Abstract
DNA methylation is believed to regulate gene expression during adulthood in response to the constant changes in environment. The methylome is therefore proposed to be a biomarker of health through age. ANGPTL2 is a circulating pro-inflammatory protein that increases with age and prematurely in patients with coronary artery diseases; integrating the methylation pattern of the promoter may help differentiate age- vs. disease-related change in its expression. We believe that in a pro-inflammatory environment, ANGPTL2 is differentially methylated, regulating ANGPTL2 expression. To test this hypothesis we investigated the changes in promoter methylation of ANGPTL2 gene in leukocytes from patients suffering from post-acute coronary syndrome (ACS). DNA was extracted from circulating leukocytes of post-ACS patients with cardiovascular risk factors and from healthy young and age-matched controls. Methylation sites (CpGs) found in the ANGPTL2 gene were targeted for specific DNA methylation quantification. The functionality of ANGPTL2 methylation was assessed by an in vitro luciferase assay. In post-ACS patients, C-reactive protein and ANGPTL2 circulating levels increased significantly when compared to healthy controls. Decreased methylation of specific CpGs were found in the promoter of ANGPTL2 and allowed to discriminate age vs. disease associated methylation. In vitro DNA methylation of specific CpG lead to inhibition of ANGPTL2 promoter activity. Reduced leukocyte DNA methylation in the promoter region of ANGPTL2 is associated with the pro-inflammatory environment that characterizes patients with post-ACS differently from age-matched healthy controls. Methylation of different CpGs in ANGPTL2 gene may prove to be a reliable biomarker of coronary disease.
Introduction
Cardiovascular diseases (CVD) are known to be caused by the prolonged exposure to a growing list of risk factors such as tobacco use, unhealthy diet, physical inactivity, obesity, hypertension, dyslipidemia and metabolic disorders [1, 2]. CVD are characterized by a state of low-grade chronic inflammation through the increased production of pro-inflammatory mediators [3].
Angiopoietin-like 2 (ANGPTL2) is a circulating protein with pro-inflammatory properties [4–8], which levels increase with aging in the general population [6]. The early involvement of ANGPTL2 in the pathogenesis of chronic inflammatory diseases in humans is supported by the elevated plasma ANGPTL2 concentration detected in patients suffering from CVD [4–6, 9], diabetes [5, 10, 11] and obesity [5, 12, 13] alongside other classical markers of inflammation such as C-reactive protein (CRP) [14, 15]; a positive correlation between serum CRP and ANGPTL2 has previously been reported in diabetic patients [5]. In line with these previous findings, recent studies propose that plasma ANGPTL2 is a promising biomarker for inflammatory diseases such as various cancers [16–19], atherosclerosis [5, 20], diabetes [5] and heart failure [21].
The origin of circulating ANGPTL2 is however problematic. Early reports state that ANGPTL2 is mainly produced from the adipose tissue [5], but its mRNA can also be detected in other organs [22] such as the skeletal muscle, heart [5] and endothelial cells [4]. Therefore, ANGPTL2 likely has both systemic and tissue-specific activities depending if it is secreted or expressed locally. ANGPTL2 has also been found to be expressed in mouse bone marrow derived macrophages [23], infiltrating mouse [24] and human macrophages [6, 24], as well as in vitro, in human primary peritoneal macrophages (RAW264.7) [25] and macrophage-like cell line (THP-1) [26]. Therefore, although ANGPTL2 could be used as a biomarker of inflammation like CRP, it is unlikely that ANGPTL2 is associated with a specific disorder. A more refined parameter characterizing ANGPTL2 would, therefore, provide more information of the health status of patients.
In this regards, it is well established that aging [27] and environmental stimuli, including risk factors for CVD [28], induce epigenetic changes such as DNA methylation that modify gene expression. The consequences of DNA methylation on gene transcription vary with their locations within the gene and they are highly specific of a cell type [29, 30]. In general, methylation of the promoter region has been shown to decrease gene expression [31], while in the gene body, methylation can induce up or down regulation of the expression [32, 33]. In mammalian cells, methylation is predominantly found on cytosines preceding a guanine called CpG dinucleotide. ANGPTL2 has been shown to be increasingly methylated in ovarian cancer [34] and myelodysplastic syndrome [35], while ANGPTL2 promoter methylation is decreased in osteosarcoma [36]. Taken together, these studies reveal a potential role of DNA methylation in ANGPTL2 expression. ANGPTL2 methylation has not been studied in CVD, despite considerable evidence now showing that DNA methylation is associated with inflammation [37–39] and atherosclerosis [28, 40]. CVD are associated with both global [41] and gene-specific [40, 42, 43] differentiated methylation profiles, notably in leukocytes. These epigenetic changes are also linked to known CVD risk factors such as smoking [44–46], hypertension [47, 48] and obesity [49, 50]. Hence, blood DNA methylation quantification is emerging as a powerful diagnostic tool that has been shown to predict all-cause mortality [51].
The aim of our project was to test whether ANGPTL2 methylation in circulating leukocytes isolated from patients with a recent first cardiovascular event could identify differential methylation marks compared to age-matched healthy volunteers.
Materials and Methods
Participants
Fasting blood samples were collected from 33 patients (26 men / 7 women; 62±2 y) with post-acute coronary syndrome (ACS) who provided written informed consent and were recruited at the cardiovascular prevention center of the Montreal Heart Institute. Consecutive cases of post-ACS patients were recruited from September 2011 to December 2013 at the Montreal Heart Institute. Per day, an average of 3 to 4 patients was studied: 750 patients per year (3 patients x 250 days of recruitment), i.e 1500 cases within 2 years, were evaluated. Among those cases, only 2–3 patients per week were eligible, and at the end 46 patients were enrolled. Among these 46 eligible patients, 9 dropped (5 patients stopped the training program that they were supposed to follow during the study, 1 patient was already involved in another clinical study, 1 was unfit for the physical training, 1 developed de novo atrial fibrillation, 1 developed a new ACS during the study). Among the remaining 37 patients, blood was available for ANGPTL2 quantification only in 33 patients. The mean duration after ACS was 65±7 days (median of 51 days [25–249]). One patient was enrolled after a period >4 months, 249 days after the ACS. The study was approved by the Ethical Board of the Montreal Heart Institute. Post-ACS patients were hypertensive, diabetic, dyslipidemic, obese, smokers (Tables 1 and 2), and were new members of the cardiovascular prevention center. Baseline characteristics, comorbidities and the medication of the patients are presented in Table 1.
