Abstract
Group B Streptococcus (GBS) is a major causative agent of neonatal meningitis due to its ability to efficiently cross the blood-brain barrier (BBB) and enter the central nervous system (CNS). It has been demonstrated that GBS can invade human brain microvascular endothelial cells (hBMEC), a primary component of the BBB; however, the mechanism of intracellular survival and trafficking is unclear. We previously identified a two component regulatory system, CiaR/H, which promotes GBS intracellular survival in hBMEC. Here we show that a GBS strain deficient in the response regulator, CiaR, localized more frequently with Rab5, Rab7 and LAMP1 positive vesicles. Further, lysosomes isolated from hBMEC contained fewer viable bacteria following initial infection with the ΔciaR mutant compared to the WT strain. To characterize the contribution of CiaR-regulated genes, we constructed isogenic mutant strains lacking the two most down-regulated genes in the CiaR-deficient mutant, SAN_2180 and SAN_0039. These genes contributed to bacterial uptake and intracellular survival. Furthermore, competition experiments in mice showed that WT GBS had a significant survival advantage over the Δ2180 and Δ0039 mutants in the bloodstream and brain.
Introduction
Bacterial pathogens that have the capability of penetrating the central nervous system (CNS) thereby eliciting life-threatening diseases are a major human health concern. A severe outcome of bacterial infiltration of the CNS is the development of meningitis. One such pathogen, Group B Streptococcus (GBS), is a Gram-positive bacterium that is the leading cause of neonatal meningitis. Although intrapartum chemoprophylaxis is available to pregnant mothers during delivery, GBS infections among both pre-term and term infants still occurs [1]. Infants who survive meningitis suffer long-term neurological complications including developmental delays, hydrocephalus, visual impairment, deafness, cerebral palsy, and seizures [2]. GBS-induced meningitis occurs upon blood-brain barrier (BBB) penetration after a prolonged period of bacteremia [3]. Persistent blood-borne bacteria evade a variety of host defenses and have the propensity to cross the BBB, although the exact mechanism(s) of GBS-BBB transit are still being discovered. The majority of the BBB is composed of a specialized single cell layer known as human brain microvascular endothelial cells (hBMEC), which regulates passage of molecules, nutrients, and infectious agents into the brain [4]. Still, a few bacterial pathogens like GBS are able to disrupt this barrier to gain access to the CNS, resulting in inflammation, BBB permeability, and disease progression.
Much research has been devoted toward understanding the key GBS virulence factors that allow for BBB transit and breakdown. It is believed that direct invasion and subsequent transcytosis of brain endothelial cells by GBS is the critical first step for the development of meningitis [5]. Our lab has published several studies implicating multiple bacterial factors that participate in this initial invasion processes into brain endothelium including GBS surface associated proteins such as pili, lipoteichoic acid (LTA), serine rich repeat (Srr) proteins and a fibronectin binding protein, SfbA [6–10]. Following bacterial uptake, electron microscopy (EM) studies have demonstrated the presence of GBS in membrane-bound vesicles within hBMEC [11,12] suggesting the involvement of endocytic pathways, however, little is known about how GBS persists and traffics through the BBB. We have recently demonstrated that autophagy is induced in BBB endothelium during GBS infection, but that this pathway was not effective in completely eliminating intracellular GBS [12]. Thus, an understanding of how GBS resists intracellular host defenses and transits through brain endothelial cells is warranted.
To this end, we have investigated GBS trafficking within brain endothelial cells and the bacterial factors responsible for GBS survival. Endocytic trafficking is initiated upon bacterial invasion of host cells and subsequently, Rab GTPases aide in delivering these invaders to the lysosome for degradation [13]. Numerous bacterial pathogens, such as Legionella pneumophila, Mycobacterium tuberculosis, Pseudomonas aeruginosa, and Salmonella enterica are known to inhibit or disrupt endocytic trafficking to establish an intracellular niche or simply promote survival or growth [14]. To accomplish this, bacteria likely modulate gene expression to adapt to different host cellular environments, often through two component regulatory systems (TCRS). TCRS function through phosphotransfer signals from a membrane-bound sensor histidine kinase, which senses environmental changes, to subsequent activation of a cytoplasmic response regulator, with downstream transcription modulation [15]. GBS genome sequence analysis suggests multiple putative TCRS, but most of these systems are currently not well described [16]. One recent study has found that GBS encodes as many as 21 TCRS [17]. Established GBS TCRS include DltR/S, which maintains constant levels of ᴅ-alanylation in GBS LTA [18]; RgfA/C, which represses the expression of C5a peptidase [19]; CovR/S global regulatory system, which controls the expression of multiple virulence factors [20]; LiaFSR, which regulates cell wall stress and pilus expression [21]; FspS/R, which regulates fructose-6-phosphate metabolism [17]; and CiaR/H, which promotes survival in the host intracellular environment [8].
We have demonstrated previously that the CiaR response regulator promoted GBS intracellular survival in phagocytic cells and brain endothelial cells [8]. Further, a GBS mutant deficient in ciaR exhibited increased susceptibility to killing by antimicrobial peptides, lysozyme, and reactive oxygen species [8]. GBS CiaR also contributed to overall virulence potential in a murine bacterial competition model of infection [8]. Thus, we hypothesize that CiaR regulation may impact GBS intracellular trafficking in BBB endothelium. Our previous studies compared the transcriptional profiles of WT GBS and the isogenic ΔciaR mutant grown to log phase under identical conditions. Only one gene with a predicted function of purine and pyrimidine biosynthesis was upregulated more than twofold, while several genes were more dramatically down-regulated in the ΔciaR mutant [8]. The most highly down-regulated gene, SAN_2180, encodes a conserved hypothetical protein, while the second most down-regulated gene, SAN_0039, belongs to the M23/M37 family of metallopeptidases, which catalyze the hydrolysis of nonterminal peptide linkages in oligopeptides or polypeptides [22]. Here we investigate the role of these CiaR regulated genes to GBS interaction with brain endothelial cells and to virulence potential.
Materials and Methods
Cell culture
The human brain microvascular endothelial cell line was kindly provided by Kwang Sik Kim (Johns Hopkins University) and cultured as previously described [23] in RPMI 1640 (VWR) containing 10% FBS, 10% Nuserum (BD Biosciences) and 1% nonessential amino acids (Life Technologies) at 37°C with 5% CO2.
