INTRODUCTION
Emerging evidence supports a number of associations between cannabis and psychosis/psychotic disorders, including schizophrenia. Acute exposure to both cannabis and synthetic cannabinoids (CBs) (including Spice and K2) can produce a range of transient psychotomimetic symptoms, cognitive deficits, and psychophysiological abnormalities that bear a striking resemblance to symptoms of schizophrenia (D'Souza et al, 2004; Radhakrishnan et al, 2014). Furthermore, epidemiologic studies suggest that early and heavy exposure to cannabis confers a higher risk for developing a psychotic disorder such as schizophrenia (Moore et al, 2007). However, only a minority of individuals exposed to CBs appear to be vulnerable to CB-related acute or persistent psychosis outcomes.
In individuals with schizophrenia, CBs have been shown to transiently exacerbate symptoms (D'Souza et al, 2005), trigger relapse, and have negative consequences on the course of the illness (Linszen and van Amelsvoort, 2007). A number of lines of evidence suggest that schizophrenia patients are more vulnerable to some of the effects of CBs. In an experience sampling study, schizophrenia patients were more sensitive to the psychosis-inducing effects of cannabis than controls (Henquet et al, 2010). Epidemiologic studies also show that cannabis use is associated with greater negative consequences on the course and expression of schizophrenia (van Os et al, 2002). In an experimental study, despite receiving stable doses of antipsychotic medications and being clinically stable, 80% of schizophrenic patients, but only 25% of controls, experienced clinically significant psychosis (>3 points on the Positive and Negative Syndrome Scale (PANSS) positive subscale) with a low dose of delta-9-tetrahydrocannabinol (THC) (D'Souza et al, 2005). Finally, individuals who are psychosis prone as determined either psychometrically or by family history are more sensitive to the psychosis-inducing effects of cannabis (Arendt et al, 2008; GROUP, 2011). However, the basis of the enhanced vulnerability to the psychosis-inducing effects of CBs in schizophrenia patients is not clear. Several other mechanisms might explain vulnerability to THC effects including polymorphisms of genes for COMT (Henquet et al, 2006), AKT1, and DAT1 (Bhattacharyya et al, 2012, 2014), and γ-aminobutyric acid (GABA) deficits. Furthermore, it is conceivable that combinations of these factors may coexist and have additive or synergistic effects on increasing vulnerability to THC effects.
GABA deficits have been observed in the dorsolateral prefrontal cortex in schizophrenia (Lewis et al, 2005), and furthermore, there is important interplay between the CB and GABA systems (Eggan et al, 2010). Indeed, converging lines of evidence, including post-mortem (Lewis et al, 2005), genetic (reviewed by Charych et al, 2009), and brain imaging studies (Busatto et al, 1997; Verhoeff et al, 1999) suggest that dysfunction of the GABA system contributes to the pathophysiology of schizophrenia. Although there is strong support for the existence of a GABA deficit in schizophrenia, the proportion of schizophrenia patients with this deficit is not known. The limited data available suggest that only 50% of schizophrenia patients have lower GABA levels compared with the lowest level found in healthy normal controls (Yoon et al, 2010).
In several brain regions, particularly the cerebral cortex and hippocampus, CB1 receptors (CB1-Rs) are present on the axon terminals of cholecystokinin (CCK) containing GABA interneurons that target the perisomatic region of pyramidal cells (PCs) (Eggan et al, 2010). CB1-Rs are activated by endocannabinoids released postsynaptically by depolarized PCs (Wilson and Nicoll, 2002). The activation of CB1Rs inhibits the release of GABA by CCK-basket cells, leading to a disinhibition of postsynaptic PCs (Bartos and Elgueta, 2012; Klausberger et al, 2005). Thus, a CB1-R-mediated braking mechanism regulates the timing and release of GABA, and subsequently the overall inhibitory/excitatory balance in cortical networks (Farkas et al, 2010). This interplay between GABA and CB1-R systems provides a mechanism that could explain the higher vulnerability to CBs in schizophrenia. For instance, if CB1-R activation occurred in the presence of a pre-existing GABA deficit (as might be the case in schizophrenia), this could lead to further disinhibition and desynchronization of PC activity, leading to perturbations in gating, associative functions, and neurocognition, which could culminate in psychotic symptoms.
This study tested the hypothesis that if, among other mechanisms, GABA deficits contribute to the increased vulnerability of schizophrenia patients to the psychosis-exacerbating effects of CBs, then inducing a GABA deficit in healthy subjects will increase the psychosis-inducing effects of THC. As described below, a GABA deficit was pharmacologically modeled by the administration of the GABAA inverse agonist, iomazenil.
MATERIALS AND METHODS
In a four test day double-blind, placebo-controlled, randomized and counterbalanced study, healthy volunteers received iomazenil followed by THC, placebo iomazenil followed by THC, iomazenil followed by placebo THC, and placebo iomazenil followed by placebo THC. The study was conducted in the Neurobiological Studies Unit (VA Connecticut Healthcare System (VACHS), West Haven, CT). Subjects were recruited by advertisements and by word of mouth, and were paid for their participation.
Approvals
The study was approved by the institutional review boards of the VACHS and Yale University School of Medicine and was carried out in accordance with the Declaration of Helsinki (1975). The study was carried out under Investigational New Drug applications (51 671 and 75 099). Subjects were informed about the potential for adverse effects of THC, iomazenil, and the combination.
Subjects
After obtaining written informed consent, subjects (n=27) underwent a Structured Clinical Interview for DSM-IV (SCID; First et al, 2002) and were carefully screened (Supplementary Text no. 1). Women were excluded because the teratogenicity of iomazenil is not known. A detailed history of cannabis exposure was obtained. Cannabis-naive individuals were excluded to minimize any risk of promoting future cannabis use/abuse. Exclusion criteria included a personal or family history of epilepsy or seizure disorder because of the potential for iomazenil to reduce seizure threshold, a family history of a major Axis 1 disorder, and DSM-IV cannabis or other substance dependence (except nicotine). A general physical and neurological examination, electrocardiogram, and laboratory tests were also conducted. A screening electroencephalogram (EEG) was conducted to exclude subjects with any activity suggestive of seizure disorder. After screening, subjects were instructed to refrain from alcohol, caffeinated beverages, illicit drugs (other than cannabis), or prescription drugs not approved by the research team for 1 week before the study and throughout study participation. Compliance with the alcohol and drug prohibitions was formally assessed at every screening visit and on the morning of each test day ( × 4), by asking subjects and also testing breath for alcohol, and urine for drugs. Compliance with the caffeine restriction was checked by asking subjects.
