Abstract
Gαq inhibitor UBO-QIC (FR900359) is becoming an important pharmacological tool, but its selectivity against other G proteins and their subunits, especially βγ, has not been well characterized. We examined UBO-QIC's effect on diverse signaling pathways mediated via various G protein-coupled receptors (GPCRs) and G protein subunits by comparison with known Gαi inhibitor pertussis toxin. As expected, UBO-QIC inhibited Gαq signaling in all assay systems examined. However, other non-Gαq-events, e.g. Gβγ-mediated intracellular calcium release and inositol phosphate production, following activation of Gi-coupled A1 adenosine and M2 muscarinic acetylcholine receptors, were also blocked by low concentrations of UBO-QIC, indicating that its effect is not limited to Gαq. Thus, UBO-QIC also inhibits Gβγ-mediated signaling similarly to pertussis toxin, although UBO-QIC does not affect Gαi-mediated inhibition or Gαs-mediated stimulation of adenylyl cyclase activity. However, the blockade by UBO-QIC of GPCR signaling, such as carbachol- or adenosine-mediated calcium or inositol phosphate increases, does not always indicate inhibition of Gαq-mediated events, as the βγ subunits released from Gi proteins following the activation of Gi-coupled receptors, e.g. M2 and A1Rs, may produce similar signaling events. Furthermore, UBO-QIC completely inhibited Akt signaling, but only partially blocked ERK1/2 activity stimulated by the Gq-coupled P2Y1R. Thus, we have revealed new aspects of the pharmacological interactions of UBO-QIC.
Keywords: G protein, Gαq inhibitor, GPCR, Gβγsubunits, FR900359, UBO-QIC
1. Introduction
UBO-QIC (FR900359), a cyclic depsipeptide from the flowering plant Ardisia crenata sims, was found to inhibit platelet aggregation and to decrease blood pressure [1]. UBO-QIC has been recently used as a selective inhibitor of Gαq signaling [2-7].
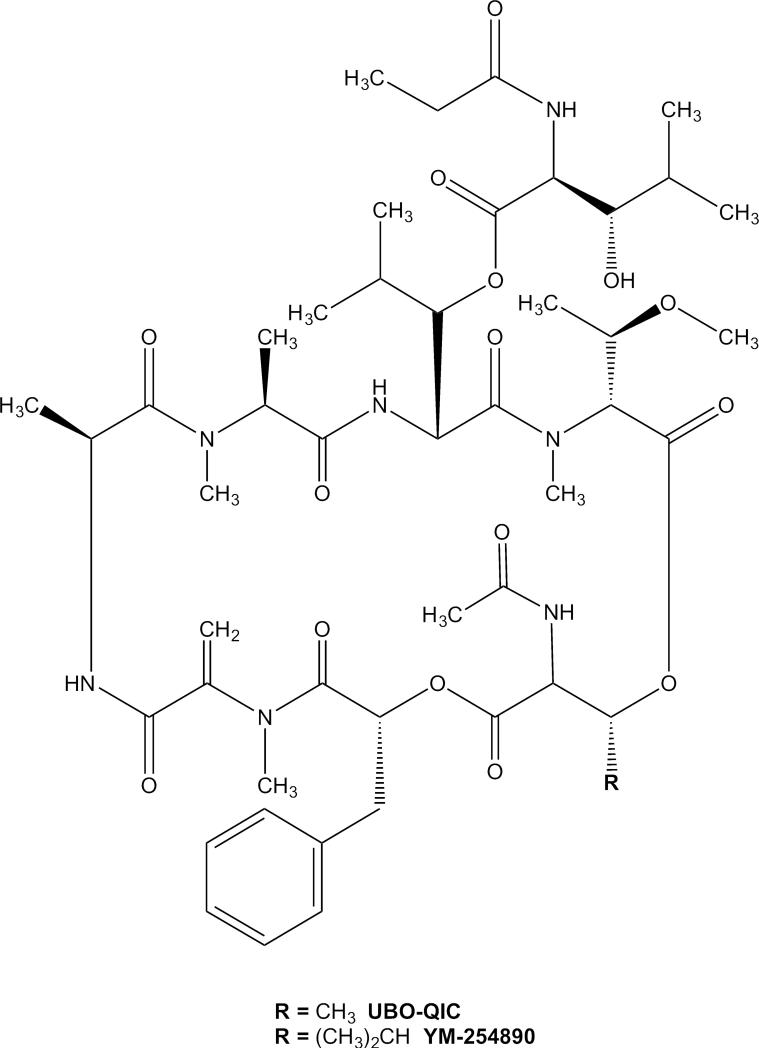
UBO-QIC is a close analog of a known Gαq inhibitor YM-254890 (Figure 1) [8-10], which is relatively well, albeit not thoroughly, characterized at G protein signaling and has been used more widely as a Gαq inhibitor [10-13]. The two depsipeptides differ only in the presence of an isopropyl group in YM-254890 in place of methyl. Nishimura et al. [14] demonstrated in an X-ray crystal structure of the complex of YM-254890 with Gαq/βγ that the depsipeptide binds to an interdomain region of the Gαq subunit, and showed that it inhibits the release of GDP from Gαq. YM-254890 has been used as a selective Gαq inhibitor, although only limited data on the selectivity of YM-254890 for Gαq over Gβγ or Gα15 subunits was reported by Takasaki et al. [9]. YM-254890 (10 μM, 5 min pretreatment) was shown to inhibit formyl peptide (fMLP)-induced Ca2+ mobilization (via Gβγ) in differentiated HL60 cells to a lesser extent than pertussis toxin (PTX, 50 ng/ml for 6 h), although it produced a much larger inhibition in UTP-mediated (via Gαq) Ca2+ release [9]. YM-254890 was also shown to have only a small effect on Gα15-mediated Ca2+ release in Chinese hamster ovary (CHO) cells expressing recombinant human fMLP receptor and Gα15. Based on that study, it was concluded that YM-254890 is selective for Gαq over Gβγ and Gα15.
Figure 1.
Chemical structures of naturally-occuring cyclic depsipeptides UBO-QIC (FR900359) and YM-254890.
The Gαq inhibitors have clearly greatly enabled the investigation of Gq-coupled receptor signaling, and UBO-QIC is becoming a widely-used tool in pharmacology due to its recent commercial availability [2-7]. However, the selectivity of UBO-QIC for Gαq over other G proteins and their subunits, especially the βγ subunit-mediated signaling following the activation of Gi-coupled receptors, has not been well characterized. Considering the fact that in many cases Gαq and Gβγ mediate similar signaling events, e.g. both Gαq and Gβγ subunits mediate phospholipase C activation or Ca2+ release [9,15-19], it is important to examine the effect of UBO-QIC on Gβγ signaling pathways. The present study explored this possibility by comparing UBO-QIC with a known Gαi inhibitor PTX.
