Abstract
When early canonical Wnt is experimentally inhibited, sea urchin embryos embody the concept of a Default Model in vivo because most of the ectodermal cell fates are specified as anterior neuroectoderm. Using this model, we describe here how the combination of orthogonally functioning anteroposterior Wnt and dorsoventral Nodal signals and their targeting transcription factors, FoxQ2 and Homeobrain, regulates the precise patterning of normal neuroectoderm, of which serotonergic neurons are differentiated only at the dorsal/lateral edge. Loss-of-function experiments revealed that ventral Nodal is required for suppressing the serotonergic neural fate in the ventral side of the neuroectoderm through the maintenance of foxQ2 and the repression of homeobrain expression. In addition, non-canonical Wnt suppressed homeobrain in the anterior end of the neuroectoderm, where serotonergic neurons are not differentiated. Canonical Wnt, however, suppresses foxQ2 to promote neural differentiation. Therefore, the three-dimensionally complex patterning of the neuroectoderm is created by cooperative signals, which are essential for the formation of primary and secondary body axes during embryogenesis.
Author Summary
The sea urchin embryo is similar to vertebrate embryos in that the default cell fate is potentially neurogenic, and normal development restricts the neural fate to the narrow area that locates at the anterior/dorsal region of the embryo. Because maintaining the default neural fate to the anterior/dorsal region is required for embryos to precisely integrate information from both the primary anterior-posterior and secondary dorsal-ventral body axes, these axes must be mutually linked by some mechanisms. In this study, we describe how the combination of orthogonally functioning signaling pathways regulates their targeting transcription factors expressing at the anterior neuroectoderm to restrict and pattern the default neurogenic region. By loss-of-function experiments using sea urchin embryos, we revealed that canonical and non-canonical Wnt pathways regulate the anterior neuroectoderm patterning along the primary axis, and TGF-ß signals control the patterning of the neuroectoderm along the secondary axis. In addition, we showed that the crosstalk between the Wnt and TGF-ß pathways was of importance in regulating the neuroectoderm patterning. As the default cell fate in some deuterostome embryos, including embryonic stem cells, is neurogenic, our findings could be widespread mechanisms to coordinate the remaining and/or suppressing developmental programs along different embryonic axes because Nodal and Wnt signals are critical in establishing early developmental polarities in many embryos.
Introduction
Embryonic cells of some animals tend to be differentiated into neuroectoderm cells/neural progenitors unless they receive an extrinsic signal, so-called default model [1,2]. This characteristic is also applicable to mammalian embryonic stem cells and induced pluripotent cells (e.g., [3,4]). Therefore, normal development in such organisms can be rephrased as molecular mechanisms that repress the initial neuroectodermal fate and drive them to be differentiated into different cell types. Transforming growth factor-ß (TGF-ß) family members are one group of well-described signaling molecules that play essential roles in determining non-neuroectodermal cell fates. Among these, Chordin and Noggin, which were initially reported as neural inducers, function in protecting the initial neuroectodermal fate at the dorsal side in vertebrates from invading bone morphogenetic protein (BMP) signals that are expressed at the ventral side and that specify a non-neuroectodermal fate [2]. Wnts, another type of secreted signaling molecule, also have functions in repressing the initial anterior neuroectodermal fate. In vertebrates, posteriorly functioning Wnt inhibits anterior neuroectoderm specification genes, such as otx2, and leads to the specification of posterior neuroectoderm [5]. Together, these secreted signaling molecules that regulate body axis formation act to suppress the initial neuroectodermal fate during early embryogenesis. However, despite a large number of these non-neuroectodermal signals, embryos still maintain the neurogenic region in its proper size and location. In addition, within the remaining initial neurogenic ectoderm, each terminal cell differentiation is precisely controlled to organize the complicated neural network, i.e., the patterning of the neurogenic ectoderm is highly sophisticated in the restricted neuroectoderm of normal embryos.
It has been suggested that the pre-signaling cell fate in sea urchin embryos is also anterior neuroectoderm, called the animal plate (AP). This is shown by an experiment in which the earliest canonical Wnt (cWnt) signal is inhibited by injecting the intracellular domain of cadherin (Δcad) to interfere with the nuclear localization of ß-catenin, resulting in most of the ectoderm of the injected embryos becoming specified as AP and differentiating into serotonergic neurons as well as other types of neurons and non-neural cells (Fig 1A: [6,7]). The expanded AP in the early cWnt-deficient embryos lacks patterning, and the serotonergic neurons are therefore dispersed throughout the AP. In contrast, the restricted AP in normal embryos differentiates into serotonergic neurons only at the dorsal/lateral edge, i.e., there are no serotonergic neurons observed at the ventral edge and central part (anterior end) of the AP (Fig 1A), even though most of the cells in the anteriorly restricted neurogenic region have the potential to be serotonergic neurons [8]. Nodal-BMP2/4, via Smad2/3-1/5/8 signaling along the dorsoventral axis, is one of the signaling networks that regulates the specification of cell fate and patterning in this region (reviewed in [9]), but their target transcription factors remain unclear. In summary, the developmental features of the AP of the sea urchin embryo are the following: 1) the serotonergic neural fate is executed only at the dorsal/lateral edge of the neuroectoderm, 2) the anterior end (i.e., the central part) of the AP does not differentiate serotonergic neurons, and 3) no serotonergic neurons appear at the ventral edge of the AP. Although information regarding the morphological and phenomenological characteristics of the development of the AP in sea urchin embryos has accumulated, the details of the molecular mechanisms that perform the intrinsic system of serotonergic neural fate specification at the dorsal/lateral edge of the neuroectoderm and that suppress the neural fate in other regions must still be defined. Thus, we have focused on the functional regulation between the signaling molecules and the transcription factors that control the patterning of the AP in the sea urchin embryo.
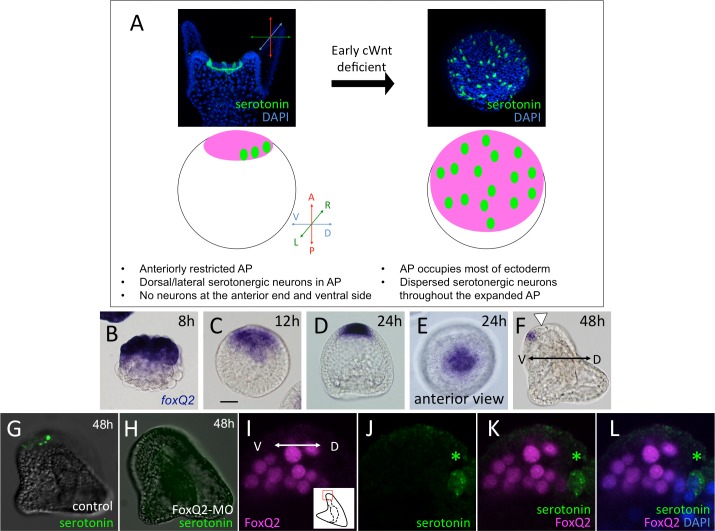
Fig 1. The pre-signaling state of most of the ectoderm is neurogenic and the region is patterned along the dorsal-ventral axis after restricted anteriorly.
(A) A brief summary of the early cWnt-deficient embryonic phenotype, in which the initial (default) neuroectoderm covers most of the embryo and a number of serotonergic neurons are differentiated. In the drawings, the pink field and green spots indicate neuroectoderm and serotonergic neurons, respectively. (B-F) foxQ2 patterns during the embryogenesis of the sea urchin, Hemicentrotus pulcherrimus. foxQ2 is initially expressed at the anterior half (B) and gradually restricted to the anterior end by the blastula/gastrula stages (C-E). (F) foxQ2 is expressed ventrally in the restricted AP region in the early pluteus stage. Left is ventral (V) and right is the dorsal (D) side. Arrowhead indicates the position where foxQ2 gene expression is missing in the AP region. (G, H) FoxQ2 is required for the development of serotonergic neurons. Without FoxQ2, serotonergic neurons are not differentiated at 48 h (H) compared to control (G). (I-L) Sagittal section of the neuroectoderm field in a prism larva. Serotonergic neuron is differentiated at the dorsal edge of the AP and never includes FoxQ2 protein in its nucleus. Bar in (F) is 20 μm.
Serotonergic neurons in the sea urchin embryo are differentiated within the AP by nearly bilateral patterning (Fig 1A: [9,10]). Among the transcription factors that are zygotically expressed in the AP, the earliest is foxQ2. Based on its expression pattern and previous experimental data, FoxQ2 is present in all AP cells during early embryogenesis (Fig 1B–1E: [11,12]), and it is essential for the specification of most of the cell types in the AP region, including the serotonergic neurons and apical tuft of Hemicentrotus pulcherrimus (Fig 1G and 1H: [8,13]) and Strongylocentrotus purpuratus [12]. However, FoxQ2 mRNA disappears from the dorsal/lateral edge of the neuroectoderm, where the serotonergic neurons are differentiated (Fig 1F, arrowhead: [13]), and the protein cannot be detected in differentiating serotonergic neurons (Fig 1G–1J). In addition, FoxQ2 plays an essential role in the formation of the apical tuft cilia through the maintenance of the ankAT-1 gene in later stages [14]. Because apical tuft cells are not serotonergic, these data suggest that FoxQ2 is first required for the specification of the most of the AP cells [12] but that the expression is subsequently suppressed in the cells at the dorsal/lateral edge of the AP, in which the serotonergic neural fate is executed. Thus, identifying the regulatory mechanisms of FoxQ2/foxQ2 patterning along the dorsoventral axis must be one of the keys to understanding how the initial neurogenic ectoderm is patterned during sea urchin embryogenesis.
Homeobrain (Hbn: LC064116 for Hemicentrotus pulcherrimus Hbn) is a paired-like homeobox gene that is classified into the homeobrain-like (hbnl) family [15]. The gene expression patterns of hbnl family members have been reported in the fruit fly [16], sandworm [15], sea urchin [17,18] and sea anemone [19], but the hbn gene has not been identified in chordate genomes. The hbn expression pattern was first investigated in Drosophila melanogaster, where it initially appears in the anterior dorsal head primordium, which forms portions of the brain, and then in the ventral nerve cord during later stages. In sandworms (Capitella sp. I), hbn expression was detected in the developing brain, as in fruit flies, and in its larval eyes. In the sea anemone Nematostella vectensis, hbn is expressed throughout the blastoderm except for around the blastopore, and its expression is excluded from the aboral pole, where the apical tuft and the subsequent neurogenic region are formed. The expression pattern of hbn in sea urchin embryos was reported during the genome sequencing of Strongylocentrotus purpuratus [17,18,20,21]. In those studies, hbn was initially expressed in the animal pole region during the early blastula stage, and, at later stages, it appeared outside of and then disappeared from the AP, where foxQ2 was expressed. Despite reports on the existence of the gene in some species, the control of its expression and its molecular function has not been investigated in all animals. Here, we focus on the function of Hbn because it is expressed in the same region as the foxQ2 gene during the early specification stage of the AP. Then, we describe the roles of Hbn in the specification of serotonergic neurons and report that the regulation of hbn and foxQ2 expression by Wnt and TGF-ß signals are essential for the precise patterning of the embryonic AP in the sea urchin H. pulcherrimus.
Results
hbn is required for the development of serotonergic neurons
In adding to FoxQ2, we focused on the function of Hbn, another AP-specific factor. hbn is initially expressed throughout the AP (Fig 2A), as previously described in different species [18]. During subsequent developmental stages, the expression of hbn progressively fades from the ventral half of the AP and appears at the dorsal/lateral ectoderm, adjacent to the AP, in the early gastrula stage (24 h) (Fig 2B and 2C). At the late gastrula stage (30 h), its expression is restricted to the dorsal/lateral ectoderm and it completely disappears from the anterior end of the AP (Fig 2D). In addition to its dorsal/lateral ectodermal expression, hbn appears at the upper lip region in the prism stage (36 h, Fig 2E, arrow), where it remains, at least until the pluteus stage (48 h Fig 2F, arrow). To compare the expression pattern of hbn with that of foxQ2 or tryptophan 5-hydroxylase (tph), a serotonin synthase gene, we employed two-color fluorescence in situ hybridization. foxQ2 and hbn expression nearly overlapped in the AP region of the unhatched blastula (Fig 2G), but hbn gradually faded from the region and a portion of the dorsal/lateral ectoderm that was adjacent to the AP began to express hbn, which resulted in the expression pattern of hbn being ‘shifted’ toward the dorsal side away from the AP (Fig 2H and 2I). By the late gastrula stage, hbn expression had completely disappeared from the foxQ2 area (Fig 2J). Double staining of hbn and tph in the pluteus stage showed that the serotonergic neurons were differentiated at the edge of the AP, which was adjacent to the hbn-expressing region (Fig 2K and 2K’).
