Abstract
Impulsivity is an important feature of multiple neuropsychiatric disorders, and individual variation in the degree of inherent impulsivity could play a role in the generation or exacerbation of problematic behaviors. Serotonin (5-HT) actions at the 5-HT2AR receptor (5-HT2AR) promote and 5-HT2AR antagonists suppress impulsive action (the inability to withhold premature responses; motor impulsivity) upon systemic administration or microinfusion directly into the medial prefrontal cortex (mPFC), a node in the corticostriatal circuit that is thought to play a role in the regulation of impulsive action. We hypothesized that the functional capacity of the 5-HT2AR, which is governed by its expression, localization, and protein/protein interactions (eg, postsynaptic density 95 (PSD95)), may drive the predisposition to inherent impulsive action. Stable high-impulsive (HI) and low-impulsive (LI) phenotypes were identified from an outbred rodent population with the 1-choice serial reaction time (1-CSRT) task. HI rats exhibited a greater head-twitch response following administration of the preferential 5-HT2AR agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) and were more sensitive to the effects of the selective 5-HT2AR antagonist M100907 to suppress impulsive action relative to LI rats. A positive correlation was observed between levels of premature responses and 5-HT2AR binding density in frontal cortex ([3H]-ketanserin radioligand binding). Elevated mPFC 5-HT2AR protein expression concomitant with augmented association of the 5-HT2AR with PSD95 differentiated HI from LI rats. The observed differential sensitivity of HI and LI rats to 5-HT2AR ligands and associated distinct 5-HT2AR protein profiles provide evidence that spontaneously occurring individual differences in impulsive action reflect variation in the cortical 5-HT2AR system.
INTRODUCTION
High impulsivity cuts across multiple diagnostic categories of neural disorders, including attention deficit hyperactivity disorder, autism, schizophrenia, and substance use disorders (American Psychiatric Association, 2013). A major challenge for maximizing therapeutic approaches to these disorders is understanding the extent to which variation in the degree of inherent impulsivity predicts, promotes, or exacerbates problematic behaviors or therapeutic outcomes. Impulsivity is commonly dichotomized into impulsive choice (preference for small immediate rewards over large delayed rewards) and impulsive action (inability to withhold a prepotent motor response) (Evenden, 1999; Moeller et al, 2001). Here, we focus on impulsive action, or motor impulsivity, that may be measured in such tasks as the continuous performance test, the Go/No-Go task, and the family of choice serial reaction time (CSRT) tasks. In these tasks, an inappropriate response that occurs before a signal (ie, premature response) constitutes the measure of impulsive action (Dougherty et al, 2000, 2002; Winstanley, 2011). Importantly, variable degrees of inherent impulsive action within outbred rodent populations are reliably identified in the CSRT tasks (Anastasio et al, 2014; Besson et al, 2013; Caprioli et al, 2014; Dalley et al, 2007; Economidou et al, 2012). The employment of such translationally focused models may lead to a greater appreciation of the neurobiological drivers underlying individual differences in motor impulsivity.
There is a limited, but growing, appreciation of the specific neurobiological drivers of individual differences underlying impulsive action (Anastasio et al, 2014; Besson et al, 2013; Caprioli et al, 2014; Dalley et al, 2007; Economidou et al, 2012). Particular attention has been given to prefrontal cortex (PFC) and its striatal connections. The medial aspect of the rodent PFC (mPFC) plays a complex and important role in impulsive action, as demonstrated by lesion, reversible inactivation, and genetic manipulation (Anastasio et al, 2014; Chudasama et al, 2003; Muir et al, 1996; Narayanan et al, 2006). Catecholamine and serotonin (5-HT) neurotransmission are important neuromodulators of such corticostriatal dynamics and have been implicated as key modulators of motor impulsivity (for review, see Cunningham and Anastasio, 2014; Dalley et al, 2002; Eagle and Baunez, 2010; Fineberg et al, 2010; Jupp et al, 2013).
Serotonin actions at the G protein-coupled 5-HT2A receptor (5-HT2AR) promote impulsive action based upon extensive pharmacological studies with 5-HT2AR ligands. Specifically, systemically administered preferential 5-HT2AR agonists increase whereas selective 5-HT2AR antagonists reduce impulsive action (Anastasio et al, 2011; Cunningham et al, 2013; Fletcher et al, 2007; Koskinen et al, 2000b; Robinson et al, 2008; Winstanley et al, 2004b), effects that are recapitulated by intra-mPFC infusion (Passetti et al, 2003; Winstanley et al, 2003; Wischhof et al, 2011). The 5-HT2AR in corticostriatal circuits may also drive individual differences in impulsive action given that 5-HT2AR binding density is elevated in frontal cortical regions of the selectively bred Roman high-avoidance rat (Klein et al, 2014). It remains unknown whether this finding extends beyond selective breeding to explain natural variation in impulsive action among genetically heterogeneous rodents.
The present study was designed to gain insights into the nature and origins of individual differences in impulsive action and the involvement of the mPFC 5-HT2AR in mediating these patterns of behavior in an outbred rodent population. The 5-HT2AR is expressed at high density in the mPFC (Lopez-Gimenez et al, 1997; Pazos et al, 1985; Pompeiano et al, 1994) and is primarily localized to cytoplasmic compartments within dendritic shafts and only rarely localized to plasma membranes in cerebral cortex (Cornea-Hebert et al, 1999, 2002). The postsynaptic density protein of 95 kDa (PSD95) is a PSD scaffolding protein that complexes with the 5-HT2AR (Becamel et al, 2002; Becamel et al, 2004; Xia et al, 2003); this interaction is essential to membrane expression/trafficking of the 5-HT2AR and its signaling capacity as well as the pharmacological response to 5-HT2AR ligands in vivo (Abbas et al, 2009). We propose that the functional capacity of the 5-HT2AR in mPFC, which is governed by its expression, subcellular localization, and macromolecular protein complex composition, may drive the predisposition to high inherent impulsive action.
We hypothesized that high-impulsive (HI) rats, identified based upon levels of impulsive action (premature responses) in the 1-CSRT task, would be more sensitive than low-impulsive (LI) rats to the pharmacological effects of a preferential 5-HT2AR agonist and/or selective 5-HT2AR antagonist as assessed by the 5-HT2AR-mediated head-twitch response and 1-CSRT task performance. We further hypothesized that HI rats, relative to LI rats, would display greater cortical 5-HT2AR binding density and mPFC synaptosomal protein levels concomitant with an augmented 5-HT2AR/PSD95 association. The following set of experiments provides the first evidence that spontaneously occurring individual differences in impulsive action in outbred rats reflect variation in the cortical 5-HT2AR system.
MATERIALS AND METHODS
General Methods
Animals
Male, Sprague–Dawley rats (n=167; Harlan, Houston, TX) weighing 250–275 g upon arrival were housed two per cage under a 12-h light–dark cycle with controlled temperature (21–23 °C) and humidity (40–50%). Animals were acclimatized for 7 days to the colony room before the start of handling and experimental procedures. During the 1-CSRT task acquisition and maintenance, rats were food restricted to 90% free-feeding weight; water was available ad libitum except during daily operant sessions. Rats were weighed daily to ensure that their body weights were maintained at 90% of free-feeding levels. All experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (2011) and with the University of Texas Medical Branch Institutional Animal Care and Use Committee approval.
