Summary
Background
Hereditary hemorrhagic telangiectasia (HHT) is a genetic disease that leads to multiregional angiodysplasia. Severe recurrent epistaxis is the most common presentation, frequently leading to severe anemia. Multiple therapeutic approaches have been tried, but they are largely palliative with variable results.We aimed to assess the efficacy of thalidomide in reducing epistaxis in patients with HHT refractory to standard therapy.
Methods
HHT patients with severe recurrent epistaxis refractory to mini-invasive surgical procedures were included in an open label, phase II, prospective, non-randomized, single-centre study. Thalidomide was administered at a starting dose of 50 mg/day orally. In the event of no response, thalidomide dosage was increased by 50 mg/day every four weeks until response to a maximum dose of 200 mg/day. After response achievement, patients were treated for eight to16 additional weeks. Monthly follow-up was based on the epistaxis severity score and transfusion need, with adverse events being reported (ClinicalTrials.gov Identifier: NCT01485224).
Findings
Thirty-one patients, mean age 62∙6 (SD 11∙1) years, were enrolled (median follow-up 15∙9 months, 25th-75th 10∙1-22∙3). Treatment induced cessation of bleeding in three cases (9∙7%) and a significant decrease in all epistaxis parameters in 28 cases (90∙3%). Twenty-five patients (80∙7%) obtained remission with 50 mg/day of thalidomide, five (16∙1%) with 100 mg/day and one (3∙2%) with 150 mg/day. Treatment significantly increased hemoglobin levels (p<0∙001), and abolished or greatly decreased the transfusion need (p<0∙001).Only nonserious, grade I, adverse effects were observed, including constipation and drowsiness. Median time to relapse after the end of therapy was 6∙4 months. No correlation was found between genetic or clinical features and response to thalidomide or toxicity.
Interpretation
Low-dose thalidomide is safe and very effective in reducing epistaxis in HHT patients, allowing for a rapid, often durable clinical improvement.
Funding
Telethon Foundation
Introduction
Hereditary hemorrhagic telangiectasia (HHT), also known as Rendu-Osler-Weber syndrome, is an autosomal-dominantly inherited vascular malformation syndrome characterized by telangiectasias and large arteriovenous malformations (AVMs). With a prevalence between 1:5000 and 1:8000 in different populations,1 it is one of the most frequent forms of inherited disorder.
The two major genes involved in HHT are ENG, coding for endoglin, a Transforming Growth Factor beta (TGFβ) receptor type III, and ACVRL1, coding for activin A receptor type II-like kinase 1, a TGFβ/ Bone Morphogenetic Proteins (BMPs) receptor type I. Mutations in ENG lead to HHT1 (OMIM 187300) while mutations in ACVRL1 lead to HHT2 (OMIM 600376); about 85% of HHT patients present a disease causing mutation in either of these genes.2 Mutations in additional genes as MADH4/SMAD4 and BMP9/GDF2 are found in 2% or less of cases.3,4 Although pathogenesis of HHT is still poorly defined, it has been suggested that mutations in ENG or ACVRL1 which are mostly expressed on endothelial cells cause through haploinsufficiency an unbalance in TGFβ/BMPs signal pathways. This leads to decreased TGFβ activation and increased vascular endothelial growth factor (VEGF) production5 that, in turn, result in excessive proliferation and migration of endothelial cells with reduced vessel maturation and thinning of the vascular wall. Clinical manifestations of HHT derive from vascular malformations, which range from small telangiectasias in the nasal, oral and gastrointestinal mucosa to large AVMs in the lung, brain and liver.1 Recurrent and severe epistaxis caused by the rupture of nasal telangiectasias is the most common presentation of HHT. Less frequently, rupture of telangiectasias in the digestive tract causes gastrointestinal bleeding. Hemorrhages usually worsen with age and lead to severe anemia requiring intravenous iron and blood transfusions. Also large AVMs can cause morbidity because of rupture or arteriovenous blood shunting.
While transcatheter embolotherapy with occluder devices is a well-established and effective treatment for large AVMs in the lung and brain, local treatment is difficult for largely disseminated telangiectasias and no medical therapy for preventing their rupture has been approved.6
Since angiogenesis has been implicated in the pathogenesis of HHT,5 it has been suggested that anti-angiogenic substances may be effective in the treatment of vascular malformations. Interestingly, bleeding inhibition has been observed in HHT patients who received thalidomide as an anti-angiogenic agent for cancer therapy.7,8 Other authors have reported that thalidomide treatment induced vessel maturation by enhancing pericyte and vascular smooth muscle cell coverage in an animal model of HHT and reduced severe nosebleeds in six out of seven HHT patients.9
Based on this evidence, we designed this study to confirm the effectiveness of thalidomide in preventing epistaxis and to identify the lowest effective dose in patients with severe forms of HHT refractory to local therapy.
Patients and methods
Study design
This was an open label, phase II, prospective, dose-finding, single arm, non-randomized, single centre study (EudraCT 2011-004096-36, ClinicalTrials.gov Identifier: NCT01485224). It was approved by the Ethical Committee of IRCCS Policlinico San Matteo Foundation, Pavia, Italy and all patients signed informed consent before treatment.
The primary endpoint was to evaluate the efficacy of thalidomide as percentage of patients showing a decrease in the frequency, intensity and/or duration of epistaxis. For each epistaxis parameter three different levels of severity were considered during a period of four weeks (table 1).10 Secondary endpoints were requirement of blood transfusions, minimum dose of thalidomide required to reduce bleeding, time to response, time to relapse after the end of treatment, safety and tolerability of the drug. We also programmed to evaluate size and number of telangiectasias by endoscopy of nasal mucosa (recording images of the size and localization of telangiectasias) at baseline and every eight weeks thereafter.
