Abstract
Adenosine is a major signaling nucleoside that orchestrates cellular and tissue adaptation under energy depletion and ischemic/hypoxic conditions by activation of four G protein-coupled receptors (GPCR). The regulation and generation of extracellular adenosine in response to stress are critical in tissue protection. Both mouse and human studies reported that extracellular adenosine signaling plays a beneficial role during acute states. However, prolonged excess extracellular adenosine is detrimental and contributes to the development and progression of various chronic diseases. In recent years, substantial progress has been made to understand the role of adenosine signaling in different conditions and to clarify its significance during the course of disease progression in various organs. These efforts have and will identify potential therapeutic possibilities for protection of tissue injury at acute stage by upregulation of adenosine signaling or attenuation of chronic disease progression by downregulation of adenosine signaling. This review is to summarize current progress and the importance of adenosine signaling in different disease stages and its potential therapeutic effects.
Keywords: adenosine signaling, disease, hypoxia, therapy
Metabolism of Adenosine
Adenosine is ubiquitously produced in almost all of the cells in our bodies under physiological condition and further produced under hypoxia or energy depletion condition. As a building block and a critical intermediate metabolite of nucleic acids, adenosine is a key signaling molecule that orchestrates the cellular response to hypoxia, energy depletion, and tissue damage by activation of its G protein-coupled receptors (GPCR) on multiple cell types (59). Under normal physiological conditions, both extracellular and intracellular adenosine levels are in the nanomolar range. However, under stress conditions, ATP is released from injured cells such as endothelial cells (8), neutrophils (36), and glial cells (9) via transmembrane protein channels including pannexins (135) or connexins (36, 135) and subsequently dephosphorylated to extracellular adenosine by ecto-nucleotidases including CD39, which converts ATP to ADP/AMP and CD73, which converts AMP to adenosine (22, 34). Under pathological conditions, extracellular adenosine concentrations can reach the millimolar range (94). Generation of extracellular adenosine through these pathways is the major source of extracellular adenosine production under hypoxia-induced injury. In addition, extracellular adenosine is regulated by adenosine deaminase (ADA), which is responsible for the degradation of extracellular adenosine to inosine (134). Moreover, extracellular adenosine signaling is terminated by equilibrative nucleoside transporters (ENTs), which are involved in the cellular uptake of adenosine. Once inside the cell, adenosine is metabolized by three enzymes, adenosine kinase (ADK), S-adenosylhomocytesine hydrolase (SAHH), and adenosine deaminase (ADA). ADA catalyzes the irreversible conversion of adenosine to inosine. SAHH converts adenosine to adenosylhomocysteine (AdoHcy). ADK phosphorylates adenosine to AMP and is critical for regulating intracellular levels of adenosine and maintaining intracellular levels of adenine nucleotides (94). Intracellular adenosine homeostasis is also maintained by bidirectional equilibrative nucleoside transporters (ENTs) on the plasma membrane through facilitated diffusion of adenosine in the direction of the concentration gradient (Fig. 1) (70).
Fig. 1.
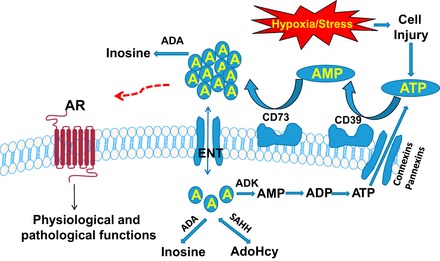
Metabolism of adenosine signaling. Cells release ATP through connexins or pannexins channels under hypoxia and other stress conditions. The accumulation of extracellular ATP is dephosphorylated to adenosine (A) by 2 ecto-nucleotidases including CD39 and CD73. Adenosine can further be metabolized by adenosine deaminase (ADA) to inosine or functions as a signaling molecule by activation of its adenosine receptors (AR) on multiple cell types. Once uptake by equilibrative nucleoside transporters (ENTs), adenosine is further metabolized by adenosine kinase (ADK) to AMP, adenosine deaminase (ADA) to inosine, or S-adenosylhomocytesine hydrolase (SAHH) to adenosylhomocysteine (AdoHcy).
Adenosine Signaling via Adenosine Receptors
Increases in extracellular adenosine in turn elicit various responses on target cells by engaging cell surface adenosine receptors both in physiological and pathological conditions (44). As GPCRs, adenosine receptors all have a single polypeptide chain that is a structural motif forming seven transmembrane helices. There are four adenosine receptors (ADORA1, ADORA2A, ADORA2B, and ADORA3), and each receptor has a cellular or tissue specific distribution and distinct affinity for adenosine(33). ADORA1, ADORA2A, and ADORA3 have a high affinity to extracellular adenosine, while ADORA2B has the lowest affinity to extracellular adenosine. Thus ADORA2B is normally activated under pathological conditions due to excess accumulation of extracellular adenosine. ADORA1 and ADORA3 adenosine receptors are coupled to adenylyl cyclase by the inhibitory G-protein subunit (Gαi) and thereby can lower intracellular levels of the second messenger cyclic adenosine monophosphate (cAMP). In contrast, the ADORA2A and ADORA2B adenosine receptors are commonly coupled to adenylyl cyclase by the stimulatory G-protein subunit (Gαs) and therefore can induce intracellular cAMP levels. Therefore, signaling through adenosine receptors plays important roles in the regulation of intracellular cAMP and thereby regulates multiple cellular functions including vasodilation in endothelial cells, neurotransmitter release from neuronal cells, neutrophil chemotaxis, and vascular smooth muscle cell relaxation (63, 64) (Fig. 2). In addition, other signaling molecules including phospholipase C (PLC), calcium, nitric oxide (NO), reactive oxygen species (ROS), phosphatidylinositol 3-kinase (PI3K)-AKT, extracellular signal-protein kinase (ERK), and mitogen-activated protein kinases (MAPKs) are implicated functioning downstream of adenosine receptors and subsequently regulating multiple cellular functions. For example, activation of ADORA2A stimulates the PLC pathway and adenylate cyclase pathway (47). ADORA2A signaling is also engaged in modulation of neutrophil function by regulating production of ROS (23, 119). By modulation of NO production via vascular endothelial cells, adenosine through ADORA2A receptor functions as a potent vasodilator involved in tissue blood flow and cellular homeostasis (55, 80). In addition, shear stress-mediated elevation of adenosine activates ADORA2B, subsequently contributes to endothelial NO synthase phosphorylation via PI3K-AKT, and further generates NO (122). Both pharmacological and genetic studies show that adenosine ADORA2B induces inflammatory cytokine interleukin 6 (IL-6) and contributes to the renal fibrosis (24). The activation of ADORA3 triggers MAPK and contributes to the critical role of cell growth, survival, and differentiation (104). Other studies reported that activation of ADORA3 modulates the proliferation of melanoma cells by regulation of ERK pathway (Fig. 2) (86). Thus activation of adenosine receptors is involved in multiple cellular function via multiple downstream signaling cascade.