1-choice serial reaction time task
Procedures occurred in standard five-hole nose-poke operant chambers equipped with a houselight, food tray, and an external pellet dispenser capable of delivering 45 mg pellets (Bio-Serv, Frenchtown, NJ) housed within a ventilated and sound-attenuated chamber (MedAssociates, St Albans, VT). The 1-CSRT task methodology has been described in detail previously (Anastasio et al, 2011, 2013, 2014; Cunningham et al, 2013). Briefly, in the pretraining stage, rats were habituated to the test chamber; a nose-poke into the singly illuminated center hole resulted in the delivery of one food pellet into the magazine on the opposite wall of the chamber and simultaneous illumination of the magazine light. Subsequent training stages consisted of daily sessions of 100 trials to be completed in a maximum of 30 min; each training stage implemented an incrementally shorter stimulus duration (final stage: 0.5 s) with a 5-s limited hold and an intertrial interval (ITI) of 5 s (ITI5). A maximum of 100 correct responses in a session resulted in a maximum of 100 reinforcers delivered; incorrect or premature responses or omissions resulted in a 5-s timeout period and a reduction in potential reinforcers obtained. Advancement to the next training stage required rats to meet acquisition criteria: ≥50 correct responses, >80% accuracy (correct responses/(correct+incorrect) × 100) and <20% omissions (omitted responses/trials completed × 100) (Anastasio et al, 2011).
The total number of responses (premature, correct, incorrect, and omissions) as well as the latency to collect the reinforcer were recorded. Premature responses were used to assess impulsive action. The number of reinforcers earned provides a measure of task competency and a secondary assessment of impulsive action, whereas percent accuracy is a general indication of attentional capacity. Percent omissions indicate failures of detection of the visual stimuli in the center hole as well as motivation to perform the task. Latency to collect the reinforcer provides an additional indicator of motivation.
Identification of impulsive action phenotype
After meeting stability criteria for the final training stage over five consecutive ITI5 sessions (with <20% variability over last three sessions, days ∼25–30), an ITI8 challenge session was conducted in which the ITI was 8 s for the entirety of the session (Anastasio et al, 2014; Dalley et al, 2002). Following ITI8 challenge, rats were restabilized on ITI5 sessions (<20% variability over three sessions) before a second ITI8 challenge. HI and LI rats were defined as the upper and lower quartile, respectively, of premature responses averaged over ITI8 challenge sessions. Rats were again restabilized on ITI5 maintenance sessions before subsequent experimentation.
Research Design
Cohort 1: inherent impulsive action predicts response to a 5-HT2AR agonist in the head-twitch assay
Following phenotype identification on the 1-CSRT task (Figure 1, see experimental timeline), rats (n=28) were kept in home cages for 3 days under maintenance of food restriction so as not to perturb 5-HT2AR sensitivity (Serafine and France, 2014). The preferential 5-HT2AR agonist (−)-2,5-dimethoxy-4-iodoamphetamine (DOI; Sigma-Aldrich, St Louis, MO) was dissolved in vehicle (sterile saline). Following injection with DOI (1 mg/kg) or vehicle (1 ml/kg, s.c.), rats were immediately placed into transparent cages (43 cm by 26 cm by 19 cm) and video recorded in high definition (HDR-XR550V; Sony, Tokyo, Japan) for 30 min. Head twitches were operationally defined as a rapid rotational head movement (Canal et al, 2013) and scored manually by a blinded reviewer over a 15-min period beginning 10 min after injection. Two outliers were removed from analysis using Cook's method (n=26 rats analyzed).
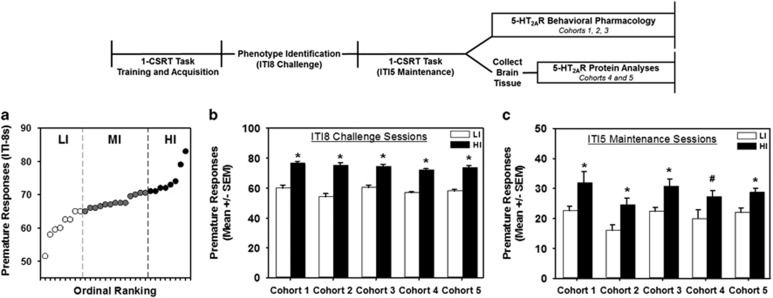
Figure 1.
1-CSRT task separates impulsive action phenotypes. (a) Ordinal ranking of a representative cohort (cohort 3) based on premature responses averaged over two ITI8 challenge sessions. Low-impulsive (LI, n=8, open circles) and high-impulsive rats (HI, n=8, closed circles) were defined by lower and upper quartiles, respectively, from mid-impulsive rats (MI, n=13, gray circles). (b) HI rats (black bars) exhibit elevated premature responses vs LI rats (white bars) in all cohorts during performance in ITI8 challenge sessions and (c) during stable performance in ITI5 maintenance sessions, as averaged over 2 to 3 days before subsequent experimentation (*p<0.05 vs LI, #p=0.06).
Cohorts 2 and 3: inherent impulsive action predicts sensitivity to 5-HT2AR ligands in the 1-CSRT task
Following the ITI8 challenge session (n=32 rats, each cohort) and ITI5 restabilization (Figure 1, see experimental timeline), 1-CSRT task performance was evaluated after administration of the 5-HT2AR agonist DOI (cohort 2) or 5-HT2AR antagonist M100907 (cohort 3; synthesized by Kenner Rice, National Institute on Drug Abuse, Bethesda, MD). Vehicle (sterile saline (cohort 2); 1% Tween-80 in sterile saline (cohort 3); 1 ml/kg), DOI (0.01, 0.03, and 0.1 mg/kg; s.c.), or M100907 (0.001, 0.01, and 0.1 mg/kg; i.p.) were injected 30 min before commencement of 1-CSRT task sessions under ITI5 conditions. Within each cohort, each rat received all doses of DOI (cohort 2) or M100907 (cohort 3) in a balanced, pseudo-randomized order. Rats underwent five daily 1-CSRT task sessions per week; rats were treated with vehicle the day before drug treatments and received only one drug treatment per week. An additional ITI8 challenge session was conducted following completion of drug testing to confirm identification of the impulsive action phenotype. Two rats from cohort 2 and three rats from cohort 3 were excluded from analysis because of failure to maintain stable performance (cohort 2: n=30 rats analyzed, cohort 3: n=29 rats analyzed).
Cohort 4: inherent impulsive action predicts 5-HT2AR density in whole frontal cortex
At 2 to 3 days following behavioral testing, rats (n=30) were anesthetized with chloral hydrate (400 mg/kg, i.p.) and brains were extracted (Figure 1, see experimental timeline). The olfactory bulbs were discarded, and frontal cortex was isolated by a single coronal cut rostral to striatum (+3 mm from bregma) (Paxinos and Watson, 1998) and frozen at −80 °C until use. Frontal cortices were processed by crude synaptosomal fractionation that enriches presynaptic and postsynaptic compartments (Gylys et al, 2000). Samples were homogenized in sucrose buffer (0.32 M sucrose with 10 μl/ml each of protease and phosphatase inhibitors; Sigma-Aldrich) using a glass-teflon homogenizer. Samples were centrifuged at 12 000 g for 15 min, and pellets were washed twice in Tris buffer (Tris HCL (50 mM), MgCl2 (10 mM), and protease and phosphatase inhibitors (10 μl/ml), pH 7.4 at 4 °C). Finally, samples were resuspended in Tris buffer. Protein concentrations were determined by BCA assay (Thermo Scientific, Rockford, IL), and samples were diluted to 1 mg/ml and stored at −80 °C until assayed (<2 weeks).
