Supplemental Digital Content is available in the text.
Keywords: animal model, antibiotic, brain–gut microbiota axis, ischemic stroke, microbiota depletion
Abstract
Background and Purpose—
Antibiotics disturbing microbiota are often used in treatment of poststroke infections. A bidirectional brain–gut microbiota axis was recently suggested as a modulator of nervous system diseases. We hypothesized that gut microbiota may be an important player in the course of stroke.
Methods—
We investigated the outcome of focal cerebral ischemia in C57BL/6J mice after an 8-week decontamination with quintuple broad-spectrum antibiotic cocktail. These microbiota-depleted animals were subjected to 60 minutes middle cerebral artery occlusion or sham operation. Infarct volume was measured using magnetic resonance imaging, and mice were monitored clinically throughout the whole experiment. At the end point, tissues were preserved for further analysis, comprising histology and immunologic investigations using flow cytometry.
Results—
We found significantly decreased survival in the middle cerebral artery occlusion microbiota-depleted mice when the antibiotic cocktail was stopped 3 days before surgery (compared with middle cerebral artery occlusion specific pathogen-free and sham-operated microbiota-depleted mice). Moreover, all microbiota-depleted animals in which antibiotic treatment was terminated developed severe acute colitis. This phenotype was rescued by continuous antibiotic treatment or colonization with specific pathogen-free microbiota before surgery. Further, infarct volumes on day one did not differ between any of the experimental groups.
Conclusions—
Conventional microbiota ensures intestinal protection in the mouse model of experimental stroke and prevents development of acute and severe colitis in microbiota-depleted mice not given antibiotic protection after cerebral ischemia. Our experiments raise the clinically important question as to whether microbial colonization or specific microbiota are crucial for stroke outcome.
Stroke is the second leading cause of death worldwide and the most frequent cause of long-term disability in adults in developed countries.1 Despite progress in understanding the pathophysiology of damage of this devastating disease, all efforts to establish pharmacological brain-protective strategies based on this knowledge have been futile. This has led stroke researchers to shift focus from neuroprotection to other modifiable determinants of outcome, in particular complications. Taken together, complications, such as infections, increased intracranial pressure, and sarcopenia explain at least 20% of the overall outcome of stroke patients.2 Infections, particularly pneumonia, are the most common complication after stroke, contributing to increased mortality and worsening the neurological outcome. A substantial number of stroke patients are treated with antibiotics, often including combinations of broad-spectrum antimicrobial agents. Effects of this treatment on commensal microbiota have been neglected and the impact of microbiota on stroke outcome has not been investigated to date.
Commensal microbiota, in particular that of the gut, has recently entered center stage in biomedicine due to the advances in DNA sequencing and bioinformatics. Gut microbiota not only defends the host from invading pathogens, but also stimulates angiogenesis,3 regulates fat storage4 and controls gut permeability.5 This was demonstrated by sequencing whole microbiotic genomes (microbiomes), by using germ-free (ie, abiotic) animal models and selectively colonizing with specific microorganisms. Specific microbiota is required for proper organ development, including the immune system and brain, and in many other physiological functions. Consequently, germ-free mice develop altered structures of the brain, blood–brain barrier, and immune organs6,7 and are resistant to diet-induced obesity because of changes in metabolic profiles.4 Disturbances in the gut microbiota have been linked to several pathologies, including inflammatory bowel disease,8 obesity,9 type I diabetes mellitus,10,11 as well as neurological conditions, such as multiple sclerosis, Guillain–Barré syndrome, nociceptive pain or stress, anxiety,12 and neurodevelopmental disorders.13
Little to nothing is known, however, about the modulating impact of gut microbiota on acute central nervous system (CNS) injury in stroke or, in reverse, the impact of stroke on the composition or functional profile of microbiota. This is surprising, because there are important reasons to suspect a relevant interplay between the lesioned brain in stroke and the microbiome, particularly the gut microbiome. Stroke causes immunodepression, which renders the organism susceptible to bacterial infections.14–16 Indeed, infections after stroke have a major impact on stroke outcome. Pneumonia, for example, is the most frequent cause of death in acute stroke.1 Immunodepression after stroke leads to a breakdown of epithelial barriers,17,18 making the gut an important potential source of invading bacteria. Stroke modulates the activity of the autonomic nervous system, which contributes to immunodepression, but also affects gut motility and permeability.19,20 Further, because of their increased susceptibility to infection, many stroke patients are treated with potent combinations of antibiotics, with possibly drastic consequences for the commensal bacterial microbiota.
We speculate that gut microbiota may be an important direct or indirect modulating factor for stroke outcome by (1) causing infection, (2) modulating the immune or autonomic nervous system and thus other complications (eg, sarcopenia), or (3) exerting metabolic or humoral effects on the brain. As a first step to test this hypothesis, we choose to study the effect of extensively depleting commensal microbiota on stroke outcome in a well-characterized murine model of focal cerebral ischemia, in a clinically relevant setting, specifically by using combinations of potent antibiotics. These broad-spectrum antibiotic-treated animals were termed as microbiota-depleted, and they did not harbor any bacteria cultivatable on standard microbiological media.21 We decided to choose this previously established model to avoid confounders present in germ-free animals, such as deficiencies in the immune system or altered brain physiology, biochemistry, and anatomy.7,22,23 In our study, we found no effect of microbiota depletion on the brain lesion (infarct volume), but we did find a protective effect of gut microbiota on survival.
Materials and Methods
Detailed description of materials and methods can be found in the online-only Data Supplement.
Animals and Housing
Female C57BL/6J mice (Forschungseinrichtung für Experimentelle Medizin, FEM, Charité Berlin, Germany) after microbiota depletion by quintuple antibiotic treatment21 were placed in autoclaved, individually ventilated cages lined with autoclaved chip bedding and kept on a 12-hour light/dark cycle with ad libitum access to food (autoclaved, standard chow, complete feed for rats and mice maintenance; Sniff, Soest, Germany) and autoclaved water. Mice were 11 to 28 weeks old at the time of the experiment. All experiments were conducted in accordance with the European directive on the protection of animals used for scientific purposes and approved by Landesamt für Gesundheit und Soziales, Berlin, Germany.