2. Materials and Methods
2.1. Materials
[[(1R,2R,3S,4R,5S)-4-[6-Amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid monoester trisodium salt (MRS2365) was from Tocris (St. Louis, MO). N6-Cyclopentyladenosine (CPA), carbachol, PTX and 2-methylthioadenosine 5′-diphosphate trisodium salt (2MeSADP), adenosine-5′-N-ethyluronamide (NECA) were from Sigma (St. Louis, MO). UBO-QIC was purchased from University of Bonn (Germany). IP-One Tb HTRF kit was from Cisbio Bioassays (Bedford, MA). AlphaScreen cAMP kit, SureFire p-ERK1/2 (Thr202/Tyr204) Assay Kit and AlphaScreen SureFire p-Akt 1/2/3 (p-Ser473) Assay Kit were purchased from PerkinElmer (Waltham, MA). HEK293 and DDT1-MF2 were from ATCC (Mannasas, VA); CHO cell lines stably expressing the human A1AR, A2AAR, and A2BAR, and human M3 and M2 muscarinic acetylcholine receptors were made at the Laboratory of Bioorganic Chemistry, NIDDK (Bethesda, MD). 1321N1 astrocytoma cells expressing either the human P2Y1R or P2Y12R were from T. K. Harden (University of North Carolina, Chapel Hill, NC); all other reagents were from standard commercial sources and of analytical grade.
2.2. Inositol 1-phosphate Assay
Inositol 1-phosphate (IP-1), a metabolite of inositol trisphosphate, which is downstream of signaling by Gαq or Gβγ subunits, was detected using the IP-One Tb HTRF kit (Cisbio Bioassays, Bedford, MA), as described elsewhere earlier [20]. Cells were grown in 96-well plates overnight before the pretreatment with UBO-QIC (100 nM) for 30 min before the addition of agonists followed by additional 30 min incubation. Assay plates were read on a Mithras LB940 reader (Berthold Technologies, Oak Ridge, TN) or a PerkinElmer (Waltham, MA) EnSpire plate reader using a time-resolved fluorescence ratio (665/620 nm).
2.3. Intracellular calcium mobilization
Cells were grown overnight in 100 μl of media in 96-well black plates at 37°C at 5% CO2. Cells were pretreated with various concentrations of UBO-QIC or GO6983 (10 μM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonists. The calcium assay kit was used as directed without washing cells, and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were incubated with 100 μl dye/probenecid for 60 min at room temperature. The compound plate was prepared using dilutions of various compounds in Hank's Buffer (pH 7.4). Samples were run in duplicate or triplicate using a FLIPR TETRA High Throughput Cellular Screening System (Molecular Devices, Sunnyvale, CA) at room temperature. Cell fluorescence (excitation at 485 nm; emission at 525 nm) was monitored following exposure to the compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.4. Activation of extracellular-signal-regulated kinase 1/2 (ERK1/2) and Akt1/2/3
For the stimulation of ERK1/2 activity, the method used was as previously described [21,22]. Briefly, CHO or 1321N1 astrocytoma cells (30,000 cells/100 μl) were seeded in a 96-well plate in complete growth medium. After cell attachment, medium was removed and cells were serum-starved overnight in medium without fetal bovine serum. Cells were pretreated with UBO-QIC (100 nM) or GO6983 (10 μM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonists. Agonists were prepared in Hank's buffered salt solution, and cells were stimulated for 5 min. Medium was removed and cells were lysed with 1x Lysis Buffer (20 μl) (PerkinElmer AlphaScreen SureFire p-ERK1/2 (Thr202/Tyr204) Assay Kit) (PerkinElmer, Waltham, MA). Lysate (4 μl/well) was transferred to a 384-well ProxiPlate Plus (PerkinElmer). Reagents were added according the manual from the manufacturer, and the plate was measured using an EnVision multilabel reader using standard AlphaScreen settings. For the stimulation of Akt1/2/3 activity, the procedures were essentially the same as that of the ERK1/2 activity, except that the stimulation time for the P2Y1 receptor is 20 min. The Akt activity was measured using AlphaScreen SureFire p-Akt 1/2/3 (p-Ser473) Assay Kit (PerkinElmer, Waltham, MA).
2.5. Cyclic AMP accumulation assay
Cells were cultured in DMEM medium containing 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin and 2 μmol/ml glutamine. For the assay of 3′,5′-cyclic adenosine monophosphate (cAMP), cells were plated in 96-well plates in 100 μl medium overnight and were first treated with UBO (300 nM) for 30 min or PTX (200 ng/ml) overnight, before the treatment with agonists and/or test compounds in the presence of phosphodiesterase inhibitor rolipram (10 μM). The reaction was terminated by removal of the supernatant, and cells were lysed upon the addition of 50 μl of lysis buffer (0.3% Tween-20). For determination of production of cAMP, an AlphaScreen cAMP kit was used according to manufacturer's instructions (PerkinElmer, Waltham, MA).
2.6. Statistical and data analyses
Functional parameters were calculated using Prism 6.0 software (GraphPAD, San Diego, CA). Data were expressed as mean ± standard error. Statistical significance of the differences was assessed using a Student's t test (between two conditions) or a One-Way Analysis of Variance (ANOVA) followed by Bonferroni's multiple comparison tests (between multiple conditions). Differences yielding P values < 0.05 were considered as statistically significant.
3. Results
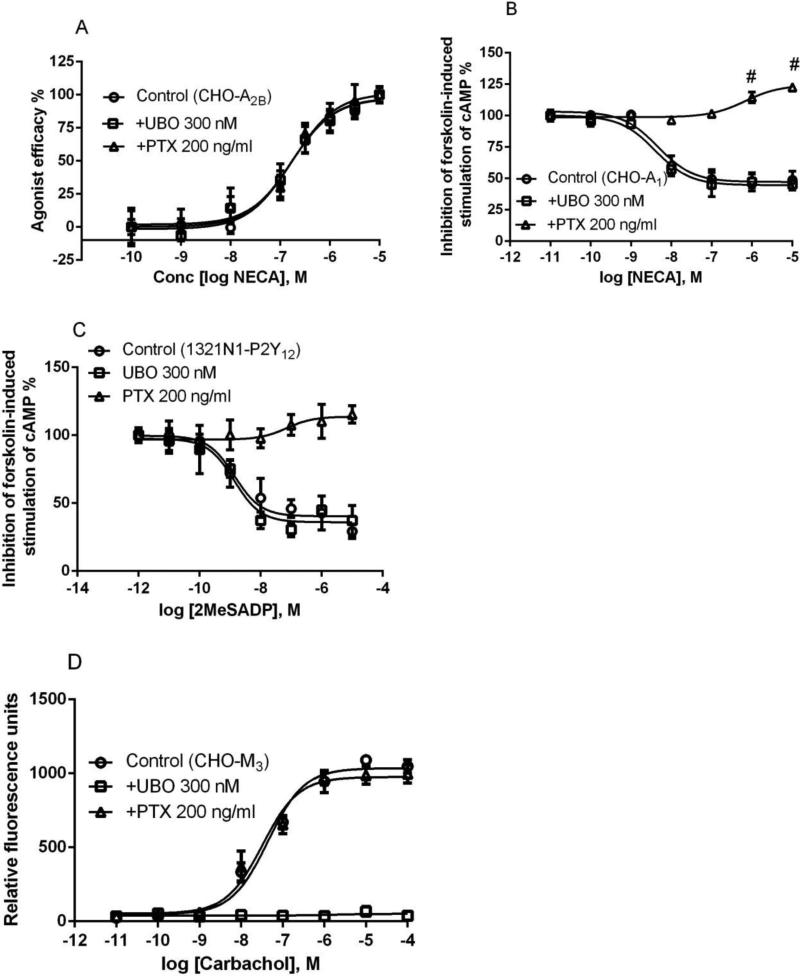
We first tested the effect of UBO-QIC on Gαs-mediated events. UBO-QIC did not show any effect on cAMP accumulation induced by nonselective adenosine receptor (AR) agonist NECA in CHO cells stably expressing the Gs-coupled human A2BAR (Figure 2a). The EC50 values (nM) of NECA in the absence and presence of UBO-QIC (300 nM) were 156 ± 27 and 169 ± 18 nM, respectively, which were not significantly different (P>0.05, Student's test). The Gαi inhibitor PTX also did not substantially affect the concentration-response curve of NECA (Figure 2a). The EC50 value of NECA in the presence of 200 ng/ml PTX (overnight incubation) was 172 ± 36 nM, which was not significantly different from that in the absence (P>0.05, Student's t-test). UBO-QIC also did not affect cAMP accumulation induced by A2AAR-selective agonist CGS21680 in CHO cells stably expressing the recombinant human A2AAR (data not shown).