Bacterial strains and growth conditions
Streptococcus agalactiae (GBS) wild-type (WT) clinical isolate COH1 (serotype III) [24] and the isogenic ΔciaR mutant described previously [8] were used for these studies. Isogenic mutants of COH1 in genes SAN_2180 and SAN_0039 were created by in-frame allelic replacement with a chloramphenicol resistance gene (cat) cassette using a previously described method [7]. Briefly, two flanking regions of target genes were amplified by PCR from COH1 genomic DNA, using 5'flank-F-2180: 5'- TAGCCATAACAGGAGATCCGACTA -3', 5'flank-F-0039: 5'- CCAACAGACTACTCAATCGCTTCAGC -3' and 5'flank-R-2180: 5'- TTTTATACCTCCCTTTCTCAA -3', 5'flank-R-0039: 5'- AGAATTAATATAATGAAGTGCTCAAACACTTG -3'; 3'flank-F-2180: 5'- TACTGATACAATACTAAGAA-3', 3'flank-F-0039: 5'-TCCAGTAAAGTGTGATATTATAGTCTC-3' and 3'flank-R-2180: 5'-TAGAGGAGGACACTGAATGACAAC -3', 3'flank-R-0039: 5'-CGTAGTCACAGGAACTGCTGG -3'. A cat cassette with complementary regions of target genes was amplified from as previously described [7] primers, Cat-F-2180: 5'-GAGAAAGGGAGGTATAAAAATGGAGAAAAAAATCACTGGATATACCACCGTTGA-3', Cat-F-0039: 5'-AGCACTTCATTATATTAATTCTATGGAGAAAAAAAT CACTGGATATACCACCGTTGA-3' and Cat-R-2180: 5'-TTCTTAGTATTGTATCAGTATTACGCCCCGCCCTGCCACTCATCGCAGTACTGTTGTA-3', Cat-R-0039: 5'-GTCCGAGACTATAATATCATTACGCCCCGCCCTGCCACTCAT CGCAGTACTGTTGTA-3'. The construct was then amplified with a pair of nested primers, Nest-F-XhoI-2180: 5'- CCGCTCGAGTCCCAGGAGCGACTAGTGTTTATG-3', Nest-F-XhoI-0039: 5'-CCGCTCGAGGATGATATTGAGACAGCTTG-3' and Nest-R-XbaI-2180: 5'- GCTCTAGAGGCTGGTATTGGGGACGGTATTTC-3', Nest-R-XbaI-0039: 5'- GCTCTAGACAGCGGCAACAGAAGCTGGT-3'; and then was cloned into the pHY304 vector. For complementation studies, full-length target genes were amplified using the following sets of primers, F-KpnI-2180: 5'- GGGGTACCGTATCGAATACTCACTT -3', R-SacI-2180: 5'- CGAGCTCCTCCATTATAGGAGGTT -3'; F-KpnI-0039: 5'- GGGGTACCTCATCAAGGTGAGTACTT -3' and R-SacI-0039: 5'- CGAGCTCATGAATCAATACCTCAAA -3' and cloned into pDCErm. Deletion mutant strains were transformed with the recombinant plasmids for generation of complemented strain. GBS strains were grown in Todd-Hewitt broth (THB, Difco) at 37°C and growth was evaluated by monitoring OD600. For antibiotic selection, 2 μg/ml chloramphenicol and 5 μg/ml erythromycin was incorporated in the growth medium when required. GFP-expressing GBS strains were created as previously described [9,12].
Microscopy
Coverslips with GBS overnight culture were air-dried, heat fixed, and then subjected to a standard Gram stain protocol. Images were taken using a Zeiss upright microscope with an attached Axiocam Icc3 camera. For electron microscopy, 1mL (107 CFU) of bacterial cells suspended in PBS was fixed in a cocktail of 2% gluteraldehyde and 1% osmium tetroxide in PBS for 10 minutes. The solution was then passed through a 0.4μm polycarbonate filter to collect bacterial cells and rinsed with 4mL of water. In order to dry the samples, the filters were taken through a series of increasing concentrations of ethanol (50, 75, 85, 95, 100%) before being placed in a Tousimis SAMDRI-790 critical point drying machine. The dried filters were mounted onto SEM sample stubs with a piece of double-sided carbon tape before applying a 6nm layer of platinum with a Quorom Q150ts high-resolution coater. Samples were viewed using an FEI FEG450 scanning electron microscope.
In vitro infection assays
To determine the total number of cell surface-adherent or intracellular bacteria, hBMEC monolayers were grown to confluence in growth medium containing 10% FBS, 10% Nu-serum, and 1% non essential amino acids in 24-well tissue culture-treated plates. Bacteria were grown to mid-log phase and used to infect cell monolayers as described previously [23]. Briefly, hBMEC monolayers were incubated with GBS at 37°C with 5% CO2 for 30 min. To assess adherent bacteria, cells were washed five times with phosphate-buffered saline (PBS) to remove non-adherent bacteria, then trypsinized with 0.1 ml of trypsin-EDTA solution and lysed with addition of 0.4 ml of 0.025% Triton X-100 by vigorous pipetting. To assess intracellular bacteria GBS were incubated with hBMEC for 2h, cells were washed three times with PBS and 1 ml of media containing 100 μg/ml of gentamicin and 5 μg/ml of penicillin was added to each well to kill extracellular bacteria. Lysates were then serially diluted and plated on THB agar to enumerate bacterial colony-forming units (CFU). Bacterial adherence and invasion was calculated as (recovered CFU/original inoculum CFU)×100%. GBS intracellular survival experiments were performed as described above except that intracellular bacteria was enumerated at indicated time points.
Immunofluorescence staining
GFP-expressing GBS strains were used to infect hBMEC monolayers. Following a 2h incubation and antibiotic treatment at indicated time points, hBMEC were fixed with 4% paraformaldehyde. Cells were then lysed with 0.1% Triton X-100, blocked with 10% FBS, and incubated overnight with antibodies (Cell Signaling Technology) to Rab5 (1:100), Rab7 (1:100), and LAMP1 (1:100). Cells were then washed and incubated with secondary antibodies (1:500) conjugated to Alexa-Fluor 594 (Life Technologies). Samples were visualized using a Zeiss Axiovert 200 inverted fluorescence microscope (Carl Zeiss). At least 100 cells per treatment were counted and all experiments were performed in triplicate.
Lysosomal isolation
hBMEC were grown in 75 cm2 flasks at 37°C with 5% CO2 until confluence was achieved, and subsequently infected with WT and mutant strains of COH1 GBS at an MOI = 10 for 2 hours. After infection, cells were incubated with media containing penicillin (5μg/mL) and gentamycin (100μg/mL) for either 1 or 12 hours to eliminate extracellular bacteria. Cells were then washed with DPBS and subjected to lysosomal isolation using the Lysosomal Enrichment kit for Tissue and Cultured Cells according to manufacturer’s instructions (Thermo-Fisher). Briefly, ~200mg of cells were harvested with trypsin and centrifuged for 2 min at 850 × g. Lysosome enrichment reagent A containing a protease inhibitor cocktail (CalBioChem) was added to pelleted cells and subjected to a 2 min incubation on ice. After incubation, cells were then sonicated 15 times to lyse the cells and Lysosome enrichment reagent B containing a protease inhibitor cocktail was then added to the cells. Cells were then centrifuged for 10 min at 500 × g at 4°C. The supernatant was then collected and the final concentration was altered to 15% with OptiPrep Cell Separation Media. The samples were then loaded on discontinuous OptiPrep gradients varying from 30%, 27%, 23%, 20% to 17% in a 13.2 mL ultracentrifugation tube (Beckman-Coulter) and centrifuged in a SW 41 Ti rotor at 145,000 × g for 2 hours at 4°C. After centrifugation, the lysosomal fraction was isolated from the top gradient and washed using 2 volumes of DPBS in a microcentrifuge at 17,000 × g for 30 min at 4°C to remove OptiPrep media. Lysosomal pellets were then washed with Gradient Dilution Buffer at 17,000 × g for 30 at 4°C. Pellets were then re-suspended in 0.1% Triton X‒100 and plated on Todd Hewitt Agar to enumerate bacterial CFU. For lysotracker staining, pellets were re-suspended in PBS and stained with Lysotracker Red (Life Technologies) for 15 minutes and imaged using a Zeiss Axiovert 200 inverted fluorescence microscope (Carl Zeiss).