Generation of Microbiota-Depleted Mice
Eight-week old female C57BL/6J mice (Forschungseinrichtung für Experimentelle Medizin, FEM, Charité Berlin, Germany) harboring a conventional microbiota were transferred to autoclaved cages and treated with quintuple antibiotic cocktail consisting of ampicillin (1 g/L; Ratiopharm), vancomycin (500 mg/L; Cell Pharm), ciprofloxacin (200 mg/L; Bayer Vital), imipenem (250 mg/L; MSD), and metronidazole (1 g/L; Fresenius) in the drinking water available ad libitum according to the previously published protocol.21 The microbiological status of mice was controlled every week as described previously.21 Cultural and molecular methods revealed that the intestinal microbiota was virtually depleted 8 weeks after broad-spectrum antibiotic treatment.21,24
Experimental Stroke (Middle Cerebral Artery Occlusion)
Surgical procedures were conducted under microorganism-reducing conditions (sterile gown, gloves, instruments, surgical hand wash before operations, nonsterile helpers during surgery). Middle cerebral artery occlusion (MCAo; experimental focal cerebral ischemia) was performed according to the standard operating procedure from the Department of Experimental Neurology, Charité Berlin, Germany.25
Magnetic Resonance Imaging
Infarct volume was assessed using magnetic resonance imaging (Bruker 7T PharmaScan 70/16) on day one after MCAo.
Flow Cytometric Analysis of Spleens, Mesenteric Lymph Nodes, and Peyer’s Patches
Cells were phenotyped on LSR Fortessa flow cytometer with FACSDiva software (BD Biosciences, Heidelberg, Germany) using antibodies specified in the online-only Data Supplement. Data were analyzed with FlowJo software (Tree Star Inc, Ashland, OR).
Microbiological Investigation of Fecal Samples
Cultural analyses of aerobic, microaerophilic, and anaerobic bacterial species abundant in the fecal samples were performed as described previously.21
Hematoxylin and Eosin Staining of Intestinal Samples
Swiss rolls of intestinal segments were isolated during necropsy,26 immersion-fixed in 4% paraformaldehyde, and embedded in paraffin. 5-μm-thick sections were cut, dewaxed, stained with hematoxylin and eosin following standard protocols.
Methods to Prevent Bias and Exclusion Criteria
Cages with animals were randomly assigned to experimental groups. Operations, and daily health examination were performed unblinded because of the microorganism-reducing conditions required during surgery and handling. The exclusion criteria were (1) unsuccessful stroke, based on magnetic resonance imaging investigation in the experiments I and II or histological assessment in the experiment III, and (2) death on day of the surgery (Table I in the online-only Data Supplement).
Statistics
Statistics were performed using SPSS Statistics (IBM SPSS Statistics for Macintosh, Version 20.0.; IBM Corp, Armonk, NY). Sample size calculations (G*Power 3.127) assumed effect size Cohen’s d=1 for comparison primarily between conventionally colonized MCAo animals and microbiota-depleted MCAo mice using t test. For the calculation, we implemented α=0.05 and β=0.8 for all experiments and drop-out rate of 10% because of MCAo and 5% to the sham surgery. Total sample sizes were n=57 in experiment I, n=46 in experiment II, and n=27 in experiment III. The original data of this study is available online: http://dx.doi.org/10.6084/m9.figshare.1476224.
Results
Extensive Depletion of Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Does Not Affect Volume of the Ischemic Brain Lesion 1 Day After Experimental Stroke
In 3 series of experiments, we used microbiota-depleted mice generated by an 8-week broad-spectrum antibiotic regimen.24 In the first experimental set, we aimed to assess long-term outcome of focal cerebral ischemia (MCAo) using this model (Figure 1A). Considering the immunomodulatory properties of antibiotics28,29 and their possible neuroprotective or neurotoxic effects,30 we stopped the antibiotic treatment in the AB(+/−) groups 72 hours before surgical intervention. Additionally, we introduced a specific pathogen-free (SPF) AB(+/−) control group treated with the quintuple antibiotic cocktail only for 48 hours up to 72 hours before operation and conventionally colonized mice without any antibiotic treatment SPF AB(−/−). To further characterize effects of the antibiotic regime and extensive depletion of commensal microbiota on the outcome of focal cerebral ischemia in the next experimental series, we additionally investigated the AB(+/+) groups, in which the treatment with antibiotic cocktail was continued up to the end of the experiments, and the groups recolonized with intestinal microbiota derived from SPF AB(−/−) littermates. We performed microbiological investigations of fecal samples in all experimental series and did not find any cultivatable microorganisms in samples from microbiota-depleted AB(+/−) and microbiota-depleted AB(+/+) mice at the time point of surgery.
Figure 1.