Fig. 2.
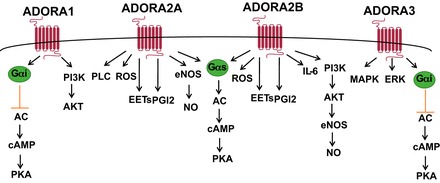
Adenosine receptor-mediated signaling pathways. Extracellular adenosine functions as signaling molecule by engaging cell surface adenosine receptors (ADORA1, ADORA2A, ADORA2B, and ADORA3). ADORA1 and ADORA3 adenosine receptors are coupled to adenylyl cyclase (AC) by the inhibitory G-protein subunit (Gαi) and thereby can lower intracellular levels of the second messenger cyclic adenosine monophosphate (cAMP). In contrast, the ADORA2A and ADORA2B adenosine receptors can induce AC by the stimulatory G-protein subunit (Gαs) and therefore can induce intracellular cAMP levels. Activation of both ADORA1 and ADORA2B stimulates phosphatidylinositol 3-kinase (PI3K)/AKT pathway, and activation of both ADORA2A and ADORA2B induces release of ROS, EETs, and PGI2. PKA, protein kinase A; PLC, phospholipase C; ROS, reactive oxygen species; EETs, epoxyeicosatrienoic acids; PGI2, prostacyclin; eNOS, endothelial NO synthase; NO, nitric oxide; IL-6, interleukin 6; MAPK, mitogen-activated protein kinases; ERK, extracellular signal-protein kinase.
Adenosine Signaling in Physiological and Pathological Conditions
Adenosine is involved in numerous critical physiological processes via activation of its adenosine receptors including modulation of nervous system, immune response, vascular function and metabolism (64, 93). Adenosine-mediated biological function is mainly dependent on activation of adenosine receptors, and responses of these cell surface receptors are predominantly determined by adenosine concentrations. Since adenosine levels are generally lower than 1 μM under physiological condition, most function of adenosine signaling is through activation of ADORA1, ADORA2A, or ADORA3 adenosine receptors, which have EC50 values between 0.01 and 1 μM. In contrast, activation of ADOAR2B requires a higher adenosine concentrations, which generally exist under pathophysiological conditions (46). With the development and generation of various adenosine receptors agonists or antagonists and four adenosine receptor knockout mouse models, adenosine signaling has been demonstrated as an essential player under pathophysiological conditions by modulation of inflammation, ischemic tissue injury, fibrosis, and tissue remodeling (38, 91, 109).
Beneficial Role of Adenosine Signaling During Acute States
Recent studies indicate that extracellular adenosine functions as a signaling molecule that plays an essential role in adaptation to stress especially hypoxia (10, 91, 108). Extracellular adenosine is induced during limited oxygen availability or acute injury, and adenosine is critical for hypoxia adaptation, maintenance of cellular function, and protection of hypoxia-induced tissue injury. Under acute hypoxic conditions, adenosine plays various beneficial roles including vasodilatory effect, antivascular endothelial leakage, and anti-inflammatory response (28, 35, 60, 67, 78).
Beneficial role in acute heart injury.
The beneficial role of adenosine in acute stage was initially found in cardiovascular system showing that adenosine functions as a potent vasodilator increasing blood flow to coronary arteries (106). Later on, adenosine was implicated to play a generally protective role in the heart by regulation of heart rate, coronary flow, contraction, inflammatory control, and tissue remodeling (127). All four adenosine receptors are known to be involved in coronary flow. Generally, previous studies (12, 73, 103) identified the expression of ADORA1 in atrial muscle cells and adenosine exerts its cardiac electrophysiologic effects mainly through the activation of ADORA1 that leads to a reduction in contraction rate (Table 1). The adenosine ADORA2A receptor is the major receptor subtype responsible for coronary blood flow regulation in endothelial-dependent and -independent manner (5), and a previous study (7) reported that adenosine increase coronary flow via vasodilation by promotion of prostacyclin release. Additional studies (135) have shown that adenosine via ADORA2A contributes to coronary reactive hyperemia by promoting the ROS release. Regadenoson (Lexiscan), a specific ADORA2A agonist, was approved by FDA and utilized for diagnosis of myocardial perfusion imaging (89). In addition, the Eltzschig group (32) demonstrated that CD73-mediated adenosine signaling via the ADORA2B is important in cardioprotection by ischemic preconditioning. However, Chen and colleagues (84) reported that selective inhibition of adenosine ADORA3 receptor significantly attenuate pressure overload-induced left ventricular hypertrophy and dysfunction. These results suggest that selective CD73 agonists and ADORA2B agonists are potential therapeutic possibilities for myocardial ischemia, and specific ADORA3 antagonists may be a novel strategy to counteract pressure overload-induced left ventricular hypertrophy and dysfunction (32, 84).
Table 1.
