Abstract
Helminths have evolved numerous pathways to prevent their expulsion or elimination from the host to ensure long-term survival. During infection, they target numerous host cells, including macrophages, to induce an alternatively activated phenotype, which aids elimination of infection, tissue repair, and wound healing. Multiple animal-based studies have demonstrated a significant reduction or complete reversal of disease by helminth infection, treatment with helminth products, or helminth-modulated macrophages in models of allergy, autoimmunity, and sepsis. Experimental studies of macrophage and helminth therapies are being translated into clinical benefits for patients undergoing transplantation and those with multiple sclerosis. Thus, helminths or helminth-modulated macrophages present great possibilities as therapeutic applications for inflammatory diseases in humans. Macrophage-based helminth therapies and the underlying mechanisms of their therapeutic or curative effects represent an under-researched area with the potential to open new avenues of treatment. This review explores the application of helminth-modulated macrophages as a new therapy for inflammatory diseases.
Introduction
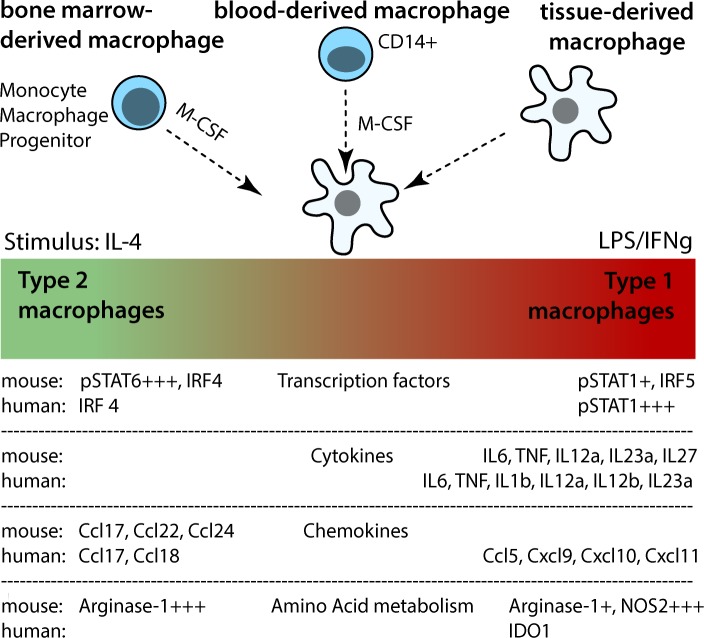
Regulation of macrophage activity and function is essential to balance tissue homeostasis, driving or resolving inflammation in most disease processes. The inflammatory or anti-inflammatory activities of macrophages are shaped in a tissue- and signal-specific manner, enabling macrophages to induce various activation patterns and develop specific functional programs (Fig 1) [1,2].
Fig 1. Origin and activation spectrum of murine and human macrophages.
Modified from Murray et al. [1] and focused on M1 and M2 macrophages only.
A recent study in airway hyperreactivity has demonstrated that local macrophages acquire an alternatively activated phenotype (AAM) with regulatory aspects that prevent the development of pathology by inducing antigen-specific CD4+ FoxP3+ T regulatory (Treg) cells [3]. In a skin allergy model, monocytes that are recruited to the site of inflammation express high levels of the typical AAM markers arginase-1 (arg-1), chitinase-like proteins (CLP), and programmed death-ligand (PD-L)2 and reduce inflammation [4]. Hence, the anti-inflammatory and immunoregulatory functions of macrophages could be harnessed for inflammatory disorders, implying that studies to understand their maintenance and stability in vivo are essential.
Helminths typically induce T helper (Th)2 responses but have also developed multiple ways to regulate the host immune system to ensure their long-term survival in the host. This regulation can affect bystander allergic or autoimmune diseases, and it has become clear that the presence or absence of helminths in humans has a major influence on the prevalence of such diseases. According to the hygiene hypothesis, improvements in public health have reduced incidences of bacterial, viral, and parasitic diseases, which correlate with an increase in chronic autoimmune inflammatory and allergic disorders. Epidemiological studies demonstrate the inverse relationship between helminth infections and inflammatory bowel disease (IBD) [5] or allergies [6,7]. Multiple experimental studies in mice recapitulate this negative correlation and show disease improvement with concurrent helminth infections, allowing underlying mechanisms to be unravelled.
Several immune cells become activated in helminth infection, with Tregs, regulatory B (Breg) cells, and AAM representing master regulators of pathology [8]. This review focuses on helminth-induced immunoregulatory macrophages, which can protect against unrelated inflammation and parasite-induced tissue damage [8–10].
Abundant evidence demonstrates the potential of immunosuppressive, macrophage-targeted therapies in the treatment of renal disease, diabetes, inflammatory diseases, and transplantation rejection. In a chronic inflammatory renal disease model, macrophages polarized in vitro with interleukin (IL)-4 and IL-13 ameliorate disease severity and injury after transfer into mice with the disease [11]. In diabetic mice, transfer of macrophages treated with a combination of IL-4, IL-10, and transforming growth factor (TGF)-β protects up to 80% from the condition [12]. M2 macrophages reduce proinflammatory Th1 and Th17 responses and disease severity in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS) [13]. Similarly, M2 macrophages can protect from septic shock in a model of cecal ligation and puncture [14]. These studies show great promise for the application of macrophages in chronic diseases.
Helminth-Modulated Macrophages
Macrophages are key innate immune cells that encounter helminths upon initial infection. The macrophage immunoregulatory phenotypes that develop during helminth infection divert anti-helminth immunity to induce host tolerance, parasite survival, and repair of any tissue injury caused by larvae or eggs [15,16].