Radioligand binding and analysis were conducted according to published protocols with modifications (Clarke et al, 2001). Assay buffer (50 mM Tris, 10 mM MgCl2, pH 7.4, at 4 °C), prazosin (100 nM final concentration; Sigma-Aldrich), and [3H]-ketanserin (50.3 Ci/mmol; 10 nM final concentration) (PerkinElmer, Waltham, MA) were sequentially added to glass tubes to a total volume of 450 μl. Prazosin was added to mask α1-adrenergic receptors, and nonspecific binding was determined by the addition of M100907 (10 μM final concentration). Reactions commenced with the addition of 50 μg protein and proceeded with constant agitation for 1 h at room temperature. Reactions were terminated using a cell harvester (Brandel, Gaithersburg, MD) by rapid filtration through GF/C glass filters presoaked in 0.3% polyethylenimine (Sigma-Aldrich). Filters were dried overnight, transferred to vials containing 5 ml scintillant (RPI, Mount Prospect, IL), equilibrated overnight, and counted with a LS6500 scintillation counter (Beckman Coulter, Indianapolis, IN). Specific binding was averaged across three independent experiments in which samples were measured in triplicate. Two samples were lost during tissue handling (n=28 samples analyzed).
The crude synaptosomal protein extracted from whole frontal cortex of HI and LI rats (above) was modified by the addition of 0.5% NP-40 and 1 mM dithiothreiotol (DTT). 5-HT2AR protein levels were assessed using the Wes automated western blotting system (ProteinSimple, San Jose, CA) that utilizes capillary electrophoresis-based immunodetection for higher resolution, sensitivity, and reproducibility (even at low sample concentrations) relative to traditional immunoblotting techniques (Liu et al, 2013). Wes reagents (biotinylated molecular weight marker, streptavidin-HRP fluorescent standards, luminol-S, hydrogen peroxide, sample buffer, DTT, stacking matrix, separation matrix, running buffer, wash buffer, and matrix removal buffer, secondary antibodies, antibody diluent, and capillaries) were obtained from the manufacturer (ProteinSimple) and used according to the manufacturer's recommendations. The 5-HT2AR antibody (LS-C172270, 1 : 500; LifeSpan Biosciences, Seattle, WA) or pan-cadherin antibody (AB6528, 1 : 1000; Abcam, Cambridge, MA) were diluted with ProteinSimple antibody diluent.
Equal amounts of protein (3 μg) were combined with 0.1 × sample buffer and 5 × master mix (200 mM DTT, 5 × sample buffer, 5 × fluorescent standards), gently mixed, and then denatured at 95 °C for 5 min. The denatured samples, biotinylated ladder, antibody diluent, primary antibodies, HRP-conjugated secondary antibodies, chemiluminescent substrate, and wash buffer were dispensed to designated wells in a pre-filled microplate (ProteinSimple). Separation electrophoresis (375 V, 31 min, 25 °C) and immunodetection in the capillaries were fully automated using the following settings: separation matrix load for 200 s, stacking matrix load for 14 s, sample load for 7 s, antibody diluent for 30 min, primary antibody incubation for 60 min, secondary antibody incubation for 30 min, and chemiluminescent signal exposure for 30, 120, 240, and 480 s. Data analyses were performed using the Compass Software (ProteinSimple) and 5-HT2AR immunoreactivity normalized to pan-cadherin immunoreactivity.
Cohort 5: impulsive action phenotypes exhibit distinct 5-HT2AR expression profiles in the mPFC
At 2 to 3 days following behavioral testing, rats (n=30) were anesthetized (chloral hydrate solution (400 mg/kg)), and brains were extracted (Figure 1, see experimental timeline). The mPFC (containing infralimbic, prelimbic, and anterior cingulate cortex) was microdissected immediately over ice, flash frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA and protein extraction. Crude synaptosomal protein was prepared (Anastasio et al, 2010) with one modification. Immediately following initial homogenization, 10% of sample volume was transferred to 500 μl of TRI Reagent for RNA isolation (Life Technologies, Grand Island, NY). RNA was stored at −80 °C until assayed (see Supplementary Materials and Methods). For immunoprecipitation experiments, mPFC samples (n=8, 4 HI and 4 LI) were drawn from a cohort that underwent identical 1-CSRT task training and phenotype identification (previously published) (Anastasio et al, 2014).
Immunoblotting
Equal amounts of crude synaptosomal protein prepared from the mPFC (Anastasio et al, 2010, 2014) were separated by SDS-PAGE and transferred to a PVDF membrane for blotting with 5-HT2AR antibody (AB16028, 1 : 1000; Abcam) or pan-cadherin antibody (AB6528, 1 : 10 000; Abcam). Membranes were incubated with mouse IgG IRDye 800 (1 : 10 000) or rabbit IgG IRDye 680 (1 : 10 000) for detection by Odyssey Imaging System (LI-COR, Lincoln, NE). The integrated intensity of each band was analyzed with the Odyssey Software and 5-HT2AR immunoreactivity normalized to pan-cadherin immunoreactivity.
Co-immunoprecipitation
Postsynaptic density (PSD95) antibody (MAB1598, 10 μg; Millipore, Billerica, MA) was covalently crosslinked onto protein A/G resin as previously described with minor modifications (Anastasio et al, 2010). Crude synaptosomal protein (200 μg) prepared from the mPFC was incubated with the antibody-crosslinked resin for 48 h at 4 °C with constant shaking. The eluted antigen was subjected to cold acetone precipitation and the protein sample centrifuged at 15 000 g for 10 min at 4 °C. The precipitated protein was resuspended in 2 × loading buffer and subjected to SDS-PAGE. Immunoblotting for 5-HT2AR and PSD95 (1 : 1000) was performed as described above. One sample was lost during processing.
Statistical analyses
Measures of 1-CSRT task performance were assessed by Student's t-test and one-way ANOVA as appropriate, and pair-wise preplanned comparisons between phenotypes or cohorts were assessed by Tukey's test. The effects of DOI and M100907 on 1-CSRT task performance were analyzed by two-way repeated-measures ANOVA; the effects of treatment vs vehicle within phenotype were assessed by Dunnett's procedure. The DOI-induced head-twitch response, [3H]-ketanserin binding, and 5-HT2AR expression data were assessed by Student's t-test and Pearson's correlation. Data were collected blinded to the experimenter, and analyses were performed in SAS (version 9.3; Cary, NC) with an experiment-wise error rate of α=0.05.
RESULTS
HI and LI Rats Are Reliably Identified in the 1-CSRT Task
Phenotypic classification of rats in the CSRT tasks has proven useful toward evaluating the neurobiology underlying individual differences in impulsive action. Five cohorts of outbred rats were stratified for the impulsive action phenotype using the 1-CSRT task. Figure 1a demonstrates the separation of rats in a representative cohort (cohort 3) by upper (HI, n=8) and lower (LI, n=8) quartiles of premature responding during ITI8 challenge sessions. In all cohorts, HI rats exhibited significantly greater premature responses (Figure 1b; p<0.0001), earned fewer reinforcers (Supplementary Table S1; p<0.0001), and omitted fewer trials (Supplementary Table S1; p<0.01) than LI rats during ITI8 challenge sessions. No differences were observed in accuracy or latency to collect the reinforcer (Supplementary Table S1). Elevated premature responding stably persisted in HI vs LI rats under ITI5 maintenance conditions (Figure 1c; p<0.05). Differences between HI and LI rats in reinforcers earned and accuracy under ITI5 conditions did not reach statistical significance (Supplementary Table S2). In a single cohort (cohort 2), fewer omissions were observed in HI relative to LI rats (Supplementary Table S2, p<0.05); however, the number of omissions for either groups was substantially below the set training criterion of <20% omissions (see Materials and Methods) (Anastasio et al, 2011). No differences in premature responses, reinforcers earned, omissions, or accuracy were observed within phenotypes between the cohorts (Supplementary Table S2). Population variability in premature responding under prolonged ITI8 challenge conditions in the 1-CSRT task allows stratification of rats as HI or LI in part because the sensitivity to detect differences is enhanced and premature responding is encouraged (Anastasio et al, 2014; Besson et al, 2013; Caprioli et al, 2014; Dalley et al, 2007; Economidou et al, 2012). The differential levels of premature responses on the ITI8 that direct phenotypic identification mirror subsequent performance under standard ITI5 maintenance conditions that stably endures for months of daily sessions and with little variability between separate cohorts of rats (Anastasio et al, 2014).