Figure 2.
Selectivity of UBO-QIC for Gαq- versus Gαs- and Gαi-mediated signaling. A. NECA-induced cAMP accumulation in CHO cells expressing the recombinant human A2BAR (Gαs). B. NECA-induced inhibition of forskolin-stimulated (10 μM) accumulation of cAMP in CHO cells expressing the recombinant human A1AR (Gαi). Cells were treated with agonist for 20 min and forskolin for 10 min. C. 2MeSADP-induced inhibition of forskolin-stimulated (10 μM) accumulation of cAMP in 1321N1 astrocytoma cells expressing the human P2Y12 receptors. D. Carbachol-inducd intracellular calcium mobilization in CHO cells expressing the recombinant human M3 muscarinic receptor (Gαq). For all assays, cells were pretreated with UBO-QIC (300 nM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonists. Results are expressed as mean ± SEM and are from at least three independent experiments performed in duplicate or triplicate. The EC50 values from agonist response curves are listed in the text. #Significantly different from control or lower concentrations of NECA in the presence of PTX (P<0.05, One-Way ANOVA with post-hoc test).
In addition to its effect on Gs coupling, we also examined the effect of UBO-QIC on Gαi coupling. Figure 2b shows that UBO-QIC has no effect on NECA-induced inhibition of forskolin-stimulated cAMP accumulation in CHO cells stably expressing the recombinant human A1 ARs. The EC50 values of NECA in the presence and absence of UBO-QIC (300 nM) were 4.9 ± 1.6 and 5.6 ± 1.7 nM, respectively, which were not significantly different (P>0.05, Student's test). As a control, PTX (200 ng/ml) blocked the effect of NECA (Figure 2b). It is noted that, in the presence of PTX, NECA actually stimulates cAMP accumulation, which is due to the fact that A1ARs can also couple to Gs protein in addtition to Gi protein coupling, a phenomenon observed previously by Cordeaux et al. [26]. In addition to the A1AR, we also tested the effect of UBO on another Gi-coupled receptor, P2Y12. Figure 2c shows that UBO (300 nM) did not produce any effect on 2MeSADP-induced inhibition of forskolin-stimulated cAMP accumulation, although PTX (200 ng/ml) completely blocked the effect of 2MeSADP. The EC50 values of 2MeSADP in the presence and absence of UBO are 1.6 ± 0.3 and 1.7 ± 0.5 nM, respectively, which are not significantly different (P>0.05, Student's test).
We then examined the effect of UBO-QIC on M3 muscarinic receptor-mediated Gαq coupling. Figure 2D shows that carbachol concentration-dependently induced intracellular calcium mobilization in CHO cells stably expressing the recombinant human M3 receptor corresponding to an EC50 value of 56.8 ± 12.7 nM. Unlike its lack of effect on the stimulation or inhibition of cAMP production, UBO-QIC (300 nM) completely blocked the effect of carbachol on Ca2+ transients. By contrast, PTX 200 ng/ml has no effect (EC50 = 46.6 ± 15.9 nM). Thus, the above experiments confirmed the selectivity of UBO-QIC for Gαq in comparison to the Gαi and Gαs proteins.
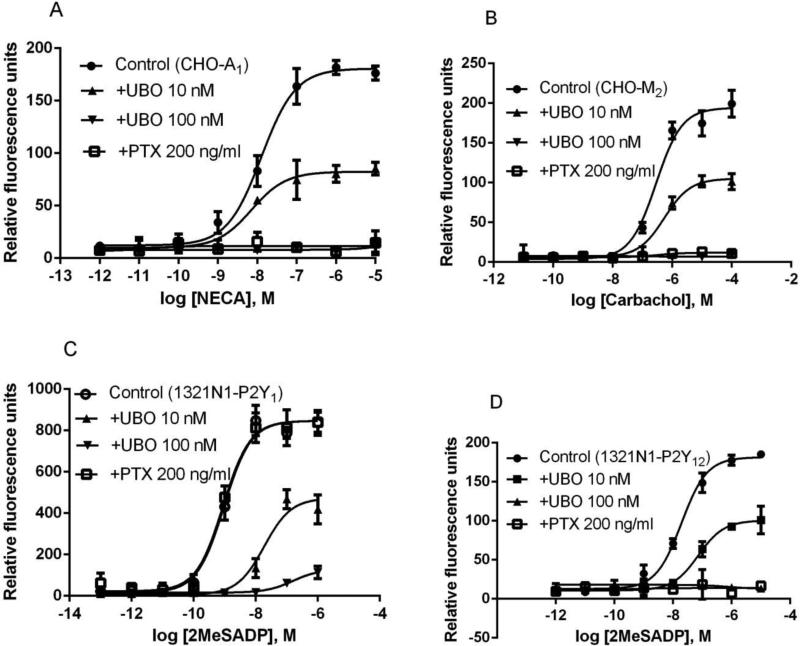
Since both Gαq and Gβγ subunits can mediate calcium mobilization [15,16], we further examined the potential effect of UBO-QIC on one Gq-coupled receptor, the P2Y1 receptor (P2Y1R) for extracellular nucleotides, and on three Gi-coupled receptors, the A1AR, P2Y12R and M2 muscarinic receptor. Surprisingly, it was found that UBO-QIC, in a concentration-dependent manner, inhibited calcium mobilization induced by all receptors tested (Figure 3). At 10 nM, UBO-QIC produced a substantial inhibition, and at 100 nM, the calcium responses were completely blocked (Figure 3). Higher concentrations (300 nM and 1000 nM) also completely blocked agonist effects (data not shown). As a comparison, PTX (200 ng/ml) also completely blocked responses to stimulation of M2, P2Y12R, and A1AR, but not P2Y1R-mediated calcium mobilization. In CHO cells expressing the human A1AR, the EC50 value of NECA to induce calcium transients was 10.6 ± 2.9 nM. UBO-QIC at the concentration of 10 nM diminished the maximum effect of NECA by ~60%. Carbachol concentration-dependently elicited calcium mobilization in CHO cells expressing the M2 receptor, corresponding to an EC50 value of 252 ± 43 nM. UBO-QIC (10 nM) reduced the maximal effect of carbachol by ~50%. Similarly, UBO-QIC (10 nM) blocked by ~50% of the maximum effect of 2MeSADP-induced calcium transients in 1321N1 astrocytoma cells expressing either the P2Y1R (EC50=1.1 ± 0.3 nM) or P2Y12R (EC50=21.6 ± 6.4 nM) (Figure 3). Thus, UBO-QIC blocks other intracellular signaling, in addition to Gαq.