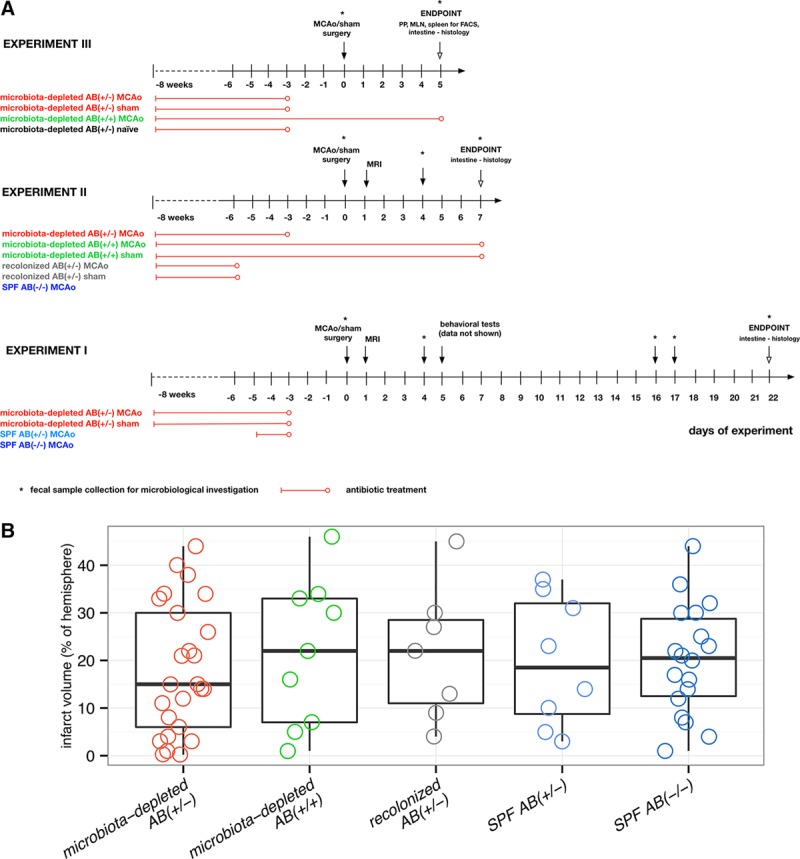
Experimental setup and infarct volume. A, Experimental setup (3 independent experiments). Experimental groups: microbiota-depleted AB(+/−) MCAo/sham, with antibiotic treatment stopped 72 h before surgery; microbiota-depleted AB(+/+) MCAo/sham, with antibiotic treatment during the entire experiment; recolonized AB(+/−) MCAo/sham, microbiota-depleted mice recolonized with SPF microbiota; antibiotic treatment was stopped 48 h before recolonization; microbiota-depleted AB(+/−) naïve, microbiota-depleted animals without any surgical intervention with antibiotic cocktail stopped 72 h before the experiment; SPF AB(−/−) MCAo, conventionally colonized (specific pathogen-free microbiota) mice without any antibiotic treatment, subjected to MCAo surgery; SPF AB(+/−) MCAo, SPF mice with antibiotic treatment for 48 h up to 72 h before surgery. B, Infarct volume assessed by MRI at day one after MCAo did not differ between investigated groups. Microbiota-depleted AB(+/−) n=25, microbiota-depleted AB(+/+) n=9, recolonized AB(+/−) (microbiota-depleted recolonized with SPF microbiota) n=7, SPF AB(+/−) (SPF with antibiotic treatment for 48 h) n=8, SPF AB(−/−) n=18. Box plot with whiskers minimum to maximum. No statistically significant differences were found when comparing all experimental groups (Kruskal Wallis test with Dunn’s post hoc) or when comparing microbiota-depleted AB(+/−) with SPF AB(−/−) mice Mann–Whitney test). FACS indicates flow cytometric analysis; MCAo, middle cerebral artery occlusion; MLN, mesenteric lymph nodes; MRI, magnetic resonance imaging; PP, Peyer’s patches; and SPF, specific pathogen-free.
Because bacterial metabolites, products, and antigens may have contributed directly or via interactions with the immune system to the development of the ischemic lesion, we assessed the infarct volume by magnetic resonance imaging on day one after focal cerebral ischemia in the first 2 series of experiments (Figure 1A). We did not find any statistically significant differences in stroke volume 24 hours after MCAo between any of the groups under investigation (Figure 1B).
Extensive Depletion of Gut Microbiota Decreases Survival After Experimental Stroke
In the first series of experiments, in which the antibiotic treatment was terminated 72 hours before operation, microbiota-depleted mice subjected to sham operation and MCAo surprisingly developed acute and severe diarrhea 5 to 6 days after surgery. Survival rate in the microbiota-depleted AB(+/−) MCAo group was significantly lower than that of SPF AB(−/−) mice. It was also lower than that in microbiota-depleted sham-operated animals (Figure 2A) that showed intestinal symptoms similar to those in the MCAo mice (weight loss, diarrhea, crouched position; Figure IA in the online-only Data Supplement). We were able to reproduce this finding in the second set of experiments, in which symptoms in the microbiota-depleted AB(+/−) MCAo group started 6 days after surgery (Figure 2B and Figure IC in the online-only Data Supplement). We strictly monitored all mice in the experiment, checking general well-being every 4 hours. Within ≈4 hours after onset of diarrhea, affected mice displayed symptoms resembling shock: crouched position, rough fur, lethargy, and difficulties in breathing.31 These symptoms are indicative of an acute progression of systemic sequelae, and the affected animals were euthanized in compliance with the humane end points in our experiments.
Figure 2.
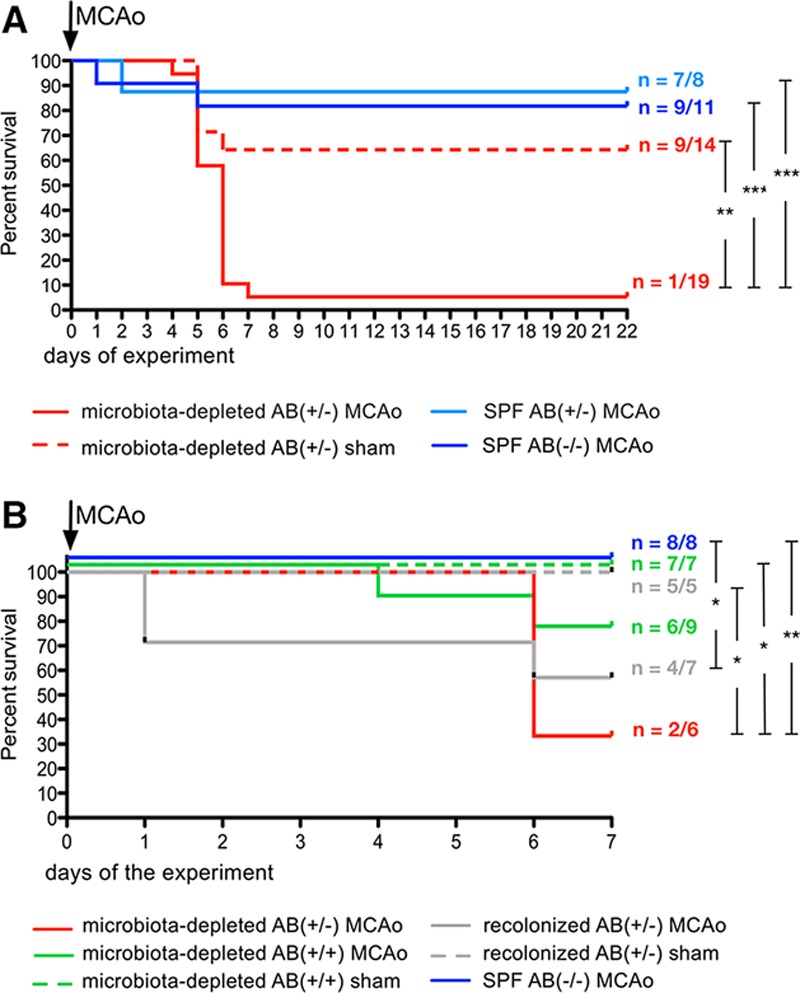