Drugs
The preparation, formulation, and storage of THC solution and control are reported elsewhere (D'Souza et al, 2004) (Supplementary Text no. 2). THC at a dose of 0.015 mg/kg (1.05 mg in a 70 kg individual) was administered intravenously over 10 min into a rapidly flowing intravenous infusion of normal saline.
Iomazenil (Ro 16-0154) is an iodine analog of the benzodiazepine receptor (BZR)-competitive antagonist flumazenil. Some of its pharmacologic properties are comparable to those of the BZR-competitive antagonist flumazenil (Beer et al, 1990). However, unlike flumazenil, which blocks the effects of BZR agonists but lacks intrinsic pharmacological effects (Hunkeler et al, 1981), inverse agonists interfere with the function of the BZR in coupling the GABAA receptor and chloride channel, and have intrinsic pharmacological effects opposite to those of BZR agonists (Tallman and Gallager, 1985). For example, the BZR inverse agonist FG-7142 is anxiogenic in healthy human subjects (Dorow et al, 1983; Horowski and Dorrow, 2002). BZR inverse agonist drugs bind to extrasynaptic GABAA receptors (Liang et al, 2004). Iomazenil has high affinity and selectivity for BZRs (Kd=0.5 nM) (Johnson et al, 1990). In in vitro models, it inhibits the binding of the weak BZR inverse agonist [3H]Ro15-4513 to γ2- and δ-subunit-containing GABAA receptors with IC50 of 20–30 nM (R Olsen, personal communication, 26 January 2007). Iomazenil produces a net deficit in GABA function; in preclinical studies, it has been shown to behave as a BZR-competitive antagonist with inverse agonist effects (Beer et al, 1990; Hoffmann-La Roche; Schubiger and Hasler, 1989), and in clinical studies, it has been shown to have anxiogenic effects, and proconvulsant effects at higher doses (Randall, 1995; personal communication). Consistent with a role of GABA deficits in the pathophysiology of psychosis, iomazenil has been shown to increase the psychotomimetic effects of the 5-HT2 partial agonist 1-(m-chlorophenyl)piperazine (m-CPP) in healthy subjects (D'Souza et al, 2006) and schizophrenia patients are more vulnerable to the propsychotic effects of iomazenil (Ahn et al, 2011). Iomazenil was administered intravenously at a dose of 3.7 μg/kg over 10 min.
Blood was sampled repeatedly and assayed for THC and 11-nor-Δ-9-THC-9-COOH (THC-COOH) using gas chromatography mass spectrometry (Shaw et al, 1991) to rule out that any hypothesized worsening of effects with the combination of the two drugs was not merely a result of iomazenil increasing THC levels.
Behavioral and Subjective Measures
Psychosis-relevant symptoms were assessed using the PANSS (Kay et al, 1989). Perceptual alterations were measured using the Clinician Administered Dissociative Symptoms Scale (CADSS) (Bremner et al, 1998). ‘High' and other subjective effects associated with cannabis intoxication were measured using a self-reported visual analog scale (VAS) (0–100). These assessments were administered at baseline (−60 min), +70 min and +240 min timepoints, where timepoint 0 min denotes the beginning of the THC infusion. The baseline rating was to assess the predrug state. Subsequent ratings covered the entire time period between the current and past timepoint, for example, +70 covered the time period from baseline to +70 min. The same research coordinators rated all 4 test days for each subject. Inter-rater reliability sessions were conducted every 1–2 months over the time period that this study was conducted (~4 years) and, for example, intraclass correlation coefficients for the PANSS were consistently >0.85 (Table 1).
Table 1. Schedule of Test Day.
| Time | Procedure |
|---|---|
| Screening (~4 weeks before test day) | • Medical and psychiatric history, SCID, cannabis/drug/alcohol use, confirmation of history with collateral |
| • Lifetime marijuana use assessment | |
| • Chemistry, hematology, urine toxicology, EKG, vital signs, height, and weight | |
| • Baseline safety EEG to rule out risk for seizures | |
| −120 | • Confirm adherence to prestudy prohibitions: |
| • Last use of tobacco, cannabis, alcohol, caffeine, other drugs, medications, and supplements | |
| • Breathalyzer | |
| • Urine toxicology | |
| • Confirm that subjects have not had any recent psychosocial stressors? | |
| • Confirm that subject has fasted since midnight? | |
| • Vital signs: blood pressure and heart rate | |
| • Standard light breakfast | |
| • Insert two IV lines: identify arms for iomazenil and THC infusions | |
| −90 | • Set up EEG cap |
| • Vital signs: blood pressure and heart rate | |
| −60 | • Ratings: PANSS, CADSS, and VAS |
| • Blood sampling for THC/THC-COOH assay | |
| −15 | • Vital signs: blood pressure and heart rate |
| −10 | • Intravenous iomazenil 3.7 μg/kg over 10 min |
| 0 | • Intravenous THC (0.015 mg/kg) or placebo over 10 min |
| +5 | • Vital signs: blood pressure and heart rate |
| +10 | • Vital signs: blood pressure and heart rate |
| • Blood sampling for THC/THC-COOH assay | |
| +25 | • Event-related potentials: P300 |
| • Vital signs: blood pressure and heart rate | |
| +45 | • Vital signs: blood pressure and heart rate |
| +70 | • Ratings: PANSS, CADSS, and VAS |
| +80 | • Vital signs: blood pressure and heart rate |
| • Blood sampling for THC/THC-COOH | |
| +240 | • Vital signs: blood pressure and heart rate |
| • Ratings: PANSS, CADSS, and VAS | |
| • Blood sampling for THC/THC-COOH assay | |
| • Safety check list: | |
| ○ MMSE | |
| ○ Field sobriety test | |
| ○ Exit interview | |
| ○ Discharge instructions |
Abbreviations: SCID, Structured Clinician Interview; EEG, electroencephalography; NPO, nil per oral; PANSS, Positive and Negative Syndrome Scale; CADSS, Clinician Administered Dissociative Symptoms Scale; VAS, Visual Analog Scale; MMSE, Mini Mental State Examination. Safety follow-up: 1 and 3 months after last test day for cannabis use and psychiatric symptoms.
General Procedure and Test Days
Test days were separated by 3 days to minimize carryover effects given the half-life of THC. Subjects fasted overnight, reported to the test facility around 0800 hours, and were provided a standard breakfast. Urine toxicology was conducted on the morning of each test day to rule out recent illicit drug use. In-study safety procedures are described elsewhere (D'Souza et al, 2004). Behavioral and subjective ratings, vital signs, and blood sampling were repeated several times before and after drug administration, while psychophysiological data were collected only once per test day. A field sobriety test, mental state examination, and exit interview were conducted at the end of each test day. Prospective safety assessments were performed at 1 and 3 months after the last test session and after they had received payment for participation to query their use of cannabis.