Figure 3.
Intracellular calcium mobilization mediated via the Gi-coupled A1AR (A) and M2 muscarinic receptor (B), both expressed in CHO cells, and the Gq-coupled P2Y1 (C) and Gi-coupled P2Y12 nucleotide receptors (D) expressed in 1321N1 astrocytoma cells. Cells were pretreated with UBO-QIC (10 and 100 nM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonists. Results were expressed as mean ± SEM from 3 independent experiments performed in duplicate.
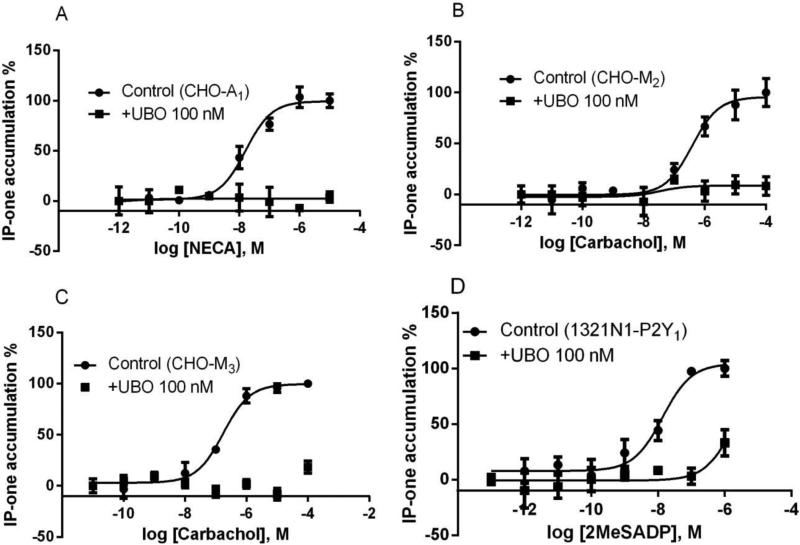
In addition to the effect of UBO-QIC on calcium mobilization, we also performed the assays of IP1 production to confirm that UBO-QIC blocks the function mediated via both Gq- and Gi-coupled receptors. It has been reported that production of inositol phosphates or calcium mobilization are mediated both via Gq-coupled receptors and Gi-coupled receptors (via Gβγ subunits) [15,17-19, 23]. Figure 4 shows that, at 100 nM, UBO-QIC almost completely blocked the agonist responses mediated via two Gq-coupled receptors (M3 and P2Y1) and two Gi-coupled receptors (A1 and M2). The EC50 values (nM) of NECA (A1), carbachol (M2), carbachol (M3), and 2MeSADP (P2Y1) were 15.8 ± 4.2, 351 ± 86, 11.6 ± 2.1, and 162 ± 18, respectively.
Figure 4.
Stimulation of IP1 production via activation of Gi-coupled A1AR (A) and M2 muscarinic receptor (B) expressed in CHO cells, or Gq-coupled M3 muscarinic receptors in CHO cells (C) and Gq-coupled P2Y1 nucleotide receptor in 1321N1 astrocytoma cells (D). Cells were pretreated with UBO-QIC (100 nM) for 30 min and PTX (200 ng/ml) overnight before the addition of agonists. Results were expressed as mean ± SEM from 3-4 separate experiments performed in duplicate.
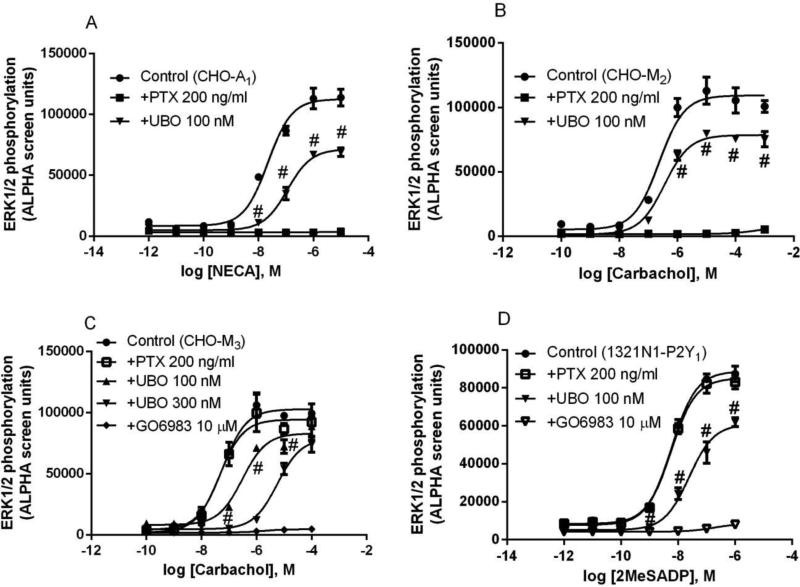
Activation of most Gαq- and Gαi-coupled receptors results in stimulation of ERK1/2 activity [21,22,24,25]. We tested the effect of UBO-QIC on ERK1/2 activation mediated through various GPCRs. In an initial experiment, it was found that UBO-QIC, at the concentration of 10 nM, which substantially diminished calcium mobilization, produced little if any effect on ERK1/2 activation. Figure 5 shows that at 100 nM, UBO-QIC only partially (around 30% or less) blocks the maximal agonist effect in ERK1/2 phosphorylation mediated via P2Y1, A1AR, or M2 receptor. In the case of M3 receptor, even at the concentration of 300 nM, UBO-QIC did not diminish the maximum agonist response by more than 30%. In all cases, UBO-QIC produced a somewhat non-parallel right shift of the agonist concentration-response curves, which was at odds with its effects in calcium mobilization or IP1 production. The broad-spectrum inhibitor of protein kinase C (PKC) GO6983 [27] completely blocked ERK1/2 phosphorylation mediated via M3 and P2Y1 receptors (Figure 5c,d). As expected, treatment with PTX inhibited ERK1/2 phosphorylation mediated via Gi-coupled A1 and M2 but not Gq-coupled P2Y1 and M3 receptors (Figure 5).