In vivo competition assay
Animal experiments were approved by the Institutional Animal Care and Use Committee at San Diego State University (protocol APF 13-07-011D) and performed using accepted veterinary standards. Animals are housed 4 mice/cage per NIH standard space requirement in Micro-Isolater cages with contact bedding. The light cycle is 12/12 (light from 6am-6pm) and cages are changed 3 times/week. Mice are fed a standard rodent diet (Purina) using a free feed system with fresh food added weekly. During the experiment animals were monitored visually at least twice a day for signs of disease such as ruffled fur, lethargy or agitation and moribund appearance. Those animals showing the first signs of disease will be monitored a minimum of four times a day for worsening signs. Animal suffering from any of the symptoms: severe lethargy or agitation, moribund appearance, failure to right oneself after 5 seconds, may be defined as moribund and will be humanely sacrificed prior to the experimental endpoint. The method of euthanasia used was an overdose of CO2 followed cervical dislocation. In our studies no animals died prior to the experimental endpoint. 8-week-old male CD1 mice (Charles River Laboratories) were injected intravenously with 2×108 bacteria at a 1:1 ratio of WT and either one of the isogenic mutant strains. After 72 hours, mice were euthanized and blood and brain were collected to enumerate bacterial CFU. PCR was performed to confirm the presence or absence of targeted genes on recovered CFU. Primers 2180_F: 5'-AGAGCACGTTATCCTTTCGCT-3' and 2180_R: 5'-TCCGCCAAAACGTGCAACAT-3'; and primers 0039_F: 5'- GAGCCAACTTTTCTTGGATGAC-3' and 0039_R: 5'- ACTAGATTGATTCTGTACAGGA-3' were used for screening. Experiments were repeated twice (5 mice/group).
Statistical analysis
GraphPad Prism version 6.0 was used for statistical analyses and statistical significance was accepted at p < 0.05 *, p< 0.05; **, p< 0.005; ***, p< 0.0005; ****, p< 0.00005).
Results
Characterization of CiaR regulated genes
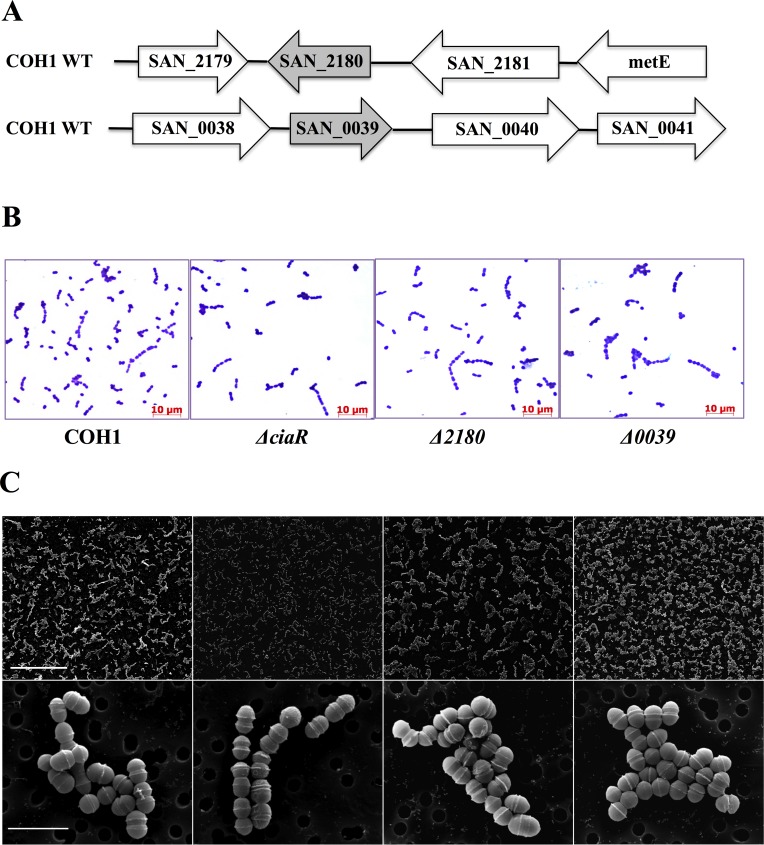
Our data indicate that the response regulator CiaR may play a role in survival and trafficking within brain endothelial cells. Thus, we hypothesize that CiaR-regulated genes may impact GBS intracellular survival and the efficient trafficking of GBS through brain endothelium. Previous microarray analysis of the WT and ΔciaR mutant identified the two most highly regulated genes, SAN_2180 and SAN_0039 [8] (Fig 1A). To characterize the impact of SAN_2180 and SAN_0039 on GBS interaction with human brain microvascular endothelial cells (hBMEC) and virulence, we generated isogenic knockout strains using in-frame allelic substitution of either gene with a chloramphenicol acetyltransferase (cat) resistance cassette using a method described previously [7] and as described in Materials and Methods. Both constructed mutants exhibited similar growth rates in THB compared to the WT parental strain (data not shown) and similar morphology as observed by Gram stain and electron microscopy (Fig 1B and 1C), although the ΔciaR mutant appeared to grow in shorter chains.
Fig 1. Morphology of GBS WT and mutant strains.
A. Diagrammatic representation of the genetic locus surrounding SAN_2180 and SAN_0039 in the WT COH1 strain. Analysis of WT, ΔciaR, Δ2180 and Δ0039 mutant strains by Gram staining (scale bar = 10 μm), B, and Scanning electron microscopy (top panel scale bar = 50 μm; bottom panel scale bar = 2μm), C.