Survival analyses. A, Kaplan–Meier curve from experiment I. Extensive depletion of microbiota significantly decreases survival after cerebral ischemia. Microbiota-depleted mice after experimental stroke without antibiotic protection have significantly lower survival rate than do sham-operated animals and SPF AB(−/−) mice. Numbers indicate surviving animals/animals included in the experiment in the investigated group. Statistically significant differences were found between microbiota-depleted AB(+/−) MCAo vs microbiota-depleted AB(+/−) sham (P=0.002; Chi square =9.402); microbiota-depleted AB(+/−) MCAo vs SPF AB(−/−) MCAo (P<0.001; Chi square =16.526); and microbiota-depleted AB(+/−) MCAo vs SPF AB(+/−) group (P<0.001; Chi square =12.163) using log-rank (Mantel–Cox) test. B, Kaplan–Meier curve from experiment II. Survival rate of microbiota-depleted animals subjected to MCAo is improved by continuous antibiotic treatment or recolonization before experimental stroke with SPF microbiota. Numbers indicate surviving animals/animals included in the experiment in the investigated group. Statistically significant differences were found in comparing SPF AB(−/−) MCAo vs recolonized AB(+/−) MCAo (P=0.044; Chi square =4.048), microbiota-depleted AB(+/−) MCAo vs recolonized AB(+/−) sham (P=0.029; Chi square =4.762); microbiota-depleted AB(+/−) MCAo vs microbiota-depleted AB(+/+) sham (P=0.013; Chi square =6.222); microbiota-depleted AB(+/−) MCAo vs SPF AB(−/−) MCAo (P=0.008; Chi square =6.933) with log-rank (Mantel–Cox) test. MCAo indicates middle cerebral artery occlusion; and SPF, specific pathogen-free.
Non-Neurological Symptoms in Microbiota-Depleted Mice Undergoing Surgery Are Linked to Severe Colitis
We next examined histopathologic changes in hematoxylin and eosin–stained intestinal samples derived from microbiota-depleted and SPF animals. Remarkably, we observed acute multifocal to segmental erosive–ulcerative and necrotizing colitis in those microbiota-depleted animals, which during the experiment had displayed apparent clinical colitis symptoms, such as weight loss and diarrhea.32 Conversely, we did not find any histopathologic abnormalities in intestinal samples from SPF AB(−/−), SPF mice after short antibiotic treatment =SPF AB(+/−), or in samples from animals recolonized with conventional microbiota (Figure 3). Although during the experiments microbiota-depleted mice were kept in individually ventilated cages with autoclaved equipment and were handled under microorganism-reducing conditions (until day 5 in the experiment I because of behavioral testing planned from day 5, data not shown, and during the entire experiment II and III), we suspected possible microbial contamination and spontaneous recolonization of microbiota-depleted animals during experimental procedures. To empirically test this right from the first experiment, we divided surviving animals into 2 groups: with antibiotic treatment starting on day 16 (vancomycin 5 g/L in the drinking water ad libitum, n=5) and without antibiotic intervention (n=5). As early as one day after starting the treatment, we noted an increase in body weight in the treated group and incipient resolution of symptoms (Figure IB in the online-only Data Supplement). In some stool samples collected from microbiota-depleted mice on different days of the experiment (day 4–17), several microorganism such as Clostridium species (spp.), Bacillus spp., and Staphylococci were detected, whereas other samples were culture-negative (Table II in the online-only Data Supplement).
Figure 3.
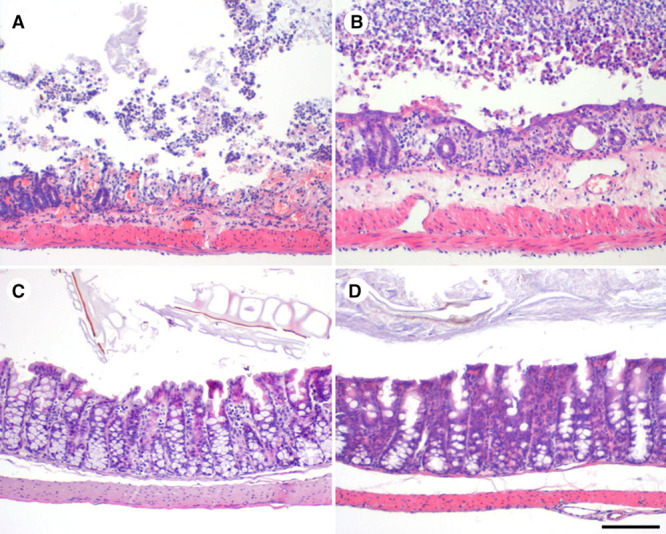
Histopathologic examination of intestinal samples. Depletion of microbiota before experimental stroke leads to the development of acute and severe colitis in the first week after cerebral ischemia. Histopathology of representative intestinal samples (hematoxylin and eosin staining; bar =100 μm) showed severe, acute erosive to ulcerative colitis present in microbiota-depleted AB(+/−) mice subjected to MCAo (A) and sham operation (B). In mice with continuous antibiotic treatment (C, microbiota-depleted AB(+/+) sham mouse) or recolonization with SPF microbiota (D, microbiota-depleted recolonized AB(+/−) MCAo mouse), colitis was prevented. MCAo indicates middle cerebral artery occlusion; and SPF, specific pathogen-free.