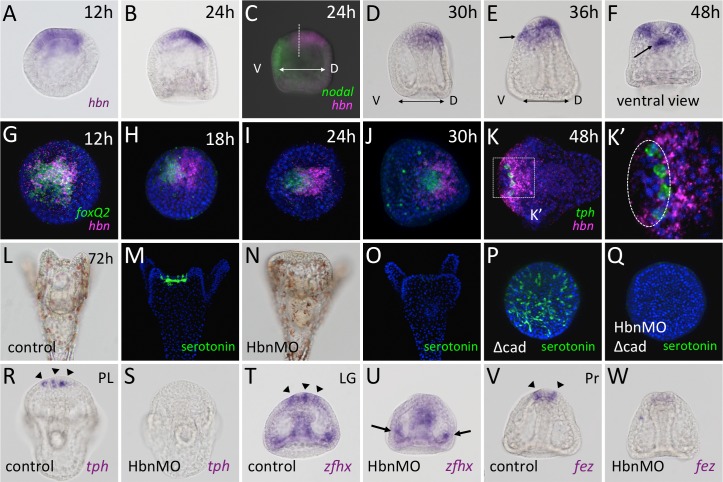
Fig 2. Homeobrain is required for the development of serotonergic neurons.
(A-K’) Spatiotemporal expression pattern of hbn. Left is ventral (V) and right is the dorsal (D) side. Compared with foxQ2 expression, the expression of hbn is ‘shifted’ towards the dorsal side of AP. nodal is the ventral marker. Arrows in (E, F) indicate hbn expression in the stomodeum. Dot-lined circle in (K’) indicates the AP region. Serotonergic neurons in control embryos (L, M) are missing in Hbn morphants (N, O). (P, Q) Serotonergic neurons are not differentiated in the Δcad and Hbn-MO co-injected embryos. Hbn is an upstream gene of tph, tryptophan 5-hydroxylase (R, S), zfhx (T, U), and fez (V, W). Arrowheads in (R, T, V) indicate the precursors of serotonergic neurons. Arrows in (U) indicate lateral ganglion neurons.
Hbn morphants developed into pluteus larvae without detectable defects in morphology or developmental timing, except for a defect in the elongation of the anterolateral arms at 72 h (cf. Fig 2N with 2L) and at 96 h (cf. S1C with S1A Fig). In addition, Hbn morphants have significantly fewer serotonergic neurons than normal embryos while non-serotonergic neurons at the AP and ciliary band are almost normal (Fig 2M, 2O, S1A–S1D and S1G Fig). Because the development of serotonergic neurons is affected by several signals from outside of the AP [9], we employed a Δcad-injected embryo to accentuate Hbn function under conditions where all other known signals were eliminated [22,23]. A Δcad-injected embryo, in which the initially fated AP contains a greatly increased number of serotonergic neurons (Fig 2P), is an appropriate system to analyze the intrinsic function of genes that are expressed within it, as was previously reported [6]. When Hbn was knocked down in Δcad-injected embryos, the development of serotonergic neurons was strongly inhibited, as was observed in normal morphants (Fig 2P and 2Q). These phenotypes were specific because they were also obtained when using a second morpholino (Hbn-MO2) that targeted a non-overlapping sequence in the mRNA (S1E and S1F Fig), and the microinjection of an mRNA encoding Hbn protein partially rescued the morpholino knockdown effect under the early cWnt-deficient condition (S2A–S2J Fig). These results indicate that Hbn is required for the development of serotonergic neurons in the AP.
To identify the step in which Hbn is involved during the development of serotonergic neurons, we examined Hbn morphants for the expression of tph, which is a terminal differentiation marker, and zinc finger homeobox 1 (zfhx1; [8]) and forebrain embryonic zinc finger (fez), which are early neural markers [14]. In Hbn morphants, tph was not expressed in the neuroectoderm (Fig 2R and 2S, arrowheads show tph-cells in the control), which indicated that the transcription of tph required Hbn function. zfhx1 and fez are downstream of FoxQ2 but are independent of each other. Hbn morphants expressed neither of these genes in the AP (Fig 2T–2W, arrowheads show the expression patterns of each gene in the control embryos). As expected, zfhx1 expression in the lateral ganglion was not affected in Hbn morphants (Fig 2U, arrows). In adding to the previous report, which showed that the entire AP region had the potential to produce serotonergic neurons [8], these data indicated that Hbn is required for the specification of serotonergic neurons.
TGF-ß signals regulate the dorsoventral patterning of foxQ2 and hbn
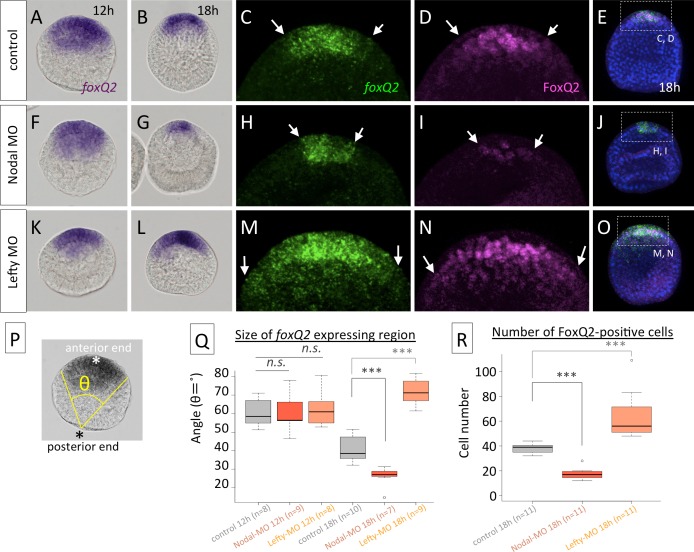
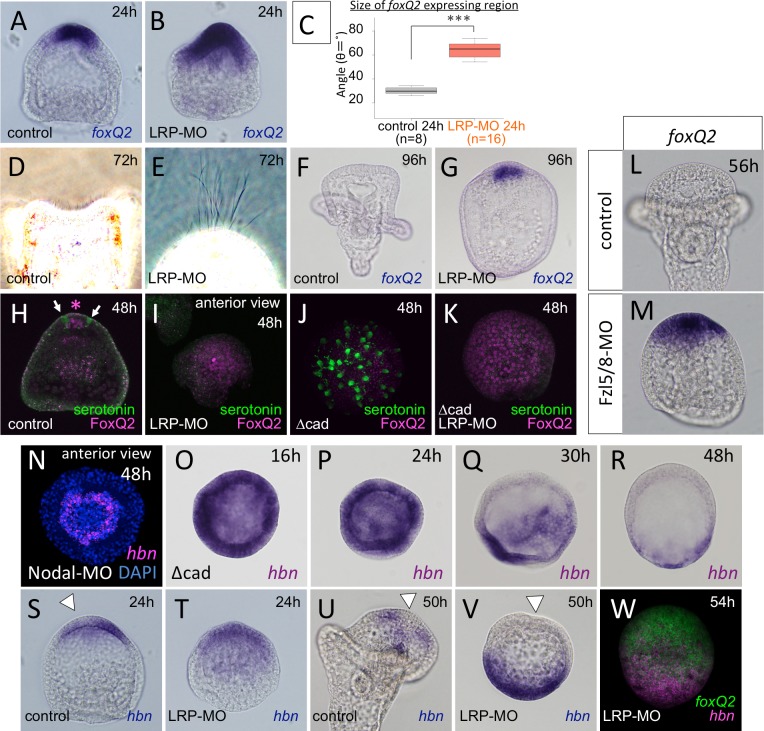
The change in the foxQ2 expression pattern along the dorsoventral axis suggested that its expression may be regulated by or depend upon TGF-ß signals because cell fate specification along the secondary, dorsal-ventral axis of sea urchin embryos was determined by TGF-ß family members such as Nodal and BMP2/4 [23–25]. Therefore, we examined whether the Nodal pathway is involved in the regulation of foxQ2 expression throughout development. In Nodal morphants, the size of the foxQ2 region was smaller than that of control embryos at the hatched blastula stage (18 h) (cf. Fig 3G with 3B, quantification of foxQ2 region was shown in P, Q), but they were invariant in unhatched blastulae (12 h) (Fig 3A, 3F and 3Q). The protein localization of FoxQ2 in hatched blastula also showed the same size as its mRNA pattern, and the immunochemical signal in Nodal morphants was weak (cf. Fig 3H–3J with 3C–3E, between arrowheads), which indicated that Nodal is required for maintaining foxQ2 expression during the blastula stages. In contrast, in Lefty morphants, in which Nodal proteins are located throughout the ectoderm [26], the size of the AP in the hatched blastula stage was significantly wider than that in control embryos (Fig 3L–3O and 3Q), which indicated that misexpressed Nodal interferes with the restriction of the neuroectoderm during blastula stages. The difference in the foxQ2-mRNA positive region in controls, Nodal morphants and Lefty morphants measured with the angle from posterior pole was supported by the data, in which we counted the number of FoxQ2-protein positive cells in 18 h stages (Fig 3R). Based on these data, Nodal maintains the expression of foxQ2 during blastula stages, and this mimics the process that occurs on the ventral side of the AP during normal development.
Fig 3. Nodal maintains foxQ2 expression.
foxQ2 mRNA and FoxQ2 protein patterns in control embryos (A-E), Nodal morphants (F-J) and Lefty morphants (K-O). Arrows in (C, H, M) and (D, I, N) indicate the edge of the foxQ2-mRNA and FoxQ2-protein region, respectively. (P) The image shows how the foxQ2-expressing area was measured with the angle (θ) from the posterior end. (Q) Quantification of the size of the foxQ2 region in the control (A, B), Nodal morphants (F, G) and Lefty morophants (K, L). The foxQ2 region in Nodal morphants and Lefty morphants is significantly narrower and wider, respectively, than that in the control at 18 h, whereas they are all similar at 12 h. (R) Quantification of the number of FoxQ2-protein positive cells in the control (E), Nodal morphants (J) and Lefty morphants (O). n.s. not significant, ***P<0.001, Student’s t-test. foxQ2 expression pattern is almost asymmetrical during all stages shown in this figure.
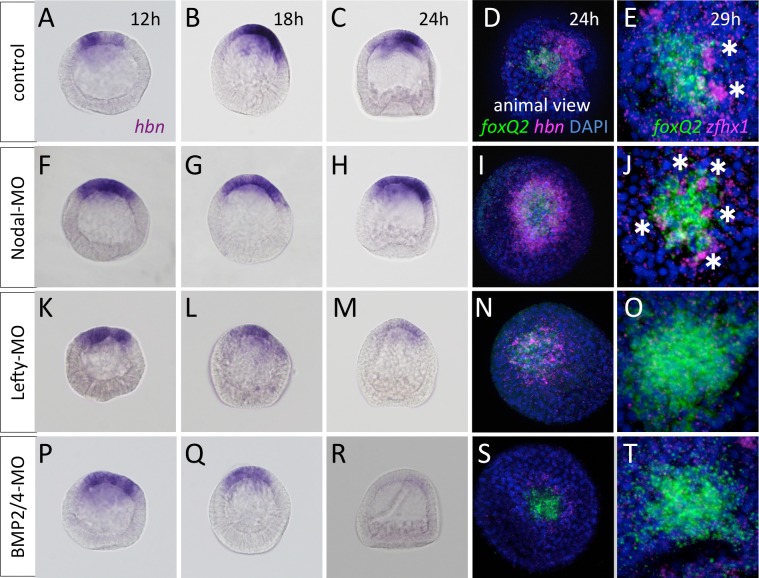
Next, to investigate what controls hbn expression along the secondary axis, we observed its pattern in the embryos, in which the TGF-ß signals responsible for secondary axis formation were disturbed [23,25]. In Nodal morphants, hbn expression was shifted uniformly to the AP-adjacent region by the early gastrula stage (24 h) (cf. Fig 4H, 4I with 4C, 4D), which suggests that Nodal suppresses the expression of hbn on the ventral side of normal embryos. However, quantitative PCR (qPCR) data indicated that the amount of hbn mRNA was not significantly changed in the morphants or even in Nodal-misexpressed embryos (S3 Fig), suggesting that the function of a strong hbn inducer was missing in both Nodal morphants and misexpressed embryos. As a result of the uniform shifting of hbn, zfhx1-positive cells were distributed around the foxQ2 region in Nodal morphants (Fig 4I and 4J, asterisks), whereas in normal embryos, the precursor cells of serotonergic neurons were present at the dorsal edge of the AP (Fig 4D and 4E). In Lefty or BMP2/4 morphants, in which the dorsal ectoderm is missing and the ventral ectoderm surrounds the AP [24,26], but in which hbn expression at the unhatched blastula stage (12 h) is not changed (cf. Fig 4K, 4P with 4A), the expression patterns became obscure after hatching and never showed clear shifting towards the edge of the AP region, unlike in control or Nodal morphants (Fig 4K–4N and 4P–4S). These results suggest that Lefty and BMP2/4 are required for maintaining the strong expression of hbn after the hatched blastula stage. In fact, because the significant decrease of hbn mRNA was observed only in BMP2/4 morphants by qPCR (S3D Fig), we concluded that BMP2/4 is essential for the maintenance of hbn on the dorsal side. Furthermore, when the clear shifting of hbn expression towards the edge of the AP was almost entirely missing in these morphants, no zfhx1 expression was observed at the AP region (Fig 4N, 4O, 4S and 4T), resulting in the loss of all serotonergic neurons, as previously reported [27]. Taken together, the findings show that Hbn plays a role in an intrinsic system that determines the initial neural fate at the dorsal/lateral edge of the AP and that its expression patterns are highly regulated by TGF-ß signals along the dorsoventral axis.