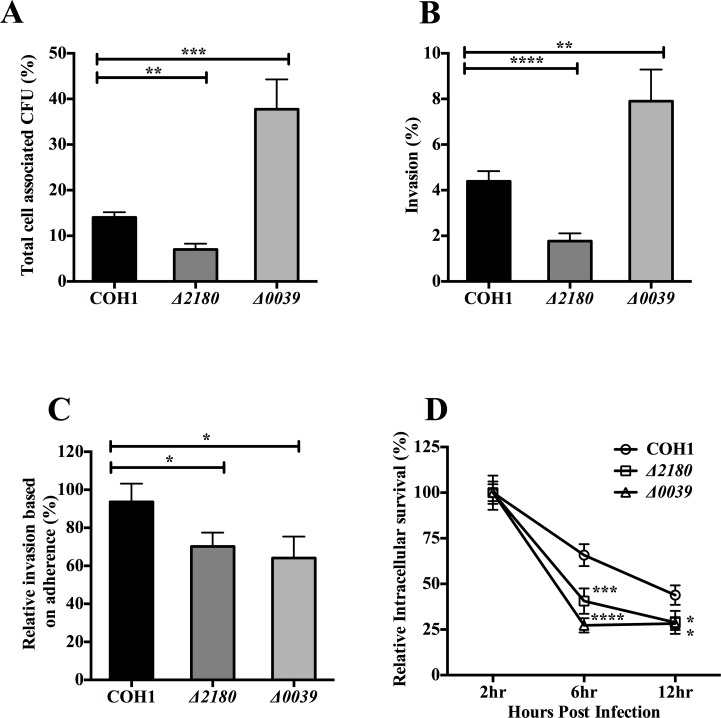
We have previously shown that the ΔciaR mutant exhibited decreased survival in hBMEC [8], thus we characterized the interactions of the Δ2180 and Δ0039 mutants with hBMEC, specifically the adherent and invasive capabilities as well as the ability to survive and persist intracellularly. The Δ2180 mutant exhibited a ~2-fold and significant decrease in hBMEC adherence and invasion compared with the WT parent strain (P < 0.005 and P < 0.00005, respectively) (Fig 2A and 2B). Interestingly, the Δ0039 mutant displayed increased adherence and invasion into hBMEC (Fig 2A and 2B), however, when calculating the percentage of the hBMEC-associated GBS that had invaded the intracellular compartment both mutants exhibited decreased invasive capability compared to the WT strain (Fig 2C). These data indicate that both SAN_2180 and SAN_0039 contribute to GBS uptake into hBMEC. To examine whether these genes impact intracellular survival, we infected hBMEC with WT and mutant strains for 2 hours, incubated with extracellular antibiotics and at 2, 6, and 12 hours post antibiotic treatment, the intracellular pool was quantified as described in Materials and Methods. The percent of invasive bacteria recovered over time is shown relative to the first time point for each strain (Fig 2D). We observed a gradual decrease in intracellular WT bacteria over time as we have demonstrated for GBS in hBMEC previously [7]. However, the level of intracellular organisms over time for each of the mutant strains was significantly less compared to the WT strain (Fig 2D).
Fig 2. Adherence, invasion, and intracellular survival of GBS in brain endothelial cells are influenced by SAN_2180 and SAN_0039.
A. Adherence to hBMEC by WT and mutant strains (MOI = 1). B. Invasion of hBMEC by WT and mutant strains (MOI = 1). C. Relative percentage of the hBMEC-associated GBS, WT and mutant strains, that had invaded the intracellular compartment (MOI = 1). D. Intracellular survival of WT and mutant GBS strains in hBMEC over time. Statistical analysis performed was a Two-way ANOVA with a Bonferroni’s multiple comparisons test and the data represents mean ± S.D. p < 0.05 *, p < 0.0005 ***, p < 0.00005 ****.
Characterization of GBS intracellular trafficking in brain endothelial cells
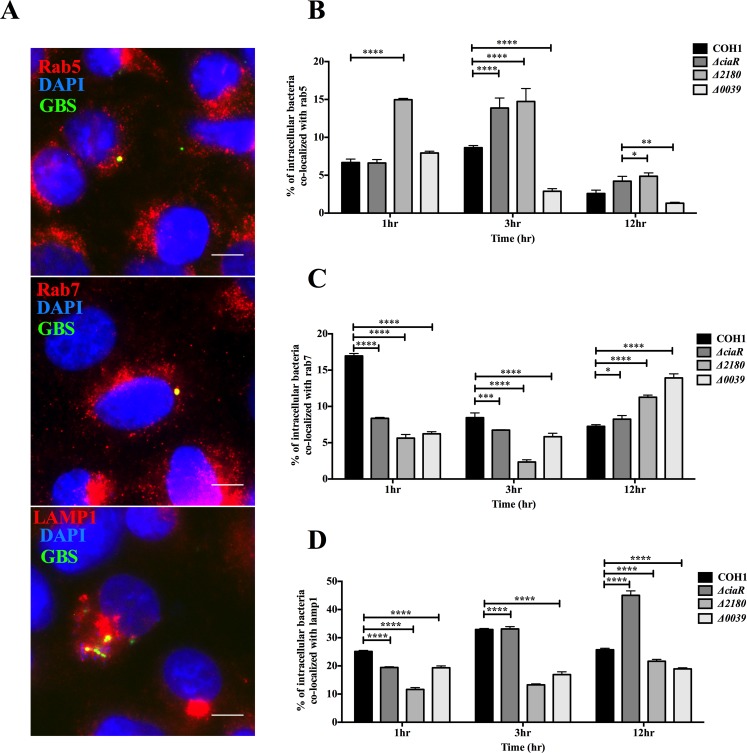
Efficient trafficking of bacteria through endothelial barriers is a hallmark of the development of a multitude of vascular diseases. Endocytic trafficking consists of labeling by Rab GTPases, small guanine nucleotide binding proteins responsible for vesicular trafficking of cargo, and selectively transporting intracellular pathogens to the lysosome [13,25]. Rab5 is a monomeric GTPase known to be involved in early endocytic trafficking, while Rab7 acts later in the endocytic pathway to regulate lysosomal fusion [26]. Rab5 and Rab7 specifically label early and late endosomes, respectively, and subsequently traffic cargo or pathogens to the lysosome, which is labeled by lysosomal associated membrane protein 1 (LAMP1). We investigated the association of WT GBS and the ΔciaR, Δ2180 and Δ0039 mutants with Rab5, Rab7, and LAMP1 labeled compartments. Infection of hBMEC with GFP-GBS strains was carried out for 2h as described in Materials and Methods, then at various time points post antibiotic treatment, cells were processed and stained for endosomal and lysosomal markers. Representative images following WT GBS infection 1h post antibiotic treatment show GBS localizing with each marker during the infection time (Fig 3A). To quantify co-localization over time, for each time point, we counted triplicate biological samples, at least 100 cells with intracellular bacteria and evaluated Rab5, Rab7, and LAMP1 localization with GBS WT and mutant strains (Fig 3B–3D). At early time points there were either similar amounts of WT and ΔciaR mutant GBS that localized with endosomal and lysosomal-labeled vesicles or in some cases there were higher amounts of WT GBS. However at later time points, significantly more of the ΔciaR mutant was associated with both Rab and LAMP1 markers. These findings demonstrate that GBS associates with vesicles involved in the endocytic pathway, with 25–30% of intracellular bacteria localizing with the lysosome.
Fig 3. CiaR regulation influences GBS intracellular trafficking in brain endothelial cells.
A. hBMEC monolayers were infected with WT COH1 GFP expressing GBS for 2 hours (MOI = 10) and then stained with antibodies to Rab5, Rab7, and LAMP1 following the indicated time points as described in Materials and Methods. Representative images of triplicate experiments demonstrate co-localization (yellow) of GBS (green) with each marker (red), Scale Bar, 5μm. B, C, D. Percentages of co-localization of Rab5, Rab7, and LAMP1 with GFP expressing GBS WT and mutant strains after various time points post infection during antibiotic treatment. At least 100 cells containing intracellular GBS were counted for each time point in triplicate. Statistical analysis performed was a Two-way ANOVA with a Bonferroni’s multiple comparisons test and the data represents mean ± S.D. p < 0.05 *, p < 0.0005 ***, p < 0.00005 ****.