Continuous Antibiotic Treatment or Recolonization With Microbiota From SPF Littermates Improves the Outcome After Cerebral Ischemia
To elucidate potential pathogenic mechanisms of colitis development after stroke in microbiota-depleted AB(+/−) mice, we next investigated whether continuous preventative antibiotic treatment or restoration of commensal microbiota in microbiota-depleted mice could rescue the observed phenotype. We recolonized 2 groups of microbiota-depleted mice slated for MCAo or sham operation with intestinal microbiota from SPF AB(−/−) littermates. We observed that this intervention restored main bacterial groups of the gut microbiota. Bacteroides/Prevotella spp. and Enterococci appeared to recolonize the intestinal niche earlier than did other bacterial families and increased total bacterial load. Furthermore, recolonized animals had lower counts of the Enterobacteriaceae and Staphylococci, albeit the differences were <2 log and thus not considered to be biologically relevant (Figure II in the online-only Data Supplement). We did not observe bacterial growth in samples from microbiota-depleted AB(+/+) or in the microbiota-depleted AB(+/−) mice: only 2 samples were positive for Staphylococci at the end point (Figure 4A–4C). When comparing survival rates among microbiota-depleted mice, we observed that antibiotic treatment during the experiment, as well as recolonization with intestinal microbiota from SPF AB(−/−) littermates protected the mice from colitis and improved survival rate. Mortality rate in the recolonized MCAo group was still higher than in the SPF AB(−/−) MCAo group; nevertheless, recolonized animals did not develop symptoms of colitis (Figures 2B and 3 and Figure IC in the online-only Data Supplement). Furthermore, in our experiments, mortality on the first day after stroke appeared to be linked primarily to the severity of ischemic damage.
Figure 4.
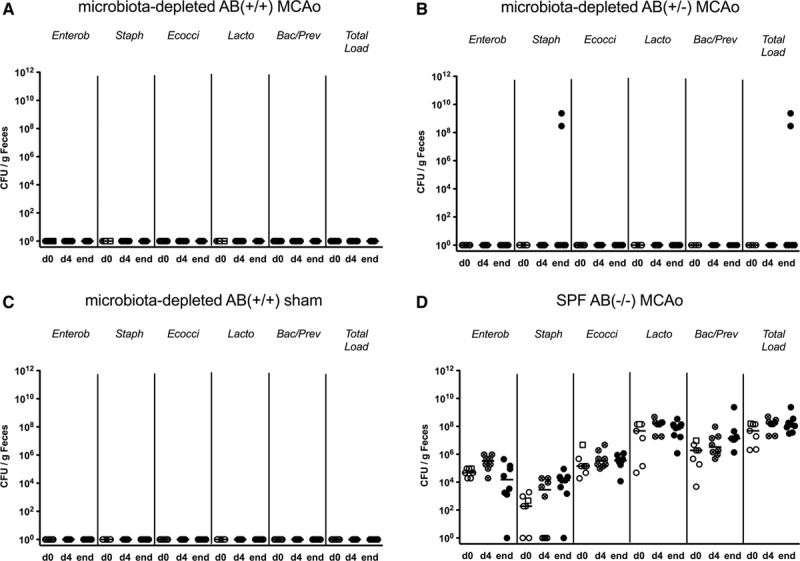
Microbiological analyses of fecal samples from experiment II. Analysis of fecal samples from (A) microbiota-depleted AB(+/+) MCAo animals, (B) microbiota depleted AB(+/-) MCAo, (C) microbiota-depleted AB(+/+) sham and (D) SPF AB(-/-) MCAo mice. Most microbiota-depleted animals remain culture-negative for 7 days of the experiment. Samples were collected on day of the surgery (d0), day 4 (d4), and at the end point (day 7 and day 6 for animals that have reached humane end points), respectively. Data presented as individual points and median. Samples from excluded animals (exclusion on day 1) are marked with squares. Bac/Prev indicates Bacteroides/Prevotella spp.; Ecocci, Enterococci; Enterob, Enterobacteriaceae; Lacto, Lactobacilli; MCAo, middle cerebral artery occlusion; SPF, specific pathogen-free; Staph, coagulase-negative Staphylococci; and Total load, total bacterial count are presented as the colony-forming units (CFU) per gram feces.
Microbiota-Depleted MCAo Mice Show Systemic Immunodepression on Day 5 After Cerebral Ischemia
In a third set of experiments, we investigated whether depletion of the intestinal microbiota might influence immune parameters after focal cerebral ischemia. We assessed main immune cell populations and percentages of interferon gamma (IFNγ) and interleukin 17 (IL-17) secreting cells after ex vivo stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin from spleens, mesenteric lymph nodes, and Peyer’s patches. Assessment took place on day 5 after surgery in microbiota-depleted AB(+/+) MCAo, AB(+/−) MCAo, AB(+/−) sham, and AB(+/−) naïve animals. Overall, we observed that cell counts of T cells (including CD4+ and CD8+ T cells subsets), B cells, and T cell receptor γδ+ (TCRγδ+) cells in spleens from mice subjected to cerebral ischemia tended to be lower than cell counts in naïve and sham-operated groups. In the AB(+/+) MCAo group, numbers of B cells and CD8+ cytotoxic T cells, as well as percentages of IFNγ-producing CD8+, TCRγδ+, and CD4+ helper T cells were significantly lower compared with sham-operated animals (Figures 5 and 6 and Figure IIIA in the online-only Data Supplement). Moreover, in Peyer’s patches, B cell numbers and percentage of IFNγ-producing TCRγδ+ cells were significantly decreased in the AB(+/+) MCAo mice compared with the sham-group (Figure 5 and Figure IIIB in the online-only Data Supplement). In contrast, in mesenteric lymph nodes, we observed significantly increased numbers of B cells in AB(+/+) MCAo animals (Figure 5) as compared with the naïve group and higher percentages of IL-17-producing CD4+ and TCRγδ+ cells than in sham-operated animals (Figure 6 and Figure IIIC in the online-only Data Supplement).
Figure 5.