The beneficial role of adenosine signaling in acute states
| Organs/Adenosine Receptors | Functions | Cell Types | References |
|---|---|---|---|
| Heart | |||
| ADORA1 | Slow heart rate | Atrial muscle cells | 12, 73, 103 |
| ADORA2A | Vasodilation | Endothelial cells | 5, 7, 89 |
| ADORA2B | Ischemic preconditioning | — | 32 |
| Lung | |||
| ADORA2A | Anti-inflammation | Immune cells | 31 |
| ADORA2B | Vascular barrier function | Endothelial cells | 29, 101 |
| ADORA3 | Anti-inflammation | Eosinophils/neutrophils | 31 |
| Kidney | |||
| ADORA1 | Anti-inflammation/apoptosis | Immune cells | 66, 72, 76, 77 |
| ADORA2A | Anti-inflammation | Immune cells | 25, 92 |
| ADORA2B | Vascular barrier function | Endothelial cells | 51 |
| Brain | |||
| ADORA1 | Inhibit excitatory transmission | Synapse | 27, 45, 120 |
| ADORA2A | Increase cerebral blood flow | Endothelial/glial cells | 9,74 |
| ADORA3 | Antiapoptosis | — | 15 |
| Cochlea | |||
| ADORA1 | Antioxidants | Cochlear hair cell | 57, 58, 126 |
| Obesity | |||
| ADORA2A | Promote thermogenesis | Brown adipose tissue | 49 |
| Liver | |||
| ADORA2A | Anti-inflammation | Immune cells | 26 |
| Skin | |||
| ADORA2A | Would healing | Endothelial cells/immune cells | 88 |
| Intestine | |||
| ADORA2B | Anti-inflammation | Immune cells | 21, 37, 53, 54 |
Beneficial role in acute lung injury.
Acute lung injury (ALI) is defined as pulmonary edema and severe hypoxia. Multiple factors including pneumonia, aspiration or lung contusion or indirect injury such as sepsis, severe trauma, or blood transfusion cause ALI. Approximately 200,000 patients develop ALI in the US annually. However, due to the lack of understanding the molecular mechanism involved in the development and progression of ALI, no effective therapeutic options are available. Several groups reported that adenosine serves beneficial functions on features of ALI such as enhancing alveolar-capillary barrier function and dampening inflammation and substantially protects against ALI resulting from hypoxia or ischemia (31, 112). Follow-up genetic and pharmacological studies reported that the adenosine-mediated beneficial role in ALI is via ADORA2B in a CD73-dependent manner (30, 101). Therefore, these studies provide potential development of adenosine-based therapies for the treatment of ALI (1, 29, 31).
Beneficial role in acute kidney injury.
Acute kidney injury (AKI), characterized as the rapid dysfunction of kidney, is currently the leading cause of mortality and morbidity in hospitalized patients; therefore, effective therapeutic strategies are urgently needed. Among multiple factors, renal ischemia is the most common cause of AKI. Previous studies indicated that all four adenosine receptors are expressed in the kidney and are involved in the progression of AKI (4). Particularly, several studies reported that the adenosine ADORA1 receptor signaling protects the kidney from ischemia-reperfusion injury (66, 72, 76, 77). Linder's group (25, 92) showed that adenosine ADORA2A receptor signaling prevents ischemia-induced injury via modulation of inflammatory cells. Additional studies showed that adenosine ADORA2A coupled with epoxyeicosatrienoic acids (EETs) plays an important role in the regulation of preglomerular microvascular tone (19), adenosine-mediated induction of EETs via ADORA2A is required for maintenance of normal renal function under high dietary salt intake, and ADORA2A and EETs are therapeutic targets for salt-sensitive hypertension (61). In addition, pharmacological and genetic studies demonstrated that adenosine ADORA2B signaling is involved in renal protection during preconditioning (51). In contrast, activation of the ADORA3 is implicated as detrimental during renal ischemia (76).
Beneficial role in brain.
As an important signaling molecule, adenosine coupling with its specific receptor functions as an upstream neuromodulator of neurotransmitters involved in the homeostasis and modulation of multiple brain function (17, 95). For example, previous studies have demonstrated that adenosine is present in the extracellular fluid in brain, its level is dramatically induced in the condition of hypoxia or ischemia, and it subsequently plays a critical role through activation of its specific receptors. Although all four adenosine receptors are expressed in the mouse forebrain, ADORA1 and ADORA2A have the highest abundance in the brain. Thus those two adenosine receptors play critical roles in the brain function, while ADORA2B and ADORA3 have a relatively modest impact on brain function (45, 50). It is found that ADORA1 is located presynaptically, postsynaptically, and nonsynaptically in the brain (45) and mainly underlies effect of adenosine in neuronal circuits by selectively depressing excitatory synaptic transmission (27). Both pharmacological evidence and genetic ADORA1 knockout mouse studies demonstrated neuroprotective role of ADORA1 in ischemia/hypoxia models of brain injury (120). In contrast, ADORA2A is demonstrated to have a widespread distribution in the brain (45). Chen and other groups suggested that adenosine signaling via ADORA2A is neuroprotective under different pathological conditions (120) including hypoxia (52), ischemia (75, 83), and hypoglycemia (11). Mechanistic studies demonstrated that adenosine is involved in brain repair through activation of ADORA2A in glial cells, which promotes the effects of cytokines release including induction of oxygenase 2, NOS activity, NO production, and upregulation of nerve growth factor expression (9). More importantly, adenosine plays a protective effect in the brain through ADORA2A by controlling brain vascular function through endothelial cells. For instance, ADORA2A plays a beneficial role in preventing brain ischemia by induction of cerebral blood flow (CBF) in multiple conditions including energy failure, tissue acidosis, imbalance of ion homeostasis, and cytotoxic edema (97, 107). In addition, Winn and colleagues (74) found that the capacity to modulate CBF in response to hypotension was significantly impaired in ADORA2A knockout mice and treatment with the extracellular adenosine transporter inhibitor dipyridamole significantly increases circulating adenosine concentrations and subsequently improves CBF in mice, indicating the importance of adenosine ADORA2A in physiologic vascular regulation of CBF. Furthermore, both pharmacological and genetic studies demonstrated that ADORA2A stimulates proliferation of Schwann cells (111). Other studies indicated that adenosine is one of the mediators of cerebral vasodilation by triggering release of ROS via both ADORA2A and ADORA2B in brain (48). Additional studies showed that specific activation of other adenosine receptors contributes to the adenosine-mediated neuroprotective effects as well. For instance, preclinical study showed that the ADORA3-specific agonist prevents ischemic brain injury through suppression of apoptosis in wild-type mice, but not in the ADORA3-deficient mice (15). Clinical human studies demonstrated that adenosine plays a role of vasodilatation in the cerebral circulation, which can be applied for investigation of cerebrovascular perfusion capacity in patients with carotid occlusive disease (48). Overall, adenosine signaling via its specific receptors plays an important role in brain function and modulating adenosine signaling is likely an effective treatment for brain ischemic injury and damage.
Beneficial role in multiple organ damage at acute states.
