Abstract
The healthy aging process affects the ability to learn and remember new facts and tasks. Prior work has shown that motor learning can be adversely affected by non-motor deficits, such as time. Here we investigated how age, and a dual task influence the learning and forgetting of a new walking pattern. We studied healthy younger (<30 yo) and older adults (>50 yo) as they alternated between 5-minute bouts of split-belt treadmill walking and resting. Older subjects learned a new walking pattern at the same rate as younger subjects, but forgot some of the new pattern during the rest breaks. We tested if forgetting was due to reliance on a cognitive strategy that was not fully engaged after rest breaks. When older subjects performed a dual cognitive task to reduce strategic control of split-belt walking, their adaptation rate slowed, but they still forgot much of the new pattern during the rest breaks. Our results demonstrate that the healthy aging process weakens motor memories during rest breaks and that this phenomenon cannot be explained solely by reliance on a conscious strategy in older adults.
Introduction
The ability to recall motor skills is important for our everyday lives. Anecdotally, we know there are certain motor skills we never forget after they are mastered, such as how to ride a bike or drive a car. However, studies of other types of learning (e.g., declarative learning) demonstrate that memories can be weakened as time elapses (See (Backman et al. 2001) for review). Age has been shown to be an important factor for declarative memory; healthy older subjects forget things more easily than younger ones (see (LaVoie and Cobia 2007) for review).
Does healthy aging affect our ability to recall motor memories? Specifically, we asked how motor memories created through adaptation are influenced by age, time and dual task demands. The effects of healthy aging have previously been studied in both skill tasks (i.e., learning tasks that require the acquisition of a new pattern of muscle activations (Krakauer 2009; Robertson et al. 2004)) and in adaptive learning (Anguera et al. 2011). Some studies have shown that motor learning is similar between young and old subjects (Bock and Schneider 2002; Roller et al. 2002; Huang and Ahmed, 2014), while others show degradation of learning in older healthy adults (Anguera et al. 2011; Fernandez-Ruiz et al. 2000; Jordan 1978; McNay and Willingham 1998; Warabi et al. 1986; Wright and Payne 1985; Huang & Ahmed 2014). One explanation for the discrepancies in the literature is the extent to which different motor learning tasks engage explicit strategies. Explicit learning can be impaired in older compared to younger adults, whereas implicit, non-strategic, recalibration mechanisms may remain intact (Bock 2005; McNay and Willingham 1998). Thus, one hypothesis is that motor learning tasks that can involve more cortical, strategic planning should show greater differences due to aging (Anderson et al. 1998; Anguera et al. 2011).
Here we investigated age-related effects on both the ability to adapt to a walking perturbation and the ability to recall the walking pattern following rest breaks during learning. Adaptation is an error-driven process that adjusts existing sensorimotor mappings of well-learned movements to account for new, predictable demands (Martin et al. 1996). Walking is a behavior that relies less on cortical processing compared with other motor learning tasks that are typically studied in aging (e.g. reaching, finger sequencing). Our well-characterized walking adaptation paradigm perturbs subjects via a split-belt treadmill by driving one leg faster than the other (Reisman et al. 2005). We first asked if there were differences between young and older subjects in the rate and extent of their adaptation. We then asked if the passage of time weakened the learned motor pattern in young and older healthy adults. Finally, we used a dual task to reduce any explicit or strategic components to the walking adaptation, since those processes might be degraded during healthy aging. Our results suggest that aging is associated with a loss of motor memory over short time periods that cannot be explained by a reliance on explicit or strategic processes.
Materials and Methods
Subjects
Thirty healthy volunteers (11 males, 19 females) participated in this study. All subjects gave informed written consent before participating. The protocols were approved by the Johns Hopkins Institutional Review Board.
Experimental protocol
Split-belt walking adaptation was studied using a custom-built treadmill (Woodway, Waukesha, WI). The treadmill had two separate belts driven by independent motors – these belts could be driven at the same speed (“tied-belts”) or at different speeds (“split-belts”). Speed commands for each belt were sent to the treadmill through a custom MATLAB (MathWorks, Natick, MA) computer interface. Subjects were positioned in the middle of the treadmill with one leg on each belt and wore a safety harness that was suspended from the ceiling. The safety harness was adjusted such that it would catch subjects if they fell, but it did not support their body weight while they stood. At the beginning of each trial, subjects were not informed of the upcoming speeds of the treadmill belts and were told to refrain from looking down at the belts. Subjects held onto a ground-referenced rail while the belts were moving. During breaks, subjects remained on the treadmill (either standing or seated).