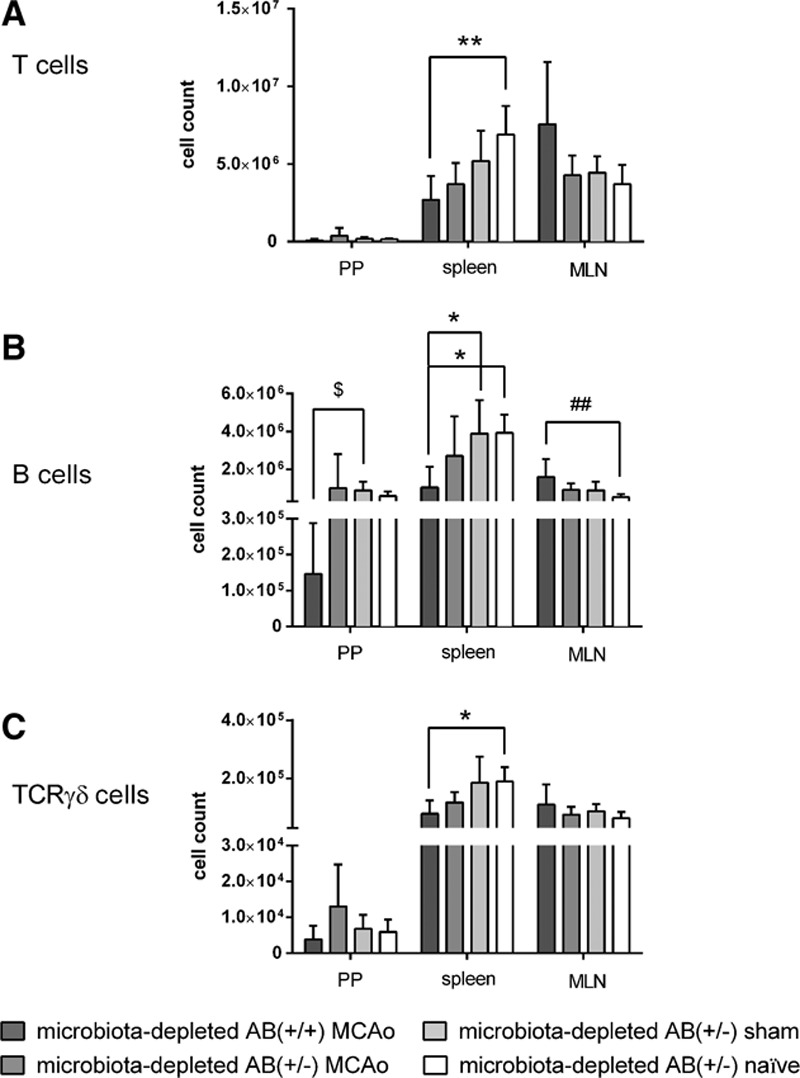
Flow cytometric analysis of immune cell populations from Peyer’s Patches, spleen, and mesenteric lymph nodes (MLN) on day 5 after cerebral ischemia. Cell counts for (A) T cells, (B) B cells, and (C) T cell receptor γδ+ (TCRγδ+) cells. Microbiota-depleted mice show systemic immunodepression an day 5 after cerebral ischemia. Data are expressed as mean+standard deviation (SD). No statistically significant differences were found between group with continuous antibiotic treatment, and group with antibiotics stopped before surgery. Microbiota-depleted AB(+/+) MCAo n=8 (n=7 for Peyer’s patches), microbiota-depleted AB(+/−) MCAo n=7, microbiotadepleted AB(+/−) sham n=6, and microbiota-depleted AB(+/−) naïve n=6. Statistical analyses comparing the groups within one lymphatic organ were conducted using Kruskal–Wallis Test with Dunn’s post hoc. MCAo indicates middle cerebral artery occlusion; and TCR, T cell receptor. Significance levels are marked as follows: Peyer’s patches (PP) $P≤0.05; $$P≤0.01; $$$P≤0.001; spleen *P≤0.05; **P≤0.01; ***P≤0.001; mesenteric lymph nodes (MLN) #P≤0.05; ##P≤0.01; ### P≤0.001.
Figure 6.
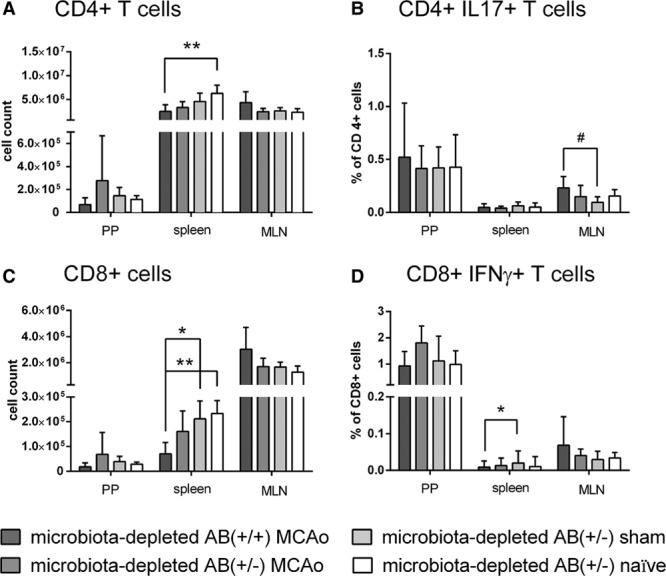
Flow cytometric analysis of immune cell populations from Peyer’s Patches, spleen, and mesenteric lymph nodes (MLN) on day 5 after cerebral ischemia. Microbiota-depleted mice show systemic immunodepression an day 5 after cerebral ischemia. Cell counts for (A) CD4+ cells and (C) CD8+ cells. (B) Percentage of CD4+ cells producing interleukin 17 and (D) percentage of CD8+ cells producing interferon gamma. Data are expressed as mean+standard deviation (SD). No statistically significant differences were found between group with continuous antibiotic treatment, and group with antibiotics stopped before surgery. Microbiota-depleted AB(+/+) MCAo n=8 (n=7 for Peyer’s patches), microbiota-depleted AB(+/-) MCAo n=7, microbiota-depleted AB(+/-) sham n=6, and microbiota-depleted AB(+/-) naïve n=6. Statistical analyses comparing the groups within one lymphatic organ were conducted using Kruskal–Wallis Test with Dunn’s post hoc. MCAo indicates middle cerebral artery occlusion. Significance levels are marked as follows: Peyer’s patches (PP) $P≤0.05; $$P≤0.01; $$$P≤0.001; spleen *P≤0.05; **P≤0.01; ***P≤0.001; mesenteric lymph nodes (MLN) #P≤0.05; ##P≤0.01; ### P≤0.001.
Discussion
We aimed at investigating the effects of microbiota depletion with broad-spectrum antibiotics on the outcome of experimental stroke. We hypothesized that uncompromised gut microbiota acts as an important modulator of stroke outcome. We applied a well-characterized murine model of experimental stroke in animals after depletion of microbiota with broad-spectrum antibiotics frequently used in intensive care medicine. Our main findings were that the absence of cultivatable microbiota at the time of induction of focal cerebral ischemia (1) does not affect infarct sizes 1 day later and (2) induces excessive mortality manifesting between days 5 and 7 (3) and that this mortality was prevented by continuous antibiotic treatment or by the recolonization of microbiota-depleted mice with a complex conventional intestinal microbiota from SPF littermates. This is the first demonstration that gut microbiota can affect outcome after acute CNS injury.