EEG Recording
EEG data were collected in an acoustically shielded booth, and recording was carried out with the commercially available Active Two Acquisition System (Biosemi, The Netherlands). A sampling rate of 1024 Hz was used, with online low-pass filter of 256 Hz to prevent aliasing of high frequencies. A 64-channel electrode cap according to the extended 10-20 system was used, along with additional electrodes to record the vertical and horizontal electro-oculogram. All electrodes were referenced during recording to a common-mode signal electrode between POz and PO3 and then subsequently re-referenced to the nose offline.
EEG Task
A three-stimulus auditory oddball task adapted from our previous study was used (D'Souza et al, 2012). Briefly, a random series of infrequent (8.33%) ‘target' tones (1000 Hz sine wave), frequent (83.33%) ‘standard' tones (20, 30, or 40 Hz click trains), and infrequent distractor sounds (8.33%) were presented with a 1250 interstimulus interval in three separate blocks. Distractors included a set of novel everyday natural and manmade sounds (Friedman et al, 1993). Target tones and standard click trains were 500 ms in duration, whereas distractor stimuli ranged in duration from 175 to 250 ms. All stimuli were presented binaurally using at an intensity of 80 dB SPL.
In each block, participants were asked to press a response key to the target stimuli with the index finger of their preferred hand. Each block was comprised of 15 targets, 15 distractor stimuli, and 150 standards (20 Hz standard click trains for Block 1, 30 Hz standard click trains for Block 2, and 40 Hz standard click trains for Block 3). The order of blocks was randomized for each test day. To maximize event-related potential (ERP) signal-to-noise-ratio, target and distractor stimuli were averaged from all three blocks. Thus, in total, there were 45 targets, 45 distractors, and 450 standards.
EEG Signal Processing
EEG data were first bandpass filtered from 0.1 to 100 Hz (24 dB/oct) and notch filtered at 60 Hz. The recorded EEG was then segmented into epochs consisting of a 100 ms baseline and ending 1000 ms after stimulus onset. Ocular movement correction was applied using Gratton's algorithm (Gratton et al, 1983). After baseline correction, any trial with a voltage >±100 μV was excluded from analysis. Finally, the data were low-pass filtered (12 Hz cutoff, 24 dB/oct) and single trials were averaged before peak detection of ERPs.
For target and novel stimuli, the P300b and P300a, respectively, were identified as the largest positive voltage peak between 250 and 400 ms after stimulus onset using an automated algorithm. To assess primary sensory processing and registration, the N100 component to both target and novel stimuli was examined. The N100 was defined as the largest negative voltage peak between 50 and 150 ms after stimulus onset. For statistical analysis, data were used for each component where amplitude was largest as described previously (Pz for P300b; Cz for P300a; Cz for N100) (D'Souza et al, 2012). Processing and analysis of distractor stimuli will be reported elsewhere. All EEG processing was performed using commercially available software (Analyzer 2.0; Brain Products GmbH, Gilching, Germany).
Statistical Analysis
Initially, data were examined descriptively using means, SDs, and graphs. Each outcome was assessed visually for normality using histograms and normal probability plots. As THC peak effects were captured 70 min after drug administration, and because at other timepoints there was little difference from the minimum score, each behavioral and subjective outcome was expressed as peak change from baseline using methods described elsewhere (D'Souza et al, 2012). The data were analyzed using linear mixed models with drug condition: (1) active iomazenil, placebo THC, (2) active iomazenil, active THC, (3) placebo iomazenil, active THC, and (4) placebo iomazenil, placebo THC as a within-subjects factor. Tukey's post-hoc procedure was used to determine significant pair-wise group differences between each of the four conditions. The correlation between repeated measures on an individual was modeled using random-effects and/or structured variance–covariance matrices. The best fitting variance–covariance structure was determined using information criteria. In the above models, potential covariates (eg, frequency of and days since last use of cannabis) were entered into the model in turn but were not significant and dropped for parsimony. The mixed-effects approach is advantageous as it is unaffected by randomly missing data and allows greater flexibility in modeling the correlation structure of repeated-measures data (Gueorguieva and Krystal, 2004). Models similar to above were used to analyze P300a and P300b data. All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC) (Table 2).
Table 2. Sample Demographics.
| Mean | SD | |
|---|---|---|
| Age (years) | 25.44 | 7.41 |
| Weight (kg) | 80.98 | 12.19 |
| Height (cm) | 178.87 | 6.96 |
| IQ (NART) | 116.85 | 5.27 |
| Psychosis proneness | ||
| Wisconsin Psychosis Proneness | 27.30 | 10.75 |
| SPQ (Schizotypal Personality Questionnaire) | 5.87 | 5.12 |
| Average number of alcoholic drinks per week | 5.8 | 5.9 |
| Cannabis use | ||
| Age at first use | 17.27 | 2.35 |
| Frequency of use in past 30 days (no. of days of use) | 6.22 | 7.73 |
| Lifetime frequency of use (no. of days of use) | 296.75 | 266.26 |
| Total n | ||
| Handedness (no.) | 22 | |
| Right | ||
| Left | 4 | |
| Ambidextrous | 1 | |
| Cigarette smoker | 4 | |
| Yes | ||
| No | 23 | |
| No. of subjects who ever tried drugs other than cannabis | ||
| None | 7 | |
| Hallucinogens | 15 | |
| Stimulants | 8 | |
| Opiates | 2 | |
| Inhalants | 0 | |
*None of the subjects met the criteria for abuse or dependence of alcohol and illicit drugs of abuse.
RESULTS
Of the 27 male subjects who were enrolled in the study, 21 completed all 4 test days. EEG data was collected in 23 subjects. The demographic characteristics of the sample are listed in Tables 2 and 3.