Murine macrophages that develop in helminth infections express arg-1, resistin-like molecule (RELM)-α, CLPs, mannose receptor C type (MRC)-1 [17], and proliferate in situ [18–20]. In murine models of Schistosoma mansoni infection, arg-1–positive macrophages suppress IL-12 and IL-23 production [21]. In Nippostrongylus brasiliensis infection, gut macrophages express arg-1, RELM-α, and Ym1 in an IL-4– and IL-13–dependent manner [22], and their depletion allows parasite persistence. Interestingly, neutrophils can also promote the development of M2 macrophages, which subsequently adhere to helminth larvae, increasing their mortality; these macrophages can transfer protection to naïve animals [23]. In human filarial infections, different monocyte phenotypes exist depending on the individual’s disease status. In asymptomatic Brugia malayi infection, monocytes express typical M2 markers, which can be recapitulated by stimulation of human monocytes with filarial antigen or live microfilariae in vitro [24–26].
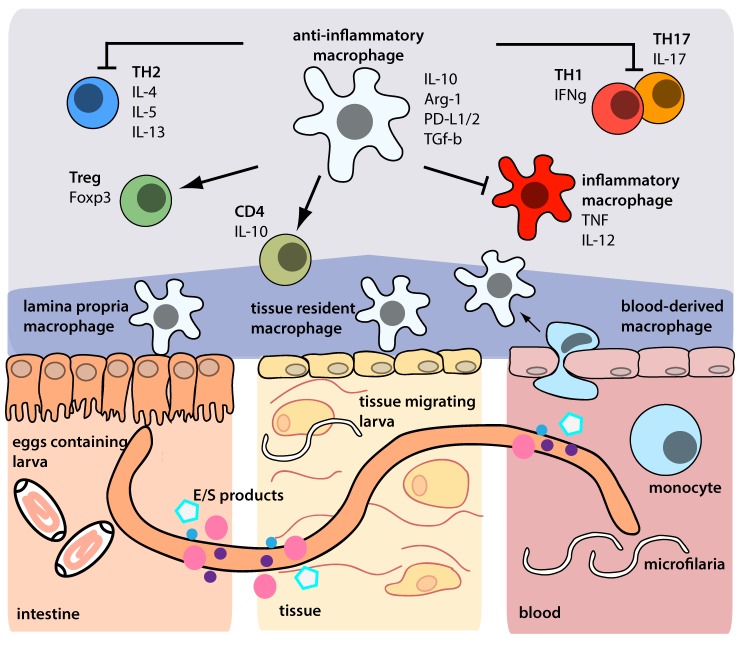
Defined helminth products can also act on macrophages to induce specific regulatory phenotypes; great efforts have been made to identify helminth products with therapeutic potential [27]. A clear example of this is the filarial molecule ES-62 from Acanthocheilonema viteae, which targets macrophages to repress IL-12 in cells exposed to lipopolysaccharide (LPS) and interferon (IFN)-γ [28,29]. A cysteine protease inhibitor from A. viteae (AvCystatin) is recognised and taken up by macrophages to induce phosphorylation of the mitogen-activated protein kinase signalling pathways ERK1/2 and p38, resulting in IL-10 production [30]. These macrophages also express arg-1, PD-L1, and PD-L2, promote IL-10 production in CD4+ T cells in a cell contact–dependent manner, and protect against allergy and colitis upon adoptive transfer [9]. In summary, helminths modulate macrophages to develop distinct phenotypes and functions that reduce or prevent host immunopathology by inducing regulatory cell populations or diverting proinflammatory effector cells (Fig 2).
Fig 2. Anti-inflammatory macrophages derived from the intestine, tissue, or blood, stimulated by helminths or their products, and their mechanism of action.
This cell population may be taken advantage of to develop new therapeutic agents and treat unrelated inflammatory diseases (Box 1).
Box 1. Characteristics of Selected Inflammatory Diseases and Widely Used Animal Models
Allergy: Strong Th2 responses in mucosal tissues or skin to environmental and food antigens involving eosinophils, mast cells, and IgE. Animal model: Allergic airway inflammation using sensitization and challenge with model allergens (ovalbumin).
Inflammatory Bowel Disease (IBD): Autoimmune disease. Ulcerative colitis is characterized by a dominant CD4+ Th1 response of the colon.
Crohn‘s Disease can occur through the entire length of the gastrointestinal tract and is typically associated with an excess of Th2 cytokines. Animal model: spontaneous development of colitis in IL-10–deficient mice or in T and B cell–deficient mice upon transfer of antigen-experienced T cells. Chemical-induced colitis is based on disruption of the intestinal barrier and T cell response against autologous proteins.
Diabetes: Type 1 diabetes (T1D) occurs early in life and is immunologically driven, primarily by a strong CD8+ T cell response that destroys pancreatic β cells. Type 2 Diabetes (T2D) is associated with lifestyle and nutrition factors.
Animal model: Nonobese diabetic (NOD) mice develop symptoms of T1D spontaneously at about 12 weeks of age.
Multiple Sclerosis (MS): Complex demyelinating inflammatory disorder of the central nervous system involving humoral and cellular (Th1 and Th17) immune responses. Animal model: Experimental autoimmune encephalomyelitis (EAE) is induced by injection of myelin-oligodendrocyte glycoprotein and adjuvants and mirrors major aspects of the complex pathophysiology of MS.
Rheumatoid Arthritis (RA): Autoimmune disease causing inflammation and destruction of the joints. It is a systemic disease that exhibits extra-articular manifestations as well. Animal model: Collagen-induced arthritis (CIA). Tissue injection of collagen together with complete Freud‘s adjuvants in susceptible mouse strains.
Sepsis: A serious medical condition characterized by dysregulated systemic inflammatory responses towards microbial stimuli followed by immunosuppression. Animal model: Bolus injection of Toll-like receptor agonists or cecal ligation and puncture, which mimics the polymicrobial sepsis observed in human disease.
