Abstract
Refinement of micro- and nanofabrication in the semiconductor field has led to innovations in biomedical technologies. Nanotopography, in particular, shows great potential in facilitating drug delivery. The flexibility of fabrication techniques has created a diverse array of topographies that have been developed for drug delivery applications. Nanowires and nanostraws deliver drug cytosolically for in vitro and ex vivo applications. In vivo drug delivery is limited by the barrier function of the epithelium. Nanowires on microspheres increase adhesion and residence time for oral drug delivery, while also increasing permeability of the epithelium. Low aspect ratio nanocolumns increase paracellular permeability, and in conjunction with microneedles increase transdermal drug delivery of biologics in vivo. In summary, nanotopography is a versatile tool for drug delivery. It can deliver directly to cells or be used for in vivo delivery across epithelial barriers. This editorial highlights the application of nanotopography in the field of drug delivery.
Keywords: drug delivery, nanofabrication, nanotopography, oral drug delivery, siRNA, surfaces, transdermal drug delivery
1. Introduction
Innovations in the semiconductor industry have expanded the repertoire and pushed the limits of resolution of micro- and nanofabrication. Nanotopography is a sub-micron-textured surface where the surface features have at least one dimension on the nanoscale. Cells innately interact and respond to micro- and nanoscale cues presented by the extracellular matrix by changing gene expression, cellular morphology and adhesion.[1] The range of available topography geometry and fabrication methods has led to applications in multiple modalities of drug delivery, including intracellular and transcellular delivery, both via oral and transdermal delivery methods. Nanotopography, therefore, represents a versatile new tool in the field with the potential to improve upon harsher or more complex chemical and physical delivery techniques.
2. Intracellular delivery
Intracellular delivery of drugs, ranging from small RNAs up to macromolecules, holds powerful therapeutic potential. Nanotechnology has already shown promise in targeted drug delivery for a number of disease models.[2] Within this field, several distinct nanotopographies have demonstrated robust intracellular delivery of drug via different modalities. First, Dalby et al. developed nanotopography that increases drug delivery by activating endocytosis.[3] Fabrication of the nanocolumns was achieved through colloidal lithography, whereby a colloidal mask was electrostatically adsorbed followed by ion beam etching to create nanopillar topography. Fibroblasts in contact with the nanopillars showed increased endocytic activity, demonstrated by an increase in clathrin and dynamin localization around columns. Analyzing fibroblast phenotype by transmission electron microscopy revealed that cells seeded on these structures increased vesicle formation and attempted to internalize the columns. These findings suggest that cell contact with nanocolumns activates endocytosis. Echoing this finding, Teo et al. have found that cellular interaction with nanotopography can encourage drug uptake by micropinocytosis.[4] Teo developed nano- and micropillars using a stamping process called nanoimprint lithography. Both human mesenchymal stem cells (hMCSs) and monkey kidney fibroblasts increased internalization of dextran while interacting with either nano- or micropillars. However, transfection efficiency was highest in hMSCs cultured on nanopillars, demonstrating that this is a cell and structure-scale dependent effect. Moreover, Solanki et al. demonstrated that nanotopography improves the efficiency of siRNA uptake in stem cells.[5] Solanki coated a monolayer of self-assembled silica nanoparticles with both laminin and siRNA. Neural stem cells showed maximal gene knockdown in cells seeded on 100 nm silica particles. Interestingly, this technique was shown to be generalizable to siRNA delivery to other mammalian cell types.