Adenosine was reported to be beneficial under stress conditions in various organs and tissues through different adenosine receptors (81, 88, 115, 117). Several studies reported that adenosine plays an otoprotective role in the auditory system to counteract intense noise exposure via activation of ADORA1 (57, 58, 126). Cronstein's group (88) demonstrated that adenosine ADORA2A signaling plays beneficial role in skin by promoting would healing and angiogenesis. Colgan and colleagues (21, 37, 53, 54) used pharmacologic and genetic approaches to show that adenosine signaling via the ADORA2B receptor attenuates tissue injury and inflammation in mucosal organs during intestinal ischemia and colitis. Gnad et al. (49) demonstrated that adenosine stimulates brown adipose tissue thermogenesis via ADORA2A, and the ADORA2A-selective agonist prevents high fat diet-induced obesity in mice. Linden's group (26) reported that elevated adenosine protects against ischemic reperfusion liver injury via ADORA2A signaling. In addition, they showed that ADORA2A signaling prevents pulmonary inflammation in a sickle cell disease (SCD) mouse model by reducing invariant natural killer cells. Therefore, the FDA-approved ADORA2A-specific agonist regadenoson is currently utilized to conduct a clinical trial in the treatment of patients with SCD (42, 43).
Summary for beneficial role of adenosine signaling in acute states.
Adenosine is induced under stress conditions including hypoxia, ischemia, or inflammation. Elevated adenosine subsequently activates four widely expressed adenosine receptors and attenuates tissue injury or promote regeneration of damage tissues.
Detrimental Role of Adenosine Signaling in Chronic Disease States
Although elevated adenosine signaling shows beneficial effects in various organs in response to acute stress or injury, numerous examples indicate that prolonged excessive adenosine signaling is detrimental and contributes to the development and progression of certain chronic disease states.
Elevated adenosine contributes to sickling and progression of SCD.
SCD is a devastating genetic hemolytic disorder associated with high morbidity and mortality worldwide. Adenosine is well known to be induced under hypoxic conditions, and SCD patients are under a chronic state of hypoxia. By using high throughput metabolomic screening combined with a multidisciplinary approach, Zhang et al. (114, 133) identified the adenosine signaling via erythrocyte ADORA2B causing induction of 2,3-bisphosphoglycerate, increasing deoxygenation of sickle hemoglobin, and subsequently triggering sickling and disease progression in SCD. They found that polyethylene glycol-modified adenosine deaminase (PEG-ADA) treatment significantly decreased circulating adenosine levels in the SCD Berkeley mice, and subsequently reduced sickling and improved multiorgan damage, which was reflected by less vascular damage and vascular congestion in liver and lung, attenuated splenomegaly, and decreased proteinuria. Similar improvement was also seen in SCD Berkeley mice treated with PSB1115, an ADORA2B-specific antagonist. These findings demonstrated that elevated plasma adenosine via activation of ADORA2B receptor on erythrocyte leads to RBC sickling, hemolysis, and multitissue damage in sickle cell transgenic mice. Suppression of adenosine ADORA2B signaling by PEG-ADA or an ADORA2B receptor specific antagonist reduced the disease phenotype, thereby revealing potential therapeutic possibilities for SCD (134).
Differential role of adenosine signaling in priapism and erectile dysfunction.
Priapism is defined as persistent penile erection without sexual excitation. The condition stems from a persist relaxation of corpus cavernosal smooth muscle cells, therefore allowing continued engorgement of the corpus cavernosum and persistent unwanted erection. Priapism is a painful pathological condition and it carries a risk of fibrosis that may cause permanent damage to the penis and ultimately erectile dysfunction (90). By using two independent lines of mutant mice including adenosine deaminase-deficient mice and SCD transgenic mice, Mi et al. (87) reported an unexpected discovery that excess adenosine in the penis, coupled with elevated ADORA2B signaling, contributes to priapism. Follow-up mechanistic studies demonstrated that adenosine ADORA2B signaling-mediated prolonged penile erection is via cAMP and cGMP activation (87). These findings provide evidence that excess extracellular adenosine contributes to development of priapism via adenosine ADORA2B receptor and adenosine-mediated therapeutic strategies including PEG-ADA and ADORA2B antagonists are likely novel effective therapeutic treatments for priapism (121-125).
Persistently elevated placental adenosine is pathogenic for preeclampsia.
Preeclampsia (PE), a gestation-specific hypertensive syndrome, has a high incidence of mother and infant morbidity and mortality. The placentas that link mothers and fetuses are newly formed organs during pregnancy. Impairment in placental development and function is one of the major factors contributing to the pathogenesis of PE. However, the molecular basis responsible for placental impairment-mediated PE has not been fully understood. Intriguingly, previous studies reported that adenosine levels are significantly elevated in the maternal or fetal circulation of PE patients compared with normal pregnant women and are correlated with disease severity (39, 129). Another earlier study found that elevated adenosine in PE patients is correlated to Th1/Th2 imbalance (130). In vitro studies indicated that elevated adenosine is related to increased platelet aggregation and P-selectin expression (128). Genetic and pharmacologic studies revealed that chronic elevated adenosine is a previously unrecognized key factor contributing to PE. Mechanistic studies demonstrated that chronic elevated placental CD73-mediated accumulation of placental adenosine coupled with excess ADORA2B contributes to the features of PE including hypertension, proteinuria, and small gestational age for fetuses (62). Therefore, these studies implicate the novel therapeutic approach by using adenosine-based strategies including PEG-ADA, CD73 inhibitor, and ADORA2B antagonist to prevent features of PE and attenuate the morbidity and mortality of PE in humans.
Sustained elevated adenosine causes chronic lung disease.
Chronic lung diseases include pulmonary hypertension and pulmonary fibrosis. Pulmonary hypertension is a common complication of interstitial lung diseases, and pulmonary fibrosis is a component of various interstitial pneumonias (116). These disorders are defined by severity of inflammation, abnormal fibroblast proliferation, and extracellular matrix deposition, which cause distortion of pulmonary architecture and pulmonary dysfunction. To elucidate the role of adenosine signaling in the pathophysiology of chronic lung diseases, multiple animal models were used (137). In particular, Blackburn's group (6) utilized two independent animal models including ADA knockout mice and bleomycin-induced mice models. They demonstrated that chronic elevation of adenosine results in severe features of chronic lung injury such as airspace enlargement, fibrosis, cardinal signs of chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF) (6, 20, 71, 102, 113), dysfunction of gas exchange, and the development of hallmarks of pulmonary hypertension (40, 71). Mechanistically, ADORA2B signaling was found to be correlated with elevation of pulmonary hypertension factors such as IL-6, matrix metalloproteins, and endothelin-1 (71, 113, 136). Furthermore, pharmacologic or genetic suppression of ADORA2B abolished the progression of air space enlargement(113), fibrosis (20) and pulmonary hypertension (69, 71, 131).