Application of Helminth-Modulated Macrophages in Autoimmune Diseases
It is important to establish whether, once differentiated, the regulatory phenotype of helminth-modulated macrophages is stable enough to treat chronic diseases. We aim to instigate a discussion by reviewing current data on these macrophages in the treatment of inflammatory diseases (Fig 3).
Fig 3. Experimental evidence and clinical trials highlighting the potential of helminth and macrophage therapy.
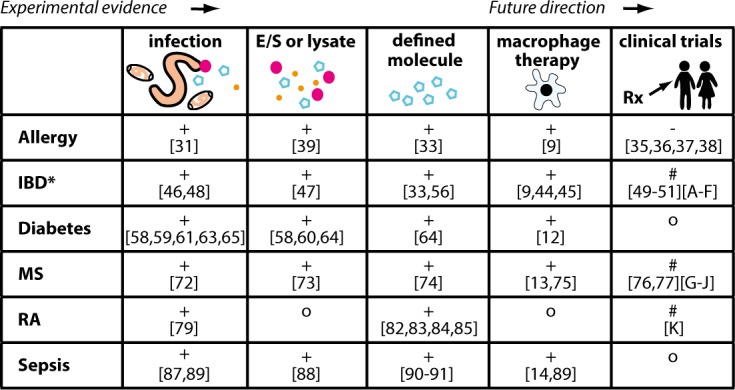
+ = positive outcome,— = no improved outcome, # = under investigation, o = no data available, * and coeliac disease. IBD: Inflammatory bowel disease, MS: Multiple sclerosis, RA: Rheumatoid arthritis. [A]: NCT01279577, [B]: NCT01576471, [C]: NCT01434693, [D]: NCT01953354, [E]: NCT01433471, [F]: NCT01661933, [G]: NCT014113243, [H]: NCT00645749, [I]: NCT01006941, [J]: NCT01470521, [K]: DHRS0005323.
Helminths and macrophages in allergy and asthma
Allergies are driven by dysregulated Th2 responses, predicting that helminth infection might exacerbate these inflammatory disorders. Nevertheless, the strong regulatory mechanisms employed by helminths suppress Th1- and Th2-mediated diseases. We have previously reviewed helminth infections that mediate protection in allergy-related experimental animal models [31]; we describe here those that illustrate macrophages as potential therapeutic targets for these and other diseases.
Lung macrophages are key players in asthma and develop a defined activation status that modulates adaptive immune responses by local T cells. Despite the fact that lung macrophages are involved in fibrogenesis in asthma [32], it has been shown that tissue-resident macrophages can induce FoxP3+ Treg cells [3]. In a murine model of ovalbumin (OVA)-induced airway hyperreactivity, treatment with AvCystatin reduced eosinophil lung recruitment and production of OVA-specific immunoglobulin (Ig)E, total IgE, and allergen-specific IL-4, thereby diminishing disease symptoms. Depleting IL-10 or macrophages reversed these antiallergic effects, implicating the therapeutic potential of macrophages in this model [33,34]. In fact, transfer of AvCystatin-treated macrophages to mice with airway hyperreactivity suppressed clinical disease symptoms [9].
A recent clinical trial focused on helminth therapy in rhinitis [35–37], in which patients were treated with Trichuris suis ova. While an antiparasitic immune response developed in these patients, neither a redirection of allergen-specific immune responses nor a therapeutic effect was achieved. Similarly, experimental hookworm infection did not lead to improved outcomes in a clinical trial with patients suffering from asthma [38]. However, allergic mice treated with excretory/secretory (E/S) products from T. suis had reduced allergic airway hyperreactivity after challenge [39], which might be a reflection of the route of application or the amount of helminth-derived immunomodulatory molecules available in this setting. Thus, promising preclinical data need to be translated to show definitive clinical benefits for patients with allergic disorders.
Helminths and macrophages in inflammatory bowel disease and coeliac disease
Distortion of the intestinal barrier and immune response to intestinal bacteria can lead to IBD, including ulcerative colitis and Crohn's disease. Tissue macrophages are present in high numbers in the intestine and present a good target for helminth therapy because of their multiple activation states. In healthy individuals, lamina propria macrophages maintain intestinal homeostasis by inducing Tregs [40,41], while in active IBD, macrophages contribute to pathology by expressing multiple proinflammatory cytokines [42]. M1 macrophages invading the intestinal tissue drive the disruption of the epithelial barrier through dysregulation of tight junction proteins and epithelial apoptosis [43]. In contrast, patients with inactive Crohn's disease have higher levels of M2 macrophages [44], which are also important in inducing protection against IBD in mice [45]. Thus, helminth-induced M2 macrophages and Tregs may contribute to protection against IBD. Mice infected with Hymenolepis diminuta [46] and treated with the adult worm extract [47] or treated with IL-4/IL-13–differentiated M2 macrophages [44] have significantly reduced pathology in experimentally induced colitis; this protective effect is abrogated when IL-10 [46] or macrophages [44] are depleted. Murine infection with S. mansoni prevents colitis in a macrophage-dependent but IL-4- and IL-13–independent manner, representing another population of suppressive macrophages [48].
Intriguingly, experimental hookworm infection combined with gluten microchallenge induces tolerance in patients with coeliac disease, an autoimmune disease resulting from gluten intolerance [49]. The contribution of macrophages was not evaluated in this setting.
Multiple clinical trials are investigating the use of T. suis ova therapy in IBD and have shown moderate success (see Fig 3) [50,51]. The safety of this treatment, threatened by the colonization and invasion of the host by T. suis, has been much debated and requires treated patients to be monitored closely [52–55]. An alternative approach would be to administer characterized helminth products such as AvCystatin or transgenic probiotic bacteria expressing helminth immunomodulators, which lead to diminished disease scores in murine IBD models by reducing numbers of inflammatory macrophages [33,56]. Nevertheless, there are currently no clinical trials addressing the role of helminth-modulated macrophages in protection against IBD. Future studies should translate the encouraging experimental evidence into clinical benefits for patients.