Table 3. Behavioral and Subjective Effects of THC and Iomazenil Mean (SD) Scores on the PANSS, CADSS, and VAS Across Drug Condition and Time, Along With Post-Hoc Contrasts.
| Condition | Timepoint | N | Mean | SD | Significant post-hoc contrasts |
|---|---|---|---|---|---|
| PANSS total score | |||||
| IOM−/THC− | −60 | 23 | 28.83 | 0.78 | IOM+/THC+ vs IOM−/THC+ (p=0.038) |
| 70 | 23 | 33.26 | 6.33 | ||
| 240 | 23 | 28.17 | 6.64 | IOM+/THC+ vs IOM+/THC− (p=0.003) | |
| IOM+/THC− | −60 | 20 | 28.65 | 0.59 | |
| 70 | 20 | 38.1 | 8.84 | IOM+/THC+ vs IOM−/THC− (p<0.0001) | |
| 240 | 20 | 28.5 | 0.69 | ||
| IOM−/THC+ | −60 | 23 | 28.83 | 0.78 | IOM−/THC+ vs IOM−/THC− (p=0.005) |
| 70 | 23 | 40.83 | 7.69 | ||
| 240 | 23 | 28.96 | 1.3 | ||
| IOM+/THC+ | −60 | 23 | 28.7 | 0.63 | |
| 70 | 21 | 46.19 | 9.79 | ||
| 240 | 21 | 29.19 | 1.89 | ||
| CADSS patient-rated | |||||
| IOM−/THC− | −60 | 23 | 0.17 | 0.49 | |
| 70 | 23 | 1.26 | 3.09 | IOM+/THC+ vs IOM+/THC− (p=0.029) | |
| 240 | 23 | 0.04 | 0.21 | ||
| IOM+/THC− | −60 | 20 | 0 | 0 | IOM+/THC+ vs IOM−/THC− (p=0.001) |
| 70 | 20 | 4.65 | 7.11 | ||
| 240 | 20 | 0.15 | 0.49 | IOM−/THC+ vs IOM−/THC− (p=0.022) | |
| IOM−/THC+ | −60 | 23 | 0.04 | 0.21 | |
| 70 | 23 | 6.22 | 6.56 | ||
| 240 | 23 | 0.04 | 0.21 | ||
| IOM+/THC+ | −60 | 23 | 0.04 | 0.21 | |
| 70 | 21 | 9.14 | 8.05 | ||
| 240 | 21 | 0.14 | 0.48 | ||
| CADSS clinician-rated | |||||
| IOM−/THC− | −60 | 23 | 0 | 0 | IOM+/THC+ vs IOM−/THC− (p=0.003) |
| 70 | 23 | 0.91 | 1.65 | ||
| 240 | 23 | 0 | 0 | IOM−/THC+ vs IOM−/THC− (p=0.06) | |
| IOM+/THC− | −60 | 20 | 0.05 | 0.22 | |
| 70 | 20 | 1.65 | 2.16 | ||
| 240 | 20 | 0 | 0 | ||
| IOM−/THC+ | −60 | 23 | 0 | 0 | |
| 70 | 23 | 2.96 | 3.61 | ||
| 240 | 23 | 0 | 0 | ||
| IOM+/THC+ | −60 | 23 | 0 | 0 | |
| 70 | 21 | 2.95 | 2.22 | ||
| 240 | 21 | 0 | 0 | ||
| VAS anxious | |||||
| IOM−/THC− | −60 | 23 | 3.62 | 7.57 | IOM+/THC+vs THC alone (p=0.039) |
| 70 | 23 | 2.78 | 8.4 | ||
| 240 | 23 | 0.32 | 0.79 | IOM+/THC+ vs IOM−/THC− (p=0.014) | |
| IOM+/THC− | −60 | 20 | 4.26 | 9.21 | |
| 70 | 20 | 16.37 | 25.89 | IOM+/THC− vs IOM−/THC− (p=0.053) | |
| 240 | 20 | 1.63 | 5.4 | ||
| IOM−/THC+ | −60 | 23 | 3.68 | 8.94 | |
| 70 | 23 | 4.61 | 8.36 | ||
| 240 | 23 | 0.3 | 0.85 | ||
| IOM+/THC+ | −60 | 23 | 1.72 | 3.92 | |
| 70 | 21 | 16.78 | 24.82 | ||
| 240 | 21 | 0.25 | 0.64 | ||
| VAS high | |||||
| IOM−/THC− | −60 | 23 | 0.5 | 2.08 | IOM+/THC+ vs IOM+/THC− (p=0.0001) |
| 70 | 23 | 7.38 | 19.12 | ||
| 240 | 23 | 0.93 | 2.46 | IOM+/THC+ vs IOM−/THC− (p<0.0001) | |
| IOM+/THC− | −60 | 19 | 0.42 | 1.17 | |
| 70 | 20 | 11.68 | 23.08 | IOM−/THC+ vs IOM+/THC− (p=0.004) | |
| 240 | 20 | 0.08 | 0.18 | ||
| IOM−/THC+ | −60 | 23 | 0.32 | 1.06 | IOM−/THC+ vs IOM−/THC− (p=0.0001) |
| 70 | 23 | 36.32 | 29.86 | ||
| 240 | 23 | 0.52 | 1.2 | ||
| IOM+/THC+ | −60 | 23 | 0.53 | 1.69 | |
| 70 | 21 | 44.33 | 33.03 | ||
| 240 | 21 | 0.38 | 0.8 | ||
Plasma Level of THC and THC-COOH
There were no significant effects of iomazenil on plasma levels of THC (drug condition × time, p=0.46) and THC-COOH (drug condition × time, p=0.18) (Supplementary Figure 1).
Behavioral and Subjective Effects
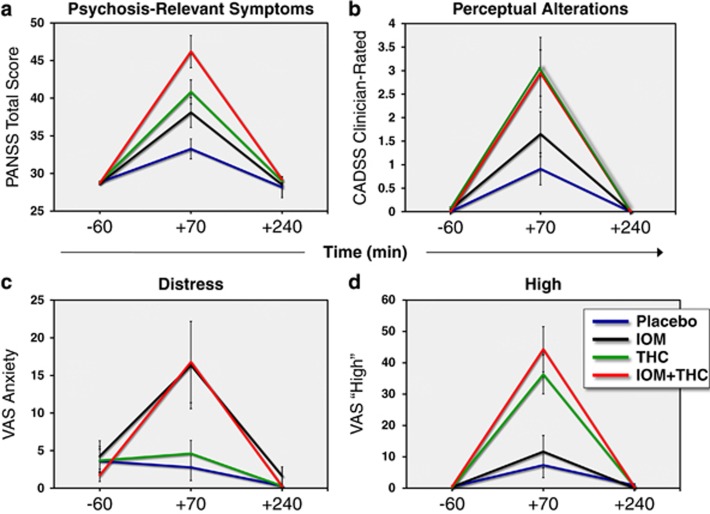
For all behavioral and subjective measures, the main analysis of interest was the contrast between the conditions of combined iomazenil and THC (+IOM+THC), and THC alone (−IOM+THC). Mean (SD) scores on the PANSS (Figure 1), CADSS, and VAS for each drug condition and timepoint are presented in Table 3. Note that even though the statistical analyses were for peak change from baseline, in the figures, all timepoints are shown.