Table 1:
Levels of severity of epistaxis parameters10
| Grade | Epistaxis parameter | ||
|---|---|---|---|
| Frequency | Intensity | Duration | |
| 1 | Less than one episode/week |
Slight stains on the handkerchief |
Less than 10 min |
| 2 | At least one episode/week |
Soaked handkerchief | From 10 to 30 min |
| 3 | More than one episode/day |
Bowl or similar utensil necessary |
Over 30 min |
In case of no epistaxis during the last four weeks, zero point was awarded to each parameter
We searched for correlations between the mutations responsible for HHT and response to treatment. Since it has been shown that cytochrome P450 (CYP)2C subfamily polymorphisms partly explain the variability of thalidomide in vivo concentration by altering its metabolism,11 we also searched for correlations between CYP2C19 and CYP2C9 polymorphisms and both efficacy and possible side effects of this drug.
Patient selection
Patients with diagnosis of HHT, according to the Curaçao criteria,12 with severe recurrent epistaxis (grade 2-3 of any epistaxis parameter according to Pagella et al.,10 during the last month), and refractory to mini-invasive surgical procedures, older than 17 years, and able to sign written informed consent, were included in the study. Women of childbearing potential had to agree to follow acceptable birth control methods to avoid conception throughout the study and for four weeks following the date of the last dose of thalidomide, to have negative serum pregnancy test obtained within 48 hours prior to the first dose of thalidomide, and to declare intention to undergo pregnancy tests periodically while on the study medication. Also males with female partner of childbearing potential had to agree to use an effective method of contraception throughout the study and for one week following the date of the last dose of thalidomide. The estimated life expectancy of each patient had to be greater than ten months.
Pregnant or lactating women were excluded. Neurological diseases, psychiatric illness, active cardiovascular disease, and high risk for thromboembolic events (diabetes or uncontrolled infections, malignancy, immobility, prior history of thromboembolic events, use of erythropoietic agents or other agents such as hormone replacement therapy, central venous catheter, anti-cardiolipin, or anti-beta2 glycoprotein antibodies) also excluded patients. Patients with hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption were also excluded since thalidomide capsules contain lactose.
Treatment plan
Eligible patients received thalidomide at a starting dose of 50 mg/day by mouth at bedtime for four weeks. In the event of no response, thalidomide dosage was progressively increased by 50 mg/day every four weeks until complete or partial response, to a maximum dose of 200 mg/day. Then, treatment was continued as follows: eight additional weeks after the achievement of complete response, 16 additional weeks after the achievement of partial response, 24 weeks in case of no response. A maximum dose of 100 mg/day was used in patients older than 74 years. For details of dose reductions/interruptions see appendix. The concomitant use of recombinant human erythropoietin or combined hormonal contraceptive was not allowed during the study. Medications causing drowsiness and medications potentially associated with peripheral neuropathy had to be used with caution. After the end of treatment, patients were followed for at least 52 weeks.Clinical evaluation and laboratory tests were performed at baseline, every four weeks during treatment, and for at least 52 weeks after the end of treatment; in particular the following parameters were monitored: epistaxis severity, blood counts, number of blood transfusions, endoscopy of nasal mucosa (size and number of telangiectasias), symptoms and complains. Epistaxis frequency, intensity, and duration grades during the previous four weeks were recorded before treatment by direct interview, and afterwards, monthly, also by telephone interview.10
Criteria of evaluation
The primary endpoint was computed as percentage of patients showing a reduction to grade 1-2 at least in one of the epistaxis parameters. The difference between baseline scores and frequency, intensity, and duration scores evaluated at each time point during treatment was computed. If any of these was more than zero, the patient was considered as responder. A complete responder was defined as a patient with all three epistaxis scores equal zero. Reduction in the severity of any bleeding parameter less than complete response did represent partial response. Both complete and partial response had to be maintained for at least four weeks. Failure to achieve at least a partial response was defined as no response, whereas relapse after complete or partial response was defined as the regression from complete response to any other degree of response or return from partial response to pretreatment severity of bleeding parameters.
Assessment of toxicity was performed with medical interview, physical examination, and laboratory tests, including thyroid-stimulating hormone levels. Adverse events and/or adverse drug reactions were recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (http://ctep.cancer.gov/reporting/ctc.html).
Treatment compliance was assessed with medical interview and as the remaining pill count.
Molecular biology studies
DNA extraction was performed using the GenElute™ Blood Genomic DNA Kit (Sigma-Aldrich, St Louis, MO, USA).
Mutational analysis of the two main genes responsible for HHT, ENG and ACVRL1, was performed by direct sequence analysis.
Three CYP450 (CYP2C19 and CYP2C9) variant sequences were examined: CYP2C19*2, a transition (+681, G to A) in exon 5 which produces an aberrant splice site; CYP2C9*2, a transition (+430, C to T) in exon 3 which produces an amino acid change (Arg to Cys), and CYP2C9*3, a transition (+1075, A to C) in exon 7 which produces an amino acid change (Ile to Leu). The DNA samples were analyzed using polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method.13,14
Statistical analysis
Sample size calculation was performed according to the Fleming’s single stage procedure. We assumed that the treatment would yield responses in about 75% of patients and that, given also a possible high rate of side effects of the drug, a proportion of response below 50% would be of no interest. Recruiting 31 patients would allow to have a power of the trial of 90% with a one-sided alpha of 5%.
All subjects who satisfied the inclusion/exclusion criteria, who received any study drug, and who participated in at least one post-baseline assessment represented the full analysis population. This population was used as the primary efficacy population. All subjects who received any dose study treatment but excluding subjects who drop out prior to receiving any treatment were used as primary safety population.
All continuous variables were summarized using the following descriptive statistics: n (non-missing sample size), mean, standard deviation (SD), median, 25th and 75th percentiles. The frequency and percentages (based on the non-missing sample size) of observed levels were reported for all categorical measures.
The overall number and percent of responders to treatment were computed together with binomial 95% confidence interval (95% CI). The cumulative relapse-free survival after therapy termination was plotted on a Kaplan Meier curve.
The mean number of blood transfusions over the study time was computed together with a Poisson 95% CI, and the change in the monthly number of transfusions was analyzed with a repeated measures negative binomial regression model. The difference between baseline and end-of-treatment hemoglobin levels was compared with the paired Student t test. A linear regression model for repeated measures was fitted to describe changes over time.