Figure 5.
Stimulation of ERK1/2 activity in CHO cells expressing the A1AR (A), M2 (B) or M3 (D) muscarinic receptor, and in 1321N1 astrocytoma cells expressing the P2Y1R (C). Cells were pretreated with UBO-QIC (100 nM) or GO6983 (10 μM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonists (5 min incubation). Data are mean ± SEM from three experiments performed in duplicate. #Significantly different from the corresponding Control values in the absence of UBO (P<0.05).
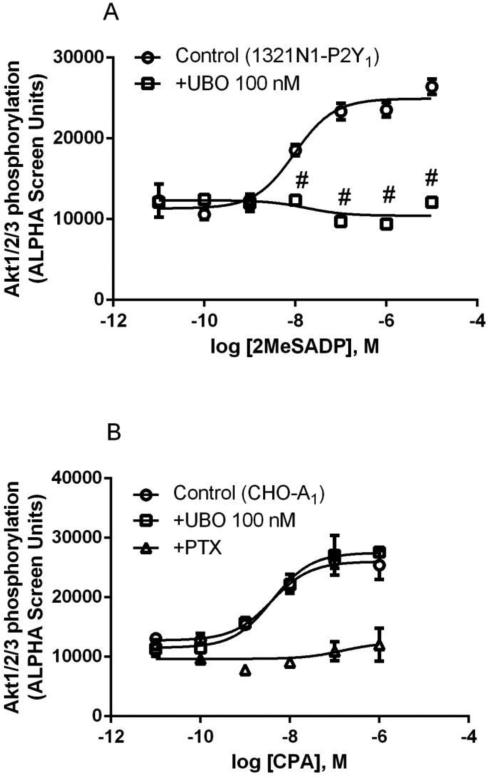
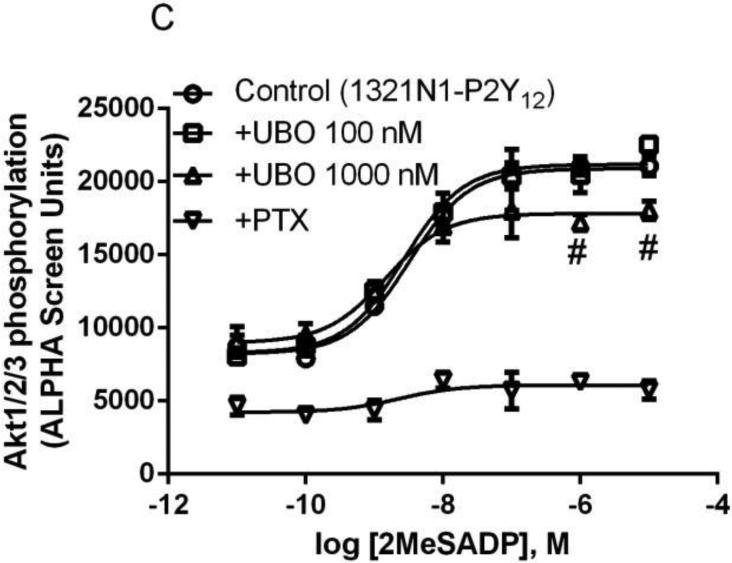
In addition to stimulation of ERK1/2 activity, both Gq- and Gi-coupled receptors are known to modulate PI3K-Akt signaling pathway [28]. Therefore, we examined the effect of UBO-QIC on Akt activity following the activation by both the Gq-coupled P2Y1R and the Gi-coupled A1AR or P2Y12R. Figure 6 shows that 2MeSADP-induced Akt1/2/3 phosphorylation in 1321N1 cells exressing the P2Y1R was completely inhibited by UBO-QIC (100 nM). By contrast, UBO-QIC (100 nM) had no effect on A1AR or the P2Y12R, although PTX (200 ng/ml) completely blocked the Akt1/2/3 activity stimulated by both receptors. At a higher concentration (1.0 μM), UBO-QIC showed a modest but significant inhibition of the maximum effect of 2MeSADP-stimulated Akt activity (P<0.05 compared with corresponding values in the absence of UBO; Figure 6c). Thus, the pattern of inhibition of Akt activity by UBO-QIC is different from that in other signaling pathways.
Figure 6.
Effect of UBO-QIC on the Akt1/2/3 phosphorylation stimulated by Gαq-coupled P2Y1R (A, 1321N1 cells) or Gi-coupled A1AR (B, CHO cells) or P2Y12R (C, 1321N1 cells). A. Cells were pretreated with UBO-QIC (100 nM) for 30 min before the addition of agonists. B. Cells were pretreated with UBO-QIC (100 nM) for 30 min or PTX (200 ng/ml) overnight. C. Cells were pretreated with UBO-QIC (100 nM and 300 nM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonist. Results are expressed as mean ± SEM from 3 independent experiments performed in duplicate. #Significantly different from corresponding control values (P<0.05).
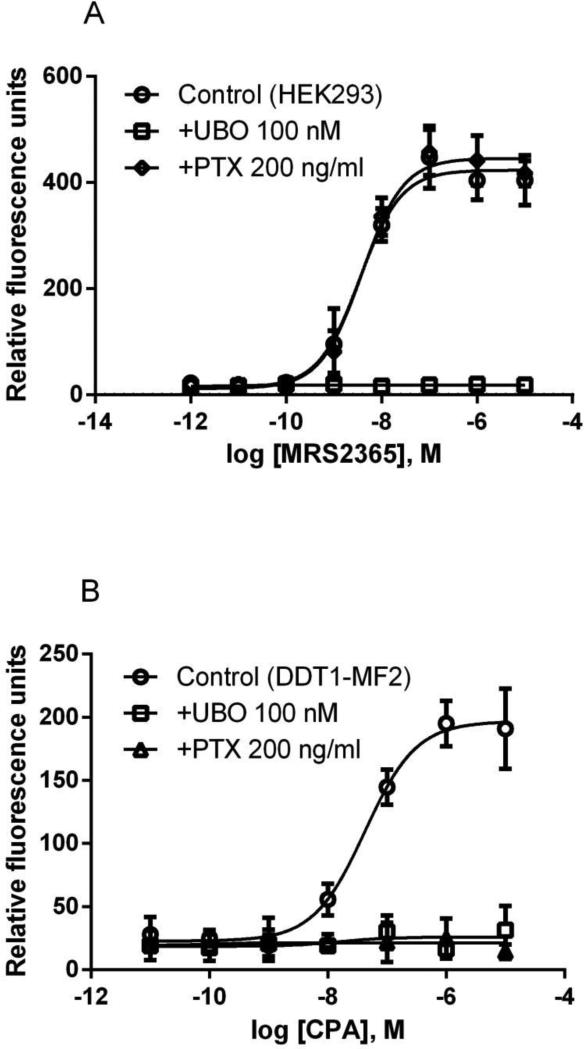
To further demonstrate the inhibitory effect of UBO-QIC on both Gαq and non-Gαq-mediated events in a relatively natural system, we examined its effect on calcium mobilization induced by a selective A1AR agonist CPA in DDT1-MF2 (Syrian hamster smooth muscle) cells known to express an A1AR endogenously that is sensitive to PTX [29]. Figure 7 shows that CPA-induced intracellular calcium mobilization in DDT1-MF2 cells is completely blocked by both UBO-QIC and PTX. By comparison, UBO-QIC but not PTX antagonizes calcium transients mediated by a P2Y1R-selective agonist MRS2365 [30] in HEK293 cells that endogenously express the human P2Y1R (Figure 7).