Table 1. Baseline parameters of post-ACS patients.
| Post-ACS patients (n = 33) | |
|---|---|
| Age (years) | 62±2 |
| Men | 26 (79%) |
| Family history | 16/33 (49%) |
| Actual Percutaneous transluminal coronary angioplasty | 33/33 (100%) |
| Actual Myocardial infarction | 29/33 (88%) |
| Actual Unstable angina | 4/33 (21%) |
| Hypertension | 21/33 (64%) |
| Type II diabetes | 4/33 (12%) |
| Dyslipidemia | 27/33(82%) |
| Obesity | 21/33 (64%) |
| Smoking | 5/33 (15%) |
| Ex smoking | 19/33 (58%) |
| Medication | |
| Statins | 32/33 (97%) |
| Aspirin | 32/33 (97%) |
| β-blockers | 28/33 (85%) |
| Angiotensin Converting Enzyme inhibitors | 26/33 (79%) |
| Clopidogrel/Pasugrel | 24/33 (73%) |
| Nitrates | 14/33 (42%) |
| Calcium channel blockers | 2/33 (6%) |
| Angiotensin II receptor antagonists | 3/33 (9%) |
Data are mean ± SEM of n participants.
Table 2. Anthropometric, hemodynamic and metabolic parameters of participants.
| Young healthy controls | n | Age-matched healthy controls | n | Post-ACS patients | n | |
|---|---|---|---|---|---|---|
| Age (years) | 28±1 | 20 | 61±2 (20) | 20 | 62±2 | 33 |
| Men | 5 | 4 | 26 | |||
| VO2max (ml/min/kg LBM) | 54.8±2.4 | 20 | 44.6±2.0 | 20 | 29.7±1.0 *, a | 32 |
| BMI (kg/m2) | 21.6±0.4 | 20 | 23.8±0.5 | 20 | 28.1±0.8 *, a | 33 |
| Body fat (%) | 17.2±1.5 | 20 | 25.2±1.6 * | 20 | 28.2±1.2 * | 32 |
| SAP (mm Hg) | 112±3 | 19 | 118±3 | 20 | 122±3 * | 33 |
| DAP (mm Hg) | 68±2 | 19 | 73±1 | 20 | 69±1 | 33 |
| Heart rate (bpm) | 67±2 | 20 | 65±2 | 20 | 65±2 | 33 |
| Glucose (mM) | 4.8±0.1 | 19 | 5.0±0.1 | 19 | 5.4±0.1 *, a | 33 |
| Insulin (pM) | 34.7±3.3 | 19 | 38.4±3.0 | 17 | 79.2±9.4 *, a | 33 |
| TG (mM) | 0.71±0.07 | 20 | 1.04±0.13 | 19 | 1.09±0.07 * | 33 |
| Total Cholesterol (mM) | 4.2±0.1 | 20 | 4.8±0.2 * | 19 | 3.0±0.1 *, a | 33 |
| LDL (mM) | 2.3±0.1 | 20 | 2.9±0.2 * | 17 | 1.5±0.1 *, a | 33 |
| HDL (mM) | 1.6±0.1 | 20 | 1.6±0.1 | 18 | 1.0±0.1 *, a | 33 |
| CRP (mg/L) | 0.89±0.25 | 20 | 0.99±0.20 | 15 | 2.20±0.46 * | 31 |
Data are mean ± SEM of (n) participants.
*: p<0.05 versus Young healthy controls,
a: p<0.05 versus Age-matched controls (Kruskal-Wallis test).
BMI, Body mass index; SAP, Systolic arterial pressure; DAP, Diastolic arterial pressure; TG, Triglycerides; LDL, Low-density lipoprotein; HDL, High-density lipoprotein; CRP, C-reactive protein.
Blood samples were collected in EDTA and heparin tubes from post-ACS patients and from 20 young (28±1 years) healthy and 20 age-matched (61±2 years) healthy volunteers recruited in a previous study [20]. Control healthy volunteers had no comorbidities and no medication; baseline characteristics, inclusion and exclusion criteria for these healthy volunteers have been previously reported [20].
Inclusion criteria for the post-ACS patients were the following: 1) men or women aged ≥18 years; 2) with previous ACS (unstable angina, or non-ST elevation myocardial infarction (NSTEMI), or ST elevation myocardial infarction (STEMI) with the presence of 2/3 criteria i.e. typical chest pain, electrocardiographic ischemic change, or elevated troponin T; 3) complete revascularization defined as no major epicardial coronary artery or bypass graft with a residual diameter stenosis > 50% and no residual left main stenosis > 40%; 4) left ventricular ejection fraction > 40%; 5) stable doses of medication during the 4 weeks prior to enrolment (STEMI patients must be on a stable dose of β-blocker); 6) able to perform a maximal cardiopulmonary exercise test; 7) capacity and willingness to sign informed consent.
Exclusion criteria for the post-ACS patients were: 1) recent (< 6 months) coronary bypass surgery; 2) incomplete revascularisation; 3) left ventricular ejection fraction (LVEF) < 40%; 4) significant valvular heart disease defined as mitral stenosis, grade III-IV mitral insufficiency, moderate-severe aortic stenosis, moderate-severe aortic insufficiency; 5) uncontrolled hypertension defined as blood pressure >180/110 mmHg; 6) significant resting ECG abnormalities including left bundle branch block, non-specific intraventricular conduction delay, left ventricular hypertrophy and resting ST-segment depression; 7) chronic atrial fibrillation; 8) pacemaker or implantable cardioverter defibrillator; 9) low functional capacity on baseline maximal exercise test (<5 METs); 10) any contra-indication to exercise training or any condition limiting effort to a greater degree than the CAD (such as neurologic disease, peripheral artery disease, osteoarthritis). The information concerning the presence of inflammatory disorders is not available.
The anthropometric, hemodynamic and metabolic parameters of the controls and the post-ACS patients are summarized in Table 2. The research protocol was approved by the Research Ethics and New Technology Development Committee of the Montreal Heart Institute. Following collection, ice-blood samples were centrifuged at 4°C and plasma was stored at -80°C. ANGPTL2 concentration was quantified by a commercial enzymatic immunoassay kit, as previously described [20].
DNA extraction and bisulfite conversion
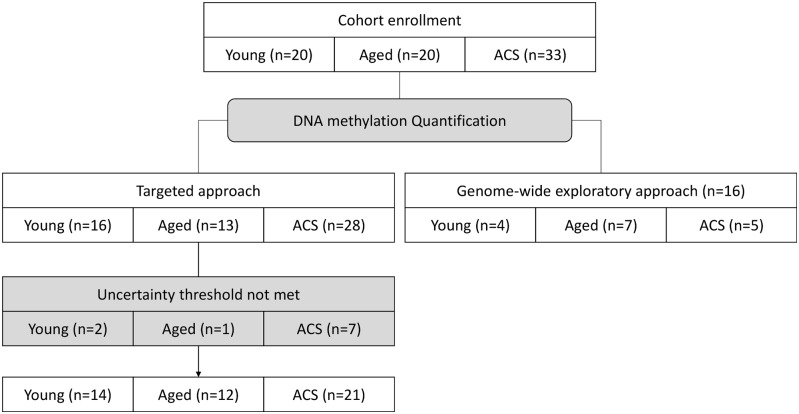
In all available samples (n = 20 young healthy controls, n = 20 age-matched healthy controls and n = 33 post-ACS patients) (Fig 1), total DNA was isolated from whole blood using a Qiagen DNeasy Blood & tissue kit following the manufacturer’s instructions. No selection of white blood cells was done, DNA was isolated from the whole white blood cells population. After extraction, DNA was quantified by NanoDrop (Thermo Scientific NanoDrop products, Wilmington, DE). DNA was then converted by bisulfite reaction using the EZ DNA Methylation-Gold kit (Zymo Research, Irvine, CA) following the manufacturer’s protocol.