The association of mutation and polymorphisms of cytochromes CYP2C19 and CYP2C9 with response was assessed with the Fisher’s exact test. The association with relapse was assessed with the log-rank test.
The number (%) of adverse events was tabulated at each time point.
Patients dropping out or interrupting the study drug did represent a protocol deviation. They were described individually and were considered as failures for the primary endpoint.
Stata 13∙1 (StataCorp, College Station, TX) was used for computation. Data, code (Stata do-file) and documents were stored in the following directory on the professional computer of the study statistician (CK) [: D:…\THALI\analysis].
Role of the funding source
The sponsor of the study was the IRCCS Policlinico San Matteo Foundation, Pavia, Italy. The study was an academic non-profitable study sponsored by a non-profit scientific foundation and carried out with the purpose of improving the clinical practice as an integral part of healthcare. There was no commercial support for the trial. The costs of the study were covered with research funds from the Telethon Foundation, a leading Italian charity organization investing in the research of genetic diseases (Telethon Grant number GGP13036). The sponsor had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and all authors approved the manuscript for submission for publication. The corresponding author had final responsibility for the decision to submit for publication.
Results
Thirty-one patients, 20 males and 11 females, mean age 62∙6 (SD 11∙1) years, were enrolled from December 1, 2011, to May 12, 2014 (table 2). Their clinical features and previous treatments for epistaxis are reported in table 2. Various types of mutations were observed in either ACVRL1 gene [25 cases (80∙6%)] or ENG gene [four cases (12∙9%)]. Median follow-up was 15∙9 months (25th-75th 10∙1-22∙3). Fourteen patients (45∙1%) were receiving iron therapy. During the study period no other medications for epistaxis were used by patients.
Table 2:
Presenting characteristics of the patients
| Number of patients | 31 |
| Age in years, mean (SD) | 62·6 (11·1) |
| M/F, n (%) | 20 (64·6)/11 (35·4) |
| Epistaxis severity grade 2/3, n pts (%) | 5 (16·1)/26 (83·9) |
| RBC transfusion dependence, n pts (%) | 23 (74·2) |
| Previous treatments, n pts (%) | |
| Argon plasma coagulation | 17 (54·8) |
| Electrocautery | 15 (48·4) |
| Embolization | 6 (19·3) |
| Laser coagulation | 2 (6·4) |