Figure 7.
MRS2365-induced intracellular calcium mobilization in HEK293 cells expressing an endogenous P2Y1R (A) and CPA- induced intracellular calcium mobilization in DDT1-MF2 cells with an endogenous A1AR (B). Cells were pretreated with UBO-QIC (100 nM) for 30 min or PTX (200 ng/ml) overnight before the addition of agonist. All data are in relative fluorescence units and are expressed as mean ± SEM from 3 experiments performed in triplicate.
4. Discussion
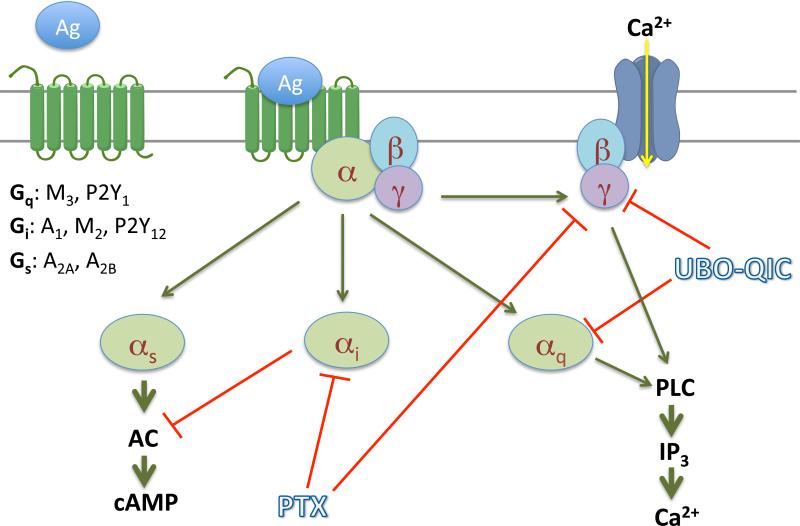
The present study clearly demonstrated that UBO-QIC is a potent inhibitor of Gβγ-mediated signaling events following the activation of Gi-coupled receptors, although UBO-QIC is selective for Gαq in comparison to Gαi- or Gαs-mediated inhibition or stimulation of cAMP accumulation (Figure 8). Thus, caution is needed when using UBO-QIC to define Gαq coupling of certain GPCRs since Gαq and Gβγ often mediate similar downstream signaling events.
Figure 8.
GPCR signaling pathways and their interaction with inhibitors.
The Gαq coupling of GPCRs has been probed in several recent publications using UBO-QIC to block production of inositol phosphates [6], calcium release [5,7,31] or GTPγS binding [2]. Inamdar et al. [4] reported that UBO-QIC is a selective Gαq inhibitor based on studies using Gαq knockout murine platelets. The authors concluded that UBO-QIC has no effect on Gαi subunits based on the results that UBO-QIC has no effect on phosphorylation of protein kinase B (Akt), which is downstream of Gαi. The authors also concluded that the Gα12/13 pathway was not involved, because platelet shape remained intact in Gαq knockout mice. However, the effect of UBO-QIC on Gβγ-mediated events was not explored in those previous studies. In addition to clear demonstration of the blockade by UBO-QIC of both Gβγ and Gαq-mediated events, results from the present study also indicate that the weak inhibition or even lack of inhibition by UBO-QIC of some pathways, such as ERK1/2 and Akt, is not an evidence that Gαq is not involved as these events are often mediated via multiple upstream signaling molecules. For example, Figure 5 shows that, even at 300 nM, UBO-QIC only diminished about 30% of the maximal effect of carbachol, although it shifted the concentration-response curve to the right. The concentrations of the agonists used in this assay were critical for the interpretation of the percentage inhibition by UBO-QIC. Consistent with the present study, Wauson et al. [3] also showed that UBO-QIC only partially reduced ERK1/2 stimulation mediated via Gαq-coupled T1R1/T1R3 heterodimeric taste receptor in the MIN6 pancreatic β-cell derived line. It is noted that in addition to Gα, βγ subunits released both from Gq/11 and Gi classes of G proteins can stimulate ERK1/2 although via a different phosphorylation site and lead to different downstream events [32]. It is intriguing to know if UBO-QIC can distinguish downstream events mediated by different βγ subunits. This could be examined by using βγ Gi over-expression system or using Gi protein mutants which may obtain more direct and less disputable results. It should be interesting to elucidate the identity of βγ subunit complexes involved in the signaling cascade that are sensitive to UBO-QIC. However, this may not be an easy task given the fact that at least 5 β-subunits and 11 γ-subunits have been identified. We do not have evidence as to how UBO-QIC affects only βγ subunits released from Gi, but not the Gαi protein. It has been reported that a small molecule 12155 binds directly the Gβγ, but without affecting Gαi subunit in the Gi heterotrimer [33].
The present study demonstrated a different pattern of inhibition by UBO-QIC for ERK1/2 and Akt activity mediated by the same class of receptors. For example, UBO-QIC completely blocked P2Y1R-mediated Akt activity but only partially inhibited ERK1/2 activity. The reason for this difference could be due to the fact that Akt and ERK1/2 activity are mediated via different upstream mechanisms directed under the same receptor. Murga et al. [28] showed that PKC did not seem to link Gαq to Akt but PI3 kinase could link a receptor to Akt phosphorylation, although the conclusion was only based on results from individual Gq-coupled receptors. GPCR-mediated ERK1/2 activity has been extensively explored, which could be mediated via various Gα, Gβγ, and β-arrestins [24], and PKC is often involved in ERK1/2 activity meditated via various G protein subunits. On the other hand, it has been shown that blockade of G protein signaling by PKC inhibitors such as GO6983 may enhance β-arrestin-mediated signaling, and the inhibition of arrestin-mediated signaling, such as by siRNA, may promote signaling mediated via G protein subunits [34]. It is not clear, if the activity of other G protein subunits or β-arrestins are changed following the blockade of Gαq by UBO-QIC, which could be similar or different for some signaling events blocked by inhibitors of other signaling molecules, such as PKC and PI3 kinase.