Fig 4. TGF-ß signals regulate the dorsoventral pattern of hbn.
(A-E) hbn is shifted toward the dorsal side, where serotonergic neurons are differentiated (E) in control embryos. (F-J) hbn surrounds the foxQ2 region and the serotonergic neurons are differentiated at the same region (J). Clear shifting toward the edge of the AP and strongly localized expression at later stages is not observed in Lefty morphants (K-O) or BMP2/4 morphants (P-T). Both have no serotonergic neurons. Asterisks indicate the precursors of serotonergic neurons, which express zfhx1.
Canonical Wnt pathways are required for the disappearance FoxQ2 from the neurogenic AP, with precise timing
The next question was what further restricts the foxQ2 region further to the anterior end without Nodal signaling (Fig 3G and 3H). To investigate this question, we focused on the cWnt pathway because the inhibition of the early cWnt pathway interfered not only with AP restriction but also with AP patterning, resulting in the serotonergic neurons being differentiated in a dispersed manner (Fig 1A). Because the effect of an exogenous cadherin fragment (Δcad) on blocking cWnt [22] might be short-lived due to the short half-life of the injected mRNA, we blocked the function of low-density lipoprotein receptor-related protein 6 (LRP6: LC064120 for H. pulcherrimus LRP6), a co-receptor that acts with the Wnt receptor Frizzled (Fzl) to mediate the cWnt pathway. Based on previous reports, LRP6 mRNA is expressed maternally, lasts throughout embryogenesis [28] and is present in all cells during the early stages [29]. The same observations were made in H. pulcherrimus embryos (S4A–S4F Fig). Although the localization of LRP6 mRNA was uniform in embryos, the protein was missing in the ingressed and future mesodermal region at the mesenchyme blastula stage (S4G and S4H Fig). In LRP6 morphants, the protein-detection level was significantly decreased (S4I and S4J Fig; each image was captured in the same microscopic condition), and mesenchyme ingression was normal but no endoderm invagination was observed (Fig 5A and 5B). This result occurred because the mRNA and likely, protein of LRP6 were present maternally, and LRP6-MO could not block the early cWnt, unlike Δcad injection. In LRP6 morphants, the foxQ2 expressing region was significantly wider than that of control embryos at 24 h (Fig 5A–5C), and the dorsal-ventral polarity in the ectoderm was normal based on nodal and hnf6 expression patterns (S4K–S4O Fig; [23]). The morphant retained the apical tuft, which should disappear by 72 h during normal development (Fig 5D and 5E; [13]), and, intriguingly, its essential regulatory gene, foxQ2, was also still detected at 96 h (Fig 5F and 5G). This result indicates that LRP6-mediated cWnt signaling is required for suppressing foxQ2 expression in the AP.
Fig 5. Canonical Wnt signal regulates the patterning of anterior neuroectoderm.
In contrast to control embryos (A), LRP6 morphant had a wider foxQ2 region (B). (C) Quantification of the size of the foxQ2 region in control (A) and LRP6 morphants (B). ***P<0.001, Student’s t-test. LRP6 morphants retain the apical tuft at 72 h (E) and foxQ2 (G) at 96 h, whereas both are diminished in control embryos at those times (D, F). The differentiation of serotonergic neurons is delayed and not observed at 48 h in LRP6 morphants (H, I). Arrows in (H) indicate serotonergic neurons. Asterisk shows foxQ2 protein. This delay is independent of the presence or absence of Δcad mRNA injection (J, K). (L, M) foxQ2 is maintained in Fz5/8 morphants. (N) hbn pattern in Nodal morphants. (O-R) Spatiotemporal expression pattern of hbn in Δcad-injected embryos. hbn mRNA is maintained at the anterior end until at least 24 h. The clearance occurs without early cWnt and TGF-ß signals even though the timing is delayed. hbn clearance is not observed in LRP6 morphant at 24 hr (S, T), but it occur by 50 h (U, V). (W) Double fluorescent in situ hybridization with hbn and foxQ2 to confirm hbn clearance from the anterior end. White arrowheads indicate the location of hbn clearance.
Despite the wider foxQ2 region, LRP6 morphants had no differentiated serotonergic neurons at 48 h (Fig 5H and 5I). This result was likely derived from the maintenance of FoxQ2 in each cell in the expanded AP. This was confirmed in 48 h Δcad-embryos and Δcad-LRP morphants because a number of serotonergic neurons and no FoxQ2 protein were observed in the former, while strong FoxQ2 signal and no serotonergic neurons were observed in the latter (Fig 5J and 5K). Taken together, these results indicate that an LRP6-mediated signal seems to be involved in the disappearance of FoxQ2 from the AP and for the precise differentiation timing of serotonergic neurons.
Among the Frizzled receptors for the Wnt pathway in sea urchin embryos, it was reported that Fzl1/2/7 and Fzl5/8 are expressed in the ectoderm [30], and Fzl5/8 is likely the only Fzl receptor whose function we can analyze during the modification of the restricted AP region because Fzl1/2/7 morphants lose the entire AP from the very beginning of its formation [31]. Thus, we investigated whether Fzl5/8 mediates the LRP6-based cWnt pathway that controls the disappearance of FoxQ2 from AP cells. In Fzl5/8 morphants at 56 h, the expression of foxQ2 was maintained (Fig 5M), whereas control embryos lost the foxQ2 message at this stage (Fig 5L). This result suggested that Fzl5/8 functions in controlling the cWnt at the AP region.
The disappearance of hbn from the anterior end occurred normally in Nodal morphants, and the hbn-expressing region surrounded the central part of the AP (Fig 5N), which suggests that the disappearance of hbn expression is independent of the dorsoventral axis formation by TGF-ß signals (Fig 4). To confirm this finding and to investigate the involvement of the cWnt pathway as an upstream factor of TGF-ß signals during hbn clearance, we employed Δcad-embryos, which lack all known zygotic signals including early cWnt and TGF-ß signals [22,23]. In these embryos, hbn is expressed throughout the entire region during the early stages (Fig 5O and 5P). Then, hbn disappears from the central portion of the AP by 30 h (Fig 5Q; [18]). The hbn-negative region is progressively expanded, and the hbn-expressing region is observed only in the squamous epithelia in the posterior half of 48 h Δcad-embryos (Fig 5R). This result supports previous data from a different species, S. purpuratus [18]. This disappearance pattern of hbn in Δcad-injected embryos was spatially similar to that observed in the anterior end area of normal embryos, which suggests that the spatial control of the disappearance of hbn expression from the anterior end of the AP is independent of cWnt and TGF-ß signals.
Focusing on the hbn expression pattern in Δcad-embryos in detail, we found that the disappearance of the gene from the anterior end was delayed. In normal embryos, hbn began to be diminished from the anterior end of the AP by 18 h (Fig 2H), but it did not disappear until 30 h in Δcad-embryos (Fig 5Q). In addition, because the cWnt pathway likely regulates the disappearance of foxQ2 as mentioned above, we investigated the function of LRP6 on hbn regulation. hbn gene expression remained in the entire anterior half in LRP6 morphants at 24 h, at when the clearance of the gene was quite obvious in the controls (Fig 5S and 5T). However, hbn had disappeared from the AP by 50 h, as in normal embryos (Fig 5U and 5V). This was confirmed by the result from double fluorescent in situ hybridization for foxQ2 (Fig 5W). In addition, the disappearance of hbn from the AP region is independent of the absence of LRP6 function (S4P Fig). Based on these observations, the LRP-mediated cWnt signal is not required for the disappearance of hbn from the AP, but it is required for the control of the timing of its clearance.
Wnt7 functions as one of the ligands in the cWnt pathway at the neurogenic AP
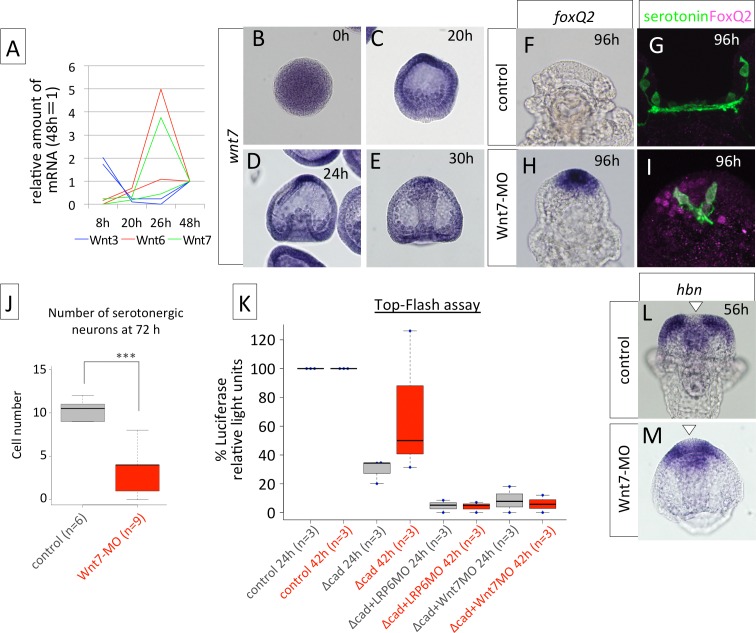
To find the ligands for cWnt signaling in suppressing foxQ2, we focused on later-expressed Wnts in this study because the early Wnts that function in endomesoderm formation might have indirect effects on AP regulation. Based on the temporal expression profile previously reported, Wnt3 (LC064118 for H. pulcherrimus Wnt3), Wnt6 (LC0641198 for H. pulcherrimus Wnt6) and Wnt7 (LC064119 for H. pulcherrimus Wnt7) are expressed relatively late [28,32]. In H. pulcherrimus, wnt3 is expressed during the cleavage stage but not after the blastula stage, according to qPCR, whereas wnt6 and wnt7 were expressed after hatching (Fig 6A). Based on perturbation experiments, it is suggested that Wnt7 functions as a ligand for the cWnt pathway, and Wnt6 for non-cWnt pathways, and those reasons will be explained in this section for Wnt7 and in the next section for Wnt6. wnt7 was expressed broadly at 20 h and was abundantly expressed in the AP. The broad expression of wnt7 and its strong expression in the AP were invariable until 30 h (Fig 6B–6E).
Fig 6. Wnt7 is a ligand for cWnt pathway that regulates the patterning of anterior neuroectoderm.
(A) Quantitative PCR data revealing wnt3, wnt6 and wnt7 during sea urchin embryogenesis. The data from two independent batches are shown. (B-E) wnt7 expression patterns during sea urchin embryogenesis. In the Wnt7 morphants, foxQ2 mRNA (H) and protein (I) are remained until late stages, e.g., 96 h, whereas they are not expressed in the control (F, G). The number of serotonergic neurons is significantly smaller in Wnt7 morphants at 72 h (J) and 96 h (I) than in controls (G, J). ***P<0.001, Student’s t-test. (K) Top-Flash assay revealed that LRP6 and Wnt7 are involved in the canonical Wnt pathway at the AP region. (L, M) hbn clearance occurs in Wnt7 morphant, indicating that hbn is not a strong target of the cWnt pathway. White arrowheads indicate the location of hbn clearance.
In Wnt7 morphants, foxQ2 mRNA was still expressed in the AP region even at 96 h (Fig 6F and 6H) and FoxQ2 protein remained to be detected in AP cell nuclei at the same stage (Fig 6G and 6I). Because FoxQ2 persisted, the differentiation of serotonergic neurons was extremely delayed. The number of serotonergic neurons at 72 h was significantly smaller than that in controls (Fig 6J). Because FoxQ2 persistence and missing serotonergic neurons were similar characteristics observed in LRP6 morphants, these results suggest that Wnt7 functions as a ligand of the cWnt pathway in mediating the differentiation of serotonergic neurons through the suppression of FoxQ2 expression.