| Septodermoplasty | 3 (9·7) |
| Arterial ligation | 1 (3·2) |
| Organ involvement, n pts (%) | |
| Telangiectasias | |
| Nose* | 31 (100) |
| Skin | 29 (93·6) |
| Gastrointestinal tract* | 9 (29·0) |
| Large arteriovenous malformations | |
| Lung** | 9 (29·0) |
| Liver*** | 13 (41·9) |
| CNS** | 1 (3·2) |
identified by endoscopy;
identified by computed tomography;
identified by ecography
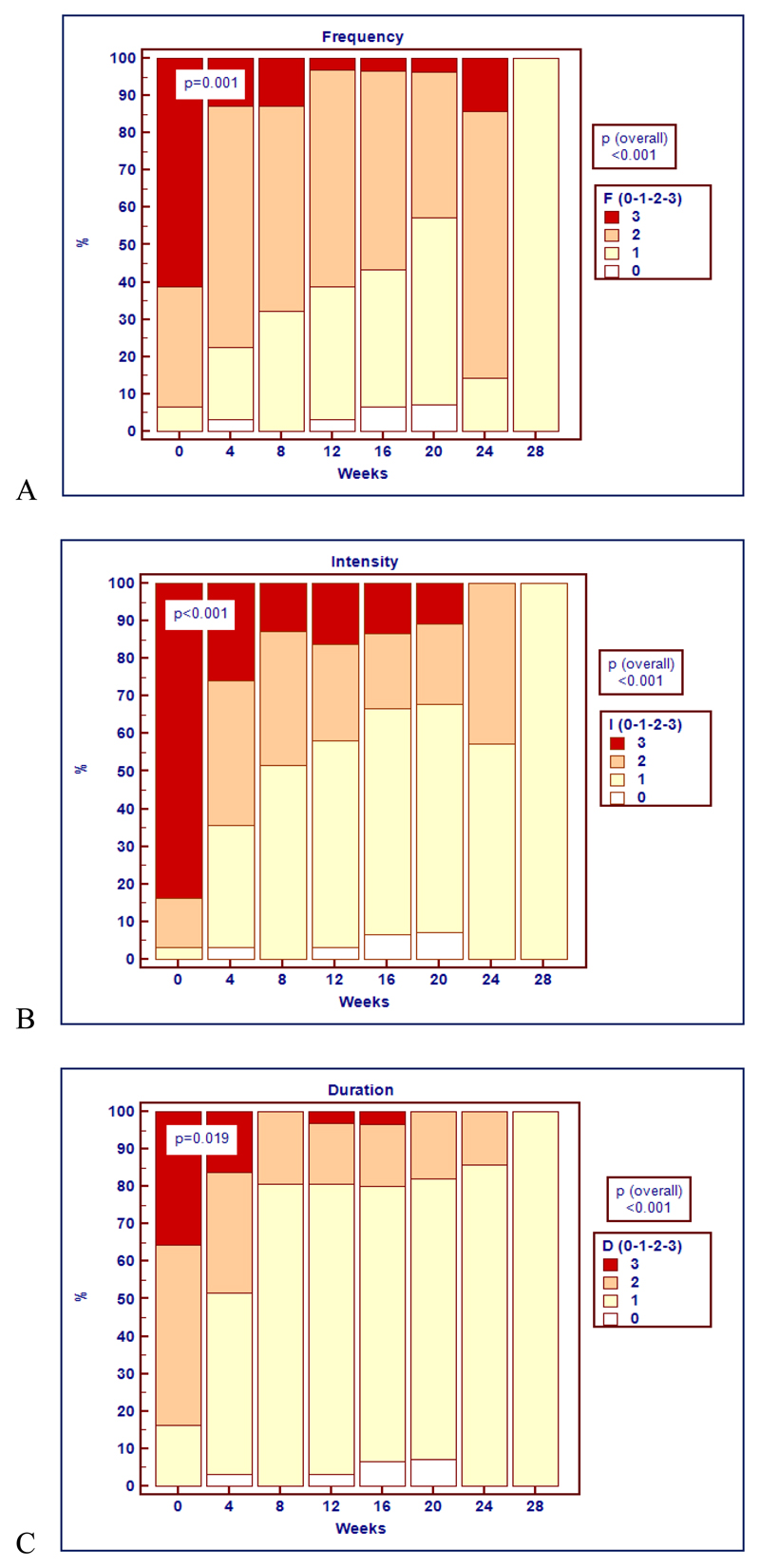
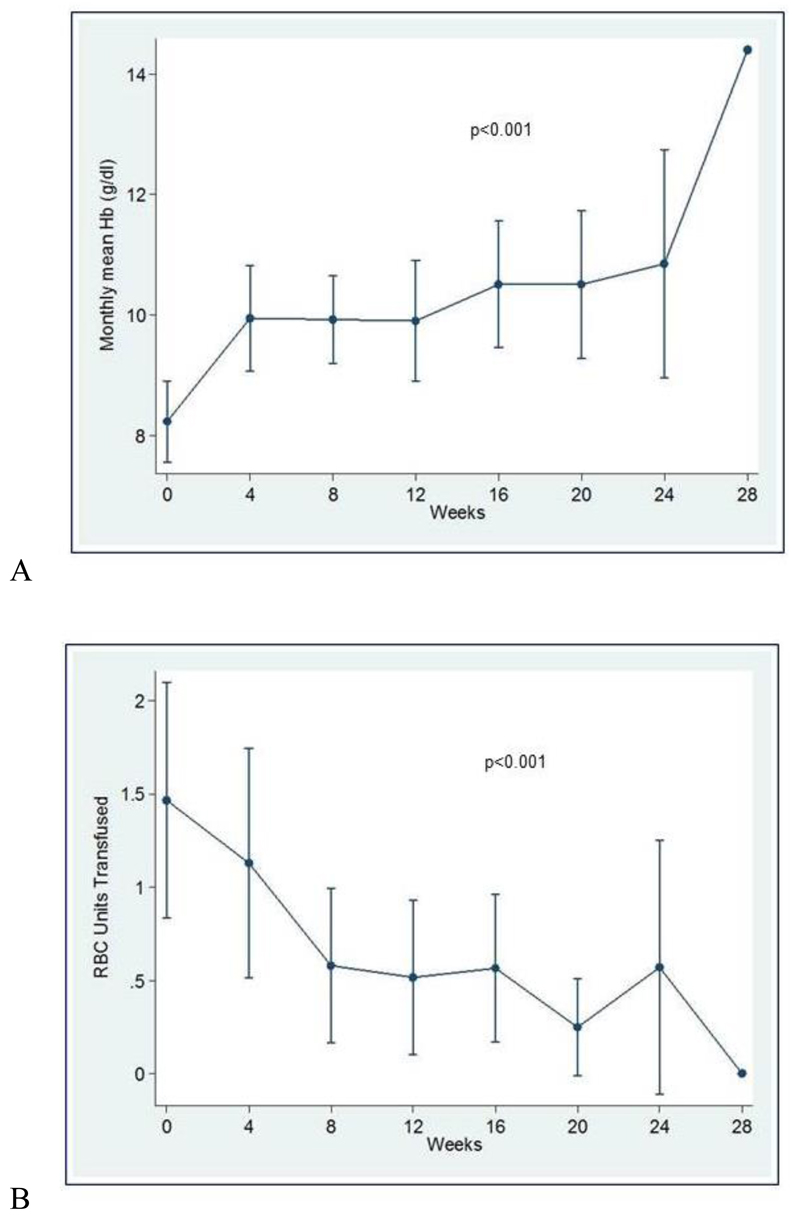
All patients were evaluated for response. Treatment induced cessation of bleeding in three cases (9∙7%) and a significant decrease in all epistaxis parameters in 28 cases (90∙3%) (table 3 and figure 1). Twenty-five patients (80∙7%) obtained remission with 50 mg/day of thalidomide, five (16∙1%) with 100 mg/day and one (3∙2%) with 150 mg/day. Treatment significantly increased hemoglobin levels (p<0∙001) with a maximum increase of 2∙27 g/dL, 95% CI 1∙13-3∙42 (figure 2A), and abolished or greatly decreased the transfusion need (p<0∙001) with a maximum decrease of 1∙77 monthly transfused RBC units, 95% CI 0∙70-2∙84 (figure 2B). Twenty of the 23 transfusion dependent patients became transfusion independent. During thalidomide treatment, the dose of iron therapy was not changed except for three patients, in whom iron therapy was discontinued because of the rapid improvement of the hemoglobin level.
Table 3:
Response to therapy
| Date of the first enrolment | December 1, 2011 |
| Date of the last enrolment | May 12, 2014 |
| Follow-up (months), median (25th-75th) | 15·9 (10·1-22·3) |
| Number of patients evaluated for response/enrolled to the study | 31/31 |
| Number of responders (%, 95% CI) | 31 (100, 89-100) |
| Complete response, n pts (%, 95% CI) | 3 (9·7, 2·0-25·7) |
| Number of patients who became transfusion-independent (%, 95% CI) | 20/23 (86·9, 66·4-97·2) |
| Time to response: 4 weeks/8 weeks/12 weeks, n pts (%) | 25 (80·7)/5 (16·1)/1 (3·2) |
| Dose of thalidomide administered (mg) | Min: 50; Max: 150 |
Figure 1:
Description over time of the percentages of patients with each score (0, 1, 2, 3) for epistaxis parameters: (A) frequency, F; (B) intensity, I; (C) duration, D. A significant decrease in all epistaxis parameters was recorded in all patients (p<0∙001). Statistically significant differences in the distribution of the scores for epistaxis frequency (p=0∙001), intensity (p<0∙001), and duration (p=0∙019) were observed within four weeks after starting thalidomide.
