http://go.brandeis.edu/projectorscope1-0).
Footnotes
Supplemental Video S1 for this article is available online at the Journal website.
REFERENCES
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol 51: 16–31, 1984. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156, 2007. [DOI] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54: 205–218, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit's retina. J Physiol 178: 477–504, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci USA 108: 7595–7600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais B, Cooper LN, Shouval H. Formation of direction selectivity in natural scene environments. Neural Comput 12: 1057–1066, 2000. [DOI] [PubMed] [Google Scholar]
- Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17: 2112–2127, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8: 1263–1268, 2005. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis 10: 433–436, 1997. [PubMed] [Google Scholar]
- Braitenberg V, Schüz A. Anatomy of the Cortex: Statistics and Geometry. Berlin: Springer, 1991. [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459: 663–667, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman B, Stryker MP. Development of orientation selectivity in ferret visual cortex and effects of deprivation. J Neurosci 13: 5251–5262, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens JM, Ritter NJ, Roy A, Miller JM, Van Hooser SD. The laminar development of direction selectivity in ferret visual cortex. J Neurosci 32: 18177–18185, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BR, Moeller S, Tsao DY. Specialized color modules in macaque extrastriate cortex. Neuron 56: 560–573, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremieux J, Orban GA, Duysens J, Amblard B. Response properties of area 17 neurons in cats reared in stroboscopic illumination. J Neurophysiol 57: 1511–1535, 1987. [DOI] [PubMed] [Google Scholar]
- Crook JM, Eysel UT, Machemer HF. Influence of GABA-induced remote inactivation on the orientation tuning of cells in area 18 of feline visual cortex: a comparison with area 17. Neuroscience 40: 1–12, 1991. [DOI] [PubMed] [Google Scholar]
- Davison IG, Ehlers MD. Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron 70: 82–94, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, Hagiwara A, Bhalla US, Murthy VN, Albeanu DF. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci 13: 1404–1412, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feidler JC, Saul AB, Murthy A, Humphrey AL. Hebbian learning and the development of direction selectivity: the role of geniculate response timing. Network 8: 195–214, 1997. [Google Scholar]
- Ferster D, Chung S, Wheat H. Orientation selectivity of thalamic input to simple cells of cat visual cortex. Nature 380: 249–252, 1996. [DOI] [PubMed] [Google Scholar]
- Garkun Y, Maffei A. Cannabinoid-dependent potentiation of inhibition at eye opening in mouse V1. Front Cell Neurosci 8: 46, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci 9: 2432–2442, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjorgjieva J, Clopath C, Audet J, Pfister JP. A triplet spike-timing-dependent plasticity model generalizes the Bienenstock-Cooper-Munro rule to higher-order spatiotemporal correlations. Proc Natl Acad Sci USA 108: 19383–19388, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabska-Barwinska A, Ng BS, Jancke D. Orientation selective or not?—Measuring significance of tuning to a circular parameter. J Neurosci Methods 203: 1–9, 2012. [DOI] [PubMed] [Google Scholar]
- Grossman N, Poher V, Grubb MS, Kennedy GT, Nikolic K, McGovern B, Berlinguer Palmini R, Gong Z, Drakakis EM, Neil MA, Dawson MD, Burrone J, Degenaar P. Multi-site optical excitation using ChR2 and micro-LED array. J Neural Eng 7: 16004, 2010. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Channelrhodopsin-2 localised to the axon initial segment. PLoS One 5: e13761, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimel JA, Van Hooser SD, Nelson SB. Laminar organization of response properties in primary visual cortex of the gray squirrel (Sciurus carolinensis). J Neurophysiol 94: 3538–3554, 2005. [DOI] [PubMed] [Google Scholar]
- Honda M, Urakubo H, Tanaka K, Kuroda S. Analysis of development of direction selectivity in retinotectum by a neural circuit model with spike timing-dependent plasticity. J Neurosci 31: 1516–1527, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JC, Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci 360: 837–862, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Elyada Y, Van Hooser SD, Jiang J, Nelson A, Fitzpatrick D. Probing the function of circuits in visual cortex using optogenetics with spatial light modulator control (Abstract). SFN Annual Meeting 2011: 270.213/II213, 2011. [Google Scholar]