Stroke may interfere with normal gut microbiota–host interaction on many levels. After a brain lesion, the CNS engages in intense signaling with the immune system, resulting in a decrease of immune cell numbers and their functionality. This is termed stroke-induced immunodepression.14–16 Altered systemic immunity after CNS lesion may lead to the breakdown of mucosal barriers and to the translocation of bacteria and their products (such as bacterial cell wall constituents or toxins) to the host blood stream or lymphatic organs, further impacting the immune system and providing costimulation in deleterious immune–brain cell interaction. Furthermore, a direct impact of brain lesions on bacterial microbiota is expected through the effects of the sympathetic nervous system, as well as the vagus nerve: acute brain lesions affect the outflow of the autonomic nervous system. This may reduce gut motility and increase its permeability via the enteric nervous system and ultimately lead to bacterial translocation, infection, and sepsis. Antibiotic therapy in patients with brain lesions further disturbs the composition or even eradicates commensal bacterial communities and produces bacterial fragments, which may act as toxins and costimulants. Moreover, changes in nutrition, which might affect the gut microbiome diversity,33 are often a consequence of acute stroke, with dysphagia and unconsciousness mandating parenteral or tube feeding.
To date, only few studies have addressed the effects of brain lesions on the gut and its microbiota. In an experimental model of stroke, stress prior MCAo induced bacterial translocation and contributed to negative outcome.17 Schulte-Herbrüggen et al demonstrated in a mouse model of stroke similar to the one used in our study that 24 hours after focal cerebral ischemia, T- and B cell counts are reduced in the Peyer’s patches.34 Swidsinski et al found signs of ulcerative colitis and dramatic changes in gut microbiota composition in stool samples from patients with acute stroke,35 Hayakawa et al described a sudden decrease in commensal organisms and an increase in potentially harmful bacteria after severe insults, as is seen in cerebral vascular disease.36 Karlson et al reported that patients with symptomatic atherosclerosis have altered gut metagenome with enrichment in the Collinsella genus and reduced levels of β-carotene in serum.37
A prediction based on these previous reports would suggest a gut–brain interaction after stroke with possible negative effects on the acute outcome post ischemia. Our findings did not provide evidence for such deleterious effects, in particular on infarct volume, although minor to moderate effects could not be ruled out given the inherent high variability of modeling stroke in rodents and limitations in sample size. In addition, delayed (beyond day one) effects on infarct volume or recovery could not be ruled out in view of the high morbidity and mortality of the microbiota-depleted animals after MCAo.
In contrast to a putative deleterious role of a depleted gut microbiota after stroke, we found that a complex conventional intestinal microbiota (or continuous antibiotic treatment) protects the host, whereas a compromised physiological resistance to colonization after completed antibiotic treatment may lead to severe illness and even death.38 It is well known that stroke induces a long-lasting immunosuppression with lymphopenia and altered cellular immune function, impairing antibacterial defense in a conventionally colonized host. Prass et al demonstrated previously that MCAo animals have reduced counts of T, NK, and B cell subsets in spleen and reduced cytokine excretion (eg, IFNγ and TNFα) as measured by ex vivo tests as early as 12 hours after experimental stroke.39 After MCAo in wild-type animals not treated with antibiotics, T and B cell counts were significantly reduced in Peyer’s patches compared with sham-operated animals, while no differences were found for natural killer cells and macrophages. Moreover, no significant changes in intraepithelial and lamina propria lymphocytes subsets were observed after cerebral ischemia compared with controls.34 In our experiments, MCAo animals showed reduced counts of T and B cells and a reduced percentage of IFNγ secreting lymphocytes on day 5 after stroke as compared with naïve and sham mice. This was observed mainly in spleens and to lesser extent in Peyer’s patches. Because we found no significant differences between microbiota-depleted AB(+/−) and AB(+/+) MCAo animals in immune cell numbers or function in secondary lymphoid organs, antibiotic treatment is unlikely to have mitigated depressed immunity in the gut. Moreover, survival rates of microbiota-depleted AB(+/−) sham-operated animals were higher compared with microbiota-depleted AB(+/−) MCAo group, suggesting that stroke-induced immunodepression–mediated systemic immune response syndrome might contribute to poor stroke outcome in the microbiota-depleted state.
Although the immunodepression observed after MCAo may promote bacterial infections, it does not provide a direct mechanistic explanation for the development of severe colitis in the absence of continuous antibiotic treatment. In a murine model of chronic psychosocial stress, which is known to lead to spontaneous colitis, Reber et al recently observed that animals rapidly developed mucosal immunosuppression and epithelial barrier defects associated with increased bacterial load in intestinal tissue shortly after stress onset. Later development of colitis was associated with hyperreactivity of mucosal immune cells in the elevated presence of bacterial antigens, both of which can be prevented by prolonged antibiotic treatment before and during chronic stress.40 Whether similar mechanisms are operational in our model of MCAo-associated colitis needs to be further elucidated.
Our study in a murine model of stroke in which we simulated the rather unphysiological scenario of virtually depleted microbiota, nevertheless raises some intriguing clinical questions because intestinal microbiota may contribute to stroke outcome in antibiotic-treated patients. Our findings indicate that the presence of an uncompromised complex intestinal microbiota may be critical for the outcome after cerebral ischemia. In the clinical scenario, considering pooled poststroke bacterial infection rate of 30% in the first week after stroke onset,1 it is probable that many stroke patients are treated with antibiotics. Representative data of large stroke populations are missing. Nevertheless, in the recently published large PASS trial investigating the effect of preventive antibiotics in acute stroke on 3-month outcome, 20% of stroke patients in the control group were treated with antibiotics.41 Because ischemic brain lesion in many of these patients produced an immunodepressed state, they may be prone to recolonization by facultative or obligatory bacterial pathogens. This would further increase their risk of infection (in particular pneumonia).
We speculate that understanding gut microbiota–brain cross talk will contribute to a better understanding of the pathophysiology of acute CNS disorders and related complications and may lead to the improvement of current clinical practice or entirely new treatment strategies.
Acknowledgments
We thank Sabine Kolodziej, Yvonne Amoneit, Silvia Schulze, and Gernot Reifenberger for excellent technical assistance and Catherine Aubel for proofreading the article.