Figure 2:
(A) Description over time of mean hemoglobin values (95% CI). A significant increase of hemoglobin levels was recorded during thalidomide treatment (p<0∙001). (B) Description over time of mean transfusion requirement (95% CI). Thalidomide treatment significantly decreased the mean number of monthly transfused RBC units (p<0∙001).
The evaluation of the recordings obtained during rhinoscopy did not allow us to highlight marked differences in morphology and number of telangiectasias of the nasal mucosa, before and after treatment (data not shown). However, the examination was strongly limited at enrolment by the presence of crusting that prevented in obtaining optimal images to be compared with those after treatment.
Only nonserious, grade 1, adverse effects were observed during treatment, including mild constipation and drowsiness (table 4). In no patient thalidomide had to be discontinued. In three cases thalidomide dosages were temporarily reduced because of paresthesias (two cases), marked asthenia and dizziness (one case); in five cases a slight elevation of TSH was observed, in the absence of clinical symptoms. No thromboembolic event was recorded.
Table 4:
Safety endpoints at each time point.
| Toxicity (grade 1) | Cumulative number of patients (%) | ||||||
|---|---|---|---|---|---|---|---|
| 4 w | 8 w | 12 w | 16 w | 20 w | 24 w | 28 w | |
| Constitutional symptoms | |||||||
| Asthenia | 0/31 (0) | 2/31 (6·4) | 2/31 (6·4) | 3/30 (10·0) | 3/28 (10·7) | 3/7 (42·8) | 0/1 (0) |
| Peripheral edema | 2/31 (6·4) | 5/31 (16·1) | 7/31 (22·6) | 8/30 (26·7) | 7/28 (25·0) | 0/7 (0) | 0/1 (0 |
| Gastrointestinal | |||||||
| Constipation | 9/31 (29·0) | 15/31 (48·4) | 19/31 (61·3) | 21/30 (70·0) | 20/28 (71·4) | 4/7 (57·1) | 1/1 (100) |
| Vomiting | 0/31 (0) | 1/31 (3·2) | 0/31 (0) | 0/31 (0) | 0/31 (0) | 0/31 (0) | 0/31 (0) |
| Neurologic | |||||||
| Dizziness | 3/31 (9·7) | 4/31 (12·6) | 5/31 (16·1) | 5/30 (16·7) | 5/28 (17·8) | 1/7 (14·3) | 0/1 (0) |
| Drowsiness | 2/31 (6·4) | 5/31 (16·1) | 6/31 (19·3) | 6/30 (20·0) | 6/28 (21·4) | 1/7 (14·3) | 0/1 (0) |
| Peripheral neuropathy | 0/31 (0) | 0/31 (0) | 1/31 (3·2) | 2/30 (6·7) | 3/28 (10·7) | 1/7 (14·3) | 0/1 (0) |
| Psychiatric | |||||||
| Depression | 0/31 (0) | 0/31 (0) | 1/31 (3·2) | 1/30 (3·3) | 1/28 (3·6) | 0/7 (0) | 0/1 (0) |
| Cardiovascular | |||||||
| Bradycardia | 0/31 (0) | 1/31 (3·2) | 1/31 (3·2) | 1/30 (3·3) | 1/28 (3·6) | 1/7 (14·3) | 0/1 (0) |
| Hematologic | |||||||
| Leukopenia | 1/31 (3·2) | 1/31 (3·2) | 1/31 (3·2) | 2/30 (6·7) | 2/28 (7·1) | 1/7 (14·3) | 0/1 (0) |
| TSH elevation | 2/31 (6·4) | 3/31 (9·7) | 4/31 (12·9) | 4/30 (13·3) | 5/28 (17·9) | 1/7 (14·3) | 0/1 (0) |
w: weeks of therapy
All adverse events were grade 1
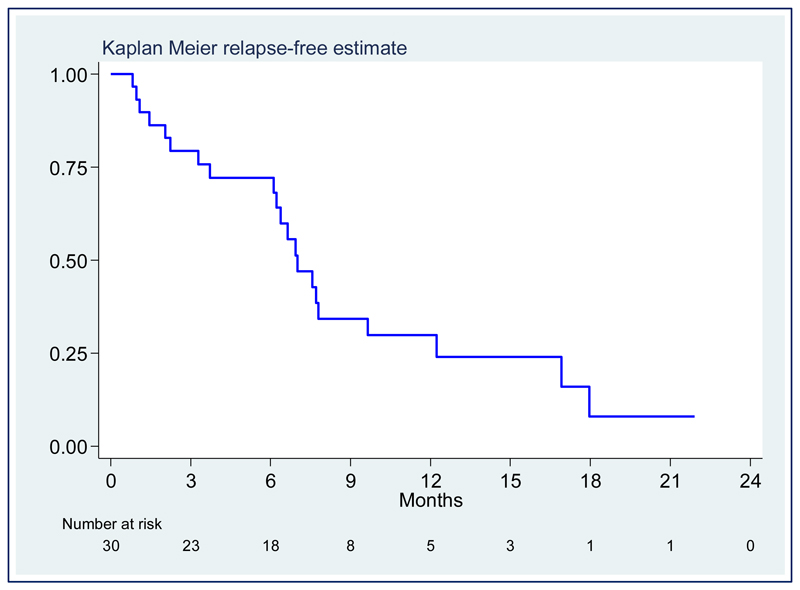
Thirty patients completed the treatment. A patient, who achieved partial remission after eight weeks of treatment at a dose of 100 mg/day thalidomide, noticed progressive worsening of epistaxis in the following weeks and underwent surgical treatment. An 80-year-old male patient suddenly died a month after the end of treatment for unknown reasons. A relationship with therapy could not be completely excluded, although no side effects had been recorded during treatment. At a median follow-up of 14∙1 months (25th-75th 10∙9-21∙9) after the end of therapy, eight cases (26∙7%) maintained remission, whereas 21 (70%) relapsed with a median relapse-free survival of 7∙0 months, 95% CI 6.1-9.6 (figure 3). No correlation was found between clinical features, such as age, gender, epistaxis severity, and time to response, response duration or toxicity (data not shown). Neither causative gene mutations nor CYP2C19/CYP2C9 polymorphisms were significantly related to response to therapy or toxicity (appendix).