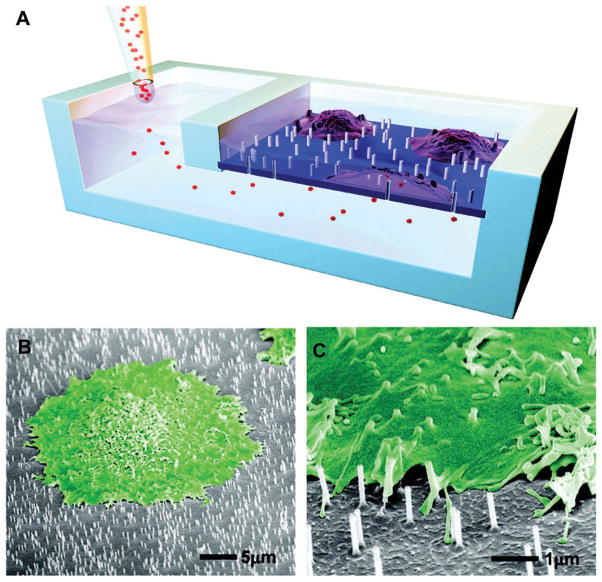
Moreover, nanotopography, specifically nanowires and nanotubes which demonstrate robust regulation of cell growth and material biocompatibility, [6,7] can also directly deliver a drug intracellularly.[8,9] Silicon nanowires grown by either chemical vapor deposition or reactive ion etching have both been shown to penetrate the cell membrane while maintaining membrane integrity. The target molecule can be loaded on to nanowires by surface modification and delivered directly into the cytosol of cells. Shalek et al. demonstrated the delivery of a small molecule, siRNA, peptides and proteins, as well as co-delivery of siRNA and protein.[8] Similarly VanDersarl et al., inspired by gap junctions and other stable conduits for intracellular communication, have developed nanostraws that can directly deliver drug into the cytosol (Figure 1).[10] Nanostraws of tunable diameter and length were created by coating filter membranes with alumina and then selectively etching away the membrane. Nanostraws directly delivered small molecules, proteins and plasmids without endocytosis or adenovirus vectors. Furthermore, these connections were stable for several days, negating the need for repeat penetrations.
Figure 1. Nanostraws provide direct and permanent cytosolic access by piercing the cellular membrane.
(A) A schematic of the device used to deliver drug to cells cultured on nanostraws. (B, C) Scanning electron microscopy of cells cultured on nanostraws. Cells are falsely colored green.
Reproduced with permission from [10].
3. Transepithelial delivery
3.1 Oral drug delivery
While direct intracellular delivery is a powerful technique, it requires direct access to cells or tissues of interest. Systemic drug delivery requires circumventing the epithelium, a substantial barrier that separates the body from its external environment. Nanotopography has shown promise in improving transepithelial drug delivery in two ways, by increasing the time and concentration gradient across the epithelium or by perturbation of cell–cell attachments like tight junctions. Fischer et al. have demonstrated that silicon nanowires, grown by a vapor–liquid–solid method on glass microbeads, can increase adhesion to epithelium in vitro and in vivo.[11] In the context of oral drug delivery, this increased adhesion of drug loaded microbeads to the intestine increased the residence time and concentration gradient, which drives drug diffusion across the intestine. Under in vitro mucus flow, microbeads coated with longer nanowires had significantly longer median survival times and resisted higher shear than those coated with shorter nanowires or nanowires coated in a mucoadhesive, lectin. The force needed to dislodge the nanowire-coated beads increased 100-fold compared to non-coated controls. In vivo, nanowire-coated microbeads increased residence time in the gastrointestinal tract by 10-fold compared to non-coated controls. Nanowires additionally have been found to modulate the barrier function of epithelium. With silicon nanowires grown on silica microbeads, Uskokovié et al. demonstrated that, in addition to adhesion, barrier function of the epithelium was also disrupted. In an intestinal cell line, Caco-2, nanowires increased permeability, as measured by transport of fluorescein-Na, and decreased barrier function, as measured by transepithelial resistance. [12] Specifically, the nanowires disrupted the tight junctions, the major determinant of paracellular permeability. For a more in-depth review on platforms for oral drug delivery, see Fox et al. [13].