Figure 1.
Mean (±SEM) data for each drug condition at baseline (−60 min), +70 min, and +240 min timepoints for the psychosis-relevant symptoms ((a) Total Positive and Negative Syndrome Scale (PANSS)), perceptual alterations ((b) patient-rated Clinician Administered Dissociative Symptoms Scale (CADSS)), distress ((c) VAS Anxiety), and high ((d) VAS High). Placebo, blue line; iomazenil, black line; THC, green line and the combination of iomazenil; and THC: red line. Note that the figure shows mean (SD) data from all timepoints, whereas the analyses were conducted on the peak change from baseline.
Positive and Negative Syndrome Scale
There was a significant drug condition effect (F3,58=12.93, p<0.0001) on the peak change from baseline in Total PANSS scores. Relative to placebo, the combination of iomazenil and THC (IOM+/THC+) produced significant increases (padj <0.0001) in Total PANSS scores. Relative to placebo, THC alone (IOM−/THC+) produced significant increases (padj=0.005) in Total PANSS scores, while iomazenil did not. Furthermore, there were significantly (padj=0.04) greater increases in Total PANSS scores for the IOM+/THC+ condition compared with IOM−/THC+ (Figure 1a).
Clinician Administered Dissociative Symptoms Scale
There was a significant drug condition effect (F3,59=5.3, p<0.003) on the peak change from baseline in clinician-rated CADSS subscale scores. However, relative to placebo, only IOM+/THC+ produced a significant increase in the clinician-rated CADSS subscale scores (padj=0.003), while the increase for IOM−/THC+ trended towards significance (padj=0.06). The difference between IOM+/THC+ and IOM−/THC+ was not significant.
There was a significant drug condition effect (F3,58=5.82, p<0.0015) on the peak change from baseline in patient-rated CADSS subscale scores. Relative to placebo, IOM+/THC+ (padj <0.0006) and IOM−/THC (padj=0.02) produced significant increases in patient-rated CADSS scores (Figure 1b). Although the change in patient-rated CADSS scores was greater on the IOM+/THC+ relative to IOM−/THC (unadjusted p=0.054), the difference was not statistically significant after Tukey's adjustment (padj=0.2).
Feeling States (VAS)
‘Anxious'. There was a significant drug condition effect (F3,58=4.82, p<0.0046) on the peak change from baseline in self-reported ‘anxious' scores. Relative to placebo, only IOM+/THC+ produced greater increases in VAS ‘anxious' scores (padj=0.01) (Figure 1c). Furthermore, IOM+/THC+ produced greater increases in VAS ‘anxious' scores relative to IOM−/THC+ (padj=0.04).
‘High'. There was a significant drug condition effect (F3,57=14.27, p<0.0001) on the peak change from baseline in self-reported ‘high' or ‘stoned' scores. Relative to placebo, both IOM−/THC+ (padj=0.0001) and IOM+/THC+ (padj<0.0001), but not iomazenil alone, produced significant increases in VAS ‘high' scores (Figure 1d). Furthermore, there were no significant differences between the IOM−/THC+ and IOM+/THC+ conditions.
EEG Measures
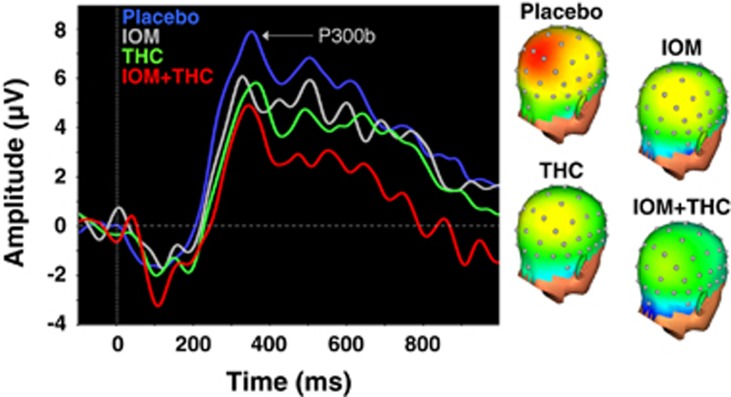
P300b
Relative to placebo, only IOM+/THC+ produced a significant (p=0.023) decrease in target P300b amplitude at Pz (Figure 2; Table 4). Furthermore, there was a significant linear trend (F1,44=6.02, p=0.018) for drug condition (IOM−/THC−>IOM+/THC−>IOM−/THC+>IOM+/THC+). There were no significant effects of any of the drug conditions on target P300b latency measured at Pz (Table 4).
Figure 2.
(Left) Grand-averaged target P300b waveforms at electrode Pz for EEG runs across drug conditions. (Right) Topographic voltage maps from the peak grand-averaged P300b for EEG runs across drug conditions. Placebo, blue line; iomazenil, gray line; THC, green line and the combination of Iomazenil; and THC, red line.
Table 4. Electrophysiological Effects of THC and Iomazenil.