Figure 3:
Kaplan Meier plot of relapse-free survival after therapy termination.
Discussion
To our knowledge, this is the first prospective study analyzing the efficacy of thalidomide in a consistent group of patients with HHT. Our results indicate that low-dose thalidomide may be safe and very effective in preventing severe epistaxis in patients who did not benefit from traditional modalities of treatment, allowing for a rapid and often durable clinical improvement.
Despite knowledge of HHT pathogenesis greatly expanded in recent years, treatment of affected patients did not improve in proportion. In particular, the control of epistaxis still represents a major problem because as many as 90% of affected individuals experience recurrent nose bleedings by age 30 years.15 Moreover, nose bleeding worsens with age, causing repeated hospitalizations, chronic anemia with severe iron deficiency and transfusion dependency. Various treatment options allow otorhinolaryngologists to control nose bleedings, but these interventions often lose efficacy over time and patients eventually require risky or debilitating treatments, as embolization of the nasal vasculature or nose closure.6
Thus, the need for medical treatments to prevent epistaxis is compelling, but unfortunately this request was not yet fulfilled. Systemic administration of antifibrinolytic agents has been used empirically since a long time, but two recent prospective clinical trials concluded that their efficacy was small and did not result in increases of hemoglobin levels.16,17 Contrasting results have been obtained with hormone therapy. Case reports and small case series suggested that systemic estrogens, estrogens plus progesterone and progesterone alone may be effective,18 but a randomized clinical trial found no difference in bleeding between subjects receiving estrogen or placebo.19 Moreover, severe side effects have been reported, especially in men and postmenopausal women, and hormone therapy lost much of its attractiveness. Finally, nine of ten patients receiving the antiestrogenic agent tamoxifen in a double-blind placebo-controlled clinical trial judged subjectively that both the frequency and intensity of nose bleeding were reduced during treatment, while only three of 11 subjects receiving placebo reported favorable responses. However, hemoglobin concentration and the need for blood transfusions were not different in the two groups of patients, this rising doubt about the real effectiveness of this treatment.20
Advancing knowledge about the pathophysiology of HHT vascular malformations opened new perspectives of medical treatment and made attractive the possibility of intervening in the regulation of the fine mechanisms of angiogenesis not only to change already formed abnormalities, but also to prevent their formation. Since VEGF levels have been shown to be highly increased in HHT and are implicated in its pathogenesis, bevacizumab, a humanized recombinant monoclonal antibody against VEGF, has been used both systemically and topically. Its systemic administration to 25 patients for 2∙5 months reduced duration of epistaxes but did not significantly change their frequency.21 Moreover, two recent controlled trials did not identify any significant benefit by submucosal injection or nasal spray administration of bevacizumab,22,23 and therefore contradicted previous uncontrolled studies reporting efficacy of local administration of this drug.24–27 Thus, the efficacy of intra-nasal bevacizumab in HHT seems to be limited or even uncertain.
Another molecule of potential interest for HHT treatment is thalidomide. It was introduced in late 1950s to prevent nausea during pregnancy, but it was withdrawn from the market because of teratogenicity.28 However, subsequent disclosure that thalidomide has immunomodulatory, anti-inflammatory and anti-angiogenic activity stimulated clinical trials in immune and inflammatory disorders, as well as in various malignancies. Based on results of these studies, thalidomide was approved by the US FDA for Erythema Nodosum Leprosum and multiple myeloma. The observation that thalidomide inhibited bleeding in a few HHT individuals who received this drug as an anti-angiogenic cancer therapy7,8 encouraged its administration to other HHT subjects, and five studies reported favorable effect for the treatment of nose bleeding in 17 of 20 patients receiving doses of thalidomide from 50 to 200 mg/daily.9,29 Importantly, treatment was well tolerated and the single notable side effect was deep vein thrombosis in one subject receiving 100 mg of thalidomide daily.30
Our study is in support of the efficacy of thalidomide in HHT, as all 31 treated patients had their nose bleeding reduced. More importantly, the average hemoglobin levels were increased and transfusion requirement was reduced. Of note, no difference in response was observed in patients with mutations in ENG or ACVRL1 as well as in subjects with different CYP450 polymorphisms, given the small numbers. However, the study was not powered to detect such associations. Another interesting finding was that 25 of 31 patients obtained remission with 50 mg of thalidomide daily, a dose much lower than that used in all other conditions for which this drug has been approved or used experimentally. This is probably the reason why thalidomide was very well tolerated in our case series. In particular, no patient had deep vein thrombosis, the side effect most feared in subjects receiving this drug for onco-hematological disorders. Also peripheral neuropathy, another frequently described side effect of thalidomide, was observed in only two patients. It was mild and disappeared upon dosage reduction. Despite these reassuring data, we cannot conclude that HHT subjects receiving low-dose thalidomide are not at risk of thromboembolic events and severe neuropathy, since patients with high thrombotic risk and preexisting neurological disorders have been excluded from our study.
Understanding the mechanism of action of thalidomide in HHT would be an important achievement because it would provide opportunities for the development of more effective therapies, but it is still a matter of debate. Decades of investigation identified a number of biological effects of thalidomide. It suppresses Tumor Necrosis Factor alpha (TNFα) and affects generation of pro-inflammatory cytokines. Moreover, it inhibits VEGF and basic Fibroblast Growth Factor production, thus antagonizing angiogenesis and modifying bone marrow micoenvironment.31 While it has been previously hypothesized that thalidomide benefits HHT patients by direct inhibition of endothelial cell proliferation and migration, more recent data supported the hypothesis that it modulates the activation of mural cells, enhancing both their proliferation and ability to embrace blood vessels.9 In other words, thalidomide makes HHT vessels more firm and less prone to breaking. We have no data directly supporting this hypothesis, but our finding that the median time to relapse after the end of treatment was more than six months fits with this hypothesis. In fact, once thalidomide promoted formation of more firm vessel walls, the reduction of bleeding is expected to last even after the drug is discontinued.