Although our data so far have suggested that the cWnt pathway is involved in AP patterning through suppressing foxQ2 expression, nuclear ß-catenin was not observed in the region until at least the 8th cleavage stage [22]. Because the antibody that recognizes nuclear ß-catenin in H. pulcherrimus is not available, we performed a TCF-luciferase reporter system (Top-Flash) assay to measure the level of cWnt signal [33,34]. Δcad-injected embryos have only approximately 30% Top-Flash activity compared to controls at 24 h (Fig 6K). This decreased activity tends to recover as the embryos grow due to the degradation of exogenous Δcad-mRNA and/or protein. However, without LRP6 or Wnt7 functions, Top-Flash activity remains low, and the scores of the activity are significantly lower than those in controls at both 24 h and 42 h (Fig 6K). These data support that in the AP region cWnt functions through the Wnt7-LRP6 pathway in suppressing foxQ2 expression. In contrast, Wnt7 morphants had normal clearance of hbn expression from the anterior end (Fig 6L and 6M), supporting the idea that cWnt is not involved in controlling hbn expression patterns.
Because one of the interesting questions in this study is that of which signaling pathway crosstalks with Nodal signaling during the regulation of AP patterning (Fig 3), a cWnt pathway regulated by Wnt7 might be the candidate. In fact, when the Wnt7 function was blocked, the foxQ2 region was wider than in normal embryos at the blastula stage (S5A, S5B and S5D Fig). The excessive restriction of the foxQ2 region that was observed in Nodal morphants (S5A and S5C Fig) was rescued in Nodal-Wnt7 double morphants (S5A and S5E Fig), suggesting that Wnt7 is the factor that restricts foxQ2 expression to the anterior end and that the Nodal signal inhibits a Wnt7-mediated signaling pathway during the blastula stages.
Wnt6-mediated non-cWnt pathway is required for hbn disappearance from the anterior end of the AP
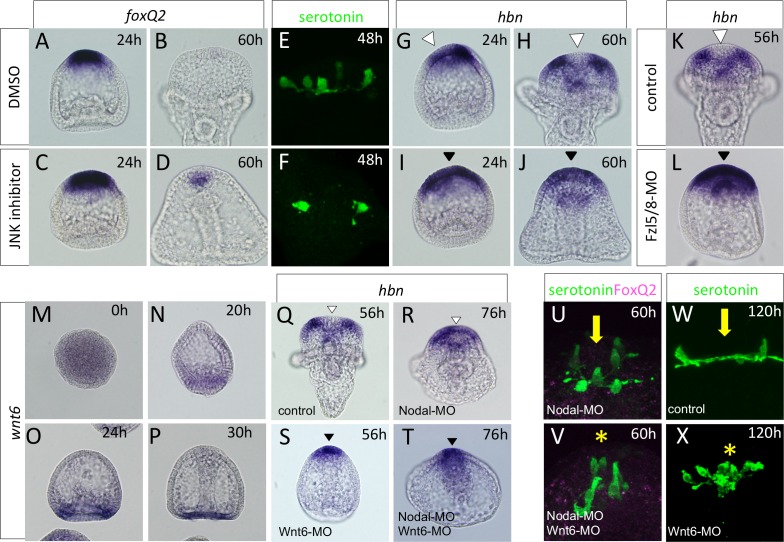
We next focused on the function of non-cWnt signals on AP patterning. As it is downstream of early cWnt signals from the posterior side, a c-Jun N-terminal kinase (JNK) signal functions in the restriction of the AP to the anterior end [31]. To examine whether a JNK signal also plays a role in AP patterning, we applied a JNK inhibitor from 2–4 cell stages and analyzed the expression patterns of foxQ2 at the desired stages. As was previously reported, the restriction of foxQ2 to the anterior end was inhibited in the absence of JNK function at 24 h (Fig 7A and 7C). foxQ2 disappearance from the AP was delayed in JNK-inhibited embryos, but the remaining signal was weak, and its area was very small at 60 h (Fig 7B and 7D). Unlike the cWnt pathway, the JNK pathway seem to be weakly involved in the maintenance of foxQ2 expression because the timing of the initial differentiation of the serotonergic neurons was slightly delayed (48 h, Fig 7E and 7F), but a number of serotonergic neurons were differentiated in the expanded AP one day later [31]. We next focused on the function of non-cWnt signals on hbn expression, and used a JNK inhibitor and analyzed the expression patterns of hbn. hbn clearance from the anterior end at 24 h was not observed in JNK-inhibited embryos (Fig 7G and 7I, arrowheads). In addition, the clearance was intriguingly not observed in JNK-inhibited embryos, even in the later stages (cf. Fig 7J with 7H; arrowheads). Together, these results suggest that a JNK signal acts as a part of non-cWnt signaling and that it mainly plays a role in the clearance of hbn from the anterior end of the AP.
Fig 7. Wnt6 is a ligand for non-cWnt pathway that regulates the patterning of hbn expression.
(A, C) foxQ2 expression in the control (DMSO-treated) and JNK inhibitor treated embryos at 24 h. (B, D) foxQ2 expression in the control (B) and in JNK inhibitor treated embryo (D) at 60 h. A trace level of foxQ2 was expressed in JNK inhibitor treated embryos, whereas foxQ2 is completely diminished in the control at this stage. (E, F) The serotonergic neurons are differentiated at 48 h with or without JNK function. The AP region is magnified. (G-J) hbn clearance does not occur in JNK inhibitor-treated embryos. (K, L) hbn remains at the anterior end of AP in Fzl5/8 morphants at 56 h. (M-P) wnt6 was strongly expressed in the veg2 endoderm region at 20 h, and afterward it was maintained in the vegetal plate until at least 30 h. (Q, S) hbn does not disappear from the anterior end and is not expressed in the non-AP region in Wnt6 morphants, and this is independent of the presence or absence of Nodal (R, T). (U-X) Without Wnt6 function, serotonergic neurons are differentiated at the anterior end of the AP. To visualize the phenotype more clearly during early stages, Nodal-MO was simultaneously injected. Arrow in (U, W) indicates the anterior end that misses serotonergic neurons. Asterisk in (V, X) shows that the serotonergic neurons are present at the central part of AP. White and black arrowheads indicate the location of hbn absence and presence, respectively.
As mentioned above, Fzl5/8 is likely the only Fzl receptor whose function we can analyze during the modification of the restricted AP region [31]. Here, we investigated whether Fzl5/8 mediates the non-cWnt pathway that controls the clearance of hbn from the central part of the AP. In Fzl5/8 morphants at 56 h, the expression of hbn was maintained (Fig 7L) whereas all control embryos had no hbn mRNA (Fig 7K). This result suggested that Fzl5/8 mediates the non-cWnt signal at the AP region.
To investigate the ligands for non-cWnt signaling in hbn patterning, we focused on Wnt6 because Wnt7 was not involved in the regulation of hbn expression (Fig 6). wnt6 is expressed in the veg2 endoderm region and is not obvious at the ectoderm (Fig 7M–7P). The morphology of Wnt6 morphants did not resemble a normal pluteus stage even at 56 h and had a straight archenteron and no pluteus arms (Fig 7Q and 7S). Focusing on the hbn expression pattern, we found that it did not disappear from the central part of the AP region in Wnt6 morphants (Fig 7S). This result was confirmed by experiments in embryos that were doubly injected with Δcad and Wnt6-MO, in which hbn was broadly expressed in the expanded AP (S6I and S6K Fig). To clarify this finding, we employed Nodal morphants; without Nodal function, hbn “shifting” to the periphery of the AP is more obvious (Fig 7R). The morphants, in which Nodal and Wnt6 were simultaneously knocked down, showed no hbn disappearance from the central part of the AP (Fig 7T). This result clearly indicated that Wnt6 is required for hbn suppression in the AP. Because hbn was maintained in the AP in Wnt6 morphants, serotonergic neurons were differentiated not at the edge of the region but at the anterior end of the AP (cf. Fig 7V, asterisk with U, arrow). These data are supported by the serotonergic neural patterns in later stage, in which the differentiated serotonergic neurons gather at the anterior end in Wnt6 morphants even if there is no Nodal inhibition (Fig 7W and 7X). In addition, the nuclear localization of FoxQ2 had already disappeared at 60 h, as in normal embryos (Fig 7U and 7V), indicating that Wnt6 is not strongly involved in the regulation of the FoxQ2 expression pattern, which is mediated by LRP6/cWnt signaling. Although it is difficult to distinguish, these results suggest that Wnt6 functions as one of the players in the non-cWnt pathways that regulate AP patterning, especially in the control of hbn expression.
Because the expression patterns of foxQ2 and hbn are complementary after the gastrula stage in normal embryos, FoxQ2 is another candidate for suppressing hbn expression. To examine this possibility, we investigated hbn expression patterns in FoxQ2 morphants. The disappearance of hbn occurred normally in the morphants (S7A–S7F Fig), which suggests that FoxQ2 and its downstream genes do not regulate the suppression of hbn expression at the AP in later embryos. Furthermore, in Hbn morphants, the expression pattern of foxQ2 was the same as that in normal embryos (S7G–S7J Fig), indicating that FoxQ2 and Hbn are mutually independent.
Discussion
Here, we reveal the molecular mechanisms that control the patterning of the anteriorly restricted neurogenic AP ectoderm along the anteroposterior and dorsoventral axes in the sea urchin embryo, i.e., how embryos pattern the initial neuroectoderm to let the specific neurons differentiate only at the correct location (Fig 8). Although a number of genes that are expressed at the neurogenic ectoderm were identified during the genome sequencing project of S. purpuratus [17,18,35], those analyses were not sufficient to explain the molecular pathways that regulate AP formation and neural differentiation. Our data show that the expression of transcription factors inside AP must be precisely controlled by the intrinsic and/or extrinsic TGF-ß and Wnt molecules and that this regulation is essential for the development of AP and neurons. We also reveal the function of Hbn in specifying the initial neuroectodermal fate. To our knowledge, this is the first study revealing the molecular function of Hbn in any animals, although the expression pattern of hbn has been reported in several species. With Hbn function, we must consider the function of FoxQ2 in the specification and differentiation of neurogenic AP. FoxQ2 is initially required for the specification of most of cell types in the AP by the mesenchyme blastula stage [12], but it is not required later for neural differentiation because its function is to maintain apical tuft gene expression [13]. Therefore, the key to understanding the molecular mechanisms that maintain and suppress the initial neuroectodermal fate is the regulation that controls these two transcription factors in sea urchin embryos.
Fig 8. Models for anterior neuroectoderm patterning.
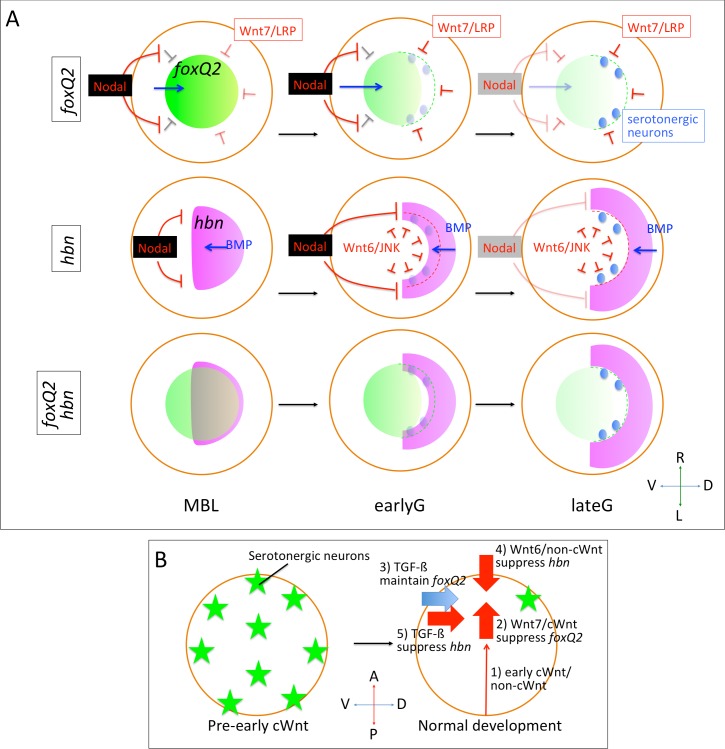
(A) Schematic images of signaling pathways regulating foxQ2 and hbn expression. Anterior views. foxQ2 (green) is suppressed by cWnt mediated by Wnt7/LRP6 from the blastula to gastrula stages. Nodal inhibits the signal from the ventral side. Serotonergic neurons (blue) begin to be differentiated in the foxQ2-missing area, i.e., the dorsal/lateral edge of the AP. hbn (magenta) expression shifts towards the dorsal/lateral edge of the AP. Nodal and non-cWnt mediated by Wnt6/JNK signals regulate the suppression, whereas BMP2/4 promotes its expression. The dotted line indicates the dorsal border of the AP. MBL, mesenchyme blastula; early G, early gastrula; late G, late gastrula. (B) Summary of signaling pathways regulating the serotonergic neural fate at the dorsal/lateral edge of the AP. Before receiving extrinsic signals beginning from early cWnt, the cell fate of most cells in sea urchin embryos are the AP, which differentiates a number of serotonergic neurons. However, under normal conditions, five signals restrict its fate to a small region.