The experimental paradigm was the same for all subjects (Figure 1B). Subjects were naïve to the task and began the experiment with three one minute tied-belt trials (1.0 m/s, 0.5 m/s, 1.0 m/s). Then everyone was exposed to three five-minute exposures to split belts (0.5 ms/ and 1.0 m/s, with each subject's dominant leg on the slow belt). After each split-belt trial, subjects had a five-minute rest break. Subjects were allowed to either sit or stand on the treadmill without walking during these breaks. Once subjects completed the third exposure to the split-belts, they received another break and then were de-adapted on tied belts at 0.5 m/s for five minutes.
Figure 1.
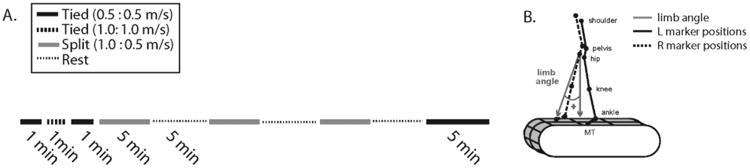
A. Diagram of marker location and limb angle convention. B. Experimental paradigm showing the periods of split-belt waking in gray lines and tied walking in black. All subjects sat or stood for five minutes between adaptation blocks.
First, we tested for the effect of age on locomotor adaptation and forgetting. All participants were classified based on age. Subjects in the ‘Younger’ group were less than 30 years of age (N=10, mean age=22.5 years, standard deviation=2.6 years). Subjects in the ‘Older’ group were over 50 years of age (N=10, mean age=54.9, standard deviation=2.8 years).
Then, we investigated the role of conscious processes on adaptation and forgetting of healthy older adults. We compared our ‘Older’ group to a new group of healthy older adults, ‘Older Distraction’ (N=10, mean age=52.8, standard deviation=5.8 years). While the ‘Older’ subjects were given no instructions during adaptation, the subjects in the ‘Older Distraction’ group were given a dual-task to complete during their split-belt adaptation periods (Malone and Bastian 2010). The ‘Older Distraction’ group watched a television program unrelated to walking and were instructed to count the number of times a particular word was said using a hand-held counter. Additionally, they were asked to focus their attention on the television program so that they could answer questions about the program's visual scenes after the adaptation block finished. Therefore, these subjects were distracted by both audio and visual stimuli.
Data collection
Kinematic data were collected at 100 Hz using Optotrak (Northern Digital, Waterloo, ON). Infared-emitting markers were placed bilaterally over the toe (fifth metatarsal head), ankle (lateral malleolus), knee (lateral femoral epicondyle), hip (greater trochanter), pelvis (iliac crest), and shoulder (acromion process) (Figure 1a). Voltages reflecting treadmill belt speeds were recorded directly from treadmill motor output at 1000 Hz. Marker position and analog data (treadmill belt speeds) were synchronized and sampled simultaneously using Optotrak software. Heel strike times were approximated using the maximum angle of the limb (Figure 1A); toe-off time was approximated to be the minimum limb angle.
Data Analysis
In this study, our primary measurement was step length symmetry, which has previously been shown to adapt robustly to split-belt walking (Choi and Bastian 2007; Choi et al. 2009; Malone and Bastian 2010; Reisman et al. 2005; Reisman et al. 2007; Reisman et al. 2009; Vasudevan and Bastian 2010). Step symmetry (SS) was defined as the normalized difference between the step lengths (SL) of the ‘fast’ and ‘slow’ leg:
A positive step symmetry value means that the fast step was larger than the slow step, and vice versa for negative values. A value of 0 indicates symmetry. Normalization by dividing by the sum of the step lengths was done so subjects who take different size steps could be compared. We chose this as our primary measure since our previous work suggested that it is a “global” measure of walking coordination (Malone and Bastian 2010).