Fig 1. Flowchart illustrating the "n" values between groups throughout the study.
DNA methylation quantification
A genome-wide exploratory DNA methylation quantification protocol assay was performed on bisulfite-converted DNA using the Infinium Human Methylation 450 BeadChip Kit (Illumina, San Diego, CA) to obtain the methylation status of >485 000 CpG sites across the genome, as previously described [52]. This exploratory approach was performed in a small number of subjects (n = 4 young healthy controls, n = 7 aged healthy controls and n = 5 post-ACS patients) (Fig 1). We normalized probe intensities using the ARRm software [Fortin JP, Greenwood CMT, Labbe A: ARRmNormalization: Adaptive Robust Regression normalization for Illumina methylation data. In R package 1.0.0 edition; 2013.]. We removed probes that target a genomic region containing SNPs based on dbSNP version 137 (N = 82,694).
After this genome-wide exploratory DNA methylation quantification, fine mapping DNA methylation was then quantified by EpiTYPER assay (Sequenom, San Diego, CA), as previously described [53]. Gene-specific primers required for the assay are presented in Table 3. These experiments were performed at the McGill University and Génome Québec Innovation Centre, Montréal, Canada. This targeted approach was performed in all the remaining patients (n = 16 young healthy controls, n = 13 aged healthy controls and n = 28 post-ACS patients) (Fig 1). During the targeted approach, some samples showed undetectable methylation ratios because the maximum level of uncertainty was not met. In other words, any data with an estimated error larger than the uncertainty threshold (which is a value for the maximum amount of error accepted) was excluded and not displayed: some undetectable data were excluded in n = 2 young healthy controls, n = 1 aged healthy controls and n = 7 post-ACS patients. The fine targeted DNA methylation mapping was therefore performed in a total of n = 14 young healthy controls, n = 12 aged healthy controls and n = 21 post-ACS patients (Fig 1).
Table 3. EpiTYPER primer sequences.
ANGPTL2-specific bisulfite primers required for PCR amplification.
| Primer | Sequence |
|---|---|
| Forward | aggaagagagTTTATTTTTAAATTTTGGGGAAAGG |
| Reverse | cagtaatacgactcactatagggagaaggctCTCCAAAATCCTAAACTCAATTCAA |
Cloning of pCpG free-ANGPTL2 vector
Constructions were done using the CpG free plasmid pCpGfree-promoter (Invivogen, San Diego, CA) as the backbone to study enhancer methylation. The ANGPTL2 CpG region was amplified using forward 5’-TAAGCTCCTTCCCACGTGACCTCACAGAGTCG-3’ and reverse 5’-GATCCGACTCTGTGAGGTCACGTGGGAAGGAGCTTATGCA-3’ primers and subsequently inserted in the backbone using NsiI and BamHI restriction sites as previously described [54, 55].
In vitro methylation, transient transfection and luciferase assay
Cloned vectors were isolated by Qiagen QIAprep Spin Miniprep kit (Qiagen). M. SssI CpG methyltransferase (New England Biolabs, Frankfurt, Germany) was used for in vitro methylation according to manufacturer’s instructions. Methylated DNA was then purified using the QIAquick gel extraction kit (Qiagen) and quantified by NanoDrop (Thermo Scientific NanoDrop products, Wilmington, DE). Methylation was confirmed by digestion with the methylation-sensitive restriction enzymes HhaI and HpaII. HEK293 cells grown to confluence on 96-well plates were transfected with the pCpG free-Gpx1 vector using Lipofectamine 2000 (Invitrogen). Twenty-four hours after transfection, luciferase activity was measured with the QUANTI-Luc reagent (Invivogen, San Diego, CA) by luminescence detection. Promoter activity was normalized to the total amount of protein measured by a Bradford assay (Biorad, Hercules, CA) [54, 55].
Statistical Analysis
Results are presented as mean±SEM of (n) participants. One-way ANOVA (with Bonferonni post-test) or Kruskal-Wallis test (with Dunn post-test), unpaired t-test or Mann Whitney test were used where applicable, depending on Gaussian distributions, to test the difference between groups (Graph Pad Prism). A p-value of p<0.05 was considered statistically significant.
Results
Increased circulating ANGPTL2 concentration in post-ACS patients
In accordance with our previously published data [4, 20], plasma ANGPTL2 concentration was higher in post-ACS patients (3.35 ± 0.67 ng/mL, n = 33) when compared to age-matched controls (1.80 ± 0.42 ng/mL, n = 20) (Fig 2). The post-ACS patients also display higher circulating CRP levels (2.20 ± 0.46 mg/L, n = 31) in comparison to young healthy controls (0.89 ± 0.25 mg/L, n = 20) (Table 2), illustrating the presence of a pro-inflammatory environment in these patients with various CVD risk factors (Table 1).
Fig 2. Increased ANGPTL2 in post-ACS patients.
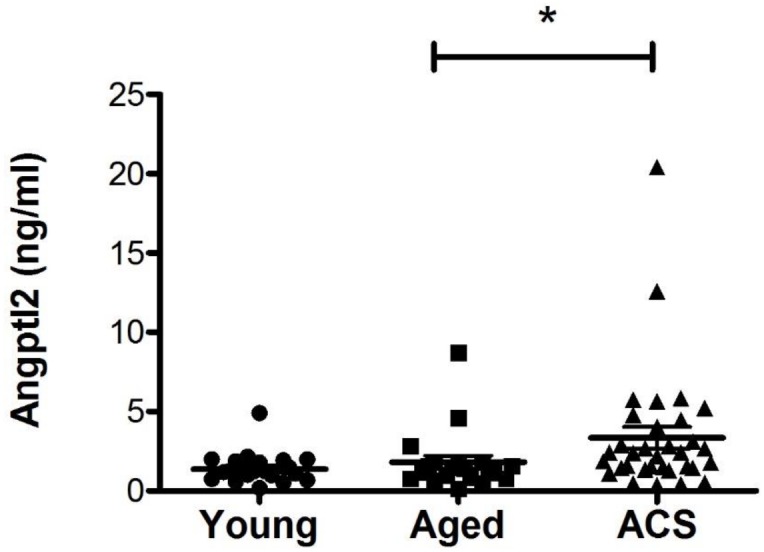
Fasting ANGPTL2 levels in the plasma of patients with post-acute coronary syndrome (ACS) (n = 33) compared to age-matched (n = 20) and young (n = 20) healthy controls. Data are mean ± SEM of (n) participants, *: p<0.05 vs Age-matched controls (Kruskal-Wallis test).