TGF-ß signals regulate the dorsoventral patterning of the neurogenic ectoderm
It was previously reported that serotonergic neurons in the neurogenic AP of the sea urchin embryo are formed at the dorsal/lateral edges of the region [9,10] and that the differentiation of serotonergic neurons at the ventral side is suppressed by Nodal, which is expressed in the ventral ectoderm [6]. In this study, we revealed that hbn expression is suppressed by Nodal on the ventral side but maintained by BMP2/4 on the dorsal side. hbn expression is eliminated from the animal pole, likely by the non-cWnt pathway mediated by Wnt6/JNK after the blastula stage, which will be discussed below, and its pattern forms a horseshoe-like shape (Fig 8A). This pattern was not reported in another sea urchin, S. purpuratus [18], but, in H. pulcherrimus, it is clear that the expression is missing at the ventral side of the normal AP. The loss of Nodal function supports this observation because Nodal morphants have a ring-like shape of hbn expression around the neurogenic ectoderm (Fig 4). Because the expression of foxQ2 is also under the control of secondary axis formation by the Nodal and BMP2/4 pathways (this study; [14]), we need to know whether Nodal and/BMP2/4 regulation is direct or indirect by further experiments, including chromatin immunoprecipitation analysis aimed at uncovering the cis-regulatory modules of foxQ2 and hbn.
In vertebrates, TGF-ß signaling also functions in the neural plate patterning along the dorsoventral body axis [36]. For example, bmp2 and bmp7 expressing outside of the neural plate are necessary for the development of noradrenergic neurons through the induction of the homeodomain protein, phox2a, in zebrafish embryos [37]. Nodal, on the other hand, is required for suppressing the precocious acquisition of forebrain characteristics in mouse embryos [38]. Our data indicated that sea urchin embryos use the similar mechanisms to pattern the neuroectoderm, controlling the timing and location of the differentiation of serotonergic neurons. In addition, because there are other types of neurons present on the ventral side of the AP in sea urchin embryos [7], future investigations regarding the relationship between in-cell factors characterizing those neurons and TGF-ß signaling coming from the outside of the neurogenic AP region will lead us to understand the conserved mechanisms of neural patterning throughout the animal kingdom.
Canonical and non-canonical Wnt signals regulate the anteroposterior patterning of the neurogenic ectoderm
Persistent FoxQ2 and apical tufts in the AP region in LRP6 morphants strongly indicate that the cWnt pathway is required to suppress FoxQ2 and exert a neural fate, although it has been reported that early cWnt, visualized with the nuclear localization of ß-catenin, was observed only at the posterior half of the embryo until the gastrula stage [22]. In addition, our results suggest that Wnt7 works as a ligand in the LRP-cWnt pathway in the AP that suppresses FoxQ2 with precise timing (Fig 6). Although we could not rule out the possibility that Wnt7 functions indirectly from outside of the AP of normal embryos, based on its expression patterns, the strong expression of wnt7 at the thickened AP of normal (Fig 6) and Δcad embryos (S6E and S6H Fig) suggested that it plays an intrinsic role in FoxQ2 suppression within the AP. The difference between LRP6 morphants (abundant FoxQ2 and no serotonergic neurons) and Δcad embryos (less FoxQ2 and a number of serotonergic neurons) might be attributed to the lifetime length of exogenous Δcad mRNA and/or protein. Because the injected mRNA can last approximately 24 hr (e.g., S8A and S8B Fig), only early cWnt but not later cWnt is suppressed in Δcad embryos. This idea was well supported by Top-Flash assay (Fig 6). Of course, we cannot completely rule out the possibility that Δcad alone is not sufficient to block all cWnt, even during the early stages. The future TOP-Flash assay during the early stages can answer this question. Because foxQ2 expression is at a gradient from anterior tip to periphery (Fig 1; [11]), the area of the biggest effect of foxQ2 removal by Wnt7/cWnt might be the edge of the AP region, resulting in serotonergic neurons starting the differentiation process at the position. A previous report [31] showed that foxQ2 was expressed at the posterior half, where this gene is never detected by in situ hybridization in normal embryos, when Axin was misexpressed, and they implicated that foxQ2 is originally expressed throughout the embryo and early cWnt at the posterior half suppressed it. Thus, it is expected that the mechanisms suppressing foxQ2 expression were also applied in the AP region after it is restricted to the anterior end.
The results from JNK inhibition led us to consider that non-cWnt is involved in neurogenesis in the AP of the sea urchin embryos (Fig 7). Additionally, data regarding its ligand, Wnt6, supported our JNK result (Fig 7). However, it is still not clear how Wnt6 mediates non-cWnt signaling in the AP region. Because it has been reported that wnt6 is zygotically expressed at the vegetal plate and functions in endomesoderm formation [30,39], it is possible that the signal is indirect. However, our data using Δcad and Wnt6-MO indicated that Wnt6 could function within the AP region even though the expression of mRNA at that location is faint (S6 Fig). More detailed studies of its distribution and function with protein level will be necessary to understand the complete mechanisms of Wnt6 function. In contrast to the Wnt7 data, the normal disappearance of FoxQ2 in Wnt6 morphants indicated that Wnt6 did not function as a ligand of the cWnt pathway in the AP. The non-cWnt includes pathways other than JNK, planar cell polarity (PCP) and Ca2+ pathways [40], suggesting that those pathways are also involved in AP patterning in the sea urchin embryo. In fact, as a ligand for the non-cWnt pathway, Wnt6 might not be sufficient because JNK inhibition led to some foxQ2 remaining in the AP, indicating that the JNK pathway weakly acts in suppressing FoxQ2 and affects the precise control of the timing and the location of serotonergic neurons (Fig 7). It was reported that the JNK pathway functions in restricting the AP region, represented by foxQ2 and hbn expression, to the anterior end during blastula stages [31], but the Wnt6 morphant had no expanded AP and tended to have a more restricted hbn region, supporting the idea that other ligands for non-cWnt signaling function during anterior neuroectoderm formation.
Our results suggest that cWnt and non-cWnt signaling function in repressing FoxQ2 and Hbn, respectively, but we cannot completely rule out the possibility that each pathway affects both types of repression. This is because LRP6 morphants showed slightly delayed hbn clearance and JNK inhibition allowed some foxQ2 to remain in the AP region. Because cWnt and non-cWnt antagonize each other in some biological processes [41], this cross-reaction might be normal in AP formation in sea urchin embryos. We must also consider the functional combination of frizzled receptors, secreted frizzled-related protein, and Dickkopfs (Dkks) to determine the complete involvement of the Wnt pathways in AP patterning. In fact, the restriction of the AP to the anterior end is managed through their combination with other Wnt ligands, such as Wnt1 and Wnt8 [31], and the patterning of anterior structure, including neuroectoderm, is also regulated those factors in other deuterostomes [42–44]. In contrast, both LRP6 and Wnt7 morphants failed to finish the restriction of the AP region by the blastula stages (Figs 5 and 6), similar to Wnt1 and Wnt8 morphants [31], suggesting that those factors affect the early cWnt events that restrict AP to the anterior end. Our data using Fzl5/8 morphants suggested that this frizzled functions as a receptor for both cWnt and non-cWnt signals as was observed in other systems [45]. This relationship strongly supports the idea that cWnt and non-cWnt cross-react with each other through sharing the frizzled receptor during AP patterning, although we cannot rule out that other types of frizzled, which we did not analyze in this study, are more essential for each pathway. Adding to our knowledge of the involvement of these molecules, biochemical analyses to reveal ligand-receptor associations will be conducted in the future to understand the complete pathway regulating AP formation in the sea urchin embryo.
As mentioned in the Introduction, the anterior neural fate in vertebrates is restricted by Wnt signaling from the posterior side [5]. Posterior Wnt signals are also reported in invertebrates, such as sea urchins [31,46,47] and amphioxus [48,49]. These species commonly use Wnt signals to establish posterior identities during early development. In this study, we found that wnt7 is expressed in the AP region and required for the differentiation of serotonergic neurons as a ligand for the cWnt pathway (Fig 6), indicating that the sea urchin embryos utilize cWnt signaling at both the posterior and anterior ends. In addition, anterior and posterior cWnt share a function, repressing foxQ2 expression (in this study, [12,31]). The simple mechanism that cWnt suppresses foxQ2 with shifting the functional timing, early at the posterior and later at the anterior ends, enables embryos to have a complicated body plan along the anterior-posterior body axis: FoxQ2 initially specifies the AP region only at the anterior end and later it disappears from the AP to let the serotonergic neurons differentiate within it. We accidentally found this both-end cWnt signaling because we blocked the early cWnt at the posterior end using exogenous mRNA encoding Δcad, which has a short life-time. If we permanently and completely inhibit some of the components of the cWnt pathway, it might be difficult to recognize the later functioning anterior cWnt. It is possible that this type of anterior cWnt commonly functions in other systems during early development because in vertebrates it was reported that the wnt7 family was expressed in the developing anterior neuroectoderm region [50].
Crosstalk between TGF-ß and Wnt signals during patterning of the neurogenic ectoderm
One of the most interesting findings in this study is that the dorsoventral Nodal pathway might interfere with the anteroposterior Wnt pathway during the embryogenesis of the sea urchin. Because a number of studies previously revealed the molecular mechanisms of cell fate specification along these embryonic axes in many species, we have now accumulated the information to determine, for example, how the anteroposterior axis is formed by the bicoid gradient in the fly [51–53] or how left-right asymmetry is created by Nodal flow in mice [54,55]. However, because embryos must control cell fate specification along all three body-axes in our three-dimensional world with precise timing, the formation of the body axes should not be independent of each other. Thus, the information from processes that occur along each axis should be integrated with a high degree of sophistication and affect cell-fate specification during each step of embryogenesis. We have previously reported that a single transcription factor links anteroposterior-dorsoventral axis formation in the sea urchin embryo and that it regulates the timing of the onset of specification of the secondary axis downstream of primary axis formation [12]. By combining our results with previous reports, we propose the following five combinational signaling steps that regulate serotonergic neuron formation at the dorsal/lateral edge of the AP in the sea urchin embryo (Fig 8A and 8B): 1) posterior cWnt/non-cWnt signaling restricts the AP, which is specified by early FoxQ2, to the anterior end [6,31], 2) Wnt7/cWnt suppresses late FoxQ2, which induces the apical tuft cilia and represses neural fate, at the edge of the restricted AP along the anterior-posterior axis, 3) dorsoventral Nodal suppresses Wnt7/cWnt to maintain late FoxQ2 at the ventral side, 4) Wnt6/non-cWnt suppresses the neural specifier Hbn, preventing its expression in the anterior end, and 5) BMP2/4 strongly maintains the expression of neural specifier Hbn at the dorsal side whereas Nodal suppresses it at the ventral side. After the AP is restricted, the regulation of the expression of two opposing functional transcription factors, the neural specifier Hbn and late neural suppressor FoxQ2, is accomplished by the molecular mechanisms of neural patterning in the AP. Crosstalk between Wnts along the primary axis and Nodal along the secondary axis is carried out during the process of suppressing the serotonergic neural fate at the ventral side of the AP. Although suppressing the specifier of neurons, Hbn, at the ventral side seems to be sufficient, maintaining the suppressor, FoxQ2, within the same region is a great supporting system for embryos to ensure the removal of the neural fate.
Our data suggested that the effect of Nodal suppressing cWnt at the AP region seems to reach to the dorsal edge (Fig 3). However, because it is reported that Nodal can diffuse to short range in AP region [56], we do not know how the Nodal pathway controls the sizing of the entire AP through interactions with cWnt signaling in the AP. As wnt7 is expressed abundantly in the AP (Fig 6), the Nodal pathway might affect its expression regulation even though Nodal itself can bind the receptor in a few rows at the ventral edge of the AP. In addition, the downstream factors of Nodal signaling, e.g., Lefty and BMP2/4, can diffuse to the AP region [12,14,23], and they might interact with cWnt pathway directly or indirectly to regulate the size control of the AP.