The Preferential 5-HT2AR Agonist DOI Elicits Greater Head-Twitch Responses in HI vs LI Rats
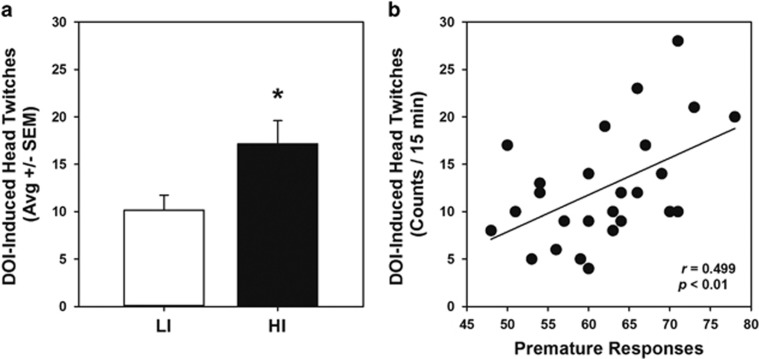
Variation in the functional capacity of the 5-HT2AR may contribute to individual differences in impulsive action, such that elevated signaling through the 5-HT2AR may underlie the predisposition to high impulsive action. We first sought to evaluate the pharmacological responsiveness of the 5-HT2AR system in HI and LI rats (cohort 1) using a nonoperant behavioral measure routinely employed to assess 5-HT2AR function in vivo. Many 5-HT2AR agonists induce a head-twitch response in rodents that is dependent upon 5-HT2AR activation (Canal et al, 2013). We hypothesized that the preferential 5-HT2AR agonist DOI would elicit a greater head-twitch response in HI relative to LI rats. Administration of DOI (1 mg/kg) produced a greater head-twitch response in HI rats than LI rats (Figure 2a; p<0.01). Regression analysis of individual rats revealed a positive correlation between DOI-induced head twitches and premature responses (Figure 2b; r=0.499, p<0.01). No head twitches were observed following vehicle administration (data not shown).
Figure 2.
Inherent impulsive action predicts the DOI-elicited head-twitch response. Upon completion of 1-CSRT task training and phenotypic identification (cohort 1), injection of DOI (1 mg/kg, s.c.) resulted in head-twitches that were quantified over a 15-min period. (a) HI rats (n=7, black bar) exhibited a greater number of head twitches than LI rats (n=7, white bar; *p<0.05 vs LI-VEH). (b) There was a positive correlation between the number of head-twitches and premature responses during phenotype identification on the ITI8 challenge in the 1-CSRT task (r=0.499; p<0.01).
The Preferential 5-HT2AR Agonist DOI Increases Impulsive Action in the 1-CSRT Task
Several studies have demonstrated that systemic administration of the preferential 5-HT2AR agonist DOI elicits modest increases in premature responses in the 5-CSRT task (Koskinen et al, 2000a; Koskinen and Sirvio, 2001; Wischhof and Koch, 2012). Given the observation that the DOI-induced head-twitch response pharmacologically dissociated HI and LI rats (Figure 2a), we next sought to determine whether the differential behavioral effects of DOI would be recapitulated in the 1-CSRT task (cohort 2). Vehicle (VEH)-treated HI rats demonstrated elevated premature responses relative to vehicle-treated LI rats (Figure 3a; p<0.05), consistent with baseline task performance (Figure 1c and Supplementary Table S2). DOI increased premature responses (Figure 3a); there was a main effect of phenotype on premature responses (F1,15=19.24, p<0.001) and a main effect of treatment (F3, 45=13.54, p<0.0001), but no treatment × phenotype interaction (F3, 45=0.38, n.s.) was observed. In HI rats, a main effect of treatment was observed (F3, 35=5.31, p<0.01); planned comparisons showed that only the 0.03 mg/kg dose of DOI increased premature responses vs vehicle (145.3±6.5% of vehicle performance; p<0.05). Similarly, only 0.03 mg/kg of DOI increased premature responses in LI rats (180.7±7.2% of vehicle performance; main effect: F3, 28=4.84, p<0.01; Dunnett's, p<0.05). The effects of DOI to increase premature responding was accompanied by a decrease in reinforcers earned (Supplementary Figure S1A; main effect: F3, 45=10.35, p<0.0001). No main effect of phenotype on reinforcers earned was observed (F1,15=3.53, n.s.), nor was there a significant treatment × phenotype interaction (F3, 45=0.11, n.s.). No main effect of treatment was observed for omissions (Supplementary Figure S1B) or accuracy (data not shown). Thus, whereas HI rats exhibit increased DOI-induced head-twitch response relative to LI rats, the DOI-mediated increase in impulsive action was parallel between HI and LI rats.
Figure 3.
HI rats are more sensitive to the behavioral effects of M100907, but not DOI, in the 1-CSRT task. Following 1-CSRT task training and phenotypic identification, the effects of DOI (0.01, 0.03, and 0.1 mg/kg; cohort 2) and M100907 (0.001, 0.01, and 0.1 mg/kg; cohort 3) were each evaluated in separate cohorts of rats under ITI5 conditions. In both cohorts, baseline levels of premature responses in HI rats administered vehicle (VEH; closed circles, upper dotted line) were significantly higher than the vehicle baseline in LI rats (open circles, lower dashed line; *p<0.05 vs LI-VEH). (a) In both HI (n=9) and LI rats (n=7), DOI significantly increased premature responses at 0.03 mg/kg (#p<0.05 vs HI-VEH, *p<0.05 vs LI-VEH). (b) In HI rats (n=8), M100907 significantly suppressed premature responses at 0.01 and 0.1 mg/kg (#p<0.05 vs HI-VEH), below the vehicle baseline of LI rats (n=8). Only the highest dose of M100907 (0.1 mg/kg) significantly suppressed premature responses in LI rats (*p<0.05 vs LI-VEH).
HI Rats Are More Sensitive to the Suppressive Effects of the Selective 5-HT2AR Antagonist M100907 than LI in the 1-CSRT Task
Selective blockade of the 5-HT2AR consistently reduces impulsive action after systemic administration, measured predominantly in 1- or 5-CSRT tasks (Anastasio et al, 2011; Cunningham et al, 2013; Fletcher et al, 2007; Koskinen et al, 2000b; Robinson et al, 2008; Winstanley et al, 2004b). In another cohort of rats (cohort 3), we tested the hypothesis that HI rats would exhibit greater sensitivity to the suppressive effects of the 5-HT2AR antagonist M100907 on impulsive action compared with LI rats. Vehicle-treated HI rats demonstrated elevated premature responses relative to vehicle-treated LI rats (Figure 3b; p<0.05). M100907 dose-dependently decreased premature responses (Figure 3b); there was no main effect of phenotype on premature responses (F1,14=1.13, n.s.), but a main effect of treatment (F3, 42=25.89, p<0.0001) and a significant treatment × phenotype interaction (F3, 42=3.68, p<0.05) were observed. In HI rats, a main effect of treatment was observed (F3, 28=8.99, p<0.001); planned comparisons showed that both the 0.01 and 0.1 mg/kg doses of M100907 decreased premature responses vs vehicle (68.6±8.0% and 36.3±7.6% of vehicle performance, respectively; p<0.05). However, only the highest dose of 0.1 mg/kg M100907 significantly suppressed premature responses in LI rats (58.9±7.1% of vehicle performance; main effect: F3, 28=4.24, p<0.05; Dunnett's, p<0.05). Thus, HI rats are more sensitive to the effects of M100907 to suppress impulsive action by one order of magnitude.