| Condition | N | Mean | SD | Only significant contrasts |
|---|---|---|---|---|
| P3b amplitude (Pz) | ||||
| IOM−/THC− | 17 | 10.23 | 5.81 | +IOM+THC vs PLA+PLA: DF=44, t-value=2.34, p=0.024 |
| IOM+/THC− | 16 | 9.24 | 6.32 | |
| IOM−/THC+ | 19 | 8.33 | 6.36 | |
| IOM+/THC+ | 16 | 7.73 | 5.48 | |
| P3b latency (Pz) | ||||
| IOM−/THC− | 17 | 328.97 | 35.8 | |
| IOM+/THC− | 16 | 325.46 | 26.95 | |
| IOM−/THC+ | 19 | 342.17 | 21.38 | |
| IOM+/THC+ | 16 | 327.55 | 24.69 | |
| P3a amplitude (Cz) | ||||
| PLA+PLA | 17 | 11.18 | 7.16 | IOM+THC vs PLA+PLA: DF=44, t-value=0.18, p=0.08 |
| IOM+PLA | 16 | 8.55 | 4.56 | PLA+THC vs PLA+PLA: DF=44, t-value=1.79, p=0.08 |
| PLA+THC | 19 | 7.79 | 6.4 | |
| IOM+THC | 16 | 8.29 | 5.36 | |
| P3a latency (Cz) | ||||
| IOM−/THC− | 17 | 325.98 | 23.2 | |
| IOM+/THC− | 16 | 320.92 | 29.03 | |
| IOM−/THC+ | 19 | 322.18 | 28.5 | |
| IOM+/THC+ | 16 | 312.96 | 18.46 | |
| Target N100 amplitude (Cz) | ||||
| IOM−/THC− | 17 | −5.97 | 3.53 | |
| IOM+/THC− | 16 | −5.56 | 3.32 | |
| IOM−/THC+ | 19 | −5.37 | 2.38 | |
| IOM+/THC+ | 16 | −6.99 | 3.86 | |
| Target N100 latency (Cz) | ||||
| IOM−/THC− | 17 | 103.36 | 11.06 | |
| IOM+/THC− | 16 | 107.38 | 16.77 | |
| IOM−/THC+ | 19 | 108.95 | 14.01 | |
| IOM+/THC+ | 16 | 109.3 | 13.59 | |
| Novel N100 amplitude (Cz) | ||||
| IOM−/THC− | 17 | −6.13 | 4 | |
| IOM+/THC− | 16 | −5.89 | 3.93 | |
| IOM−/THC+ | 19 | −5.36 | 2.55 | |
| IOM+/THC+ | 16 | −5.91 | 2.9 | |
| Novel N100 latency (Cz) | ||||
| IOM−/THC− | 17 | 107.12 | 14.24 | |
| IOM+/THC− | 16 | 111.94 | 10.34 | |
| IOM−/THC+ | 19 | 106.64 | 18.48 | |
| IOM+/THC+ | 16 | 106.36 | 12.56 | |
P300a
Relative to IOM−/THC−, both IOM+/THC+ and IOM−/THC+ produced trend level (p=0.08) reductions in P3a amplitude measured at Cz. Furthermore, there was a trend towards a linear trend (F1,44=3.11, p=0.085) for drug condition (IOM−/THC−>IOM+/THC−>IOM−/THC+>IOM+/THC+). Relative to IOM−/THC−, only IOM+/THC+ produced a trend levels reduction in (p=0.08) P300a latency measured at Cz (Table 4).
There were no correlations between the effects of IOM+/THC+ or IOM−/THC+ on PANSS outcomes and both P3b and P3a amplitudes (Supplementary Table 1).
N100
There were no significant effects of THC, IOM, or their combination on N100 amplitude or latency for both target and novel stimuli measured at Cz.
Safety
There were no serious adverse events that occurred on test days. Prospective poststudy safety assessments (described in Supplementary Text no. 3) did not reveal any evidence, suggestive of an increase in cannabis use or psychosis outcomes.
DISCUSSION
This is the first study to our knowledge that examines the effects of THC in the backdrop of a pharmacologically induced GABA deficit in humans. The results showed that pretreatment with IOM exacerbated several of the behavioral, subjective, and psychophysiological effects of THC in healthy humans. When pretreated with IOM, THC induced significantly greater psychosis-relevant symptoms, as captured by the Total PANSS, compared with the THC-alone condition. Similarly, only with IOM pretreatment did THC significantly increase perceptual alterations as captured by the patient-rated CADSS. Furthermore, only when pretreated with IOM did THC induce distress as captured by VAS ‘anxiety' scores. Finally, only the combination of IOM+THC reduced THC-induced P300b amplitude.
The combination of IOM and THC did not increase measures of euphoria (VAS ‘high') compared with THC alone. Furthermore, there were no differences in THC blood levels across conditions. Taken together, these findings suggest a pharmacodynamic rather than a pharmacokinetic interaction.
The close interplay between the CB and GABAergic systems in several brain regions provides a mechanistic framework to understand the study findings. In the cerebral cortex and hippocampus, presynaptic CB1-Rs primarily inhibit the release of GABA from CCK− interneurons (Eggan et al, 2010). IOM likely causes a net reduction in GABAA function across various interneuron types (eg, PV, SST, CCK, and so on). However, as PV-positive interneurons are thought to be the primary GABAergic subtype involved in psychosis (Glausier et al, 2014; Lewis et al, 2012), it is tempting to speculate if IOM exacerbates the psychotomimetic effects of THC by disrupting inputs from PV cells onto PCs. Indeed, several lines of evidence suggest that CB1-R-positive CCK interneurons and PV cells work in concert to modulate the synchronized output of PCs (Bartos and Elgueta, 2012; Klausberger et al, 2005). Put another way, THC-induced activation of CB-1Rs on the axon terminals of CCK containing GABA neurons reduces GABA release, resulting in disinhibition of PC activity. If this were to occur in the presence of a GABA deficit, as might be produced by IOM (and as might occur in schizophrenia), it would lead to further disinhibition and desynchronization of PC activity, which would lead to the perturbation of gating and associative functions and culminate in psychotic symptoms.
Pretreatment with IOM has been shown to enhance the psychotomimetic effects of serotonergic (m-CPP) (D'Souza et al, 2006), dopaminergic (amphetamine) (K Ahn, personal communication), glutamatergic (ketamine) (H Gunduz-Bruce, personal communication), and now cannabinoidergic (THC) agents. Furthermore, when administered alone, IOM has been shown to produce small increases in psychosis in schizophrenia patients but not in healthy controls (Ahn et al, 2011). Collectively, these findings highlight the contributions of GABAergic deficits to the pathophysiology of schizophrenia.
The combination of IOM and THC also caused the largest reductions in the P300b. Exogenous cannabinoids have been shown to reduce the amplitude of the P300b (D'Souza et al, 2012; Roser et al, 2008). Reductions in P300 amplitude and increased latencies have been observed in a number of other neuropsychiatric disorders including schizophrenia (reviewed in Bramon et al, 2004; Jeon and Polich, 2003). Both GABAergic and glutamatergic systems have been strongly implicated in contributing to the P300 (Watson et al, 2009). In fact, it is now recognized that ERPs such as the P300 are generated by inhibitory and excitatory postsynaptic potentials, which are primarily driven by the release of glutamate and GABA (Luck et al, 2011). It is likely that normal P300 generation requires an optimal level of inhibitory–excitatory balance, and any perturbation above or below the optimum range can disrupt the neural networks involved in context updating, working memory, and the allocation of attentional resources. Future research is needed to determine how GABA-CB effects on the P300 are related to the psychosis-enhancing effects of IOM and THC.
These results lend support to the hypothesis that a pharmacologically induced GABA deficit would enhance the psychosis-relevant effects of THC in healthy adults. These data suggest that the enhanced vulnerability to cannabis and THC in schizophrenia patients (D'Souza et al, 2005) may be explained by underlying GABA deficits. Although admittedly speculative, it may be inferred that psychosis-prone individuals who appear sensitive to the psychosis-inducing effects of cannabis may also have GABAergic deficits. However, to our knowledge, the functional state of the GABA system has not been examined in psychosis prone individuals.