In S. purpuratus, it was indirectly implicated that Nodal regulates the expression of foxQ2 by controlling transcription factors, Not, and Emx. Their relationships seem to be complicated along the spatiotemporal patterning [57,58], and none of them have yet been analyzed in H. pulcherrimus. However, our data on Nodal loss-of-function and gain-of-function are quite reproducible during those stages (Fig 3), and even in S. purpuratus Nodal morphants had a smaller AP, judging from the distribution of serotonergic neurons [18], supporting the idea that Nodal maintains the foxQ2 expression in the sea urchin embryos. The precise cis-regulatory analysis of the foxQ2 expression pattern in the future will lead us to understand the detailed molecular mechanisms of how TGF-ß signals control AP patterning.
Exerting neural fate through FoxQ2 and Hbn functions
Because we have not yet succeeded in completely dissociating single cell-cultures, we cannot, strictly speaking, conclude that the default fate of sea urchin cells is the neuroectoderm or neurons. However, if the earliest known signal, cWnt, which functions in the posterior half at the beginning of the 8–16 cell-stage [59], is blocked, almost the entire region develops into neurogenic AP [31], suggesting that the initial or pre-signaling fate of sea urchin cells is anterior neuroectoderm. Within this expanded pre-signaling AP, embryos differentiate a number of serotonergic neurons that are scattered throughout the AP, in which other cells are produced with long, immotile, apical tuft cilia [12,13]. Removing Notch signaling from the AP promotes an increased number and the clustering of serotonergic neurons, indicating that lateral inhibition in the AP is another signal that inhibits the serotonergic neural fate in the sea urchin embryo [8]. FoxQ2 is required to exert the serotonergic neural fate initially [12]. However, FoxQ2 is a bifunctional transcription factor that is required early for the specification of most of the cell types in the anterior neuroectoderm, and then it must be removed from the cell late, which subsequently takes on a serotonergic neural fate [8,12–14]. As an initial specifier, the function of FoxQ2 might be similar to that of Zfp521 in mouse embryos. Zfp521 is zygotically expressed by a cell-intrinsic mechanism to exert the initial neural fate [60]. In sea urchins, however, the initial specifier is substituted with a second one, Hbn, because the function of FoxQ2 becomes another one that is against serotonergic neural differentiation after the blastula stage. Taken together, the unknown mechanisms that initially induce foxQ2 and/or hbn at the anterior end of the sea urchin embryo are the substances that determine the initial cell fate, which will be clarified in the future through the analysis of cis-elements of the foxQ2 and hbn genes. So far, none of functional data of Hbn in other systems have been published, but understanding these mechanisms will lead us to answer the question of what is truly the default cell fate in the sea urchin embryo as well as in other organisms.
Materials and Methods
Animals and embryo culture
Embryos of Hemicentrotus pulcherrimus were collected around Shimoda Marine Research Center, University of Tsukuba, and around the Marine and Coastal Research Center, Ochanomizu University. The divergence time between H. pulcherrimus used in this study and S. purpuratus used in most previously described studies was estimated to be 7.2–14 million years ago [61], and the developmental time-course, gene expression patterns, and reported phenotypes in gene-knockdown and/or misexpressed experiments are almost the same. The gametes were collected by the intrablastocoelar injection of 0.5 M KCl, and embryos were cultured in glass beakers or plastic dishes that contained filtered natural seawater (FSW) at 15°C. Cell-permeable JNK inhibitor I, (L)-Form (Merck Millipore, Billerica, MA, USA), was used at 50 μM from the two-cell stage to desired stages [31]. For the control experiment, we added same volume of dimethyl sulfoxide (DMSO), which is used for dissolving the JNK inhibitor.
Whole-mount in situ hybridization and immunohistochemistry
In whole-mount in situ hybridization, embryos were fixed with 3.7% formaldehyde-sea water (SW) overnight at 4°C. After 7 x 7 min washes in MOPS buffer (0.1 M MOPS, pH 7.0, 0.5 M NaCl, 0.1% Tween-20), MOPS buffer was substituted with hybridization buffer (HB: 70% formamide, 0.1 M MOPS, pH 7.0, 0.5 M NaCl, 0.1% Tween-20, 1% BSA), and specimens were pre-hybridized at 50°C for 1 h. Subsequently, pre-hybridization HB was substituted with fresh HB containing Dig-labeled RNA probes (0.4 ng/μl final concentration), and samples were incubated at 50°C for 5–7 days. After washing in MOPS buffer for 7 min x 7 times at room temperature (RT), for 1 h x 3 times at 50°C, and for 7 min x 2 times at RT, samples were blocked with 1–5% skim milk (Nacalai Tesque, Tokyo, Japan) in MOPS buffer for 1 h at RT and thereafter incubated with anti-Dig antibody conjugated with alkaline phosphatase (Roche, Basel, Switzerland; 1:1,500 dilution) overnight at 4°C. Tissue was washed with MOPS buffer for a half day with several buffer exchanges. Dig signal was detected with NBT/BCIP (Promega, Madison, WI, USA). For two-color fluorescent in situ hybridization, Dig-labeled and FITC-labeled probes were simultaneously applied to HB and detected with anti-Dig and anti-FITC POD-conjugated antibodies, respectively (Roche), followed by the Tyramide signal amplification plus system (TSA-plus; Perkin Elmer, Waltham, MA, USA). After blocking in 1–5% skim milk, specimens were incubated with 1:1,000 diluted anti-Dig POD-conjugated antibody for 1 h at RT, washed with MOPS buffer for 7 min x 7 times at RT, and treated with tetramethylrhodamine TSA-plus for 10 min at RT. Then, samples were washed three times with MOPS buffer, and the remaining POD function was quenched by 0.5% sodium azide in MOPS buffer for 30 min at RT. After washing, we repeated treatment of the samples with anti-FITC antibody and the FITC TSA-plus system. The size of the foxQ2-expressing region is quantified with the angle from the posterior end (Fig 3P). The angle was measured using ImageJ and Student’s t-test was applied to each quantification to judge whether their differences were significantly meaningful. The graph was drawn with software R [62].
In whole-mount immunohistochemistry, embryos were fixed with 3.7% formaldehyde-SW for 10 min at RT. After washing with PBST (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.76 mM KH2PO4, pH 7.4, 0.1% Tween-20) for 7 min x 7 times, samples were blocked with 1–5% lamb serum in PBST for 1 h at RT and incubated with primary antibodies (dilutions: serotonin (Sigma-Aldrich, St. Louis, MO, USA) 1:2,000, synB [7] 1:100, LRP6 (Sigma) 1:1,000, FoxQ2 [14] 1:100 and c-myc (Sigma) 1:1,000) overnight at 4°C. Antibodies were washed off with PBST for 7 min x 7 times, and the samples were incubated with the secondary antibodies (1:2,000 diluted anti-rabbit IgG conjugated with Alexa 488 and/or 1:2,000 diluted anti-mouse IgG conjugated with Alexa 568 (Thermo Fisher Scientific, Waltham, MA, USA)) for 2 h at RT. The specimens were observed using a Zeiss Axio Imager.Z1 that was equipped with an Apotome system (Zeiss, Oberkochen, Germany) and an Olympus FV10i confocal laser scanning microscope (Olympus, Tokyo, Japan). The optical sections were stacked and analyzed using ImageJ and Adobe Photoshop. Panels and drawings for the figures were made using Microsoft PowerPoint. The number of FoxQ2-positive cells was counted under the fluorescent microscope (IX70, Olympus). Student t-test was applied on each quantification to judge whether their differences were significantly meaningful.
Microinjection of morpholino antisense oligonucleotides (MO) and mRNAs
The morpholino (Gene Tools, Philomath, OR, USA) sequences and the in-needle concentration with 24% glycerol were as follows:
Hbn-MO1 (0.7 mM): 5’- AAAATGAACGGAACAAGTCCAGTGT -3’,
Hbn-MO2 (2.0 mM): 5’- TAGGAGAACCAACGACCGCCGTCAT -3’,
Nodal-MO (0.2 mM): 5’- AGATCCGATGAACGATGCATGGTTA -3’,
Lefty-MO (0.4 mM): 5’- AGCACCGAGTGATAATTCCATATTG -3’,
FoxQ2-MO (0.2 mM): 5’- TCATGATGAAATGTTGGAACGAGAG -3’,
BMP2/4-MO (0.4 mM): 5’- GACCCCAATGTGAGGTGGTAACCAT -3’,
LRP6-MO1 (1.9 mM): 5’- GAAAGGTTTCAAGGCAGCCCATTTC -3’,
LRP6-MO2 (1.5 mM): 5’- TGCCGTTGACTAAATATCATCTACA -3’,
Wnt6-MO1 (3.8 mM): 5’- ACGTGTCCACTCCATCTTGTAATAC -3’,
Wnt6-MO2 (1.9 mM): 5’- TCGTCCAGCGATTTAATAAAGAGCT -3’,
Wnt7-MO1 (3.8 mM): 5’- ATAACCACACCAAgTTgggCCgCAT -3’, and
Wnt7-MO2 (1.9 mM): 5’- GCTCAGCGATGCCCGATGGATAAAA -3’.
Two non-overlapping morpholinos that blocked the translation of Hbn, LRP6, Wnt6 and Wnt7 were used to confirm the specificity of their function. For negative control experiments, we injected 24% glycerol into eggs.
mRNAs were synthesized from linearized plasmids using the mMessage mMachine kit (Thermo Fisher Scientific) and injected at the indicated concentrations in 24% glycerol in needles: hbn-mRNA (0.1 μg/μl), Δ-cadherin (0.3–0.6 μg/μl; [22]), and myc-mRNA (0.1 μg/μl). Microinjections into fertilized eggs and into one blastomere at the two-cell stage were performed as previously described [13].
Quantitative PCR
Quantitative PCR (qPCR) was performed as previously described [13,63] with some modifications. The total RNA from 100 embryos of H. pulcherrimus was isolated, and reverse transcription was performed using the Realtime Ready Cell Lysis kit and Transcriptor Universal cDNA Master (Roche). GoTaq qPCR Master Mix (Promega) was used for PCR carried out with a Thermal Cycler Dice Real Time system (Takara, Shiga, Japan). Primer pairs used for qPCR were the following:
Wnt3-qF1; 5’- TATATCCGGCAAACAGGTCC -3’,
Wnt3-qR1; 5’- TCTTCTCCCTCGGAACTGAA -3’,
Wnt6-qF1; 5’- GACCTGCTGGAAGAAAATGC -3’,
Wnt6-qR1; 5’- GGGCTGTTTGACCGTATCAT -3’,
Wnt7-qF1; 5’- CATGGTGTTTCAGGTTCGTG -3’,
Wnt7-qR1; 5’- TCCTAGTTCGTTTGGCCTTG -3’,
COI-qF1; 5’- CCGCATTCTTGCTCCTTCTT -3’, and
COI-qR1; 5’- TGCTGGGTCGAAGAAAGTTG -3’.
The relative concentrations of each mRNA were normalized with mitochondrial COI Ct values.
Luciferase assay
Top-Flash plasmid M50 Super 8xTOPFlash (Addgene plasmid # 12456) and M51 Super 8xFOPFlash (TOPFlash mutant) (Addgene plasmid # 12457) were gifts from Dr. Randall Moon. DNA fragments containing TCF/LEF-binding sites with Firefly Luciferase gene were amplified by KOD-Fx DNA polymerase (TOYOBO, Tokyo, Japan) with RVprimer3 and EBV_rev_primer set and injected at 20 ng/μl in a needle into the fertilized eggs with a carrier EcoRV-digested H. pulcherrimus genomic DNA at 10 ng/μl. The signal was obtained from 20–40 embryos for each experiment (three independent batches) using the Bright-Glo Luciferase Assay System (Promega). The luminescence was detected with the LB941 Multimode Reader TriStar (Berthold Technologies GmbH & Co.KG, Bad Wildbad, Germany) for 60 sec. The Top-Flash signal was normalized to the Fop-Flash level for each experiment.
Supporting Information
(A) Brightfield image of control embryo at 96 h. (B) Serotonin (green) and synB (magenta) in (A). (C) Transmission image in Hbn morphants. (D) Serotonin and synB in Hbn morphants. (E, F) Serotonin and synB in control and HbnMO-2 embryos, respectively, at 72 h. (G) Number of serotonergic neurons in control embryo and Hbn morphants at 72 h. Independent of batches, the number of serotonergic neurons is significantly decreased in Hbn morphants. ***P<0.001, Student’s t-test.