Exploratory discovery of ANGPTL2 methylation sites
We selected a total of 16 patients from the young controls (n = 4), age-matched controls (n = 7) and post-ACS patients (n = 5) for this genome-wide exploratory DNA methylation analysis. Out of the >485 000 probes included in the genome-wide quantification, only 6 probes were associated with the ANGPTL2 gene: 1 probe (cg09427311) was distributed in the promoter region of the ANGPTL2 gene, 2 probes (cg08076018 and cg13662634) were distributed in the 5’ region transcription start site and 3 probes were located in the gene body (cg11213150, cg14281592 and cg13508369) (Table 4). Significant levels of DNA methylation were detected in all 6 probes (Fig 3). However, due to the low number of individuals among each group, no statistical difference was observed between controls and post-ACS groups (data not shown).
Table 4. Exploratory probe coordinates.
Genomic localisation of probes covering ANGPTL2 CpG sites analyzed by the Infinium HumanMethylation450 exploratory assay, as provided by the manufacturer.
| Probe ID | Coordinates |
|---|---|
| cg08076018 | chr9:128924901 |
| cg09427311 | chr9:128925551 |
| cg11213150 | chr9:128924278 |
| cg13508369 | chr9:128923847 |
| cg13662634 | chr9:128924769 |
| cg14281592 | chr9:128924134 |
Fig 3. Detectable ANGPTL2 methylation profile.
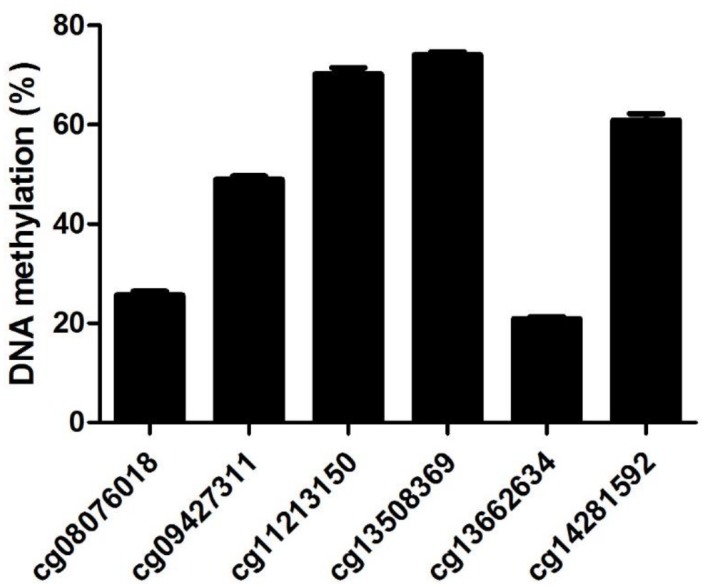
Quantification of various methylation sites located in the ANGPTL2 gene identified following a preliminary genome-wide exploratory assay. DNA samples were pooled from a small number of participants taken from all three groups. Data are mean ± SEM of a total of 16 patients from the young controls (n = 4), age-matched controls (n = 7) and post-ACS patients (n = 5).
Fine mapping DNA methylation was then quantified by EpiTYPER assay in larger groups; we chose to proceed with the investigation of probe cg09427311, since it is the only one located in the promoter region of ANGPTL2 gene and that it is sufficiently far from the other probes to allow targeting with specific primers for downstream fine mapping analysis.
Post-ACS patients have decreased ANGPTL2 methylation
Using a targeted approach, we proceeded to the fine mapping analysis of the unique CpGs (Fig 4) surrounding the previously identified ANGPTL2 methylation site covered by the selected probe cg09427311.
Fig 4. Fine mapping of ANGPTL2 methylation profile.
Identification (CpG1-6) and localization of CpGs targeted for DNA methylation quantification. The arrow represents the CpG previously characterized by probe cg09427311 during the exploratory analysis.
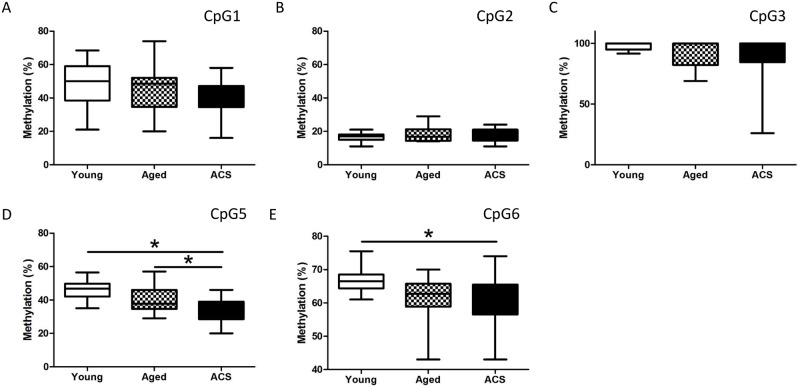
Of the 6 CpGs found within the 344 bp region amplified by specific primers, 2 CpGs (CpG5 and CpG6) show differential methylation between groups (Fig 5). Methylation of CpG5 was significantly decreased in post-ACS patients (34.7 ± 1.4%; p < 0.05, n = 21) when compared to young (45.8 ± 1.5%, n = 14) and aged-matched (40.6 ± 2.3%, n = 12) control groups. However, no difference was observed between young and age-matched control groups (Fig 5D). Compared to young controls (66.6 ± 0.9%, n = 14), methylation of CpG6 was also lower in post-ACS (60.4 ± 1.5%; p < 0.05, n = 21), but no significant difference was observed with age-matched controls (61.4 ± 2.0%, n = 12). Again, methylation of CpG6 was similar between both control groups (Fig 5E).
Fig 5. Hypomethylation of CpG5 and CpG6 in post-ACS patients.
Methylation percentage of the methylation sites (A) CpG1, (B) CpG2, (C) CpG3, (D) CpG5 and (E) CpG6, previously identified in Fig 4. DNA was isolated from leukocytes of post-ACS patients (n = 21), age-matched (n = 12) and young (n = 14) healthy controls. Box and whiskers plot of (n) participants, *: p<0.05 (Kruskal-Wallis test).
These results suggest that CpG5 methylation is sensitive to the disease state since a significant hypomethylation is detected in post-ACS patients when compared to both healthy groups. Conversely, it is unclear which factor regulates CpG6 methylation due to the lack of discrepancy between the post-ACS group and the age-matched controls or between the young and age-matched controls.
On the other hand, no significant variations in the methylation levels among groups were detected for CpG1, CpG2 and CpG3 (Fig 5A–5C). The remaining CpG4 could not be analyzed due to a limitation of this assay: the EpiTYPER technology relies on a mass spectroscopy analysis of CpG-containing DNA fragments by uracil-specific cleavage and the CpG4 fragment was too small to be reliably detected by mass spectrometry (data not shown).