3.2 Transdermal drug delivery
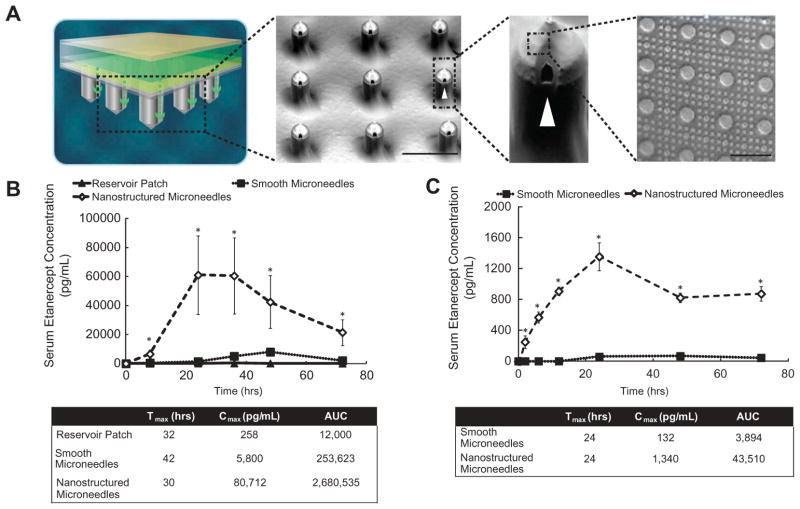
This effect on barrier function is not limited to nanowire geometry. Kam et al. used nanocolumns to improve epithelial permeability to high molecular weight therapeutics. Nanoimprint lithography was used to create low aspect ratio nanocolumns. Caco-2 monolayers exposed to nanocolumns increased permeability to fluorescein isothiocyanate (FITC)-labeled bovine serum albumin and FITC-labeled IgG.[14] This increase in permeability was not due to penetration of the cells, but by an active cellular response to the nanotopography. Subsequent studies by Walsh et al. using the same topography demonstrated that this phenomenon could be generalized to keratinocytes. Furthermore, this nanotopography, in combination with microneedles, was able to increase trans-dermal drug delivery across epidermis in two animal models (Figure 2).[15] Microneedles were used to pierce the dead cells of the stratum corneum, putting the nanostructures in contact with the keratinocytes. Nanostructured microneedles were able to increase the maximum serum concentration of antibody-based therapeutics to 30 and 24 times that of uncoated needles in rats and rabbits, respectively. Hence, nanostructures in conjunction with microneedles are a novel method to deliver high molecular weight therapeutics across the skin where previously, injection was the predominant method of delivery.
Figure 2. Nanotopography mediates transdermal drug delivery of high molecular weight therapeutics in vivo.
(A) A schematic of the transdermal drug delivery device. Scanning electron microscopy shows microneedles are coated with nanotopography. (B) Nanostructured microneedles deliver significantly more IgG-based therapeutic than smooth microneedles in rats. (C) Nanostructured microneedles deliver significantly more IgG-based therapeutic in rabbits compared to therapeutic delivered by a smooth microneedle device.
Reproduced with permission from [15].
4. Expert opinion
It is well established that cells sense and interact with topography on the nanoscale and this can have profound effects on gene expression and cell morphology. More recently nanotopography has shown its potential as a tool for drug delivery, by direct intracellular delivery, activation of endocytosis, manipulation of residence time or facilitation of transepithelial delivery. Further investigation is however warranted. The field would benefit from a systematic investigation of nanostructure properties that disrupt epithelial barrier function for pattern optimization. Elucidation of the biological mechanism by which this occurs would also need further innovation in the field by allowing for structure-based design of nanotopographies. Also investigation of how, why and if these phenomena are generalized to other cell types would greatly expand in vivo applications. All these developments would hopefully result in drug delivery modalities and devices that use topography in conjunction with or as a replacement for chemical or other physical methods to increase patient comfort and compliance. With nanofabrication techniques decreasing the limitations of resolution, fabrication is becoming cheaper and a wider array of nanotopography patterns is available. Within the next decade, we should see drug delivery devices that leverage nanotopography in clinical testing and hopefully on the market. Despite the surge of research into microneedle systems in the 1990s, currently only one microneedle device is approved by the FDA. Soluvia, a device with a single microneedle, has been approved to deliver the Fluzone influenza vaccine. Another device, MicronJet with four microneedles, has been granted FDA clearance. There are approximately 40 ongoing clinical trials leveraging microneedle-based technologies for vaccine or small molecule delivery. None of these devices include nanotopography in their design, which may be a crucial addition that would allow painless delivery of biologics and other large molecular weight therapeutics via a microneedle platform.