Demonstration that a course of low-dose thalidomide is effective in transiently reducing epistaxis in HHT opens new perspective and interesting questions concerning the possibility to identify treatment schedules for permanently reducing epistaxis: continuous treatment with further reduced dosages might be effective in this respect without significant side effects. Alternatively, the same result could be achieved by treatments given in cycles rather than continuously. Another important question that needs an answer is whether thalidomide is also effective for preventing gastrointestinal bleeding of HHT and whether it has any effect on AVMs and on cardiac output or pulmonary hypertension.
Our study has some weaknesses. The most important one is that it was not randomized, since the rarity of HHT and the lack of a drug certainly effective to which compare the effect of thalidomide discouraged us from carrying out a controlled study. HHT is one of the most frequent forms of inherited disorder, but we have studied just a small subset of patients, those with phenotypically severe disease, refractory to previous standard treatments, who did not have contraindications to the use of a potentially toxic drug such as thalidomide. In this setting, we designed a single-arm study with a 90% power. The second weaknesses is that we were unable to evaluate the effect of thalidomide on telangiectasias of the nasal mucosa. Finally, we did not formally investigated the effect of thalidomide on the quality of life. However, reduction of epistaxis was reported by the patients themselves, and this was certainly and directly connected to their perceived quality of life.
Our findings should be validated by further studies with large patient populations, longer follow-up, and evaluating also the benefit on quality of life. Anyway, the suggestion that thalidomide may be effective in reducing epistaxis in HHT patients is expected to promote the search for additional drugs with even better safety and efficacy profiles.
Supplementary Material
Acknowledgments
This trial was funded by Telethon Foundation (Grant number GGP13036). We wish to thank all patients, their families and physicians for participation in the study, and the Associazione Fondazione HHT “Onilde Carini” (www.hht.it) and HHT Onlus (www.hhtonlus.com). Data from this study were presented in part at the 19th Congress of the European Hematology Association; Milan, Italy; June 12-15, 2014.
Footnotes
Contributors
RI contributed to the conception and design of the study, to patient enrolment and treatment, to analysis and interpretation of data, and writing the manuscript. FQ collected clinical data and participated in the data analysis and interpretation. CK performed statistical analysis and contributed to the design of the study, the writing of the protocol, and the data interpretation. FP, FC, EM, and GS contributed to patient screening and recruitment, data collection and interpretation, and performed endoscopy of nasal mucosa. FO, SP, PG, and CO performed molecular biology studies, collected data, and participated in the data analysis and interpretation. FB and RB collected data and contributed to data analysis and interpretation. MB contributed to study design, analysis and interpretation of data. CD contributed to study design, coordination of molecular biology studies, analysis and interpretation of data, and reviewing the manuscript. CLB contributed to study conception and design, clinical trial coordination, data analysis and interpretation, writing and reviewing the manuscript.
Declaration of interests
The authors declare no potential conflict of interest.
Contributor Information
Rosangela Invernizzi, Department of Internal Medicine, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Federica Quaglia, Department of Internal Medicine, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Caherine Klersy, Service of Biometry and Clinical Epidemiology, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Fabio Pagella, Department of Otorhinolaryngology, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Federica Ornati, Department of Cardio-thorax-vascular, Unit of Cardiology, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy, and Department of Molecular Medicine, General Biology, and Medical Genetics Unit, University of Pavia, Pavia, Italy.
Francesco Chu, Department of Otorhinolaryngology, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Elina Matti, Department of Otorhinolaryngology, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Giuseppe Spinozzi, Department of Otorhinolaryngology, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Sara Plumitallo, Department of Molecular Medicine, General Biology, and Medical Genetics Unit, University of Pavia, Pavia, Italy.
Pierangela Grignani, Department of Legal Medicine and Public Health, University of Pavia, Pavia, Italy.
Carla Olivieri, Department of Molecular Medicine, General Biology, and Medical Genetics Unit, University of Pavia, Pavia, Italy.
Raffaella Bastia, Department of Internal Medicine, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Francesca Bellistri, Department of Internal Medicine, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Cesare Danesino, Department of Molecular Medicine, General Biology, and Medical Genetics Unit, University of Pavia, Pavia, Italy.
Marco Benazzo, Department of Otorhinolaryngology, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
Carlo L Balduini, Department of Internal Medicine, University of Pavia, IRCCS Policlinico San Matteo Foundation, Pavia, Italy.