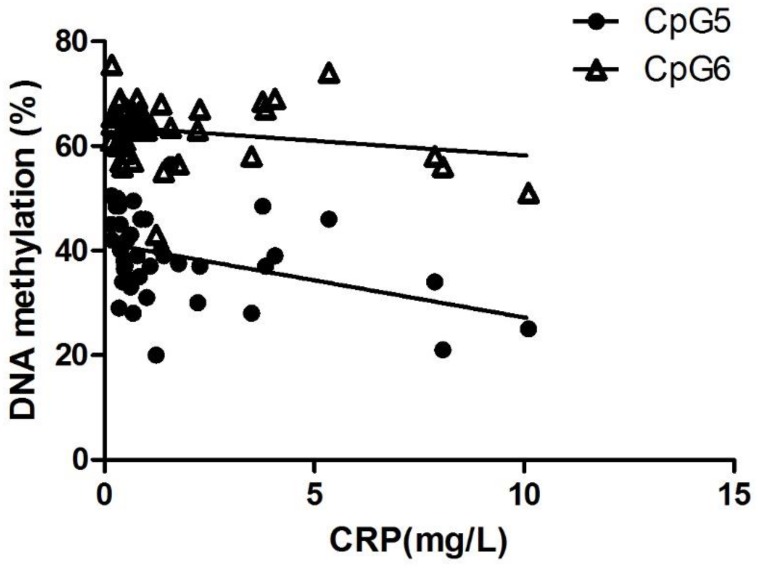
ANGPTL2 methylation is correlated with CRP levels
Interestingly, CpG5, but not CpG6 methylation negatively correlates (p<0.05) with circulating CRP levels when all participants were considered (Fig 6), suggesting that ANGPTL2 hypomethylation is associated with high levels of CRP, establishing a link between ANGPTL2 methylation and inflammation. For this reason, only CpG5 was considered for further analysis.
Fig 6. CpG5 methylation is inversely correlated with CRP concentration.
Negative correlation between plasma CRP concentrations and CpG5 (p = 0.0096, r = -0.395, n = 42) and CpG6 (p = 0.1731, r = -0.214, n = 42) methylation in all participants (n = 14 young healthy controls, n = 9 age-matched healthy controls, n = 19 post-ACS patients).
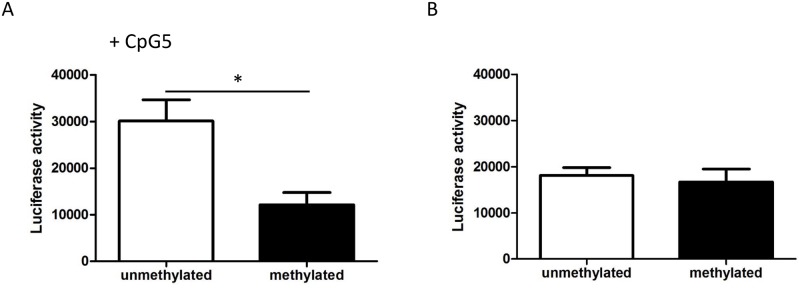
In vitro methylation decreases ANGPTL2 promoter activity
To assess the impact of CpG5 methylation on ANGPTL2 expression, we used an in vitro methylation luciferase assay as previously described [54–56]. A 32 bp promoter sequence containing CpG5 was inserted into the pCpGfree-basic vector, upstream of a hEF-1α CpG-free promoter. This construct was then methylated in vitro and subsequently transfected in HEK293 cells where luciferase activity was measured as an indicator of promoter activity. In vitro methylation of the construct containing the ANGPTL2 promoter fragment significantly reduced promoter activity as shown by a decrease of 60% (p < 0.05) in luciferase activity (Fig 7A). Conversely, methylation of the vector lacking the CpG5 sequence did not alter promoter activity (Fig 7B). These results suggest a potential molecular regulatory role of CpG5 methylation on ANGPTL2 expression.
Fig 7. In vitro methylation of ANGPTL2 decreases promoter activity.
In vitro methylation of ANGPTL2 target region containing CpG5 inhibited transcriptional activity, as measured by a luciferase reporter assay. Luciferase activity of methylated (M.SssI treated) and unmethylated constructs (A) containing the CpG5 site or (B) without. The assay was repeated 3 times and data are mean ± SEM. *: p<0.05 versus unmethylated (Unpaired t-test).
Discussion
In this study, we show for the first time that ANGPTL2 methylation pattern varies in post-ACS patients and that this methylation pattern is independent of aging. During the course of the study we also identified a novel regulatory region in the ANGPTL2 promoter, the CpG5, which is hypomethylated in association with the pro-inflammatory environment in these patients. We found that in vitro methylation of CpG5 induced a lower transcriptional activity. Therefore, differential CpG5 methylation pattern may identify patients at risk of a first cardiovascular event.
Previous studies suggest that ANGPTL2 expression is regulated by DNA methylation. For example, hypermethylation of CpGs located in ANGPTL2 gene have been reported in various ovarian cancer cell lines, where ANGPTL2 is silenced [34] and in bone marrow samples from patients suffering from primary myelodysplastic syndrome [35]. In contrast, hypomethylation of ANGPTL2 promoter has been observed in human osteosarcoma cell lines, proportionally with the increase in ANGPTL2 expression and progression of the disease when these cells were injected in mice [36]. In our study, we observed a decrease in ANGPTL2 methylation in the CpG5 region in post-ACS patients. In our hands, human leukocytes do not produce detectable levels of ANGPTL2 and mRNA levels were at the low detection limit (data not shown). Nonetheless, our data suggest that methylation of CpG may represent a mechanism of regulation that could in part account for the elevated circulating levels of ANGPTL2 in these patients. Indeed, we have demonstrated that two specific methylation sites, CpG5 and CpG6, are less methylated in diseased patients when compared to young healthy controls. However, only CpG5 significantly differed from the age-matched control group and significantly correlated with CRP levels, suggesting that potential methylation sites are differently sensitive to various stimuli such as age and the disease state. This is also supported by the observation that methylation in surrounding CpGs (CpG1-3) do not significantly vary in any of our groups. The amplitudes of the changes in methylation levels observed in our study (>5 to 10%) are in line with what is typically observed in studies conducted on white blood cells in a pro-inflammatory context [57, 58]. In addition, a previously published study conducted on cord blood cells in association with maternal obesity revealed that ANGPTL2 methylation differed by less than 5% across body mass index categories [59]. Aberrant methylation patterns have been extensively studied in the context on chronic inflammatory diseases. In cancer, global DNA methylation measured from the blood can be used as a biomarker for cancer risk [60]. In CVD, low-density lipoproteins exert their effect on endothelial cells through changes in DNA methylation [61, 62] and atherosclerosis is characterized by a global state of hypomethylation [63]. Therefore, it could be speculated that changes in methylation patterns could reflect the health status of the patients and be much more specific of the pathology involved compared to the circulating levels of the protein.
Indeed, as previously mentioned, our group [4, 20] and others [5, 12, 13] have demonstrated that circulating ANGPTL2 concentration is increased in a pro-inflammatory context in a proportional manner to the severity of the disease. In our study, only a small difference was observed in Angplt2 plasma concentrations of post-ACS patients when compared to age-matched healthy controls, reflecting a lesser or shorter cumulative inflammatory burden compared to that of patients with known CVD and a longer history of cardiovascular events [20]. We can hypothesize that in the presence of a more severe inflammatory environment such as in patients with established CVD, which would be highlighted by higher ANGPTL2 levels, changes in ANGPTL2 methylation may become detectable in other CpGs. Such graded methylation has been previously reported in cancer: methylation of ANGPTL2 varies proportionally with tumour metastasis [36].