(TIF)
(A, B) Similar pattern of neurons in Δcad embryos with or without Hbn mRNA. (C) Without Homeobrain, the serotonergic neurons are not differentiated in Δcad embryos. (D) Exogenous Hbn can partially rescue the Hbn morphant phenotype. (E) Schematic image of the experimental procedure used in 2-cell injections to confirm the sufficiency of Hbn mRNA in the differentiation of serotonergic neurons. (F, G) Control of 2-cell injection experiment. No serotonergic neurons are differentiated in the myc-mRNA alone-injected side of Δcad-Hbn morphants. (H, I) The rescued serotonergic neurons are present at the exogenous Hbn-injected side. (J) The number of serotonergic neurons counted in the half of the embryos in each experiment. The number of serotonergic neurons in the Hbn-mRNA injected side is significantly increased over that in the Hbn-mRNA negative side, but the number does not reach that of the control. *P<0.05, ***P<0.001, Student’s t-test.
(TIF)
(A-C) in situ hybridization reveals that misexpressed Nodal suppresses hbn, and qPCR data shows the tendency, but the difference is not significant (<0.5, >2.0) (D). hbn mRNA in BMP2/4 morphants is only significantly decreased, indicating that BMP2/4 is required for hbn expression.
(TIF)
Almost all cells express LRP6 message in Hemicentrotus pulcherrimus from unfertilized egg until at least 25 h (A-C). (D-F) Negative control using sense RNA probe for LRP6. (G-J) LRP signal in normal (G-I) and LRP morphants (J). (G) Epifluorescent image of (H). (I) and (J) are stacked images of confocal microscopy and captured under the same microscopic conditions with the same exposure time. (K-O) LRP6 morphants have a normal dorsoventral body axis. Ventral marker, nodal (M), and ciliary band marker, hnf6 (N, O), are normally expressed in LRP6 morphants as they are in the control (K, L). (P) hbn disappearance occurs normally in Δcad-LRP6 morphants at 50 h.
(TIF)
(A) When Wnt7 is missing, the significant decrease of the foxQ2 region in Nodal morphants is never occurred, indicating that Wnt7 is the factor that suppresses foxQ2 in the AP region.foxQ2 expression patterns in the control (B), Nodal morphants (C), Wnt7 morphant (D) and a Nodal-Wnt7 morphant (E). ***P<0.001, Student’s t-test.
(TIF)
(A-D) The expression pattern of wnt6 in the control (A, C) and Δcad-embryos (B, D). wnt6 is slightly expressed at the thickened ectodermal region in Δcad-injected embryos. (E-H) The expression pattern of wnt7 in the control (E, G) and Δcad-embryos (F, H). (I-K) The expression pattern of hbn in Δcad (I), Δcad-Wnt6MO (J) and Δcad-Wnt7MO embryos (K). At this stage, hbn is expressed only at the posterior squamous ectoderm and has disappeared from thickened region in Δcad (I) and Δcad-Wnt7MO embryos (K). In contrast, the disappearance of hbn is inhibited in Δcad-Wnt6MO embryos (J). Asterisk (*) in (I) indicates the position of squamous ectoderm expressing hbn. (L-O) Wnt7 and Wnt6 morphant phenotypes described in the text are reproducible using the second non-overlapped morpholinos for each. foxQ2 is remains in Wnt7-MO2 morphants at 65 h (M) but not in the control (L). The disappearance of the hbn gene from the anterior end of the AP is robust in the control at 65 h (N), but the gene expression is not cleared from the place in Wnt6-MO2 morphants (O). White and black arrowheads indicate the location of hbn absence and presence, respectively.
(TIF)
hbn expression pattern in the control (A, B, C) and FoxQ2 morphants (D, E, F). foxQ2 expression pattern in the control (G, H) and Hbn morphants (I, J). These results indicate that their expression is mutually independent. MBL, mesenchyme blastula; early G, early gastrula.
(TIF)
mRNA encoding Hbn was injected into fertilized eggs and detected with in situ hybridization using the Hbn probe. The exogenous mRNA was detected at 16 h in the whole body (A), but by 24 h only the endogenous hbn was detected (B).
(TIF)
Acknowledgments
We thank Drs. Robert Burke, Yoko Nakajima Randall Moon, and David McClay for essential reagents. We thank Mrs. Y. Tsuchiya, T. Sato, H, Shinagawa, and Y. Yamada, Shimoda Marine Research Center, for collecting and keeping the adult sea urchins.
Data Availability
All DNA sequence data are available from GenBank database (accession numbers LC064116, LC064120, LC064118, LC064119, LC064117).
Funding Statement
This work is supported by JSPS KAKENHI (Grant-in Aid for Scientific Research C: No. 25440101), Takeda Science Foundation, and Kishimoto Foundation to SY. JY was a Postdoctoral Fellows of JSPS with research grant (RPD:No.2640015). These funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.De Robertis EM, Sasai Y (1996) A common plan for dorsoventral patterning in Bilateria. Nature 380: 37–40. Available: http://www.ncbi.nlm.nih.gov/pubmed/8598900. Accessed 29 June 2015. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz-Sanjuán I, Brivanlou AH (2002) Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 3: 271–280. Available: http://www.ncbi.nlm.nih.gov/pubmed/11967557. Accessed 7 January 2015. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, et al. (2005) Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8: 288–296. Available: http://www.ncbi.nlm.nih.gov/pubmed/15696161. Accessed 29 June 2015. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Zhang S-C (2011) Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci 68: 3995–4008. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3206177&tool=pmcentrez&rendertype=abstract. Accessed 12 June 2015. 10.1007/s00018-011-0770-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiecker C, Niehrs C (2001) A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128: 4189–4201. Available: http://www.ncbi.nlm.nih.gov/pubmed/11684656. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 6.Yaguchi S, Yaguchi J, Burke RD (2006) Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development 133: 2337–2346. Available: http://www.ncbi.nlm.nih.gov/pubmed/16687447. Accessed 8 October 2013. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima Y, Kaneko H, Murray G, Burke RD (2004) Divergent patterns of neural development in larval echinoids and asteroids. Evol Dev 6: 95–104. Available: http://www.ncbi.nlm.nih.gov/pubmed/15009122. [DOI] [PubMed] [Google Scholar]
- 8.Yaguchi J, Angerer LM, Inaba K, Yaguchi S (2012) Zinc finger homeobox is required for the differentiation of serotonergic neurons in the sea urchin embryo. Dev Biol 363: 74–83. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3288183&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. 10.1016/j.ydbio.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angerer LM, Yaguchi S, Angerer RC, Burke RD (2011) The evolution of nervous system patterning: insights from sea urchin development. Development 138: 3613–3623. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3152920&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. 10.1242/dev.058172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaguchi S, Kanoh K, Amemiya S, Katow H (2000) Initial analysis of immunochemical cell surface properties, location and formation of the serotonergic apical ganglion in sea urchin embryos. Dev Growth Differ 42: 479–488. Available: http://www.ncbi.nlm.nih.gov/pubmed/11041489. [DOI] [PubMed] [Google Scholar]
- 11.Tu Q, Brown CT, Davidson EH, Oliveri P (2006) Sea urchin Forkhead gene family: phylogeny and embryonic expression. Dev Biol 300: 49–62. Available: http://www.ncbi.nlm.nih.gov/pubmed/17081512. Accessed 22 May 2014. [DOI] [PubMed] [Google Scholar]
- 12.Yaguchi S, Yaguchi J, Angerer RC, Angerer LM (2008) A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev Cell 14: 97–107. Available: http://www.ncbi.nlm.nih.gov/pubmed/18194656. Accessed 8 October 2013. 10.1016/j.devcel.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 13.Yaguchi S, Yaguchi J, Wei Z, Shiba K, Angerer LM, et al. (2010) ankAT-1 is a novel gene mediating the apical tuft formation in the sea urchin embryo. Dev Biol 348: 67–75. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2976814&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. 10.1016/j.ydbio.2010.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaguchi S, Yaguchi J, Wei Z, Jin Y, Angerer LM, et al. (2011) Fez function is required to maintain the size of the animal plate in the sea urchin embryo. Development 138: 4233–4243. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3171223&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. 10.1242/dev.069856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fröbius AC, Seaver EC (2006) Capitella sp. I homeobrain-like, the first lophotrochozoan member of a novel paired-like homeobox gene family. Gene Expr Patterns 6: 985–991. Available: http://www.ncbi.nlm.nih.gov/pubmed/16765105. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 16.Walldorf U, Kiewe A, Wickert M, Ronshaugen M, McGinnis W (2000) Homeobrain, a novel paired-like homeobox gene is expressed in the Drosophila brain. Mech Dev 96: 141–144. Available: http://www.ncbi.nlm.nih.gov/pubmed/10940637. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 17.Burke RD, Angerer LM, Elphick MR, Humphrey GW, Yaguchi S, et al. (2006) A genomic view of the sea urchin nervous system. Dev Biol 300: 434–460. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1950334&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Z, Yaguchi J, Yaguchi S, Angerer RC, Angerer LM (2009) The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136: 1179–1189. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2685935&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. 10.1242/dev.032300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazza ME, Pang K, Reitzel AM, Martindale MQ, Finnerty JR (2010) A conserved cluster of three PRD-class homeobox genes (homeobrain, rx and orthopedia) in the Cnidaria and Protostomia. Evodevo 1: 3 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2938728&tool=pmcentrez&rendertype=abstract. Accessed 3 July 2015. 10.1186/2041-9139-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cameron RA, Samanta M, Yuan A, He D, Davidson E (2009) SpBase: The sea urchin genome database and web site. Nucleic Acids Res 37: 750–754. 10.1093/nar/gkn887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs R a, et al. (2006) The genome of the sea urchin Strongylocentrotus purpuratus. Science 314: 941–952. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3159423&tool=pmcentrez&rendertype=abstract. Accessed 21 September 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Logan CY, Miller JR, Ferkowicz MJ, McClay DR (1999) Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126: 345–357. Available: http://www.ncbi.nlm.nih.gov/pubmed/9847248. [DOI] [PubMed] [Google Scholar]
- 23.Duboc V, Röttinger E, Besnardeau L, Lepage T (2004) Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev Cell 6: 397–410. Available: http://www.ncbi.nlm.nih.gov/pubmed/15030762. [DOI] [PubMed] [Google Scholar]
- 24.Lapraz F, Besnardeau L, Lepage T (2009) Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol 7: e1000248 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2772021&tool=pmcentrez&rendertype=abstract. Accessed 16 September 2013. 10.1371/journal.pbio.1000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saudemont A, Haillot E, Mekpoh F, Bessodes N, Quirin M, et al. (2010) Ancestral regulatory circuits governing ectoderm patterning downstream of Nodal and BMP2/4 revealed by gene regulatory network analysis in an echinoderm. PLoS Genet 6: e1001259 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3009687&tool=pmcentrez&rendertype=abstract. Accessed 24 September 2013. 10.1371/journal.pgen.1001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duboc V, Lapraz F, Besnardeau L, Lepage T (2008) Lefty acts as an essential modulator of Nodal activity during sea urchin oral-aboral axis formation. Dev Biol 320: 49–59. Available: http://www.ncbi.nlm.nih.gov/pubmed/18582858. Accessed 8 October 2013. 10.1016/j.ydbio.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 27.Yaguchi S, Yaguchi J, Angerer RC, Angerer LM, Burke RD (2010) TGFβ signaling positions the ciliary band and patterns neurons in the sea urchin embryo. Dev Biol 347: 71–81. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2950233&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. 10.1016/j.ydbio.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei Z, Angerer RC, Angerer LM (2006) A database of mRNA expression patterns for the sea urchin embryo. Dev Biol 300: 476–484. Available: http://www.ncbi.nlm.nih.gov/pubmed/17007833. Accessed 2 May 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamateris RE, Rafiq K, Ettensohn CA (2010) The expression and distribution of Wnt and Wnt receptor mRNAs during early sea urchin development. Gene Expr Patterns 10: 60–64. Available: http://www.ncbi.nlm.nih.gov/pubmed/19853669. Accessed 3 July 2015. 10.1016/j.gep.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 30.Robert N, Lhomond G, Schubert M, Croce JC (2014) A comprehensive survey of wnt and frizzled expression in the sea urchin Paracentrotus lividus. Genesis 52: 235–250. 10.1002/dvg.22754 [DOI] [PubMed] [Google Scholar]