The initial perturbation was defined as the average of the first five strides of each exposure. Plateau values were calculated by averaging the last 30 strides of each exposure. After-effects were defined as the average of the first five strides of de-adaptation. To assess the influence of time, we calculated the amount of ‘forgetting’ as the perturbation after a rest break relative to the plateau in performance before the break. Rates of adaptation and de-adaptation were quantified using epochs of five strides for the first 50 strides in adaptation and de-adaptation.
Statistical analysis
Repeated measures ANOVAs were used to compare adaptation and de-adaptation rates between the groups. One way ANOVAs were used to compare baseline values, initial perturbation, after effects and plateaus across groups. Two-way repeated measures ANOVAs were used to “forgetting” across groups. Post-hoc analyses were performed using Fisher's LSD test. Linear regressions were used to investigate the relationship between break 1 and break 2 forgetting for each group. Statistica (StatSoft, Tulsa, OK) was used for all statistical analysis and the alpha level was set at p = 0.05.
Results
All subjects, regardless of group assignment, were able to complete the walking task without difficulty.
We first checked if there were any differences in baseline walking across groups, and found no difference in step symmetry in the last 30 s of the second ‘slow’ tied period, (F(2,27) =0.57, p=0.57). Subjects' mean baseline values from the last 30 s of this second ‘slow’ tied period were subtracted from all subsequent data, thus referencing all data to each subject's initial baseline. Figure 2 shows our results for step symmetry across exposures. We saw typical adaptation behavior in the first exposure, where subjects in all groups were initially perturbed by the split-belt and returned towards symmetric walking with increasing stride number. All groups were similarly perturbed upon the first exposure to the split-belts (F(2,27)=0.782, p=0.47). By the end of the first five-minute exposure, however, they reached different plateaus (F(2,27)=7.17, p=0.003); the ‘Older Distraction’ group was significantly more asymmetric than the ‘Older’ group (p<0.01) and the ‘Younger’ group (p<0.01). However, the ‘Younger’ and ‘Older’ group were not significantly different at the end of exposure 1 (p=0.63).
Figure 2.
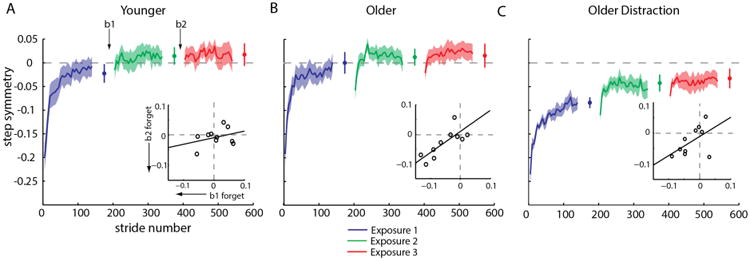
Comparison of step symmetry adaptation across groups. Group adaptation curves (smoothed by 5 strides) and shaded standard error regions are shown. Plateau values for each exposure are plotted. Inset shows the correlation between forgetting during break 1 and 2 for each group. A. ‘Younger’ subjects showed no forgetting during ‘rest’ breaks. Notice that the first point of the subsequent exposure is not different from the plateau preceding it. B. ‘Older’ subjects demonstrated time-related forgetting. Upon re-exposure to the split-belts, the ‘Older’ group showed fast re-adaptation transients. There is a significant positive correlation between the forgetting during break 1 and 2 for ‘Older’ subjects. C. The ‘Older Distraction’ group shows a slower adaptation rate in exposure 1, but forgetting still occurred during the ‘rest’ breaks. They also never achieve complete symmetry during adaptation.
We assessed the adaptation rate for each group using a repeated measures ANOVA on the first 50 strides, dividing the curves into epochs of five strides (Figure 3). The analysis revealed an effect of epoch (F(9,243)=54.07, p<0.001) and a group effect (F(2,27)=5.65, p<0.01), but not an epoch by group interaction (F(18,243)=1.38, p=0.138). Post hoc analysis of the Group effect showed that the ‘Older Distraction’ group was significantly different from both the ‘Older’ (p<0.01) and ‘Younger’ (p=0.02) groups; however, the ‘Older’ and ‘Younger’ groups were not different form each other (p=0.49). This indicates that the ‘Older Distraction’ group adapted to the split-belts, but they adapted at an overall slower rate and therefore were more asymmetric at the end of the first exposure (Figure 3A & Figure 2C). In contrast, the ‘Younger’ and ‘Older’ groups adapted similarly to the perturbation and adapted the same amount during the first exposure; therefore, we concluded that behavior during the first exposure was the same across these two groups.