Excess adenosine plays an important role in chronic kidney disease.
Chronic kidney disease (CKD) is a worldwide devastating disease including kidney injury, progression to renal fibrosis, and end-stage renal failure. The reactive treatments rarely restore normal kidney function, and preventive approaches to limit renal fibrosis are lacking due to poorly understood underlying mechanism for its progression. Zhang et al. (132) found that, in three independent lines of mice, one with a genetic deficiency in ADA, one with angiotensin II-infused mice and another with surgically manipulated unilateral ureteral obstruction, chronic elevations of renal adenosine level contribute to hallmarks of CKD including severe kidney injury, fibrosis, and hypertension. Moreover, follow-up studies demonstrated that the adenosine-mediated detrimental role in CKD is in an ADORA2B receptor-dependent manner and that inhibition of adenosine ADORA2B signaling attenuates the progression and features of CKD (24). Taken together, these studies identified the pathophysiological role; determined molecular basis of chronic elevated adenosine signaling in CKD, hypertension, and renal fibrosis; and highlighted novel adenosine-based therapeutic possibilities (132).
Adenosine signaling in multiple chronic conditions.
Adenosine signaling is known in the prevention of acute tissue injury under most pathophysiological conditions. In contrast, excess elevation of adenosine has been indicated in the progression and development of chronic diseases states. In particular, a previous study (93) reported that accumulation of extracellular adenosine activates cAMP pathway through ADORA2B and subsequently induces the apoptosis of arterial smooth muscle cells and contributes to the pathogenesis of atherosclerosis or restenosis. Chan and colleagues (13, 41) reported that the ADORA2A antagonist significantly prevents the development of dermal fibrosis in the model of excess elevated tissue adenosine and ADORA2A-deficient mice are protected from bleomycin-induced dermal fibrosis, which implicates the detrimental role of adenosine in the development of skin disorder (Table 2). Other studies demonstrated that adenosine via the ADORA2A receptor contributes to the pathogenesis of hepatic fibrosis, which suggests a novel therapeutic strategy in the treatment of hepatic cirrhosis (14). In addition, Chen and colleagues reported that mice lacking ADORA2A display reduced brain damage postfocal ischemia (16), and pharmacologic studies using ADORA2A antagonists showed that ADORA2A blockade confers the protective effect in brain ischemia animal models by regulation of glutamate release, excitotoxicity, and generation of oxidents (85, 96), indicating a neuroprotective role of ADORA2A blockade. Recently, both human epidemiologic studies and animal results suggest that inactivation of ADORA2A plays a neuroprotective role to prevent neuronal degeneration (18, 68, 105). Thereby, blockade of ADORA2A is considered as a leading nondopaminergic drug for Parkinson's disease (PD) patients, and several ADORA2A antagonists have entered phase II and III clinical trials for advanced PD patients (3, 65, 79, 110). In addition, several perspective studies reported that with increased consumption of caffeine, a common adenosine antagonist, reduced risk of developing multiple diseases including PD disease (2, 99), Alzheimer's disease (82, 98), chronic liver disorder (56), and diabetes (100, 118).
Table 2.
The detrimental role of adenosine signaling in chronic states
| Adenosine Receptors/Organs | Functions | References |
|---|---|---|
| ADORA2A | ||
| Skin disorder | Dermal fibrosis | 13, 41 |
| Hepatic dysfunction | Hepatic fibrosis and hepatic cirrhosis | 14 |
| Brain damage | Glutamate release, excitotoxicity, and generation of oxidents | 16, 85, 96 |
| Parkinson's disease | Neuronal degeneration | 2, 3, 65, 79, 99, 105 |
| ADORA2B | ||
| Sickle cell disease | Erythrocyte sickling and multiple organ damage in sickle cell disease | 114, 133, 134 |
| Priapism | Priapism and penis fibrosis | 87, 90, 121–125 |
| Preeclampsia | Preeclampsia including proteinuria, hypertension small fetus | 62 |
| Chronic pulmonary disease | Pulmonary fibrosis, hypertension | 6, 20, 71, 102 |
| Chronic kidney disease | Renal fibrosis and hypertension | 24, 132 |
Conclusion
Acutely accumulated extracellular adenosine is considered a beneficial metabolite involved in cellular and tissue adaptation under energy depletion and ischemic/hypoxic conditions. However, prolonged excess extracellular adenosine is detrimental and contributes to development and progression of various chronic diseases. Therefore, it is critical to define the specific roles of adenosine signaling during the course of disease progression in various organs. Understanding the differential roles of adenosine signaling will provide potential therapeutic possibilities for protection of tissue injury at acute stage by upregulation of adenosine signaling or attenuation of chronic disease progression by downregulation of adenosine signaling.
GRANTS
This work was supported by National Institute of Health Grants HL-119549 (to Y. Xia), DK-083559 (to Y. Xia), and HL-1135-74 (to Y. Xia) and American Heart Association Grant 12IRG9150001 (to Y. Xia).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.L. and Y.X. conception and design of research; H.L. prepared figures; H.L. drafted manuscript; H.L. and Y.X. edited and revised manuscript; H.L. and Y.X. approved final version of manuscript.