ANGPTL2 is often associated with markers of inflammation such as CRP [64, 65], IL-6 and TNF-α [4, 66] and although it is not always clear which comes first, it is acknowledged that ANGPTL2 participates in a pro-inflammatory loop by being sensitive to inflammation and in turn, further promotes inflammatory pathways. In our study, ANGPTL2 methylation at CpG5 is inversely correlated with CRP. Furthermore, CpG5 and CpG6 methylation is decreased in leukocytes from post-ACS patients who also happen to have higher levels of circulating ANGPTL2 and CRP; this suggests that a pro-inflammatory environment may favour the production of ANGPTL2 in part by decreasing DNA methylation in the relevant producing cells. An interesting finding by Sasaki and al. [26] indeed states that ANGPTL2 can act in an autocrine manner. Their work shows that treatment of macrophage-like cells with ANGPTL2 increases its own expression in a dose-dependent manner [26]. Taking these results together, we can hypothesize that in addition of inflammation per se, ANGPTL2 could induce its own expression through a DNA methylation mechanism.
Limitations of the study
The present study allows us to observe the methylation changes of ANGPTL2 in a context of mild inflammatory stress in optimally treated post-ACS patients. Our group has previously demonstrated that patients with chronic documented coronary artery disease exhibit greater signs of inflammation through slightly higher circulating ANGPTL2 (3.35 ± 0.67 ng/mL for post-ACS versus 5.74 ± 0.75 ng/mL for CAD) [20]. It would be interesting to study ANGPTL2 methylation under such conditions.
Following the exploratory experiment aiming to identify methylation candidates, we narrowed our target CpGs down to 6 potential regulatory CpGs. Hence, DNA methylation quantification approaches covering broader ANGPTL2-related CpGs and regulatory regions should be considered, especially when considering patients with longer history of risk factors and longer history of cardiovascular events. Therefore, other regulatory methylation sites previously characterized by others [34–36] in different pathological contexts could be included in future studies enrolling patients with CVD. This would allow us to determine how these epigenetic marks can differ when comparing various types of inflammatory diseases. Based on the limited literature on the subject of ANGPTL2 methylation, we observe contrasting results; in cancer cells, researchers have reported a decrease in ANGPTL2 methylation resulting in an increased ANGPTL2 expression with the progression of the disease [36] while others [34, 35] have shown the opposite. These illustrate how epigenetic mechanisms can vary within the same type of disease by taking into consideration the cell type.
Leukocytes represent an accessible and reliable way to obtain DNA with little discomfort to the patient. However, it is a mixed population of cells in which methylation patterns may be different [67]. Although immune cells express ANGPTL2 [6, 23, 24] and are likely not the main contributor to the circulating pool of ANGPTL2 [5], further studies should isolate the mixed leukocytes population.
In conclusion, reduced leukocyte DNA methylation in the promoter region of ANGPTL2 is associated with the pro-inflammatory environment that characterizes post-ACS patients differently from age-matched healthy controls. Importantly, our data suggest that methylation of different CpGs in ANGPTL2 may prove to be a reliable biomarker of coronary disease. Replication of our study in a wider range of CpGs in patients with different combination of risk factors for CVD and a history of cardiovascular events should validate the usefulness of methylation patterns in ANGPTL2 as a biomarker for a better risk assessment of future cardiovascular events.
Data Availability
All relevant data have been included in the paper. Due to ethical restrictions, individual data from the current study are available upon request from the corresponding author. The corresponding author Eric Thorin may be contacted at eric.thorin@umontreal.ca.
Funding Statement
The work was funded by the Canadian Institute for Health Research (ET). Students were supported through fellowship (VT) and scholarship (SL) of the Canadian Institute for Health Research. GL holds a Canada Research Chair. Clinical research (JL, MG, DH, MJ, A Nigam) and basic research (ET, NTT) teams are supported by an annual grant of the Foundation of the Montreal Heart Institute. Clinical research in prevention is supported by the EPIC Foundation, the prevention center of the Montreal Heart Institute.
References
- 1.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–60. 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daskalopoulou SS, Rabi DM, Zarnke KB, Dasgupta K, Nerenberg K, Cloutier L, et al. The 2015 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2015;31(5):549–68. 10.1016/j.cjca.2015.02.016 . [DOI] [PubMed] [Google Scholar]
- 3.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. . [DOI] [PubMed] [Google Scholar]
- 4.Farhat N, Thorin-Trescases N, Mamarbachi M, Villeneuve L, Yu C, Martel C, et al. Angiopoietin-like 2 promotes atherogenesis in mice. J Am Heart Assoc. 2013;2(3):e000201 10.1161/JAHA.113.000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, et al. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10(3):178–88. 10.1016/j.cmet.2009.08.003 . [DOI] [PubMed] [Google Scholar]
- 6.Horio E, Kadomatsu T, Miyata K, Arai Y, Hosokawa K, Doi Y, et al. Role of endothelial cell-derived angptl2 in vascular inflammation leading to endothelial dysfunction and atherosclerosis progression. Arterioscler Thromb Vasc Biol. 2014;34(4):790–800. 10.1161/ATVBAHA.113.303116 . [DOI] [PubMed] [Google Scholar]
- 7.Thorin-Trescases N, Thorin E. Angiopoietin-like-2: a multifaceted protein with physiological and pathophysiological properties. Expert Rev Mol Med. 2014;16:e17 10.1017/erm.2014.19 . [DOI] [PubMed] [Google Scholar]
- 8.Kadomatsu T, Endo M, Miyata K, Oike Y. Diverse roles of ANGPTL2 in physiology and pathophysiology. Trends Endocrinol Metab. 2014;25(5):245–54. 10.1016/j.tem.2014.03.012 . [DOI] [PubMed] [Google Scholar]
- 9.Oike Y, Tabata M. Angiopoietin-like proteins—potential therapeutic targets for metabolic syndrome and cardiovascular disease. Circ J. 2009;73(12):2192–7. . [DOI] [PubMed] [Google Scholar]
- 10.Li Q, Gong W, Yang Z, Lu B, Yang Y, Zhao W, et al. Serum Angptl2 levels are independently associated with albuminuria in type 2 diabetes. Diabetes Res Clin Pract. 2013;100(3):385–90. 10.1016/j.diabres.2013.03.028 . [DOI] [PubMed] [Google Scholar]