- Huang X, Elyada YM, Bosking WH, Walker T, Fitzpatrick D. Optogenetic assessment of horizontal interactions in primary visual cortex. J Neurosci 34: 4976–4990, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Shape and arrangement of columns in cat's striate cortex. J Physiol 165: 559–568, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey AL, Saul AB. Strobe rearing reduces direction selectivity in area 17 by altering spatiotemporal receptive-field structure. J Neurophysiol 80: 2991–3004, 1998. [DOI] [PubMed] [Google Scholar]
- Humphrey AL, Saul AB, Feidler JC. Strobe rearing prevents the convergence of inputs with different response timings onto area 17 simple cells. J Neurophysiol 80: 3005–3020, 1998. [DOI] [PubMed] [Google Scholar]
- Jonas JB, Schmidt AM, Muller-Bergh JA, Schlotzer-Schrehardt UM, Naumann GO. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci 33: 2012–2018, 1992. [PubMed] [Google Scholar]
- Kalil RE, Chase R. Corticofugal influence on activity of lateral geniculate neurons in the cat. J Neurophysiol 33: 459–474, 1970. [DOI] [PubMed] [Google Scholar]
- Kennedy H, Orban GA. Response properties of visual cortical neurons in cats reared in stroboscopic illumination. J Neurophysiol 49: 686–704, 1983. [DOI] [PubMed] [Google Scholar]
- Law MI, Zahs KR, Stryker MP. Organization of primary visual cortex (area 17) in the ferret. J Comp Neurol 278: 157–180, 1988. [DOI] [PubMed] [Google Scholar]
- Leifer AM, Fang-Yen C, Gershow M, Alkema MJ, Samuel AD. Optogenetic manipulation of neural activity in freely moving Caenorhabditis elegans. Nat Methods 8: 147–152, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci 9: 676–681, 2006. [DOI] [PubMed] [Google Scholar]
- Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature 456: 952–956, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456: 189–194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Amir Y, Harel M, Grinvald A. Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. Proc Natl Acad Sci USA 90: 10469–10473, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek M, Kager M, Van Hooser SD. Robust quantification of orientation selectivity and direction selectivity. Front Neural Circuits 8: 92, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain 120: 701–722, 1997. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Thompson ID, Tolhurst DJ. Receptive field organization of complex cells in the cat's striate cortex. J Physiol 283: 79–99, 1978a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon JA, Thompson ID, Tolhurst DJ. Spatial summation in the receptive fields of simple cells in the cat's striate cortex. J Physiol 283: 53–77, 1978b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci 12: 1308–1316, 2009. [DOI] [PubMed] [Google Scholar]
- Muresan RC, Jurjut OF, Moca VV, Singer W, Nikolic D. The oscillation score: an efficient method for estimating oscillation strength in neuronal activity. J Neurophysiol 99: 1333–1353, 2008. [DOI] [PubMed] [Google Scholar]
- Naka KI, Rushton WA. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185: 536–555, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JL, Yanagawa Y, Obata K, Callaway EM. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161: 441–450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S, Toth L, Sheth B, Sur M. Orientation selectivity of cortical neurons during intracellular blockade of inhibition. Science 265: 774–777, 1994. [DOI] [PubMed] [Google Scholar]
- Nikolenko V, Watson BO, Araya R, Woodruff A, Peterka DS, Yuste R. SLM microscopy: scanless two-photon imaging and photostimulation with spatial light modulators. Front Neural Circuits 2: 5, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg S, Pandarinath C. Retinal prosthetic strategy with the capacity to restore normal vision. Proc Natl Acad Sci USA 109: 15012–15017, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol 51: 78–109, 1976. [DOI] [PubMed] [Google Scholar]
- Packer AM, Peterka DS, Hirtz JJ, Prakash R, Deisseroth K, Yuste R. Two-photon optogenetics of dendritic spines and neural circuits. Nat Methods 9: 1202–1205, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer AM, Russell LE, Dalgleish HW, Hausser M. Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo. Nat Methods 12: 140–146, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandarinath C, Carlson ET, Nirenberg S. A system for optically controlling neural circuits with very high spatial and temporal resolution. Proc IEEE Int Symp Bioinformatics Bioeng 2013: 14026461, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442, 1997. [PubMed] [Google Scholar]
- Penfield W, Jasper HH. Epilepsy and the Functional Anatomy of the Human Brain (1st ed). Boston, MA: Little, Brown, 1954. [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci 10: 663–668, 2007. [DOI] [PubMed] [Google Scholar]
- Rao SC, Toth LJ, Sur M. Optically imaged maps of orientation preference in primary visual cortex of cats and ferrets. J Comp Neurol 387: 358–370, 1997. [DOI] [PubMed] [Google Scholar]