REFERENCES
- 1.Alencar AK, Pereira SL, Montagnoli TL, Maia RC, Kummerle AE, Landgraf SS, Caruso-Neves C, Ferraz EB, Tesch R, Nascimento JH, de Sant'Anna CM, Fraga CA, Barreiro EJ, Sudo RT, Zapata-Sudo G. Beneficial effects of a novel agonist of the adenosine A2A receptor on monocrotaline-induced pulmonary hypertension in rats. Br J Pharmacol 169: 953–962, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 50: 56–63, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bara-Jimenez W, Sherzai A, Dimitrova T, Favit A, Bibbiani F, Gillespie M, Morris MJ, Mouradian MM, Chase TN. Adenosine A(2A) receptor antagonist treatment of Parkinson's disease. Neurology 61: 293–296, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22: 14–20, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther 284: 1066–1073, 1998. [PubMed] [Google Scholar]
- 6.Blackburn MR, Volmer JB, Thrasher JL, Zhong H, Crosby JR, Lee JJ, Kellems RE. Metabolic consequences of adenosine deaminase deficiency in mice are associated with defects in alveogenesis, pulmonary inflammation, and airway obstruction. J Exp Med 192: 159–170, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blass KE, Forster W, Zehl U. Coronary vasodilation: interactions between prostacyclin and adenosine. Br J Pharmacol 69: 555–559, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodin P, Burnstock G. Increased release of ATP from endothelial cells during acute inflammation. Inflamm Res 47: 351–354, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ 17: 1071–1082, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouma MG, van den Wildenberg FA, Buurman WA. The anti-inflammatory potential of adenosine in ischemia-reperfusion injury: established and putative beneficial actions of a retaliatory metabolite. Shock 8: 313–320, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Calabresi P, Centonze D, Pisani A, Bernardi G. A possible mechanism for the aglycemia-induced depression of glutamatergic excitation in the striatum. J Cereb Blood Flow Metab 17: 1121–1126, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Carr CS, Hill RJ, Masamune H, Kennedy SP, Knight DR, Tracey WR, Yellon DM. Evidence for a role for both the adenosine A1 and A3 receptors in protection of isolated human atrial muscle against simulated ischaemia. Cardiovasc Res 36: 52–59, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Chan ES, Fernandez P, Merchant AA, Montesinos MC, Trzaska S, Desai A, Tung CF, Khoa DN, Pillinger MH, Reiss AB, Tomic-Canic M, Chen JF, Schwarzschild MA, Cronstein BN. Adenosine A2A receptors in diffuse dermal fibrosis: pathogenic role in human dermal fibroblasts and in a murine model of scleroderma. Arthritis Rheum 54: 2632–2642, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, Friedman SL, Cronstein BN. Adenosine A(2A) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol 148: 1144–1155, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res 84: 1848–1855, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19: 9192–9200, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JF, Lee CF, Chern Y. Adenosine receptor neurobiology: overview. Int Rev Neurobiol 119: 1–49, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci 21: RC143, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol 141: 441–448, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol 175: 1937–1946, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol 74: 153–175, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5’-nucleotidase (CD73). Purinergic Signal 2: 351–360, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronstein BN, Rosenstein ED, Kramer SB, Weissmann G, Hirschhorn R. Adenosine; a physiologic modulator of superoxide anion generation by human neutrophils. Adenosine acts via an A2 receptor on human neutrophils. J Immunol 135: 1366–1371, 1985. [PubMed] [Google Scholar]
- 24.Dai Y, Zhang W, Wen J, Zhang Y, Kellems RE, Xia Y. A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J Am Soc Nephrol 22: 890–901, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest 112: 883–891, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol 286: G285–G293, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Dunwiddie TV, Haas HL. Adenosine increases synaptic facilitation in the in vitro rat hippocampus: evidence for a presynaptic site of action. J Physiol 369: 365–377, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood 111: 2024–2035, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckle T, Fullbier L, Wehrmann M, Khoury J, Mittelbronn M, Ibla J, Rosenberger P, Eltzschig HK. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol 178: 8127–8137, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118: 3301–3315, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 24: 298–306, 2009. [DOI] [PubMed] [Google Scholar]
- 32.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Eltzschig HK. Extracellular adenosine signaling in molecular medicine. J Mol Med (Berl) 91: 141–146, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13: 852–869, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 17: 1391–1401, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, Weissmuller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99: 1100–1108, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets 13: 1267–1277, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espinoza J, Espinoza AF, Power GG. High fetal plasma adenosine concentration: a role for the fetus in preeclampsia? Am J Obstet Gynecol 205: 485 e424–487, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 45: 1–15, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez P, Trzaska S, Wilder T, Chiriboga L, Blackburn MR, Cronstein BN, Chan ES. Pharmacological blockade of A2A receptors prevents dermal fibrosis in a model of elevated tissue adenosine. Am J Pathol 172: 1675–1682, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field JJ, Lin G, Okam MM, Majerus E, Keefer J, Onyekwere O, Ross A, Campigotto F, Neuberg D, Linden J, Nathan DG. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood 121: 3329–3334, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Field JJ, Nathan DG, Linden J. Targeting iNKT cells for the treatment of sickle cell disease. Clin Immunol 140: 177–183, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14: 1315–1323, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol 63: 191–270, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol 61: 443–448, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Fresco P, Diniz C, Goncalves J. Facilitation of noradrenaline release by activation of adenosine A(2A) receptors triggers both phospholipase C and adenylate cyclase pathways in rat tail artery. Cardiovasc Res 63: 739–746, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Gebremedhin D, Weinberger B, Lourim D, Harder DR. Adenosine can mediate its actions through generation of reactive oxygen species. J Cereb Blood Flow Metab 30: 1777–1790, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gnad T, Scheibler S, von Kugelgen I, Scheele C, Kilic A, Glode A, Hoffmann LS, Reverte-Salisa L, Horn P, Mutlu S, El-Tayeb A, Kranz M, Deuther-Conrad W, Brust P, Lidell ME, Betz MJ, Enerback S, Schrader J, Yegutkin GG, Muller CE, Pfeifer A. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516: 395–399, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808: 1380–1399, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med 5: e137, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gribkoff VK, Bauman LA. Endogenous adenosine contributes to hypoxic synaptic depression in hippocampus from young and aged rats. J Neurophysiol 68: 620–628, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol 186: 4367–4374, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting Edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol 182: 3965–3968, 2009. [DOI] [PubMed] [Google Scholar]