The number of reinforcers earned increased significantly only in HI rats following M100907 administration (Supplementary Figure S2A). No main effect of phenotype on reinforcers earned was observed (F1, 14=0.17, n.s.), but a main effect of treatment (F3, 42=11.34, p<0.0001) and a significant treatment × phenotype interaction (F3, 42=3.43, p<0.05) were observed. In HI rats, a main effect of treatment on reinforcers earned was observed (F3, 28=4.94, p<0.01); 0.1 mg/kg M100907 significantly increased reinforcers earned (126.6±5.1% of vehicle performance; p<0.05). A main effect of treatment on reinforcers earned in LI rats was not observed (F3, 28=0.52, n.s.), indicating that M100907 preferentially enhanced task performance in HI, but not LI, rats. No main effect of treatment was observed for accuracy or latency to collect the reinforcer (data not shown), but there was a nonsignificant trend for increased omissions (Supplementary Figure S2B; main effect: F3, 42=2.58, p=0.066).
Impulsive Action Phenotypes Exhibit Distinct 5-HT2AR Protein Profiles in Whole Frontal Cortex
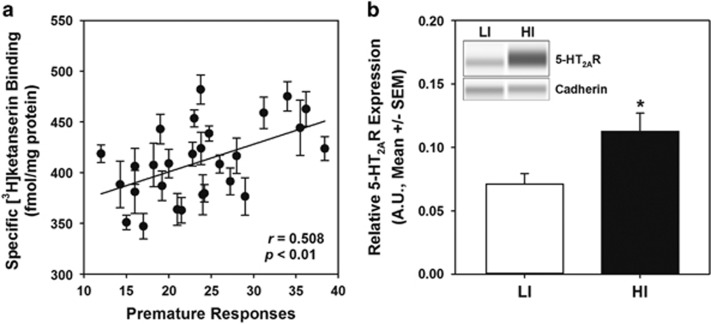
HI rats were more sensitive to the effects of the 5-HT2AR ligands than LI rats in two independent behavioral measures. Differential sensitivity to 5-HT2AR ligands may be explained by differences in 5-HT2AR density in frontal cortex (Leysen and Pauwels, 1990), the brain region with the highest 5-HT2AR density (Pazos et al, 1985; Pompeiano et al, 1994). In a separate cohort of rats (cohort 4), we tested the hypothesis that inherent impulsive action is associated with greater 5-HT2AR binding sites in frontal cortex. Single-point radioligand binding with the 5-HT2AR antagonist [3H]-ketanserin was conducted to estimate 5-HT2AR binding sites at saturation (Muguruza et al, 2013) in a crude synaptosomal fraction of the whole frontal cortex; specific binding of 410.7±7.0 fmol/mg protein (average±SEM) is consistent with previous reports (Leysen et al, 1982). A significant difference in [3H]-ketanserin binding was not observed in frontal cortex of HI and LI rats (HI: 418.4±33.1 fmol/mg, LI: 409.6±41 fmol/mg; n.s.); a regression analysis of individual rats revealed a positive correlation between specific [3H]-ketanserin binding and premature responses (Figure 4a; r=0.508, p<0.01). There was a relatively wide distribution of premature responses for this cohort under ITI5 maintenance conditions (Supplementary Figure S3). Although ITI8 challenge sessions generally allow more facile stratification of phenotypes, premature responding under ITI8 and ITI5 conditions equally reflect a failure to appropriately withhold a response. Thus, the absence of a difference in [3H]-ketanserin binding sites between HI and LI groups does not diminish the biological significance of the correlation between [3H]-ketanserin binding and impulsive action across the entire cohort (Figure 4a).
Figure 4.
Inherent impulsive action predicts 5-HT2AR binding density and protein expression in whole frontal cortex. (a) Following 1-CSRT task training and phenotypic identification (cohort 4), whole frontal cortex was collected and a crude synaptosomal preparation analyzed for single-point radioligand binding ([3H]-ketanserin, 10 nM) to approximate saturation binding. Specific [3H]-ketanserin binding positively correlated with premature responses during stable ITI5 performance in the 1-CSRT task (n=28; r=0.508, p<0.01). Error bars (±SEM) represent results from three independent experiments run in triplicate. (b) The subset of HI and LI samples from the same frontal cortex crude synaptosomal preparations were then analyzed by capillary gel-based immunodetection. Quantitation of 5-HT2AR immunoreactivity (normalized to cadherin loading control) revealed elevated 5-HT2AR protein expression in HI (n=6, black bar) relative to LI rats (n=6, white bar; *p<0.05 vs. LI). The inset presents representative electrophoretic bands. Arbitrary units (AU) of normalized densitometry are presented (see Materials and Methods).
We next sought an alternative assessment of 5-HT2AR protein abundance to confirm the association between frontal cortex 5-HT2AR binding density and inherent impulsive action. The same frontal cortex crude synaptosomal protein employed for [3H]-ketanserin binding was evaluated by capillary gel-based immunodetection that offers greater sensitivity and quantitation over traditional immunoblotting (Liu et al, 2013). Higher 5-HT2AR immunoreactivity was observed in the whole frontal cortex sample of HI rats relative to LI rats (Figure 4b; p<0.05). These results suggest that phenotypic variation in 5-HT2AR protein profiles in whole frontal cortex associates with levels of motor impulsivity.
Impulsive Action Phenotypes Exhibit Distinct 5-HT2AR Protein Profiles in mPFC
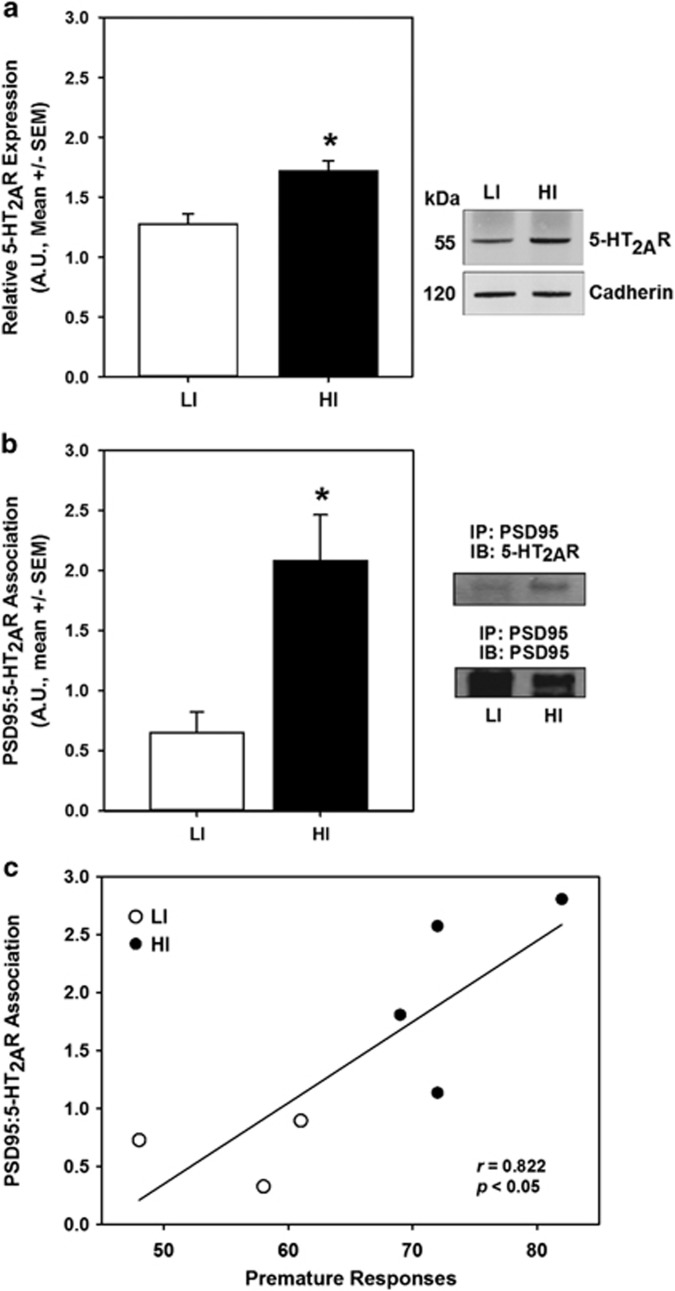
The relatively large section of frontal cortex necessary for radioligand binding experiments is a functionally heterogeneous structure containing regions that may not contribute to inhibitory control as reflected in the 1-CSRT task. The mPFC is a subregion of the frontal cortex known to modulate impulsive action (Dalley et al, 2004), and microinfusion of the 5-HT2AR agonist DOI into the mPFC is sufficient to increase impulsive action and elicit the head-twitch response (Willins and Meltzer, 1997; Wischhof et al, 2011). Thus, we next sought to determine whether the 5-HT2AR protein expression level was higher in HI relative to LI rats, specifically within the mPFC subregion of the frontal cortex. Immunoblots performed on crude synaptosomal fractions of mPFC revealed elevated 5-HT2AR expression in HI vs LI rats (Figure 5a; p<0.05). To determine whether differential regulation of 5-HT2AR expression occurs at the level of transcription, qRT-PCR was performed to measure Htr2a mRNA; no difference in Htr2a mRNA abundance was observed in mPFC extracts of HI and LI rats (Supplementary Figure S4). Immunoblots were conducted to evaluate 5-HT2AR protein expression in total mPFC homogenate to address the discordance between Htr2a mRNA levels and crude synaptosomal 5-HT2AR protein levels; no difference in 5-HT2AR protein expression between HI and LI rats was observed in the total mPFC homogenate (data not shown). These results suggest differential regulation of 5-HT2AR protein expression in the synaptosomal compartment of the mPFC in HI and LI rats.