Generally the Δ2180 mutant strain exhibited similar localization with Rab5 and Rab7 positive vesicles as the ΔciaR mutant, except at the early time point where we observed that the Δ2180 mutant co-localized more with rab5 (Fig 3B and 3C). It is also notable that the WT strain co-localized more than other strains with rab7 and LAMP1 positive vesicles at the early time point. However, both Δ2180 and Δ0039 mutants co-localized less with LAMP1 positive cells when compared to WT and the ΔciaR mutant strain, particularly at later time points (Fig 3D). Thus, overall CiaR regulation and specific regulated genes may influence GBS trafficking through endocytic compartments in different ways.
Recovery of GBS from lysosomes isolated from brain endothelial cells
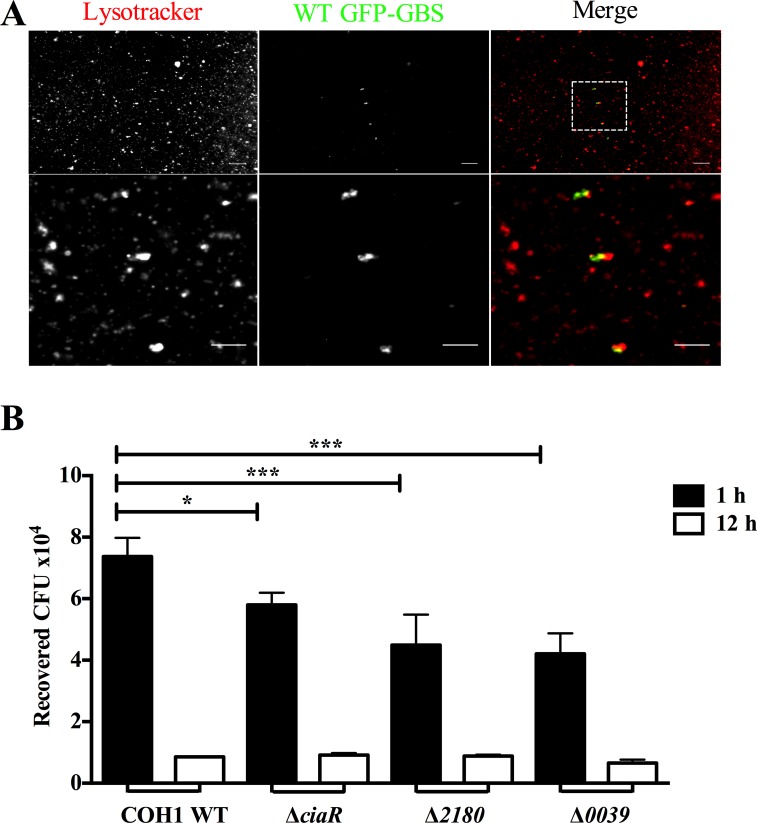
Our results suggest that GBS may regulate genes to prevent or limit endocytic trafficking to the lysosome. Although we observed that there was actually less co-localization of both Δ2180 and Δ0039 mutants with lysosomal markers at later time points, it is possible that these mutants are still readily trafficked to acidic compartments, but exhibit increased sensitivity to lysosomal killing. Thus, we investigated whether we could recover viable intracellular GBS from lysosomes isolated from brain endothelial cells. We used a lysosomal enrichment protocol that employs differential centrifugation to enrich for lysosomes based on size and density. Following hBMEC infection with WT and mutant GBS strains for 2 hours, cells were treated with antibiotics, and lysosomes were subsequently isolated at early (1 hour) and late (12 hours) time points. Western blot analysis was performed to confirm that recovered lysosomes were positive for LAMP1 (data not shown). We first used Lysotracker, which stains acidic vesicles, and found that WT GBS could be visualized within acidified lysosomes (Fig 4A). Lysosomes were also lysed in 0.1% Triton X-100 and lysates plated on THB agar to enumerate viable bacteria. Markedly more WT GBS was recovered from the lysosome fraction than any of the mutant strains at the early time point post infection (Fig 4B). Furthermore, fewer viable GBS was recovered from lysosomes at the later time point. These data further indicate that GBS does indeed traffic to the lysosome and that CiaR, SAN_2180, and SAN_0039 contribute to initial GBS trafficking and survival.
Fig 4. GBS recovery from Lysosomes.
A. Isolated lysosomes infected with WT COH1 GFP expressing GBS were subjected to staining with Lysotracker Red (0.5μM) and visualized using fluorescence microscopy. Scale Bar, 5 μm B. hBMEC were infected with WT or mutant GBS strains for 2 hours (MOI = 10) and subjected to lysosomal isolation by differential centrifugation after 1 or 12 hours post antibiotic treatment. Lysosomal pellets were plated to enumerate the amount of recovered viable CFU. Statistical analysis performed was a One-way ANOVA with a Tukey’s multiple comparisons test and the data represents mean ± S.D. p < 0.05 *, p < 0.005 **.
CiaR regulated genes contribute to GBS virulence
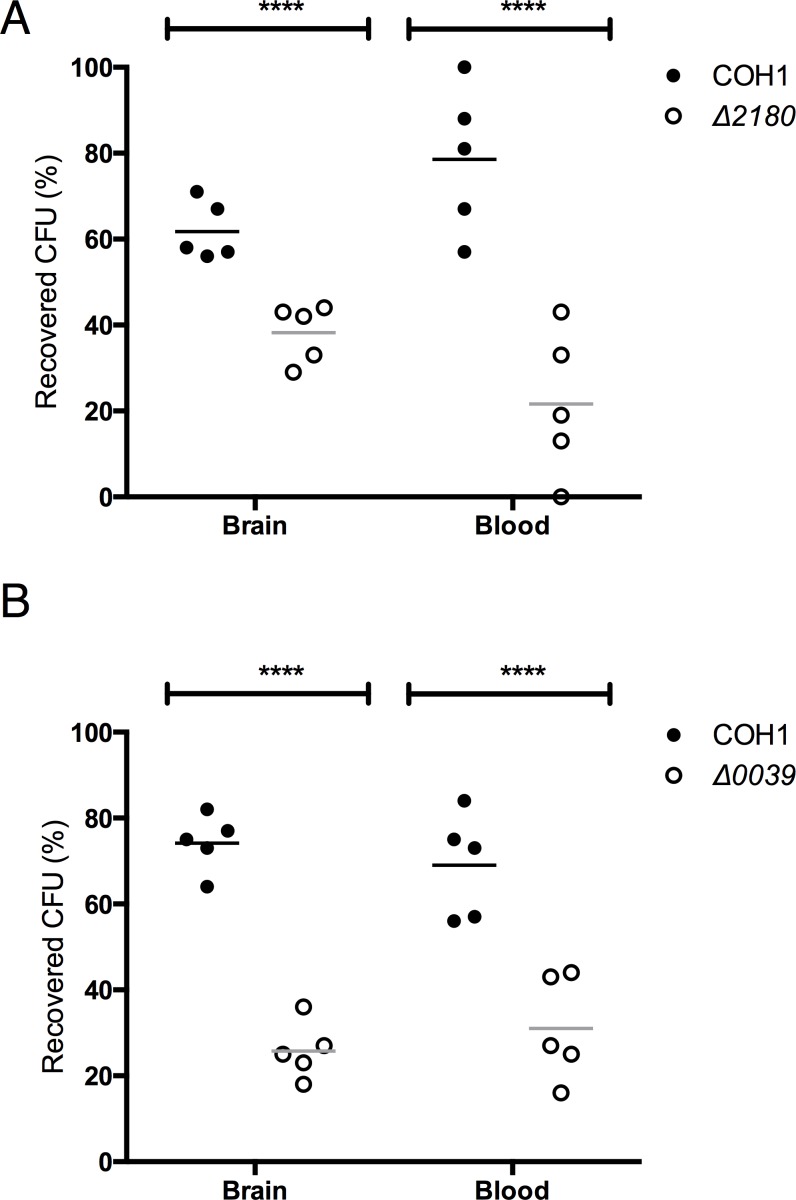
We have demonstrated previously that CiaR promotes bacterial fitness and overall virulence in a mouse model of GBS infection [8]. To similarly examine whether the CiaR regulated genes contribute to virulence in vivo, we employed the same bacterial competition model as described previously [8,27]. Mice were challenged intravenously with equal amounts (2 × 108 CFU) of WT COH1 and either Δ2180 or Δ0039 isogenic mutants. At the experimental end point (72h), mice were euthanized and blood and brains were collected for the enumeration of surviving bacteria. PCR-based screening was used to distinguish between the WT and mutant strains. Data is expressed as the percentage of WT or mutant GBS recovered compared to total recovered CFU. Consistently more WT GBS than the Δ2180 or Δ0039 mutant strains were recovered from the blood and brain (Fig 5A and 5B). This is consistent with previous results with the ΔciaR mutant [8], and suggests that both CiaR regulated genes contribute to bacterial fitness and virulence.