- 55.Hein TW, Belardinelli L, Kuo L. Adenosine A(2A) receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J Pharmacol Exp Ther 291: 655–664, 1999. [PubMed] [Google Scholar]
- 56.Higdon JV, Frei B. Coffee and health: a review of recent human research. Crit Rev Food Sci Nutr 46: 101–123, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res 179: 21–32, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res 113: 198–206, 1997. [DOI] [PubMed] [Google Scholar]
- 59.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 509: 310–317, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Idzko M, Ferrari D, Riegel AK, Eltzschig HK. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 124: 1029–1037, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imig JD. Adenosine2A receptors and epoxyeicosatrienoic acids: a recipe for salt and blood pressure regulation. Hypertension 54: 1223–1225, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iriyama T, Sun K, Parchim NF, Li J, Zhao C, Song A, Hart LA, Blackwell SC, Sibai BM, Chan LN, Chan TS, Hicks MJ, Blackburn MR, Kellems RE, Xia Y. Elevated placental adenosine signaling contributes to the pathogenesis of preeclampsia. Circulation 131: 730–741, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal 8: 419–436, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov 5: 247–264, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A2A antagonists, and Parkinson's disease. Parkinsonism Relat Disord 15: 406–413, 2009. [DOI] [PubMed] [Google Scholar]
- 66.Joo JD, Kim M, Horst P, Kim J, D'Agati VD, Emala CW Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol 293: F1847–F1857, 2007. [DOI] [PubMed] [Google Scholar]
- 67.Joyner MJ, Casey DP. Muscle blood flow, hypoxia, and hypoperfusion. J Appl Physiol (1985) 116: 852–857, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalda A, Yu L, Oztas E, Chen JF. Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson's disease. J Neurol Sci 248: 9–15, 2006. [DOI] [PubMed] [Google Scholar]
- 69.Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, Molina JG, Bunge R, Bruckner BA, Xia Y, Johnston RA, Loebe M, Zeng D, Seethamraju H, Belardinelli L, Blackburn MR. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 49: 1038–1047, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karmouty-Quintana H, Xia Y, Blackburn MR. Adenosine signaling during acute and chronic disease states. J Mol Med (Berl) 91: 173–181, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karmouty-Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD, Hemnes A, Grenz A, Eltzschig HK, Blackwell TS, Xia Y, Johnston RA, Zeng D, Belardinelli L, Blackburn MR. The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J 26: 2546–2557, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim M, Chen SW, Park SW, D'Agati VD, Yang J, Lee HT. Kidney-specific reconstitution of the A1 adenosine receptor in A1 adenosine receptor knockout mice reduces renal ischemia-reperfusion injury. Kidney Int 75: 809–823, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One 4: e6784, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, Winn HR. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab 30: 808–815, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Latini S, Bordoni F, Corradetti R, Pepeu G, Pedata F. Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br J Pharmacol 128: 1035–1044, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3 receptors. Am J Physiol Renal Physiol 278: F380–F387, 2000. [DOI] [PubMed] [Google Scholar]
- 77.Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol 15: 102–111, 2004. [DOI] [PubMed] [Google Scholar]
- 78.Leuenberger UA, Gray K, Herr MD. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J Appl Physiol (1985) 87: 2218–2224, 1999. [DOI] [PubMed] [Google Scholar]
- 79.LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson's disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 63: 295–302, 2008. [DOI] [PubMed] [Google Scholar]
- 80.Li JM, Fenton RA, Cutler BS, Dobson JG Jr. Adenosine enhances nitric oxide production by vascular endothelial cells. Am J Physiol Cell Physiol 269: C519–C523, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Linden J. Adenosine in tissue protection and tissue regeneration. Mol Pharmacol 67: 1385–1387, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol 156: 445–453, 2002. [DOI] [PubMed] [Google Scholar]
- 83.Lloyd HG, Lindstrom K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int 23: 173–185, 1993. [DOI] [PubMed] [Google Scholar]
- 84.Lu Z, Fassett J, Xu X, Hu X, Zhu G, French J, Zhang P, Schnermann J, Bache RJ, Chen Y. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation 118: 1713–1721, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG, Pedata F. The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res 1073–1074: 470–480, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, Maclennan S, Borea PA. A3 adenosine receptor activation inhibits cell proliferation via phosphatidylinositol 3-kinase/Akt-dependent inhibition of the extracellular signal-regulated kinase 1/2 phosphorylation in A375 human melanoma cells. J Biol Chem 280: 19516–19526, 2005. [DOI] [PubMed] [Google Scholar]
- 87.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, Xia Y. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest 118: 1491–1501, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol 160: 2009–2018, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muller CE, Jacobson KA. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta 1808: 1290–1308, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ning C, Wen J, Zhang Y, Dai Y, Wang W, Zhang W, Qi L, Grenz A, Eltzschig HK, Blackburn MR, Kellems RE, Xia Y. Excess adenosine A2B receptor signaling contributes to priapism through HIF-1alpha mediated reduction of PDE5 gene expression. FASEB J 28: 2725–2735, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414: 916–920, 2001. [DOI] [PubMed] [Google Scholar]
- 92.Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol Renal Physiol 277: F404–F412, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Peyot ML, Gadeau AP, Dandre F, Belloc I, Dupuch F, Desgranges C. Extracellular adenosine induces apoptosis of human arterial smooth muscle cells via A(2b)-purinoceptor. Circ Res 86: 76–85, 2000. [DOI] [PubMed] [Google Scholar]
- 94.Phatarpekar PV, Wen J, Xia Y. Role of adenosine signaling in penile erection and erectile disorders. J Sex Med 7: 3553–3564, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phillis JW. Adenosine in the control of the cerebral circulation. Cerebrovasc Brain Metab Rev 1: 26–54, 1989. [PubMed] [Google Scholar]
- 96.Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res 705: 79–84, 1995. [DOI] [PubMed] [Google Scholar]