- 11.Doi Y, Ninomiya T, Hirakawa Y, Takahashi O, Mukai N, Hata J, et al. Angiopoietin-like protein 2 and risk of type 2 diabetes in a general Japanese population: the Hisayama study. Diabetes Care. 2013;36(1):98–100. 10.2337/dc12-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muramoto A, Tsushita K, Kato A, Ozaki N, Tabata M, Endo M, et al. Angiopoietin-like protein 2 sensitively responds to weight reduction induced by lifestyle intervention on overweight Japanese men. Nutr Diabetes. 2011;1:e20 10.1038/nutd.2011.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng QX, Wen L, Chen XY, Zhong HJ. Association of serum angiopoietin-like protein 2 and epinephrine levels in metabolically healthy but obese individuals: and evidence. Exp Ther Med. 2013;5(6):1631–6. 10.3892/etm.2013.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–20. 10.1056/NEJMoa1107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):e584–636. 10.1161/CIR.0b013e3182051b4c . [DOI] [PubMed] [Google Scholar]
- 16.Ide S, Toiyama Y, Shimura T, Kawamura M, Yasuda H, Saigusa S, et al. Angiopoietin-Like Protein 2 Acts as a Novel Biomarker for Diagnosis and Prognosis in Patients with Esophageal Cancer. Ann Surg Oncol. 2015. 10.1245/s10434-014-4315-0 . [DOI] [PubMed] [Google Scholar]
- 17.Shimura T, Toiyama Y, Tanaka K, Saigusa S, Kitajima T, Kondo S, et al. Angiopoietin-like Protein 2 as a Predictor of Early Recurrence in Patients After Curative Surgery for Gastric Cancer. Anticancer Res. 2015;35(9):4633–9. . [PubMed] [Google Scholar]
- 18.Toiyama Y, Tanaka K, Kitajima T, Shimura T, Imaoka H, Mori K, et al. Serum angiopoietin-like protein 2 as a potential biomarker for diagnosis, early recurrence and prognosis in gastric cancer patients. Carcinogenesis. 2015. 10.1093/carcin/bgv139 . [DOI] [PubMed] [Google Scholar]
- 19.Toiyama Y, Tanaka K, Kitajima T, Shimura T, Kawamura M, Kawamoto A, et al. Elevated serum angiopoietin-like protein 2 correlates with the metastatic properties of colorectal cancer: a serum biomarker for early diagnosis and recurrence. Clin Cancer Res. 2014;20(23):6175–86. 10.1158/1078-0432.CCR-14-0007 . [DOI] [PubMed] [Google Scholar]
- 20.Larouche JF, Yu C, Luo X, Farhat N, Guiraud T, Lalonge J, et al. Acute High-Intensity Intermittent Aerobic Exercise Reduces Plasma Angiopoietin-Like 2 in Patients With Coronary Artery Disease. Can J Cardiol. 2015. 10.1016/j.cjca.2015.01.038 . [DOI] [PubMed] [Google Scholar]
- 21.Huang CL, Wu YW, Wu CC, Hwang JJ, Yang WS. Serum Angiopoietin-Like Protein 2 Concentrations Are Independently Associated with Heart Failure. PLoS One. 2015;10(9):e0138678 10.1371/journal.pone.0138678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim I, Moon SO, Koh KN, Kim H, Uhm CS, Kwak HJ, et al. Molecular cloning, expression, and characterization of angiopoietin-related protein. angiopoietin-related protein induces endothelial cell sprouting. J Biol Chem. 1999;274(37):26523–8. . [DOI] [PubMed] [Google Scholar]
- 23.Swain L, Wottawa M, Hillemann A, Beneke A, Odagiri H, Terada K, et al. Prolyl-4-hydroxylase domain 3 (PHD3) is a critical terminator for cell survival of macrophages under stress conditions. J Leukoc Biol. 2014;96(3):365–75. 10.1189/jlb.2HI1013-533R . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32(6):1400–9. 10.1161/ATVBAHA.112.247866 . [DOI] [PubMed] [Google Scholar]
- 25.Lee HJ, Kim JH, Kim JH, Martinus RD, Park SH. Angiopoietin-like protein 2, a chronic inflammatory mediator, is a new target induced by TGF-beta1 through a Smad3-dependent mechanism. Biochem Biophys Res Commun. 2013;430(3):981–6. 10.1016/j.bbrc.2012.11.127 . [DOI] [PubMed] [Google Scholar]
- 26.Sasaki Y, Ohta M, Desai D, Figueiredo JL, Whelan MC, Sugano T, et al. Angiopoietin Like Protein 2 (ANGPTL2) Promotes Adipose Tissue Macrophage and T lymphocyte Accumulation and Leads to Insulin Resistance. PLoS One. 2015;10(7):e0131176 10.1371/journal.pone.0131176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20(8):350–8. 10.1016/j.tig.2004.06.009 . [DOI] [PubMed] [Google Scholar]
- 28.Wierda RJ, Geutskens SB, Jukema JW, Quax PH, van den Elsen PJ. Epigenetics in atherosclerosis and inflammation. J Cell Mol Med. 2010;14(6A):1225–40. 10.1111/j.1582-4934.2010.01022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, et al. Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res. 2013;23(3):555–67. 10.1101/gr.147942.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deaton AM, Webb S, Kerr AR, Illingworth RS, Guy J, Andrews R, et al. Cell type-specific DNA methylation at intragenic CpG islands in the immune system. Genome Res. 2011;21(7):1074–86. 10.1101/gr.118703.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenet F, Moh M, Funk P, Feierstein E, Viale AJ, Socci ND, et al. DNA methylation of the first exon is tightly linked to transcriptional silencing. PLoS One. 2011;6(1):e14524 10.1371/journal.pone.0014524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball MP, Li JB, Gao Y, Lee JH, LeProust EM, Park IH, et al. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nat Biotechnol. 2009;27(4):361–8. 10.1038/nbt.1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aran D, Toperoff G, Rosenberg M, Hellman A. Replication timing-related and gene body-specific methylation of active human genes. Hum Mol Genet. 2011;20(4):670–80. 10.1093/hmg/ddq513 . [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi R, Tsuda H, Kozaki K, Kanai Y, Kasamatsu T, Sengoku K, et al. Frequent inactivation of a putative tumor suppressor, angiopoietin-like protein 2, in ovarian cancer. Cancer Res. 2008;68(13):5067–75. 10.1158/0008-5472.CAN-08-0062 . [DOI] [PubMed] [Google Scholar]
- 35.Lee S, Kwon HC, Kim SH, Oh SY, Lee JH, Lee YS, et al. Identification of genes underlying different methylation profiles in refractory anemia with excess blast and refractory cytopenia with multilineage dysplasia in myelodysplastic syndrome. Korean J Hematol. 2012;47(3):186–93. 10.5045/kjh.2012.47.3.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odagiri H, Kadomatsu T, Endo M, Masuda T, Morioka MS, Fukuhara S, et al. The secreted protein ANGPTL2 promotes metastasis of osteosarcoma cells through integrin alpha5beta1, p38 MAPK, and matrix metalloproteinases. Sci Signal. 2014;7(309):ra7 10.1126/scisignal.2004612 . [DOI] [PubMed] [Google Scholar]
- 37.Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev. 2015;21:26–41. . [PubMed] [Google Scholar]
- 38.Obata Y, Furusawa Y, Hase K. Epigenetic modifications of the immune system in health and disease. Immunol Cell Biol. 2015;93(3):226–32. 10.1038/icb.2014.114 . [DOI] [PubMed] [Google Scholar]
- 39.Yara S, Lavoie JC, Levy E. Oxidative stress and DNA methylation regulation in the metabolic syndrome. Epigenomics. 2015;7(2):283–300. 10.2217/epi.14.84 . [DOI] [PubMed] [Google Scholar]