Helminths and macrophages in diabetes
Both environmental and genetic factors play a role in the development of diabetes, and incidences of this condition have increased dramatically in the past 30 years in developed and newly industrialized countries [57]. Studies have demonstrated an inverse correlation between diabetes (type 1 [T1D] and type 2 [T2D]) and helminth infections [58]. Helminth products have also been demonstrated to reduce incidences of diabetes in animal models [58–61]. Early studies suggested that macrophages could exacerbate T1D, in which macrophage depletion ameliorated disease [62]. As T1D is a Th1-driven disease, it is likely that the macrophages involved are classically activated, which could be redirected by a helminth infection. Nonobese diabetic (NOD) mice infected with Heligmosomoides polygyrus have augmented numbers of Tregs and Th2 responses as well as an infiltration of M2 macrophages and increased IL-10 expression in the pancreatic lymph nodes [63]. Injection of schistosome egg antigen into NOD mice induces arg-1 and RELM-α expression in macrophages and modulates T cell responses [64]. In another diabetes model, infection with Taenia crassiceps attenuates disease in two different mouse strains and is accompanied by high levels of IL-4 and M2 macrophages [65]. To date, there are no clinical trials examining the application of helminths or macrophages in T1D, indicating an open area for future research.
Helminths and macrophages in multiple sclerosis
MS is an inflammatory autoimmune disorder driven by dysregulated Th1 and Th17 responses, resulting in a demyelinating disease that affects the central nervous system (CNS). Environmental and genetic factors may be involved in disease onset [66]. As MS progresses, acute inflammatory lesions develop when the integrity of the blood–brain barrier is disturbed, with CD4+ Th1, Th17 cells, and CD8+ cells becoming activated by mature dendritic cells [67].Various studies have demonstrated that helminth-infected patients with MS have fewer relapses and inflammatory changes than uninfected patients, while removal of helminth infection exacerbates MS disease [68–70].
Different helminth species have been studied for their ability to modulate unwanted inflammatory responses in MS [71]. Mice with EAE immunised with S. mansoni eggs have lower disease severity; clinical scores and cellular infiltrates are reduced, and CD11b+ macrophages isolated from the CNS show decreased IL-12 expression [72]. Schistosomal egg antigen and a single schistosome glycan were also effective in protecting mice against EAE [73,74]. The importance of M2 macrophages that produce IL-10 and protect mice from developing EAE has also been described [75].
While no clinical trials currently exist that use macrophages to treat MS, trials using T. suis ova (TSO) or hookworm larvae are underway or already present results from a small cohort of patients (Fig 3). While both studies show that TSO is safe, the therapeutic effect is ambiguous: one study reports a decrease in the number of CNS lesions observed by magnetic resonance imaging [76] while a comparable study did not detect clinical improvement [77].
Helminths and macrophages in rheumatoid arthritis
Multiple experimental helminth-based treatment strategies have been tested in rheumatoid arthritis (RA), a chronic inflammatory disorder [78]. While the exact disease cause is unknown, dysregulated immune responses are important, as high levels of tumour necrosis factor (TNF) and IL-1β have been detected in inflamed synovial membranes. T cells from synovial tissue express Th1- and Th17-associated cytokines and activate neighbouring macrophages that release large amounts of TNF and IL-1β. These and other macrophage-derived proinflammatory cytokines drive much of the inflammation and implicate macrophages as key players in disease [79]. Current treatments include nonsteroidal anti-inflammatory drugs, which can have potentially detrimental long-term side effects [80]. ES-62 shows great potential to treat dysregulated inflammatory disorders [81]. ES-62 prevents collagen-induced arthritis when injected into mice by downregulating IL-17 and MyD88 [82] and restoring levels of IL-10-producing B cells and reducing intra-articular plasma cell infiltration [83]. Introduction of ES-62 in a coculture of T cells from patients with RA and macrophage cell lines significantly reduced macrophage TNF expression compared with ES-62–untreated cells [84]. A synthetic analogue of ES-62 prevented experimental arthritis and inhibited macrophage-derived IL-1β [85]. Numerous therapies for RA are in preclinical or clinical trials, which aim to neutralise or inhibit many macrophage-related disease-driving mechanisms [86]. However, as yet, only one clinical trial assesses helminth infection as a potential therapy for RA (Fig 3).
Helminths and macrophages in systemic inflammation
Recently, it was shown that helminths and their products can decrease the prevalence of sepsis and improve the outcome of systemic bacterial infection and inflammation [87–91]. Epidemiological data demonstrated a lower prevalence of filarial infection in patients with sepsis than in healthy individuals, suggesting that preexisting helminth infection prevents sepsis development [87]. Fundamental evidence demonstrating that helminth-modulated macrophages improve sepsis came from a murine experimental filarial infection, in which gene expression profiles of macrophages modulated by Litosomosoides sigmodontis illustrated decreased Toll-like receptor (TLR) responsiveness. Transfer of macrophages from L. sigmodontis–infected mice into naïve recipients improved sepsis outcome in a TLR2-dependent but AAM-independent manner [89]. Macrophages from patients with sepsis expressed reduced sepsis-inducing inflammatory cytokines after treatment with Trichinella spiralis E/S products [88]. Similarly, a T. spiralis cathepsin B–like protein ameliorates intestinal ischemia/reperfusion injury, a model for systemic inflammation, by promoting a switch from M1 to M2 macrophages [91]. Furthermore, a single helminth molecule from Fasciola hepatica (fatty acid–binding protein; FABP or Fh12) can suppress serum inflammatory cytokines in a septic shock model. This was accompanied by suppression of proinflammatory cytokines and nitric oxide synthase-2 (NOS2) in macrophages [90] and demonstrates the potential of macrophages in this disease setting.