Figure 5.
Inherent impulsive action predicts synaptic protein expression in mPFC. (a) Following 1-CSRT task training and phenotypic identification (cohort 5), the mPFC was collected for biochemical analysis. Immunoblot for the 5-HT2AR and cadherin loading control was performed using crude synaptosomal protein from the mPFC (inset, representative blot). Densitometric quantitation revealed elevated 5-HT2AR protein expression in HI (n=7, black bar) relative to LI rats (n=7, white bar; p<0.05). (b) Immunoprecipitation (IP) of mPFC crude synaptosomes with α-PSD95 antibody followed by immunoblots (IB) for 5-HT2AR and PSD95 (right, representative blots) revealed an increased 5-HT2AR/PSD95 association in HI (n=4, black bar) relative to LI rats (n=3, white bar; p<0.05). (c) There was a correlation between premature responses in the 1-CSRT task and densitometric quantitation of 5-HT2AR/PSD95 association (r=0.822; p<0.05). Arbitrary units (AU) of normalized densitometry are presented (see Materials and Methods).
Ultrastructural studies within the mPFC have localized the majority of 5-HT2AR expression to postsynaptic elements (Miner et al, 2003), and the abundant scaffolding protein PSD95 complexes with the 5-HT2AR to positively regulate membrane trafficking and signaling (Abbas et al, 2009; Xia et al, 2003). Thus, we hypothesized that the difference in 5-HT2AR protein expression between HI and LI rats in a crude synaptosomal fraction of the mPFC (Figure 5a) may be attributed to differences in the association of 5-HT2AR and PSD95. We performed immunoprecipitation of mPFC crude synaptosomal fractions with anti-PSD95 antibody and subsequently immunoblotted with anti-5-HT2AR antibody to determine 5-HT2AR/PSD95 association in HI and LI rats. Greater 5-HT2AR co-immunoprecipitated with PSD95 in HI rats than LI rats (Figure 5b; p<0.05); a positive correlation was observed between premature responses and 5-HT2AR/PSD95 association (Figure 5c; r=0.822, p<0.05). No differences were observed in total PSD95 protein expression (data not shown).
DISCUSSION
The present study provides the first indication that spontaneously occurring individual differences in impulsive action in genetically heterogeneous outbred rats reflect variation in the cortical 5-HT2AR system. We discovered that HI rats exhibited higher sensitivity to the effects of the preferential 5-HT2AR agonist DOI (5-HT2AR-mediated head-twitches) and to the effects of the selective 5-HT2AR antagonist M100907 (suppression of premature responses). Our finding that HI action tracks with high cortical 5-HT2AR density, particularly within the mPFC, is consistent with microinfusion studies that validate the importance of the 5-HT2AR in the mPFC for both generating the head-twitch response (Willins and Meltzer, 1997) and mediating impulsive action (Passetti et al, 2003; Winstanley et al, 2003; Wischhof et al, 2011; but see Robinson et al, 2008). Furthermore, the higher level of 5-HT2AR/PSD95 association in the mPFC of HI rats relative to LI rats suggests that factors that control the functional status of the 5-HT2AR in mPFC may drive in part the predisposition to inherent impulsive action. Our observations, concordant across multiple independent cohorts of animals, represent a unique contribution to an emergent body of research indicating that individual differences in motor impulsivity are related to specific neurobiological distinctions within corticostriatal circuits.
Substantial evidence supports the complex role of serotonergic tone within corticostriatal networks in the control of motor impulsivity. Premature responding positively correlates with 5-HT release in the PFC (Dalley et al, 2002); however, depletion of 5-HT, which results in a compensatory upregulation of the 5-HT2AR (Heal et al, 1985), increases impulsive action (Harrison et al, 1997; Winstanley et al, 2004a, 2004b). In addition, 5-HT2AR-expressing neurons in the mPFC project to the dorsal raphe to regulate the activity of midbrain 5-HT neurons (Vazquez-Borsetti et al, 2009). Differences between HI and LI rats in 5-HT release during demands on inhibitory control, 5-HT2AR signaling capacity, and 5-HT2AR-mediated cortical-raphe feedback may dynamically interact to account for the effects of the selective 5-HT2AR antagonist M100907 to potently and efficaciously ‘normalize' high motor impulsivity. Furthermore, the dose-dependent increase in reinforcers earned in high, but not low, impulsive rats suggests that M100907 may act as a ‘cognitive enhancer' selectively in HI rats (Robbins, 2002; Robbins et al, 1997). Taken together, these findings support the conclusion that the enhanced sensitivity of HI rats to M100907 is not simply attributable to putative floor effects on premature responses in LI rats, but that the cortical network discerns shifts in serotonergic modulation via the 5-HT2AR to mitigate impulsive responding.
The proposal that HI rats exhibit higher sensitivity to 5-HT2AR ligands relative to LI rats is further substantiated by our observation that the preferential 5-HT2AR agonist DOI elicited greater head-twitches in HI vs LI rats. The positive correlation between premature responses and DOI-induced head-twitches ensures that differences in 5-HT2AR ligand sensitivity are continuous across individual differences in impulsive action, irrespective of phenotypic grouping by the experimenter. The DOI-induced head-twitch response is potently and efficaciously blocked by selective 5-HT2AR antagonists; however, DOI additionally exhibits a high affinity for, and partial agonist actions, at the 5-HT2CR (McClue et al, 1989; Porter et al, 1999; Smith et al, 1998). The 5-HT2AR and 5-HT2CR exhibit oppositional effects on premature responding in CSRT tasks (Cunningham et al, 2013; Fletcher et al, 2007; Robinson et al, 2008; Winstanley et al, 2004b) and competing actions of DOI at these receptors upon systemic administration may complicate interpretation of its effects in relatively complex behavioral paradigms (Nichols, 2014), such as the CSRT tasks. The nonoperant, and presumably involuntary, DOI-induced head-twitch circumvents these complications and may provide a more specific index of 5-HT2AR functional capacity in vivo.