Sources of Funding
Dr Winek received a stipend from the International Max Planck Research School for Infectious Diseases and Immunology (IMPRS-IDI) and the Sonnenfeld-Stiftung. The study was financially supported through the German Research Foundation (Exc257; SFB633, SFB-TR84), the Federal Ministry of Education and Research (01EO0801), and the European Community’s Seventh Framework Programme (FP7/2007–2013; grant agreement no. 201024).
Disclosures
None.
Supplementary Material
Footnotes
Guest Editor for this article was Eng H. Lo, PhD.
Current address for C.C.: Immunology Department, The Weizmann Institute of Science, Rehovot, Israel.
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.115.011800/-/DC1.
References
- 1.Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurol. 2010;9:105–118. doi: 10.1016/S1474-4422(09)70266-2. doi: 10.1016/S1474-4422(09)70266-2. [DOI] [PubMed] [Google Scholar]
- 3.Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci U S A. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 7.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AK, Ling F, Kaas A, Funda DP, Farlov H, Buschard K. Diabetes preventive gluten-free diet decreases the number of caecal bacteria in non-obese diabetic mice. Diabetes Metab Res Rev. 2006;22:220–225. doi: 10.1002/dmrr.609. doi: 10.1002/dmrr.609. [DOI] [PubMed] [Google Scholar]
- 11.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 15.Dirnagl U, Klehmet J, Braun JS, Harms H, Meisel C, Ziemssen T, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2 suppl):770–773. doi: 10.1161/01.STR.0000251441.89665.bc. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 16.Chamorro Á, Meisel A, Planas AM, Urra X, van de Beek D, Veltkamp R. The immunology of acute stroke. Nat Rev Neurol. 2012;8:401–410. doi: 10.1038/nrneurol.2012.98. doi: 10.1038/nrneurol.2012.98. [DOI] [PubMed] [Google Scholar]
- 17.Caso JR, Hurtado O, Pereira MP, García-Bueno B, Menchén L, Alou L, et al. Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am J Physiol Regul Integr Comp Physiol. 2009;296:R979–R985. doi: 10.1152/ajpregu.90825.2008. doi: 10.1152/ajpregu.90825.2008. [DOI] [PubMed] [Google Scholar]
- 18.Tascilar N, Irkorucu O, Tascilar O, Comert F, Eroglu O, Bahadir B, et al. Bacterial translocation in experimental stroke: what happens to the gut barrier? Bratisl Lek Listy. 2010;111:194–199. [PubMed] [Google Scholar]
- 19.Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gómez-Choco M, et al. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 22.Sommer F, Bäckhed F. The gut microbiota–masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 23.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kühl AA, et al. Novel murine infection models provide deep insights into the “ménage à trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirnagl U Members of the MCAO-SOP Group. Standard operating procedures (SOP) in experimental stroke research: SOP for middle cerebral artery occlusion in the mouse. Nature Precedings. Acessed October 13, 2015. doi: 10.1038/npre.2010.3492.2. [Google Scholar]
- 26.Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 27.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 28.Tauber SC, Nau R. Immunomodulatory properties of antibiotics. Curr Mol Pharmacol. 2008;1:68–79. [PubMed] [Google Scholar]
- 29.Schubert S, Andresen BH, Bähr V, Fischer L, Stamp R, Stricker G, et al. The immunomodulatory effects of antibiotics: in vitro and ex vivo investigations of 21 substances by means of the lymphocyte transformation test. Zentralbl Bakteriol. 1996;284:402–438. doi: 10.1016/s0934-8840(96)80116-2. [DOI] [PubMed] [Google Scholar]
- 30.Stock ML, Fiedler KJ, Acharya S, Lange JK, Mlynarczyk GS, Anderson SJ, et al. Antibiotics acting as neuroprotectants via mechanisms independent of their anti-infective activities. Neuropharmacology. 2013;73:174–182. doi: 10.1016/j.neuropharm.2013.04.059. doi: 10.1016/j.neuropharm.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 31.Lu JY, Sadri N, Schneider RJ. Endotoxic shock in AUF1 knockout mice mediated by failure to degrade proinflammatory cytokine mRNAs. Genes Dev. 2006;20:3174–3184. doi: 10.1101/gad.1467606. doi: 10.1101/gad.1467606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Knight R. Dietary effects on human gut microbiome diversity. Br J Nutr. 2015;113(suppl):S1–S5. doi: 10.1017/S0007114514004127. doi: 10.1017/S0007114514004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulte-Herbrüggen O, Quarcoo D, Meisel A, Meisel C. Differential affection of intestinal immune cell populations after cerebral ischemia in mice. Neuroimmunomodulation. 2009;16:213–218. doi: 10.1159/000205514. doi: 10.1159/000205514. [DOI] [PubMed] [Google Scholar]
- 35.Swidsinski A, Loening-Baucke V, Krüger M, Kirsch S. Central nervous system and the colonic bioreactor: analysis of colonic microbiota in patients with stroke unravels unknown mechanisms of the host defense after brain injury. Intest Res. 2012;10:332–342. [Google Scholar]
- 36.Hayakawa M, Asahara T, Henzan N, Murakami H, Yamamoto H, Mukai N, et al. Dramatic changes of the gut flora immediately after severe and sudden insults. Dig Dis Sci. 2011;56:2361–2365. doi: 10.1007/s10620-011-1649-3. doi: 10.1007/s10620-011-1649-3. [DOI] [PubMed] [Google Scholar]
- 37.Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3:1245. doi: 10.1038/ncomms2266. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reber SO, Peters S, Slattery DA, Hofmann C, Schölmerich J, Neumann ID, et al. Mucosal immunosuppression and epithelial barrier defects are key events in murine psychosocial stress-induced colitis. Brain Behav Immun. 2011;25:1153–1161. doi: 10.1016/j.bbi.2011.03.004. doi: 10.1016/j.bbi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Westendorp WF, Vermeij JD, Zock E, Hooijenga IJ, Kruyt ND, Bosboom HJ, et al. PASS investigators. The Preventive Antibiotics in Stroke Study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385:1519–1526. doi: 10.1016/S0140-6736(14)62456-9. doi: 10.1016/S0140-6736(14)62456-9. [DOI] [PubMed] [Google Scholar]