- Reichardt W. Autocorrelation, a principle for the evaluation of sensory information by the central nervous system. In: Sensory Communication, edited by Rosenblith WA. Cambridge, MA: MIT Press, 1961, p. 303–317. [Google Scholar]
- Rickgauer JP, Deisseroth K, Tank DW. Simultaneous cellular-resolution optical perturbation and imaging of place cell firing fields. Nat Neurosci 17: 1816–1824, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach DL, Shapley RM, Hawken MJ. Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci 22: 5639–5651, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter NJ, Roy A, Osik JJ, Van Hooser SD. The rapid emergence of direction selectivity across all layers of ferret primary visual cortex. Neuroscience Meeting Planner 2013: 259–04., 2013. [Google Scholar]
- Rockland KS, Lund JS, Humphrey AL. Anatomical binding of intrinsic connections in striate cortex of tree shrews (Tupaia glis). J Comp Neurol 209: 41–58, 1982. [DOI] [PubMed] [Google Scholar]
- Sakai S, Ueno K, Ishizuka T, Yawo H. Parallel and patterned optogenetic manipulation of neurons in the brain slice using a DMD-based projector. Neurosci Res 75: 59–64, 2013. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Britten KH, Newsome WT. Cortical microstimulation influences perceptual judgements of motion direction. Nature 346: 174–177, 1990. [DOI] [PubMed] [Google Scholar]
- Sato TK, Hausser M, Carandini M. Distal connectivity causes summation and division across mouse visual cortex. Nat Neurosci 17: 30–32, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul AB, Humphrey AL. Spatial and temporal response properties of lagged and nonlagged cells in cat lateral geniculate nucleus. J Neurophysiol 64: 206–224, 1990. [DOI] [PubMed] [Google Scholar]
- Saul AB, Humphrey AL. Temporal frequency tuning of direction selectivity in cat visual cortex. Vis Neurosci 8: 365–372, 1992. [DOI] [PubMed] [Google Scholar]
- Shon AP, Rao RP, Sejnowski TJ. Motion detection and prediction through spike-timing dependent plasticity. Network 15: 179–198, 2004. [PMC free article] [PubMed] [Google Scholar]
- Smith GB, Sederberg A, Elyada YM, Van Hooser SD, Kaschube M, Fitzpatrick D. The development of cortical circuits for motion discrimination. Nat Neurosci 18: 252–261, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirman JN, Crane MM, Husson SJ, Gottschalk A, Lu H. A multispectral optical illumination system with precise spatiotemporal control for the manipulation of optogenetic reagents. Nat Protoc 7: 207–220, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez H, Koch C, Douglas R. Modeling direction selectivity of simple cells in striate visual cortex within the framework of the canonical microcircuit. J Neurosci 15: 6700–6719, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser SD, Escobar GM, Maffei A, Miller P. Emerging feed-forward inhibition allows the robust formation of direction selectivity in the developing ferret visual cortex. J Neurophysiol 111: 2355–2373, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser SD, Heimel JA, Chung S, Nelson SB. Lack of patchy horizontal connectivity in primary visual cortex of a mammal without orientation maps. J Neurosci 26: 7680–7692, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser SD, Li Y, Christensson M, Smith GB, White LE, Fitzpatrick D. Initial neighborhood biases and the quality of motion stimulation jointly influence the rapid emergence of direction preference in visual cortex. J Neurosci 32: 7258–7266, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Reyn CR, Breads P, Peek MY, Zheng GZ, Williamson WR, Yee AL, Leonardo A, Card GM. A spike-timing mechanism for action selection. Nat Neurosci 17: 962–970, 2014. [DOI] [PubMed] [Google Scholar]
- Watson BO, Nikolenko V, Araya R, Peterka DS, Woodruff A, Yuste R. Two-photon microscopy with diffractive optical elements and spatial light modulators. Front Neurosci 4: 29, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weliky M, Bosking WH, Fitzpatrick D. A systematic map of direction preference in primary visual cortex. Nature 379: 725–728, 1996. [DOI] [PubMed] [Google Scholar]
- Wenisch OG, Noll J, Hemmen JL. Spontaneously emerging direction selectivity maps in visual cortex through STDP. Biol Cybern 93: 239–247, 2005. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Runyan CA, Wang FL, Sur M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488: 343–348, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Fajardo O, Shum J, Zhang Scharer YP, Friedrich RW. High-resolution optical control of spatiotemporal neuronal activity patterns in zebrafish using a digital micromirror device. Nat Protoc 7: 1410–1425, 2012. [DOI] [PubMed] [Google Scholar]
- Zorzos AN, Scholvin J, Boyden ES, Fonstad CG. Three-dimensional multiwaveguide probe array for light delivery to distributed brain circuits. Opt Lett 37: 4841–4843, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.