References
- 1.Shovlin CL. Hereditary hemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 2010;24:203–19. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 2.McDonald J, Wooderchak-Donahue W, VanSant Webb C, Whitehead K, Stevenson DA, Bayrak-Toydemir P. Hereditary hemorrhagic telangiectasia: genetics and molecular diagnostics in a new era. Front Genet. 2015;6:1. doi: 10.3389/fgene.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallione CJ, Repetto GM, Legius E, et al. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4) Lancet. 2004;363:852–59. doi: 10.1016/S0140-6736(04)15732-2. [DOI] [PubMed] [Google Scholar]
- 4.Wooderchak-Donahue WL, McDonald J, O'Fallon B, et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am J Hum Genet. 2013;93:530–37. doi: 10.1016/j.ajhg.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadick H, Hage J, Goessler U, et al. Does the genotype of HHT patients with mutations of the ENG and ACVRL1 gene correlate to different expression levels of the angiogenic factor VEGF? Int J Mol Med. 2008;22:575–80. [PubMed] [Google Scholar]
- 6.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. J Med Genet. 2011;48:73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 7.Kurstin R. Using thalidomide in a patient with epithelioid leiomyosarcoma and Osler-Weber-Rendu disease. Oncology (Williston Park) 2002;16:21–24. [PubMed] [Google Scholar]
- 8.Pérez-Encinas M, Rabunal Martinez MJ, Bello Lopez JL. Is thalidomide effective for the treatment of gastrointestinal bleeding in hereditary hemorrhagic telangiectasia? Haematologica. 2002;87:ELT34. [PubMed] [Google Scholar]
- 9.Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med. 2010;16:420–28. doi: 10.1038/nm.2131. [DOI] [PubMed] [Google Scholar]
- 10.Pagella F, Colombo A, Matti E, et al. Correlation of severity of epistaxis with nasal telangiectasias in hereditary hemorrhagic telangiectasia (HHT) patients. Am J Rhinol Allergy. 2009;23:52–58. doi: 10.2500/ajra.2009.23.3263. [DOI] [PubMed] [Google Scholar]
- 11.Ando Y, Fuse E, Figg WD. Thalidomide metabolism by the CYP2C subfamily. Clin Cancer Res. 2002;8:1964–73. [PubMed] [Google Scholar]
- 12.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiecta (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000;91:66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Ando Y, Price DK, Dahut WL, Cox MC, Reed E, Figg WD. Pharmacogenetic associations of CYP2C19 genotype with in vivo metabolisms and pharmacological effects of thalidomide. Cancer Biol Ther. 2002;1:669–73. doi: 10.4161/cbt.318. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JA. CYP2C9 polymorphisms and CYP2C9*2 genotyping primers. Br J Clin Pharmacol. 2002;53:409–10. doi: 10.1046/j.1365-2125.2002.01572-7.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lesca G, Olivieri C, Burnichon N, et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med. 2007;9:14–22. doi: 10.1097/gim.0b013e31802d8373. [DOI] [PubMed] [Google Scholar]
- 16.Gaillard S, Dupuis-Girod S, Boutitie F, et al. Tranexamic acid for epistaxis in hereditary hemorrhagic telangiectasia patients: a European cross-over controlled trial in a rare disease. J Thromb Haemost. 2014;12:1494–502. doi: 10.1111/jth.12654. [DOI] [PubMed] [Google Scholar]
- 17.Geisthoff UW, Seyfert UT, Kübler M, Bieg B, Plinkert PK, König J. Treatment of epistaxis in hereditary hemorrhagic telangiectasia with tranexamic acid - a double-blind placebo-controlled cross-over phase IIIB study. Thromb Res. 2014;134:565–71. doi: 10.1016/j.thromres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Jameson JJ, Cave DR. Hormonal and antihormonal therapy for epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope. 2004;114:705–09. doi: 10.1097/00005537-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Vase P. Estrogen treatment of hereditary hemorrhagic telangiectasia. A double-blind controlled clinical trial. Acta Med Scand. 1981;209:393–96. doi: 10.1111/j.0954-6820.1981.tb11614.x. [DOI] [PubMed] [Google Scholar]
- 20.Yaniv E, Preis M, Hadar T, Shvero J, Haddad M. Antiestrogen therapy for hereditary hemorrhagic telangiectasia: a double-blind placebo-controlled clinical trial. Laryngoscope. 2009;119:284–88. doi: 10.1002/lary.20065. [DOI] [PubMed] [Google Scholar]
- 21.Dupuis-Girod S, Ginon I, Saurin JC, et al. Bevacizumab in patients with hereditary hemorrhagic telangiectasia and severe hepatic vascular malformations and high cardiac output. JAMA. 2012;307:948–55. doi: 10.1001/jama.2012.250. [DOI] [PubMed] [Google Scholar]
- 22.Dupuis-Girod S, Ambrun A, Decullier E, et al. ELLIPSE Study: a phase I study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia. MAbs. 2014;6:794–99. doi: 10.4161/mabs.28025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riss D, Burian M, Wolf A, Kranebitter V, Kaider A, Arnoldner C. Intranasal submucosal bevacizumab for epistaxis in hereditary hemorrhagic telangiectasia: a double-blind, randomized, placebo-controlled trial. Head Neck. 2015;37:783–87. doi: 10.1002/hed.23655. [DOI] [PubMed] [Google Scholar]
- 24.Simonds J, Miller F, Mandel J, Davidson TM. The effect of bevacizumab (Avastin) treatment on epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope. 2009;119:988–92. doi: 10.1002/lary.20159. [DOI] [PubMed] [Google Scholar]
- 25.Guldmann R, Dupret A, Nivoix Y, Schultz P, Debry C. Bevacizumab nasal spray: noninvasive treatment of epistaxis in patients with Rendu Osler disease. Laryngoscope. 2012;122:953–55. doi: 10.1002/lary.23230. [DOI] [PubMed] [Google Scholar]
- 26.Karnezis TT, Davidson TM. Treatment of hereditary hemorrhagic telangiectasia with submucosal and topical bevacizumab therapy. Laryngoscope. 2012;122:495–97. doi: 10.1002/lary.22501. [DOI] [PubMed] [Google Scholar]
- 27.Rohrmeier C, Sachs HG, Kuehnel TS. A retrospective analysis of low dose, intranasal injected bevacizumab (Avastin) in hereditary hemorrhagic telangiectasia. Eur Arch Otorhinolaryngol. 2012;269:531–36. doi: 10.1007/s00405-011-1721-9. [DOI] [PubMed] [Google Scholar]
- 28.Speirs AL. Thalidomide and congenital abnormalities. Lancet. 1962;1:303–05. doi: 10.1016/s0140-6736(62)91248-5. [DOI] [PubMed] [Google Scholar]
- 29.Franchini M, Frattini F, Crestani S, Bonfanti C. Novel treatments for epistaxis in hereditary hemorrhagic telangectasia: a systematic review of the clinical experience with thalidomide. J Thromb Thrombolysis. 2013;36:355–57. doi: 10.1007/s11239-012-0840-5. [DOI] [PubMed] [Google Scholar]
- 30.Penaloza A, Vekemans MC, Lambert C, Hermans C. Deep vein thrombosis induced by thalidomide to control epistaxis secondary to hereditary haemorrhagic telangiectasia. Blood Coagul Fibrinolysis. 2011;22:616–18. doi: 10.1097/MBC.0b013e32834a040c. [DOI] [PubMed] [Google Scholar]
- 31.Thalgott J, Dos-Santos-Luis D, Lebrin F. Pericytes as targets in hereditary hemorrhagic telangiectasia. Front Genet. 2015;6:37. doi: 10.3389/fgene.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.