A number of G protein inhibitors have been reported previously. For example, PTX inhibits the α subunit of Gi proteins by locking subunits in a GDP-bound inactive state [35], but also affected release of βγ subunits [17,20,36]. GRK2i, a peptide analog of G-protein receptor kinase (GRK), blocks only Gβγ signaling thus rendering it a useful tool to discern between Gα and βγ pathways [4,15]. However, it is not clear if UBO-QIC interacts with Gβγ in the same way as GRK2i or not. It is also not clear if YM-254890 or UBO-QIC inhibits signaling events mediated by βγ subunits released from Gαq [32], although YM-254890 was proposed to inhibit Gq proteins by inhibiting the release of GDP from the α subunits [14]. In addition to the G protein inhibitors, G protein activators were also found to activate multiple G proteins or G protein subunits. For example, Pasteurella multocida toxin (PMT) was initially found to activate Gq and G12/13, but it was found later that it also activates Gαi, and Gβγ subunits are needed for Gα signaling [37]. We do not have a structural explanation of the interaction of UBO-QIC with Gβγ, as it was noted that although the binding region is close to Gβγ in the X-ray structure, the related depsipeptide YM-254890 bound to Gα in the absence of the Gβγ subunits [14].
As mentioned in the Introduction section, compared with UBO-QIC, YM-254890 is a relatively well-characterized Gαq inhibitor [8,10-13]. However, even for YM-254890, only very limited data are available concerning its selectivity against some G proteins, such as G15 and Gβγ [9].
Our findings are supported by a very recent report by Kukkonen [38], who raised similar doubts about the Gαq selectivity and showed that UBO-QIC at the concentration of 1 μM could inhibit Gα16 in addition to Gαq [35]. The author cautioned that “further studies are required to establish its profile with respect to the different Gq-family proteins”, and we agree.
In summary, UBO-QIC is an extremely useful inhibitor of Gαq signaling, but has additional effects that need to be considered in interpreting pharmacological data. The present study clearly demonstrated that UBO-QIC blocked both Gαq and Gβγ-mediated signaling following the activation of Gq- and Gi-coupled receptors.
Acknowledgements
Supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD. We thank Jianxin Hu and Jürgen Wess (NIDDK, Bethesda, MD) for helpful discussions and supplying cell lines. We thank T. K. Harden (Univ. of North Carolina, Chapel Hill, NC) for cell lines.
Abbreviations
- BAY60-6583 (LUF6210)
2-[[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy)phenyl]-2-pyridinyl]thio]-acetamide
- cAMP
3′,5′-cyclic adenosine monophosphate
- CGS21680
2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamidoadenosine
- CHO
Chinese hamster ovary
- CPA
N6-cyclopentyladenosine
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular-signal-regulated kinase
- fMLP
formyl peptide
- GO6983
3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione
- GPCR
G protein-coupled receptor
- HEK
human embryonic kidney
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- IP1
inositol 1-phosphate
- 2MeSADP
2-methylthioadenosine 5′-diphosphate trisodium salt
- MRS2365
[[(1R,2R,3S,4R,5S)-4-[6-amino-2-(methylthio)-9H-purin-9-yl]-2,3-dihydroxybicyclo[3.1.0]hex-1-yl]methyl] diphosphoric acid monoester
- NECA
adenosine-5′-N-ethyluronamide
- PTX
pertussis toxin
- UBO-QIC
L-threonine, (3R)-N-acetyl-3-hydroxy-L-leucyl-(αR)-α-hydroxybenzenepropanoyl-2, 3-didehydro-N-methylalanyl-L-alanyl-N-methyl-L-alanyl-(3R)-3-[[(2S,3R)-3-hydroxy-4-methyl-1-oxo-2-[(1-oxopropyl)amino]pentyl]oxy]-L-leucyl-N,O-dimethyl-, (7→1)-lactone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fujioka M, Koda S, Morimoto Y, Biemann K. Structure of FR900359, a cyclic depsipeptide from Ardisia crenata sims. J Org Chem. 1988;53(12):2820–5. [Google Scholar]
- 2.Carr R, 3rd, Koziol-White C, Zhang J, Lam H, An SS, Tall GG, Panettieri RA, Jr, Benovic JL. Interdicting Gq Activation in Airway Disease by Receptor-Dependent and Receptor-Independent Mechanisms. Mol Pharmacol. 2015 doi: 10.1124/mol.115.100339. pii: mol.115.100339. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wauson EM, Guerra ML, Dyachok J, McGlynn K, Giles J, Ross EM, Cobb MH. Differential Regulation of ERK1/2 and mTORC1 Through T1R1/T1R3 in MIN6 Cells. Mol Endocrinol. 2015;29(8):1114–22. doi: 10.1210/ME.2014-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inamdar V, Patel A, Manne BK, Dangelmaier C, Kunapuli SP. Characterization of UBOQIC as a G q inhibitor in platelets. Platelets. 2015;26(8):771–8. doi: 10.3109/09537104.2014.998993. [DOI] [PubMed] [Google Scholar]
- 5.Hennen S, Wang H, Peters L, Merten N, Simon K, Spinrath A, Blättermann S, Akkari R, Schrage R, Schröder R, Schulz D, Vermeiren C, Zimmermann K, Kehraus S, Drewke C, Pfeifer A, König GM, Mohr K, Gillard M, Müller CE, Lu QR, Gomeza J, Kostenis E. Decoding signaling and function of the orphan G protein-coupled receptor GPR17 with a small-molecule agonist. doi: 10.1126/scisignal.2004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jacobsen SE, Nørskov-Lauritsen L, Thomsen AR, Smajilovic S, Wellendorph P, Larsson NH, Lehmann A, Bhatia VK, Bräuner-Osborne H. Delineation of the GPRC6A receptor signaling pathways using a mammalian cell line stably expressing the receptor. J Pharmacol Exp Ther. 2013;347(2):298–309. doi: 10.1124/jpet.113.206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpinsky-Semper D, Volmar CH, Brothers SP, Slepak VZ. Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release. Mol Pharmacol. 2014;85(5):758–68. doi: 10.1124/mol.114.091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taniguchi M, Nagai K, Arao N, Kawasaki T, Saito T, Moritani Y, Takasaki J, Hayashi K, Fujita S, Suzuki K, Tsukamoto S. YM-254890, a novel platelet aggregation inhibitor produced by Chromobacterium sp. QS3666. J Antibiot (Tokyo) 2003;56(4):358–63. doi: 10.7164/antibiotics.56.358. [DOI] [PubMed] [Google Scholar]
- 9.Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, Kobori M. A novel Galphaq/11-selective inhibitor. J Biol Chem. 2004;279(46):47438–45. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- 10.Uemura T, Kawasaki T, Taniguchi M, Moritani Y, Hayashi K, Saito T, Takasaki J, Uchida W, Miyata K. Biological properties of a specific Galphaq/11 inhibitor, YM-254890, on platelet functions and thrombus formation under high-shear stress. Br J Pharmacol. 2006;148(1):61–9. doi: 10.1038/sj.bjp.0706711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisaoka-Nakashima K, Miyano K, Matsumoto C, Kajitani N, Abe H, Okada-Tsuchioka M, Yokoyama A, Uezono Y, Morioka N, Nakata Y, Takebayashi M. Tricyclic Antidepressant Amitriptyline-induced Glial Cell Line-derived Neurotrophic Factor Production Involves Pertussis Toxin-sensitive Gαi/o Activation in Astroglial Cells. J Biol Chem. 2015;290(22):13678–91. doi: 10.1074/jbc.M114.622415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ando K, Obara Y, Sugama J, Kotani A, Koike N, Ohkubo S, Nakahata N. P2Y2 receptor-Gq/11 signaling at lipid rafts is required for UTP-induced cell migration in NG 108-15 cells. J Pharmacol Exp Ther. 2010;334(3):809–19. doi: 10.1124/jpet.110.167528. [DOI] [PubMed] [Google Scholar]