Macrophages in Cell Therapy: A Potential Treatment Option
For macrophage-based therapies, one must consider the possibility of phenotype reversion after transfer. The phenotype and function of a particular macrophage subset develops from the combined integration of tissue-specific and environmental cues, such as inflammation or infection, which can lead to epigenetic imprinting [19,92]; however, the stability of the therapeutic macrophage phenotype must be determined.
Murine studies have shown that transferred macrophages can block pathology independently of the perturbed environment they encounter [9,12,13,45,75,89]. One particular macrophage subset can confer protection upon transfer in mice and humans. Murine macrophages stimulated with IFN-γ have significant anti-inflammatory characteristics, mitigating colitis and prolonging allograft survival [93,94]. Human macrophages stimulated with IFN-γ in vitro and administered to patients undergoing renal transplant significantly reduced the required dose of immunosuppressant drugs and improved transplanted kidney function [95]. These macrophages conferred immunosuppression on T cells, which was partly mediated by the indoleamine 2,3-dioxygenase and likely induced nutrient deficiencies in alloreactive T cells [94]. Although the mechanism of action of these macrophages is different to that of helminth-induced macrophages, it exemplifies how these powerful cells can redirect undesired immune responses in disease settings.
The macrophage population that bears sufficient therapeutic function in a given environment must be carefully evaluated. Alongside macrophages, other immune cells are involved in helminth-derived immunomodulation. The application of one helminth-modulated cell population cannot represent the full spectrum of immunomodulation compared with a chronic helminth infection, which can induce changes in microbiota [38,96], mediating a therapeutic effect [97], but it might be enough to reset the diseased environment to homeostasis.
What Does the Future Hold for Helminth-Based Therapies?
The studies discussed herein demonstrate the potential of helminth infections and, in particular, helminth-induced macrophages to treat inflammatory disorders; in some cases, clinical trials are already underway. However, the mode of application must be addressed to determine the safest and most effective route for patients. Is it best to treat the patient with a patent infection or with isolated stages (e.g., eggs)? Is it best to apply specific helminth-derived products (e.g., AvCystatin, ES-62, T. spiralis cathepsin B–like protein) or to stimulate in vitro and reinfuse a patient’s own macrophages? Live infections provide a rapid path to clinical trials compared with identifying and characterising defined products. Nevertheless, live infections remain infectious, and can induce pathological consequences in the host, especially in immunocompromised individuals [98]. In contrast, defined products can be produced recombinantly in high quantities at relatively low costs. Defined products allow efficient site-directed and prolonged application, e.g., through the use of carriers like probiotic bacteria that colonize and release the molecules in targeted tissues [56]. Generating transgenic auxotrophic strains that release powerful helminth products will enable the use of such techniques without risking contamination of the environment. However, helminth products themselves may be immunogenic, and thus, a further therapeutic alternative is the synthesis of small-molecule analogues, as described for ES-62. New targets identified by large-scale technologies (proteomics, metabolomics, genomics) combined with bioinformatics aid the discovery of novel pathways and molecules that can translate helminth–or helminth product–derived immunomodulating strategies into efficient therapies [27].
The experimental models that illustrate the prospect of helminth-modulated, macrophage-based therapies provide hope that safe and effective treatments for humans are a viable option. The abilities of macrophages to regulate T and B cell function and cytokine production highlight this innate cell population as a powerful tool in therapy development. However, the stability of transferred macrophages must be established. The fact that clinical trials employing the regulatory effects of helminths or immune-suppressive macrophages are underway is extremely encouraging and indicates that research in this direction should be pursued.
Funding Statement
This work was supported by the German Research Foundation: GRK 1673 (SS and SH) http://www.vetmed.fu-berlin.de/en/einrichtungen/sonstige/grk1673/, SFB 650 (SH) http://www.sfb650.charite.de/, and HA2542/3-2 (SH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity. 2014. July 17;41(1):14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultze JL, Freeman T, Hume DA, Latz E. A transcriptional perspective on human macrophage biology. Semin Immunol. 2015. February;27(1):44–50. 10.1016/j.smim.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 3. Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210(4):775–88. 10.1084/jem.20121849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38(3):570–80. 10.1016/j.immuni.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 5. Weinstock J V, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15(1):128–33. 10.1002/ibd.20633 [DOI] [PubMed] [Google Scholar]
- 6. Cooper PJ. The potential impact of early exposures to geohelminth infections on the development of atopy. Clin Rev Allergy Immunol. 2004;26(1):5–14. [DOI] [PubMed] [Google Scholar]
- 7. Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M. Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep. 2010;10(1):3–12. 10.1007/s11882-009-0085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43(3–4):301–10. 10.1016/j.ijpara.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 9. Ziegler T, Rausch S, Steinfelder S, Klotz C, Hepworth MR, Kühl AA, et al. A novel regulatory macrophage induced by a helminth molecule instructs IL-10 in CD4+ T cells and protects against mucosal inflammation. J Immunol. 2015. February 15;194(4):1555–64. 10.4049/jimmunol.1401217 [DOI] [PubMed] [Google Scholar]