These data add to a growing body of evidence from epidemiologic and experimental studies that have identified other factors that modulate the acute response to cannabis and THC, respectively. Caspi et al, (2005) demonstrated that polymorphisms of the gene encoding catechol-O-methyltransferase (COMT), which is critical for the removal of dopamine (DA) in the prefrontal cortex, influenced the risk for psychosis outcomes in later life, following cannabis exposure in adolescence. Henquet et al, (2006) then showed in an experimental study that polymorphisms of the gene encoding COMT-mediated differential sensitivity to the acute psychotomimetic effects of THC (Henquet et al, 2006). Similarly, epidemiologic studies have shown that variation in the gene for protein kinase C (AKT1), an integral component of the DA signaling cascade, influences the risk of associated psychosis outcomes with cannabis use (Di Forti et al, 2012; van Winkel et al, 2011a; van Winkel et al, 2011b). In an experimental study, Bhattacharyya et al, (2012) showed that polymorphisms of the genes for AKT1 and the dopamine transporter (DAT1) influence the psychotomimetic and neurophysiological response to THC. Finally, in a recent study, Bhattacharyya et al, (2014) also showed that the AKT1 genotype mediates the sensitivity to THC-induced impairments in psychomotor control.
Strengths, Limitations, and Conclusions
The intravenous route of drug administration, and weight-adjusted doses address the inter- and intraindividual variability associated with oral or smoked THC. The randomized, placebo-controlled, double-blind, repeated-measure, 2 (active or placebo THC) × 2 (active or placebo iomazenil), within-subjects design is both powerful and efficient. The behavioral and cognitive outcome measures were complemented by a psychophysiological measure that allows a more proximal index of neuronal activity and interaction of CB and GABA systems. Further, ERPs afford near-perfect temporal resolution that is not afforded by other approaches. However, while the proposed study may have limited social relevance, as cannabis is not typically used intravenously, the strengths of the intravenous paradigm outweigh its limited social relevance. Finally, the study was not powered to evaluate interactive effects on all the subscales of the outcome measures or the influence of cannabis exposure on the interactions between IOM and THC.
Future Directions
Whether these findings can be generalized to women needs further study. These findings need replication in a larger sample that is adequately powered to examine the outcome measures in further detail, for example, items of the PANSS. The animal literature shows that GABA and CB systems modulate gamma range (γ)-band neural oscillations, which are thought to have a key role in a number of processes that are altered in schizophrenia (Uhlhaas et al, 2009), including sensory registration, the integration and binding of perceptual features, associative learning, and conscious awareness; it will be important to study the interplay between CB and GABA systems on (γ)-band neural oscillations in humans. The development and availability of reliable and valid in vivo methods to determine GABAergic deficits in schizophrenia will permit a more direct approach to determining the contribution of GABAergic deficits to vulnerability to the psychosis-inducing effects of THC in schizophrenia. Finally, the availability of drugs that target specific GABA interneurons will permit a more refined testing of the hypothesis.
Funding and Disclosure
RR, PS, MC, JE, AS, BP, and AS report no financial relationships with commercial interests. RR has received funding from APIRE/Janssen Pharmaceutical Resident Psychiatric Research Scholar and is supported by R25MH071584. MC is currently employed at Inventiv Health and is a contract employee at Bristol Myers Squibb. In the past 3 years, she has been an employee at Pfizer and owned Pfizer stocks as part of her 401k. MR has in the past 3 years or currently receives research grant support administered through Yale University School of Medicine from Eli Lilly Inc. DCD has in the past 3 years or currently receives research grant support administered through Yale University School of Medicine from AbbVieand Pfizer Inc.; he is a consultant for Bristol Meyers Squibb and Johnson and Johnson.
Acknowledgments
We wish to acknowledge support from the (1) National Institute of Mental Health, (2) National Institute of Alcoholism and Alcohol Abuse (NIAAA), (3) National Institute of Drug Abuse, (4) Department of Veterans Affairs, and (5) the Yale Center for Clinical Investigation (YCCI). This research project was funded in part by grants from NIMH(R21 MH086769 to DCD). We also thank Angelina Genovese, RNC, MBA; Michelle San Pedro, RN.; Elizabeth O'Donnell, RN; Brenda Breault, RN, BSN; Sonah Yoo, RPh; Rachel Galvan, RPh; and Willie Ford of the Neurobiological Studies Unit at the VA Connecticut Healthcare System, West Haven Campus for their central contributions to the success of this project. Finally, this manuscript is dedicated to the memory of our dear friend and colleague, the late Dr R Andrew Sewell.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahn K, Gil R, Seibyl J, Sewell RA, D'Souza DC (2011). Probing GABA receptor function in schizophrenia with iomazenil. Neuropsychopharmacology 36: 677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt M, Mortensen PB, Rosenberg R, Pedersen CB, Waltoft BL (2008). Familial predisposition for psychiatric disorder: comparison of subjects treated for cannabis-induced psychosis and schizophrenia. Arch Gen Psychiatry 65: 1269–1274. [DOI] [PubMed] [Google Scholar]
- Bartos M, Elgueta C (2012). Functional characteristics of parvalbumin- and cholecystokinin-expressing basket cells. J Physiol 590: 669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer HF, Blauenstein PA, Hasler PH, Delaloye B, Riccabona G, Bangerl I et al (1990). In vitro and in vivo evaluation of iodine-123-Ro 16-0154: a new imaging agent for SPECT investigations of benzodiazepine receptors. J Nucl Med 31: 1007–1014. [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Prata D et al (2012). Preliminary report of biological basis of sensitivity to the effects of cannabis on psychosis: AKT1 and DAT1 genotype modulates the effects of delta-9-tetrahydrocannabinol on midbrain and striatal function. Mol Psychiatry 17: 1152–1155. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Iyegbe C, Atakan Z, Martin-Santos R, Crippa JA, Xu X et al (2014). Protein kinase B (AKT1) genotype mediates sensitivity to cannabis-induced impairments in psychomotor control. Psychol Med 44: 3315–3328. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S (2004). Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res 70: 315–329. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS et al (1998). Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 11: 125–136. [DOI] [PubMed] [Google Scholar]
- Busatto GF, Pilowsky LS, Costa DC, Ell PJ, David AS, Lucey JV et al (1997). Correlation between reduced in vivo benzodiazepine receptor binding and severity of psychotic symptoms in schizophrenia. Am J Psychiatry 154: 56–63. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Cannon M, McClay R, Harrington H et al. (2005). Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 57: 1117–1127. [DOI] [PubMed] [Google Scholar]