Figure 3.
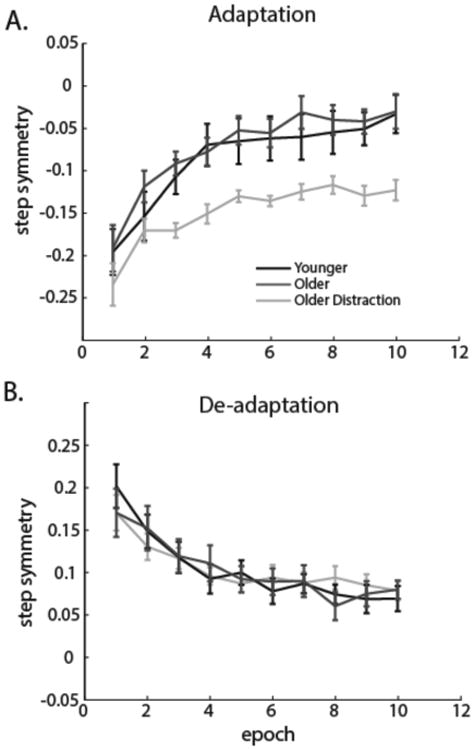
Adaptation and de-adaptation rates are shown. Epochs of five strides are shown for the first 50 strides in adaptation and de-adaptation. No age-related differences were found for the rate of adaptation (A) or de-adaptation (B). There was significant slowing in the adaptation rate for the ‘Older Distraction’ group compared to the ‘Older’ group (A), but not in the de-adaptation rate (B).
Although initial adaptation behavior during Exposure 1 was the same across the ‘Younger’ and ‘Older’ groups, we found differences in how time affected the learned locomotor pattern during the breaks. We quantified the ‘forgetting’ as the difference between the step symmetry plateau of the previous exposure and the initial perturbation (i.e., first five strides, first point in Figure 2) of the following exposure. These ‘forgetting’ values are shown for Breaks 1 and 2 in Figure 4. Repeated measures ANOVA showed, we found there was a significant group effect (F(2,27)= 3.34, p=0.048), but not an epoch effect (F(1,27)=0.28, p=0.60) or an epoch by group interaction effect (F(2,27)=2.83, p=0.08). Investigating these differences further with post hoc tests, we found there was a significant difference between ‘Younger’ and ‘Older’ for the first break (p<0.01), but not the second (p=0.22). Notice that the ‘Younger’ group immediately returns to the learned walking pattern (i.e., no change in step symmetry after Break 1), whereas the ‘Older’ group shows increased step symmetry errors at the beginning of exposure 2.
Figure 4.
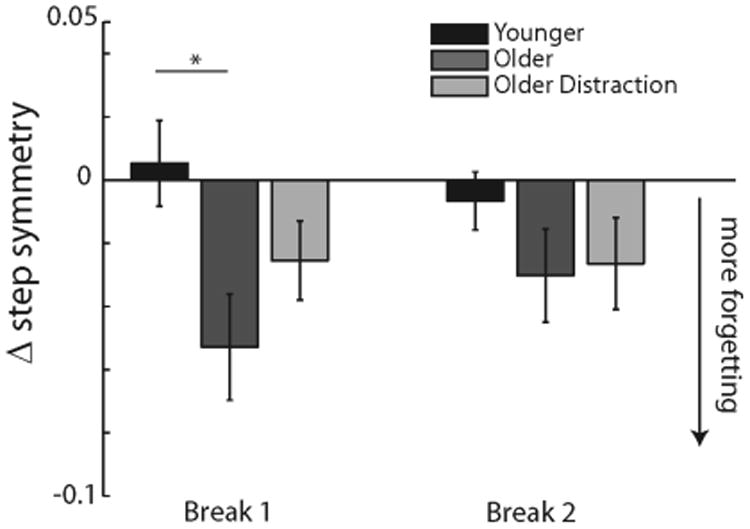
Forgetting values. The ‘Older’ healthy subjects had significantly more forgetting during break 1 than the ‘Younger’ subjects. Distraction did not significantly reduce the amount of forgetting found in the healthy older adults. * p<0.05
Given that older subjects adapted more slowly when conscious efforts were reduced and attention was diverted away from adaptation, we next tested how forgetting was affected. Comparing the ‘Older’ and ‘Older Distraction’ group with post hoc tests, we saw a slight decreased in forgetting; however, the ‘Older Distraction’ group was not significantly different than the ‘Older’ group for either break 1 (p=0.10) or break 2 (p=0.85). Neither was the ‘Older Distraction’ different than the ‘Younger’ group (p=0.09 for break 1 and p=0.31 for break 2).