- 40.Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790(9):886–91. 10.1016/j.bbagen.2009.02.008 . [DOI] [PubMed] [Google Scholar]
- 41.Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, et al. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49(8):1292–6. . [DOI] [PubMed] [Google Scholar]
- 42.Webster AL, Yan MS, Marsden PA. Epigenetics and cardiovascular disease. Can J Cardiol. 2013;29(1):46–57. 10.1016/j.cjca.2012.10.023 . [DOI] [PubMed] [Google Scholar]
- 43.Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34(4):883–901. 10.1016/j.mam.2012.08.001 . [DOI] [PubMed] [Google Scholar]
- 44.Bouwland-Both MI, van Mil NH, Tolhoek CP, Stolk L, Eilers PH, Verbiest MM, et al. Prenatal parental tobacco smoking, gene specific DNA methylation, and newborns size: the Generation R study. Clin Epigenetics. 2015;7(1):83 10.1186/s13148-015-0115-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer M, Linsel G, Fink B, Offenberg K, Hahn AM, Sack U, et al. A varying T cell subtype explains apparent tobacco smoking induced single CpG hypomethylation in whole blood. Clin Epigenetics. 2015;7(1):81 10.1186/s13148-015-0113-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burris HH, Baccarelli AA, Byun HM, Cantoral A, Just AC, Pantic I, et al. Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal BMI, gestational age, and birth weight. Epigenetics. 2015:1–9. 10.1080/15592294.2015.1078963 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato N, Loh M, Takeuchi F, Verweij N, Wang X, Zhang W, et al. Trans-ancestry genome-wide association study identifies 12 genetic loci influencing blood pressure and implicates a role for DNA methylation. Nat Genet. 2015. 10.1038/ng.3405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Gong L, Tan Y, Hui R, Wang Y. Hypertensive epigenetics: from DNA methylation to microRNAs. J Hum Hypertens. 2015;29(10):575–82. 10.1038/jhh.2014.132 . [DOI] [PubMed] [Google Scholar]
- 49.Mansego ML, Milagro FI, Zulet MA, Moreno-Aliaga MJ, Martinez JA. Differential DNA Methylation in Relation to Age and Health Risks of Obesity. Int J Mol Sci. 2015;16(8):16816–32. 10.3390/ijms160816816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS, Members of Epi S. Epigenetics and human obesity. Int J Obes (Lond). 2015;39(1):85–97. 10.1038/ijo.2014.34 . [DOI] [PubMed] [Google Scholar]
- 51.Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25 10.1186/s13059-015-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bibikova M, Barnes B, Tsan C, Ho V, Klotzle B, Le JM, et al. High density DNA methylation array with single CpG site resolution. Genomics. 2011;98(4):288–95. 10.1016/j.ygeno.2011.07.007 . [DOI] [PubMed] [Google Scholar]
- 53.Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci U S A. 2005;102(44):15785–90. 10.1073/pnas.0507816102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen A, Duquette N, Mamarbachi M, Thorin E. Epigenetic regulatory effect of exercise on glutathione peroxidase 1 expression in the skeletal muscle of severely dyslipidemic mice PLoS One. 2016;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen A, Leblond F, Mamarbachi M, Geoffroy S, Thorin E. Age-Dependent Demethylation of Sod2 Promoter in the Mouse Femoral Artery. Oxidative Medicine and Cellular Longevity. 2016;2016:1–6. 10.1155/2016/8627384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra P, Soni V, Kumar A, Anbazhagan AN, Dudeja A, Saksena S, et al. Epigenetic modulation of intestinal cholesterol transporter Niemann-Pick C1-like 1 (NPC1L1) gene expression by DNA methylation. J Biol Chem. 2014;289(33):23132–40. 10.1074/jbc.M113.546283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nestor CE, Barrenas F, Wang H, Lentini A, Zhang H, Bruhn S, et al. DNA methylation changes separate allergic patients from healthy controls and may reflect altered CD4+ T-cell population structure. PLoS Genet. 2014;10(1):e1004059 10.1371/journal.pgen.1004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics. 2011;6(7):828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu X, Chen Q, Tsai HJ, Wang G, Hong X, Zhou Y, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: exploration of early life origins of disease. Environ Mol Mutagen. 2014;55(3):223–30. 10.1002/em.21827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woo HD, Kim J. Global DNA hypomethylation in peripheral blood leukocytes as a biomarker for cancer risk: a meta-analysis. PLoS One. 2012;7(4):e34615 10.1371/journal.pone.0034615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim YR, Kim CS, Naqvi A, Kumar A, Kumar S, Hoffman TA, et al. Epigenetic upregulation of p66shc mediates low-density lipoprotein cholesterol-induced endothelial cell dysfunction. Am J Physiol Heart Circ Physiol. 2012;303(2):H189–96. 10.1152/ajpheart.01218.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitra S, Khaidakov M, Lu J, Ayyadevara S, Szwedo J, Wang XW, et al. Prior exposure to oxidized low-density lipoprotein limits apoptosis in subsequent generations of endothelial cells by altering promoter methylation. Am J Physiol Heart Circ Physiol. 2011;301(2):H506–13. 10.1152/ajpheart.00252.2011 . [DOI] [PubMed] [Google Scholar]
- 63.Lund G, Zaina S. Atherosclerosis: an epigenetic balancing act that goes wrong. Curr Atheroscler Rep. 2011;13(3):208–14. 10.1007/s11883-011-0174-3 . [DOI] [PubMed] [Google Scholar]
- 64.Toiyama Y, Inoue Y, Shimura T, Fujikawa H, Saigusa S, Hiro J, et al. Serum Angiopoietin-like Protein 2 Improves Preoperative Detection of Lymph Node Metastasis in Colorectal Cancer. Anticancer Res. 2015;35(5):2849–56. . [PubMed] [Google Scholar]
- 65.Wang Z, Zheng H, Chen H, Lin X, Chen J, Wang L, et al. Elevated Serum Angiopoietin-like Protein 2 in Patients with Acute Coronary Syndrome. Arch Med Res. 2015;46(4):257–64. 10.1016/j.arcmed.2015.05.003 . [DOI] [PubMed] [Google Scholar]
- 66.Wang JY, Xiao HB, Sun ZL, Zhang DS. Angiopoietin-like protein 2 may mediate the inflammation in murine mastitis through the activation of interleukin-6 and tumour necrosis factor-alpha. World J Microbiol Biotechnol. 2015;31(8):1235–40. 10.1007/s11274-015-1873-7 . [DOI] [PubMed] [Google Scholar]
- 67.Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlen SE, Greco D, et al. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One. 2012;7(7):e41361 10.1371/journal.pone.0041361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data have been included in the paper. Due to ethical restrictions, individual data from the current study are available upon request from the corresponding author. The corresponding author Eric Thorin may be contacted at eric.thorin@umontreal.ca.