Footnotes
Declaration of interest
All authors receive funding from the NIH. They have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Bibliography
- 1*.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem Int Ed Engl. 2009;48(30):5406–5415. doi: 10.1002/anie.200805179. General review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. doi: 10.1021/nn900002m. Available from: http://pubs.acs.org/doi/pdf/10.1021/nn900002m. General review. [DOI] [PubMed] [Google Scholar]
- 3**.Dalby MJ, Yarwood SJ, Riehle MO, et al. Increasing fibroblast response to materials using nanotopography: morphological and genetic measurements of cell response to 13-nm-high polymer demixed islands. Exp Cell Res. 2002;276(1):1–9. doi: 10.1006/excr.2002.5498. Relevant research in the field. [DOI] [PubMed] [Google Scholar]
- 4**.Teo BKK, Goh S-H, Kustandi TS, et al. The effect of micro and nanotopography on endocytosis in drug and gene delivery systems. Biomaterials. 2011;32(36):9866–9875. doi: 10.1016/j.biomaterials.2011.08.088. Relevant research in the field. [DOI] [PubMed] [Google Scholar]
- 5**.Solanki A, Shah S, Yin PT, et al. Nanotopography-mediated reverse uptake for siRNA delivery into neural stem cells to enhance neuronal differentiation. Sci Rep. 2013;3:1553. doi: 10.1038/srep01553. Relevant research in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Tan AW, Pingguan-Murphy B, Ahmad R, et al. Review of titania nanotubes: fabrication and cellular response. Ceram Int. 2012;38(6):4421–4435. doi: 10.1016/j.ceramint.2012.03.002. General review. [DOI] [Google Scholar]
- 7*.Correa-Duarte MA, Wagner N, Rojas-Chapana J, et al. Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett. 2004;4(11):2233–2236. doi: 10.1021/nl048574f. General review. [DOI] [Google Scholar]
- 8**.Shalek AK, Robinson JT, Karp ES, et al. Vertical silicon nanowires as a universal platform for delivering biomolecules into living cells. Proc Natl Acad Sci U S A. 2010;107(5):1870–1875. doi: 10.1073/pnas.0909350107. Relevant research in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.McKnight TE, Melechko AV, Hensley DK, et al. Tracking gene expression after DNA delivery using spatially indexed nanofiber arrays. Nano Lett. 2004;4(7):1213–1219. doi: 10.1021/nl049504b. Relevant research in the field. [DOI] [Google Scholar]
- 10**.VanDersarl JJ, Xu AM, Melosh NA. Nanostraws for direct fluidic intracellular access. Nano Lett. 2012;12(8):3881–3886. doi: 10.1021/nl204051v. Relevant research in the field. [DOI] [PubMed] [Google Scholar]
- 11**.Fischer KE, Nagaraj G, Hugh Daniels R, et al. Hierarchical nanoengineered surfaces for enhanced cytoadhesion and drug delivery. Biomaterials. 2011;32(13):3499–3506. doi: 10.1016/j.biomaterials.2011.01.022. Relevant research in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Uskokovié V, Lee PP, Walsh LA, et al. PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials. 2012;33(5):1663–1672. doi: 10.1016/j.biomaterials.2011.11.010. Relevant research in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13*.Fox CB, Kim J, Le LV, et al. Micro/nanofabricated platforms for oral drug delivery. J Control Release. 2015 doi: 10.1016/j.jconrel.2015.07.033. General review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14**.Kam KR, Walsh LA, Bock SM, et al. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano Lett. 2013;13(1):164–171. doi: 10.1021/nl3037799. Relevant research in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15**.Walsh L, Ryu J, Bock S, et al. Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism. Nano Lett. 2015;15(4):2434–2441. doi: 10.1021/nl504829f. Relevant research in the field. [DOI] [PMC free article] [PubMed] [Google Scholar]