- 10. Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, et al. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012. January 15;18(2):260–6. 10.1038/nm.2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72(3):290–9. [DOI] [PubMed] [Google Scholar]
- 12. Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, et al. Adoptive Transfer of Immunomodulatory M2 Macrophages Prevents Type 1 Diabetes in NOD Mice. Diabetes. 2012; 61(11):2881–92. 10.2337/db11-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin H, Yeh WI, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A. 2012;109(13):5004–9. 10.1073/pnas.1117218109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh P, Dejager L, Amand M, Theatre E, Vandereyken M, Zurashvili T, et al. DUSP3 Genetic Deletion Confers M2-like Macrophage-Dependent Tolerance to Septic Shock. J Immunol. 2015. May 15;194(10):4951–62. 10.4049/jimmunol.1402431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gause WC, Wynn TA, Allen JE. Type 2 immunity and wound healing: evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol. 2013;13(8):607–14. 10.1038/nri3476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esser-von Bieren J, Volpe B, Sutherland DB, Bürgi J, Verbeek JS, Marsland BJ, et al. Immune antibodies and helminth products drive CXCR2-dependent macrophage-myofibroblast crosstalk to promote intestinal repair. PLoS Pathog. 2015. March;11(3):e1004778 10.1371/journal.ppat.1004778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. 10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 18. Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32(10):470–7. 10.1016/j.it.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–8. 10.1126/science.1204351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–95. 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herbert DR, Orekov T, Roloson A, Ilies M, Perkins C, O’Brien W, et al. Arginase I suppresses IL-12/IL-23p40-driven intestinal inflammation during acute schistosomiasis. J Immunol. 2010;184(11):6438–46. 10.4049/jimmunol.0902009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao A, Urban JF Jr., Anthony RM, Sun R, Stiltz J, van Rooijen N, et al. Th2 cytokine-induced alterations in intestinal smooth muscle function depend on alternatively activated macrophages. Gastroenterology. 2008;135(1):217–25 e1. 10.1053/j.gastro.2008.03.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014. October;15(10):938–46. 10.1038/ni.2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199(12):1827–37. 10.1086/599090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Semnani RT, Mahapatra L, Moore V, Sanprasert V, Nutman TB. Functional and phenotypic characteristics of alternative activation induced in human monocytes by interleukin-4 or the parasitic nematode Brugia malayi. Infect Immun. 2011;79(10):3957–65. 10.1128/IAI.05191-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Regan NL, Steinfelder S, Venugopal G, Rao GB, Lucius R, Srikantam A, et al. Brugia malayi microfilariae induce a regulatory monocyte/macrophage phenotype that suppresses innate and adaptive immune responses. PLoS Negl Trop Dis. 2014. October;8(10):e3206 10.1371/journal.pntd.0003206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shepherd C, Navarro S, Wangchuk P, Wilson D, Daly NL, Loukas A. Identifying the immunomodulatory components of helminths. Parasite Immunol. 2015. June;37(6):293–303. 10.1111/pim.12192 [DOI] [PubMed] [Google Scholar]
- 28. Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167(2):940–5. [DOI] [PubMed] [Google Scholar]
- 29. Goodridge HS, Marshall FA, Wilson EH, Houston KM, Liew FY, Harnett MM, et al. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113(4):491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klotz C, Ziegler T, Figueiredo AS, Rausch S, Hepworth MR, Obsivac N, et al. A helminth immunomodulator exploits host signaling events to regulate cytokine production in macrophages. PLoS Pathog. 2011;7(1):e1001248 10.1371/journal.ppat.1001248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Danilowicz-Luebert E, O’Regan NL, Steinfelder S, Hartmann S. Modulation of specific and allergy-related immune responses by helminths. J Biomed Biotechnol. 2011;2011:821578 10.1155/2011/821578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, Loddenkemper C, et al. A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol. 2008;180(6):4265–72. [DOI] [PubMed] [Google Scholar]
- 34. Danilowicz-Luebert E, Steinfelder S, Kuhl AA, Drozdenko G, Lucius R, Worm M, et al. A nematode immunomodulator suppresses grass pollen-specific allergic responses by controlling excessive Th2 inflammation. Int J Parasitol. 2013;43(3–4):201–10. 10.1016/j.ijpara.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 35. Bager P, Arnved J, Ronborg S, Wohlfahrt J, Poulsen LK, Westergaard T, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. 2010;125(1):123 10.1016/j.jaci.2009.08.006 [DOI] [PubMed] [Google Scholar]
- 36. Bager P, Kapel C, Roepstorff A, Thamsborg S, Arnved J, Ronborg S, et al. Symptoms after ingestion of pig whipworm Trichuris suis eggs in a randomized placebo-controlled double-blind clinical trial. PLoS ONE. 2011;6(8):e22346 10.1371/journal.pone.0022346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bourke CD, Mutapi F, Nausch N, Photiou DM, Poulsen LK, Kristensen B, et al. Trichuris suis ova therapy for allergic rhinitis does not affect allergen-specific cytokine responses despite a parasite-specific cytokine response. Clin Exp Allergy. 2012;42(11):1582–95. 10.1111/j.1365-2222.2012.04063.x [DOI] [PubMed] [Google Scholar]
- 38. Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. 2010. February;40(2):299–306. 10.1111/j.1365-2222.2009.03433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ebner F, Hepworth MR, Rausch S, Janek K, Niewienda A, Kühl A, et al. Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy. 2014. November;69(11):1489–97. 10.1111/all.12496 [DOI] [PubMed] [Google Scholar]
- 40. Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8(10):1086–94. [DOI] [PubMed] [Google Scholar]