Fig 5. GBS SAN_2180 and SAN_0039 contribute to overall bacterial virulence.
The recovered bacterial CFU in blood and brains were analyzed 72 h after intravenous injection with equal amounts of WT and mutant GBS strains into CD1 mice. Bacteria were enumerated on THA plates with serial dilutions and the bacterial colonies from the same dilution were distinguished by colonies PCR between WT and mutant strains. A. The percentage of recovered CFU in blood and brains from WT and the Δ2180 mutant strain. B. The percentage of recovered CFU in blood and brains from WT and the Δ0039 mutant strain. Statistical analysis performed was an unpaired t-test and the data represents mean ± S.D. p < 0.00005 ****.
Discussion
Penetration of the BBB likely requires GBS to invade microvascular endothelial cells and transcytose through cells, exiting basolaterally to breach the CNS. It is known that GBS can persist within brain endothelial cells for up to 24 hours post infection, however, there is no net increase in bacterial replication, and the intracellular pool actually decreases over time [7,11]. This is likely due to the ability of the host cell to limit GBS intracellular growth by using various forms of antibacterial defense including transit to the lysosomal compartment for subsequent degradation. We observed that intracellular WT GBS readily acquired markers of endosomal maturation, as GBS associated with early and late endosomes, and approximately 25–30% of intracellular organisms localized with acidified lysosomal vesicles (Fig 3). Our results presented here suggest that GBS may use the response regulator, CiaR, to prevent endocytic trafficking to the lysosome. A GBS mutant deficient in CiaR exhibited increased localization with vesicles in the endocytic pathway including Rab5, Rab7 and LAMP1 positive cells. Rab GTPase modifications have been identified during bacterial infection and Rab5 modulation by numerous pathogens has proven to be an efficient strategy to promote intracellular replication or persistence [28]. Additionally, bacteria such as Helicobacter pylori and Mycobacterium bovis may modulate Rab7 endosomal maturation in order to establish a protective intracellular niche during infection [29,30]. Interestingly, following infection with the ΔciaR mutant less viable bacteria was recovered from lysosomes isolated from hBMEC. This is likely due to the increased sensitivity of the ΔciaR mutant to the hostile environment of the phagolysosome, namely antimicrobial peptides and reactive oxygen species, which we have demonstrated previously [8]. The reduced recovery of the Δ2180 and Δ0039 mutant strains from lysosomes likely also reflects an increased sensitivity to lysosomal killing as we observed these mutants, like ΔciaR, were more sensitive to antimicrobial peptides, lysozyme, and H2O2 (data not shown).
While GBS is not thought of as a classic intracellular pathogen, GBS survival in phagocytic cells has been reported [31,32]. Most research has focused on understanding the virulence factors responsible for GBS persistence in human macrophages and neutrophils. The pore forming β-hemolysin/cytolysin (β-h/c) encoded by cylE is a major virulence factor contributing to GBS disease progression [3]. Interestingly, cylE deletion results in the loss of β-h/c activity and the carotenoid pigment. It has been shown that cylE contributed to enhanced GBS survival within phagocytes that was attributed to the ability of carotenoid to shield GBS from oxidative damage [33]. However, other reports suggest that the ß-h/c did not impact intracellular survival in macrophages (Cumley et al., 2012) or even that the absence of the β-h/c enabled increased GBS survival in professional phagocytes [34]. It is possible that these results reflect differences in GBS strains, β-h/c production, and/or host cells and cell lines, and requires further investigation. Other GBS cell associated factors reported to impact survival in phagocytic cells include pili [27] and the capsule polysaccharide [35,36]. An additional TCRS, the CovR/S global regulator that regulates many genes including cylE, has also been shown to be required for intracellular survival in macrophages [37]. That study suggests that CovR/S mediates a transcriptional response stimulated by the acidic environment in the phagolysosome that mediates survival [37]. Less is known about GBS survival in epithelial or endothelial cells. Interestingly, we have previously infected hBMEC with a GBS ΔcovR mutant to assess bacterial uptake and survival, and were not able to recover viable ΔcovR bacteria from the intracellular compartment [20]. However, at this point we cannot conclude whether CovR regulation is required for GBS invasion into brain endothelial cells, or if it regulates intracellular survival.