We then asked if there was any consistency in the amount that individuals forgot during breaks 1 and 2 via correlation (Figure 2, insets). There was no correlation for the ‘Younger’ group. (p= 0.37), which is not surprising since they did not forget much during the breaks. The ‘Older’ group showed a clear positive correlation (p<0.01, r = 0.77), such that individuals who forgot during break 1 also forgot a proportional amount during break 2. The ‘Older Distraction’ group showed similar behavior to the ‘Older’ group, though this did not reach significance (p=0.11) (Figure 2 insets). Despite the ‘forgetting’ exhibited during the rest breaks, all groups eventually plateaued to similar step symmetry values by the end of Exposure 3 (F(2,27)=1.76, p=0.19).
In de-adaptation we did not see differences across our groups (Figure 3B). All groups showed similar after-effects (F(2,27)=0.51, p=0.61). Repeated measures ANOVA revealed a significant epoch effect in de-adaptation (F(9,243)=37.15, p<0.001) but no group effect (F(2,27)=0.01, p=0.99) and no epoch by group interaction (F(18,273)=1.22, p=0.24). In other words, all groups stored and de-adapted the walking pattern at similar rates.
Discussion
We have shown that healthy aging does not affect the rate of adaptation for locomotor learning. It should be noted that our older subjects were, on average, in their early 50s, which would not normally be considered elderly (i.e. 65 and older). Yet, we found clear differences in their behavior compared to young adult participants. Healthy older adults forget some of the learned locomotor pattern during rest breaks, whereas younger adults did not. We also demonstrated that this forgetting is not due solely to degraded strategic control, since distracting older subjects during adaptation slowed down learning but did not abolish forgetting.
First, we investigated how healthy older adults adapted their walking pattern compared to younger adults. We found that both young and older subjects adapted and de-adapted at similar rates, and stored comparable amounts, assessed as after-effects. This contrasts with a prior study of prism goggle adaptation, where they found that healthy older subjects adapted arm movements significantly more slowly than younger subjects but retained the learned calibration longer (Fernandez-Ruiz et al. 2000). In a more recent study, researchers showed that older adults adapted reaching movements less to a force-field perturbation (Huang and Ahmed 2014). Our work differs from these studies in two ways. First, walking differs from reaching in terms of the behavior and the neural circuits involved. Walking is a bilateral behavior and the consequence of failure (i.e., falling) is significant. It is also controlled by lower motor centers compared to reaching, which may change the dynamics of this learning. Second, the elderly subjects studied by Fernandez-Ruiz (2000) and Huang (2014) were older than those tested in our study (theirs averaged 64 and 73 years old; ours averaged 55 and 53 years old). It might be that an older population tested using our paradigm would adapt more slowly and to a lesser extent.