- Charych EI, Liu F, Moss SJ, Brandon NJ (2009). GABA(A) receptors and their associated proteins: implications in the etiology and treatment of schizophrenia and related disorders. Neuropharmacology 57: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Iyegbe C, Sallis H, Kolliakou A, Falcone MA, Paparelli A et al (2012). Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol Psychiatry 72: 811–816. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G et al (2005). Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry 57: 594–608. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N et al (2012). Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacology 37: 1632–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Gil RB, Zuzarte E, MacDougall LM, Donahue L, Ebersole JS et al (2006). gamma-Aminobutyric acid-serotonin interactions in healthy men: implications for network models of psychosis and dissociation. Biol Psychiatry 59: 128–137. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT et al (2004). The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29: 1558–1572. [DOI] [PubMed] [Google Scholar]
- Dorow R, Horowski R, Paschelke G, Amin M (1983). Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet 2: 98–99. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Melchitzky DS, Sesack SR, Fish KN, Lewis DA (2010). Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience 169: 1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas I, Kallo I, Deli L, Vida B, Hrabovszky E, Fekete C et al (2010). Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology 151: 5818–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders—Non-Patient Edition. American Psychiatric Association: Washington, DC. [Google Scholar]
- Friedman D, Simpson G, Hamberger M (1993). Age-related changes in scalp topography to novel and target stimuli. Psychophysiology 30: 383–396. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Fish KN, Lewis DA (2014). Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry 19: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55: 468–484. [DOI] [PubMed] [Google Scholar]
- GROUP (2011). (Genetic, Risk Outcome in Psychosis, Investigators) Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis: an analysis of patient–sibling and sibling–control pairs. Arch Gen Psychiatry 68: 138–147. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH (2004). Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 61: 310–317. [DOI] [PubMed] [Google Scholar]
- Henquet C, Rosa A, Krabbendam L, Papiol S, Fananas L, Drukker M et al (2006). An experimental study of catechol-o-methyltransferase Val158Met moderation of delta-9-tetrahydrocannabinol-induced effects on psychosis and cognition. Neuropsychopharmacology 31: 2748–2757. [DOI] [PubMed] [Google Scholar]
- Henquet C, van Os J, Kuepper R, Delespaul P, Smits M, Campo JA et al (2010). Psychosis reactivity to cannabis use in daily life: an experience sampling study. Br J Psychiatry 196: 447–453. [DOI] [PubMed] [Google Scholar]
- Hoffman-La Roche – Data on File.
- Horowski R, Dorrow R (2002). Anxiogenic, not psychotogenic, properties of the partial inverse benzodiazepine receptor agonist FG 7142 in man. Psychopharmacology (Berl) 162: 223–224. [DOI] [PubMed] [Google Scholar]
- Hunkeler W, Mohler H, Pieri L, Polc P, Bonetti EP, Cumin R et al (1981). Selective antagonists of benzodiazepines. Nature 290: 514–516. [DOI] [PubMed] [Google Scholar]
- Jeon YW, Polich J (2003). Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology 40: 684–701. [DOI] [PubMed] [Google Scholar]
- Johnson EW, Woods SW, Zoghbi S, McBride BJ, Baldwin RM, Innis RB (1990). Receptor binding characterization of the benzodiazepine radioligand 125I-Ro16-0154: potential probe for SPECT brain imaging. Life Sci 47: 1535–1546. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP (1989). The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. Br J Psychiatry Suppl 59–67. [PubMed]
- Klausberger T, Marton LF, O'Neill J, Huck JH, Dalezios Y, Fuentealba P et al (2005). Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci 25: 9782–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Curley AA, Glausier JR, Volk DW (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci 35: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci 6: 312–324. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I (2004). Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther 310: 1234–1245. [DOI] [PubMed] [Google Scholar]
- Linszen D, van Amelsvoort T (2007). Cannabis and psychosis: an update on course and biological plausible mechanisms. Curr Opin Psychiatry 20: 116–120. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Mathalon DH, O'Donnell BF, Hamalainen MS, Spencer KM, Javitt DC et al (2011). A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biol Psychiatry 70: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M et al (2007). Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370: 319–328. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Wilkinson ST, D'Souza DC (2014). Gone to Pot—A review of the association between cannabis and psychosis. Front Psychiatry 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall P (1995) .Iomazenil effects in PTSD patients and controls. In: personal communication D'Souza C (ed).
- Roser P, Juckel G, Rentzsch J, Nadulski T, Gallinat J, Stadelmann AM (2008). Effects of acute oral Delta(9)-tetrahydrocannabinol and standardized cannabis extract on the auditory P300 event-related potential in healthy volunteers. Eur Neuropsychopharmacol 18: 569–577. [DOI] [PubMed] [Google Scholar]
- Schubiger P, Hasler P (eds) (1989). Iomazenil and other brain receptor tracers for SPECT. Proceedings of the Sixth Bottstein Colloquim. Wurenlingen/Villigen, Switzerland. [Google Scholar]
- Shaw LM, Edling-Owens J, Mattes R (1991). Ultrasensitive measurement of delta-9-tetrahydrocannabinol with a high energy dynode detector and electron-capture negative chemical-ionization mass spectrometry. Clin Chem 37: 2062–2068. [PubMed] [Google Scholar]
- Tallman JF, Gallager DW (1985). The GABA-ergic system: a locus of benzodiazepine action. Annu Rev Neurosci 8: 21–44. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Lima B, Melloni L, Neuenschwander S, Nikolic D et al (2009). Neural synchrony in cortical networks: history, concept and current status. Front Integr Neurosci 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H (2002). Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol 156: 319–327. [DOI] [PubMed] [Google Scholar]
- van Winkel; Genetic Risk and Outcome of Psychosis (GROUP) Investigators (2011. a). Family-based analysis of genetic variation underlying psychosis-inducing effects of cannabis: sibling analysis and proband follow-up. Arch Gen Psychiatry 68: 148–157. [DOI] [PubMed] [Google Scholar]
- van Winkel R, van Beveren NJ, Simons C; Genetic Risk and Outcome of Psychosis (GROUP) Investigators (2011. b). AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology 36: 2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeff NP, Soares JC, D'Souza CD, Gil R, Degen K, Abi-Dargham A et al (1999). [123I]Iomazenil SPECT benzodiazepine receptor imaging in schizophrenia. Psychiatry Res 91: 163–173. [DOI] [PubMed] [Google Scholar]
- Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH (2009). Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol 12: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2002). Endocannabinoid signaling in the brain. Science 296: 678–682. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD et al (2010). GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci 30: 3777–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.