Two-component regulatory systems allow bacteria to adapt to changing environmental conditions. CiaR/H is not fully characterized in GBS, but it has been linked to stress tolerance and host defense resistance similar to the role of CiaR/H in Streptococcus mutans [38] and Streptococcus pnuemoniae [39]. Interestingly, the S. pneumoniae CiaR homologue has also been described to be involved in β-lactam resistance and lytic capabilities [40,41]. CiaR-deficient GBS displayed decreased intracellular survival in neutrophils, macrophages, and brain microvascular endothelial cells and was more susceptible to killing by antimicrobial peptides and reactive oxygen species, suggesting CiaR/H as a vital element for environmental stress tolerance [8]. Previously, our group identified a subset of genes that are down-regulated in a CiaR- deficient mutant. One gene, SAN_0039, encodes a putative metallopeptidase exhibiting a high degree of homology (70% similarity, 56% identity for 91%protein coverage) to a protein called Zoocin A (zooA) [8]. Zoocin A is produced by S. zooepidemicus (Group C Streptococcus) which has a bacteriolytic effect on several other Streptococcal species [42]. Zoocin A has two functional domains, an N-terminal catalytic domain and a C-terminal substrate-binding or target recognition domain [43,44]. Zoocin A has been determined to act as a ᴅ-alanyl-l-alanine endopeptidase which hydrolyses the cross bridge of peptidoglycan of certain Streptococcus species [45]. Utilization of peptidoglycan hydrolases for both peptidoglycan rearrangement and pathogenicity in host cells has been described in several bacteria. S. pneumoniae, Listeria monocytogenes, and Staphylococcus aureus employ differential acetylation strategies to obtain resistance to lysozyme [46–48]. Another peptidoglycan hydrolase, known as IspC, has been identified in L. monocytogenes as being essential for virulence in vivo, and crossing the blood-cerebrospinal fluid barrier [49]. The attenuated virulence of an IspC deficient mutant may be partly due to the reduced surface expression or display of other known or putative virulence factors [49]. We observed that while the GBS Δ0039 mutant exhibited increased adherence to hBMEC, invasion into cells was reduced, suggesting a defect in a surface factor(s) that modulates bacterial uptake. Future studies using proteomic analysis of GBS WT and the Δ0039 mutant strain will be of interest to determine if the GBS peptidoglycan hydrolase contributes indirectly to host cell interaction and virulence by modulating surface targeting mechanisms that affect other GBS factors. We should note that overexpression of the 0039 gene was toxic to GBS making complementation experiments impossible. This is consistent with what has been observed for Zoocin A (R. Simmonds, personal communication).
Another GBS gene, SAN_2180, was the most highly down-regulated gene in the ΔciaR mutant [8]. Characterization of a Δ2180 mutant demonstrated that this gene contributes to bacterial uptake, a phenotype that was complemented by reintroducing the WT gene back into the Δ2180 mutant (data not shown). The Δ2180 mutant also exhibited decreased survival within brain endothelial cells as well as decreased virulence potential in vivo. Like the ΔciaR mutant, the Δ2180 mutant more readily localized with endosomal and lysosomal marked cells, but was not readily isolated from lysosomes even at early times points likely due to an increased sensitivity to antimicrobial factors. Thus this factor may be the primary CiaR regulated gene responsible for the observed phenotype of the ΔciaR mutant, although future studies with a double mutant strain would help clarify this. Protein sequence analysis using BLAST predicted that the SAN_2180 protein belongs to the proteins of unknown function family, DUF1003, but shares homology (60% similarity, 42% identity for 93% protein coverage) to a protein in Lactococcus lactis involved in acid tolerance and multistress tolerance [8,50]. Additionally, the SAN_2180 protein sequence is homologous to cyclic nucleotide-binding proteins present in other Streptococcus species such as Streptococcus urinalis (84% identity for 100% protein coverage), Streptococcus parasanguinis (69% identity for 98% protein coverage) and Streptococcus gallolyticus (60% identity for 98% protein coverage). Cyclic nucleotide-binding proteins are important for binding intracellular messengers such as cyclic AMP [51]. Modulation of host cAMP levels has been proven to be a novel bacterial mechanism to engage inflammatory responses and disease progression [52]. However, further characterization of the SAN_2180 encoded protein is needed to elucidate specific mechanisms responsible for its role in GBS invasion and intracellular survival.
In summary our data suggest that GBS may modulate gene expression through the TCRS CiaR/H to promote intracellular survival. This may impact trafficking to the lysosome as at later time points we observed only 25% of intracellular WT bacteria localizing with LAMP1 positive vesicles compared to 45% in the absence of CiaR regulation. Interestingly, we have not observed GBS free in the cytoplasm of hBMEC, even at later time points [12], suggesting that surviving GBS likely traffics through endosomes in brain endothelial cells to promote transcytosis across the BBB. However further experimentation is required to fully characterize the fate of all GBS-containing endosomes and how a percentage of GBS bacteria may avoid the lysosomal compartment and transit through the brain endothelium in order to breech the CNS. Our data suggest that the specific CiaR regulated genes, SAN_2180 and SAN_0039, may not independently explain the phenotype of the CiaR deficient mutant, but still provide interesting new bacterial targets that may further inform the mechanisms of BBB penetration as well as the development of preventative therapies.
Acknowledgments
We thank Kwang Sik Kim and Monique Stins (Johns Hopkins) for providing hBMEC and the SDSU Electron Microscopy Core Facility, Dr. Steve Barlow director.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the Rees Stealy Research Foundation/SDSU Heart Institute Scholarship to A.S.C., the San Diego Chapter ARCS Scholarship to K.A.P., and NIH/NINDS R01-NS051247 to K.S.D.
References
- 1.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, et al. (2011) Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics 127: 817–826. 10.1542/peds.2010-2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libster R, Edwards KM, Levent F, Edwards MS, Rench MA, et al. (2012) Long-term outcomes of group B streptococcal meningitis. Pediatrics 130: e8–15. 10.1542/peds.2011-3453 [DOI] [PubMed] [Google Scholar]
- 3.Maisey HC, Doran KS, Nizet V (2008) Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med 10: e27 10.1017/S1462399408000811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eigenmann DE, Xue G, Kim KS, Moses AV, Hamburger M, et al. (2013) Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies. Fluids Barriers CNS 10: 33 10.1186/2045-8118-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doran KS, Nizet V (2004) Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol Microbiol 54: 23–31. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee A, Kim BJ, Carmona EM, Cutting AS, Gurney MA, et al. (2011) Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat Commun 2: 462 10.1038/ncomms1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, et al. (2005) Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest 115: 2499–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quach D, van Sorge NM, Kristian SA, Bryan JD, Shelver DW, et al. (2009) The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses. J Bacteriol 191: 2023–2032. 10.1128/JB.01216-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu R, Kim BJ, Paco C, Del Rosario Y, Courtney HS, et al. (2014) Identification of a group B streptococcal fibronectin binding protein, SfbA, that contributes to invasion of brain endothelium and development of meningitis. Infect Immun 82: 2276–2286. 10.1128/IAI.01559-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Sorge NM, Quach D, Gurney MA, Sullam PM, Nizet V, et al. (2009) The group B streptococcal serine-rich repeat 1 glycoprotein mediates penetration of the blood-brain barrier. J Infect Dis 199: 1479–1487. 10.1086/598217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nizet V, Kim KS, Stins M, Jonas M, Chi EY, et al. (1997) Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun 65: 5074–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutting AS, Del Rosario Y, Mu R, Rodriguez A, Till A, et al. (2014) The role of autophagy during group B Streptococcus infection of blood-brain barrier endothelium. J Biol Chem 289: 35711–35723. 10.1074/jbc.M114.588657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117. [DOI] [PubMed] [Google Scholar]