- 31.Range RC, Angerer RC, Angerer LM (2013) Integration of canonical and noncanonical Wnt signaling pathways patterns the neuroectoderm along the anterior-posterior axis of sea urchin embryos. PLoS Biol 11: e1001467 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3545869&tool=pmcentrez&rendertype=abstract. Accessed 16 September 2013. 10.1371/journal.pbio.1001467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui M, Siriwon N, Li E, Davidson EH, Peter IS (2014) Specific functions of the Wnt signaling system in gene regulatory networks throughout the early sea urchin embryo. Proc Natl Acad Sci U S A 111: E5029–E5038. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4250154&tool=pmcentrez&rendertype=abstract. Accessed 3 July 2015. 10.1073/pnas.1419141111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, et al. (1997) Constitutive transcriptional activation by a b-catenin-Tcf complex in APC-/- colon carcinoma. Science (80-) 275: 1784–1787. [DOI] [PubMed] [Google Scholar]
- 34.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT (2003) Zebrafish Prickle, a Modulator of Noncanonical Wnt/Fz Signaling, Regulates Gastrulation Movements. Curr Biol 13: 680–685. 10.1016/S [DOI] [PubMed] [Google Scholar]
- 35.Poustka AJ, Kühn A, Groth D, Weise V, Yaguchi S, et al. (2007) A global view of gene expression in lithium and zinc treated sea urchin embryos: new components of gene regulatory networks. Genome Biol 8: R85 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1929154&tool=pmcentrez&rendertype=abstract. Accessed 8 October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wurst W, Bally-Cuif L (2001) Neural plate patterning: upstream and downstream of the isthmic organizer. Nat Rev Neurosci 2: 99–108. Available: http://www.ncbi.nlm.nih.gov/pubmed/11253000. [DOI] [PubMed] [Google Scholar]
- 37.Guo S, Brush J, Teraoka H, Goddard a, Wilson SW, et al. (1999) Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron 24: 555–566. 10.1016/S0896-6273(00)81112-5 [DOI] [PubMed] [Google Scholar]
- 38.Camus A, Perea-Gomez A, Moreau A, Collignon J (2006) Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev Biol 295: 743–755. 10.1016/j.ydbio.2006.03.047 [DOI] [PubMed] [Google Scholar]
- 39.Croce J, Range R, Wu S-Y, Miranda E, Lhomond G, et al. (2011) Wnt6 activates endoderm in the sea urchin gene regulatory network. Development 138: 3297–3306. 10.1242/dev.058792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komiya Y, Habas R (2008) Wnt signal transduction pathways. Organogenesis 4: 68–75. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2634250&tool=pmcentrez&rendertype=abstract. Accessed 12 January 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuzugullu H, Benhaj K, Ozturk N, Senturk S, Celik E, et al. (2009) Canonical Wnt signaling is antagonized by noncanonical Wnt5a in hepatocellular carcinoma cells. Mol Cancer 8: 90 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2770486&tool=pmcentrez&rendertype=abstract. Accessed 3 July 2015. 10.1186/1476-4598-8-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, et al. (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362. Available: http://www.ncbi.nlm.nih.gov/pubmed/9450748. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi TP (2001) Heads or tails: Wnts and anterior-posterior patterning. Curr Biol 11: R713–R724. Available: http://www.ncbi.nlm.nih.gov/pubmed/11553348. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 44.Kim S-H, Shin J, Park H-C, Yeo S-Y, Hong S-K, et al. (2002) Specification of an anterior neuroectoderm patterning by Frizzled8a-mediated Wnt8b signalling during late gastrulation in zebrafish. Development 129: 4443–4455. Available: http://www.ncbi.nlm.nih.gov/pubmed/12223403. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 45.Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, et al. (2010) Canonical and noncanonical wnts use a common, Grumolato.pdf. Genes Dev 24: 2517–2530. 10.1101/gad.1957710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson EH, Rast JP, Oliveri P, Ransick A, Calestani C, et al. (2002) A genomic regulatory network for development. Science 295: 1669–1678. Available: http://www.ncbi.nlm.nih.gov/pubmed/11872831. [DOI] [PubMed] [Google Scholar]
- 47.Davidson EH (2010) Emerging properties of animal gene regulatory networks. Nature 468: 911–920. Available: http://www.ncbi.nlm.nih.gov/pubmed/21164479. Accessed 16 September 2013. 10.1038/nature09645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu J-K, Satou Y, Holland ND, Shin-I T, Kohara Y, et al. (2007) Axial patterning in cephalochordates and the evolution of the organizer. Nature 445: 613–617. 10.1038/nature05472 [DOI] [PubMed] [Google Scholar]
- 49.Holland LZ, Panfilio K a., Chastain R, Schubert M, Holland ND (2005) Nuclear β-catenin promotes non-neural ectoderm and posterior cell fates in amphioxus embryos. Dev Dyn 233: 1430–1443. 10.1002/dvdy.20473 [DOI] [PubMed] [Google Scholar]
- 50.Beretta CA, Brinkmann I, Carl M (2011) All four zebrafish Wnt7 genes are expressed during early brain development. Gene Expr Patterns 11: 277–284. Available: http://www.ncbi.nlm.nih.gov/pubmed/21300182. Accessed 3 July 2015. 10.1016/j.gep.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 51.Driever W, Nüsslein-Volhard C (1988) A gradient of bicoid protein in Drosophila embryos. Cell 54: 83–93. Available: http://www.ncbi.nlm.nih.gov/pubmed/3383244. Accessed 24 May 2015. [DOI] [PubMed] [Google Scholar]
- 52.Driever W, Nüsslein-Volhard C (1989) The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature 337: 138–143. Available: http://www.ncbi.nlm.nih.gov/pubmed/2911348. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
- 53.Driever W, Nüsslein-Volhard C (1988) The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell 54: 95–104. Available: http://www.ncbi.nlm.nih.gov/pubmed/3383245. Accessed 27 May 2015. [DOI] [PubMed] [Google Scholar]
- 54.Hamada H, Meno C, Watanabe D, Saijoh Y (2002) Establishment of vertebrate left-right asymmetry. Nat Rev Genet 3: 103–113. Available: 10.1038/nrg732. Accessed 16 October 2014. [DOI] [PubMed] [Google Scholar]
- 55.Hirokawa N, Tanaka Y, Okada Y, Takeda S (2006) Nodal flow and the generation of left-right asymmetry. Cell 125: 33–45. Available: http://www.ncbi.nlm.nih.gov/pubmed/16615888. Accessed 11 September 2014. [DOI] [PubMed] [Google Scholar]
- 56.Yaguchi S, Yaguchi J, Burke RD (2007) Sp-Smad2/3 mediates patterning of neurogenic ectoderm by nodal in the sea urchin embryo. Dev Biol 302: 494–503. Available: http://www.ncbi.nlm.nih.gov/pubmed/17101124. Accessed 8 October 2013. [DOI] [PubMed] [Google Scholar]
- 57.Li E, Materna SC, Davidson EH (2012) Direct and indirect control of oral ectoderm regulatory gene expression by Nodal signaling in the sea urchin embryo. Dev Biol 369: 377–385. Available: 10.1016/j.ydbio.2012.06.022. 10.1016/j.ydbio.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li E, Cui M, Peter IS, Davidson EH (2014) Encoding regulatory state boundaries in the pregastrular oral ectoderm of the sea urchin embryo. Proc Natl Acad Sci 111: E906–E913. Available: http://www.pnas.org/content/111/10/E906.abstract. 10.1073/pnas.1323105111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kenny a P, Kozlowski D, Oleksyn DW, Angerer LM, Angerer RC (1999) SpSoxB1, a maternally encoded transcription factor asymmetrically distributed among early sea urchin blastomeres. Development 126: 5473–5483. Available: http://www.ncbi.nlm.nih.gov/pubmed/10556071. [DOI] [PubMed] [Google Scholar]
- 60.Kamiya D, Banno S, Sasai N, Ohgushi M, Inomata H, et al. (2011) Intrinsic transition of embryonic stem-cell differentiation into neural progenitors. Nature 470: 503–509. Available: http://www.ncbi.nlm.nih.gov/pubmed/21326203. Accessed 17 June 2015. 10.1038/nature09726 [DOI] [PubMed] [Google Scholar]
- 61.Lee YH (2003) Molecular phylogenies and divergence times of sea urchin species of Strongylocentrotidae, Echinoida. Mol Biol Evol 20: 1211–1221. 10.1093/molbev/msg125 [DOI] [PubMed] [Google Scholar]
- 62.Team RC (2015) R: A language and environment for statistical computing. Available: URL http://www.R-project.org/.
- 63.Ransick A (2004) Detection of mRNA by in situ hybridization and RT-PCR. Methods Cell Biol 74: 601–620. Available: http://www.ncbi.nlm.nih.gov/pubmed/15575623. Accessed 3 July 2015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Brightfield image of control embryo at 96 h. (B) Serotonin (green) and synB (magenta) in (A). (C) Transmission image in Hbn morphants. (D) Serotonin and synB in Hbn morphants. (E, F) Serotonin and synB in control and HbnMO-2 embryos, respectively, at 72 h. (G) Number of serotonergic neurons in control embryo and Hbn morphants at 72 h. Independent of batches, the number of serotonergic neurons is significantly decreased in Hbn morphants. ***P<0.001, Student’s t-test.
(TIF)
(A, B) Similar pattern of neurons in Δcad embryos with or without Hbn mRNA. (C) Without Homeobrain, the serotonergic neurons are not differentiated in Δcad embryos. (D) Exogenous Hbn can partially rescue the Hbn morphant phenotype. (E) Schematic image of the experimental procedure used in 2-cell injections to confirm the sufficiency of Hbn mRNA in the differentiation of serotonergic neurons. (F, G) Control of 2-cell injection experiment. No serotonergic neurons are differentiated in the myc-mRNA alone-injected side of Δcad-Hbn morphants. (H, I) The rescued serotonergic neurons are present at the exogenous Hbn-injected side. (J) The number of serotonergic neurons counted in the half of the embryos in each experiment. The number of serotonergic neurons in the Hbn-mRNA injected side is significantly increased over that in the Hbn-mRNA negative side, but the number does not reach that of the control. *P<0.05, ***P<0.001, Student’s t-test.
(TIF)
(A-C) in situ hybridization reveals that misexpressed Nodal suppresses hbn, and qPCR data shows the tendency, but the difference is not significant (<0.5, >2.0) (D). hbn mRNA in BMP2/4 morphants is only significantly decreased, indicating that BMP2/4 is required for hbn expression.
(TIF)
Almost all cells express LRP6 message in Hemicentrotus pulcherrimus from unfertilized egg until at least 25 h (A-C). (D-F) Negative control using sense RNA probe for LRP6. (G-J) LRP signal in normal (G-I) and LRP morphants (J). (G) Epifluorescent image of (H). (I) and (J) are stacked images of confocal microscopy and captured under the same microscopic conditions with the same exposure time. (K-O) LRP6 morphants have a normal dorsoventral body axis. Ventral marker, nodal (M), and ciliary band marker, hnf6 (N, O), are normally expressed in LRP6 morphants as they are in the control (K, L). (P) hbn disappearance occurs normally in Δcad-LRP6 morphants at 50 h.
(TIF)
(A) When Wnt7 is missing, the significant decrease of the foxQ2 region in Nodal morphants is never occurred, indicating that Wnt7 is the factor that suppresses foxQ2 in the AP region.foxQ2 expression patterns in the control (B), Nodal morphants (C), Wnt7 morphant (D) and a Nodal-Wnt7 morphant (E). ***P<0.001, Student’s t-test.
(TIF)
(A-D) The expression pattern of wnt6 in the control (A, C) and Δcad-embryos (B, D). wnt6 is slightly expressed at the thickened ectodermal region in Δcad-injected embryos. (E-H) The expression pattern of wnt7 in the control (E, G) and Δcad-embryos (F, H). (I-K) The expression pattern of hbn in Δcad (I), Δcad-Wnt6MO (J) and Δcad-Wnt7MO embryos (K). At this stage, hbn is expressed only at the posterior squamous ectoderm and has disappeared from thickened region in Δcad (I) and Δcad-Wnt7MO embryos (K). In contrast, the disappearance of hbn is inhibited in Δcad-Wnt6MO embryos (J). Asterisk (*) in (I) indicates the position of squamous ectoderm expressing hbn. (L-O) Wnt7 and Wnt6 morphant phenotypes described in the text are reproducible using the second non-overlapped morpholinos for each. foxQ2 is remains in Wnt7-MO2 morphants at 65 h (M) but not in the control (L). The disappearance of the hbn gene from the anterior end of the AP is robust in the control at 65 h (N), but the gene expression is not cleared from the place in Wnt6-MO2 morphants (O). White and black arrowheads indicate the location of hbn absence and presence, respectively.
(TIF)
hbn expression pattern in the control (A, B, C) and FoxQ2 morphants (D, E, F). foxQ2 expression pattern in the control (G, H) and Hbn morphants (I, J). These results indicate that their expression is mutually independent. MBL, mesenchyme blastula; early G, early gastrula.
(TIF)
mRNA encoding Hbn was injected into fertilized eggs and detected with in situ hybridization using the Hbn probe. The exogenous mRNA was detected at 16 h in the whole body (A), but by 24 h only the endogenous hbn was detected (B).
(TIF)
Data Availability Statement
All DNA sequence data are available from GenBank database (accession numbers LC064116, LC064120, LC064118, LC064119, LC064117).