- 41. Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014. July;260(1):102–17. 10.1111/imr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bar-On L, Zigmond E, Jung S. Management of gut inflammation through the manipulation of intestinal dendritic cells and macrophages? Semin Immunol. 2011;23(1):58–64. 10.1016/j.smim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 43. Lissner D, Schumann M, Batra A, Kredel L- I, Kühl AA, Erben U, et al. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis. 2015. July;21(6):1297–305. 10.1097/MIB.0000000000000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hunter MM, Wang A, Parhar KS, Johnston MJ, Van Rooijen N, Beck PL, et al. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138(4):1395–405. 10.1053/j.gastro.2009.12.041 [DOI] [PubMed] [Google Scholar]
- 45. Weisser SB, Brugger HK, Voglmaier NS, McLarren KW, van Rooijen N, Sly LM. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J Leukoc Biol. 2011;90(3):483–92. 10.1189/jlb.0311124 [DOI] [PubMed] [Google Scholar]
- 46. Hunter MM, Wang A, Hirota CL, McKay DM. Neutralizing anti-IL-10 antibody blocks the protective effect of tapeworm infection in a murine model of chemically induced colitis. J Immunol. 2005;174(11):7368–75. [DOI] [PubMed] [Google Scholar]
- 47. Johnston MJ, Wang A, Catarino ME, Ball L, Phan VC, MacDonald JA, et al. Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect Immun. 2010;78(3):1364–75. 10.1128/IAI.01349-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith P, Mangan NE, Walsh CM, Fallon RE, McKenzie AN, van Rooijen N, et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J Immunol. 2007;178(7):4557–66. [DOI] [PubMed] [Google Scholar]
- 49. Croese J, Giacomin P, Navarro S, Clouston A, McCann L, Dougall A, et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. 2015. February;135(2):508–16.e5. 10.1016/j.jaci.2014.07.022 [DOI] [PubMed] [Google Scholar]
- 50. Summers RW, Elliott DE, Urban JF, Thompson RA, Weinstock J V. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005. April;128(4):825–32. [DOI] [PubMed] [Google Scholar]
- 51. Summers RW, Elliott DE, Urban JF Jr., Thompson R, Weinstock J V. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54(1):87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kradin RL, Badizadegan K, Auluck P, Korzenik J, Lauwers GY. Iatrogenic Trichuris suis infection in a patient with Crohn disease. Arch Pathol Lab Med. 2006;130(5):718–20. [DOI] [PubMed] [Google Scholar]
- 53. Summers RW, Elliott DE, Weinstock J V. Why Trichuris suis should prove safe for use in inflammatory bowel diseases. Inflamm Bowel Dis. 2005;11(8):783–4. [DOI] [PubMed] [Google Scholar]
- 54. Van Kruiningen HJ, West AB. Potential danger in the medical use of Trichuris suis for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(5):515. [DOI] [PubMed] [Google Scholar]
- 55. Van Kruiningen HJ, West AB. Iatrogenic Trichuris suis infection. Arch Pathol Lab Med. 2007;131(2):180 [DOI] [PubMed] [Google Scholar]
- 56. Whelan RA, Rausch S, Ebner F, Günzel D, Richter JF, Hering NA, et al. A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol Ther. 2014. October;22(10):1730–40. 10.1038/mt.2014.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Z, Liu Q, Bleich D, Salgame P, Gause WC. Regulation of type 1 diabetes, tuberculosis, and asthma by parasites. J Mol Med. 2010;88(1):27–38. 10.1007/s00109-009-0546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hussaarts L, García-Tardón N, van Beek L, Heemskerk MM, Haeberlein S, van der Zon GC, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. 2015. July;29(7):3027–39. 10.1096/fj.14-266239 [DOI] [PubMed] [Google Scholar]
- 59. Cooke A, Tonks P, Jones FM, O’Shea H, Hutchings P, Fulford AJ, et al. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21(4):169–76. [DOI] [PubMed] [Google Scholar]
- 60. Zaccone P, Burton O, Miller N, Jones FM, Dunne DW, Cooke A. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur J Immunol. 2009;39(4):1098–107. 10.1002/eji.200838871 [DOI] [PubMed] [Google Scholar]
- 61. Aravindhan V, Mohan V, Surendar J, Rao MM, Ranjani H, Kumaraswami V, et al. Decreased prevalence of lymphatic filariasis among subjects with type-1 diabetes. Am J Trop Med Hyg. 2010;83(6):1336–9. 10.4269/ajtmh.2010.10-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jun HS, Yoon CS, Zbytnuik L, van Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189(2):347–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Q, Sundar K, Mishra PK, Mousavi G, Liu Z, Gaydo A, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009;77(12):5347–58. 10.1128/IAI.01170-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zaccone P, Burton OT, Gibbs S, Miller N, Jones FM, Dunne DW, et al. Immune modulation by Schistosoma mansoni antigens in NOD mice: effects on both innate and adaptive immune systems. J Biomed Biotechnol. 2010;2010:795210 10.1155/2010/795210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Espinoza-Jimenez A, Rivera-Montoya I, Cardenas-Arreola R, Moran L, Terrazas LI. Taenia crassiceps infection attenuates multiple low-dose streptozotocin-induced diabetes. J Biomed Biotechnol. 2010;2010:850541 10.1155/2010/850541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8(9):913–9. [DOI] [PubMed] [Google Scholar]
- 67. Simmons SB, Pierson ER, Lee SY, Goverman JM. Modeling the heterogeneity of multiple sclerosis in animals. Trends Immunol. 2013;34(8):410–22. 10.1016/j.it.2013.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis. Ann Neurol. 2007;61(2):97–108. [DOI] [PubMed] [Google Scholar]
- 69. Correale J, Farez MF. The impact of parasite infections on the course of multiple sclerosis. J Neuroimmunol. 2011;233(1–2):6–11. 10.1016/j.jneuroim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 70. Correale J, Farez MF. Parasite infections in multiple sclerosis modulate immune responses through a retinoic acid-dependent pathway. J Immunol. 2013;191(7):3827–37. 10.4049/jimmunol.1301110 [DOI] [PubMed] [Google Scholar]
- 71. Elliott DE, Weinstock J V. Helminth-host immunological interactions: prevention and control of immune-mediated diseases. Ann N Y Acad Sci. 2012;1247:83–96. 10.1111/j.1749-6632.2011.06292.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sewell D, Qing Z, Reinke E, Elliot D, Weinstock J, Sandor M, et al. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int Immunol. 2003;15(1):59–69. [DOI] [PubMed] [Google Scholar]