Despite the fact that our subjects were younger than those typically studied in aging, there was clear forgetting during rest breaks: healthy older adults forgot some of the learned walking pattern whereas younger subjects did not. Because adaptation involves both conscious and subconscious processes (Malone and Bastian 2010), we thought that our older subjects might be relying more heavily on conscious processes for controlling their walking pattern, particularly in challenging situations like on a split-belt treadmill. Indeed it is known that as people age, they increase activation of cerebral cortical areas such as multisensory cortices and dorsolateral prefrontal cortex for sensorimotor tasks (Heuninckx et al. 2005) and locomotion (Zwergal et al. 2010). This suggests that they use more cognitive strategies in performing sensorimotor tasks. We thought that the ‘forgetting’ we observed could be due to older subjects relying on more explicit strategies that they disengaged during the breaks. In particular, one recent study used a two-state multi-rate model of adaptive learning (Smith et al. 2006) to show that although younger and older subjects seemed to adapt equally well to a force-field reaching adaptation task, the underlying learning dynamics differed (Trewartha et al. 2014). Specifically, older adults had decreased retention of the slow learning component, but perhaps some older adults could use the fast component to help compensate for this impairment since this was similar between older and younger adults (Trewartha et al. 2014). Additionally, they did show that the fast component was correlated with an explicit memory task (Trewartha et al. 2014), which suggests that older adults might rely on more conscious strategies to drive adaptation since they have impairments in more subcortical processes. However, while possibly true for other tasks, we do not believe the forgetting seen here is due to older subjects relying on explicit strategies in locomotor adaptation; when we distracted healthy older subjects we slowed down the adaptation rate, but the forgetting remained.
Here we saw that distraction affected adaptation rate but not the de-adaptation rate, which is slightly different from a prior study (Malone and Bastian 2010), where we showed that distraction slowed both adaptation and de-adaptation rate. One possible reason for this finding is that the subjects in this study received a smaller split-belt perturbation (2:1 vs. 3:1), which allows subjects to adapt and de-adapt more quickly. It is possible that we could not see differences in the de-adaptation rate because subjects de-adapted too quickly here.
In this work, we cannot pin down which underlying neural changes make motor memories of older subjects less resistant to time. One possible site of neural changes is in the cerebellum, since locomotor adaptation requires cerebellar integrity (Morton and Bastian 2006). Age-related Purkinje cell loss has been demonstrated (Ogata et al. 1984; Rogers et al. 1984; Woodruff-Pak 2006; Woodruff-Pak et al. 2010), in addition to atrophy of the Purkinje cell soma (Ogata et al. 1984) and reduction and atrophy of dendritic arborizations (Quackenbush et al. 1990; Zhang et al. 2006). Additionally, neural changes that occur in the cerebral cortex (e.g., motor cortex or prefrontal cortex) could also be responsible for the age-related forgetting that we see. Although numerous brain areas show age-related reductions (Fjell and Walhovd 2010), frontal lobes show a rapid rate of white matter volume decrease compared to other brain areas (Allen et al. 2005; Madden et al. 2009).
In sum, this study demonstrates that aging can affect motor memories on a short time scale (i.e. over the passage of minutes), without impairing the rate or extent of motor learning. This deficit does not appear to be due to an increased reliance on strategic control of walking in the older subjects. Future studies will be aimed at understanding how motor memories in healthy older adults and patient populations are affected by longer periods of times (i.e., days to week), which is important for designing rehabilitation techniques.
Acknowledgments
This work was supported by NIH grant R01 HD048741 and R37 NS090610.