The functional capacity of the 5-HT2AR protein in mPFC is governed in part by its expression that, in the present studies, is elevated in HI vs LI rats, and also by other features such as subcellular localization and trafficking. The positive correlation between premature responses and 5-HT2AR density assessed by [3H]-ketanserin binding in whole frontal cortex provides biochemical evidence that mechanistically supports our behavioral pharmacological observations. The quantity of tissue required to conduct receptor binding assays necessitated the use of the whole frontal cortex that comprises not only the mPFC, but also cortical subregions that putatively could have limited or opposing functional involvement in inhibitory control. Thus, 5-HT2AR density determined in whole frontal cortex may underestimate differences between impulsive action phenotypes within functionally relevant frontal cortical subregions such as the mPFC that mediates DOI-induced head-twitches (Willins and Meltzer, 1997) and motor impulsivity (Passetti et al, 2003; Winstanley et al, 2003; Wischhof et al, 2011). Interestingly, we found that HI rats expressed higher 5-HT2AR protein expression assessed by immunodetection methods in the whole frontal cortex as well as the mPFC. As differential 5-HT2AR expression was observed neither at the mRNA level nor within total homogenate fractions, targeted localization of the 5-HT2AR to the functionally relevant synaptic compartment may be an important neurobiological determinant of inhibitory control. Association of the 5-HT2AR with PSD95 is critically important for 5-HT2AR signaling and membrane targeting as PSD95 deletion substantially reduced the effects of 5-HT2AR agonists and antagonists at cellular and behavioral levels (Abbas et al, 2009; Xia et al, 2003). Excitingly, HI rats exhibited a significant elevation in the association of the 5-HT2AR and PSD95 in the mPFC, supporting the concept that a higher level of 5-HT2AR translocation to the functionally relevant postsynaptic density (Abbas et al, 2009) may be an important neurobiological determinant of HI action.
The correlational analyses in the present experiments support an interpretation that functional capacity of the 5-HT2AR protein in mPFC is important in setting the tone for the differential sensitivity to systemically administered 5-HT2AR ligands between HI and LI rats. Given the clear bidirectional effects of pharmacological 5-HT2AR manipulation on motor impulsivity (agonists promote, antagonists suppress), it is unlikely that our observations of elevated 5-HT2AR density in HI rats reflect a molecular response to the consequences of highly impulsive behavior. Nevertheless, a strictly causal relationship between 5-HT2AR density and individual differences in motor impulsivity cannot be definitively deduced from these experiments. Genetic manipulation of 5-HT2AR through virally mediated overexpression (Herin et al, 2013) or knockdown of the 5-HT2AR in the mPFC will facilitate a more conclusive determination of causal directionality in the association between 5-HT2AR density and phenotypic impulsive action.
The present data add to the small, but growing, body of research to suggest that phenotypic variance in motor impulsivity is under the control of a complex basal or constitutive balance within corticostriatal circuitry. Individual differences in impulsive action have now been associated with expression patterns of the dopamine D1 receptor (Simon et al, 2013), D2/3 receptor (Besson et al, 2013; Dalley et al, 2007; Simon et al, 2013), γ-aminobutyric acid (GABA), and dendritic markers (Caprioli et al, 2014) in the nucleus accumbens as well as the 5-HT2AR (present results) and the 5-HT2CR in mPFC (Anastasio et al, 2014). The precise manner in which these systems coordinate at the level of the neuron and/or circuit remains poorly understood, and it is parsimonious to suggest that these neurobiological factors interact to fine-tune behavioral control afforded by the corticostriatal network (Jupp et al, 2013). For example, in a recent study, we reported that genetic loss of the 5-HT2CR in the mPFC resulted in increased motor impulsivity (Anastasio et al, 2014), concomitant with augmented 5-HT2AR-mediated control over impulsive action and increased 5-HT2AR expression in the mPFC (Anastasio et al, unpublished observations). Given that the constitutive knockout of the 5-HT2AR results in upregulation of 5-HT2CR control over the excitability of mPFC neurons (Beique et al, 2007), these data suggest that a 5-HT2AR/5-HT2CR interaction at the level of the mPFC may rheostatically control impulsive action (Cunningham and Anastasio, 2014). Future studies will be required to intuit the coordinated serotonergic, dopaminergic, and GABAergic control of the corticostriatal circuitry that contributes to phenotypic impulsive action (Besson et al, 2013; Caprioli et al, 2014; Dalley et al, 2007; Jupp et al, 2013; Simon et al, 2013).
Determination of the neurobiological substrates of individual differences in motor impulsivity remains a critical step toward development of personalized therapeutic strategies to curb problematic impulsive tendencies associated with neuropsychiatric disorders. Our observations are a significant contribution toward this goal and stand among few studies to reveal the neurobiological underpinnings of individual differences in motor impulsivity. Given the evolutionary value of natural variation in the predisposition toward impulsive behavior (Stevens et al, 2005), examination of such individual differences affords a strategic approach toward identification of the specific factors that underlie the impulsive phenotype (Jupp et al, 2013) and may collectively lead to refined and optimized treatment of neuropsychiatric disorders characterized by extreme, disadvantageous impulsive behavior.
FUNDING AND DISCLOSURE
Dr Cunningham is a consultant for Arena Pharmaceuticals. Dr Moeller is a consultant for Boehringer-Ingelheim. All other authors declare no conflict of interest.
Acknowledgments
We thank Dr Charles D Nichols and Dr Kenneth M Johnson for providing assistance and advice for the radioligand binding studies. This work was supported by NIDA grants F30 DA034488 (to LHLF), T32 007287 (to LHLF), P20 DA024157 (to KAC), P50 DA0333935 (to KAC), K05 DA020087 (to KAC), and K99 DA033374 (to NCA), and the Center for Addiction Research at the University of Texas Medical Branch. A portion of this work was supported by the Intramural Research Programs of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG et al (2009). PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. JNeurosci 29: 7124–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5.
- Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng YJ et al (2013). Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J Neurosci 33: 1615–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG et al (2010). Serotonin 5-HT(2C) receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem 113: 1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG et al (2011). Serotonin (5-hydroxytryptamine) 5-HT2A receptor: association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol 22: 248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ et al (2014). Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology 39: 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Alonso G, Galeotti N, Demey E, Jouin P, Ullmer C et al (2002). Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J 21: 2332–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Gavarini S, Chanrion B, Alonso G, Galeotti N, Dumuis A et al (2004). The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J Biol Chem 279: 20257–20266. [DOI] [PubMed] [Google Scholar]
- Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R (2007). Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA 104: 9870–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A et al (2013). Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology 38: 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D (2013). Support for 5-HT2C receptor functional selectivity in vivo utilizing structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head-twitch response model. Neuropharmacology 70: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B et al (2014). Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry 75: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW (2003). Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res 146: 105–119. [DOI] [PubMed] [Google Scholar]
- Clarke WP, Berg KA, Gould G, Frazer A (2001). Characterization of 5-HT((1)A,B) and 5-HT((2)A,C) serotonin receptor binding. Curr Protoc Pharmacol Chapter 1: Unit 1.23. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L (1999). Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol 409: 187–209. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Watkins KC, Roth BL, Kroeze WK, Gaudreau P, Leclerc N et al (2002). Similar ultrastructural distribution of the 5-HT(2A) serotonin receptor and microtubule-associated protein MAP1A in cortical dendrites of adult rat. Neuroscience 113: 23–35. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC (2014). Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76(Pt B): 460–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE et al (2013). Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci 4: 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW (2004). Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28: 771–784. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K et al (2007). Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315: 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW (2002). Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology 26: 716–728. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Marsh DM, Moeller FG (2000). A comparison between adults with conduct disorder and normal controls on a continuous performance test: differences in impulsive response characteristics. Psychol Record 50: 203–219. [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW (2002). Immediate and delayed memory tasks: a computerized behavioral measure of memory, attention, and impulsivity. Behav Res Methods Instrum Comp 34: 391–398. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C (2010). Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev 34: 50–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW (2012). Norepinephrine and dopamine modulate impulsivity on the five-choice serial reaction time task through opponent actions in the shell and core sub-regions of the nucleus accumbens. Neuropsychopharmacology 37: 2057–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL (1999). Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol 13: 180–192. [DOI] [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A et al (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology 35: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA (2007). Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 195: 223–234. [DOI] [PubMed] [Google Scholar]
- Gylys KH, Fein JA, Cole GM (2000). Quantitative characterization of crude synaptosomal fraction (P-2) components by flow cytometry. J Neurosci Res 61: 186–192. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW (1997). Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 133: 329–342. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Philpot J, Molyneux SG, Metz A (1985). Intracerebroventricular administration of 5,7-dihydroxytryptamine to mice increases both head-twitch response and the number of cortical 5-HT2 receptors. Neuropharmacology 24: 1201–1205. [DOI] [PubMed] [Google Scholar]
- Herin DV, Bubar MJ, Seitz PK, Thomas ML, Hillman GR, Tarasenko YI et al (2013). Elevated expression of serotonin 5-HT(2A) receptors in the rat ventral tegmental area enhances vulnerability to the behavioral effects of cocaine 1. Front Psychiatry 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Caprioli D, Dalley JW (2013). Highly impulsive rats: modelling an endophenotype to determine the neurobiological, genetic and environmental mechanisms of addiction. Dis Model Mech 6: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AB, Ultved L, Adamsen D, Santini MA, Tobena A, Fernandez-Teruel A et al (2014). 5-HT(2A) and mGlu2 receptor binding levels are related to differences in impulsive behavior in the Roman Low- (RLA) and High- (RHA) avoidance rat strains. Neuroscience 263: 36–45. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Haapalinna A, Sirvio J (2003). Alpha-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol 92: 214–225. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT et al (2000. a). Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology 39: 471–481. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Sirvio J (2000. b). The 5-HT(2) receptor activation enhances impulsive responding without increasing motor activity in rats. Pharmacol Biochem Behav 66: 729–738. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Sirvio J (2001). Studies on the involvement of the dopaminergic system in the 5-HT2 agonist (DOI)-induced premature responding in a five-choice serial reaction time task. Brain Res Bull 54: 65–75. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Niemegeers CJ, Van Nueten JM, Laduron PM (1982). [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol 21: 301–314. [PubMed] [Google Scholar]
- Leysen JE, Pauwels PJ (1990). 5-HT2 receptors, roles and regulation. Ann NY Acad Sci 600: 183–193. [DOI] [PubMed] [Google Scholar]
- Liu S-B, Sardi S, Sonom B, Zocco D, McSweeney R, Fraser AD et al (2013). The application of a novel nanovolume capillary electrophoresis-based protein analysis system in personalized & translational medicine research. J Bioanal Biomed S3–004 doi:10.4172/1948-593X.S3-004.
- Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT (1997). Selective visualization of rat brain 5-HT2A receptors by autoradiography with [3H]MDL 100,907. Naunyn Schmiedebergs Arch Pharmacol 356: 446–454. [DOI] [PubMed] [Google Scholar]
- McClue SJ, Brazell C, Stahl SM (1989). Hallucinogenic drugs are partial agonists of the human platelet shape change response: a physiological model of the 5-HT2 receptor. Biol Psychiatry 26: 297–302. [DOI] [PubMed] [Google Scholar]
- Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR (2003). Ultrastructural localization of serotonin2A receptors in the middle layers of the rat prelimbic prefrontal cortex. Neuroscience 116: 107–117. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC (2001). Psychiatric aspects of impulsivity. Am J Psychiatry 158: 1783–1793. [DOI] [PubMed] [Google Scholar]
- Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, Gonzalez-Maeso J (2013). Dysregulated 5-HT(2A) receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol 23: 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW (1996). The cerebral cortex of the rat and visual attentional function: dissociable effects of mediofrontal, cingulate, anterior dorsolateral, and parietal cortex lesions on a five-choice serial reaction time task. Cereb Cortex 6: 470–481. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M (2006). Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience 139: 865–876. [DOI] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SD et al (2014). N-Benzyl-5-methoxytryptamines as potent serotonin 5-HT2 receptor family agonists and comparison with a series of phenethylamine analogues. ACS Chem Neurosci; e-pub ahead of print 29 December 2014. doi:10.1021/cn500292d. [DOI] [PMC free article] [PubMed]
- Passetti F, Dalley JW, Robbins TW (2003). Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology (Berl) 165: 136–145. [DOI] [PubMed] [Google Scholar]
- Paxinos W, Watson C (1998) The Rat Brain in Stereotaxic Coordinates, 4th edn. Academic Press: San Diego, CA. [Google Scholar]
- Pazos A, Cortes R, Palacios JM (1985). Quantitative autoradiographic mapping of serotonin receptors in rat brain. II. Serotonin-2 receptors. Brain Res 346: 231–249. [DOI] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G (1994). Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Mol Brain Res 23: 163–178. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF et al (1999). Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol 128: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW (2002). The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163: 362–380. [DOI] [PubMed] [Google Scholar]
- Robbins TW, McAlonan G, Muir JL, Everitt BJ (1997). Cognitive enhancers in theory and practice: studies of the cholinergic hypothesis of cognitive deficits in Alzheimer's disease. Behav Brain Res 83: 15–23. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER et al (2008). Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology 33: 2398–2406. [DOI] [PubMed] [Google Scholar]
- Serafine KM, France CP (2014). Restricted access to standard or high fat chow alters sensitivity of rats to the 5-HT(2A/2C) receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane. Behav Pharmacol 25: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Beas BS, Montgomery KS, Haberman RP, Bizon JL, Setlow B (2013). Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur J Neurosci 37: 1779–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Canton H, Barrett RJ, Sanders-Bush E (1998). Agonist properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol Biochem Behav 61: 323–330. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Hallinan EV, Hauser MD (2005). The ecology and evolution of patience in two New World monkeys. Biol Lett 1: 223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Borsetti P, Cortes R, Artigas F (2009). Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cereb Cortex 19: 1678–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY (1997). Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther 282: 699–706. [PubMed] [Google Scholar]
- Winstanley CA (2011). The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol 164: 1301–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW (2003). Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 167: 304–314. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW (2004. a). Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology 29: 1331–1343. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW (2004. b). 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176: 376–385. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Hollensteiner KJ, Koch M (2011). Impulsive behaviour in rats induced by intracortical DOI infusions is antagonized by co-administration of an mGlu2/3 receptor agonist. Behav Pharmacol 22: 805–813. [DOI] [PubMed] [Google Scholar]
- Wischhof L, Koch M (2012). Pre-treatment with the mGlu2/3 receptor agonist LY379268 attenuates DOI-induced impulsive responding and regional c-Fos protein expression. Psychopharmacology (Berl) 219: 387–400. [DOI] [PubMed] [Google Scholar]
- Xia Z, Gray JA, Compton-Toth BA, Roth BL (2003). A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem 278: 21901–21908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.