- 73. Zheng X, Hu X, Zhou G, Lu Z, Qiu W, Bao J, et al. Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J Neuroimmunol. 2008. February;194(1–2):107–14. 10.1016/j.jneuroim.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 74. Zhu B, Trikudanathan S, Zozulya AL, Sandoval-Garcia C, Kennedy JK, Atochina O, et al. Immune modulation by Lacto-N-fucopentaose III in experimental autoimmune encephalomyelitis. Clin Immunol. 2012. March;142(3):351–61. 10.1016/j.clim.2011.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tierney JB, Kharkrang M, La Flamme AC. Type II-activated macrophages suppress the development of experimental autoimmune encephalomyelitis. Immunol Cell Biol. 2009;87(3):235–40. 10.1038/icb.2008.99 [DOI] [PubMed] [Google Scholar]
- 76. Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, Cook TD, et al. Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler. 2011;17(6):743–54. 10.1177/1352458511398054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Voldsgaard A, Bager P, Garde E, Åkeson P, Leffers AM, Madsen CG, et al. Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult Scler. 2015. November;21(13):1723–9. 10.1177/1352458514568173 [DOI] [PubMed] [Google Scholar]
- 78. Matisz CE, McDougall JJ, Sharkey KA, McKay DM. Helminth parasites and the modulation of joint inflammation. J Parasitol Res. 2011;2011:942616 10.1155/2011/942616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–42. [DOI] [PubMed] [Google Scholar]
- 80. Pincus T, Marcum SB, Callahan LF, Adams RF, Barber J, Barth WF, et al. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: I. Nonsteroidal antiinflammatory drugs. J Rheumatol. 1992;19(12):1874–84. [PubMed] [Google Scholar]
- 81. Pineda MA, Lumb F, Harnett MM, Harnett W. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. Mol Biochem Parasitol. January;194(1–2):1–8. 10.1016/j.molbiopara.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 82. Pineda MA, McGrath MA, Smith PC, Al-Riyami L, Rzepecka J, Gracie JA, et al. The parasitic helminth product ES-62 suppresses pathogenesis in CIA by targeting of the IL-17-producing cellular network at multiple sites. Arthritis Rheum. 2012; 64(10):3168–78. 10.1002/art.34581 [DOI] [PubMed] [Google Scholar]
- 83. Rodgers DT, Pineda MA, McGrath MA, Al-Riyami L, Harnett W, Harnett MM. Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints. Immunology. 2014. March;141(3):457–66. 10.1111/imm.12208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171(4):2127–33. [DOI] [PubMed] [Google Scholar]
- 85. Rzepecka J, Pineda MA, Al-Riyami L, Rodgers DT, Huggan JK, Lumb FE, et al. Prophylactic and therapeutic treatment with a synthetic analogue of a parasitic worm product prevents experimental arthritis and inhibits IL-1β production via NRF2-mediated counter-regulation of the inflammasome. J Autoimmun. 2015. June;60:59–73. 10.1016/j.jaut.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li J, Hsu HC, Mountz JD. Managing macrophages in rheumatoid arthritis by reform or removal. Curr Rheumatol Rep. 2012;14(5):445–54. 10.1007/s11926-012-0272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Panda M, Sahoo PK, Mohapatra A Das, Dutta S kanti, Thatoi PK, Tripathy R, et al. Decreased prevalence of sepsis but not mild or severe P. falciparum malaria is associated with pre-existing filarial infection. Parasit Vectors. 2013. January;6:203 10.1186/1756-3305-6-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Du L, Liu L, Yu Y, Shan H, Li L. Trichinella spiralis excretory-secretory products protect against polymicrobial sepsis by suppressing MyD88 via mannose receptor. Biomed Res Int. 2014. January;2014:898646 10.1155/2014/898646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gondorf F, Berbudi A, Buerfent BC, Ajendra J, Bloemker D, Specht S, et al. Chronic filarial infection provides protection against bacterial sepsis by functionally reprogramming macrophages. PLoS Pathog. 2015. January;11(1):e1004616 10.1371/journal.ppat.1004616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Martin I, Cabán-Hernández K, Figueroa-Santiago O, Espino AM. Fasciola hepatica fatty acid binding protein inhibits TLR4 activation and suppresses the inflammatory cytokines induced by lipopolysaccharide in vitro and in vivo. J Immunol. 2015. April 15;194(8):3924–36. 10.4049/jimmunol.1401182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu W-F, Wen S-H, Zhan J-H, Li Y-S, Shen J-T, Yang W-J, et al. Treatment with Recombinant Trichinella spiralis Cathepsin B-like Protein Ameliorates Intestinal Ischemia/Reperfusion Injury in Mice by Promoting a Switch from M1 to M2 Macrophages. J Immunol. 2015. July 1;195(1):317–28. 10.4049/jimmunol.1401864 [DOI] [PubMed] [Google Scholar]
- 92. Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2015. December 17;17(1):18–25. 10.1038/ni.3325 [DOI] [PubMed] [Google Scholar]
- 93. Brem-Exner BG, Sattler C, Hutchinson JA, Koehl GE, Kronenberg K, Farkas S, et al. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J Immunol. 2008;180(1):335–49. [DOI] [PubMed] [Google Scholar]
- 94. Riquelme P, Tomiuk S, Kammler A, Fandrich F, Schlitt HJ, Geissler EK, et al. IFN-gamma-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol Ther. 2013;21(2):409–22. 10.1038/mt.2012.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011. September 1;187(5):2072–8. 10.4049/jimmunol.1100762 [DOI] [PubMed] [Google Scholar]
- 96. Rausch S, Held J, Fischer A, Heimesaat MM, Kühl AA, Bereswill S, et al. Small Intestinal Nematode Infection of Mice Is Associated with Increased Enterobacterial Loads alongside the Intestinal Tract. Allen IC, editor. PLoS ONE. 2013. September 10;8(9):e74026 10.1371/journal.pone.0074026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zaiss MM, Rapin A, Lebon L, Dubey LK, Mosconi I, Sarter K, et al. The Intestinal Microbiota Contributes to the Ability of Helminths to Modulate Allergic Inflammation. Immunity. 2015. October 27;43(5):998–1010. 10.1016/j.immuni.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012. August;25(4):458–63. 10.1097/QCO.0b013e3283551dbd [DOI] [PMC free article] [PubMed] [Google Scholar]