Footnotes
Conflict of Interest: None
Reference List
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Craik FI, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: 1. Evidence from divided attention costs. Psychol Aging. 1998 Sep;13(3):405–23. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2011 Jan;23(1):11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Bock O. Components of sensorimotor adaptation in young and elderly subjects. Exp Brain Res. 2005;160:259–263. doi: 10.1007/s00221-004-2133-5. [DOI] [PubMed] [Google Scholar]
- Bock O, Schneider S. Sensorimotor adaptation in young and elderly humans. Neurosci Biobehav Rev. 2002;26:761–767. doi: 10.1016/s0149-7634(02)00063-5. [DOI] [PubMed] [Google Scholar]
- Backman L, Small BJ, Wahlin A. Aging and memory: cognitive and biological perspectives. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. San Diego, CA: Academic Press; 2001. pp. 349–377. [Google Scholar]
- Choi JT, Bastian AJ. Adaptation reveals independent control networks for human walking. Nat Neurosci. 2007;10:1055–1062. doi: 10.1038/nn1930. [DOI] [PubMed] [Google Scholar]
- Choi JT, Vining EP, Reisman DS, Bastian AJ. Walking flexibility after hemispherectomy: split-belt treadmill adaptation and feedback control. Brain. 2009;132:722–733. doi: 10.1093/brain/awn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Hall C, Vergara P, Diiaz R. Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Brain Res Cogn Brain Res. 2000;9:223–226. doi: 10.1016/s0926-6410(99)00057-9. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21:187–221. doi: 10.1515/revneuro.2010.21.3.187. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HJ, Ahmed AA. Older adults learn less, but still reduce metabolic cost, during motor adaptation. J Neurophysiol. 2014;111:135–144. doi: 10.1152/jn.00401.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan T. Age differences in visual and kinesthetic short-term memory. Percept Mot Skills. 1978;46:667–674. doi: 10.2466/pms.1978.46.2.667. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol. 2009;629:405–421. doi: 10.1007/978-0-387-77064-2_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie DJ, Cobia DJ. Recollecting, recognizing, and other acts of remembering: an overview of human memory. J Neurol Phys Ther. 2007;31:135–144. doi: 10.1097/NPT.0b013e31814a63e8. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–435. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: Effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(Pt 4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- McNay EC, Willingham DB. Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn Mem. 1998;4:411–420. doi: 10.1101/lm.4.5.411. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci. 2006;26:9107–9116. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R, Ikari K, Hayashi M, Tamai K, Tagawa K. Age-related changes in the Purkinje's cells in the rat cerebellar cortex: a quantitative electron microscopic study. Folia Psychiatr Neurol Jpn. 1984;38:159–167. doi: 10.1111/j.1440-1819.1984.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Quackenbush LJ, Ngo H, Pentney RJ. Evidence for nonrandom regression of dendrites of Purkinje neurons during aging. Neurobiol Aging. 1990;11:111–115. doi: 10.1016/0197-4580(90)90043-y. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol. 2005;94:2403–2415. doi: 10.1152/jn.00089.2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain. 2007;130:1861–1872. doi: 10.1093/brain/awm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–744. doi: 10.1177/1545968309332880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Miall RC. Current concepts in procedural consolidation. Nat Rev Neurosci. 2004;5:576–582. doi: 10.1038/nrn1426. [DOI] [PubMed] [Google Scholar]
- Rogers J, Zornetzer SF, Bloom FE, Mervis RE. Senescent microstructural changes in rat cerebellum. Brain Res. 1984;292:23–32. doi: 10.1016/0006-8993(84)90886-2. [DOI] [PubMed] [Google Scholar]
- Roller CA, Cohen HS, Kimball KT, Bloomberg JJ. Effects of normal aging on visuo-motor plasticity. Neurobiol Aging. 2002;23:117–123. doi: 10.1016/s0197-4580(01)00264-0. [DOI] [PubMed] [Google Scholar]
- Seidler RD. Differential effects of age on sequence learning and sensorimotor adaptation. Brain Res Bull. 2006 Oct 16;70(4-6):337–46. doi: 10.1016/j.brainresbull.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Trewartha KM, Garcia A, Wolpert DM, Flanagan JR. Fast but fleeting: adaptive motor learning processes associated with aging and cognitive decline. J Neurosci. 2014 Oct 1;34(40):13411–21. doi: 10.1523/JNEUROSCI.1489-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol. 2010;103:183–191. doi: 10.1152/jn.00501.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warabi T, Noda H, Kato T. Effect of aging on sensorimotor functions of eye and hand movements. Exp Neurol. 1986;92:686–697. doi: 10.1016/0014-4886(86)90309-2. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS. Stereological estimation of Purkinje neuron number in C57BL/6 mice and its relation to associative learning. Neuroscience. 2006;141:233–243. doi: 10.1016/j.neuroscience.2006.03.070. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, Comalli DM, Kennard JA, Agelan A, Thompson RF. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 2010;107:1624–1629. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright BM, Payne RB. Effects of aging on sex differences in psychomotor reminiscence and tracking proficiency. J Gerontol. 1985;40:179–184. doi: 10.1093/geronj/40.2.179. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hua T, Zhu Z, Luo X. Age-related changes of structures in cerebellar cortex of cat. J Biosci. 2006;31:55–60. doi: 10.1007/BF02705235. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Linn J, Xiong G, Brandt T, Strupp M, Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]