- 13.Taboubi S, Milanini J, Delamarre E, Parat F, Garrouste F, Pommier G, Takasaki J, Hubaud JC, Kovacic H, Lehmann M. G alphaq/11-coupled P2Y2 nucleotide receptor inhibits human keratinocyte spreading and migration. FASEB J. 2007;21(14):4047–58. doi: 10.1096/fj.06-7476com. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura A, Kitano K, Takasaki J, Taniguchi M, Mizuno N, Tago K, Hakoshima T, Itoh H. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc Natl Acad Sci U S A. 2010;107(31):13666–71. doi: 10.1073/pnas.1003553107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koch WJ, Hawes BE, Inglese J, Luttrell LM, Lefkowitz RJ. Cellular expression of the carboxyl terminus of a G protein-coupled receptor kinase attenuates G beta gamma-mediated signaling. J Biol Chem. 1994;269(8):6193–7. [PubMed] [Google Scholar]
- 16.Ethier MF, Madison JM. Adenosine A1 receptors mediate mobilization of calcium in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 2006;35(4):496–502. doi: 10.1165/rcmb.2005-0290OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickenson JM, Hill SJ. Involvement of G-protein betagamma subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur J Pharmacol. 1998;355(1):85–93. doi: 10.1016/s0014-2999(98)00468-3. [DOI] [PubMed] [Google Scholar]
- 18.Katz A, Wu D, Simon MI. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992;360(6405):686–9. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- 19.Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-beta 2 by G protein beta gamma-subunits. Nature. 1992;360(6405):684–6. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- 20.Violin JD, DeWire SM, Yamashita D, Rominger DH, Nguyen L, Schiller K, Whalen EJ, Gowen M, Lark MW. Selectively engaging β-arrestins at the angiotensin II type 1 receptor reduces blood pressure and increases cardiac performance. J Pharmacol Exp Ther. 2010;335(3):572–9. doi: 10.1124/jpet.110.173005. [DOI] [PubMed] [Google Scholar]
- 21.Gao ZG, Verzijl D, Zweemer A, Ye K, Göblyös A, IJzerman AP, Jacobson KA. Functionally biased modulation of A3 adenosine receptor agonist efficacy and potency by imidazoquinolinamine allosteric enhancers. Biochem Pharmacol. 2011;82(6):658–68. doi: 10.1016/j.bcp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao ZG, Balasubramanian R, Kiselev E, Wei Q, Jacobson KA. Probing biased/partial agonism at the G protein-coupled A2B adenosine receptor. Biochem Pharmacol. 2014;90(3):297–306. doi: 10.1016/j.bcp.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haske TN, DeBlasi A, LeVine H. An intact N terminus of the gamma subunit is required for the Gbetagamma stimulation of rhodopsin phosphorylation by human beta-adrenergic receptor kinase-1 but not for kinase binding. J Biol Chem. 1996;271(6):2941–8. doi: 10.1074/jbc.271.6.2941. [DOI] [PubMed] [Google Scholar]; Sci Signal. 2013;6(298):ra93. doi: 10.1126/scisignal.2004350. doi: 10.1126/scisignal.2004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent beta-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Nat Acad Sci USA. 2003;100(19):10782–7. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulte G, Fredholm BB. Human adenosine A1, A2A, A2B, and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol. 2000;58(3):477–82. [PubMed] [Google Scholar]
- 26.Cordeaux Y, IJzerman AP, Hill SJ. Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br J Pharmacol. 2004 Nov;143(6):705–14. doi: 10.1038/sj.bjp.0705925. Epub 2004 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C μ by various inhibitors. Differentiation from protein kinase c isozymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 28.Murga C, Laguinge L, Wetzker R, Cuadrado A, Gutkind JS. Activation of Akt/protein kinase B by G protein-coupled receptors. A role for alpha and beta gamma subunits of heterotrimeric G proteins acting through phosphatidylinositol-3-OH kinase γ. J Biol Chem. Jul 24. 1998;273(30):19080–5. doi: 10.1074/jbc.273.30.19080. [DOI] [PubMed] [Google Scholar]
- 29.Fredholm BB, Assender JW, Irenius E, Kodama N, Saito N. Synergistic effects of adenosine A1 and P2Y receptor stimulation on calcium mobilization and PKC translocation in DDT1 MF-2 cells. Cell Mol Neurobiol. 2013;23(3):379–400. doi: 10.1023/A:1023644822539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J Pharm Exp Therap. 2004;311:1038–43. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaima K, Deguchi J, Matsuno Y, Kaneda T, Hirasawa Y, Morita H. Vasorelaxant effect of FR900359 from Ardisia crenata on rat aortic artery. J Nat Med. 2013;67(1):196–201. doi: 10.1007/s11418-012-0644-0. [DOI] [PubMed] [Google Scholar]
- 32.Gutkind JS, Offermanns S. A new Gq-initiated MAPK signaling pathway in the heart. Dev Cell. 2009;16(2):163–4. doi: 10.1016/j.devcel.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Surve CR, To JY, Malik S, Kim M, Smrcka AV. Dynamic regulation of neutrophil polarity and migration by the heterotrimeric G protein subunits Gαi-GTP and Gβγ. Sci Signal. 2016 Feb 23;9(416):ra22. doi: 10.1126/scisignal.aad8163. doi: 10.1126/scisignal.aad8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Ann Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. Review. [DOI] [PubMed] [Google Scholar]
- 35.Bokoch GM, Gilman AG. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984;39(2 Pt 1):301–8. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- 36.Gilman AG. G proteins: transducers of receptor-generated signals. Ann Rev Biochem. 1987;56:615–49. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 37.Preuss I, Kurig B, Nürnberg B, Orth JH, Aktories K. Pasteurella multocida toxin activates Gbetagamma dimers of heterotrimeric G proteins. Cell Signal. 2009;21(4):551–8. doi: 10.1016/j.cellsig.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Kukkonen JP. G-protein inhibition profile of the reported Gq/11 inhibitor UBO-QIC. Biochem Biophys Res Commun. 2015 Nov 22;:S0006–291X(15)30947-5. doi: 10.1016/j.bbrc.2015.11.078. doi: 10.1016/j.bbrc.2015.11.078. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]