- 97.Raichle ME. The pathophysiology of brain ischemia. Ann Neurol 13: 2–10, 1983. [DOI] [PubMed] [Google Scholar]
- 98.Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology 69: 536–545, 2007. [DOI] [PubMed] [Google Scholar]
- 99.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283: 2674–2679, 2000. [DOI] [PubMed] [Google Scholar]
- 100.Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, Hu FB. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med 140: 1–8, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol 184: 5271–5279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schneider DJ, Lindsay JC, Zhou Y, Molina JG, Blackburn MR. Adenosine and osteopontin contribute to the development of chronic obstructive pulmonary disease. FASEB J 24: 70–80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schrader J, Nees S, Gerlach E. Evidence for a cell surface adenosine receptor on coronary myocytes and atrial muscle cells. Studies with an adenosine derivative of high molecular weight. Pflügers Arch 369: 251–257, 1977. [DOI] [PubMed] [Google Scholar]
- 104.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal 15: 813–827, 2003. [DOI] [PubMed] [Google Scholar]
- 105.Schwarzschild MA. Adenosine A2A antagonists as neurotherapeutics: crossing the bridge. Prog Neurobiol 83: 261–262, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shryock JC, Snowdy S, Baraldi PG, Cacciari B, Spalluto G, Monopoli A, Ongini E, Baker SP, Belardinelli L. A2A-adenosine receptor reserve for coronary vasodilation. Circulation 98: 711–718, 1998. [DOI] [PubMed] [Google Scholar]
- 107.Siesjo BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab 1: 155–185, 1981. [DOI] [PubMed] [Google Scholar]
- 108.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5: 712–721, 2005. [DOI] [PubMed] [Google Scholar]
- 109.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol 22: 657–682, 2004. [DOI] [PubMed] [Google Scholar]
- 110.Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, Sussman NM. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 70: 2233–2240, 2008. [DOI] [PubMed] [Google Scholar]
- 111.Stevens B, Ishibashi T, Chen JF, Fields RD. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol 1: 23–34, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the A1 adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest 115: 35–43, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest 116: 2173–2182, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun K, Xia Y. New insights into sickle cell disease: a disease of hypoxia. Curr Opin Hematol 20: 215–221, 2013. [DOI] [PubMed] [Google Scholar]
- 115.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thannickal VJ, Toews GB, White ES, Lynch JP 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004. [DOI] [PubMed] [Google Scholar]
- 117.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200: 1395–1405, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tuomilehto J, Hu G, Bidel S, Lindstrom J, Jousilahti P. Coffee consumption and risk of type 2 diabetes mellitus among middle-aged Finnish men and women. JAMA 291: 1213–1219, 2004. [DOI] [PubMed] [Google Scholar]
- 119.Varani K, Gessi S, Dionisotti S, Ongini E, Borea PA. [3H]-SCH 58261 labelling of functional A2A adenosine receptors in human neutrophil membranes. Br J Pharmacol 123: 1723–1731, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta 1808: 1358–1379, 2011. [DOI] [PubMed] [Google Scholar]
- 121.Wen J, Dai Y, Zhang Y, Zhang W, Kellems RE, Xia Y. Impaired erectile function in CD73-deficient mice with reduced endogenous penile adenosine production. J Sex Med 8: 2172–2180, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wen J, Grenz A, Zhang Y, Dai Y, Kellems RE, Blackburn MR, Eltzschig HK, Xia Y. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J 25: 2823–2830, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Kellems RE, Blackburn MR, Xia Y. Adenosine deaminase enzyme therapy prevents and reverses the heightened cavernosal relaxation in priapism. J Sex Med 7: 3011–3022, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wen J, Jiang X, Dai Y, Zhang Y, Tang Y, Sun H, Mi T, Phatarpekar PV, Kellems RE, Blackburn MR, Xia Y. Increased adenosine contributes to penile fibrosis, a dangerous feature of priapism, via A2B adenosine receptor signaling. FASEB J 24: 740–749, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wen J, Xia Y. Adenosine signaling: good or bad in erectile function? Arterioscler Thromb Vasc Biol 32: 845–850, 2012. [DOI] [PubMed] [Google Scholar]
- 126.Wong AC, Guo CX, Gupta R, Housley GD, Thorne PR, Vlajkovic SM. Post exposure administration of A(1) adenosine receptor agonists attenuates noise-induced hearing loss. Hear Res 260: 81–88, 2010. [DOI] [PubMed] [Google Scholar]
- 127.Yang Z, Day YJ, Toufektsian MC, Xu Y, Ramos SI, Marshall MA, French BA, Linden J. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation 114: 2056–2064, 2006. [DOI] [PubMed] [Google Scholar]
- 128.Yoneyama Y, Suzuki S, Sawa R, Kiyokawa Y, Power GG, Araki T. Plasma adenosine levels and P-selectin expression on platelets in preeclampsia. Obstet Gynecol 97: 366–370, 2001. [DOI] [PubMed] [Google Scholar]
- 129.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Increased plasma adenosine concentrations and the severity of preeclampsia. Obstet Gynecol 100: 1266–1270, 2002. [DOI] [PubMed] [Google Scholar]
- 130.Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Power GG, Araki T. Relation between adenosine and T-helper 1/T-helper 2 imbalance in women with preeclampsia. Obstet Gynecol 99: 641–646, 2002. [DOI] [PubMed] [Google Scholar]
- 131.Zaynagetdinov R, Ryzhov S, Goldstein AE, Yin H, Novitskiy SV, Goleniewska K, Polosukhin VV, Newcomb DC, Mitchell D, Morschl E, Zhou Y, Blackburn MR, Peebles RS Jr, Biaggioni I, Feoktistov I. Attenuation of chronic pulmonary inflammation in A2B adenosine receptor knockout mice. Am J Respir Cell Mol Biol 42: 564–571, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang W, Zhang Y, Wang W, Dai Y, Ning C, Luo R, Sun K, Glover L, Grenz A, Sun H, Tao L, Colgan SP, Blackburn MR, Eltzschig HK, Kellems RE, Xia Y. Elevated ecto-5′-nucleotidase-mediated increased renal adenosine signaling via A2B adenosine receptor contributes to chronic hypertension. Circ Res 112: 1466–1478, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Dai Y, Wen J, Zhang W, Grenz A, Sun H, Tao L, Lu G, Alexander DC, Milburn MV, Carter-Dawson L, Lewis DE, Eltzschig HK, Kellems RE, Blackburn MR, Juneja HS, Xia Y. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med 17: 79–86, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Y, Xia Y. Adenosine signaling in normal and sickle erythrocytes and beyond. Microbes Infect 14: 863–873, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou X, Teng B, Tilley S, Mustafa SJ. A1 adenosine receptor negatively modulates coronary reactive hyperemia via counteracting A2A-mediated H2O2 production and KATP opening in isolated mouse hearts. Am J Physiol Heart Circ Physiol 305: H1668–H1679, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR. Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One 5: e9224, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhou Y, Schneider DJ, Blackburn MR. Adenosine signaling and the regulation of chronic lung disease. Pharmacol Ther 123: 105–116, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