- 14.Stein MP, Muller MP, Wandinger-Ness A (2012) Bacterial pathogens commandeer Rab GTPases to establish intracellular niches. Traffic 13: 1565–1588. 10.1111/tra.12000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoch JA (2000) Two-component and phosphorelay signal transduction. Curr Opin Microbiol 3: 165–170. [DOI] [PubMed] [Google Scholar]
- 16.Tettelin H, Masignani V, Cieslewicz MJ, Eisen JA, Peterson S, et al. (2002) Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc Natl Acad Sci U S A 99: 12391–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faralla C, Metruccio MM, De Chiara M, Mu R, Patras KA, et al. (2014) Analysis of two-component systems in group B Streptococcus shows that RgfAC and the novel FspSR modulate virulence and bacterial fitness. MBio 5: e00870–00814. 10.1128/mBio.00870-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poyart C, Lamy MC, Boumaila C, Fiedler F, Trieu-Cuot P (2001) Regulation of D-alanyl-lipoteichoic acid biosynthesis in Streptococcus agalactiae involves a novel two-component regulatory system. J Bacteriol 183: 6324–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spellerberg B, Rozdzinski E, Martin S, Weber-Heynemann J, Lutticken R (2002) rgf encodes a novel two-component signal transduction system of Streptococcus agalactiae. Infect Immun 70: 2434–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lembo A, Gurney MA, Burnside K, Banerjee A, de los Reyes M, et al. (2010) Regulation of CovR expression in Group B Streptococcus impacts blood-brain barrier penetration. Mol Microbiol 77: 431–443. 10.1111/j.1365-2958.2010.07215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinzing DC, Ishmael N, Dunning Hotopp JC, Tettelin H, Shields KR, et al. (2013) The two-component response regulator LiaR regulates cell wall stress responses, pili expression and virulence in group B Streptococcus. Microbiology 159: 1521–1534. 10.1099/mic.0.064444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawlings ND, Barrett AJ (1995) Evolutionary families of metallopeptidases. Methods Enzymol 248: 183–228. [DOI] [PubMed] [Google Scholar]
- 23.Doran KS, Liu GY, Nizet V (2003) Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest 112: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilkinson HW (1977) Nontypable group B streptococci isolated from human sources. J Clin Microbiol 6: 183–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10: 513–525. 10.1038/nrm2728 [DOI] [PubMed] [Google Scholar]
- 26.Wandinger-Ness A, Zerial M (2014) Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6: a022616 10.1101/cshperspect.a022616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maisey HC, Quach D, Hensler ME, Liu GY, Gallo RL, et al. (2008) A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J 22: 1715–1724. 10.1096/fj.07-093963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumell JH, Scidmore MA (2007) Manipulation of rab GTPase function by intracellular bacterial pathogens. Microbiol Mol Biol Rev 71: 636–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terebiznik MR, Vazquez CL, Torbicki K, Banks D, Wang T, et al. (2006) Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect Immun 74: 6599–6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA, et al. (1997) Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem 272: 13326–13331. [DOI] [PubMed] [Google Scholar]
- 31.Cornacchione P, Scaringi L, Fettucciari K, Rosati E, Sabatini R, et al. (1998) Group B streptococci persist inside macrophages. Immunology 93: 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valentin-Weigand P, Benkel P, Rohde M, Chhatwal GS (1996) Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun 64: 2467–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, et al. (2004) Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A 101: 14491–14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagar A, Klemm C, Hartjes L, Mauerer S, van Zandbergen G, et al. (2013) The beta-hemolysin and intracellular survival of Streptococcus agalactiae in human macrophages. PLoS One 8: e60160 10.1371/journal.pone.0060160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemire P, Houde M, Lecours MP, Fittipaldi N, Segura M (2012) Role of capsular polysaccharide in Group B Streptococccus interactions with dendritic cells. Microbes Infect 14: 1064–1076. 10.1016/j.micinf.2012.05.015 [DOI] [PubMed] [Google Scholar]
- 36.Lemire P, Houde M, Segura M (2012) Encapsulated group B Streptococcus modulates dendritic cell functions via lipid rafts and clathrin-mediated endocytosis. Cell Microbiol 14: 1707–1719. 10.1111/j.1462-5822.2012.01830.x [DOI] [PubMed] [Google Scholar]
- 37.Cumley NJ, Smith LM, Anthony M, May RC (2012) The CovS/CovR acid response regulator is required for intracellular survival of group B Streptococcus in macrophages. Infect Immun 80: 1650–1661. 10.1128/IAI.05443-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi F, Merritt J, Lux R, Shi W (2004) Inactivation of the ciaH Gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect Immun 72: 4895–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blanchette-Cain K, Hinojosa CA, Akula Suresh Babu R, Lizcano A, Gonzalez-Juarbe N, et al. (2013) Streptococcus pneumoniae biofilm formation is strain dependent, multifactorial, and associated with reduced invasiveness and immunoreactivity during colonization. MBio 4: e00745–00713. 10.1128/mBio.00745-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mascher T, Heintz M, Zahner D, Merai M, Hakenbeck R (2006) The CiaRH system of Streptococcus pneumoniae prevents lysis during stress induced by treatment with cell wall inhibitors and by mutations in pbp2x involved in beta-lactam resistance. J Bacteriol 188: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascher T, Zahner D, Merai M, Balmelle N, de Saizieu AB, et al. (2003) The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J Bacteriol 185: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akesson M, Dufour M, Sloan GL, Simmonds RS (2007) Targeting of streptococci by zoocin A. FEMS Microbiol Lett 270: 155–161. [DOI] [PubMed] [Google Scholar]
- 43.Gargis SR, Heath HE, Heath LS, Leblanc PA, Simmonds RS, et al. (2009) Use of 4-sulfophenyl isothiocyanate labeling and mass spectrometry to determine the site of action of the streptococcolytic peptidoglycan hydrolase zoocin A. Appl Environ Microbiol 75: 72–77. 10.1128/AEM.01647-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai AC, Tran S, Simmonds RS (2002) Functional characterization of domains found within a lytic enzyme produced by Streptococcus equi subsp. zooepidemicus. FEMS Microbiol Lett 215: 133–138. [DOI] [PubMed] [Google Scholar]
- 45.Gargis SR, Gargis AS, Heath HE, Heath LS, LeBlanc PA, et al. (2009) Zif, the zoocin A immunity factor, is a FemABX-like immunity protein with a novel mode of action. Appl Environ Microbiol 75: 6205–6210. 10.1128/AEM.01011-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bera A, Biswas R, Herbert S, Kulauzovic E, Weidenmaier C, et al. (2007) Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J Bacteriol 189: 280–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, et al. (2007) A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc Natl Acad Sci U S A 104: 997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vollmer W, Tomasz A (2002) Peptidoglycan N-acetylglucosamine deacetylase, a putative virulence factor in Streptococcus pneumoniae. Infect Immun 70: 7176–7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Lin M (2008) A novel cell wall-anchored peptidoglycan hydrolase (autolysin), IspC, essential for Listeria monocytogenes virulence: genetic and proteomic analysis. Microbiology 154: 1900–1913. 10.1099/mic.0.2007/015172-0 [DOI] [PubMed] [Google Scholar]
- 50.Rallu F, Gruss A, Ehrlich SD, Maguin E (2000) Acid- and multistress-resistant mutants of Lactococcus lactis: identification of intracellular stress signals. Mol Microbiol 35: 517–528. [DOI] [PubMed] [Google Scholar]
- 51.Huang K, Yanai A, Kang R, Arstikaitis P, Singaraja RR, et al. (2004) Huntingtin-interacting protein HIP14 is a palmitoyl transferase involved in palmitoylation and trafficking of multiple neuronal proteins. Neuron 44: 977–986. [DOI] [PubMed] [Google Scholar]
- 52.McDonough KA, Rodriguez A (2012) The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol 10: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.